Abstract
Glutamate is the major excitatory neurotransmitter in the central nervous system. Considerable evidence suggests that both ionotropic and metabotropic glutamate receptors are involved in pain hypersensitivity. However, glutamate receptor-based therapies are limited by side effects because the activities of glutamate receptors are essential for many important physiological functions. Here, we highlight recent key findings in molecular and cellular mechanisms of glutamate receptor regulation and their roles in triggering and sustaining pain hypersensitivity. Targeting these molecular mechanisms could form the basis for new therapeutic strategies for the treatment of chronic pain.
INTRODUCTION
Nociception refers to the neural processes for encoding and processing noxious stimuli. While acute nociception is protective as a warning system, chronic pain lacks obvious protective function and can be disabling, severe and intractable. Clinical manifestations of chronic pain include spontaneous pain, pain elicited by innocuous stimuli (allodynia) or exaggerated pain response to noxious stimuli (hyperalgesia) (see definitions on IASP homepage www.iasp-pain.org). Neuroplastic changes in both peripheral and central nervous systems are the key cellular and molecular substrates for the generation and maintenance of chronic pain (Woolf and Salter 2000;Basbaum et al., 2009;Latremoliere and Woolf 2009;Sandkuhler 2009).
Because glutamate receptors are critical for neuroplasticity in nociceptive networks, there have been considerable efforts to develop therapeutic approaches that suppress the function of these receptors. These approaches have had limited clinical success largely due to limitations from the blockade of the many normal functions of glutamate receptors in the central nervous system. Recent understanding to the molecular mechanisms that regulate glutamate receptor function and signaling provide new opportunities to selectively interfere with those mechanisms that produce pain hypersensitivity.
Nociceptive transmission normally starts from the peripheral endings of those primary sensory afferents which are activated by noxious stimuli. Action potentials generated in these nociceptive afferents are propagated into the spinal cord dorsal horn or the corresponding region in the trigeminal nuclear complex in the brain stem. At the central terminals of primary afferents, both nociceptive and non-nociceptive, glutamate is the major fast neurotransmitter which is released and mediates rapid excitatory post-synaptic potentials in dorsal horn neurons. In the presynaptic terminals glutamate is taken up into synaptic vesicles in neurons by vesicular glutamate receptor transporters (VGLUTs) (Santos et al., 2009). VGLUTs mediate the transport of glutamate from the cytoplasm into synaptic vesicles, driven primarily by the electrical component of H+ electrochemical gradient generated by H+-ATPase (Santos et al., 2009). There are three distinct isoforms of VGLUTs, with similar transport characteristics but exhibit an essentially complementary pattern of expression in primary sensory neurons (Todd et al., 2003;Neumann et al., 2008;Seal et al., 2009). VGLUT1 is predominately expressed in the terminals of heavily myelinated Aβ fibers which mediate innocuous mechanical sensation. VGLUT1 positive afferents terminate in lamina III/IV and the inner part of lamina II (IIi) in dorsal horn, forming synapses with PKCγ interneurons in dorsal horn (Todd et al., 2003;Neumann et al., 2008). VGLUT2 is predominately expressed in terminals of thinly myelinated Aδ fiber and unmyelinated C-fibers, including peptidergic and non-peptidergic, IB4 positive C-fibers. VGLUT2 positive afferents predominately mediate noxious sensations, and terminate in lamina I and II in dorsal horn (Todd et al., 2003;Alvarez et al., 2004). The most recently discovered glutamate transporter, VGLUT3, shows much more restricted distribution. VGLUT3 is expressed in a subpopulation of unmyelinated, low threshold mechanoceptor, which is non-peptidergic and IB4 negative (Seal et al., 2009). These afferents terminate in lamina I and IIi in dorsal horn (Seal et al., 2009) and under physiological circumstances appear to mediate the sensation of pleasant touch (Loken et al., 2009). Following inflammation or nerve injury, however, this population of neurons may be involved in mechanical allodynia, as VGLUT3 null mutant mice do not develop mechanical allodynia with inflammation or peripheral nerve injury (PNI) (Seal et al., 2009).
Once glutamate is released it acts on post-synaptic glutamate receptors localized on second-order neurons in the dorsal horn. Three main classes of ligand-gated, ionotropic glutamate receptors (iGluRs), the α-amino-3-hydroxy-5-methyl-4-izoxazolepropionic acid receptor (AMPAR), the kainate receptor (KAR), the N-methyl-D-aspartate receptor (NMDAR), and three groups of the G-protein coupled, metabotropic glutamate receptors (mGluRs), groups I–III, are involved in initiating and maintaining neuroplasticity (Woolf and Salter 2000;Latremoliere and Woolf 2009;Larsson 2009;Sandkuhler 2009;Larsson and Broman 2010). In the nociceptive system, glutamate receptors are expressed at peripheral, spinal and supraspinal levels. In this review, we focus on the glutamate receptors in primary sensory afferents and spinal dorsal horn neurons, with particular emphasis on the molecular and cellular mechanisms underlying the spinal synaptic plasticity in inflammatory or neuropathic pain. The roles of glutamate receptors in supraspinal nociceptive networks are reviewed in detail elsewhere (Bleakman et al., 2006;Goudet et al., 2009;Zhuo 2009).
SYNAPTIC PLASTICITY AT GLUTAMATERGIC SYNAPSES IN THE DORSAL HORN
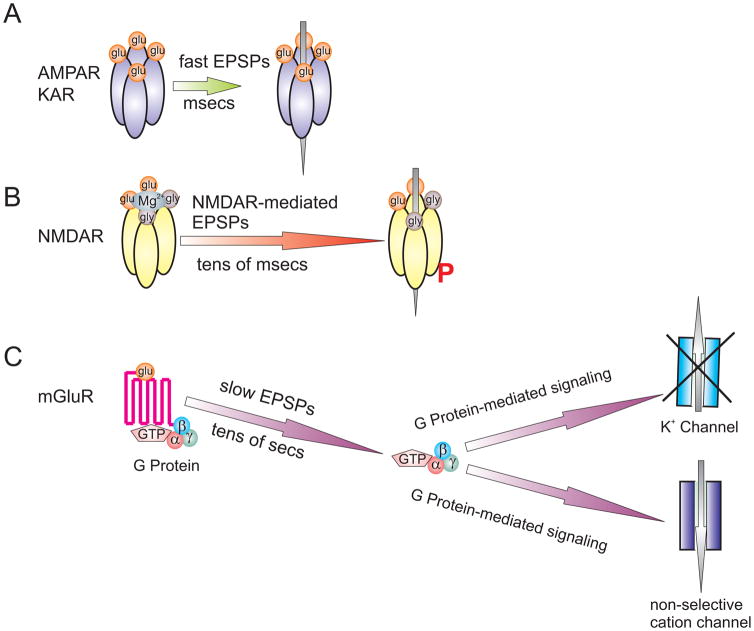
All synaptic transmission from primary sensory afferents to second-order neurons is excitatory, involving iGluRs and mGluRs, at time scales from milliseconds to tens of seconds (Fig 1) (Woolf and Salter 2000;Latremoliere and Woolf 2009;Larsson 2009;Larsson and Broman 2010). Glutamate released from primary afferents binds to receptors strategically localized on second order neurons to produce fast excitatory post-synaptic potentials (fast EPSPs), mediated by AMPARs and KARs, on the scale of milliseconds (Fig 1A). NMDARs contribute little to the fast excitatory transmission, or to acute pain, as under normal circumstance NMDARs are down regulated at resting potential by Mg2+ block at the channel pore (Mayer et al., 1984).
Fig 1.
Schematic illustration of the synaptic transmission from primary sensory afferents to second-order neurons in the dorsal horn. (A) Under normal circumstances, the fast excitatory post-synaptic potentials (fast EPSPs) are mediated by AMPARs and KARs, on the scale of milliseconds. (B) NMDARs contribute little to fast excitatory transmission as NMDARs are down regulated at resting potential by the Mg2+ block at the channel pore and receptor dephosphorylation. However, NMDARs are recruited into synaptic transmission under high frequency stimulation, producing EPSPs on a scale of tens of milliseconds. The recruitment of NMDARs involves disinhibition of Mg2+ block and receptor phosphorylation. (C) Sustained release of glutamate leads to activation of post-synaptic group I mGluRs, resulting in G protein activation. The activated G protein subunits then associate with downstream effectors to affect membrane potential by inhibiting K+ channels or facilitating non-selective cation channels, leading to slow EPSPs lasting up to tens of seconds.
Importantly, NMDARs are also down regulated biochemically by biasing of kinase/phosphatase regulatory systems towards dephosphorylation under basal physiological conditions (Huang et al., 2001;Pelkey et al., 2002). However, NMDARs are recruited into synaptic transmission under high frequency stimulation, producing EPSPs on a scale of tens of milliseconds. The recruitment of NMDARs involves disinhibition of the Mg2+ block and receptor phosphorylation (Fig 1B).
Slow excitatory depolarizing synaptic potentials (slow EPSPs) are also critically dependent upon relief of the Mg2+ block from the NMDAR channel pore. Slow EPSPs are evoked by post-synaptic G-protein coupled receptor (GPCR) activation. Upon sustained, intense noxious stimuli, primary sensory afferents release peptide neuromodulators, such as substance P (Ikeda et al., 2006) and CGRP (Schaible et al., 1994) together with glutamate presynaptically. This sustained release of peptides and glutamate leads to activation of post-synaptic GPCRs, including group I mGluRs. The binding of ligand to GPCRs induces conformational changes in the transmembrane and intracellular domains of the receptor, leading to its interaction with heterotrimeric G protein. The activated G protein subunits then associate with downstream effectors to effect membrane potential by inhibiting K+ channels or facilitating a non-selective cation conductance, leading to slow EPSPs lasting up to tens of seconds (Fig 1C). The slow EPSPs provide substantial opportunities for temporal summation of fast EPSPs, and the cumulative depolarization of the dorsal horn neuron leads to relief of the Mg2+ block in the NMDAR channel (Mayer et al., 1984). The sustained depolarization furthermore recruits voltage-gated Ca2+ currents, triggering plateau potentials mediated by Ca2+-activated non-selective cation channels (Morisset and Nagy 1999). Thus, post-synaptic Ca2+ concentration increases substantially, resulting in subsequent activation of multiple intracellular signaling pathways, leading to increased neuronal excitability and enhanced synaptic transmission. Importantly, the fast excitatory transmission mediated by AMPARs is also facilitated by signaling initiated and maintained by NMDARs. The end result of these processes is central sensitization- the enhancement in the activity of neurons and circuits in the nociceptive pathway. Thus, previously subthreshold synaptic inputs are recruited to generate increased or augmented responses (Woolf and Salter 2000;Salter 2005;Latremoliere and Woolf 2009).
