Abstract
Upregulation of N-methyl D-aspartate (NMDA) receptor function by the non-receptor protein tyrosine kinase Src has been implicated in physiological plasticity at glutamatergic synapses. Here, we highlight recent findings which suggest that aberrant Src upregulation of NMDA receptors may also be key in pathophysiological conditions. Within the nociceptive processing network in the dorsal horn of the spinal cord pathologically increased Src-upregulation of NMDA receptors is critical for pain hypersensitivity in models of chronic inflammatory and neuropathic pain. On the other hand, in the hippocampus and prefrontal cortex the physiological upregulation of NMDA receptors by Src is blocked by neuregulin 1- ErbB4 signaling, a pathway genetically implicated in the positive symptoms of schizophrenia. Thus, either over- or under-upregulation of NMDA receptors by Src may lead to pathological conditions in the central nervous system. Therefore, normalizing Src upregulation of NMDA receptors may be a novel therapeutic approach for CNS disorders, an approach without the deleterious consequences of directly blocking NMDA receptors.
Keywords: glutamate receptors, tyrosine kinase, tyrosine phosphatase, synaptic plasticity, spinal cord dorsal horn, hippocampus, prefrontal cortex
Introduction
N-methyl D-aspartate receptors (NMDARs) comprise one of the principal types of ionotropic glutamate receptor mediating fast excitatory synaptic transmission in the central nervous system (CNS). Abundant evidence indicates that NMDARs have critical roles in a diversity of physiological and pathological processes in the CNS [1;2]. The ion channel pore of the NMDAR is permeable to monovalent cations such as Na+ and K+ and to divalent cations, particularly Ca2+, and is blocked in a voltage-dependent manner by Mg2+ [3]. The pore is at the core of a heterotetrameric complex consisting of two GluN1 subunits together with two GluN2A, B, C or D subunits [2]. NMDARs are co-receptors for glutamate and glycine: glutamate binds to the GluN2 subunits and glycine to the GluN1 subunits inducing a conformational change that opens the pore conductance pathway. The subunit proteins are at the center of a multiprotein NMDAR complex comprised of signaling, scaffolding, and regulatory proteins [4], as well as auxiliary subunits [5]. Proteins within the NMDAR complex serve a diversity of functions including targeting the receptors to synaptic or extra-synaptic sites, regulating channel activity, trafficking and internalization of the receptors, and scaffolding signaling proteins that are downstream of current flow through the receptor. Thus, NMDAR activity, localization and signaling are highly regulated and tightly controlled.
Bi-directional regulation of NMDA receptor function by tyrosine phosphorylation-dephosphorylation
The activity of NMDARs is not just a direct readout of ligand binding but rather the activity is dynamically controlled by intracellular signaling pathways, as was first established through the discovery that NMDARs are upregulated by phosphorylation and down-regulated by dephosphorylation [6]. Subsequently, it was established that NMDAR channel function is subject to regulation by activity of serine/threonine kinases and phosphatases [7;8] and of protein tyrosine kinases (PTKs) and phosphotyrosine phosphatases (PTPs) [9;10]. Electrophysiologicial recordings from neurons showed that NMDAR currents are governed by a balance between phosphorylation and dephosphorylation: inhibiting endogenous PTK activity [9] or increasing PTP activity by introducing exogenous PTP [11] leads to suppression of NMDAR currents, and conversely, inhibiting endogenous PTP activity or increasing PTK activity by introducing exogenous Src causes enhancement of NMDAR currents [9]. Furthermore, exogenous Src or Fyn were found to potentiate currents mediated by recombinant NMDARs expressed in HEK293 cells [12;13]. From recordings of NMDAR single channel currents, the predominant effect of PTK activity, or of inhibiting PTPs, was found to be to increase NMDAR channel gating with no effect on NMDAR single channel conductance [11;14]. Moreover, because the effects of manipulating PTKs and PTPs were present with NMDARs in excised membrane patches, the PTK and PTP must be intimately associated with the NMDAR complex.
While these studies also showed that exogenous Src family kinases (SFKs) are sufficient to enhance NMDAR channel gating, further work was required to identify the principal endogenous PTK. Convergent lines of biochemical and electrophysiological evidence led to the conclusion that this PTK is Src kinase [14]. SFKs were implicated as endogenous enzymes that upregulate NMDAR activity through the use of a phosphopeptide SFK activator (EPQ(pY)EEIPIA peptide), which is a ligand for SFK SH2 domains, and a SFK-family function-blocking antibody (anti-cst1), which inhibits SFKs but not other PTKs. Src itself was implicated through the use of anti-src1, an inhibitory antibody, and Src40-58, an inhibitory peptide [14] that selectively inhibit this kinase but not other members of the Src kinase family. Each of these Src-specific inhibitors decreases synaptic NMDAR-mediated currents, and each produces a decrease in NMDAR channel gating. Subsequently, it was found that Src sensitizes NMDARs to intracellular Na+ [15] linking the function of these receptors to neuronal activity. More recently, it was discovered that Src is anchored in this complex via binding to the protein NADH dehydrogenase 2 (ND2) [16], previously considered only to be a mitochondrial protein.
