Abstract
Since the N-methyl-D-aspartate receptor (NMDAR) subunits were first cloned less than two decades ago, a substantial amount of research has been invested into understanding the physiological function of NMDARs in the healthy CNS and their pathological roles in a variety of neurological diseases. These include conditions resulting from acute excitotoxic insults (e.g. ischemic stroke, traumatic brain injury), diseases due to chronic neurodegeneration (e.g. Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, amyotrophic lateral sclerosis), disorders arising from sensitization of neurons (e.g. epilepsy, neuropathic pain), as well as neurodevelopmental disorders associated with NMDAR hypofunction (e.g. schizophrenia). There has been much focus on selective NMDAR antagonists which have not produced positive results in clinical trials. However, there are other NMDAR-targeted therapies used in current practice which are effective for treating certain neurological disorders. In this review, we describe the evidence for the use of these therapies and provide an overview of drugs being investigated in clinical trials. We also discuss novel NMDAR-based strategies which are emerging in clinical neurology.
INTRODUCTION
NMDARs have been the focus of much basic neuroscience research over the past couple decades. Extension of this research into preclinical studies has produced an overwhelming body of evidence that blocking or suppressing NMDARs is effective in preventing and, in some cases, allowing for reversal of pathology in various models of neurological diseases. Consequently, it has been with a great deal of frustration that earlier attempts at translating scientific knowledge of NMDARs into effective treatments for patients with neurological illness, in particular stroke and traumatic brain injury (TBI), were unsuccessful. However, there are a handful of drugs which have been used in clinical neurology for many years only later to be discovered to target the NMDAR-glutamate system, suggesting there is a role for NMDAR-based treatments for some neurological disorders. This is supported by the more recent demonstration of benefit, although modest, of the NMDAR antagonist memantine in the treatment of Alzheimer’s disease (AD). The repertoire of NMDAR-based drugs in neurology is expected to grow in the near future. This Review will provide a brief overview of the rationale for the development of NMDAR-based drugs and the pitfalls encountered in earlier trials. Our focus will be on an evidence-based review of NMDAR-targeted drugs currently used in neurological practice. We will also discuss NMDAR-targeted drugs being investigated in clinical trials and explore potential targets for novel NMDAR-based therapies.
NMDA RECEPTORS IN NEUROLOGICAL DISEAESES
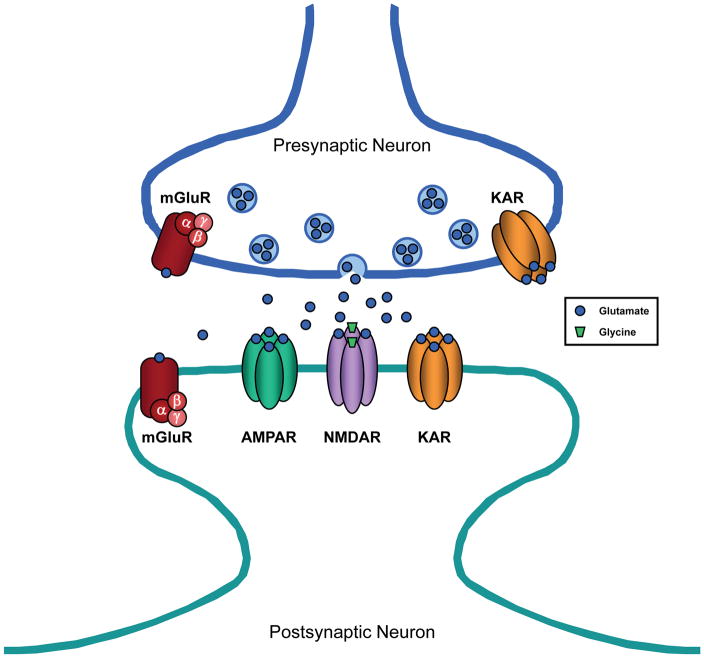
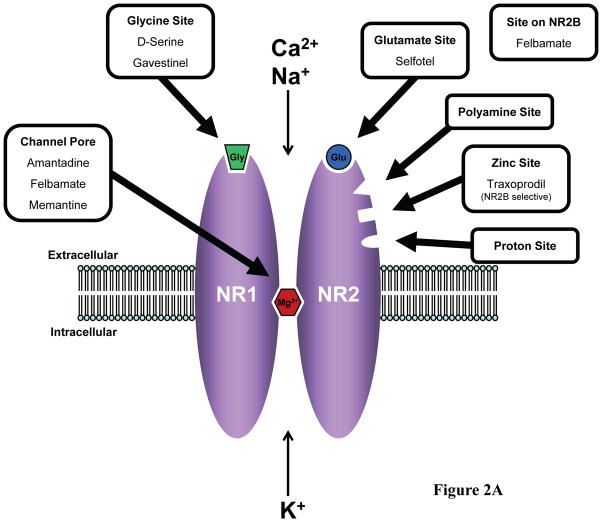
Glutamate is the major excitatory neurotransmitter in the CNS which acts on ionotropic and metabotropic glutamate receptors located at the presynaptic terminal (1) and in the postsynaptic membrane at synapses in the brain and spinal cord (Figure 1). There are three pharmacologically and molecularly distinct subtypes of ionotropic, or ion channel-containing, glutamate receptors which were originally named according to their preferred agonists: N-methyl-D-aspartate (NMDA) (Figure 2A), α-amino-3-hydroxy-5 methylisoxazole-4-proprionic acid (AMPA), and kainate (2). NMDARs are protein complexes, the core of which is composed of polypeptide subunits that form the ion channel pathway (3) (Figure 2B). The genes encoding these subunits – NR1, NR2 (NR2A, NR2B, NR2C, NR2D), and NR3 (NR3A, NR3B) – were identified just less than two decades ago (4–6). NMDARs typically contain four subunit proteins, two NR1 subunits plus two NR2 subunits and, less commonly, include an NR3 subunit. Both the NR1 and NR2 subunits contribute to the formation of the NMDAR ion channel. The NMDAR is unique in that the opening of the channel pore requires binding of two different agonists –glutamate as well as glycine (3). The glutamate binding site resides on the NR2 subunits whereas the glycine binding site is located on the NR1 subunits (Figure 2A). The NMDAR ion channel is permeable to monovalent cations, including Na+ and K+, and divalent cations, most notably Ca2+. However, there is a binding site within the channel pore for Mg2+ and, at resting membrane potential, Mg2+ binds to this site largely blocking ion flow through the channel. When the membrane is depolarized, Mg2+ is expelled from the channel allowing for greatly enhanced passage of ions. Therefore, both depolarization of the postsynaptic neuron and presynaptic release of glutamate which diffuses across the synapse to the receptors are required for maximal current flow through the NMDAR channel. The concentration of glycine at most synapses under normal conditions is generally sufficient to allow for efficient NMDAR activation upon release of glutamate from the presynaptic terminal. In recent years, it has become recognized that D-serine is also an endogenous ligand for the glycine binding site of the NMDAR and is at least as potent as glycine as a coagonist at this site (7).
Figure 1.
Excitatory synapse in the CNS. The excitatory neurotransmitter, glutamate, is released from presynaptic vesicles and diffuses across the synaptic cleft to act on two different types of receptors: ionotropic glutamate receptors, which have an intrinsic ion channel, and metabotropic glutamate receptors (mGluR), which are coupled to G proteins (α, β, and γ subunits). The three subtypes of ionotropic glutamate receptors include AMPA receptor (AMPAR), NMDA receptor (NMDAR), and kainate receptor (KAR).
Figure 2.
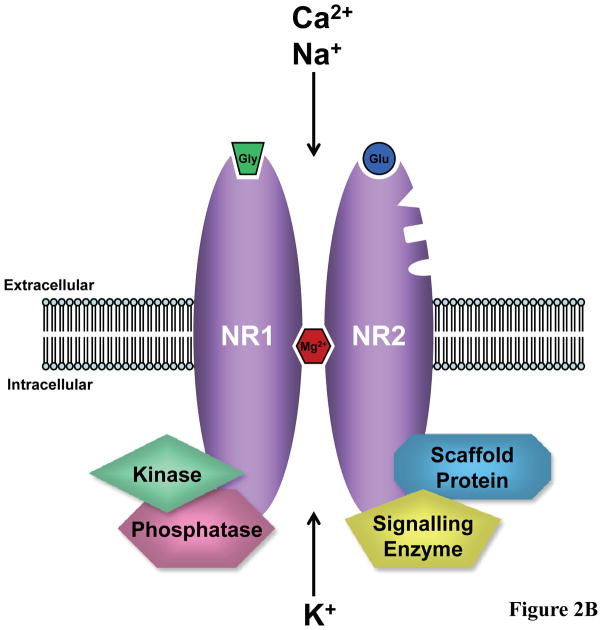
Potential sites for drug action within the NMDAR protein complex. (A) Extracellular sites include the glycine (Gly) binding site on NR1 subunits, glutamate (Glu) binding site on NR2 subunits, and binding sites within the channel pore which overlap with the site for magnesium binding (Mg2+) (2;3). D-serine is an endogenous coagonist at the glycine binding site (7). Currently used drugs or previously tested drugs which target these sites are indicated. NR2 subunits also contain sites of action for polyamines, zinc, and protons. The site of action of NR2B selective antagonists, such as traxoprodil, overlaps with a zinc binding site on NR2B subunits (138;139). (B) Intracellular targets include signalling molecules such as kinases, phosphatases, other enzymes, and scaffold proteins which are components of the NMDAR protein complex. These molecules are upstream modulators of NMDAR function or downstream effectors of NMDAR activity.
The NMDAR, over other glutamate receptor subtypes, has been a major target for drug development in neurology because preclinical research has provided a substantial amount of evidence for its role in cellular and animal models of many neurological diseases (8). The initial focus on NMDARs was based on the finding that excitotoxicity, a pathological process where neuronal injury or death occurs due to high concentrations of glutamate, results predominantly from excessive NMDAR activity with increased inflow of Ca2+ through the NMDAR channel (8). This process has been implicated in both acute ischemic stroke and TBI. Glutamate excitotoxicity is also presumed to contribute, at least partly, to neuronal loss in chronic neurodegenerative conditions, including AD and other dementias, Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and possibly multiple sclerosis (MS) and prion disease. Recent preclinical research has demonstrated that the endogenous cellular prion protein (PrPC) protects against excitotoxicity by downregulating a subpopulation of NMDARs, suggesting that progressive misfolding of PrPC into the disease-associated form of the protein (PrPSc) may result in the loss of this neuroprotective function and subsequent neurodegeneration in Creutzfeldt-Jakob disease (9). Glutamate released by neoplastic glial cells has been proposed to promote the death of neurons in areas of invasion of malignant gliomas (10). Thus, glutamate excitotoxicity may also mediate the growth of malignant gliomas, such as glioblastoma multiforme, and therapies that target the NMDAR-glutamate system could provide novel agents for the treatment of some brain tumours (11). Excessive NMDAR activity may also underlie neurological disorders characterized by hyperexcitability or sensitization of neurons, such as seizure disorders (8), neuropathic pain states (12), and some types of dyskinesias (13). In contrast, it has been proposed that underactivity of NMDARs may be associated with neurodevelopmental conditions, specifically schizophrenia (14).