The synaptic potentiation in dorsal horn underlying central sensitization may be homosynaptic or heterosynaptic (Woolf and Salter 2006). One form of homosynaptic potentiation in spinal cord is wind up, in which the action potential discharge elicited by a train of identical low frequency C-fiber strength stimuli gets larger on each successive stimulus. Windup results from the activation of NK1 and CGRP1 receptors that produce a cumulative membrane depolarization of slow EPSPs and subsequent activation of NMDARs by suppression of Mg2+ block as discussed above. The degree of relief of the Mg2+ blockade increases with increasing depolarization and thus windup is a feedforward process of progressive enhancement of current flow through NMDARs with repetitive synaptic inputs. Windup is transient, as the facilitation is lost quickly when the synaptic inputs stop and the membrane potential returns to the resting level within seconds of the end of noxious stimulation.
A distinct form of homosynaptic potentiation occurs in nociceptive dorsal horn neurons. This form of synaptic enhancement persists for hours or days after nociceptive synaptic stimulation and is thus a form of long-term potentiation (LTP)(Woolf and Salter 2000;Sandkuhler 2007). LTP is observed in NK1-expressing neurons in spinal lamina I and can be evoked by both brief periods of high frequency electrical stimulation (~100HZ)(Randic et al., 1993) or low frequency electrical stimulation (i.e. 2HZ) (Ikeda et al., 2006) at C-fiber strength (Sandkuhler 2007;Sandkuhler 2009). NMDAR (Ikeda et al., 2003) and group I mGluR (Azkue et al., 2003) activation is involved in the induction of LTP. Homosynaptic plasticity is bi-directional and under certain circumstances glutamatergic synapses in the dorsal horn may show long-term depression (LTD) (Sandkuhler 2007;Larsson 2009;Sandkuhler 2009;Larsson and Broman 2010).
In contrast to homosynaptic plasticity, in which the efficacy only of activated synapses is changed, the most prominent form of plasticity at excitatory synapses in the dorsal horn is heterosynaptic potentiation, in which activity in one set of synapses enhances activity in non-activated synapses(Woolf and Salter 2006). In fact, heterosynaptic potentiation appears to be the dominate form of use-dependent, persistent plasticity contributing to central sensitization in spinal cord nociceptive pathways (Latremoliere and Woolf 2009). Activation of group I mGluRs and subsequent release of intracellular Ca2+ from endoplasmic reticulum appears to be critical for spreading the facilitation from active synapses to neighboring non-active synapses (Fagni et al., 2000;Adwanikar et al., 2004).
Persistent potentiation of transmission at glutamatergic synapses in spinal nociceptive pathways, either homosynaptic potentiation or heterosynaptic potentiation, is critical for pain hypersensitivity. Behaviorally, homosynaptic potentiation contributes to primary hyperalgesia, whereas heterosynaptic potentiation contributes to secondary hyperalgesia and allodynia (Latremoliere and Woolf 2009). Thus, understanding the mechanisms which produce persistent synaptic potentiation in nociceptive pathways provides the opportunity to advance new therapeutic approaches.
A large body of evidence indicates that persistent synaptic plasticity at glutamatergic synapses in the CNS is critically dependent on post-translational modification of glutamate receptors by phosphorylation and alterations in the trafficking of these receptors are critical processes(Woolf and Salter 2006;Latremoliere and Woolf 2009;Larsson 2009;Larsson and Broman 2010). Here we focus on recent discoveries on the role of glutamate receptor phosphorylation and trafficking in excitatory synaptic plasticity in nociceptive pathways in the dorsal horn. Interfering with these fundamental regulatory mechanisms has been found to suppress pain hypersensitivity in models of inflammatory and neuropathic pain, and as such, these targeting these regulatory mechanisms may provide new strategies for treating chronic pain.
IONOTROPIC GLUTAMATE RECEPTORS IN NOCICEPTION AND PAIN
Ionotropic glutamate receptors are comprised of three subgroups: AMPARs, KARs and NMDARs. At the core, each of these receptors is a tetramer of the pore-forming subunits, with the four subunits co-translationally assembled as a dimer of dimers in the functional receptor. Associated with the pore-forming core is a diversity of transmembrane and intracellular proteins to produce a multiprotein receptor complex. The associated proteins participate in receptor trafficking, control receptor activity and pharmacological characteristics, and mediate signaling downstream of the receptor. The core subunits of ionotropic glutamate receptor share a common structure: a long extracellular N-terminal domain, three transmembrane segments, a reentrant pore loop, and an intracellular C-terminal domain. The N-terminal domain and the extracellular loop form the ligand-binding pocket and the carboxyl tail regulates receptor trafficking, localization and phosphorylation (Kew and Kemp 2005;Sobolevsky et al., 2009). The nomenclature for iGluRs recently put forward by the International Union of Basic and Clinical Pharmacology (IUPHAR) will be used here (Collingridge et al., 2009).
AMPA receptors: nociception and pain plasticity
AMPA receptors are encoded by four receptor subunit genes, GRIA1, GRIA2, GRIA3 and GRIA4. The functional AMPA channel is a tetramer with the assembly of one or more of these subunits. In adult animals, nearly all GluA2 subunits undergo post-transcriptional RNA editing, which significantly reduces the Ca2+ permeability of GluA2-containing AMPARs. AMPARs lacking edited GluA2, in contrast, are Ca2+ permeable and exhibit inward rectification (Fleming and England 2010). Mice deficient of GRIA1 have reduced Ca2+ permeable AMPARs with decreased AMPA currents, whereas GRIA2 deficient mice have increased Ca2+ permeable AMPARs and increased AMPA currents (Hartmann et al., 2004).
AMPARs are expressed in both peripheral and spinal nociceptive pathways (Fig 2). In DRG neurons, AMPAR subunits are expressed in the cell bodies and in the peripheral or central axons (Sato et al., 1993;Willcockson and Valtschanoff 2008). Presynaptic AMPA receptors at the central terminals of primary sensory afferents have been implicated in presynaptic inhibition of glutamate release (Lee et al., 2002).
Fig 2.
Glutamate receptors in spinal cord synapses. (A) Schematic illustration of the basal condition of the synapses between the central anxon of primary sensory afferents and dorsal horn neurons. NMDARs are blocked by Mg2+ at resting potential. (B) Intense noxious stimulation increases synaptic transmission. Presynaptically, group I mGluRs, especially mGluR5 sensitize the TRPV1 through PKA-dependent phosphorylation, resulting in increased presynaptic release of glutamate. On contrast, activation of presynaptic group II and group III mGluRs inhibits presynaptic neurotransmitter release. Postsynaptically, the phosphorylation of AMPARs, the insertion of GluA1 subunits and the internalization of GluA2 subunits lead to upregulated AMPAR-mediated synaptic efficacy and increased Ca2+ permeable AMPARs at synapses. Membrane depolarization relieves the Mg2+ block of NDMARs. NMDARs are also upregulated by receptor phosphorylation. Group 1 mGluRs enhance synaptic transmission by indirectly phosphorylation of NMDARs and increasing neuronal excitability by inhibiting K+ channel.
Postsynaptically, GluA1 and GluA2 subunit-containing AMPARs are expressed virtually in all the glutamatergic synapses in the dorsal horn, with the highest levels in laminas I-II, whereas GluA3 and GluA4 subunits are highly expressed in the ventral horn, with limited expression in superficial laminas (Nagy et al., 2004a;Polgar et al., 2008;Antal et al., 2008;Todd et al., 2009). At the cellular level, inhibitory neurons preferentially express GluR1 subunits, whereas almost all the excitatory neurons express GluR2-containing Ca2+ impermeable AMPARs (Latremoliere and Woolf 2009).
Pharmacological studies support a role of AMPARs in inflammatory pain or pain induced by tissue injury. For example, intrathecal application of AMPAR antagonists or Ca2+ permeable AMPAR antagonists significantly attenuates mechanical allodynia induced by burnt injury, thermal and mechanical hyperalgesia induced by intraplantar injection of inflammatory reagents carrageenan and complete Freund’s adjuvant (CFA), and mechanical hyperalgesia induced by post-surgery pain (Sorkin et al., 2001;Park et al., 2008;Larsson 2009). Genetic studies also indicate that GluA1 and GluA2 containing-AMPARs are involved in inflammatory pain. In acute inflammatory pain induced by the first phase of formalin test or by capsaicin, both GRIA1 and GRIA2 null mutant mice have increased nocifensive behaviors (Hartmann et al., 2004), suggesting GluA1 and GluA2 containing AMPARs are involved in the tonic pre-synaptic inhibition of neurotransmitter release from primary sensory afferents (Lee et al., 2002). While in the second phase of formalin-test, GRIA1 null mutant mice have reduced responses, and GRIA2 null mutant mice have increased responses. In a more chronic inflammatory pain model, the CFA model, mice with null mutation of GRIA2 but not GRIA1 have increased thermal and mechanical hyperalgesia. These results indicate Ca2+ permeable AMPARs are essential for inflammation induced tonic or chronic pain (Hartmann et al., 2004). Importantly, GRIA1, GRIA2 and GRIA3 null mutant mice have normal basal neurotransmission, and the basal thermal and mechanical thresholds are intact in each phenotype with the null mutant of each respective gene (Hartmann et al., 2004).
Electrophysiological studies indicate that AMPAR-mediated spinal synaptic transmission in dorsal horn neuron is enhanced by inflammation, and this enhancement is suppressed by Ca2+ permeable AMPA channel blockers (Voitenko et al., 2004;Katano et al., 2008;Luo et al., 2008). What are the molecular mechanisms underlying AMPAR-mediated enhanced synaptic efficacy? AMPARs can be phosphorylated at serine-threonine residues in the carboxyl tail of the subunits to upregulate channel activity or to change synaptic targeting of AMPARs (Lee 2006), phosphorylation of the GluA1 at the PKC/CaMKII site Ser831 or the PKA site Ser845 in the dorsal horn is observed after either plantar incision or inflammation induced by capsaicin or CFA (Fang et al., 2003a; Nagy et al., 2004a;Lu et al., 2008). Phosphorylation at Ser831 increases single-channel conductance of homomeric GluA1(Oh and Derkach 2005), whereas phosphorylation at Ser845 increases mean open probability of the channel and promotes surface insertion of GluA1 containing receptor(Esteban et al., 2003). Therefore, phosphorylation of GluA1 subunit of AMPAR not only changes channel activity per se, but also regulates receptor trafficking. Such recruitment of GluA1 subunit into dorsal horn synapses is observed following various forms of inflammation (Galan et al., 2004;Katano et al., 2008;Park et al., 2008;Pezet et al., 2008); (Larsson and Broman 2008), which is consistent with the increased phosphorylation of spinal GluA1-S845 following inflammation(Fang et al., 2003a;Fang et al., 2003b). It is known that synaptic recruitment of GluA1 is a major mechanism contributing to synaptic potentiation (Kessels and Malinow 2009). Thus, AMPA synaptic transmission is potentiated in dorsal horn neurons following inflammation through phosphorylation of GluA1 subunits.
