Abstract
Purpose
Although pediatric intensivists claim to embrace lung protective ventilation for ALI, ventilator management is variable. We describe ventilator changes clinicians made for children with hypoxemic respiratory failure, and evaluate the potential acceptability of a pediatric ventilation protocol.
Methods
Retrospective cohort study; tertiary care PICU, 1/2000–7/2007. We included mechanically ventilated children with PaO2/FiO2 (P/F) ratio <300. We assessed variability in ventilator management by evaluating actual changes to ventilator settings after an arterial blood gas (ABG). We evaluated the potential acceptability of a pediatric mechanical ventilation protocol we adapted from NIH/NHLBI ARDS Network protocols by comparing actual practice changes in ventilator settings to changes that would have been recommended by the protocol.
Results
2,719 ABGs from 402 patients were associated with 6,017 ventilator settings. Clinicians infrequently decreased FiO2, even when the PaO2 was high (>68 mmHg). The protocol would have recommended more positive end expiratory pressure (PEEP) than was used in actual practice 42% of the time in the mid PaO2 range (55 to 68 mmHg) and 67% of the time in the low PaO2 range (<55 mmHg). Clinicians often made no change to either peak inspiratory pressure (PIP) or ventilator rate (VR) when the protocol would have recommended a change, even when the pH was >7.45 with PIP ≥ 35 cm H2O.
Conclusions
There may be lost opportunities to minimize potentially injurious ventilator settings for children with ALI. A reproducible pediatric mechanical ventilation protocol could prompt clinicians to make ventilator changes that are consistent with lung protective ventilation.
Keywords: Acute Lung Injury, Clinical Protocols, Decision Support Systems, Clinical, Pediatrics, Critical Care
Introduction
Ventilator management for children with hypoxemic respiratory failure, Acute Lung Injury (ALI), and Acute Respiratory Distress Syndrome (ARDS) is variable [1]. Adult intensivists have generally accepted NIH/NHLBI ARDS Network ventilator protocols [2] which have improved outcomes for adults with ALI/ARDS [2–5], but protocol implementation is not yet widespread [6–8]. Few ventilator protocols exist for pediatric critical care, although studies of ALI in children [9, 10] have used protocols described as similar to published ARDS network protocols [2].
However, protocols developed in the adult ICU may need modification to match pediatric ICU (PICU) needs. For mechanical ventilation, pediatric intensivists commonly use different modes of ventilation than adult intensivists. There are unanswered questions regarding the weight to use for tidal volume targets, where and how to measure tidal volume, the magnitude of changes to fraction of inspired oxygen (FIO2), and the acceptable range of permissive hypercapnia [11]. Given these potential differences, we modified the ARDS Network protocol tables to develop a pediatric ALI/ARDS mechanical ventilation protocol [12].
We sought to describe usual care clinician decisions for mechanically ventilated children with ALI/ARDS, and to determine the potential applicability of our pediatric mechanical ventilation protocol. We extracted mechanical ventilation, oxygenation, and blood gas data from a PICU database that contained 7 years of information. We compared these historical clinician determined ventilator settings, with recommendations that would have been generated by the protocol.
Our clinicians agreed to use a lung protective mechanical ventilation strategy. Nevertheless, we expected variability in usual care ventilator management for children with lung injury, and inconsistency in lung protective decisions.
Methods
The Children’s Hospital Los Angeles IRB approved this study. Patients were eligible if they were endotracheally intubated and mechanically ventilated, and had at least one PaO2/FiO2 (P/F) ratio < 300 within 24 hours of intubation. Patients were excluded if they had heart failure, uncorrected cyanotic heart disease, or primary pulmonary hypertension. All patients met three of the four diagnostic criteria for ALI (acute onset of disease, P/F ratio <300, and no left ventricular dysfunction). The fourth criterion for ALI is bilateral infiltrates; we used chest radiograph information regarding infiltrates for subgroup analysis.
We examined data from January 2000 through July 2007. The reported benefits of lung protective ventilation in adults [13, 14] just prior to 2000 affected our clinical practice. During the study period, our clinicians agreed with principles of lung protective ventilation strategies, although we had no formal mechanical ventilation protocol. We sought to use pressure-limited modes of ventilation, with target peak inspiratory pressure (PIP) < 35 cm H20, ventilator rate (VR) <35 bpm, and allowed permissive hypercapnia. We sought to adjust positive end expiratory pressure (PEEP) and FiO2 to maintain SpO2 between 88–95% or PaO2 >60 mmHg. We did not commonly employ recruitment maneuvers [15]. We used three conventional ventilators: Servo 300 (Siemens, Solna, Sweden), Avea (Viasys Healthcare, Yorba Linda, CA), and Servo i (Maquet Medical, Solna, Sweden).
Data extraction
We extracted data from the electronic healthcare record and from two local computer databases (Philips CareVue ISM and Microsoft Access®). The local databases are routinely evaluated for accuracy, and have been determined to be appropriate for clinical care, quality improvement, and research. An attending pediatric radiologist read all chest x-rays, and we reviewed those reports for diagnostic criteria for ALI.