EARLIER ANTI-NMDA RECEPTOR DRUGS
The earlier and most obvious approach to the development of NMDAR-based drugs for the treatment of neurological conditions was to directly target the NMDAR itself. A number of sites for pharmacological action have been identified for the NMDAR (Figure 2A). Three major classes of NMDAR antagonists can be distinguished based on their site of action: 1) competitive NMDAR antagonists which act at the glutamate or glycine binding site, 2) non-competitive NMDAR allosteric inhibitors which act at other extracellular sites, and 3) NMDAR channel blockers which bind to sites within the NMDAR channel pore (2;3). Many compounds have been found to modulate NMDAR activity by binding to these various extracellular sites and their use in basic neuroscience research has contributed substantially to our understanding of NMDAR function. However, NMDAR-targeted drugs which have been developed as neuroprotective agents, specifically selective NMDAR antagonists, have failed in large randomized, controlled trials (RCTs) of adequate methodological quality for the majority of selected indications, primarily ischemic stroke and TBI (15). The drugs which completed RCT testing included antagonists to the glutamate site (selfotel) (16;17) and the glycine site (gavestinel) (18;19), an antagonist to the ion channel site (aptiganel) (20), and a NR2B subunit-selective antagonist (traxoprodil) (21). There were too few patients included in each of the clinical trials, except for the trials of gavestinel, to make any definitive conclusions regarding potential benefit or harm for these agents (15;22). It is postulated that the convincing findings observed in animal studies were not translated into positive results in clinical trials because therapeutic doses of these NMDAR antagonists were not reached in the patients studied (22). This may have been due to decreased brain penetrance in the case of gavestinel or, for selfotel and aptiganel, due to significant dose-limiting adverse events. Trials conducted with selfotel in stroke (16) and TBI (17) were prematurely terminated due to concerns over excess early neurological mortality in the treatment arms. Patients receiving selfotel also displayed more agitation, confusion, reduced level of consciousness, hallucinations, and hypertension than those in the placebo group (16). This finding may be confounded by the observation that patients in the selfotel group were also more frequently administered sedatives (15). The efficacy trial with aptiganel in stroke (20) was prematurely terminated as well after review of safety data. There was no significant difference between low dose aptiganel and placebo in deaths but high dose aptiganel was associated with higher mortality than placebo (p=0.06). Other side effects more common in aptiganel-treated patients included those seen with selfotel, in addition to cerebral edema and ventricular dysrhythmias (15). It has been hypothesized that glutamate is involved in an acute excitotoxic process which occurs immediately after ischemic or traumatic injury but, after this early finite time period, glutamate may then reassume its normal physiological functions, including facilitation of neuronal survival. Thus, the use of NMDAR antagonists as neuroprotective agents in stroke and TBI could be limited by the existence of a short therapeutic time window (23;24). Further commercial development of these NMDAR antagonists for these indications remains unlikely and current investment of industry in the development of other selective NMDAR antagonists for neurological indications is minimal.
CURRENT NMDA RECEPTOR-BASED THERAPIES
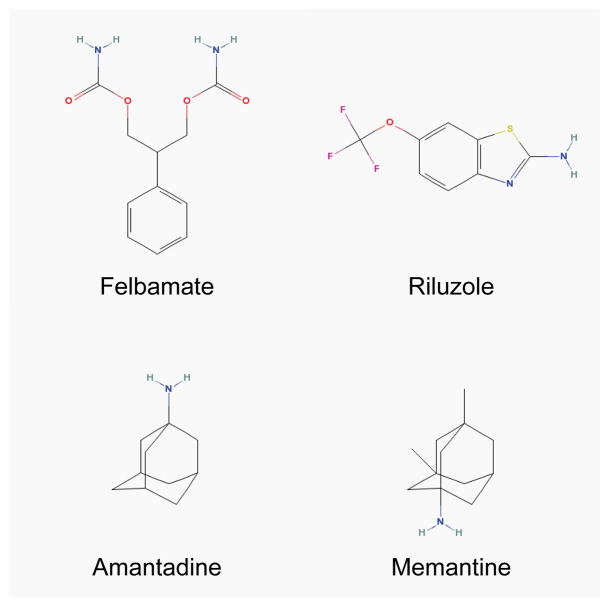
The lack of clinical success of the above NMDAR antagonists in the 1990s and early 2000s initially dampened enthusiasm for the potential of NMDAR-based therapies. Around the same time, however, previously discovered drugs which were not initially known to have anti-NMDAR properties were starting to be shown, in adequately designed RCTs, to be well tolerated and to have benefit in the treatment of certain neurological conditions. Unlike the selective NMDAR antagonists discussed above, these drugs have multiple mechanisms of action of which anti-NMDAR activity is considered key for their clinical effects. The varied mechanisms of action may explain, in part, the favourable clinical profile of these drugs – felbamate, riluzole, amantadine, and memantine (Figure 3).
Figure 3.
Chemical structures of felbamate, riluzole, amantadine, and memantine. The structures of these chemical compounds were copied from the National Library of Medicine (NLM)/National Center for Biotechnology Information (NCBI) PubChem database site (http://pubchem.ncbi.nlm.nih.gov/).
Felbamate
Felbamate was synthesized in 1955 and submitted to the antiepileptic drug (AED) development program within the epilepsy branch of the National Institute of Neurological Disorders and Stroke in 1982 (25). In this program, it was screened for anticonvulsant activity in animal models and found to have a broad anticonvulsant profile, similar to that of valproate yet appeared to have a lower level of neurotoxicity. Felbamate’s precise mechanism of action was unknown at that time. Later studies have proposed several mechanisms of action, in particular NMDAR antagonism at the glycine binding site (26;27). Others have suggested that felbamate may bind to a site within the NMDAR channel pore and act as an open channel blocker (28). This means that, for the drug to reach its binding site and reduce ion flow, the channel must first be opened by binding of glutamate and glycine, and by depolarization of the postsynaptic membrane to relieve the voltage-dependent Mg2+ block. Thus, it is thought that open channel blockers only act on active NMDARs. Recent studies indicate that felbamate is not a competitive antagonist at the glycine binding site but rather a non-competitive, allosteric inhibitor with some modest selectivity for NR2B-containing receptors which may also associate with the channel pore (29;30). Felbamate has been also shown to inhibit voltage-dependent Na+ and Ca2+ channels (31;32). It is unclear whether felbamate additionally may have a direct effect on γ-aminobutyric acid (GABA) receptors (33;34).
A series of clinical trials evaluating felbamate as a second generation AED were performed prior to its approval by the US Food and Drug Administration (FDA) in 1993 (Table 1). These included two crossover RCTs testing its efficacy as adjunctive therapy for refractory partial seizures in adults (35) (36). The smaller of the two trials found no significant difference in seizure frequency between felbamate and placebo in patients also taking carbamazepine (35). In the larger trial with patients taking carbamazepine and phenytoin, there was a statistically significant decrease in seizure frequency by 23% with the addition of felbamate versus placebo (36). Two other RCTs, comparing felbamate with valproate, evaluated the efficacy of felbamate as monotherapy for refractory partial seizures in adults (37;38). In both trials, there were significantly higher completion rates in the felbamate-treated versus valproate-treated groups (86% versus 10% (37), and 60% versus 22% (38)). Felbamate has also been studied in Lennox-Gastaut syndrome, a seizure disorder with childhood onset associated with multiple seizure types which are typically resistant to standard AEDs. In a RCT testing felbamate as an adjunctive therapy for Lennox-Gastaut syndrome, felbamate-treated patients had a statistically significant decrease in the frequency of atonic seizures (“drop attacks”) by 34% compared with a 9% decrease in placebo-treated patients (39). In addition, patients treated with felbamate had a 19% decrease in total seizure frequency versus a 4% increase with placebo. An open-label, 12-month extension of this trial found similar results in patients who converted from placebo to felbamate, as well as a sustained effect of felbamate on atonic and total seizure frequency (40).
Table 1.
Summary of published randomized, double blind, controlled trials of felbamate in patients with epilepsy.
| Study | Participants | Treatment | Primary Outcomes | Main Results | |
|---|---|---|---|---|---|
| Partial Seizures (adjunctive therapy) | Theodore et al, 1991 | N=47 ≥6 seizures during 3-week baseline period on CBZ |
FBM 2400–3000 mg/d or PLC plus CBZ | # of seizures |
|
| Leppik et al, 1991 | N=67 ≥4 seizures/month on CBZ and PHT |
FBM 2300 mg/d (mean) or PLC plus CBZ and PHT | SFR¶; SFPR†; TSFPR# |
|
|
| Partial Seizures (monotherapy) | Sachdeo et al, 1992 | N=44 ≥8 seizures during 56-day baseline period on 1–2 AEDs |
FBM 3600 mg/d (mean) or VPA 1225 mg/d (mean) | # of patients who met escape criteria‡ |
|
| Faught et al, 1993 | N=111 ≥8 seizures during 56-day baseline period on 1–2 AEDs |
FBM 3600 mg/d (mean) or VPA 1080 mg/d (mean) | # of patients who met escape criteria‡ |
|
|
| Lennox-Gastaut Syndrome (adjunctive therapy) | FBM Study Group, 1993 | N=73 Multiple seizure types; ≥90 atonic or atypical absence seizures/month on 1–2 AEDs |
FBM 3600 mg/d or PLC plus 1–2 other AEDs | # of seizures; # of atonic seizures; Caregivers’ global evaluation |
|
AED=antiepileptic drugs. CBZ=carbamazepine. FBM=felbamate. PHT=phenytoin. PLC=placebo. SFR=seizure frequency reduction. SFPR=seizure frequency percentage reduction. TSFPR=truncated seizure frequency percentage reduction. VPA=valproate.
SFR equals seizure frequency in baseline period minus seizure frequency in treatment period.
SFPR equals SFR × 100 divided by seizure frequency in baseline period.
TSFPR equals SFPR except that SFPR values less than −100 are truncated to −100.
Criteria to escape relative to baseline were: 1) 2-fold increase in monthly seizure frequency, 2) 2-fold increase in highest 2-day seizure frequency, 3) single generalized tonic-clonic seizure if none occurred during baseline, or 4) significant prolongation of generalized tonic-clonic seizures.