In addition to phosphorylation of GluA1 subunits, inflammation also induces rapid phosphorylation of GluA2 subunits at Ser880 site, in a PKC-dependent manner (Park et al., 2009). In transgenic mice expressing mutant GRIA2 which could not be phosphorylated at GluA2-Ser880, CFA-induced pain hypersensitivity is attenuated (Park et al., 2009) (Fig 2B). Furthermore, inflammation also induces GluA2 subunit internalization, as the amount of GluA2 subunits is decreased in the PSD fraction but increased in endocytic vesicle fractions of the dorsal horn (Katano et al., 2008;Park et al., 2008;Park et al., 2009). The internalization could be initiated by inflammation-induced GluA2 phosphorylation at Ser880 (Park et al., 2009), which results in the subsequent disruption of GluA2 binding to its synaptic anchoring protein, glutamate receptor interacting protein (GRIP) (Park et al., 2009), leading to subsequent GluA2 subunit endocytosis (Chung et al., 2000). Alternatively, impaired association of GluA2 with its scaffolding protein, N-ethylmaleimide-sensitive fusion (NSF) protein, could also lead to GluA2 internalization as NSF, which is required for the retention of GluA2 on synaptic membranes, is reduced in the PSD fraction of the dorsal horn following inflammation (Katano et al., 2008). Insertion of GluA1 subunits and internalization of GluA2 subunits together significantly increase Ca2+-permeable AMPARs at spinal synapses (Vikman et al., 2008), resulting in increased synaptic AMPA transmission in dorsal horn neurons, leading to inflammation-induced pain hypersensitivity (Fig 2B).
For pain hypersensitivity induced by PNI, pharmacological studies indicate that intrathecal administration of AMPAR antagonists or selective Ca2+ permeable non-NMDAR antagonists suppresses PNI-induced thermal hyperalgesia (Sorkin et al., 2001;Garry et al., 2003b). Consistent with involvement of AMPARs, thermal hyperalgesia is attenuated by a myristoylated peptides which disrupts the interaction of GluA2 with GRIP (Garry et al., 2003b). In contrast to thermal hyperalgesia, mechanical allodynia induced by PNI is resistant to AMPAR antagonists (Sorkin et al., 2001). Furthermore, neither GluA1-Ser831 nor GluA1-Ser845 is enhanced following PNI(Lu et al., 2008). GluA1 and GluA2/3 subunit immunoreactive labeling is increased in the ipsilateral superficial dorsal horn following PNI (Harris et al., 1996). The levels of GluA2 and GRIP protein are also increased in the ipsilateral dorsal horn following PNI (Garry et al., 2003b;Tomiyama et al., 2005). However, whether PNI induces AMPAR subunit trafficking in dorsal horn synapses remains to be investigated.
Converging evidence indicates that AMPA receptor phosphorylation is involved in inflammation but not PNI-induced pain hypersensitivity. Following inflammation, AMPAR-mediated synaptic transmission is enhanced in spinal dorsal horn neurons due to the phosphorylation of AMPARs, the insertion of GluA1subunits into synapses and the increase of Ca2+-permeable AMPARs at synapses. In contrast to inflammation, the cellular mechanisms of AMPAR receptor regulation in PNI-induced pain hypersensitivity need further investigation.
Kainate receptors: emerging role in nociception
The kainate family receptors (KARs) are encoded by five subunit genes, GRIK1-5. The subunits are assembled in various combinations to form the functional tetramer receptor. GluK1-3 are low affinity subunits and GluK4-5 are high affinity subunits. Compared with AMPARs and NMDARs, the understanding of KARs in nociception is largely unexplored, mainly due to the relative lack of specific pharmacological tools. However, emerging evidence suggests that KARs, especially GluK1-containing KARs, play an important role in nociception.
Kainate receptors are expressed in both peripheral and spinal nociceptive pathways (Fig 2). In DRG neurons, the GluK1subunits are expressed primarily in small-diameter non-peptidergic TRPV1 positive neurons, as well as the central axons of non-peptidergic primary afferents in superficial dorsal horn (Lee et al., 2001). In the spinal cord dorsal horn, GluK1 subunits are localized at spinothalamic tract neurons (Hegarty et al., 2007). An increase in GluK1-containing KARs is observed in spinal cord dorsal horn in models of inflammatory pain (Guo et al., 2002b) (Fig 2B). Mice deficient in GRIK1 exhibit reduced inflammation-induced nociceptive behaviors. As both phases of formalin-induced activities are suppressed in GRIK1 null mutant mice, GluK1 is involved in both acute and tonic pain hypersensitivities (Ko et al., 2005).
With the development of relatively more specific GluK1 antagonists, pharmacological studies support that KARs could be the new target for inflammatory pain treatment. Several studies demonstrate that systemic or spinal inhibition of GluK1-containing KARs reduces pain hypersensitivity induced by formalin, capsaicin, carrageenan and CFA (Guo et al., 2002b;Dominguez et al., 2005;Jones et al., 2006). In a primate PNI model, electrophysiology studies indicate that responses of spinothalamic neurons to thermal and mechanical stimuli are blocked by the GluK1 antagonist, LY382884 (Palecek et al., 2004). However, the behavioral effect of LY382884, or any other selective GluK1 antagonist, on PNI-induced pain hypersensitivity has not been investigated yet.
Thus, there is evidence indicates that KARs, especially GluK1 containing KARs, are involved in pain hypersensitivity induced by inflammation. In contrast, whether KARs are involved in PNI-induced pain hypersensitivity is still an open question. As receptor phosphorylation and trafficking are the major molecular mechanisms underlying AMPAR receptor-mediated dorsal horn synaptic potentiation following inflammation, it is not unreasonable that KARs could also be regulated by phosphorylation and trafficking after PNI.
NMDA receptors: nociception and pain plasticity
NMDARs are encoded by 7 subunit genes, GRIN1, GRIN2A-2D, and GRIN3A-GRIN3B. The functional NMDA tetramer channel has two obligatory GluN1 glycine binding subunits, and two GluN2 or GluN3 L-glutamate binding subunits (Furukawa et al., 2005;Paoletti and Neyton 2007). Binding of two glutamates and two glycines leads to conformational changes that open the channel conductance pathway and subsequent channel activation. The subunit composition determines the pharmacological and biophysical characteristic of NMDARs. For example, GluN1/GluN2A diheteromeric receptors exhibit relatively fast deactivation compared with receptors containing other GluN2 subunits (Kew and Kemp 2005). In contrast to AMPARs and KARs, NMDARs have high Ca2+ permeability with slow activation/deactivation kinetics.
NMDARs are expressed in both peripheral and central nociceptive pathways (Fig 2). Peripherally, NMDARs are abundantly expressed in both small and large DRG neuron cell bodies and their peripheral terminus and central processes (Sato et al., 1993;Marvizon et al., 2002;Carlton 2009). The messenger RNA of all four subtypes are detected in DRG neurons, with GluN2B and GluN2D most abundantly expressed in protein level (Marvizon et al., 2002;Li et al., 2006a). NMDARs are transported to central terminals of primary sensory afferents to regulate presynaptic substance P release(Liu et al., 1997);(Chen et al., 2010). A recent study suggested NMDAR-induced presynaptic SP release is dependent on NR2B subunits and is controlled by the balance of the activity of receptor tyrosine kinase and phosphotase (Chen et al., 2010). Inflammation increases GluN1 subunit expression in unmyelinated axons (Du et al., 2003). Messenger RNA and protein level of GluN2B subunits are increased in the DRG following inflammation (Li et al., 2006), with increased NMDAR current density and increased sensitivity to GluN2B selective antagonist inhibition (Du et al., 2003;Li et al., 2006b).
In the dorsal horndorsal horn, NMDARs are expressed abundantly, with GluN1 and GluN2A subunits expressed throughout the gray matter, whereas GluN2B subunits are concentrated in laminae I–II (Nagy et al., 2004b). An increase in synaptic GluN2B containing NMDARs in dorsal horn neurons is observed following inflammation and PNI (Hu et al., 2006b;Iwata et al., 2007;Katano et al., 2008;Yang et al., 2009).
Spatial/temporal deletion of the obligatory GluN1 subunit of NMDAR in lumbar dorsal horn neurons results in suppression of phase 2 nociceptive behaviors induced by intraplantar injection of formalin and inhibition of pain hypersensitivity induced by CFA (Weyerbacher et al., 2009;Garraway et al., 2009). GluN2B is the critical subunit for NMDAR-dependent pain hypersensitivity as spinal knockdown of GRIN2B suppresses the second phase of formalin-induced nociceptive behaviors (Tan et al., 2005). Furthermore, pharmacological block of NMDARs with selective GluN2B antagonists attenuates nociceptive behavior, thermal hyperalgesia and mechanical allodynia in various models of inflammation, neuropathic and cancer pain (Malmberg et al., 2003;Abe et al., 2005;Narita et al., 2007;Gu et al., 2009;Qu et al., 2009). Importantly, basal sensory thresholds and acute nociceptive pain are not affected by genetic or pharmacological inhibition of spinal NMDARs, indicating that NMDARs are essential for chronic but not acute pain.
Inflammation or peripheral nerve injury increases NMDAR-mediated synaptic transmission, resulting in increased amplitude of excitatory postsynaptic currents (EPSCs) and Ca2+ signals in dorsal horn neurons (Hu et al., 2006b;Luo et al., 2008). What is the molecular mechanism of NMDAR-mediated synaptic enhancement in dorsal horn? At resting membrane potentials, NMDARs are blocked by Mg2+ in the channel pore in a voltage-dependent manner. Inflammation or PNI-evoked presynaptic release of glutamate, SP and CGRP leads to membrane depolarization(Ikeda et al., 2006), which relieves the Mg2+ blockade (Mayer et al., 1984). The activated NMDARs then allow Ca2+ to enter into neurons. This rapid increase in intracellular Ca2+ is the initial trigger for activity-dependent plasticity. (Fig 1B, Fig 2)
When intracellular Ca2+ is increased beyond a certain level, multiple intracellular signaling pathways including PKA, PKC, Src family kinase (SFK) are activated, and NMDAR function is upregulated through serine-threonine or tyrosine phosphorylation (Latremoliere and Woolf 2009;Larsson 2009;Larsson and Broman 2010). (Fig 2B).
