Abstract
Effective treatment of infections in avascular and necrotic tissues can be challenging due to limited penetration into the target tissue and systemic toxicities. Controlled release polymer implants have the potential to achieve the high local concentrations needed while also minimizing systemic exposure. Silk biomaterials possess unique characteristics for antibiotic delivery including biocompatibility, tunable biodegradation, stabilizing effects, water-based processing and diverse material formats. We report on functional release of antibiotics spanning a range of chemical properties from different material formats of silk (films, microspheres, hydrogels, coatings). The release of penicillin and ampicillin from bulk-loaded silk films, drug-loaded silk microspheres suspended in silk hydrogels and bulk-loaded silk hydrogels was investigated and in vivo efficacy of ampicillin-releasing silk hydrogels was demonstrated in a murine infected wound model. Silk sponges with nanofilm coatings were loaded with gentamicin and cefazolin and release was sustained for 5 and 3 days, respectively. The capability of silk antibiotic carriers to sequester, stabilize and then release bioactive antibiotics represents a major advantage over implants and pumps based on liquid drug reservoirs where instability at room or body temperature is limiting. The present studies demonstrate that silk biomaterials represent a novel, customizable antibiotic platform for focal delivery of antibiotics using a range of material formats (injectable to implantable).
Keywords: silk fibroin, biomaterials, antibiotics, drug delivery systems, biological applications of polymers
Antibiotics are an essential component of infection treatment and prevention. In cases where systemic drug penetration into ischemic, avascular and necrotic tissues is limited (including treatment of abscesses,[1–3] bone infections[4] and post-operative tissue), controlled-release polymers have the potential to reduce side effects and increase efficacy by releasing antibiotics locally at a target site of interest.[5–8] Toward this goal, various biodegradable polymeric biomaterials have been explored for antibiotic delivery, including synthetic polymers like poly-(lactide-co-glycolide) and polycaprolactone, and natural polymers like collagen and chitosan. However, these polymer systems suffer from a number of drawbacks including harsh processing conditions or poor biocompatibility in the case of the synthetic polymers, and rapid degradation and poor tunability for the natural polymers.[9–11]
Silk fibroin is a biologically-derived protein polymer purified from domesticated silkworm (Bombyx mori) cocoons and has demonstrated excellent properties for drug delivery, including biocompatibility,[12–16] robust mechanical strength in various materials formats[17] and controllable rates of biodegradation to non-toxic products in vivo.18[18] The degradation time course of silk implants can be controlled from days to years via regulation of beta sheet content (crystallinity).[19–20] Silk can be processed entirely in aqueous systems using mild, ambient conditions of temperature and pressure, allowing the incorporation of sensitive compounds and proteins without loss of bioactivity.[21–22]
A variety of silk fibroin biomaterials have been explored for sustained small molecule delivery, including porous sponges, films and coatings, hydrogels and micro- and nano-particles.[20, 23–24] The release behavior of these compounds from silk carriers is tunable based on manipulation of multiple control points during the process of preparing the silk polymer, including carrier morphology, molecular weight and crystallinity/β-sheet content.[23–24] The combined diversity of material formats for drug delivery, tight control of the drug carrier features, and the unique combination of properties of the silk material (biocompatibility, biodegradation, aqueous processing) result in a broad range of silk based delivery systems for focal antibiotic delivery. Further, silk films exert a remarkable stabilizing effect on encapsulated enzymes[25–26] and antibodies,[27] suggesting improved antibiotic stability derived from encapsulation in silk as another critical benefit in using this protein material system.
The objective of the present study was to demonstrate the utility of silk-based biomaterials to address the need for focal antibiotic delivery by investigating the loading and release of a variety of antibiotics in different silk material formats. Antibiotic release from silk biomaterials was evaluated based on inhibition of Gram negative Escherichia coli ATCC 25922 and gram positive Staphylococcus aureus ATCC 25923, pathogens that are frequently isolated from surgical site infections. Silk material formats studied included bulk loaded silk films, 3D porous sponges, nanofilm coatings, bulk and microsphere loaded hydrogels and degummed silk fibers.
Implants are most susceptible to surface bacterial colonization during a 6-h post-implantation “decisive period,”[8] making the first 24 hours of antibiotic release most critical in the prevention of bacterial adhesion and long-term implant success. The first 24 hours of bacterial inhibition capability were evaluated for silk films loaded with varied concentrations of ampicillin and penicillin (0.4 mg per film and 0.2 mg per film for ampicillin loaded films and 0.8 mg per film and 0.4 mg per film for penicillin loaded films) which were either treated with methanol for 5 minutes or left untreated. No reduction in activity was seen in the methanol treated films compared with the untreated films (Table 1A and 1B), suggesting that methanol treatment did not degrade the incorporated antibiotic. The films delivered approximately half of their initial load of ampicillin within the first 24 hours of bacterial exposure. The fraction of the total load recovered was lower in untreated high loaded films than in methanol treated films, possibly because bioactivity was lost when the film was resolubilized on the agar lawn (as opposed to eluting slowly from insoluble methanol-treated films) (Table 1A and 1B).