During these clinical trials, felbamate was not associated with the adverse CNS effects of many of the other AEDs nor those of the NMDAR antagonists tested in stroke and TBI. The most commonly documented side effects with felbamate were nausea, anorexia, and insomnia. There were no severe adverse events during the trials. However, postmarketing experience revealed two rare but serious idiosyncratic reactions related to felbamate: aplastic anemia (estimated incidence of 1 in 8,000 exposures) and hepatotoxicity (estimated incidence of 1 in 26,000 exposures) (41). These unexpected adverse events have limited felbamate’s clinical usefulness. Currently, felbamate is not considered a first-line AED, and recommendations for its use are generally restricted only to patients with intractable partial seizures or Lennox-Gastaut syndrome who have failed primary AEDs (42). Felbamate remains on the market in the US but with a black box warning. It is also approved for use in some European countries but is not readily available in the UK, Canada or Australia. The risk of bone marrow suppression and liver failure has also limited the potential for studying felbamate in other neurological disorders mediated by NMDAR hyperexcitability (for example, neuropathic pain). Consequently, no clinical trials investigating felbamate are currently registered.
Riluzole
Riluzole was originally synthesized by researchers in France and early laboratory studies in the 1980s suggested it had anticonvulsant properties (43). However, it was the discovery that riluzole can interfere with glutamate neurotransmission to prevent NMDAR-mediated neuronal death in experimental models (44) which promoted its further development. The entire mechanism of its neuroprotective action has not yet been fully delineated but is due, at least in part, to multiple effects on the NMDAR-glutamate system. Firstly, riluzole has been shown to inhibit Na+ channels on glutamate-containing neurons and thereby selectively reduce presynaptic release of glutamate (45). Secondly, there is evidence to suggest that riluzole blocks NMDAR activation preventing Ca2+ entry via the channel. Riluzole either acts directly on the NMDAR, although a binding site for riluzole on the receptor has not been identified (46), or indirectly, possibly via a G-protein-dependent signalling pathway (47). Thirdly, riluzole has been found to facilitate glutamate reuptake by increasing the activity of glutamate transporters expressed on neurons and glia (48), suggesting a modulatory action on glutamate clearance from the synaptic cleft.
Riluzole has been developed for treatment of chronic neurodegenerative disorders with the most promising results found for ALS. Three RCTs comparing riluzole to placebo in patients diagnosed with probable or definite ALS have been published (49–51) (Table 2). Two of the trials studied patients aged 75 years or younger with duration of illness no greater than 5 years and minimal to moderate respiratory impairment (forced vital capacity (FVC) equal to or greater than 60% of predicted) (49;50). The primary analysis for both trials was comparison of 100 mg riluzole daily with placebo which revealed benefit in favour of riluzole on tracheostomy-free survival. The third trial investigated patients with more advanced disease and/or age (51). A difference in survival between riluzole and placebo was not detected in this heterogeneous patient population, possibly because the study’s predetermined power specifications were not met. When the data for the 100 mg/day dose of riluzole were pooled from all three trials and analyzed in a Cochrane review (52), the calculated difference in median survival for patients receiving riluzole versus placebo was 3.0 months (14.8 and 11.8 months for riluzole and placebo, respectively). The combined analysis found a statistically significant survival advantage with riluzole at 12 months with an absolute risk reduction of 9%. Thus, the number-needed-to-treat to delay one death until after 12 months is 11. Results from the Cochrane review also showed a small beneficial effect of riluzole on limb function, as well as on bulbar function which was not found in any of the individual trials. There was no correlation between site of onset (limb versus bulbar) and benefit from riluzole. Riluzole displayed no positive effect on muscle strength assessed by manual muscle testing (52). It was well tolerated and no serious adverse effects from riluzole were reported in any of the trials. The most frequently documented side effects associated with 100 mg/day dose of riluzole were asthenia, nausea, and elevated liver enzymes.
Table 2.
Summary of published randomized, double blind, placebo-controlled trials of riluzole in patients with ALS.
| Study | Participants | Treatment | Duration | Primary Outcomes | Main Results |
|---|---|---|---|---|---|
| Bensimon et al, 1994 | N=155 Age 20–75 yrs and FVC >60% predicted and onset of symptoms ≤ 5 yrs |
RLZ 100 mg/d or PLC | 573 days (median follow up) | Tracheostomy-free survival; Change in functional status |
|
| Lacomblez et al, 1996 | N=959 Age 18–75 yrs and FVC ≥ 60% predicted and onset of symptoms ≤ 5 yrs |
RLZ 50 mg/d or 100 mg/d or 200 mg/d or PLC | 548 days (median follow up) | Tracheostomy-free survival |
|
| Bensimon et al, 2002 | N=168 Age >75 yrs and/or FVC <60% predicted and/or onset of symptoms >5 yrs |
RLZ 100 mg/d or PLC | 548 days (median follow up) | Tracheostomy-free survival |
|
FVC=forced vital capacity. PLC=placebo. RLZ=riluzole.
Although the effects of riluzole in ALS are modest, it remains the only approved drug for treatment of ALS in most countries. Riluzole is seen as a first step forward in treating this devastating neurological disease with one of the most logical next steps being investigation into add-on therapies which have mechanisms of action distinct from riluzole. One RCT testing xaliproden, a drug with neurotrophic properties, as an add-on therapy to riluzole in ALS showed it to have no statistically significant benefit on survival (53). Arundic acid, a modulator of astrocyte activation, is currently being tested as an add-on therapy in a registered clinical trial (Table 5).
Table 5.
Summary of ongoing registered clinical trials of NMDAR-based therapies in neurological diseases.
| NMDAR-Based Drug | Neurological Disease | Primary Outcomes | Identifier | |
|---|---|---|---|---|
| NMDAR Antagonists | Amantadine | PD | • Effect on dyskinesias in PD vs PLC | UMIN000000780 |
| • Effect on levodopa-induced dyskinesia vs PLC | NCT00632762 | |||
|
| ||||
| FTLD | • Efficacy in FTLD vs PLC | NCT00127114 | ||
|
| ||||
| Dextromethorphan | Rett syndrome | • Effect on EEG abnormalities vs PLC | NCT00593957 | |
| • Effect on EEG abnormalities vs DNP | NCT00069550 | |||
|
| ||||
| Ketamine | CRPS | • Pain relief vs PLC | NCT00579085 | |
|
| ||||
| Cancer NeP | • Pain relief with standard management vs standard management alone | ISRCTN49116945 | ||
| • Pain relief with opioids vs opioids alone | NCT00484484 | |||
| • Intranasal ketamine for pain vs PLC | NCT00492388 | |||
| • Subcutaneous ketamine for pain vs PLC | ACTRN12607000501448 | |||
|
| ||||
| Chronic post-op NeP | • Effect on pain after major back surgery vs PLC | NCT00618423 | ||
| • Effect on pain after thoracotomy vs PLC | NCT00313378 | |||
| • Effect on pain after mastectomy vs PLC | NCT00129597 | |||
|
| ||||
| Memantine | AD | • Effect on MRS parameters in mild to moderate AD vs DNP | NCT00505167 | |
| • Effect on MRS parameters with DNP, RIV or GAL | NCT00551161 | |||
| • Effect on behaviour in severe AD | NCT00401167 | |||
| • Effect on agitation in moderate to severe AD | NCT00371059 | |||
| • Effect on agitation and ADLs vs neuroleptic | ISRCTN68407918 | |||
| • Efficacy in moderate to severe AD (MMT vs DNP vs MMT+DNP) | ISRCTN49545035 | |||
|
| ||||
| FTLD | • Effect on FDG-PET parameters | NCT00594737 | ||
| • Efficacy in FTLD vs PLC | NCT00200538, NCT00545974 | |||
|
| ||||
| DLB/PDD | • Effect on cognition in PDD vs PLC | NCT00294554 | ||
| • Efficacy in DLB and PDD vs PLC | NCT00630500, ISRCTN89624516 | |||
|
| ||||
| PD | • Effect on non-motor symptoms vs PLC | NCT00646204 | ||
|
| ||||
| HD | • Effect on cognition and behaviour in HD | NCT00652457 | ||
|
| ||||
| TBI | • Effect on cognition vs PLC | NCT00462228 | ||
|
| ||||
| ALS | • Efficacy in ALS with various doses | NCT00409721 | ||
| • Efficacy in ALS vs PLC | NCT00353665 | |||
|
| ||||
| Neramexane | AD | • Efficacy in moderate to severe AD vs PLC | NCT00090116 | |
|
| ||||
| Drugs with Multiple MOAs | Dimebon | AD | • Efficacy in mild to moderate AD vs PLC | NCT00377715 |
|
| ||||
| HD | • Efficacy in HD vs PLC | NCT00497159 | ||
|
| ||||
| Riluzole | ALS | • Decline in respiratory function with arundic acid vs with PLC | NCT00403104 | |
|
| ||||
| MS | • Effect on MRI parameters combined with Avonex in CIS | NCT00501943 | ||
|
| ||||
| Glycine Site Agonist | D-Cycloserine | Schizophrenia | • Effect on antipsychotic-resistant symptoms | UMIN000000468 |
|
| ||||
| Autism | • Safety and efficacy in children with autism vs PLC | NCT00198120 | ||
| • Efficacy as add-on to aripiprazole | NCT00198107 | |||
|
| ||||
| EAAT Upregulator | Ceftriaxone | ALS | • Efficacy in ALS vs PLC | NCT00349622 |
|
| ||||
| Signalling Protein Modulators | Bryostatin-1 | AD | • Efficacy in mild to moderate AD vs PLC | NCT00606164 |
|
| ||||
| Nefiracetam | AD | • Efficacy in mild to moderate AD vs PLC | NCT00001933 | |
AD=Alzheimer’s disease. ALS=amyotrophic lateral sclerosis. CIS=clinically isolated syndrome. CRPS=Complex regional pain syndrome. DLB=dementia with Lewy bodies. DNP=donepezil. EAAT=excitatory amino acid transporter. FTLD=frontotemporal lobar degeneration. HD=Huntington’s disease. MOAs=mechanisms of action. MS=multiple sclerosis. NeP=neuropathic pain. PD=Parkinson’s disease. PDD=Parkinson’s disease dementia. PLC=placebo. TBI=traumatic brain injury.
The effects of riluzole in ALS are presumed to be due to its neuroprotective properties resulting from its actions on the NMDAR-glutamate system (54). Unfortunately, riluzole has not been found to be effective in clinical trials investigating two other neurodegenerative disorders, HD (55) and PD (56). A preliminary trial studying 16 patients with primary progressive MS has demonstrated that treatment with riluzole is associated with a decreased rate of cervical cord atrophy but only a slight decrease in the rate of brain atrophy as determined by MRI (57). This trial suggests possible differential effects of riluzole on the spinal cord and other regions of the CNS, which may explain its lack of effect in HD and PD. It also supports the potential of riluzole to reduce neurodegeneration associated with MS. A clinical trial is currently registered to assess the effects of riluzole on MRI parameters in early MS (Table 5).