Within the NMDAR complex, Src kinase activity itself is critically regulated through the C-terminal tyrosine phosphorylation site, which is controlled through the balance of activity of R-PTPα [17] and Csk [18], which themselves are subject to regulation. In addition, some additional molecules identified in other systems that regulate Src activity also play important roles in the regulation of Src within the NMDAR complex and include the tyrosine kinase CAKβ/Pyk2 [19]. In addition to these well-characterized regulators of Src, three PSD proteins were identified to modulate Src within the NMDAR complex: RACK1[20], H-Ras [21] and PSD-95 [22]. A variety of signaling pathways have been shown by several labs to converge on Src to enhance NMDAR function and thus Src functions as a key regulatory hub in the NMDAR complex [23].
NMDAR function is not regulated by Src alone but by the balance between the activity of Src kinase and that of a protein tyrosine phosphatase which depresses NMDAR gating, reversing the effects of Src. Inhibiting PTPs pharmacologically increases NMDAR channel gating in excised membrane patches [11] and PTP activity co-immunoprecipitates with NMDARs [24] indicating that the endogenous PTP is intrinsic to the NMDAR complex. One family of PTPs that has been observed at the PSD of glutamatergic synapses is the STEP (striatal enriched tyrosine phosphatase) family [25], a family of brain-specific, non-receptor type PTPs [26]. The STEP61 isoform has been found to be a component of the NMDAR complex in spinal cord and hippocampus [27] and, therefore, is appropriately located to downregulate NMDAR function. Applying recombinant STEP to the cytoplasmic aspect of inside-out membrane patches suppressed NMDA channel gating, mimicking the effect of inhibiting Src. Similarly, recombinant STEP applied intracellularly reduced synaptic NMDAR currents. In contrast, intracellular application of a function-blocking STEP antibody or of a dominant-negative STEP produced an increase in synaptic NMDAR-mediated currents, implying that NMDAR activity is regulated by endogenous STEP. Both the reduction of NMDAR currents produced by exogenous STEP and the increase of NMDAR currents that resulted from inhibiting endogenous STEP required Src because the changes were prevented by pharmacologically inhibiting Src activity [27]. Thus, NMDAR channel function is dynamically controlled through relative activities of Src and STEP within the NMDAR complex, the activities of these enzymes themselves being subject to regulation.
Src enhancement of NMDA receptors in long-term potentiation
Long-term potentiation (LTP) refers to a group of forms of lasting enhancement of synaptic transmission, and is the predominant cellular model of learning and memory processes [28]. It is clear that induction of one main form of LTP, that is exemplified at Schaffer collateral-CA1 synapses in the hippocampus, requires substantially enhanced entry of Ca2+ through NMDARs. Depolarization-induced reduction of Mg2+-inhibition of NMDAR currents is a commonly accepted mechanism for induction of NMDAR-dependent LTP but relief of Mg2+ blockade alone may not be sufficient [29;30]. Rather, additional mechanisms to boost NMDAR currents, such as by stimulating signaling cascades, are also needed. When such cascades are engaged as a result of synaptic activity they provide a biochemical form of coincidence detection, a hallmark of synaptic theories of learning and memory, analogous to postsynaptic depolarization.
Signaling by SFKs and in particular Src itself are known to play a critical role in the induction of LTP [31;32]. Consistent with this role for SFKs is that the level of phosphorylation of Y1472 of NR2B has been found to be increased following tetanic stimulation in CA1 hippocampus [33]. Previously, tyrosine phosphorylation of GluN2B had been found to increase after LTP induction in the dentate gyrus in the hippocampus [34]. Consistent with involvement of SFK-mediated upregulation of NMDARs in LTP induction, induction of LTP in hippocampal CA1 neurons is prevented by inhibiting endogenous PTPa activity with the intracellular application of an inhibitory antibody [17]. LTP induction in CA1 hippocampus was found to be impaired in mice with a targeted deletion of PTPa and this was associated with a reduction in phosphorylation levels of Y1472 in the GluN2B C-tail in Ptpra−/− mice [35].
Looking at activating pathways upstream of Src, induction of LTP in hippocampal CA1 neurons is prevented by blocking CAKβ using a dominant negative mutant [19]. Conversely, administering CAKβ into CA1 neurons produces a lasting enhancement of AMPAR (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor) synaptic responses, mimicking and occluding LTP. This CAKβ-stimulated enhancement of synaptic AMPAR responses is prevented by blocking NMDARs, chelating intracellular Ca2+, or blocking Src. Importantly, NMDAR currents in CA1 neurons are not tonically upregulated by CAKβ-Src signaling. Rather, CAKβ, as well as Src, become activated by stimulation that produced LTP [19;36]. Evidence for the role of a Ras-Src cascade in LTP is that H-Ras−/− mice display increased PTK activity, increased tyrosine phosphorylation of GluN2A and GluN2B, and enhanced LTP in the hippocampus, [37].
In opposition to Src, STEP has also been implicated in the induction of LTP [27]. In hippocampal slices, administering STEP into CA1 neurons does not affect basal glutamatergic transmission but prevents induction of LTP. Conversely, inhibiting endogenous STEP activity with an inhibitory antibody in CA1 neurons enhanced transmission and occluded LTP induction through a mechanism dependent on NMDARs, Ca2+, and Src [27]. Thus, it has been hypothesized [29] that LTP-inducting synaptic presynaptic stimulation rapidly activates CAKβ post-synaptically which associates with and thereby activates Src, overcoming the tonic suppression of NMDAR function by STEP. This kinase-dependent upregulation may be further amplified by the rise in intracellular [Na+] that occurs during high levels of activity, as Src kinases not only increase NMDAR function they also sensitize the channels to potentiation by Na+ [15]. Coupled with depolarization-induced reduction of Mg2+ inhibition there is a dramatic boost in the influx of Ca2+ through NMDARs which sets in motion the downstream cascade that ultimately results in potentiation of synaptic AMPAR responses either by recruiting new AMPARs to the synapse or by phosphorylating existing AMPARs. The potential for involvement of SFKs in LTP has been investigated in mice with targeted deletions of these kinases. Mutant mice lacking Fyn show blunted LTP in CA1 [31] as do mice lacking Src [38].