Table 1A. Ampicillin Film Release.
High loading = 2 mg mL-1 ampicillin in a 6% (w/v) silk solution (theoretical load = 0.4 mg per film). Low loading = 1 mg mL-1 ampicillin in a 6% (w/v) silk solution (theoretical load = 0.2 mg per film)
| High Loading/Methanol treated | High Loading/Untreated | Low Loading/Methanol treated | Low Loading/Untreated | |
|---|---|---|---|---|
| Average ampicillin release (in μg) | 213.84 ± 50.52 | 176.14 ± 45.49 | 68.47 ± 9.25 | 68.33 ± 22.96 |
| Fraction of total theoretical film load released in the first 24 hours | 0.53 | 0.44 | 0.34 | 0.34 |
Table 1B. Penicillin Film Release.
High loading = 4 mg mL-1 penicillin in a 6% (w/v) silk solution (theoretical load = 0.8 mg per film). Low loading = 1 mg mL-1 penicillin in a 6% (w/v) silk solution (theoretical load = 0.4 mg per film)
| High Loading/Methanol treated | High Loading/Untreated | Low Loading/Methanol treated | Low Loading/Untreated | |
|---|---|---|---|---|
| Average penicillin release (in μg) | 345.87 ± 140.94 | 167.08 ± 24.82 | 193.98 ± 5.03 | 205.88 ± 22.58 |
| Fraction of total theoretical film load released in the first 24 hours | 0.43 | 0.21 | 0.48 | 0.51 |
To determine the minimum inhibitory penicillin loading concentration in silk films, silk solution loaded with varied penicillin concentrations (100 mg mL−1 to 0.013 mg mL−1) was cast into films and exposed to liquid cultures of E. coli and S. aureus. For both bacteria complete inhibition was found in liquid cultures exposed to films prepared with 25, 50, or 100 mg or penicillin per mL of silk (5, 10 and 20 mg of penicillin per film, respectively) (Figure 1). The 0.39 mg mL−1 and 0.05 mg mL−1 concentrations reduced OD600 of the liquid cultures to less than 5% of control culture OD600 to in E. coli and S. aureus, respectively (Figure 1). These results suggest that when prepared with sufficient drug concentrations, silk films can be used to prevent infection and totally suppress bacterial growth. Silk film coatings can achieve high local concentrations that cannot be administered systemically due to side-effects as mentioned earlier.
Figure 1. Antibiotic release from bulk-loaded silk films.
Optical density of S. aureus and E. coli liquid cultures at 600 nm (OD600) after 24 hours incubation at 37°C relative to the concentration of penicillin used in the preparation of antibiotic silk films. N=3, error bars represent standard deviations.
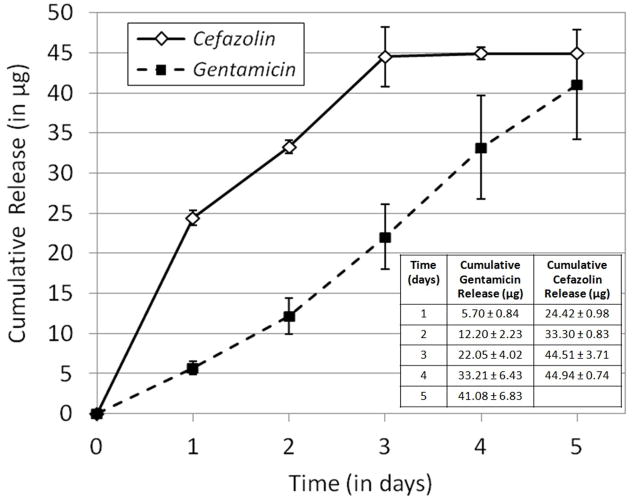
Layer-by-layer nanofilm coatings were studied as a potential strategy for functionalizing porous three-dimensional substrates with water-soluble antibiotics. Release duration was 5 days for gentamicin loaded sponges and 3 days for cefazolin loaded sponges (Figure 2, Table inset in Figure 2). For gentamicin loaded scaffolds, total loading was 107.5 ± 31.0 μg (n = 4 samples) and for cefazolin loaded scaffolds, total loading was 55.5 ± 7.0 μg (n =3 samples). The gentamicin loaded scaffolds released approx. 40 μg over 5 days and the cefazolin loaded scaffolds released approx. 45 μg over 3 days (Figure 2, Table inset in Figure 2). Gentimicin release from sponges determined based on clearance of both S. aureus and E. coli shows good agreement (Supplementary Figure 1). This suggests that nanofilm coatings can be used to achieve constant, sustained release of water-soluble drugs from porous sponge substrates. Both the silk nanofilm layer-by-layer coating process and macroscale bulk-loaded silk films could be useful for building antibiotic release into medical materials with conformal coatings that would benefit from microbial resistance, such as bandages, porous sponge packing materials, tissue engineering scaffolds and implants. Proof-of-concept data on an example application is shown in Supplementary Figure 2. We have previously described the ability to control the release behavior from silk films and nanofilm coating through manipulation of the morphology and silk material properties,[28–29] amplifying the utility of the silk delivery systems to control infections.