Amantadine
Amantadine was the first member of a class of organic molecules called aminoadamantanes to be introduced into clinical use. It was first marketed in the 1960s for prophylaxis of respiratory infections due to influenza A virus but was serendipitously discovered to have beneficial effects on extrapyramidal symptoms in a PD patient who was taking amantadine for influenza prophylaxis (58). Initially, amantadine was assumed to have its antiparkinsonian effects through direct dopaminomimetic activity based on indirect in vivo evidence (59). Later studies have demonstrated that the dominant mechanism of action for amantadine is through its NMDAR antagonistic properties, acting as an open channel blocker (60).
A recent Cochrane review has examined the efficacy of amantadine versus placebo in the treatment of PD (61). Crosby and colleagues identified six adequately designed RCTs (62–67). All six trials were conducted at single centres with a total of 215 patients receiving amantadine or placebo. Each of the trials reported a positive effect of amantadine in PD. However, the small numbers of patients per trial and suboptimal reporting of study results prevented any conclusions to be made regarding the efficacy of amantadine in the treatment of PD. Levodopa remains the mainstay of treatment for the disabling symptoms of PD (68;69). With time, the development of dyskinesias manifests as a dose-limiting side effect of levodopa therapy. These levodopa-induced-dyskinesias (LIDs) are a major challenge in the current pharmacological treatment of PD. They occur at the peak effect of each dose of levodopa and with increasing frequency with longer duration of therapy (70;71). Crosby and colleagues also examined published RCTs to assess the efficacy of amantadine in treating LIDs in patients with PD on established levodopa therapy (72). Their literature review identified three trials comparing amantadine with placebo for the treatment of LIDs with a total of 53 PD patients (73–75) (Table 3). Again, each of the individual trials reported a reduction in LIDs in those patients treated with amantadine but it was concluded from the systematic review that it was not possible to determine the efficacy of amantadine in the treatment of LIDs. All three trials were short in duration thus it is difficult to assess the long term effects of amantadine. A follow-up study of one of the trials (75) did report that the beneficial effect of amantadine on LIDs was reproducible at 1-year following the initiation of the initial trial (76).
Table 3.
Summary of published randomized, double blind, controlled trials of amantadine in Parkinson’s disease patients with levodopa-induced dyskinesias.
| Study | Participants | Treatment | Primary Outcomes | Main Results |
|---|---|---|---|---|
| Verhagen Metman et al, 1998 | N=18 | AMA 300–400 mg/d or PLC | Abbreviated UPDRS-III; AIMS (during IV levodopa challenge) |
|
| Snow et al, 2000 | N=24 | AMA 200 mg/d or PLC | Total dyskinesia score (following PO levodopa challenge) |
|
| Luginger et al, 2000 | N=11 | AMA 300 mg/d or PLC | Marconi dyskinesia rating scale (following PO levodopa challenge); patient daily diary assessment of dyskinesias using VAS |
|
AIMS=Abnormal Involuntary Movement Scale. AMA=amantadine. PLC=placebo. UPDRS-III=Unified Parkinson’s Disease Rating Scale Part III. VAS=Visual Analogue Scale.
Larger RCTs are needed to further examine the efficacy of amantadine in the treatment of PD and in the treatment of LIDs. These trials are currently underway (Table 5). In addition, amantadine is being investigated for the treatment of frontotemporal lobar degeneration (FTLD).
Memantine
Memantine, like amantadine, is a member of the aminoadamantane class of organic molecules. It was originally synthesized in the early 1960s as a potential hypoglycaemic agent but was found to be ineffective at lowering elevated blood sugar. Based on anecdotal reports of its utility in a variety of neurological diseases, memantine was first officially used in Germany for treatment of dementia in the 1980s. At around the same time, laboratory studies provided evidence for binding of memantine to NMDARs.
Memantine is an open channel blocker NMDAR antagonist with its primary site for binding overlapping with that of Mg2+. It is hypothesized that the absence of severe adverse effects results from the kinetics of its block of the NMDAR (77–79). Memantine has a relatively low affinity for the NMDAR allowing memantine to rapidly bind to and, unlike high affinity antagonists, quickly dissociate from the receptor. In addition, memantine displays pronounced voltage-dependency and, therefore, will leave the NMDAR channel upon strong postsynaptic depolarization, as occurs during normal physiological activation of NMDARs, but will remain blocking the channel pore during moderate prolonged depolarization, which occurs during chronic excitotoxic conditions (77). Therefore, it is proposed that memantine’s favourable clinical profile is also because it preserves normal synaptic activity while inhibiting excitotoxicity. Memantine has also been reported to exert effects on the cholinergic neurotransmitter system; in particular, memantine has been shown to inhibit α7 nicotinic acetylcholine receptors (nAChRs) (80). This may contribute to its favourable clinical profile as there is some evidence that α7 nAChR inhibition results in attenuation of pathological processes associated with AD, such as β-amyloid peptide-induced tau protein phosphorylation (81) and NMDAR-mediated excitotoxicity (82).
Three early RCTs conducted in Europe tested memantine in heterogeneous populations of dementia patients (83–85) (Table 4). These patients had possible AD, vascular dementia (VaD) or mixed dementia at varying stages of disease. Sample sizes were small and study duration was short, but results from all three studies showed benefit in favour of memantine compared with placebo on their primary outcome measure of clinical global impression. Development of memantine for treatment of AD started in the US in 2000 and was accompanied by six RCTs comparing memantine to placebo in patients diagnosed with probable AD (86–91) (Table 4). Three of the trials tested memantine in moderate to severe AD (86–88). In one of these trials, all participants also received the acetylcholinesterase inhibitor donepezil (87). A Cochrane review analyzing the pooled data from the three trials demonstrated that memantine had positive effects on measures of cognition, mood, behaviour, and ability to perform activities of daily living (ADLs) (92). Memantine also had a positive effect on clinical impression of change, suggesting its effects are clinically detectable. An open-label, 24-week extension of one of the trials suggested continued clinical benefit with prolongation of memantine treatment (93). Three other trials tested memantine in mild to moderate AD (89–91) with all participants in one study on concurrent, stable doses of an acetylcholinesterase inhibitor (donepezil, rivastigmine or galantamine) (91). A Cochrane review pooling data from the three trials suggested a beneficial effect of memantine on cognitive function supported by a positive but small effect in clinical impression of change (92).
Table 4.
Summary of published randomized, double blind, placebo-controlled trials of memantine in patients with dementia.
| Study | Participants | Treatment | Duration | Primary Outcomes | Main Results | |
|---|---|---|---|---|---|---|
| Dementia (AD, VaD, mixed) | Ditzler, 1991 | N=66 SCAG ≥50 |
MMT 30 mg/d or PLC | 6 wks | SCAG; SKT; ADL tests |
|
| Gortelmeyer and Erbler, 1992 | N=88 SCAG ≥50 MMSE 11–30 |
MMT 20 mg/d or PLC | 6 wks | SCAG; GBS; ADL tests; CGI |
|
|
| Winblad and Portis, 1999 | N=166 MMSE <10 |
MMT 10 mg/d or PLC | 12 wks | BGP-dep; CGI-C |
|
|
| Alzheimer’s Disease (moderate to severe) | Reisberg et al, 2003 | N=252 MMSE 3–14 |
MMT 20 mg/d or PLC | 28 wks | ADCS-ADLsev; CIBIC-plus |
|
| Tariot et al, 2004 | N=404 MMSE 5–14 |
MMT 20 mg/d or PLC plus DNP 5–10 mg/d | 24 wks | ADCS–ADL19; SIB |
|
|
| van Dyck et al, 2007 | N=350 MMSE 5–14 |
MMT 20 mg/d or PLC | 24 wks | ADCS-ADL19; SIB |
|
|
| Alzheimer’s Disease (mild to moderate) | Peskind et al, 2006 | N=403 MMSE 10–22 |
MMT 20 mg/d or PLC | 24 wks | ADAS-cog; CIBIC-plus |
|
| Bakchine and Loft, 2008 | N=470 MMSE 11–23 |
MMT 20 mg/d or PLC | 24 wks | ADAS-cog; CIBIC-plus |
|
|
| Porsteinsson et al, 2008 | N=433 MMSE 10–22 |
MMT 20 mg/d or PLC plus 1 DNP, RIV or GAL | 24 wks | ADAS-cog; CIBIC-plus |
|
|
| Vascular Dementia (mild to moderate) | Orgogozo et al, 2002 | N=321 MMSE 12–20 |
MMT 20 mg/d or PLC | 28 wks | ADAS-cog; CIBIC-plus |
|
| Wilcock et al, 2002 | N=579 MMSE 10–22 |
MMT 20 mg/d or PLC | 28 wks | ADAS-cog; CGI-C |
|
AD=Alzheimer’s disease. ADAS-cog=Alzheimer’s Disease Assessment Scale-cognitive subscale. ADCS-ADL19=Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory. ADCS-ADLsev=Alzheimer’s Disease Cooperative Study-Activities of Daily Living Inventory modified for severe dementia. BGP-dep=Behavioural Rating Scale for Geriatric Patients- ‘care dependence’ subscore. CGI=Clinical Global Impression. CGI-C=Clinical Global Impression of Change. CIBIC-plus=Clinician’s Interview-Based Impression of Change Plus Caregiver Input. DNP=donepezil. GAL=galantamine. GBS=Gottfries-Bräne-Steen Scale. MMSE=Mini-Mental State Examination. MMT=memantine. PLC=placebo. RIV=rivastigmine. SCAG=Sandoz Clinical Assessment Geriatric Scale. SIB=Severe Impairment Battery. SKT=Syndrom-Kurz-Test. VaD=vascular dementia.
Memantine was well tolerated in the above trials with no severe CNS adverse effects. There were no significant differences between memantine and placebo in withdrawal rates or in the overall incidence of adverse events. Adverse events reported from the trials included nausea, diarrhea, headache, insomnia, dizziness, confusion, and agitation. Interestingly, the adverse event most frequently reported was agitation but more commonly in the placebo group suggesting that patients taking memantine were less likely to develop agitation (92). Currently registered clinical trials include studies aimed at more clearly defining the benefit of memantine in agitated patients with probable AD using neuropsychiatric or agitation scale scores as primary outcome measures. Other registered clinical trials of note include head-to-head trials of memantine versus donepezil in AD, and trials testing whether memantine plus donepezil, with their separate actions on the glutamate and acetylcholine neurotransmitter systems, respectively, may have synergistic benefit in AD (Table 5).
Investigation into the use of memantine for the treatment of other types of dementia has been initiated. Two RCTs testing memantine in mild to moderate VaD have been completed (94;95) (Table 4). Meta-analyses of the pooled data supported a beneficial effect of memantine on cognitive function but there was no effect on the clinical impression of change, suggesting that the positive effect on cognition may not be translated into a clinically detectable benefit (92;96). Clinical trials investigating memantine in the treatment of FTLD, Parkinson’s disease dementia (PDD) and dementia with Lewy bodies (DLB), cognitive and behavioural symptoms associated with HD, as well as cognitive dysfunction associated with TBI are currently registered (Table 5). Others include trials testing the effects on memantine treatment on ALS and non-motor PD symptoms. It is hypothesized that memantine, via NMDAR antagonism, may be neuroprotective and thus slow disease progression in many of these conditions (77;97).