Src enhancement of NMDA receptors is critical for hypersensitivity in chronic pain models
Chronic pain has been labeled the silent health crisis with untreated or undertreated pain being the major cause of disability that impairs quality of life [39]. The great paradox of pain is that acute pain is a necessary defense mechanism that warns against existing or imminent damage to the body, whereas chronic pain may be so deleterious that individuals may prefer death to an existence of suffering. As a defense mechanism, acute pain is essential for survival and there has been strong evolutionary pressure for organisms to detect damaging or potentially damaging (nociceptive) stimuli in the external or internal bodily environment. By contrast, chronic pain serves no known defensive, or any other helpful, function. Neither the intensity nor the quality of chronic pain is obviously related to tissue damage, and indeed chronic pain may persist long after any tissue damage, which may have caused acute pain, has abated. As such, chronic pain has a fundamentally different neurobiological basis than does acute pain; while acute pain is produced by the physiological functioning of the normal nervous system, chronic pain is a reflection of aberrant functioning of a pathologically altered nervous system.
There are two principal types of chronic pain – inflammatory pain and neuropathic pain [40]. Inflammatory pain is initiated by tissue damage/inflammation and neuropathic pain by nervous system lesions. Inflammatory pain hypersensitivity usually returns to normal if the disease process is controlled whereas neuropathic pain persists long after the initiating event has healed. Both types of chronic pain are characterized by hypersensitivity at the site of damage and in adjacent normal tissue. Chronic pain reflects not only increases in the sensory input into the spinal cord, but also pathological amplification of these inputs within the nociceptive processing networks in the CNS [40;41]. The somatosensory gateway in the CNS is in the spinal cord dorsal horn, which is not a simple relay station. Rather, it is a complex nociceptive processing network through which inputs from the periphery are transduced and modulated by local, as well as descending, excitatory and inhibitory control mechanisms [41]. The output of this network is transmitted to areas of the CNS involved in sensory, emotional, autonomic and motor processing. Normally, the output is balanced by excitatory and inhibitory processes. But in pathological pain states the output of the dorsal horn nociceptive network is greatly increased. Major mechanisms for increased output are: i) enhancing excitatory synaptic transmission, via NMDARs [42] or ii) suppressing inhibitory mechanisms mediated by GABA and glycine receptors [43].
Upregulation of NMDARs appears to be crucial for the initiation and maintenance of the enhanced responsiveness of nociceptive neurons in the dorsal horn of the spinal cord that occurs in experimental pain models [40]. Peripheral inflammation [44] and nerve injury [45] alters NMDAR-mediated currents in superficial dorsal horn neurons. Peripheral nerve injury increases the amplitude and slows the decay phase of NMDA excitatory post-synaptic currents (EPSCs) [46], and produces prolonged facilitation of membrane currents and calcium transient induced by bath application of NMDA [45], thus potentiating glutamatergic transmission. In the dorsal horn, glutamatergic transmission might be potentiated homosynaptically, as in CA1, although the predominant form of enhancement of synaptic transmission is heterosynaptic [47]. As in CA1, NMDARs in dorsal horn neurons are regulated by CAKβ –Src signaling balanced by STEP activity in vitro. In vivo, tyrosine phosphorylation of NR2B in the spinal cord increases with models of inflammatory [48] and neuropathic pain [49]. Furthermore, peripheral nerve injury activates SFKs in lumbar spinal cord [50], and intrathecal administration of PP2, a non-selective SFK inhibitor, suppresses mechanical hypersensitivity in nerve injured mice, suggesting a role of SFK in neuropathic pain.
From studies using mice with deletion of specific SFK genes, it is known that Src [51], Fyn [49] and Lyn [52] are each essential for the development of neuropathic pain, as there is a deficit in peripheral nerve injury-induced mechanical hypersensitivity in mice lacking each of these genes. However, the role of these SFKs in neuropathic pain may be different. Spinal cord dorsal horn NR2B phosphorylation induced by peripheral nerve injury is reduced in both Src and Fyn mutant mice, indicating that NMDARs are downstream of Src and Fyn. However, Lyn is predominantly activated in microglia following PNI, and the upregulation of the ionotropic purinoceptor P2X4 in microglia is deficient in Lyn null mutant mice. As multiple signaling pathways converge on SFK in synaptic transmission [23], SFK dependent NMDAR upregulation may also serve as a convergence point in development and maintenance of chronic pain. For example, activation of EphB in the spinal cord with ephrinB2 results in prolonged hyperalgesia [53], while inhibition of EphB reduces chronic inflammatory [53] and neuropathic pain [54]. EphB activation induces phosphorylation of SFKs, resulting in phosphorylation of NR2B and amplifying NMDAR responses[23]. So, the convergence of multiple signaling pathways on SFKs allows both homosynaptic and heterosynaptic plasticity in the dorsal horn, which are likely mediated through upregulation of NMDARs by these kinases.