Figure 2. Antibiotic release from silk nanofilm coatings on silk sponges.
Cumulative release of gentamicin and cefazolin from nanofilm-coated porous silk sponges on S. aureus lawns. N=3, error bars represent standard deviation.
Systems for injectable delivery (bulk loaded silk hydrogels and silk gels loaded with drug-releasing silk microspheres) were evaluated in vitro and in vivo. Sonication of silk induces sol-gel transition of silk solution to stable, beta-sheet rich, physically crosslinked networks without requiring chemical agents.[30] Sonication-induced silk hydrogels are also characterized by highly tunable gelation behavior: conditions of hydrogel formation can be precisely controlled to ensure the silk solution remains in a liquid state long enough post-sonication to mix in other components, such as cells, microspheres or antibiotic suspensions, and inject to complete the transition to a physically crosslinked hydrogel in vivo. Total loading of the microspheres was approximately 0.5 mg of antibiotic mg of microspheres (encapsulation efficiency (determined as previously described[31]) of approx. 80%). In vitro (zone of inhibition in S. aureus lawns) bulk loaded gels exhausted their penicillin load within 48 hours and ampicillin within 72 hours (Figure 3A and 3B). Concentration of the silk hydrogel (4% (w/v) compared with 8% (w/v)) did not impact penicillin release behavior (Supplementary Figure 3). Microsphere loaded gels released at a lower daily release rate than bulk loaded systems, but exhibited longer release durations (4 days). These two different loading strategies provide different release profiles; rapid release from bulk-loaded hydrogels compared with slower, more sustained release from microspheres suspended in hydrogels. These two injectable delivery formats can be combined to achieve the desired combination of physical properties and release kinetics.
Figure 3. In vitro testing of antibiotic-releasing silk hydrogels.

Cumulative drug release from (A) penicillin and (B) ampicillin-loaded silk gels. Gels prepared either by mixing penicillin or ampicillin into the silk solution post-sonication/pre-gelation (bulk loaded) or by mixing antibiotic-loaded silk microspheres into the silk solution post-sonication/pre-gelation (microsphere loaded). N=3, error bars represent standard deviations. Where error bars are not shown, they fall into background.
The in vivo efficacy of bulk loaded ampicillin releasing silk hydrogels was evaluated in an infected murine wound model. S. aureus infected wounds treated with injections of ampicillin-releasing silk hydrogels were compared with wounds treated with injections of ampicillin in an aqueous solution, wounds which received injections of unloaded silk hydrogel and untreated wounds. For comparison, the CFU count for each treatment was divided by the CFU count for the untreated wound (Figure 4) (individual animal results without normalization are reported in Supplementary Table 1). Wounds treated with ampicillin in solution (AMP+PBS) had CFU counts that averaged 8.1 ± 5.7% of the untreated wound CFU counts. Wounds treated with ampicillin releasing silk hydrogels (AMP+SILK) had CFU counts that averaged 4.8 ± 3.7% of the untreated wound CFU counts, approximately a 20-fold reduction. Wounds receiving unloaded silk hydrogels (SILK ALONE) had CFU counts that averaged 69.5 ± 29.5% of the untreated wound CFU counts. The difference in average CFU count in wounds treated with ampicillin solution and wounds receiving unloaded silk hydrogel was significant (two tailed t-test, df =8, p<0.05), as was the difference in average CFU count in wounds treated with ampicillin releasing silk hydrogel and wounds receiving unloaded silk hydrogel (two tailed t-test, df =8, p<0.01).
Figure 4. In vivo testing of antibiotic-releasing silk hydrogels.