Memantine has been approved for treatment of moderate to severe AD in most of Europe, the US, and Canada. It has remained the only anti-dementia drug approved for more advanced stages of AD. Its use, however, is often restricted by national formularies (for example, the British National Formulary (98)) because of controversy regarding the clinical significance of the small beneficial effects found in the clinical trials and uncertainty about its cost effectiveness for public health care systems. Regardless, the demonstration of some benefit of memantine in the treatment of AD provides proof-of-principle for the role of NMDAR-targeted therapies in clinical neurology and supports further clinical trials and development of related treatment strategies.
OTHER NMDA RECEPTOR-BASED DRUGS IN CLINICAL TRIALS
In addition to the ongoing clinical trials involving the anti-NMDAR agents described above, there are a number of current trials investigating other NMDAR-targeted drugs. These include the NMDAR antagonists, ketamine and dextromethorphan, as well as D-cycloserine which is a partial agonist at the glycine binding site on NMDARs (Table 5).
NMDA Receptor Antagonists
Ketamine and dextromethorphan are NMDAR antagonists which have been used in clinical practice for many years but for non-neurological indications. Ketamine is a NMDAR open channel blocker (99) commonly used as a dissociative anaesthetic. Many preliminary trials have suggested a potential role for ketamine as an adjuvant analgesic at subanaesthetic doses (100–102). Consequently, a number of ongoing clinical trials are investigating low dose ketamine in various pain states, including neuropathic pain in cancer patients, chronic neuropathic pain which develops following surgical procedures, and complex regional pain syndrome. Dextromethorphan is a commonly used cough suppressant and its metabolite, dextrorphan, has been found to antagonize the NMDAR by binding to a site within the channel pore (103;104). Dextromethorphan is currently registered to be tested in clinical trials in children with Rett syndrome, a neurodevelopmental disorder mainly affecting females which is characterized by the development of autistic features and stereotypic hand movements after relatively normal early development (105). Epilepsy is a common and frequently challenging comorbidity for Rett syndrome patients and their families. Rett syndrome is caused by mutations in the gene encoding methyl-CpG binding protein 2 (MeCP2). MeCP2 is a transcriptional repressor which may protect against NMDAR-mediated excitotoxicity in postmitotic neurons (106); thus, neurons of Rett syndrome patients may be more susceptible to excitotoxicity. It is hypothesized that, by blocking the NMDAR, dextromethorphan may reduce EEG spike abnormalities, seizure activity, and excitotoxicity associated with this condition.
Two new NMDAR antagonists in clinical trials include neramexane and dimebon. Neramexane belongs to a recently described group of NMDAR open channel blockers known as the amino-alkyl-cyclohexanes (107). It exhibits similar kinetics and voltage-dependency as memantine, as well as comparable clinical tolerability. While there are no clinical trials currently registered to investigate neramexane, this drug is being developed for treatment of AD (108). Dimebon, a drug predominantly developed in Russia, is being evaluated in clinical trials on AD and HD. A small preliminary clinical trial performed in Moscow suggested some improvement in cognitive function and reduction in neuropsychiatric symptoms with dimebon in patients with mild to moderate AD (109). Dimebon was initially classified as an antihistamine but its mechanism of action appears to be more complex (110;111). Studies suggest dimebon blocks NMDARs but likely at a site distinct from memantine (112). It is hypothesized that dimebon, being an antagonist to the H1 histamine receptor, may act at the polyamine binding site of NMDARs which is a suspected site of interaction for histamine (113).
Glycine Site Agonists
Clinical studies examining the utility of partial agonists targeting the glycine binding site of the NMDAR have also been initiated. The antibiotic D-cycloserine is a partial glycine agonist which enhances the glutamatergic effect on NMDARs through its action on the glycine site (114;115). Early clinical trials tested the efficacy of D-cycloserine in AD (116–118) but no beneficial effect of D-cycloserine relative to placebo was observed in meta-analysis (119). More recently, D-cycloserine in addition to other NMDAR glycine agonists, such as glycine, serine, and D-serine, has been examined in the treatment of neurodevelopmental disorders, in particular schizophrenia (120). Available data from RCTs are limited and firm conclusions from meta-analysis cannot be made. Therefore, it has been suggested that additional research on glycine site agonists is needed to establish the utility of these agents in the treatment of schizophrenia (120). In a pilot study (121), treatment with D-cycloserine has been shown to be associated with improvement in the core symptoms of social impairment in patients with autism, another neurodevelopmental condition. Taken together, these preliminary studies suggest that further research into D-cycloserine as a possible treatment for certain neurodevelopmental diseases are needed to identify if glycine site agonists of the NMDAR are a viable treatment paradigm. RCTs to assess the efficacy of D-cycloserine in schizophrenia and autism are ongoing (Table 5).
EMERGING NMDA RECEPTOR-BASED STRATEGIES
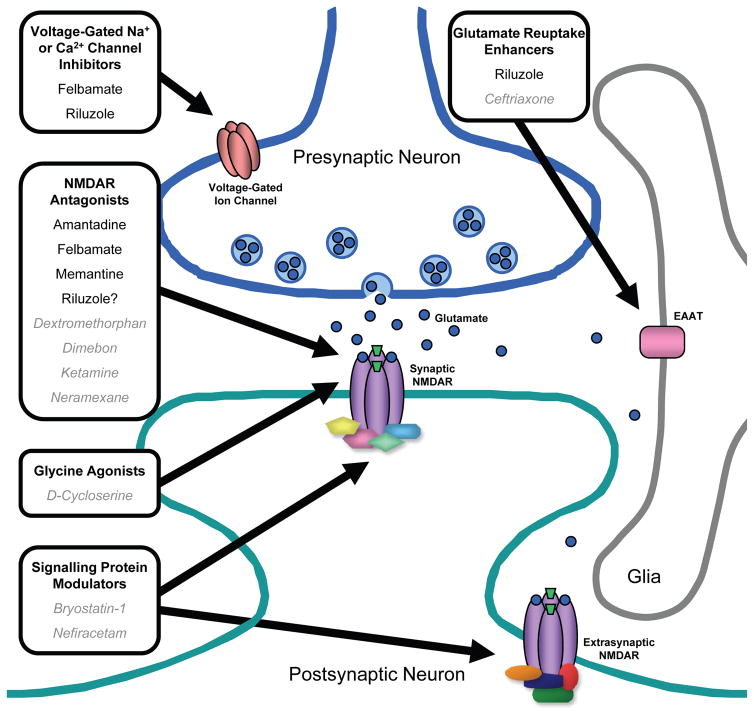
The major sites of action of the NMDAR-targeted therapies described above are on the extracellular aspect of the NMDAR itself or within its channel pore. Two interesting targets for future drug discovery and development are distinct from the NMDAR core but may allow for more selective targeting of specific receptor populations. These include: 1) the transporter systems which regulate the concentration of glutamate at the synaptic cleft, and 2) the intracellular proteins involved in NMDAR signalling pathways (Figure 4).
Figure 4.
Current and emerging NMDAR-based strategies for treatment of neurological diseases. These strategies include NMDAR antagonism, decreasing glutamate release by inhibiting presynaptic voltage-gated Na+ and/or Ca2+ channels, enhancing glutamate uptake from the synaptic cleft by excitatory amino acid transporter (EAAT) on neurons and glia, and targeting intracellular signalling molecules associated with synaptic or extrasynaptic NMDARs. Currently used drugs or drugs being studied (in gray italics) which target these sites are indicated.
Glutamate Reuptake Enhancers
The amount of glutamate available at the synapse to activate NMDARs, as well as other glutamate receptors, is regulated by a family of excitatory amino acid transporters (EAATs) (122). EAATs are analogous to serotonin transporters which are well known to be the sites of action of selective serotonin reuptake inhibitors (SSRIs). EAATs are localized to the membranes of both neurons and glia. Five members of the EAAT family have been identified (EAAT1, EAAT2, EAAT3, EAAT4, and EAAT5) with EAAT2 contributing to the bulk of glutamate transport activity in the forebrain. Therefore, compounds which could upregulate the activity of EAAT2 and/or increase its protein expression may provide novel therapeutic agents to reduce NMDAR-mediated excitotoxicity. Interestingly, a random screen of some FDA-approved drugs revealed that β-lactam antibiotics, including penicillin, amoxicillin, and ceftriaxone, were able to increase EAAT2 protein expression (123). Furthermore, ceftriaxone was shown to increase EAAT2 protein levels in a mouse model of ALS and to significantly prolong survival of these mice. The concept of using an EAAT2 upregulator for the treatment of neurodegenerative disease is currently being tested in a clinical trial of ceftriaxone in ALS patients (Table 5).
The activity of glutamate transporters determines not only the concentration of glutamate within the synaptic cleft but also the amount of glutamate which spills over to extrasynaptic sites (124). NMDARs are localized both to the synapse and to extrasynaptic regions, and these two populations of NMDARs may possess different characteristics. NMDARs at both locations can mediate excitotoxicity (125;126) but stimulation of extrasynaptic NMDARs may be more strongly associated with neuronal death whereas synaptic NMDAR activation may promote survival pathways (127). This appears to be explained by the discovery that synaptic and extrasynaptic NMDARs are associated with distinct intracellular signalling pathways: synaptic NMDAR stimulation activates a signalling pathway which upregulates prosurvival transcription factors, such as cAMP response element binding protein (CREB), resulting in expression of genes including brain derived neurotrophic factor (BDNF), whereas activation of extrasynaptic NMDARs is associated with an opposing signalling pathway that downregulates CREB and BDNF leading to neuronal death (128). Thus, decreasing glutamate spillover by increasing glutamate uptake by EAATs and thereby reducing the stimulation of the extrasynaptic population of NMDARs may prevent neuronal loss in neurological conditions associated with glutamate toxicity (for example, during the acute excitotoxic process which occurs immediately after ischemic or traumatic injury mentioned above (23;24)).