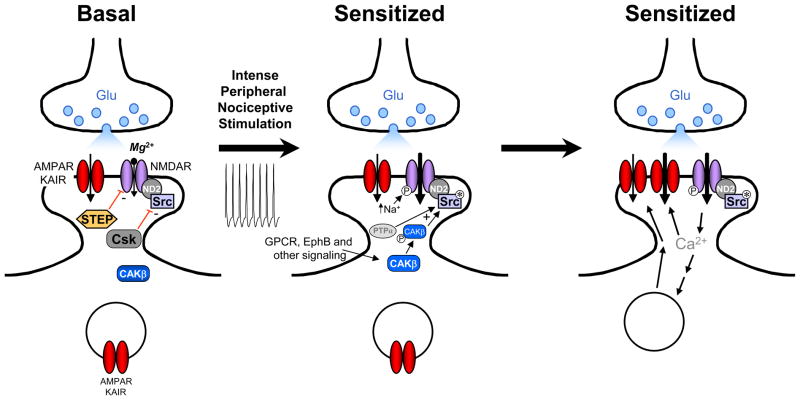
We have tested the involvement of Src-dependent phosphorylation of NMDARs in both inflammatory pain and neuropathic pain [16] by using a 10-amino-acid peptide derived from Src unique domain fused with the protein transduction domain of HIV Tat protein (Src40-49Tat), rendering the peptide membrane permeant [55]. Src40-49Tat was found to uncouple Src from ND2 anchoring within the NMDAR complex, thereby inhibiting Src-mediated upregulation of NMDARs [16]. Administering Src40-49Tat reverses inflammation- and peripheral nerve injury (PNI)-induced mechanical, thermal and cold pain hypersensitivity, without changing basal sensory thresholds and acute nociception. Furthermore, no confounding effects such as sedation, motor deficit or learning and memory impairment were observed at doses that suppress pain hypersensitivity. Importantly, there was no further depression of neuropathic or inflammation-evoked pain hypersensitivity behaviours in the Src null mice by Src40-49Tat, implying that the effect of the peptide was occluded in these animals. These findings indicate that the Src-ND2-NMDAR interaction can be interrupted in vivo, and that uncoupling Src from the NMDAR complex prevents phosphorylation-mediated enhancement of these receptors, and thereby inhibits pain hypersensitivity while avoiding the deleterious consequence of directly blocking NMDARs [1]. Thus, we hypothesize that upregulation of NMDAR function via activating signaling pathways that converge onto Src are critical in chronic pain (Figure 1).
Figure 1.
A model for the role of sensitization of nociceptive dorsal horn neuron in pain hypersensitivity. Left; under basal conditions NMDAR activity is suppressed by partial blockade of the channel by Mg2+ and by the activity of the protein tyrosine phosphatase, STEP, and the kinase, Csk. KAIR, kainate receptor. Middle; nociceptive input increases NMDAR-mediated currents by 1) relief of Mg2+ inhibition, 2) by activation of Src (Src*) via the actions of PTPa and activated CAKβ (CAKβ-P) which overcomes the suppression by STEP, and 3) by sensitizing the NMDARs to raised intracellular [Na+]. Right; upregulation of NMDAR function allows a large boost in entry of Ca2+, which binds to calmodulin (CaM) causing activation of CaMKII, not illustrated. The enhancement of glutamatergic transmission is ultimately expressed through increased numbers of AMPA/KAIRs in the postsynaptic membrane and/or enhanced AMPA/KAIR activity.
Suppression of Src enhancement of NMDA receptors by schizophrenia risk pathway neuregulin-ErbB4
Schizophrenia is a chronic and severely debilitating psychiatric illness that affects approximately 1% of the population worldwide and is characterized by hallucinations, thought disorders, deficits in attention and memory, social withdrawal and impaired social tasks [56]. Although schizophrenia is among the most prevalent of CNS disorders with one of the highest heritabilities of approximately 80% [57] it continues to be one of the least understood. A prominent mechanistic hypothesis is that hypofunction of NMDARs may underlie several of the core psychopathological features of schizophrenia including hallucinations and cognitive dysfunction [58]. The NMDAR hypofunction hypothesis of schizophrenia was based originally upon clinical observations of chronic abusers of the NMDAR antagonist phencyclidine (PCP) who have symptoms similar to those observed in schizophrenia, PCP exposure elicits thought disorder, memory disturbances, and hallucinations [59]. The observation that acute administration of another NMDA receptor antagonist, ketamine, induces similar psychopathological effects in normal healthy volunteers supports the concept that hypofunction of NMDA receptors may play a critical role in the pathophysiological phenomena observed in schizophrenia. On the surface the NMDAR hypofunction hypothesis appears reasonable as hallucinations and cognitive deficits are produced in otherwise normal adults by administering NMDAR blocking drugs. However, NMDARs are multiprotein complexes with phosphorylation-dependent and- independent functional states that are not discriminated with NMDAR blocking drugs. Thus, it may be that the pathophysiology of schizophrenia involves reduction of basal NMDAR function or of phosphorylation-mediated enhancement of NMDAR function. Evidence pointing to the latter comes from work of Hahn and colleagues who have demonstrated that the level of tyrosine phosphorylation of NMDAR subunit proteins is reduced in schizophrenics as compared with controls [60].