Multiple factors are released into the spinal dorsal horn in response to peripheral tissue inflammation and PNI, such as bradykinin (Kohno et al., 2008) and interleukin 1β (Zhang et al., 2008a;Zhang et al., 2008b). These factors can directly or indirectly activate PKC and PKA in post-synaptic dorsal horn neurons, leading the phosphorylation of GluN1 subunits. PKC- and PKA-dependent phosphorylation of Ser896 and Ser897 in GluN1 subunits in dorsal horn neurons is observed in models of inflammatory, neuropathic, cancer and visceral pain (Ultenius et al., 2006;Tian et al., 2008;Zhang et al., 2008a;Zhang et al., 2008b;Yang et al., 2009). GluN1 phosphorylation increases the amplitude of NMDAR-mediated EPSCs by increasing the opening rate of NMDAR channels, or modulating the synaptic targeting of NMDARs (Chen and Roche 2007). Besides the GluN1 subunit, increased GluN2B-Ser1303 phosphorylation is observed in capsaicin-induced pain hypersensitivity, but the significance of this phosphorylation site remains to be investigated (Zhang et al., 2005).
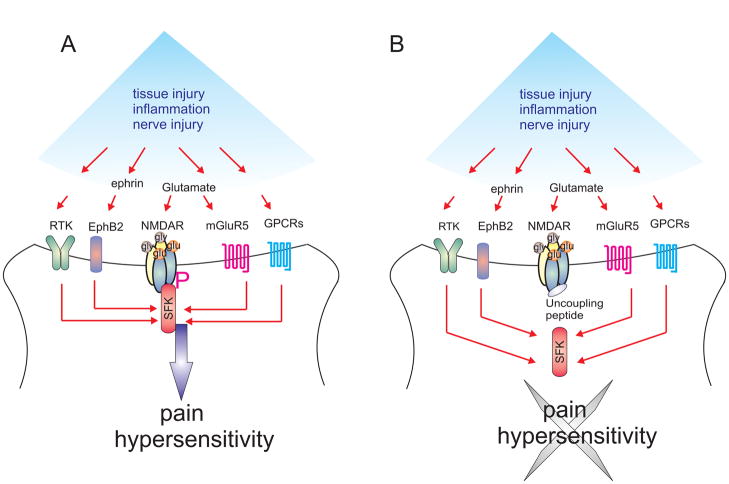
In addition to serine-threonine phosphorylation, the function of NMDARs is also regulated by the balance of activity of the protein tyrosine kinase Src and striatal enriched tyrosine phosphatase (STEP) (Salter 2005). NMDAR tyrosine phosphorylation appears critical for triggering and maintaining pain hypersensitivities induced by inflammation or nerve injury (Guo et al., 2002a;Liu et al., 2008). There are 25 tyrosine residues in the carboxyl tail of the GluN2B subunit that could be tyrosine phosphorylated (Nakazawa et al., 2001). The exact tyrosine phosphorylation sites involved in pain hypersensitivity are yet to be determined definitely, but Y1472 and Y1336 of the GluN2B subunit have been implicated (Abe et al., 2005;Slack et al., 2008;Peng et al., 2010). Inflammation-induced spinal tyrosine phosphorylation is observed within minutes following inflammation, and lasts for days. Genetic deletion of the Src gene (Liu et al., 2008) or pharmacological inhibition with the Src family kinase (SFK) inhibitor PP2 (Guo et al., 2004;Liu et al., 2008;Peng et al., 2010) suppresses inflammation-induced tyrosine phosphorylation of spinal GluN2B and reverses pain hypersensitivity. Increased tyrosine phosphorylation of spinal GluN2B subunits is also observed following nerve injury (Abe et al., 2005;Liu et al., 2008). This enhancement lasts weeks after PNI, indicating that there is a sustained and ongoing increase of activity of Src relative to that of STEP (Liu et al., 2008). Electrophysiology studies also support SFKs are involved in PNI-induced upregulation of NMDARs, as PP2 selectively suppresses the NMDAR-component EPSC evoked by Aβ-fiber stimulation in nerve-injured rats but has little effect on AMPA component EPSC (Hu et al., 2006b). Furthermore, in Src null mutant mice (Liu et al., 2008) or Fyn null mutant mice (Abe et al., 2005), PNI-induced spinal GluN2B phosphorylation and mechanical allodynia are markedly attenuated, indicating both Src and Fyn maybe involved in PNI-induced NMDAR upregulation. Importantly, the basal sensory thresholds and the first phase formalin-induced behavior are unchanged in Src null mutant mice, indicating that Src is essential for chronic pain rather than acute pain (Liu et al., 2008). Thus, biochemical, genetic, electrophysiological and behavioral evidence all point to a crucial role of SFK-mediated NMDAR upregulation in chronic pain hypersensitivity. Inflammation or nerve injury-induced activation of G protein coupled receptors (GPCRs), such as group I mGluRs and EPI receptors or receptor tyrosine kinases, such as EphB2 receptors, and intracellular signaling pathways such as IP3 and PKC all converged in Src to upregulate NMDARs (Guo et al., 2004;Abe et al., 2005;Peng et al., 2010) (Fig 3A). The phosphorylation of NMDARs by SFKs will increase channel open time and open probability, leading to increased NMDAR mediated synaptic currents(Salter 2005), thus resulting in pain hypersensitivity.
Fig 3.
Src family kinases (SFKs) are key enhancers of NMDAR-mediated pain hypersensitivity. (A) Inflammation or nerve injury activates multiple signaling pathways, including G-protein-coupled receptor (GPCR) pathways, such as mGluR5 or receptor tyrosine kinase pathways (RTK), such as EphB2, to activate SFKs which upregulates NMDAR function and results in pain hypersensitivity. (B) Uncoupling SFKs from the NMDAR complex could be a new strategy to inhibit pain without the deleterious consequence of directly blocking NMDARs.
As Src-dependent phosphorylation of NMDARs is critically involved in both inflammatory pain and neuropathic pain, a 10-amino-acid peptide derived from Src unique domain was designed as a fusion with the protein transduction domain of HIV Tat protein (Src40-49Tat), rendering the peptide membrane permeant (Liu et al., 2008). Src40-49Tat uncouples Src from the NMDA complex, therefore, inhibiting Src mediated NMDAR upregulation. Intravenous or intrathecal administration of Src40-49Tat reverses inflammation and PNI-induced mechanical, thermal and cold pain hypersensitivity, without changing basal sensory thresholds and acute chemical nociception. Furthermore, no confounding sedation, motor deficit or learning and memory impairment was observed at doses that suppress pain hypersensitivity (Liu et al., 2008). As the peptide is derived from Src unique domain, it disrupts the binding of Src unique domain with the NMDAR complex through its adaptor protein, NADH dehydrogenase subunit 2, therefore avoiding potential confounding effects of general inhibition of the SFK family (Liu et al., 2008). Thus, by uncoupling Src, from the NMDAR complex inhibits pain hypersensitivity while avoiding the deleterious consequence of directly blocking NMDARs (Bleakman et al., 2006;Childers and Baudy 2007) (Fig 3B).
In addition to the phosphorylation of NMDAR subunits, the function and cell surface expression of NMDARs are also affected by membrane-associated guanylate kinase (MAGUK) proteins. The MAGUK proteins are intracellular scaffolding proteins which are comprised of a family of four main members, PSD-95, PSD-93, SAP102 and SAP 97. PSD-95 and PSD-93 are expressed in the superficial lamina of dorsal horn, and implied in nociception through their interaction with the C-termini of GluN2 subunits (Tao and Johns 2006). Spinal knockdown of PSD-93 (Zhang et al., 2003) or PSD-95 (Tao et al., 2001) markedly reduces thermal and mechanical hypersensitivity-induced by PNI or inflammation. Furthermore, PSD-93 null mutant mice or transgenic mice expressing a truncated version of PSD-95 are protected from pain hypersensitivity induced by inflammation or PNI (Tao et al., 2003;Garry et al., 2003a). Intriguingly, the molecular mechanisms underlying PSD-93 and PSD-95 in pain hypersensitivity are different. Mice with PSD-93 deletion exhibit a marked reduction in GluN2A and GluN2B tyrosine phosphorylation and decreased synaptic expression of NMDARs, accompanied with reduced NMDAR-dependent EPSC in brain and spinal cord (Tao et al., 2003;Sato et al., 2008;Liaw et al., 2008). In contrast, the synaptic NMDAR expression and NMDAR-mediated EPSC are unaltered in PSD-95 truncated mice (Migaud et al., 1998). Therefore, the anti-hyperalgesic effect observed by disrupting NMDAR-PSD95 is likely due to blocking the downstream effectors of NMDAR activation, rather than suppressing NMDAR activity per se (Tao et al., 2008). One of the downstream effectors is nNOS, as disrupting PSD-95 association with nNOS also reduces thermal hyperalgesia induced by PNI (Florio et al., 2009).
Collectively, the function of synaptic t NMDAR in spinal dorsal horn neurons is upregulated following inflammation or PNI, likely through serine/threonine phosphorylation of GluN1 subunits and/or tyrosine phosphorylation of GluN2B subunits. Selectively dissociating NMDARs from key enhancers of receptor function could be a new strategy to inhibit pain hypersensitivity. Alternatively, disrupting NMDAR interaction with scaffolding proteins and the downstream effectors could also provide novel targets for pain treatment.
METABOTROPIC GLUTAMATE RECEPTORS IN NOCICEPTION AND PAIN
The metabotropic GluRs (mGluRs) belong to the C-subfamily of G protein-coupled receptors (GPCRs) (Ritter and Hall 2009). The predicted mGluR topology includes a large N-terminal extracellular domain, three extracellular loops, three intracellular loops and a cytoplasmic C-terminus, separated by seven highly hydrophobic regions that span the lipid bilayer. The large N-terminal portion of the receptor forms a pocket which contains the glutamate-binding site, whereas the intracellular domains physically interact with the G-proteins to initiate signal transduction events (Ferraguti and Shigemoto 2006). Eight mGlu receptors have been identified and they are segregated into three groups: group I mGluRs (mGlu1 and mGlu5) are coupled to phospholipase C, and activation of this group of receptors generally increases neuronal excitability; group II (mGlu2 and mGlu3), and group III (mGlu4-mGlu8) mGluRs are negatively coupled to adenylate cyclase and activation of these two groups of receptors generally reduces neuronal excitability (Ferraguti and Shigemoto 2006). All three groups of the mGluRs are distributed throughout the central nervous system and are involved in various forms of pain hypersensitivity.