Total S. aureus CFU counts for various wound treatments relative to total S. aureus CFU for untreated wound. Treatment groups shown: AMP+PBS (250 μg mL−1 mL-1 ampicillin in sterile phosphate buffered saline), AMP+SILK (250 μg mL-1 ampicillin bulk loaded into 4% (w/v) silk hydrogel) and SILK ALONE (unloaded 4% (w/v) silk hydrogel). N=5, error bars represent standard deviations. Data analyzed by two-tailed t-test, df =8; significance levels of individual tests are indicated: *P<0.05, **P<0.01.
The reduction in average CFU count for wounds treated with ampicillin-releasing silk hydrogel compared with a local injection of ampicillin in PBS was not statistically significant, but this is an expected result given that the wounds were analyzed 24 hours after treatment (longer durations were not investigated due to the presence of untreated control infections on every mouse). Over a short time frame, the therapeutic benefit of sustained release and/or the presence of filler material might not be detectable; in vitro data suggested bulk loaded silk hydrogels sustained ampicillin release up to 3 days and continued to suppress bacterial growth during that time frame, while the ampicillin in PBS was expected to dissipate rapidly, allowing the bacterial population that survived the initial exposure to re-populate the wound site. Future work will include longer therapeutic time frames, but the preliminary study demonstrated the feasibility of silk hydrogels for injectable antibiotic delivery. In addition, the observation that the total CFU counts for wounds receiving ampicillin injections (either in PBS or silk hydrogel) were reduced compared with the untreated wounds and wounds injected with unloaded silk hydrogel suggests that the antibacterial effect is localized.
Antibiotics with low water solubility are difficult to administer using conventional methods because the high systemic doses required cannot be prepared in aqueous solutions. To test loading and delivery of antibiotics with high methanol solubility and low water solubility from silk biomaterials, rifampicin (log P = 2.7, aqueous solubility = 2.5 mg mL−1) and erythromycin (log P = 1.2, aqueous solubility = 2.0 mg mL−1) were chosen for study. To deliver hydrophobic, alcohol soluble drugs from silk biomaterials a high-concentration solution of the compound of interest is prepared in methanol, then the silk biomaterial substrate is immersed in the drug-saturated methanol. During the methanol soaking, the compound is able to move into the polymer network of the silk until equilibrium is achieved. Once the drug loaded biomaterial is immersed in an aqueous solution, diffusion of the “trapped” drug out of the silk carrier is limited by the drug’s solubility in water. As a result, slow, sustained, constant release is achieved. Figure 5A shows a rifampicin loaded silk fiber after soaking and drying. Figure 5B and 5C show zones of clearance in an S. aureus lawn produced by a rifampicin loaded silk fiber and silk film, respectively. Plots of rifampicin loading versus concentration in the soak solution for silk sponges and silk films are shown in Figure 6A and Figure 6B, respectively.
Figure 5. Bacterial lawn growth inhibition induced by rifampicin-releasing silk biomaterials.

(A) Silk fibers after immersion in a 20 mg mL-1 rifampicin in methanol solution overnight, rinsing in distilled water and drying. (B) Representative sample agar plate showing zone of inhibition in S. aureus lawns produced by rifampicin-releasing silk fibers. (C) Representative sample agar plate showing the zones of inhibition in S. aureus lawns produced by rifampicin-releasing silk film.
Figure 6. Rifampicin loading in silk biomaterials.

(A) Rifampicin loading in silk sponges versus starting rifampicin soaking solution concentration for varied soaking solution solvents (B)Rifampicin loading in silk films versus starting rifampicin concentration in the methanol soak solution for varied film thickness (in mg of silk film)
Rifampicin-loaded sponges exhibited a release duration of 8–9 days (Figure 7B), while films exhibited burst release, >85% of the cumulative release was released within the first 24 hours (Figure 7A). Good agreement was observed between the cumulative release curves generated using ZOI data and the UV-Absorbance data, suggesting drug activity was preserved during loading and the duration of the release study (Figure 7A and 7B). Rifampicin release from films was faster than from sponges or fibers, likely due to the short path length and difference in material diffusivity. Rifampicin loaded silk fibers produced zones of clearance in S. aureus lawns for 8 days (Supplementary Figure 4).
Figure 7. Rifampicin release from silk biomaterials.
(A) Cumulative rifampicin release from silk films and (B) silk sponges. N=3, error bars represent standard deviations, where error bars are not shown they fall into background.