Amongst the genes implicated in schizophrenia are Nrg1 and Erbb4 [61] which encode the ligand-receptor pair neuregulin1 (NRG1) and ErbB4, respectively. In studies of schizophrenic individuals, NRG1 expression is increased in both the cortex [62] and hippocampus [63], where NRG1-ErbB4 signaling is excessive [60]. In the mouse, behaviour considered to correspond to that in schizophrenia in humans is found in animals overexpressing NRG1 selectively in the brain [64;64]. NRG1β, a soluble form of NRG1, has been found to block NMDAR-dependent LTP at Schaffer collateral-CA1 synapses [65–67]. Therefore, recently we investigated the effect of NRG1β-ErbB4 signaling on Src-mediated enhancement of synaptic NMDAR function and LTP at these synapses [38]. We also examined NMDAR synaptic responses in the prefrontal cortex, as both brain regions are considered critical in the cognitive dysfunction in schizophrenia.
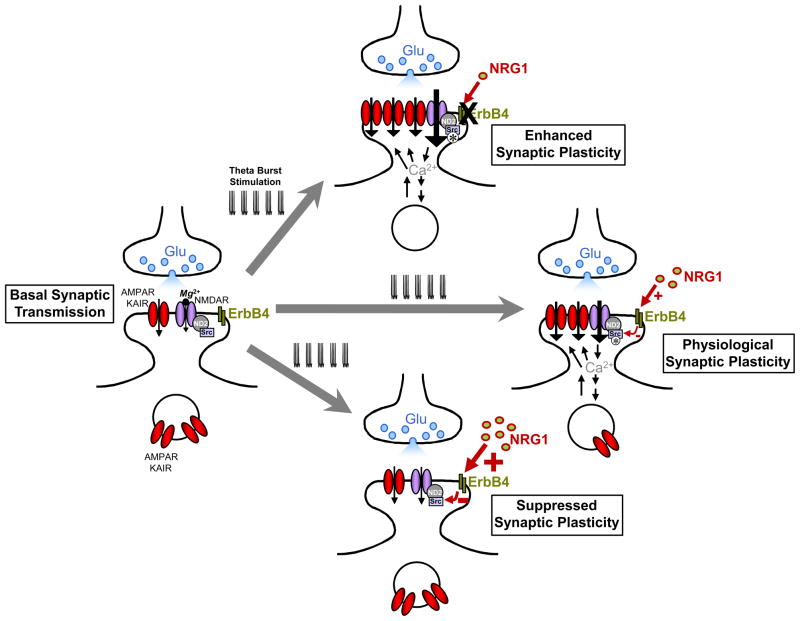
Although NRG1β-ErbB4 signaling had no effect on amplitude, time course or voltage-dependence of basal NMDAR excitatory post-synaptic currents (EPSCs), the enhancement of NMDAR EPSCs by the Src activating peptide, EPQ(pY)EEIPIA administered directly into the neurons, was blocked by NRG1β in CA1 hippocampus and prefrontal cortex. Moreover, NRG1β blocked the induction, but not the maintenance of LTP, induced by theta burst stimulation (TBS) of Schaffer collateral inputs to CA1. NRB1β also inhibited TBS-induced activation of Src and the Src-dependent increase in tyrosine phosphorylation of GluN2B. The most simple model to account for these findings is that NRG1-ErbB4 signaling leads to inhibition of the catalytic activity of Src kinase thereby blocking the enhancement of NMDAR function. As a consequence this signaling prevents downstream events that require Src-mediated enhancement of NMDARs, in the case of CA1 neurons this being preventing the induction of LTP at Schaffer collateral synapses (Figure 2).
Figure 2.
A model for regulation of LTP induction in the hippocampus by NRG1-ErbB4 signaling through suppressing Src enhancement of NMDARs. The cartoon illustrates induction of LTP by theta burst stimulations under physiological conditions (right), suppressed NRG1-ErbB4 signaling (top) and enhanced NRG1-signaling (bottom). Under physiological conditions, the NRG1-ErbB4 pathway is a partial brake on the induction of LTP as genetic or pharmacological suppression of this pathway increases the magnitude of the potentiation. Gain-of-function of NRG1-ErbB4 signaling may block induction of LTP.
Src-mediated enhancement of NMDAR currents is reversed by STEP (see above). Under basal conditions the relative activity of STEP with respect to NMDARs exceeds that of Src [27], and the balance is normally shifted so far towards the activity of STEP that NMDAR function is unaffected by acute blockade of Src. On the other hand, acute blockade of STEP, by a dominant negative protein or function blocking antibody, leads to Src-dependent enhancement of NMDAR currents indicating that there is ongoing activity of both STEP and Src, even under normal conditions. The ongoing, and relatively high, activity of STEP provides an explanation for the reduction of NMDAR currents by NRG1β once the currents had been enhanced by EPQ(pY)EEIPIA, even though NRG1β had no effect on basal NMDAR function.
An effect of NRG1-ErbB4 signaling on STEP, which would be consistent with the suppression of Src-mediated enhancement of NMDAR currents, could not account for the finding that the activity of Src activity itself is decreased by administering NRG1. Rather the effect of NRG1-ErbB4 may be mediated by one or more of the many types of regulators of Src kinase, with the decrease in Src catalytic activity caused by suppressing upstream activators, or by facilitating inhibitors, of kinase function. Potential mediators of NRG1-ErbB4 signaling include the most direct regulators of Src within the NMDAR complex, the tyrosine kinase Csk [18] and the phosphatase PTPα [17]. Another potential mediator is the prominent scaffolding protein PSD-95 which suppresses Src activity in the NMDAR complex through binding of a sequence in the PSD-95N-terminal region to the SH2 domain of Src [22]. Actions of Src in general are regulated by the coordination of its binding to substrates through SH2 or SH3 interactions. However, the effect of NRG1-ErbB4 was shown not to be due to blocking the localization of Src with NMDARs because NRG1β caused no change in the association of Src within the NMDAR complex [38]. Thus, the molecular basis of the inhibition of Src activity by NRG1-ErbB4 signaling is an important unanswered question.