Signalling Protein Modulators
A host of intracellular signalling molecules, some of which act upstream of synaptic NMDARs to regulate their activity (129) and others which act downstream as effector molecules following receptor activation (130), have been identified within the past decade (Figure 2B). These include enzymes, such as protein kinases and phosphatases, which may be targeted to alter NMDAR activity or to modulate the intracellular sequelae from Ca2+ entry via the channel in certain pathological states. In the area of oncology, the identification of enzymes that drive neoplastic transformation have allowed for the development of rationally designed cancer therapeutics that target specific signalling molecules. For example, small-molecule inhibitors, such as imatinib (Gleevac), and monoclonal antibodies, such as trastuzumab (Herceptin), have been designed to inhibit specific kinases to interrupt the intracellular signal for further tumour cell proliferation (131). A similar approach may be used for the development of NMDAR-based therapies. One potential molecular target which regulates synaptic NMDAR activity is protein kinase C (PKC). In a model of learning and memory called long-term potentiation (LTP), activation of PKC, likely via upstream activation of metabotropic glutamate receptors, leads to upregulation of NMDAR activity and enhanced LTP (132;133). Inhibition of PKC in animals impairs spatial memory (134). Therefore, PKC activators may be useful in neurological disorders associated with memory impairment, such as AD. Bryostatin-1 (135;136)and nefiracetam (137) are two molecules which can activate PKC and are being tested in patients with AD (Table 5).
CONCLUSIONS
The glutamate system is the most complex of all neurotransmitter systems in the CNS with the NMDAR being the most complex of the glutamate receptor subtypes. These layers of complexity are likely the result of the pivotal role of the NMDAR-glutamate system in a plethora of fundamental CNS functions and necessary for protection against the devastating effects of uncontrolled NMDAR-mediated neurotransmission. Despite a number of setbacks in the development of clinically useful drugs targeting the NMDAR, a number of drugs are in clinical use and our increasing knowledge of the molecular subtleties of this pervasive receptor are a sign of excitatory times ahead for the development of future drugs for use in neurological conditions.
SEARCH STRATEGY AND SELECTION CRITERIA
References for this Review were identified by searches of PubMed for peer-reviewed articles published up to June 2008. The search terms “amantadine”, “felbamate”, “memantine”, “NMDA”, “riluzole” were used. Additional articles were identified by searching the reference lists of identified articles and the authors’ own files. Only papers published in English were reviewed. Abstracts or unpublished material were excluded. Searches of public trial registries (http://clinicaltrials.gov, http://isrctn.org, http://actr.org.au, http://trialregister.nl, http://www.umin.ac.jp/ctr) were performed for ongoing clinical trials.
Acknowledgments
MWS is an International Research Scholar of the Howard Hughes Medical Institute, holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain, and is supported by a grant from the Canadian Institutes of Health Research (CIHR, grant number MT-12682). These funding sources had no role in determining the idea and outline of this Review or in the writing and revision.
Footnotes
CONTRIBUTORS
LVK wrote the initial draft of this Review. All authors participated in the writing and revision. All authors have seen and approved the final version.
CONFLICTS OF INTEREST
LVK and SKK have no conflicts of interest to declare. MWS has been a consultant for various pharmaceutical companies and a member of scientific advisory boards. MWS has received speaker’s fees and participated in meetings supported by unrestricted grants from industry. None of these declarations present a conflict of interest in relation to the content of this Review.
References
- 1.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008 Jun;9(6):423–36. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 2.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999 Mar;51(1):7–61. [PubMed] [Google Scholar]
- 3.Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007 Feb;7(1):39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–7. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- 5.Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, et al. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992 May 7;357(6373):70–4. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- 6.Monyer H, Sprengel R, Schoepfer R, Herb A, Higuchi M, Lomeli H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992 May 22;256(5060):1217–21. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 7.Wolosker H. D-serine regulation of NMDA receptor activity. Sci STKE. 2006 Oct 10;2006(356):e41. doi: 10.1126/stke.3562006pe41. [DOI] [PubMed] [Google Scholar]
- 8.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005 Feb;11(1):37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 9.Khosravani H, Zhang Y, Tsutsui S, Hameed S, Altier C, Hamid J, et al. Prion protein attenuates excitotoxicity by inhibiting NMDA receptors. J Cell Biol. 2008 May 5;181(3):551–65. doi: 10.1083/jcb.200711002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001 Sep;7(9):1010–5. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein JD, Brem H. Excitotoxic destruction facilitates brain tumor growth. Nat Med. 2001 Sep;7(9):994–5. doi: 10.1038/nm0901-994. [DOI] [PubMed] [Google Scholar]
- 12.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000 Jun 9;288(5472):1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 13.Brotchie JM. The neural mechanisms underlying levodopa-induced dyskinesia in Parkinson’s disease. Ann Neurol. 2000 Apr;47(4 Suppl 1):S105–S112. [PubMed] [Google Scholar]
- 14.Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006 Jul;26(4–6):365–84. doi: 10.1007/s10571-006-9062-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muir KW, Lees KR. Excitatory amino acid antagonists for acute stroke. Cochrane Database Syst Rev. 2003;(3):CD001244. doi: 10.1002/14651858.CD001244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of Acute Stroke Studies Involving Selfotel Treatment. ASSIST Steering Committed. Lancet. 1997 Jan 4;349(9044):32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- 17.Morris GF, Bullock R, Marshall SB, Marmarou A, Maas A, Marshall LF. Failure of the competitive N-methyl-D-aspartate antagonist Selfotel (CGS 19755) in the treatment of severe head injury: results of two phase III clinical trials. The Selfotel Investigators. J Neurosurg. 1999 Nov;91(5):737–43. doi: 10.3171/jns.1999.91.5.0737. [DOI] [PubMed] [Google Scholar]
- 18.Sacco RL, DeRosa JT, Haley EC, Jr, Levin B, Ordronneau P, Phillips SJ, et al. Glycine antagonist in neuroprotection for patients with acute stroke: GAIN Americas: a randomized controlled trial. JAMA. 2001 Apr 4;285(13):1719–28. doi: 10.1001/jama.285.13.1719. [DOI] [PubMed] [Google Scholar]
- 19.Lees KR, Asplund K, Carolei A, Davis SM, Diener HC, Kaste M, et al. Glycine antagonist (gavestinel) in neuroprotection (GAIN International) in patients with acute stroke: a randomised controlled trial. GAIN International Investigators. Lancet. 2000 Jun 3;355(9219):1949–54. doi: 10.1016/s0140-6736(00)02326-6. [DOI] [PubMed] [Google Scholar]
- 20.Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001 Dec 5;286(21):2673–82. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 21.Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005 Dec;22(12):1428–43. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- 22.Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006 Feb;6(1):53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002 Oct;1(6):383–6. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- 24.Roesler R, Quevedo J, Schroder N. Is it time to conclude that NMDA antagonists have failed? Lancet Neurol. 2003 Jan;2(1):13. doi: 10.1016/s1474-4422(03)00260-6. [DOI] [PubMed] [Google Scholar]
- 25.Graves NM. Felbamate. Ann Pharmacother. 1993 Sep;27(9):1073–81. doi: 10.1177/106002809302700913. [DOI] [PubMed] [Google Scholar]
- 26.McCabe RT, Wasterlain CG, Kucharczyk N, Sofia RD, Vogel JR. Evidence for anticonvulsant and neuroprotectant action of felbamate mediated by strychnine-insensitive glycine receptors. J Pharmacol Exp Ther. 1993 Mar;264(3):1248–52. [PubMed] [Google Scholar]
- 27.White HS, Harmsworth WL, Sofia RD, Wolf HH. Felbamate modulates the strychnine-insensitive glycine receptor. Epilepsy Res. 1995 Jan;20(1):41–8. doi: 10.1016/0920-1211(94)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Subramaniam S, Rho JM, Penix L, Donevan SD, Fielding RP, Rogawski MA. Felbamate block of the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther. 1995 May;273(2):878–86. [PubMed] [Google Scholar]
- 29.Kleckner NW, Glazewski JC, Chen CC, Moscrip TD. Subtype-selective antagonism of N-methyl-D-aspartate receptors by felbamate: insights into the mechanism of action. J Pharmacol Exp Ther. 1999 May;289(2):886–94. [PubMed] [Google Scholar]
- 30.Harty TP, Rogawski MA. Felbamate block of recombinant N-methyl-D-aspartate receptors: selectivity for the NR2B subunit. Epilepsy Res. 2000 Mar;39(1):47–55. doi: 10.1016/s0920-1211(99)00108-4. [DOI] [PubMed] [Google Scholar]
- 31.Taglialatela M, Ongini E, Brown AM, Di RG, Annunziato L. Felbamate inhibits cloned voltage-dependent Na+ channels from human and rat brain. Eur J Pharmacol. 1996 Dec 5;316(2–3):373–7. doi: 10.1016/s0014-2999(96)00802-3. [DOI] [PubMed] [Google Scholar]
- 32.Stefani A, Calabresi P, Pisani A, Mercuri NB, Siniscalchi A, Bernardi G. Felbamate inhibits dihydropyridine-sensitive calcium channels in central neurons. J Pharmacol Exp Ther. 1996 Apr;277(1):121–7. [PubMed] [Google Scholar]
- 33.Ticku MK, Kamatchi GL, Sofia RD. Effect of anticonvulsant felbamate on GABAA receptor system. Epilepsia. 1991 May;32(3):389–91. doi: 10.1111/j.1528-1157.1991.tb04667.x. [DOI] [PubMed] [Google Scholar]
- 34.Kume A, Greenfield LJ, Jr, Macdonald RL, Albin RL. Felbamate inhibits [3H]t-butylbicycloorthobenzoate (TBOB) binding and enhances Cl− current at the gamma-aminobutyric AcidA (GABAA) receptor. J Pharmacol Exp Ther. 1996 Jun;277(3):1784–92. [PubMed] [Google Scholar]
- 35.Theodore WH, Raubertas RF, Porter RJ, Nice F, Devinsky O, Reeves P, et al. Felbamate: a clinical trial for complex partial seizures. Epilepsia. 1991 May;32(3):392–7. doi: 10.1111/j.1528-1157.1991.tb04668.x. [DOI] [PubMed] [Google Scholar]