Longer release durations from silk biomaterials can be achieved through selection of the chemical properties of the antibiotic, as we demonstrate with silk sponges loaded with erythromycin (a more alcohol soluble, less water soluble antibiotic than rifampicin). Erythromycin was loaded into porous silk sponges soaked for 24 hours in solutions of erythromycin in methanol (5 mg mL−1, 50 mg mL−1 and 500 mg mL−1). Release was sustained for at least two weeks from the scaffolds prepared from 50 mg mL−1 (Figure 8A). At the highest erythromycin loading (500 mg mL−1 initial methanol soak solution), continuous release was sustained for at least 31 days (Figure 8B). Release rate did not scale linearly with preliminary erythromycin concentration, likely due to drug release being limited by the solubility of erythromycin in water. However, loading did impact release behavior and some amount of early burst release was observed. This suggests that drug concentration in the methanol soaking solution can affect the diffusion gradient and thereby manipulate release behavior, though maxima where increased loading would have no further effect on release rate due to low compound water solubility are anticipated. Different material formats (films vs. porous sponges) and initial drug concentrations in the methanol soaking solution produced different release behavior, which suggests that silk drug carriers can be customized to suit specific applications, including rapid burst release (>85% within the first 24 hours) and sustained, long-term release (up to 31 days).
Figure 8. Erythromycin release from silk biomaterials.

(A) Cumulative erythromycin release from porous silk sponges loaded by soaking in 5 mg mL-1, 50 mg mL-1 and 500 mg mL-1 erythromycin in methanol solutions over 14 days. (B) Release from sponges prepared using the highest loading concentration (500 mg mL-1) over 31 days. N=3, error bars represent standard deviations.
An effective sustained release drug carrier must preserve the bioactivity of the incorporated therapeutic during fabrication, storage and delivery in vivo. As silk can be processed using mild, aqueous processing at ambient conditions, the bioactivity of sensitive therapeutics is preserved during fabrication.[21–22] We found no loss of ampicillin or penicillin activity following the physical crosslinking of silk films (Tables 1A and 1B). Good agreement was observed between release data based on clearance zone and UV-absorbance for rifampicin releasing films and sponges (Figure 7A and 7B), further suggesting that antibiotic bioactivity is preserved during carrier fabrication. In addition to the stabilization of compounds stored in dry silk films we have reported previously,[22,25–26] here we show silk is also capable of exerting dramatic stabilization effects in more challenging conditions: erythromycin is highly unstable in aqueous media,[32] but despite being incubated at 37°C for over a month in a hydrated silk sponge, erythromycin continued to inhibit S. aureus growth for 31 days (Figure 8B).
Effective, controllable loading and release of a range of antibiotics (penicillin, ampicillin, gentamicin, cefazolin, rifampicin and erythromycin) was demonstrated in a variety of silk biomaterials, including fibers, sponges, nanofilm coatings, films, microspheres and hydrogels. The range of loading strategies, material properties, release behavior and antibiotics represented here could be applied to a variety of prophylactic and curative antibiotic therapies. In addition to adding antibiotic-eluting silk coatings to implants (including bone implants and vascular grafts) to prevent infection and biofilm formation, antibiotic-releasing silk biomaterials could be applied at the site of implant removal to aid in wound healing (particularly the highly conformal[33] and injectable, space-filling[34] silk platforms recently described). Silk fibrous materials could be loaded with antibiotics for functionalized sutures, yarns reinforcement meshes, bandages and gauze. Injectable delivery systems can provide minimally invasive local delivery and be used as degradable abscess filler materials to prevent collapse following drainage. As silk has been extensively investigated as a tissue engineering scaffold material,[35] we anticipate these antibiotic delivery strategies could also be interfaced with tissue engineering and regenerative medicine approaches to further improve wound healing outcomes. When the data presented here are combined with the remarkable mechanical features and dramatic stabilization effects of silk materials, a robust stabilization and delivery platform can be envisioned based on this approach, extending into even convenient microneedle formats.[36]
The unique properties of silk, including biocompatibility, tunable biodegradation rate, stabilizing effects, water based processing and diverse material formats make it well suited to antibiotic delivery. The present studies provide data to demonstrate that antibiotics can be controllably released from silk biomaterials to repress local bacteria growth. Multiple loading approaches were demonstrated for a broad range of silk material formats, including bulk loading, microsphere imbedding, methanol-assisted impregnation and nanofilm coating. We anticipate the variety of material formats, release profiles and antibiotics described will have broad applicability in focal antibiotic delivery for the treatment of infections that are currently difficult to prevent or cure with conventional systemic delivery alone.