Group I metabotropic glutamate receptors: nociception and pain plasticity
Group I metabotropic glutamate receptors, mGluR1 and mGluR5, are expressed in both peripheral and spinal nociceptive pathways (Fig 2). In peripheral sensory afferents, group I mGluR receptors are expressed in both myelinated and unmyelinated peripheral afferents. In DRG cell bodies, a proportion of mGluR5-positive neurons also express transient receptor potential cation channel TRPV1 (Walker et al., 2000;Zhou et al., 2001).
In the spinal cord, mGlu1 and mGlu5 receptors are expressed in both soma and dendrites of dorsal horn neurons, with mGluR5s localized in the superficial laminae, and mGluR1αs expressed throughout the rest of the dorsal horn with high levels in lamina V (Wang and Tseng 2004;Pitcher et al., 2007). The expression of group I metabotropic glutamate receptors is altered in models of inflammatory or neuropathic pain. In DRG and dorsal horn neurons, the expression level of mGluR5s is upregulated following inflammation, PNI and diabetic neuropathy (Hudson et al., 2002;Pitcher et al., 2007;Osikowicz et al., 2009;Li et al., 2009). Inflammation also leads to a recruitment of mGluR5s to the plasma membrane, and lateral movement of plasma membrane associated mGluR1αs toward the synapse (Pitcher et al., 2007).
Systemic or intrathecal administration of selective group I mGlu receptor antagonists attenuates pain hypersensitivity induced by inflammation, PNI and streptozotocin-induced diabetic neuropathy (Hudson et al., 2002;Soliman et al., 2005;Schkeryantz et al., 2007;Osikowicz et al., 2009). The systemic effect is likely mediated through both peripheral and central mechanisms. The peripheral effect is demonstrated by subcutaneous intraplantar administration of group I mGluR antagonists into the rat hindpaw. Ipsilateral, but not contralateral, paw injection reduces nociceptive responses and mechanical or thermal hyperalgesia induced by inflammation and PNI (Walker et al., 2000;Dogrul et al., 2000;Bhave et al., 2001), indicating that these effects are mediated peripheral locally. However, intrathecal administration of selective mGlu1 or mGluR5 receptor antagonists also reduces pain hypersensitivity induced by inflammation (Soliman et al., 2005) or PNI (Dogrul et al., 2000). Although a supraspinal effect could not be excluded by intrathecal injection, spinal antisense knockdown of mGluR1reduces pain hypersensitivity-induced by inflammation or PNI, further supporting that spinal mGluR1s are involved (Fundytus et al., 2002;Noda et al., 2003). Both pharmacological inhibition and genetic deletion of group I mGluRs reduces the second phase but not the first phase of formalin induced behaviors, indicating that mGluR1 and mGluR5 are selectively involved in chronic pain rather than acute pain (Karim et al., 2001;Noda et al., 2003).
Both peripheral and spinal mechanisms appear to contribute to group I mGluRs in regulating pain hypersensitivity. In primary sensory afferents, group I mGluRs are functionally coupled with TRPV1 receptors, and activation of TRPV1 in primary sensory afferents by inflammation or nerve injury leads to glutamate release from both peripheral and central terminals (Jin et al., 2009). This will subsequently activate mGluR5s, which in turn sensitize TRPV1 receptors through PLC, PGE2 and PKA pathways (Hu et al., 2002). TRPV1 receptor activation will further increase intracellular Ca2+and depolarizing primary sensory afferents, leading to increased quantal release of glutamate (Hu et al., 2002;Kim et al., 2009)(Fig 2B). There are several lines of evidence supporting that mGluR5s are functionally linked with TRPV1 receptors in the nociceptor. For example, in spinal cord slices, the presynaptic quanta release of glutamate evoked by mGluR1 and mGluR5 agonists is blocked by capsazepine, a TRPV1 receptor antagonist (Park et al., 2004;Kim et al., 2009). Furthermore, pain hypersensitivity-induced by spinal application of a group I mGluR agonist is markedly reduced in TRPV1 null mutant mice (Kim et al., 2009). At the cellular level, activation of group I mGluR evokes extracellular Ca2+ influx in TRPV1 positive DRG neurons, and this effect is blocked by a mGluR5 receptor antagonist (Kim et al., 2009). Therefore, converging evidence indicates that mGluR5 are functionally coupled with TRPV1 receptor to regulate primary sensory afferent activation and presynaptic neurotransmitter release.
Postsynaptically, activation of spinal group I mGluRs in dorsal horn neurons increases synaptic transmission through at least two pathways. First, activation of postsynaptic mGluR5 increases intracellular Ca2+ through IP3 receptor, resulting in PKC activation and subsequent Src activation. NMDARs are then subsequently phosphorylated, resulting in increased synaptic transmission (Guo et al., 2004) (Fig 2B). Secondly, activation of group I mGluRs leads to an activation of extracellular signal regulated kinase (ERK) (Adwanikar et al., 2004;Hu et al., 2007), and activation of ERK through mGluR5 will phosphorylate K+ channel subunit Kv4.2 at the S616 phosphorylation site (Hu et al., 2006a;Hu et al., 2007), resulting in increased neuronal excitability (Fig 2B). Therefore, both presynaptic and postsynaptic group I mGluRs play important roles in developing and sustaining pain hypersensitivity.
Thus, group I mGlu receptors in both primary sensory neurons and dorsal horn neurons are involved in pain hypersensitivity induced by inflammatory and PNI. Presynaptically, mGluR5 functionally couples with TRPV1 to regulate primary sensory afferents activation and presynaptic glutamate release. Postsynaptically, activation of mGluR5 leads to increased neuronal excitability and synaptic transmission by K+ channel subunit Kv4.2 phosphorylation and Src-mediated NMDAR phosphorylation.
Group II and III metabotropic glutamate receptors: emerging role in nociception
Both group II and III mGluRs are predominately expressed presynaptically in primary sensory DRG neurons and axons. For group II mGluRs, most mGluR2/mGluR3-positive cells are small diameter, TRPV1-positive neurons, and the majority of them co-express with IB4 and P2X3 (Carlton et al., 2009). For group III receptors, the presence of mGluR4 and mGluR7 have been confirmed in DRG neurons and the central terminals in lamina I and the outer zone of lamina II in the spinal cord (Carlton and Hargett 2007;Zhou et al., 2007).
Group II and III mGluRs are negatively coupled to the cAMP pathway. Activation of these receptors in primary sensory afferents inhibits nociceptor sensitization and presynaptic neurotransmitter release, thus depressing synaptic transmission in dorsal horn (Gerber et al., 2000;Du et al., 2008;Carlton et al., 2009)(Fig 2B). Pharmacological activation of group II and III mGluRs inhibits pain hypersensitivity in various models. For example, systemically or intraplantar administration of mGluR2/3 agonists reduces inflammation- and PNI-induced pain hypersensitivity (Du et al., 2008;Osikowicz et al., 2009;Chiechio et al., 2009). Systemic or spinal administration of group III mGluRs also inhibits both phases of formalin-induced nociceptive behaviors and attenuates mechanical hyperalgesia induced by inflammation or PNI (Marabese et al., 2007;Goudet et al., 2008;Dolan et al., 2009).
Collectively, these studies indicate that the activation of group II and III mGluRs localized at peripheral and central terminal of sensory afferents suppresses pain hypersensitivity induced by inflammation or PNI. Therefore, activation of group II and group III mGluRs could be novel targets for pain treatment. Further studies are necessary to understand the molecular mechanism of these two receptor groups in nociception regulation.
Conclusions
Glutamate receptors have been the target for the development of pain treatments for decades. However, despite much encouraging preclinical data, truly safe and effective glutamate-based treatments for the devastating condition of chronic pain are yet to be developed.
Recent advance in our understanding of glutamate receptor regulation in pain plasticity provides new insights for developing novel glutamate receptor based pain management strategies. There is now abundant evidence indicates that glutamate receptor phosphorylation and trafficking play important roles in pain hypersensitivity. In spinal dorsal horn synapses, inflammation-induced the serine/threonine phosphorylation of AMPAR subunits, the insertion of GluA1 subunits into the synapses and the internalization of the GluA2 subunits lead to upregulated AMPAR-mediated synaptic efficacy. Inflammation and PNI-induced membrane depolarization relieves the Mg2+ block of NDMARs and boosting up the synaptic efficacy in a non-liner manner. NMDARs are also upregulated through serine/threonine and tyrosine phosphorylation, resulting in increased NMDAR mediated synaptic potentiation. Furthermore, sustained release of presynaptic glutamate activates post-synaptic group I mGluRs, resulting G protein activation. The activated G protein subunits then associate with downstream effectors to inhibit K+ channels or to facilitate NMDARs through Src-mediated phosphorylation. The end result of AMPAR, NMDAR and group I mGluR activation is sustained increased neuronal excitability and enhanced synaptic transmission, which result in pain hypersensitivity following inflammation of PNI. Disrupting these cellular and molecular pathways provide new opportunities to inhibit pain without the side effects of blocking glutamate receptors per se.
A main challenge for the above mentioned approach is that protein kinases that upregulate glutamate receptors in dorsal horn synapse are also involved in many other physiological pathways. Therefore, general inhibition of these kinases may not be a practical solution. Several recent studies demonstrate an alternative approach to inhibit pain hypersensitivity is to disrupt the upstream regulatory signaling pathways or the downstream transduction pathways from glutamate receptors. Disrupting protein-protein interaction with peptides, peptidomimetics or small molecules is therefore, a potential strategy for developing glutamate receptor-based therapies. As we continue to discover molecular and cellular mechanisms of glutamate receptor regulation and their role in pain hypersensitivity, we expect to identify more promising targets for chronic pain management.
Acknowledgments
Work of the authors is supported by grants from the Canadian Institutes of Health Research (CIHR; grant number MT-12682) and the Neuroscience Canada Brain Repair Program to MWS. MWS is an International Research Scholar of the Howard Hughes Medical Institute and holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain. XJL was a CIHR & Ronald Melzack Pain Research Award recipient, and a fellow of Canadian Arthritis Network as well as of the Pain: Molecules to Community Strategic Training Initiative in Health Research of CIHR. We thank G.M. Pitcher, S. Quilop and J. Hicks for helpful comments on the manuscript.