The magnitude of LTP is increased by acutely blocking ErbB4 function, or in mice lacking ErbB4 [66], indicating that a physiological role of NRG1-ErbB4 signaling is to suppress LTP at Schaffer collateral synapses in CA1 hippocampus. By contrast NMDARs are not tonically suppressed by NRG1-ErbB4 signaling. The differential effect of NRG1-ErbB4 signaling on LTP induction but not on basal NMDAR currents suggests that NRG1 may be released by the increased activity caused by TBS, and there is evidence that increasing neuronal activity leads to release of NRG1 in the hippocampus [68].
The physiological function of NRG1-ErbB4 is not normally at maximum because induction of LTP can be further suppressed, even to the point of being blocked, by administering NRG1β [65]. Thus, findings from rodent models together with evidence for increased NRG1 expression and elevated NRG1-ErbB4 signaling in the hippocampus and other brain regions [61] in individuals with schizophrenia, led us to hypothesize that a critical cellular mechanism in this disorder may be gain-of-function of NRG1-ErbB4 signaling with subsequent suppression of Src-mediated enhancement of NMDARs. Because Src-mediated upregulation of NMDARs normally occurs during activity-dependent plasticity such as LTP, we hypothesize further that in excessive NRG1-ErbB4 suppression of Src/NMDAR-mediated plasticity may be a fundamental mechanism for cognitive dysfunction in schizophrenia (Figure 2). Not only was activity-dependent plasticity suppressed by NRG1β-ErbB4 signaling, but NRG1β disrupts the responses of CA1 neurons to theta-patterned input itself [38] which could contribute to alterations in oscillatory brain network activity observed in schizophrenia [69]. Thus, pathological suppression of Src-enhancement of NMDARs by excessive NRG1-ErbB4 signaling in various regions of the CNS might generally be involved in the diversity of schizophrenia symptoms.
Conclusions
The recent findings summarized above suggest that a fine balance is required in the regulation of NMDARs by Src-mediated tyrosine phosphorylation/dephosphorylation. We propose that dysregulation of Src-mediated enhancement of the NMDA receptor, which may result in the extremes of pathologically excessive or suppressed neuroplasticity, is a unifying theme for several CNS disorders. Thus, a novel therapeutic approach for such CNS disorders is through normalizing dysregulated Src enhancement of NMDARs. This is an approach with the advantage of not directly blocking NMDARs which is known to have deleterious consequences.
Acknowledgments
Work of the author is supported by grants from the Canadian Institutes of Health Research (CIHR; grant number MT-12682), the Krembil Foundation, the Ontario Neurotrauma Foundation and the Ontario Research Foundation Research Excellence Program. MWS is an International Research Scholar of the Howard Hughes Medical Institute and holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain.
References
- 1.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacDonald JF, Porietis AV, Wojtowicz JM. L-Aspartic acid induces a region of negative slope conductance in the current-voltage relationship of cultured spinal cord neurons. Brain Res. 1982;237:248–253. doi: 10.1016/0006-8993(82)90575-3. [DOI] [PubMed] [Google Scholar]
- 4.Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG. Proteomic analysis of NMDA receptor-adhesion protein signaling complexes. Nat Neurosci. 2000;3:661–669. doi: 10.1038/76615. [DOI] [PubMed] [Google Scholar]
- 5.Ng D, Pitcher GM, Szilard RK, Sertie A, Kanisek M, Clapcote SJ, Lipina T, Kalia LV, Joo D, McKerlie C, Cortez M, Roder JC, Salter MW, McInnes RR. Neto1 is a novel CUB-domain NMDA receptor-interacting protein required for synaptic plasticity and learning. PLoS Biol. 2009;7:e41. doi: 10.1371/journal.pbio.1000041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacDonald JF, Mody I, Salter MW. Regulation of N-methyl-D-aspartate receptors revealed by intracellular dialysis of murine neurones in culture. J Physiol (Lond ) 1989;414:17–34. doi: 10.1113/jphysiol.1989.sp017674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 8.Wang LY, Orser BA, Brautigan DL, MacDonald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369:230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- 9.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 10.Yu X-M, Groveman BR, Feng S, Fang XQ, Pflueger M, Lin SX, Bienkiewicz E. Src family kinases in the nervous system - current knowledge of src kinase activity regulation. FEBS J. 2011 in this issue. [Google Scholar]
- 11.Wang YT, Yu XM, Salter MW. Ca(2+)-independent reduction of N-methyl-D-aspartate channel activity by protein tyrosine phosphatase. Proc Natl Acad Sci U S A. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor-channels by protein tyrosine kinases of the src family. J Physiol (Lond) 1996;492 ( Pt 2):445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacDonald JF, Trepanier C, Jackson M. Regulation of NMDA receptors by the tyrosine kinase Fyn. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08391.x. in this issue. [DOI] [PubMed] [Google Scholar]