Materials and Methods
Materials
The bacteria strains used were E. coli ATCC 25922 and S. aureus ATCC 25923 (American Type Culture Collection, Manassas, VA). Antibiotics (penicillin G sodium salt, ampicillin sodium salt, cefazolin, gentamicin, rifampicin, erythromycin and tetracycline) were purchased from Sigma Aldrich (St. Louis, MO). Bacterial culture dishes, BD brand Luria-Bertani broth, Miller (formula per liter = 10 g tryptone, 5 g yeast extract, 10 g sodium chloride), BD brand Luria-Bertani agar, Miller (formula per liter = 10 g tryptone, 5 g yeast extract, 10 g sodium chloride, 15 g agar), Tryptic Soy broth (formula per liter = 17 g pancreatic digest of casein, 3 g papaic digest of soybean, 2.5 g dextrose, 5 g sodium chloride, 2.5 g dipotassium phosphate) and Tryptic Soy agar (formula per liter = 15 g pancreatic digest of casein, 5 g papaic digest of soybean, 5 g sodium chloride, 15 g agar) were purchased from Fisher Scientific (Pittsburgh, PA).
Silk solution and materials preparation
Silk fibroin solution was prepared from B. mori cocoons as we have previously described.[37] Briefly, cocoons were boiled for either 30 min or 60 min in a solution of Na2CO3 (0.02 M) and rinsed, then dried at ambient conditions overnight. The dried fibroin was solubilized in a aqueous LiBr (9.3 M) solution at 60°C for 2–4 h, yielding a 20% (w/v) solution. LiBr was then removed from the silk by dialyzing the solution against distilled water for 2.5 days using Slide-a-Lyzer dialysis cassettes (MWCO 3,500, Pierce Thermo Scientific Inc., Rockford, IL). Silk fibroin concentration was determined by evaporating water from a solution sample of known volume and massing using an analytical balance. Silk solutions were stored at 4–7°C before use.
Bulk antibiotic loaded silk films were prepared as previously described.[28] Microspheres were prepared according to the phospholipid template protocol previously described.[31] Water-based porous scaffolds were prepared as we have previously described.[38] Nanofilm coatings were applied using our previously described protocol,[29] modified to accommodate the coating of a 3D porous scaffold.[39] Silk hydrogels were prepared using sonication-induced gelation as we have previously described.[30] For additional details on fabrication of antibiotic loaded silk films, silk microspheres, water-based porous scaffold, silk nanofilm coatings and silk hydrogels, see Supplement.
Bacteria culture
Luria Bertani (LB) and Tryptic Soy Broth were prepared according to manufacturer’s instructions and aliquoted into 100 mm diameter Fisherbrand cell culture plates (15–20 mL per plate). Lyophilized bacteria cultures were reconstituted and expanded according to instructions provided by ATCC. To test susceptibility, liquid cultures were grown for 18–24 hours to an optical density (OD600) between 0.8 and 1 (corresponding to a viable count of approx. 107–108CFU mL−1). These cultures are referred to hereafter as “overnight cultures.”
Susceptibility testing
Active antibiotic release was quantified using a direct zone of inhibition assay based on the principle of the Kirby-Bauer Susceptibility Test;[40–41] active antibiotic is quantified by comparing zones of clearance in bacterial lawns with the zones of clearance generated by standards of known antibiotic concentration. Samples (either silk biomaterials directly, sterile filter paper disks wetted with known volumes of a standard solution of known concentration or sterile filter paper disks wetted with known volumes of experimental solution of unknown concentration) were applied directly to agar plates coated with a mixture of dilute agar (half the recommended mass of powder per liter, or 20 g L−1) and S. aureus overnight culture (5:1 volume:volume ratio), which grows to lawns overnight at 37°C. After 24 hours the zone of inhibition was measured using Image J image analysis software. The amount of drug released over 24 hours was then determined by comparison of sample zones of inhibition to the zones of inhibition of known standards on filter paper disks. Extended release studies were carried out by transferring test materials to new plates every 24 hours.
Release testing
In addition to quantifying rifampicin release from silk films and sponges using zone of clearance susceptibility testing, rifampicin release was also determined by immersing drug loaded silk materials in Dulbecco’s PBS (0.5 mL) at 37°C, removing and replacing the buffer at desired time points, and determining the amount of rifampicin released into the buffer using UV-V is spectroscopy at 475 nm.