Reference List
- Abe T, Matsumura S, Katano T, Mabuchi T, Takagi K, Xu L, Yamamoto A, Hattori K, Yagi T, Watanabe M, Nakazawa T, Yamamoto T, Mishina M, Nakai Y, Ito S. Fyn kinase-mediated phosphorylation of NMDA receptor NR2B subunit at Tyr1472 is essential for maintenance of neuropathic pain. Eur J Neurosci. 2005;22:1445–1454. doi: 10.1111/j.1460-9568.2005.04340.x. [DOI] [PubMed] [Google Scholar]
- Adwanikar H, Karim F, Gereau RW. Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol. 2004;472:257–280. doi: 10.1002/cne.20012. [DOI] [PubMed] [Google Scholar]
- Antal M, Fukazawa Y, Eordogh M, Muszil D, Molnar E, Itakura M, Takahashi M, Shigemoto R. Numbers, densities, and colocalization of AMPA-and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azkue JJ, Liu XG, Zimmermann M, Sandkuhler J. Induction of long-term potentiation of C fibre-evoked spinal field potentials requires recruitment of group I, but not group II/III metabotropic glutamate receptors. Pain. 2003;106:373–379. doi: 10.1016/j.pain.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave G, Karim F, Carlton SM, Gereau RW. Peripheral group I metabotropic glutamate receptors modulate nociception in mice. Nat Neurosci. 2001;4:417–423. doi: 10.1038/86075. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Alt A, Nisenbaum ES. Glutamate receptors and pain. Seminars in Cell & Developmental Biology. 2006;17:592–604. doi: 10.1016/j.semcdb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL. Colocalization of metabotropic glutamate receptors in rat dorsal root ganglion cells. J Comp Neurol. 2007;501:780–789. doi: 10.1002/cne.21285. [DOI] [PubMed] [Google Scholar]
- Carlton SM. Peripheral NMDA receptors revisited - Hope floats. Pain. 2009;146:1–2. doi: 10.1016/j.pain.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Du J, Zhou S. Group II metabotropic glutamate receptor activation on peripheral nociceptors modulates TRPV1 function. Brain Research. 2009;1248:86–95. doi: 10.1016/j.brainres.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marviz JCG. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiechio S, Zammataro M, Morales ME, Busceti CL, Drago F, Gereau RW, Copani A, Nicoletti F. Epigenetic modulation of mGlu2 receptors by histone deacetylase inhibitors in the treatment of inflammatory pain. Mol Pharmacol. 2009;75:1014–1020. doi: 10.1124/mol.108.054346. [DOI] [PubMed] [Google Scholar]
- Childers WE, Baudy RB. N-Methyl-d-Aspartate Antagonists and Neuropathic Pain: The Search for Relief. Journal of Medicinal Chemistry. 2007;50:2557–2562. doi: 10.1021/jm060728b. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Olsen RW, Peters J, Spedding M. A nomenclature for ligand-gated ion channels. Neuropharmacology. 2009;56:2–5. doi: 10.1016/j.neuropharm.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogrul A, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Peripheral and spinal antihyperalgesic activity of SIB-1757, a metabotropic glutamate receptor (mGLUR(5)) antagonist, in experimental neuropathic pain in rats. Neurosci Lett. 2000;292:115–118. doi: 10.1016/s0304-3940(00)01458-0. [DOI] [PubMed] [Google Scholar]
- Dolan S, Gunn MD, Biddlestone L, Nolan AM. The selective metabotropic glutamate receptor 7 allosteric agonist AMN082 inhibits inflammatory pain-induced and incision-induced hypersensitivity in rat. Behav Pharmacol. 2009 doi: 10.1097/FBP.0b013e32832ec5d1. [DOI] [PubMed] [Google Scholar]
- Dominguez E, Iyengar S, Shannon HE, Bleakman D, Alt A, Arnold BM, Bell MG, Bleisch TJ, Buckmaster JL, Castano AM, Del PM, Escribano A, Filla SA, Ho KH, Hudziak KJ, Jones CK, Martinez-Perez JA, Mateo A, Mathes BM, Mattiuz EL, Ogden AM, Simmons RM, Stack DR, Stratford RE, Winter MA, Wu Z, Ornstein PL. Two prodrugs of potent and selective GluR5 kainate receptor antagonists actives in three animal models of pain. J Med Chem. 2005;48:4200–4203. doi: 10.1021/jm0491952. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Carlton SM. Group II metabotropic glutamate receptor activation attenuates peripheral sensitization in inflammatory states. Neuroscience. 2008;154:754–766. doi: 10.1016/j.neuroscience.2008.03.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Molecular Brain Research. 2003a;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Fang L, Wu J, Zhang X, Lin Q, Willis WD. Increased phosphorylation of the GluR1 subunit of spinal cord [alpha]-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor in rats following intradermal injection of capsaicin. Neuroscience. 2003b;122:237–245. doi: 10.1016/s0306-4522(03)00526-8. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell and Tissue Research. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Fleming JJ, England PM. AMPA receptors and synaptic plasticity: a chemist’s perspective. Nat Chem Biol. 2010;6:89–97. doi: 10.1038/nchembio.298. [DOI] [PubMed] [Google Scholar]
- Florio SK, Loh C, Huang SM, Iwamaye AE, Kitto KF, Fowler KW, Treiberg JA, Hayflick JS, Walker JM, Fairbanks CA, Lai Y. Disruption of nNOS-PSD95 protein-protein interaction inhibits acute thermal hyperalgesia and chronic mechanical allodynia in rodents. Br J Pharmacol. 2009;158:494–506. doi: 10.1111/j.1476-5381.2009.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fundytus ME, Osborne MG, Henry JL, Coderre TJ, Dray A. Antisense oligonucleotide knockdown of mGluR1 alleviates hyperalgesia and allodynia associated with chronic inflammation. Pharmacol Biochem Behav. 2002;73:401–410. doi: 10.1016/s0091-3057(02)00831-6. [DOI] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Galan A, Laird JM, Cervero F. In vivo recruitment by painful stimuli of AMPA receptor subunits to the plasma membrane of spinal cord neurons. Pain. 2004;112:315–323. doi: 10.1016/j.pain.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Garraway SM, Xu Q, Inturrisi CE. siRNA-mediated knockdown of the NR1 subunit gene of the NMDA receptor attenuates formalin-induced pain behaviors in adult rats. J Pain. 2009;10:380–390. doi: 10.1016/j.jpain.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry EM, Moss A, Delaney A, O’Neill F, Blakemore J, Bowen J, Husi H, Mitchell R, Grant SG, Fleetwood-Walker SM. Neuropathic sensitization of behavioral reflexes and spinal NMDA receptor/CaM kinase II interactions are disrupted in PSD-95 mutant mice. Curr Biol. 2003a;13:321–328. doi: 10.1016/s0960-9822(03)00084-8. [DOI] [PubMed] [Google Scholar]
- Garry EM, Moss A, Rosie R, Delaney A, Mitchell R, Fleetwood-Walker SM. Specific involvement in neuropathic pain of AMPA receptors and adapter proteins for the GluR2 subunit. Mol Cell Neurosci. 2003b;24:10–22. doi: 10.1016/s1044-7431(03)00134-9. [DOI] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100:393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Goudet C, Chapuy E, Alloui A, Acher F, Pin JP, Eschalier A. Group III metabotropic glutamate receptors inhibit hyperalgesia in animal models of inflammation and neuropathic pain. Pain. 2008;137:112–124. doi: 10.1016/j.pain.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, IV, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Research Reviews. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Gu X, Wu X, Liu Y, Cui S, Ma Z. Tyrosine phosphorylation of the N-Methyl-D-Aspartate receptor 2B subunit in spinal cord contributes to remifentanil-induced postoperative hyperalgesia: the preventive effect of ketamine. Molecular Pain. 2009;5:76. doi: 10.1186/1744-8069-5-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Wei F, Zou S, Robbins MT, Sugiyo S, Ikeda T, Tu JC, Worley PF, Dubner R, Ren K. Group I metabotropic glutamate receptor NMDA receptor coupling and signaling cascade mediate spinal dorsal horn NMDA receptor 2B tyrosine phosphorylation associated with inflammatory hyperalgesia. J Neurosci. 2004;24:9161–9173. doi: 10.1523/JNEUROSCI.3422-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Guan Y, Ikeda T, Tal M, Dubner R, Ren K. Tyrosine phosphorylation of the NR2B subunit of the NMDA receptor in the spinal cord during the development and maintenance of inflammatory hyperalgesia. J Neurosci. 2002a;22:6208–6217. doi: 10.1523/JNEUROSCI.22-14-06208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zou S, Tal M, Ren K. Activation of spinal kainate receptors after inflammation: behavioral hyperalgesia and subunit gene expression. Eur J Pharmacol. 2002b;452:309–318. doi: 10.1016/s0014-2999(02)02333-6. [DOI] [PubMed] [Google Scholar]
- Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Hartmann B, Ahmadi S, Heppenstall PA, Lewin GR, Schott C, Borchardt T, Seeburg PH, Zeilhofer HU, Sprengel R, Kuner R. The AMPA receptor subunits GluR-A and GluR-B reciprocally modulate spinal synaptic plasticity and inflammatory pain. Neuron. 2004;44:637–650. doi: 10.1016/j.neuron.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Hegarty DM, Mitchell JL, Swanson KC, Aicher SA. Kainate receptors are primarily postsynaptic to SP-containing axon terminals in the trigeminal dorsal horn. Brain Res. 2007;1184:149–159. doi: 10.1016/j.brainres.2007.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Alter BJ, Carrasquillo Y, Qiu CS, Gereau RW., IV Metabotropic Glutamate Receptor 5 Modulates Nociceptive Plasticity via Extracellular Signal-Regulated Kinase Kv4.2 Signaling in Spinal Cord Dorsal Horn Neurons. J Neurosci. 2007;27:13181–13191. doi: 10.1523/JNEUROSCI.0269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Bhave G, Gereau RW., IV Prostaglandin and Protein Kinase A-Dependent Modulation of Vanilloid Receptor Function by Metabotropic Glutamate Receptor 5: Potential Mechanism for Thermal Hyperalgesia. J Neurosci. 2002;22:7444–7452. doi: 10.1523/JNEUROSCI.22-17-07444.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RW., IV The Kv4.2 Potassium Channel Subunit Is Required for Pain Plasticity. Neuron. 2006a;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Hu XD, Hu NW, Xin WJ, Zhou LJ, Zhang T, Liu XG. Inhibition of protein tyrosine kinases attenuated abeta-fiber-evoked synaptic transmission in spinal dorsal horn of rats with sciatic nerve transection. J Pharmacol Sci. 2006b;102:64–71. doi: 10.1254/jphs.fp0060492. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lu W, Ali DW, Pelkey KA, Pitcher GM, Lu YM, Aoto H, Roder JC, Sasaki T, Salter MW, MacDonald JF. CAKbeta/Pyk2 kinase is a signaling link for induction of long-term potentiation in CA1 hippocampus. Neuron. 2001;29:485–496. doi: 10.1016/s0896-6273(01)00220-3. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, McNair K, Gentry C, Fox A, Kuhn R, Winter J. Metabotropic glutamate receptor 5 upregulation in A-fibers after spinal nerve injury: 2-methyl-6-(phenylethynyl)-pyridine (MPEP) reverses the induced thermal hyperalgesia. J Neurosci. 2002;22:2660–2668. doi: 10.1523/JNEUROSCI.22-07-02660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Heinke B, Ruscheweyh R, Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Iwata H, Takasusuki T, Yamaguchi S, Hori Y. NMDA receptor 2B subunit-mediated synaptic transmission in the superficial dorsal horn of peripheral nerve-injured neuropathic mice. Brain Research. 2007;1135:92–101. doi: 10.1016/j.brainres.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Jin YH, Yamaki F, Takemura M, Koike Y, Furuyama A, Yonehara N. Capsaicin-Induced Glutamate Release Is Implicated in Nociceptive Processing Through Activation of Ionotropic Glutamate Receptors and Group I Metabotropic Glutamate Receptor in Primary Afferent Fibers. Journal of Pharmacological Sciences. 2009;109:233–241. doi: 10.1254/jphs.08262fp. [DOI] [PubMed] [Google Scholar]