- 36.Leppik IE, Dreifuss FE, Pledger GW, Graves NM, Santilli N, Drury I, et al. Felbamate for partial seizures: results of a controlled clinical trial. Neurology. 1991 Nov;41(11):1785–9. doi: 10.1212/wnl.41.11.1785. [DOI] [PubMed] [Google Scholar]
- 37.Sachdeo R, Kramer LD, Rosenberg A, Sachdeo S. Felbamate monotherapy: controlled trial in patients with partial onset seizures. Ann Neurol. 1992 Sep;32(3):386–92. doi: 10.1002/ana.410320313. [DOI] [PubMed] [Google Scholar]
- 38.Faught E, Sachdeo RC, Remler MP, Chayasirisobhon S, Iragui-Madoz VJ, Ramsay RE, et al. Felbamate monotherapy for partial-onset seizures: an active-control trial. Neurology. 1993 Apr;43(4):688–92. doi: 10.1212/wnl.43.4.688. [DOI] [PubMed] [Google Scholar]
- 39.Efficacy of felbamate in childhood epileptic encephalopathy (Lennox-Gastaut syndrome). The Felbamate Study Group in Lennox-Gastaut Syndrome. N Engl J Med. 1993 Jan 7;328(1):29–33. doi: 10.1056/NEJM199301073280105. [DOI] [PubMed] [Google Scholar]
- 40.Dodson WE. Felbamate in the treatment of Lennox-Gastaut syndrome: results of a 12-month open-label study following a randomized clinical trial. Epilepsia. 1993;34( Suppl 7):S18–S24. doi: 10.1111/j.1528-1157.1993.tb04590.x. [DOI] [PubMed] [Google Scholar]
- 41.Pellock JM, Faught E, Leppik IE, Shinnar S, Zupanc ML. Felbamate: consensus of current clinical experience. Epilepsy Res. 2006 Oct;71(2–3):89–101. doi: 10.1016/j.eplepsyres.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 42.French J, Smith M, Faught E, Brown L. Practice advisory: The use of felbamate in the treatment of patients with intractable epilepsy: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 1999 May 12;52(8):1540–5. doi: 10.1212/wnl.52.8.1540. [DOI] [PubMed] [Google Scholar]
- 43.Mizoule J, Meldrum B, Mazadier M, Croucher M, Ollat C, Uzan A, et al. 2-Amino-6-trifluoromethoxy benzothiazole, a possible antagonist of excitatory amino acid neurotransmission--I. Anticonvulsant properties. Neuropharmacology. 1985 Aug;24(8):767–73. doi: 10.1016/0028-3908(85)90011-5. [DOI] [PubMed] [Google Scholar]
- 44.Malgouris C, Daniel M, Doble A. Neuroprotective effects of riluzole on N-methyl-D-aspartate- or veratridine-induced neurotoxicity in rat hippocampal slices. Neurosci Lett. 1994 Aug 15;177(1–2):95–9. doi: 10.1016/0304-3940(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 45.Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000 Jun;26(3):671–82. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- 46.Debono MW, Le GJ, Canton T, Doble A, Pradier L. Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol. 1993 Apr 28;235(2–3):283–9. doi: 10.1016/0014-2999(93)90147-a. [DOI] [PubMed] [Google Scholar]
- 47.Hubert JP, Delumeau JC, Glowinski J, Premont J, Doble A. Antagonism by riluzole of entry of calcium evoked by NMDA and veratridine in rat cultured granule cells: evidence for a dual mechanism of action. Br J Pharmacol. 1994 Sep;113(1):261–7. doi: 10.1111/j.1476-5381.1994.tb16203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fumagalli E, Funicello M, Rauen T, Gobbi M, Mennini T. Riluzole enhances the activity of glutamate transporters GLAST, GLT1 and EAAC1. Eur J Pharmacol. 2008 Jan 14;578(2–3):171–6. doi: 10.1016/j.ejphar.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 49.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994 Mar 3;330(9):585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 50.Lacomblez L, Bensimon G, Leigh PN, Guillet P, Meininger V. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996 May 25;347(9013):1425–31. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 51.Bensimon G, Lacomblez L, Delumeau JC, Bejuit R, Truffinet P, Meininger V. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. J Neurol. 2002 May;249(5):609–15. doi: 10.1007/s004150200071. [DOI] [PubMed] [Google Scholar]
- 52.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst Rev. 2007;(1):CD001447. doi: 10.1002/14651858.CD001447.pub2. [DOI] [PubMed] [Google Scholar]
- 53.Meininger V, Bensimon G, Bradley WR, Brooks B, Douillet P, Eisen AA, et al. Efficacy and safety of xaliproden in amyotrophic lateral sclerosis: results of two phase III trials. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004 Jun;5(2):107–17. doi: 10.1080/14660820410019602. [DOI] [PubMed] [Google Scholar]
- 54.Bruijn LI, Miller TM, Cleveland DW. Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci. 2004;27:723–49. doi: 10.1146/annurev.neuro.27.070203.144244. [DOI] [PubMed] [Google Scholar]
- 55.Landwehrmeyer GB, Dubois B, de Yebenes JG, Kremer B, Gaus W, Kraus PH, et al. Riluzole in Huntington’s disease: a 3-year, randomized controlled study. Ann Neurol. 2007 Sep;62(3):262–72. doi: 10.1002/ana.21181. [DOI] [PubMed] [Google Scholar]
- 56.Jankovic J, Hunter C. A double-blind, placebo-controlled and longitudinal study of riluzole in early Parkinson’s disease. Parkinsonism Relat Disord. 2002 Mar;8(4):271–6. doi: 10.1016/s1353-8020(01)00040-2. [DOI] [PubMed] [Google Scholar]
- 57.Kalkers NF, Barkhof F, Bergers E, van SR, Polman CH. The effect of the neuroprotective agent riluzole on MRI parameters in primary progressive multiple sclerosis: a pilot study. Mult Scler. 2002 Dec;8(6):532–3. doi: 10.1191/1352458502ms849xx. [DOI] [PubMed] [Google Scholar]
- 58.Schwab RS, England AC, Jr, Poskanzer DC, Young RR. Amantadine in the treatment of Parkinson’s disease. JAMA. 1969 May 19;208(7):1168–70. [PubMed] [Google Scholar]
- 59.Danysz W, Parsons CG, Kornhuber J, Schmidt WJ, Quack G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents--preclinical studies. Neurosci Biobehav Rev. 1997 Jul;21(4):455–68. doi: 10.1016/s0149-7634(96)00037-1. [DOI] [PubMed] [Google Scholar]
- 60.Kornhuber J, Weller M, Schoppmeyer K, Riederer P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J Neural Transm Suppl. 1994;43:91–104. [PubMed] [Google Scholar]
- 61.Crosby N, Deane KH, Clarke CE. Amantadine in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(1):CD003468. doi: 10.1002/14651858.CD003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fahn S, Isgreen WP. Long-term evaluation of amantadine and levodopa combination in parkinsonism by double-blind corssover analyses. Neurology. 1975 Aug;25(8):695–700. doi: 10.1212/wnl.25.8.695. [DOI] [PubMed] [Google Scholar]
- 63.Fehling C. The effect of adding amantadine to optimum L-dopa dosage in Parkinson’s syndrome. Acta Neurol Scand. 1973;49(2):245–51. doi: 10.1111/j.1600-0404.1973.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 64.Savery F. Amantadine and a fixed combination of levodopa and carbidopa in the treatment of Parkinson’s disease. Dis Nerv Syst. 1977 Aug;38(8):605–8. [PubMed] [Google Scholar]
- 65.Silver DE, Sahs AL. Double blind study using amantadine hydrochloride in the therapy of Parkinson’s disease. Trans Am Neurol Assoc. 1971;96:307–8. [PubMed] [Google Scholar]
- 66.Walker JE, Albers JW, Tourtellotte WW, Henderson WG, Potvin AR, Smith A. A qualitative and quantitative evaluation of amantadine in the treatment of Parkinson’s disease. J Chronic Dis. 1972 Mar;25(3):149–82. doi: 10.1016/0021-9681(72)90171-3. [DOI] [PubMed] [Google Scholar]
- 67.Walker JE, Potvin A, Tourtellotte W, Albers J, Repa B, Henderson W, et al. Amantadine and levodopa in the treatment of Parkinson’s disease. Clin Pharmacol Ther. 1972 Jan;13(1):28–36. doi: 10.1002/cpt197213128. [DOI] [PubMed] [Google Scholar]
- 68.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998 Oct 8;339(15):1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 69.Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. N Engl J Med. 1998 Oct 15;339(16):1130–43. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- 70.Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord. 2005 Aug;20(8):919–31. doi: 10.1002/mds.20612. [DOI] [PubMed] [Google Scholar]
- 71.Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG. Levodopa-induced dyskinesias. Mov Disord. 2007 Jul 30;22(10):1379–89. doi: 10.1002/mds.21475. [DOI] [PubMed] [Google Scholar]
- 72.Crosby NJ, Deane KH, Clarke CE. Amantadine for dyskinesia in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(2):CD003467. doi: 10.1002/14651858.CD003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luginger E, Wenning GK, Bosch S, Poewe W. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2000 Sep;15(5):873–8. doi: 10.1002/1531-8257(200009)15:5<873::aid-mds1017>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 74.Snow BJ, Macdonald L, Mcauley D, Wallis W. The effect of amantadine on levodopa-induced dyskinesias in Parkinson’s disease: a double-blind, placebo-controlled study. Clin Neuropharmacol. 2000 Mar;23(2):82–5. doi: 10.1097/00002826-200003000-00004. [DOI] [PubMed] [Google Scholar]
- 75.Verhagen ML, Del DP, van den MP, Fang J, Mouradian MM, Chase TN. Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology. 1998 May;50(5):1323–6. doi: 10.1212/wnl.50.5.1323. [DOI] [PubMed] [Google Scholar]
- 76.Metman LV, Del DP, LePoole K, Konitsiotis S, Fang J, Chase TN. Amantadine for levodopa-induced dyskinesias: a 1-year follow-up study. Arch Neurol. 1999 Nov;56(11):1383–6. doi: 10.1001/archneur.56.11.1383. [DOI] [PubMed] [Google Scholar]
- 77.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999 Jun;38(6):735–67. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JW, Kotermanski SE. Mechanism of action of memantine. Curr Opin Pharmacol. 2006 Feb;6(1):61–7. doi: 10.1016/j.coph.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007 Oct;8(10):803–8. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 80.Aracava Y, Pereira EF, Maelicke A, Albuquerque EX. Memantine blocks alpha7* nicotinic acetylcholine receptors more potently than n-methyl-D-aspartate receptors in rat hippocampal neurons. J Pharmacol Exp Ther. 2005 Mar;312(3):1195–205. doi: 10.1124/jpet.104.077172. [DOI] [PubMed] [Google Scholar]
- 81.Ferchmin PA, Perez D, Eterovic VA, de VJ. Nicotinic receptors differentially regulate N-methyl-D-aspartate damage in acute hippocampal slices. J Pharmacol Exp Ther. 2003 Jun;305(3):1071–8. doi: 10.1124/jpet.102.048173. [DOI] [PubMed] [Google Scholar]
- 82.Wang HY, Li W, Benedetti NJ, Lee DH. Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. J Biol Chem. 2003 Aug 22;278(34):31547–53. doi: 10.1074/jbc.M212532200. [DOI] [PubMed] [Google Scholar]
- 83.Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. A double-blind, placebo controlled trial. Arzneimittelforschung. 1991 Aug;41(8):773–80. [PubMed] [Google Scholar]
- 84.Gortelmeyer R, Erbler H. Memantine in the treatment of mild to moderate dementia syndrome. A double-blind placebo-controlled study. Arzneimittelforschung. 1992 Jul;42(7):904–13. [PubMed] [Google Scholar]
- 85.Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine) Int J Geriatr Psychiatry. 1999 Feb;14(2):135–46. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 86.van Dyck CH, Tariot PN, Meyers B, Malca RE. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord. 2007 Apr;21(2):136–43. doi: 10.1097/WAD.0b013e318065c495. [DOI] [PubMed] [Google Scholar]