In vivo testing
All animal studies were conducted under protocols reviewed and approved by the Tufts University IACUC. Male BALB/c mice weighing 20–25 g were shaved on the back and depilated with Nair (Carter-Wallace Inc., New York, NY). Mice were anesthetized with an IP injection of ketamine/xylazine cocktail (90 mg/kg ketamine, 10 mg/kg xylazine) for surgery and infection. The operative area of skin was cleaned with alcohol, and subcutaneous injections containing live bacteria were given at four different sites on the shaved back of each animal. Each 100 μL injection contained 1 × 106 colony forming units (CFU) of S. aureus. After 24 hours, raised bumps were observed at the four injection sites. Each animal (n=5) received four treatments: one wound was untreated (control), one wound received an injection of ampicillin in sterile saline (100 μL, 250 μL mL−1), one wound received an injection of unloaded silk hydrogel (4% (w/v), 100 μL) and one wound received an injection of silk hydrogel (4% (w/v)) loaded with ampicillin (100 μL, 250 μL mL−1). Ampicillin concentration was selected based on the maximum MIC of ampicillin reported against clinical isolates of methicillin-resistant S. aureus (MRSA) (approx. 256 mg L−1).[42–43] Twenty-four hours after treatment (48 hours post-inoculation), animals were anesthetized with an IP injection of ketamine/xylazine cocktail (90 mg kg−1 ketamine, 10 mg kg−1 xylazine) and the infected tissue and surrounding tissues were excised and transferred to sterile 50-ml Falcon tubes containing sterile saline (10 mL). Animals were euthanized by carbon dioxide asphyxiation.
Excised tissue samples were homogenized using a T25 basic Ultra Turrax mechanical homeogenizer (IKA Works, Inc., Wilmington, NC). Bacteria in the homogenate were estimated by standard plate count methods[44] using Tryptic Soy agar plates. Colonies were counted after 24 hr of incubation at 37°C. The bacterial count was expressed as the number of colony forming units (CFU) per wound. To normalize for variability among the mice, CFU counts for each treatment modality were divided by the CFU count measured for the untreated wound and reported as a percentage.
Supplementary Material
Acknowledgments
This work was supported by the NIH (EB002520-05, EY020856, DE017207-01, EB003210) and AFOSR 9550-10-1-0172zsAJMn. The authors would like to thank Nikola Kojic and Reid McCabe for their assistance.
Footnotes
Author Contributions
Eleanor M. Pritchard and Thomas Valentin carried out in vitro release testing, storage stability testing and the majority of the writing. Bruce Panilaitis advised on in vivo experimental design and performed all aspects of the in vivo study involving animal handling. Fiorenzo Omenetto and David L. Kaplan oversaw all research and advised on experimental design and interpretation.
Competing Financial Interest Statement
The authors have no competing financial interests to declare.
References
- 1.Sauermann R, Karch R, Langenberger H, Kettenbach J, Mayer-Helm B, Petsch M, Wagner C, Sautner T, Gattringer R, Karanikas G, Joukhadar C. Antimicrob agentsch. 2005;49:4448. doi: 10.1128/AAC.49.11.4448-4454.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerding DN, Kozak AJ, Peterson LR, Hall WH. Antimicrob agentsch. 1980;17:1023. doi: 10.1128/aac.17.6.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han G, Martinez LR, Mihu MR, Friedman AJ, Friedman JM, Nosanchuk JD. PLoS One. 2009;4:e7804. doi: 10.1371/journal.pone.0007804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nandi SK, Mukherjee P, Roy S, Kundu B, De DK, Basu D. Mater Sci Eng C. 2009;29:2478. [Google Scholar]
- 5.Englander L, Friedman A. J Clin Aesthet Dermatol. 2010;3:45. [PMC free article] [PubMed] [Google Scholar]
- 6.Price JS, Tencer AF, Arm DM, Bohach GA. J Biomed Mater Res. 1996;30:281. doi: 10.1002/(SICI)1097-4636(199603)30:3<281::AID-JBM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 7.Ruszczak Z, Friess W. Adv Drug Deliv Rev. 2003;55:1679. doi: 10.1016/j.addr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Zilberman M, Elsner JJ. J Control Release. 2008;130:202. doi: 10.1016/j.jconrel.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Puppi D, Chiellini F, Piras AM, Chiellini E. Prog Polym Sci. 2010;35:403. [Google Scholar]
- 10.Siepmann F, Siepmann J, Walther M, MacRae RJ, Bodmeier R. J Control Release. 2008;125:1. doi: 10.1016/j.jconrel.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Abletshauser CB, Schneider R, Rupprecht H. J Control Release. 1993;21:149. [Google Scholar]