- Jones CK, Alt A, Ogden AM, Bleakman D, Simmons RM, Iyengar S, Dominguez E, Ornstein PL, Shannon HE. Antiallodynic and antihyperalgesic effects of selective competitive GLUK5 (GluR5) ionotropic glutamate receptor antagonists in the capsaicin and carrageenan models in rats. J PharmacolExp Ther. 2006;319:396–404. doi: 10.1124/jpet.106.105601. [DOI] [PubMed] [Google Scholar]
- Karim F, Wang CC, Gereau RW., IV Metabotropic Glutamate Receptor Subtypes 1 and 5 Are Activators of Extracellular Signal-Regulated Kinase Signaling Required for Inflammatory Pain in Mice. JNeurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katano T, Furue H, Okuda-Ashitaka E, Tagaya M, Watanabe M, Yoshimura M, Ito S. N-ethylmaleimide-sensitive fusion protein (NSF) is involved in central sensitization in the spinal cord through GluR2 subunit composition switch after inflammation. Eur J Neurosci. 2008;27:3161–3170. doi: 10.1111/j.1460-9568.2008.06293.x. [DOI] [PubMed] [Google Scholar]
- Kessels HW, Malinow R. Synaptic AMPA Receptor Plasticity and Behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JNC, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park CK, Back SK, Lee CJ, Hwang SJ, Bae YC, Na HS, Kim JS, Jung SJ, Oh SB. Membrane-Delimited Coupling of TRPV1 and mGluR5 on Presynaptic Terminals of Nociceptive Neurons. JNeurosci. 2009;29:10000–10009. doi: 10.1523/JNEUROSCI.5030-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S, Zhao MG, Toyoda H, Qiu CS, Zhuo M. Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci. 2005;25:977–984. doi: 10.1523/JNEUROSCI.4059-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M. Ionotropic Glutamate Receptors in Spinal Nociceptive Processing. Mol Neurobiol. 2009 doi: 10.1007/s12035-009-8086-8. [DOI] [PubMed] [Google Scholar]
- Larsson M, Broman J. Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci. 2008;28:7084–7090. doi: 10.1523/JNEUROSCI.5749-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Broman J. Synaptic Plasticity and Pain: Role of Ionotropic Glutamate Receptors. Neuroscientist. 2010 doi: 10.1177/1073858409349913. 1073858409349913. [DOI] [PubMed] [Google Scholar]
- Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Kong H, Manzini MC, Albuquerque C, Chao MV, MacDermott AB. Kainate receptors expressed by a subpopulation of developing nociceptors rapidly switch from high to low Ca2+ permeability. J Neurosci. 2001;21:4572–4581. doi: 10.1523/JNEUROSCI.21-13-04572.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CJ, Bardoni R, Tong CK, Engelman HS, Joseph DJ, Magherini PC, MacDermott AB. Functional Expression of AMPA Receptors on Central Terminals of Rat Dorsal Root Ganglion Neurons and Presynaptic Inhibition of Glutamate Release. Neuron. 2002;35:135–146. doi: 10.1016/s0896-6273(02)00729-8. [DOI] [PubMed] [Google Scholar]
- Lee HK. Synaptic plasticity and phosphorylation. Pharmacology & Therapeutics. 2006;112:810–832. doi: 10.1016/j.pharmthera.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006a doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Li JQ, Chen SR, Chen H, Cai YQ, Pan HL. Regulation of increased glutamatergic input to spinal dorsal horn neurons by mGluR5 in diabetic neuropathic pain. J Neurochem. 2009 doi: 10.1111/j.1471-4159.2009.06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Am J Physiol Gastrointest Liver Physiol. 2006b;291:G219–G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Liaw WJ, Zhu XG, Yaster M, Johns R, Gauda E, Tao YX. Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Molecular Pain. 2008;4:45. doi: 10.1186/1744-8069-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu XJ, Gingrich JR, Vargas-Caballero M, Dong YN, Sengar A, Beggs S, Wang SH, Ding HK, Frankland PW, Salter MW. Treatment of inflammatory and neuropathic pain by uncoupling Src from the NMDA receptor complex. Nat Med. 2008;14:1325–1332. doi: 10.1038/nm.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci. 2009;12:547–548. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sun YN, Wu X, Sun Q, Liu FY, Xing GG, Wan Y. Role of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor subunit GluR1 in spinal dorsal horn in inflammatory nociception and neuropathic nociception in rat. Brain Res. 2008;1200:19–26. doi: 10.1016/j.brainres.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Luo C, Seeburg PH, Sprengel R, Kuner R. Activity-dependent potentiation of calcium signals in spinal sensory networks in inflammatory pain states. Pain. 2008;140:358–367. doi: 10.1016/j.pain.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Gilbert H, McCabe RT, Basbaum AI. Powerful antinociceptive effects of the cone snail venom-derived subtype-selective NMDA receptor antagonists conantokins G and T. Pain. 2003;101:109–116. doi: 10.1016/s0304-3959(02)00303-2. [DOI] [PubMed] [Google Scholar]
- Marabese I, de NV, Palazzo E, Scafuro MA, Vita D, Rossi F, Maione S. Effects of (S)-3,4-DCPG, an mGlu8 receptor agonist, on inflammatory and neuropathic pain in mice. Neuropharmacology. 2007;52:253–262. doi: 10.1016/j.neuropharm.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Migaud M, Charlesworth P, Dempster M, Webster LC, Watabe AM, Makhinson M, He Y, Ramsay MF, Morris RG, Morrison JH, O’Dell TJ, Grant SG. Enhanced long-term potentiation and impaired learning in mice with mutant postsynaptic density-95 protein. Nature. 1998;396:433–439. doi: 10.1038/24790. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Ionic basis for plateau potentials in deep dorsal horn neurons of the rat spinal cord. J Neurosci. 1999;19:7309–7316. doi: 10.1523/JNEUROSCI.19-17-07309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004a;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Watanabe M, Fukaya M, Todd AJ. Synaptic distribution of the NR1, NR2A and NR2B subunits of the N-methyl-d-aspartate receptor in the rat lumbar spinal cord revealed with an antigen-unmasking technique. Eur J Neurosci. 2004b;20:3301–3312. doi: 10.1111/j.1460-9568.2004.03798.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa T, Komai S, Tezuka T, Hisatsune C, Umemori H, Semba K, Mishina M, Manabe T, Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluR epsilon 2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- Narita M, Miyoshi K, Narita M, Suzuki T. Changes in function of NMDA receptor NR2B subunit in spinal cord of rats with neuropathy following chronic ethanol consumption. Life Sciences. 2007;80:852–859. doi: 10.1016/j.lfs.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, Not Noxious, Input Activates PKC{gamma} Interneurons of the Spinal Dorsal Horn via Myelinated Afferent Fibers. JNeurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Anzai T, Ogata M, Akita H, Ogura T, Saji M. Antisense knockdown of spinal-mGluR1 reduces the sustained phase of formalin-induced nociceptive responses. Brain Res. 2003;987:194–200. doi: 10.1016/s0006-8993(03)03330-4. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- Osikowicz M, Skup M, Mika J, Makuch W, Czarkowska-Bauch J, Przewlocka B. Glial inhibitors influence the mRNA and protein levels of mGlu2/3, 5 and 7 receptors and potentiate the analgesic effects of their ligands in a mouse model of neuropathic pain. Pain. 2009;147:175–186. doi: 10.1016/j.pain.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Palecek J, Neugebauer V, Carlton SM, Iyengar S, Willis WD. The effect of a kainate GluR5 receptor antagonist on responses of spinothalamic tract neurons in a model of peripheral neuropathy in primates. Pain. 2004;111:151–161. doi: 10.1016/j.pain.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Current Opinion in Pharmacology. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Park JS, Yaster M, Guan X, Xu JT, Shih MH, Guan Y, Raja SN, Tao YX. Role of spinal cord alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in complete Freund’s adjuvant-induced inflammatory pain. Mol Pain. 2008;4:67. doi: 10.1186/1744-8069-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Voitenko N, Petralia RS, Guan X, Xu JT, Steinberg JP, Takamiya K, Sotnik A, Kopach O, Huganir RL, Tao YX. Persistent Inflammation Induces GluR2 Internalization via NMDA Receptor-Triggered PKC Activation in Dorsal Horn Neurons. JNeurosci. 2009;29:3206–3219. doi: 10.1523/JNEUROSCI.4514-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Galik J, Ryu PD, Randic M. Activation of presynaptic group I metabotropic glutamate receptors enhances glutamate release in the rat spinal cord substantia gelatinosa. Neuroscience Letters. 2004;361:220–224. doi: 10.1016/j.neulet.2003.12.075. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, Askalan R, Paul S, Kalia LV, Nguyen TH, Pitcher GM, Salter MW, Lombroso PJ. Tyrosine phosphatase STEP is a tonic brake on induction of long-term potentiation. Neuron. 2002;34:127–138. doi: 10.1016/s0896-6273(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lai CH, Tung KC, Chang JL, Lin TB. Endogenous ephrinB2 mediates colon-urethra cross-organ sensitization via Src kinase-dependent tyrosine phosphorylation of NR2B. Am J Physiol Renal Physiol. 2010;298:F109–F117. doi: 10.1152/ajprenal.00287.2009. [DOI] [PubMed] [Google Scholar]