- 14.Yu XM, Askalan R, Keil GJ, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 15.Yu XM, Salter MW. Gain control of NMDA-receptor currents by intracellular sodium. Nature. 1998;396:469–474. doi: 10.1038/24877. [DOI] [PubMed] [Google Scholar]
- 16.Gingrich JR, Pelkey KA, Fam SR, Huang Y, Petralia RS, Wenthold RJ, Salter MW. Unique domain anchoring of Src to synaptic NMDA receptors via the mitochondrial protein NADH dehydrogenase subunit 2. Proc Natl Acad Sci U S A. 2004;101:6237–6242. doi: 10.1073/pnas.0401413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei G, Xue S, Chery N, Liu Q, Xu J, Kwan CL, Fu YP, Lu YM, Liu M, Harder KW, Yu XM. Gain control of N-methyl-D-aspartate receptor activity by receptor-like protein tyrosine phosphatase alpha. EMBO J. 2002;21:2977–2989. doi: 10.1093/emboj/cdf292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J, Weerapura M, Ali MK, Jackson MF, Li H, Lei G, Xue S, Kwan CL, Manolson MF, Yang K, MacDonald JF, Yu XM. Control of excitatory synaptic transmission by C-terminal Src kinase. J Biol Chem. 2008;283:17503–17514. doi: 10.1074/jbc.M800917200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKβ/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- 20.Yaka R, Thornton C, Vagts AJ, Phamluong K, Bonci A, Ron D. NMDA receptor function is regulated by the inhibitory scaffolding protein, RACK1. Proc Natl Acad Sci U S A. 2002;99:5710–5715. doi: 10.1073/pnas.062046299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton C, Yaka R, Dinh S, Ron D. H-Ras modulates N-methyl-D-aspartate receptor function via inhibition of Src tyrosine kinase activity. J Biol Chem. 2003;278:23823–23829. doi: 10.1074/jbc.M302389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalia LV, Pitcher GM, Pelkey KA, Salter MW. PSD-95 is a negative regulator of the tyrosine kinase Src in the NMDA receptor complex. EMBO J. 2006;25:4971–4982. doi: 10.1038/sj.emboj.7601342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 24.Ali DW, Salter MW. NMDA receptor regulation by Src kinase signalling in excitatory synaptic transmission and plasticity. Curr Opin Neurobiol. 2001;11:336–342. doi: 10.1016/s0959-4388(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 25.Oyama T, Goto S, Nishi T, Sato K, Yamada K, Yoshikawa M, Ushio Y. Immunocytochemical localization of the striatal enriched protein tyrosine phosphatase in the rat striatum: a light and electron microscopic study with a complementary DNA-generated polyclonal antibody. Neuroscience. 1995;69:869–880. doi: 10.1016/0306-4522(95)00278-q. [DOI] [PubMed] [Google Scholar]
- 26.Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 28.Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 29.Kalia LV, Gingrich JR, Salter MW. Src in synaptic transmission and plasticity. Oncogene. 2004;23:8007–8016. doi: 10.1038/sj.onc.1208158. [DOI] [PubMed] [Google Scholar]
- 30.MacDonald JF, Jackson MF, Beazely MA. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit Rev Neurobiol. 2006;18:71–84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. [DOI] [PubMed] [Google Scholar]
- 31.Grant SG, O’Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 32.Lu YM, Roder JC, Davidow J, Salter MW. Src activation in the induction of long-term potentiation in CA1 hippocampal neurons. Science. 1998;279:1363–1368. doi: 10.1126/science.279.5355.1363. [DOI] [PubMed] [Google Scholar]
- 33.Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated Tyrosine Phosphorylation Sites on GluRepsilon 2 (NR2B) Subunit of the N-Methyl-D-aspartate Receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 34.Rostas JA, Brent VA, Voss K, Errington ML, Bliss TV, Gurd JW. Enhanced tyrosine phosphorylation of the 2B subunit of the N-methyl-D- aspartate receptor in long-term potentiation. Proc Natl Acad Sci U S A. 1996;93:10452–10456. doi: 10.1073/pnas.93.19.10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrone A, Battaglia F, Wang C, Dusa A, Su J, Zagzag D, Bianchi R, Casaccia-Bonnefil P, Arancio O, Sap J. Receptor protein tyrosine phosphatase alpha is essential for hippocampal neuronal migration and long-term potentiation. EMBO J. 2003;22:4121–4131. doi: 10.1093/emboj/cdg399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauri SE, Taira T, Rauvala H. High-frequency synaptic stimulation induces association of fyn and c- src to distinct phosphorylated components. NeuroReport. 2000;11:997–1000. doi: 10.1097/00001756-200004070-00020. [DOI] [PubMed] [Google Scholar]
- 37.Manabe T, Aiba A, Yamada A, Ichise T, Sakagami H, Kondo H, Katsuki M. Regulation of long-term potentiation by H-Ras through NMDA receptor phosphorylation. J Neurosci. 2000;20:2504–2511. doi: 10.1523/JNEUROSCI.20-07-02504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitcher GM, Kalia LV, Ng D, Goodfellow NM, Yee KT, Lambe EK, Salter MW. Schizophrenia susceptibility pathway neuregulin 1-ErbB4 suppresses Src upregulation of NMDA receptors. Nat Med. 2011;17:470–478. doi: 10.1038/nm.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10:287–333. doi: 10.1016/j.ejpain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 41.Salter MW. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Curr Top Med Chem. 2005;5:557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- 42.Liu XJ, Salter MW. Glutamate receptor phosphorylation and trafficking in pain plasticity in spinal cord dorsal horn. Eur J Neurosci. 