- 12.Leal-Egaña A, Scheibel T. Biotechnol Appl Biochem. 2010;55:155. doi: 10.1042/BA20090229. [DOI] [PubMed] [Google Scholar]
- 13.Tang X, Ding F, Yang Y, Hu N, Gi X. J Biomed Mater Res A. 2009;91A:166. doi: 10.1002/jbm.a.32212. [DOI] [PubMed] [Google Scholar]
- 14.Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, Zichner L, Langer R, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2005;26:147. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 15.Seo YK, Yoon HH, Song KY, Kwon SY, Lee HS, Park YS, Park JK. J Orthop Res. 2009;27:495. doi: 10.1002/jor.20752. [DOI] [PubMed] [Google Scholar]
- 16.Panilaitis B, Altman GH, Chen J, Jin HJ, Karageorgiou V, Kaplan DL. Biomaterials. 2003;24:3079. doi: 10.1016/s0142-9612(03)00158-3. [DOI] [PubMed] [Google Scholar]
- 17.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Biomaterials. 2003;24:401. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. Biomaterials. 2008;29:3415. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. Biomaterials. 2005;26:3385. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Numata K, Kaplan DL. Adv Drug Deliv Rev. 2010;62:1497. doi: 10.1016/j.addr.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vepari C, Kaplan DL. Prog Polym Sci. 2007;32:991. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence BD, Cronin-Golomb M, Georgakoudi I, Kaplan DL, Omenetto FG. Biomacromolecules. 2008;9:1214. doi: 10.1021/bm701235f. [DOI] [PubMed] [Google Scholar]
- 23.Pritchard EM, Kaplan DL. Expert Opin Drug Deliv. 2011;8:797. doi: 10.1517/17425247.2011.568936. [DOI] [PubMed] [Google Scholar]
- 24.Wenk E, Merkle HP, Meinel L. J Control Release. 2011;150:128. doi: 10.1016/j.jconrel.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, Kaplan DL. Biomacromolecules. 2009;10:1032. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Q, Wang X, Hu X, Cebe P, Omenetto F, Kaplan DL. Macromol Biosci. 2010;10:359. doi: 10.1002/mabi.200900388. [DOI] [PubMed] [Google Scholar]
- 27.Guziewicz N, Best A, Perez-Ramirez B, Kaplan DL. Biomaterials. 2011;32:2642. doi: 10.1016/j.biomaterials.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofmann S, Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. J Control Release. 2006;111:219. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Hu X, Daley A, Rabotyagova O, Cebe P, Kaplan DL. J Control Release. 2007;121:190. doi: 10.1016/j.jconrel.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Kluge JA, Leisk GG, Kaplan DL. Biomaterials. 2008;29:1054. doi: 10.1016/j.biomaterials.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. J Control Release. 2007;117:360. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 32.Brisaert M, Heylen M, Plaizier-Vercammen J. Pharm World Sci. 1996;18:182. doi: 10.1007/BF00820730. [DOI] [PubMed] [Google Scholar]
- 33.Kim D-H, Viventi J, Ams JJ, Xiao J, Vigeland L, Kim Y-S, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang K-C, Zakin MR, Litt B, Rogers JA. Nature Mater. 2010;9:511. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Wang X, Wang S, Zhao J, Xu L, Zhu C, Zeng D, Chen J, Zhang Z, Kaplan DL, Jiang X. Biomaterials. 2011;32:9415. doi: 10.1016/j.biomaterials.2011.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Kim H-J, Vunjak-Novakovic G, Kaplan DL. Biomaterials. 2006;2:6064. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 36.Tsioris K, Raja WK, Pritchard EM, Panilaitis B, Kaplan DL, Omenetto FG. Adv Mater. 2012;22:330. [Google Scholar]
- 37.Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. J Biomed Mater Res. 2001;54:139. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Biomaterials. 2005;26:2775. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- 39.Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Biomaterials. 2008;29:3609. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer AW, Perry DM, Kirby WMM. Arch Int Med. 1959;104:208. doi: 10.1001/archinte.1959.00270080034004. [DOI] [PubMed] [Google Scholar]
- 41.Boyle VJ, Fancher ME, Ross RW. Antimicrob Ag Chemother. 1973;3:418. doi: 10.1128/aac.3.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu ZQ, Zhao W-H, Hara Y, Shimamura T. J Antimicrob Chemother. 2001;28:361. doi: 10.1093/jac/48.3.361. [DOI] [PubMed] [Google Scholar]
- 43.Braga LC, Leite AA, Xavier KG, Takahashi JA, Bemquerer MP, Chartone-Souza E, Nascimento AM. Can J Microbiol. 2005;51:541. doi: 10.1139/w05-022. [DOI] [PubMed] [Google Scholar]
- 44.Saymen DG, Nathan P, Holder IA, Hill EO, Macmillan BG. Appl Microbiol. 1972;23:509. doi: 10.1128/am.23.3.509-514.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.