- 87.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004 Jan 21;291(3):317–24. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 88.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003 Apr 3;348(14):1333–41. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 89.Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer’s disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis. 2008 Feb;13(1):97–107. doi: 10.3233/jad-2008-13110. [DOI] [PubMed] [Google Scholar]
- 90.Peskind ER, Potkin SG, Pomara N, Ott BR, Graham SM, Olin JT, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry. 2006 Aug;14(8):704–15. doi: 10.1097/01.JGP.0000224350.82719.83. [DOI] [PubMed] [Google Scholar]
- 91.Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008 Feb;5(1):83–9. doi: 10.2174/156720508783884576. [DOI] [PubMed] [Google Scholar]
- 92.McShane R, Areosa SA, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;(2):CD003154. doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 93.Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006 Jan;63(1):49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 94.Orgogozo JM, Rigaud AS, Stoffler A, Mobius HJ, Forette F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300) Stroke. 2002 Jul;33(7):1834–9. doi: 10.1161/01.str.0000020094.08790.49. [DOI] [PubMed] [Google Scholar]
- 95.Wilcock G, Mobius HJ, Stoffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500) Int Clin Psychopharmacol. 2002 Nov;17(6):297–305. doi: 10.1097/00004850-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 96.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol. 2007 Sep;6(9):782–92. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 97.Lipton SA. Failures and successes of NMDA receptor antagonists: molecular basis for the use of open-channel blockers like memantine in the treatment of acute and chronic neurologic insults. NeuroRx. 2004 Jan;1(1):101–10. doi: 10.1602/neurorx.1.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kmietowicz Z. NICE proposes to withdraw Alzheimer’s drugs from NHS. BMJ. 2005 Mar 5;330(7490):495. doi: 10.1136/bmj.330.7490.495-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, et al. Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol. 1991 Jan;432:483–508. doi: 10.1113/jphysiol.1991.sp018396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000 Nov;20(5):358–73. doi: 10.1016/s0885-3924(00)00213-x. [DOI] [PubMed] [Google Scholar]
- 101.Bell RF, Eccleston C, Kalso E. Ketamine as adjuvant to opioids for cancer pain. A qualitative systematic review. J Pain Symptom Manage. 2003 Sep;26(3):867–75. doi: 10.1016/s0885-3924(03)00311-7. [DOI] [PubMed] [Google Scholar]
- 102.Bell RF, Dahl JB, Moore RA, Kalso E. Perioperative ketamine for acute postoperative pain. Cochrane Database Syst Rev. 2006;(1):CD004603. doi: 10.1002/14651858.CD004603.pub2. [DOI] [PubMed] [Google Scholar]
- 103.Franklin PH, Murray TF. High affinity [3H]dextrorphan binding in rat brain is localized to a noncompetitive antagonist site of the activated N-methyl-D-aspartate receptor-cation channel. Mol Pharmacol. 1992 Jan;41(1):134–46. [PubMed] [Google Scholar]
- 104.LePage KT, Ishmael JE, Low CM, Traynelis SF, Murray TF. Differential binding properties of [3H]dextrorphan and [3H]MK-801 in heterologously expressed NMDA receptors. Neuropharmacology. 2005 Jul;49(1):1–16. doi: 10.1016/j.neuropharm.2005.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007 Nov 8;56(3):422–37. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 106.Russell JC, Blue ME, Johnston MV, Naidu S, Hossain MA. Enhanced cell death in MeCP2 null cerebellar granule neurons exposed to excitotoxicity and hypoxia. Neuroscience. 2007 Dec 12;150(3):563–74. doi: 10.1016/j.neuroscience.2007.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Danysz W, Parsons CG, Jirgensons A, Kauss V, Tillner J. Amino-alkyl-cyclohexanes as a novel class of uncompetitive NMDA receptor antagonists. Curr Pharm Des. 2002;8(10):835–43. doi: 10.2174/1381612024607117. [DOI] [PubMed] [Google Scholar]
- 108.Rammes G, Schierloh A. Neramexane (merz pharmaceuticals/forest laboratories) IDrugs. 2006 Feb;9(2):128–35. [PubMed] [Google Scholar]
- 109.Bachurin S, Bukatina E, Lermontova N, Tkachenko S, Afanasiev A, Grigoriev V, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001 Jun;939:425–35. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 110.Lermontova NN, Redkozubov AE, Shevtsova EF, Serkova TP, Kireeva EG, Bachurin SO. Dimebon and tacrine inhibit neurotoxic action of beta-amyloid in culture and block L-type Ca(2+) channels. Bull Exp Biol Med. 2001 Nov;132(5):1079–83. doi: 10.1023/a:1017972709652. [DOI] [PubMed] [Google Scholar]
- 111.Bachurin SO, Shevtsova EP, Kireeva EG, Oxenkrug GF, Sablin SO. Mitochondria as a target for neurotoxins and neuroprotective agents. Ann N Y Acad Sci. 2003 May;993:334–44. doi: 10.1111/j.1749-6632.2003.tb07541.x. [DOI] [PubMed] [Google Scholar]
- 112.Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med. 2003 Nov;136(5):474–7. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- 113.Vorobjev VS, Sharonova IN, Walsh IB, Haas HL. Histamine potentiates N-methyl-D-aspartate responses in acutely isolated hippocampal neurons. Neuron. 1993 Nov;11(5):837–44. doi: 10.1016/0896-6273(93)90113-6. [DOI] [PubMed] [Google Scholar]
- 114.Hood WF, Compton RP, Monahan JB. D-cycloserine: a ligand for the N-methyl-D-aspartate coupled glycine receptor has partial agonist characteristics. Neurosci Lett. 1989 Mar 13;98(1):91–5. doi: 10.1016/0304-3940(89)90379-0. [DOI] [PubMed] [Google Scholar]
- 115.Chessell IP, Procter AW, Francis PT, Bowen DM. D-cycloserine, a putative cognitive enhancer, facilitates activation of the N-methyl-D-aspartate receptor-ionophore complex in Alzheimer brain. Brain Res. 1991 Nov 29;565(2):345–8. doi: 10.1016/0006-8993(91)91668-q. [DOI] [PubMed] [Google Scholar]
- 116.Schwartz BL, Hashtroudi S, Herting RL, Schwartz P, Deutsch SI. d-Cycloserine enhances implicit memory in Alzheimer patients. Neurology. 1996 Feb;46(2):420–4. doi: 10.1212/wnl.46.2.420. [DOI] [PubMed] [Google Scholar]
- 117.Tsai GE, Falk WE, Gunther J. A preliminary study of D-cycloserine treatment in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 1998;10(2):224–6. doi: 10.1176/jnp.10.2.224. [DOI] [PubMed] [Google Scholar]
- 118.Tsai GE, Falk WE, Gunther J, Coyle JT. Improved cognition in Alzheimer’s disease with short-term D-cycloserine treatment. Am J Psychiatry. 1999 Mar;156(3):467–9. doi: 10.1176/ajp.156.3.467. [DOI] [PubMed] [Google Scholar]
- 119.Laake K, Oeksengaard AR. D-cycloserine for Alzheimer’s disease. Cochrane Database Syst Rev. 2002;(2):CD003153. doi: 10.1002/14651858.CD003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tuominen HJ, Tiihonen J, Wahlbeck K. Glutamatergic drugs for schizophrenia. Cochrane Database Syst Rev. 2006;(2):CD003730. doi: 10.1002/14651858.CD003730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Posey DJ, Kem DL, Swiezy NB, Sweeten TL, Wiegand RE, McDougle CJ. A pilot study of D-cycloserine in subjects with autistic disorder. Am J Psychiatry. 2004 Nov;161(11):2115–7. doi: 10.1176/appi.ajp.161.11.2115. [DOI] [PubMed] [Google Scholar]
- 122.Shigeri Y, Seal RP, Shimamoto K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res Brain Res Rev. 2004 Jul;45(3):250–65. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 123.Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005 Jan 6;433(7021):73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 124.Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004 Jun;14(3):346–52. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 125.Sattler R, Xiong Z, Lu WY, MacDonald JF, Tymianski M. Distinct roles of synaptic and extrasynaptic NMDA receptors in excitotoxicity. J Neurosci. 2000 Jan 1;20(1):22–33. doi: 10.1523/JNEUROSCI.20-01-00022.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007 Mar 14;27(11):2846–57. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002 May;5(5):405–14. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 128.Hardingham GE, Bading H. The Yin and Yang of NMDA receptor signalling. Trends Neurosci. 2003 Feb;26(2):81–9. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 129.Salter MW, Kalia LV. Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci. 2004 Apr;5(4):317–28. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 130.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007 Dec;13(6):572–9. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sebolt-Leopold JS, English JM. Mechanisms of drug inhibition of signalling molecules. Nature. 2006 May 25;441(7092):457–62. doi: 10.1038/nature04874. [DOI] [PubMed] [Google Scholar]
- 132.Lu WY, Jackson MF, Bai D, Orser BA, MacDonald JF. In CA1 pyramidal neurons of the hippocampus protein kinase C regulates calcium-dependent inactivation of NMDA receptors. J Neurosci. 2000 Jun 15;20(12):4452–61. doi: 10.1523/JNEUROSCI.20-12-04452.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu WY, Xiong ZG, Lei S, Orser BA, Dudek E, Browning MD, et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999 Apr;2(4):331–8. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- 134.Bonini JS, Da Silva WC, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of hippocampal PKC in acquisition, consolidation and reconsolidation of spatial memory. Neuroscience. 2007 Jun 15;147(1):37–45. doi: 10.1016/j.neuroscience.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 135.Etcheberrigaray R, Tan M, Dewachter I, Kuiperi C, Van dA I, Wera S, et al. Therapeutic effects of PKC activators in Alzheimer’s disease transgenic mice. Proc Natl Acad Sci U S A. 2004 Jul 27;101(30):11141–6. doi: 10.1073/pnas.0403921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alkon DL, Sun MK, Nelson TJ. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol Sci. 2007 Feb;28(2):51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 137.Moriguchi S, Shioda N, Maejima H, Zhao X, Marszalec W, Yeh JZ, et al. Nefiracetam potentiates N-methyl-D-aspartate (NMDA) receptor function via protein kinase C activation and reduces magnesium block of NMDA receptor. Mol Pharmacol. 2007 Feb;71(2):580–7. doi: 10.1124/mol.106.027607. [DOI] [PubMed] [Google Scholar]
- 138.Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002 Jul 15;22(14):5955–65. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rachline J, Perin-Dureau F, Le GA, Neyton J, Paoletti P. The micromolar zinc-binding domain on the NMDA receptor subunit NR2B. J Neurosci. 2005 Jan 12;25(2):308–17. doi: 10.1523/JNEUROSCI.3967-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]