2010;32:278–289. doi: 10.1111/j.1460-9568.2010.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beggs S, Salter MW. Microglia-neuronal signalling in neuropathic pain hypersensitivity 2.0. Curr Opin Neurobiol. 2010;20:474–480. doi: 10.1016/j.conb.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo H, Huang LY. Alteration in the voltage dependence of NMDA receptor channels in rat dorsal horn neurones following peripheral inflammation. J Physiol. 2001;537:115–123. doi: 10.1111/j.1469-7793.2001.0115k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isaev D, Gerber G, Park SK, Chung JM, Randik M. Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5-L6 spinal nerves. NeuroReport. 2000;11:4055–4061. doi: 10.1097/00001756-200012180-00030. [DOI] [PubMed] [Google Scholar]
- 46.Iwata H, Takasusuki T, Yamaguchi S, Hori Y. NMDA receptor 2B subunit-mediated synaptic transmission in the superficial dorsal horn of peripheral nerve-injured neuropathic mice. Brain Res. 2007;1135:92–101. doi: 10.1016/j.brainres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Woolf CJ, Salter MW. Plasticity and pain: role of the dorsal horn. In: McMahon SB, Koltzenburg M, editors. Melzack and Wall’s Textbook of Pain. 5. Elsevier; 2006. pp. 91–106. [Google Scholar]
- 48.Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- 50.Katsura H, Obata K, Mizushima T, Sakurai J, Kobayashi K, Yamanaka H, Dai Y, Fukuoka T, Sakagami M, Noguchi K. Activation of Src-family kinases in spinal microglia contributes to mechanical hypersensitivity after nerve injury. J Neurosci. 2006;26:8680–8690. doi: 10.1523/JNEUROSCI.1771-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salter MW, Dong Y, Kalia LV, Liu XJ, Pitcher G. Regulation of NMDA Receptors by Kinases and Phosphatases. 2009. [PubMed] [Google Scholar]
- 52.Tsuda M, Tozaki-Saitoh H, Masuda T, Toyomitsu E, Tezuka T, Yamamoto T, Inoue K. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–58. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 53.Battaglia AA, Sehayek K, Grist J, McMahon SB, Gavazzi I. EphB receptors and ephrin-B ligands regulate spinal sensory connectivity and modulate pain processing. Nat Neurosci. 2003;6:339–340. doi: 10.1038/nn1034. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi H, Kitamura T, Sekiguchi M, Homma MK, Kabuyama Y, Konno S, Kikuchi S, Homma Y. Involvement of EphB1 receptor/EphrinB2 ligand in neuropathic pain. Spine. 2007;32:1592–1598. doi: 10.1097/BRS.0b013e318074d46a. [DOI] [PubMed] [Google Scholar]
- 55.Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gogos JA, Gerber DJ. Schizophrenia susceptibility genes: emergence of positional candidates and future directions. Trends Pharmacol Sci. 2006;27:226–233. doi: 10.1016/j.tips.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 57.Cardno AG, Sham PC, Murray RM, McGuffin P. Twin study of symptom dimensions in psychoses. Br J Psychiatry. 2001;179:39–45. doi: 10.1192/bjp.179.1.39. [DOI] [PubMed] [Google Scholar]
- 58.Balu DT, Coyle JT. Neuroplasticity signaling pathways linked to the pathophysiology of schizophrenia. Neurosci Biobehav Rev. 2011;35:848–870. doi: 10.1016/j.neubiorev.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lindsley CW, Shipe WD, Wolkenberg SE, Theberge CR, Williams DL, Jr, Sur C, Kinney GG. Progress towards validating the NMDA receptor hypofunction hypothesis of schizophrenia. Curr Top Med Chem. 2006;6:771–785. doi: 10.2174/156802606777057599. [DOI] [PubMed] [Google Scholar]
- 60.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, Bakshi K, Kamins J, Borgmann-Winter KE, Siegel SJ, Gallop RJ, Arnold SE. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee A, Macdonald ML, Borgmann-Winter KE, Hahn CG. Neuregulin 1-erbB4 pathway in schizophrenia: From genes to an interactome. Brain Res Bull. 2010;83:132–139. doi: 10.1016/j.brainresbull.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chong VZ, Thompson M, Beltaifa S, Webster MJ, Law AJ, Weickert CS. Elevated neuregulin-1 and ErbB4 protein in the prefrontal cortex of schizophrenic patients. Schizophr Res. 2008;100:270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law AJ, Lipska BK, Weickert CS, Hyde TM, Straub RE, Hashimoto R, Harrison PJ, Kleinman JE, Weinberger DR. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5′ SNPs associated with the disease. Proc Natl Acad Sci U S A. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deakin IH, Law AJ, Oliver PL, Schwab MH, Nave KA, Harrison PJ, Bannerman DM. Behavioural characterization of neuregulin 1 type I overexpressing transgenic mice. NeuroReport. 2009;20:1523–1528. doi: 10.1097/WNR.0b013e328330f6e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, Pelkey KA, Yang DJ, Xiong WC, Salter MW, Mei L. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 66.Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. NeuroReport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, Buonanno A. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proc Natl Acad Sci U S A. 2008;105:15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci U S A. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]