- Pezet S, Marchand F, D’Mello R, Grist J, Clark AK, Malcangio M, Dickenson AH, Williams RJ, McMahon SB. Phosphatidylinositol 3-Kinase Is a Key Mediator of Central Sensitization in Painful Inflammatory Conditions. JNeurosci. 2008;28:4261–4270. doi: 10.1523/JNEUROSCI.5392-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher MH, Ribeiro-da-Silva A, Coderre TJ. Effects of inflammation on the ultrastructural localization of spinal cord dorsal horn group I metabotropic glutamate receptors. J Comp Neurol. 2007;505:412–423. doi: 10.1002/cne.21506. [DOI] [PubMed] [Google Scholar]
- Polgar E, Watanabe M, Hartmann B, Grant SG, Todd AJ. Expression of AMPA receptor subunits at synapses in laminae I–III of the rodent spinal dorsal horn. Mol Pain. 2008;4:5. doi: 10.1186/1744-8069-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu XX, Cai J, Li MJ, Chi YN, Liao FF, Liu FY, Wan Y, Han JS, Xing GG. Role of the spinal cord NR2B-containing NMDA receptors in the development of neuropathic pain. Exp Neurol. 2009;215:298–307. doi: 10.1016/j.expneurol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Randic M, Jiang MC, Cerne R. Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci. 1993;13:5228–5241. doi: 10.1523/JNEUROSCI.13-12-05228.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter SL, Hall RA. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol. 2009;10:819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MW. Cellular signalling pathways of spinal pain neuroplasticity as targets for analgesic development. Curr Top Med Chem. 2005;5:557–567. doi: 10.2174/1568026054367638. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Molecular Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkuhler J. Models and Mechanisms of Hyperalgesia and Allodynia. PhysiolRev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- Santos MS, Li H, Voglmaier SM. Synaptic vesicle protein trafficking at the glutamate synapse. Neuroscience. 2009;158:189–203. doi: 10.1016/j.neuroscience.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-d-aspartate receptors. Neuroscience. 2008;153:700–708. doi: 10.1016/j.neuroscience.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible HG, Freudenberger U, Neugebauer V, Stiller RU. Intraspinal release of immunoreactive calcitonin gene-related peptide during development of inflammation in the joint in vivo--a study with antibody microprobes in cat and rat. Neuroscience. 1994;62:1293–1305. doi: 10.1016/0306-4522(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Schkeryantz JM, Kingston AE, Johnson MP. Prospects for Metabotropic Glutamate 1 Receptor Antagonists in the Treatment of Neuropathic Pain. Journal of Medicinal Chemistry. 2007;50:2563–2568. doi: 10.1021/jm060950g. [DOI] [PubMed] [Google Scholar]
- Seal RP, Wang X, Guan Y, Raja SN, Woodbury CJ, Basbaum AI, Edwards RH. Injury-induced mechanical hypersensitivity requires C-low threshold mechanoreceptors. Nature. 2009;462:651–655. doi: 10.1038/nature08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack S, Battaglia A, Cibert-Goton V, Gavazzi I. EphrinB2 induces tyrosine phosphorylation of NR2B via Src-family kinases during inflammatory hyperalgesia. Neuroscience. 2008;156:175–183. doi: 10.1016/j.neuroscience.2008.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky AI, Rosconi MP, Gouaux E. X-ray structure, symmetry and mechanism of an AMPA-subtype glutamate receptor. Nature. 2009;462:745–756. doi: 10.1038/nature08624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AC, Yu JS, Coderre TJ. mGlu and NMDA receptor contributions to capsaicin-induced thermal and mechanical hypersensitivity. Neuropharmacology. 2005;48:325–332. doi: 10.1016/j.neuropharm.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Sorkin LSPh, Yaksh TLP, Doom CMB. Pain Models Display Differential Sensitivity to Ca2+-Permeable Non-NMDA Glutamate Receptor Antagonists. [Article] Anesthesiology. 2001;95:965–973. doi: 10.1097/00000542-200110000-00028. [DOI] [PubMed] [Google Scholar]
- Tan PH, Yang LC, Shih HC, Lan KC, Cheng JT. Gene knockdown with intrathecal siRNA of NMDA receptor NR2B subunit reduces formalin-induced nociception in the rat. Gene Ther. 2005;12:59–66. doi: 10.1038/sj.gt.3302376. [DOI] [PubMed] [Google Scholar]
- Tao F, Su Q, Johns RA. Cell-permeable peptide Tat-PSD-95 PDZ2 inhibits chronic inflammatory pain behaviors in mice. Mol Ther. 2008;16:1776–1782. doi: 10.1038/mt.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao F, Tao YX, Gonzalez JA, Fang M, Mao P, Johns RA. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport. 2001;12:3251–3255. doi: 10.1097/00001756-200110290-00022. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johns RA. PDZ domains at excitatory synapses: potential molecular targets for persistent pain treatment. Curr Neuropharmacol. 2006;4:217–223. doi: 10.2174/157015906778019473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci. 2003;23:6703–6712. doi: 10.1523/JNEUROSCI.23-17-06703.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian SL, Wang XY, Ding GH. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal cord NMDA receptor phosphorylation in a rat irritable bowel syndrome model. Life Sciences. 2008;83:356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Polgar E, Watt C, Bailey ME, Watanabe M. Neurokinin 1 receptor-expressing projection neurons in laminae III and IV of the rat spinal cord have synaptic AMPA receptors that contain GluR2, GluR3 and GluR4 subunits. Eur J Neurosci. 2009;29:718–726. doi: 10.1111/j.1460-9568.2009.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama M, Furusawa K, Kamijo M, Kimura T, Matsunaga M, Baba M. Upregulation of mRNAs coding for AMPA and NMDA receptor subunits and metabotropic glutamate receptors in the dorsal horn of the spinal cord in a rat model of diabetes mellitus. Brain Res Mol Brain Res. 2005;136:275–281. doi: 10.1016/j.molbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ultenius C, Linderoth B, Meyerson BA, Wallin J. Spinal NMDA receptor phosphorylation correlates with the presence of neuropathic signs following peripheral nerve injury in the rat. Neurosci Lett. 2006;399:85–90. doi: 10.1016/j.neulet.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Vikman KS, Rycroft BK, Christie MJ. Switch to Ca2+-permeable AMPA and reduced NR2B NMDA receptor-mediated neurotransmission at dorsal horn nociceptive synapses during inflammatory pain in the rat. J Physiol. 2008;586:515–527. doi: 10.1113/jphysiol.2007.145581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voitenko N, Gerber G, Youn D, Randic M. Peripheral inflammation-induced increase of AMPA-mediated currents and Ca2+ transients in the presence of cyclothiazide in the rat substantia gelatinosa neurons. Cell Calcium. 2004;35:461–469. doi: 10.1016/j.ceca.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Walker K, Reeve A, Bowes M, Winter J, Wotherspoon G, Davis A, Schmid P, Gasparini F, Kuhn R, Urban L. mGlu5 receptors and nociceptive function II. mGlu5 receptors functionally expressed on peripheral sensory neurones mediate inflammatory hyperalgesia. Neuropharmacology. 2000;40:10–19. doi: 10.1016/s0028-3908(00)00114-3. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Tseng GF. Spinal axonal injury transiently elevates the level of metabotropic glutamate receptor 5, but not 1, in cord-projection central neurons. J Neurotrauma. 2004;21:479–489. doi: 10.1089/089771504323004629. [DOI] [PubMed] [Google Scholar]
- Weyerbacher A, Tamasdan C, Inturrisi C. The N-Methyl-D-Aspartate Receptor (NMDAR) and neuronal-glial-cytokine interactions that initiate and maintain inflammatory pain. The Journal of Pain. 2009;10:S28. [Google Scholar]
- Willcockson H, Valtschanoff J. AMPA and NMDA glutamate receptors are found in both peptidergic and non-peptidergic primary afferent neurons in the rat. Cell and Tissue Research. 2008;334:17–23. doi: 10.1007/s00441-008-0662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Plasticity and pain: role of the dorsal horn. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Elesvier, Churchill Livingstone; 2006. pp. 91–105. [Google Scholar]
- Yang X, Yang HB, Xie QJ, Liu XH, Hu XD. Peripheral inflammation increased the synaptic expression of NMDA receptors in spinal dorsal horn. Pain. 2009;144:162–169. doi: 10.1016/j.pain.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Zhang B, Tao F, Liaw WJ, Bredt DS, Johns RA, Tao YX. Effect of knock down of spinal cord PSD-93/chapsin-110 on persistent pain induced by complete Freund’s adjuvant and peripheral nerve injury. Pain. 2003;106:187–196. doi: 10.1016/j.pain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Li A, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Interleukin 1beta facilitates bone cancer pain in rats by enhancing NMDA receptor NR-1 subunit phosphorylation. Neuroscience. 2008a;154:1533–1538. doi: 10.1016/j.neuroscience.2008.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RX, Li A, Liu B, Wang L, Ren K, Zhang H, Berman BM, Lao L. IL-1ra alleviates inflammatory hyperalgesia through preventing phosphorylation of NMDA receptor NR-1 subunit in rats. Pain. 2008b;135:232–239. doi: 10.1016/j.pain.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wu J, Lei Y, Fang L, Willis WD. Protein phosphatase modulates the phosphorylation of spinal cord NMDA receptors in rats following intradermal injection of capsaicin. Brain Res Mol Brain Res. 2005;138:264–272. doi: 10.1016/j.molbrainres.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol. 2007;97:871–882. doi: 10.1152/jn.00964.2006. [DOI] [PubMed] [Google Scholar]
- Zhou S, Komak S, Du J, Carlton SM. Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res. 2001;913:18–26. doi: 10.1016/s0006-8993(01)02747-0. [DOI] [PubMed] [Google Scholar]
- Zhuo M. Plasticity of NMDA receptor NR2B subunit in memory and chronic pain. Mol Brain. 2009;2:4. doi: 10.1186/1756-6606-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]