The first P/F ratio < 300 after intubation defined the beginning of the study for each patient. We extracted all blood gas values and ventilator settings beginning at or immediately prior this, and moved forward for three days or until extubation (whichever was first). We associated ventilator settings with blood gas values based on time stamps. We determined actual clinical care changes to ventilator settings by comparing pairs of sequential ventilator settings before and after the ABG, if the time stamps for the settings were no more than 8 hours apart. We used all intubation and extubation times to calculate 28-day ventilator free days (VFDs). We limited analysis to patients supported with PC ventilation (>90% of patients).
Pediatric ALI/ARDS mechanical ventilation protocol
Our pediatric ALI/ARDS mechanical ventilation protocol was modified from ARDS Network protocol tables [2] using preliminary data and expert review by clinicians from the Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) network and the NICHD Collaborative Pediatric Critical Care Research Network (CPCCRN). We believe it represents the best available evidence and current consensus of pediatric critical care clinicians. The impact of this protocol on clinical outcomes still needs to be evaluated, but was not an objective of our current study.
The protocol contains decision tables that implement lung protective ventilation strategies through discrete, explicit steps. Oxygenation tables evaluate combinations of PEEP and FIO2 stratified into high PaO2 (>68 mmHg), mid PaO2 (55–68 mmHg) and low PaO2 (<55 mmHg) subsets. The adult protocol table bins are based on FiO2 increments of 0.1; this was reduced to 0.05 for the pediatric protocol.
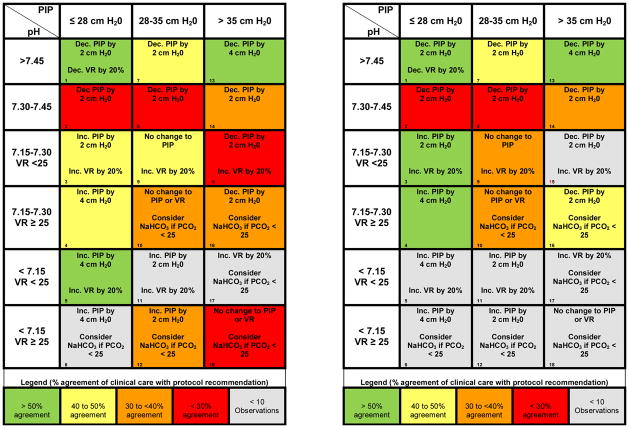
Ventilation tables describe combinations of ventilator support, stratified by pH, for four different modes of ventilation (Pressure Control (PC), Volume Control (VC), Pressure Regulated Volume Control (PRVC), and High Frequency Oscillatory Ventilation (HFOV)). We retained pH ranges from the adult protocol. For the PC mode of ventilation, used in this study, pH categories are combined with three ranges of PIP (≤28, 29–35 and >35 cm H20), and when pH is < 7.3, additional stratification is based on VR (breaths per minute, bpm). Pediatric clinicians recommended 25 bpm as a stratification point (compared to 35 bpm in the ARDS Network protocol) because they felt pressure support is frequently added to controlled modes of ventilation in pediatrics, as opposed to pure assist control modes. (Table 4)
Table 4.
Table 4(a,b) Adapted ventilation table for PC mode, with cell numbers listed in the lower left hand corner of each cell (corresponding to Table 5). For changes in ventilation (PIP or Vent Rate) each ABG/Ventilator setting combination was categorized into the boxes on the above ventilation table. The direction of change to VR and PIP which clinicians chose was compared to the direction of change of the ventilator protocol’s recommendation. Table 4a (left) uses all observations (N=2,719), while Table 4b (right) includes observations from patients with bilateral pulmonary infiltrates (ALI cohort, N=1,415). Colors represent percent agreement with ventilator protocol recommendations: >50% agreement (green), 40–50% (yellow), 30–40% (orange), < 30% (red), excluded cells (<10 observations, grey). Note that changes to PIP and VR are similar between the ALI cohort and the entire cohort, particularly when pH is >7.30.
|
Data preparation: use of the mechanical ventilation protocol
We used the pediatric mechanical ventilation protocol to group data for analysis. Our protocol tables specify combinations of blood gas and ventilator data ranges and define data bins. Each data bin contains a treatment recommendation. We grouped the usual clinical care data in the same data bins. We entered the actual clinical ABG values and ventilator settings from the database into a computer version of the pediatric ventilator protocol to determine what the protocol would have recommended for changes to PEEP, FiO2, PIP and VR. We compared the computer protocol recommendations to the actual clinical care changes recorded in the database.
Analysis
We used descriptive statistics for blood gas and ventilator settings. To examine variability of usual care regarding oxygenation, we created a box plot of FIO2, stratified by PEEP (Figure 2a). To evaluate variability in ventilation, we calculated the frequency of changes to either PIP or VR after an ABG for each data bin in the ventilation table (Tables 4, 5).
Fig. 2.
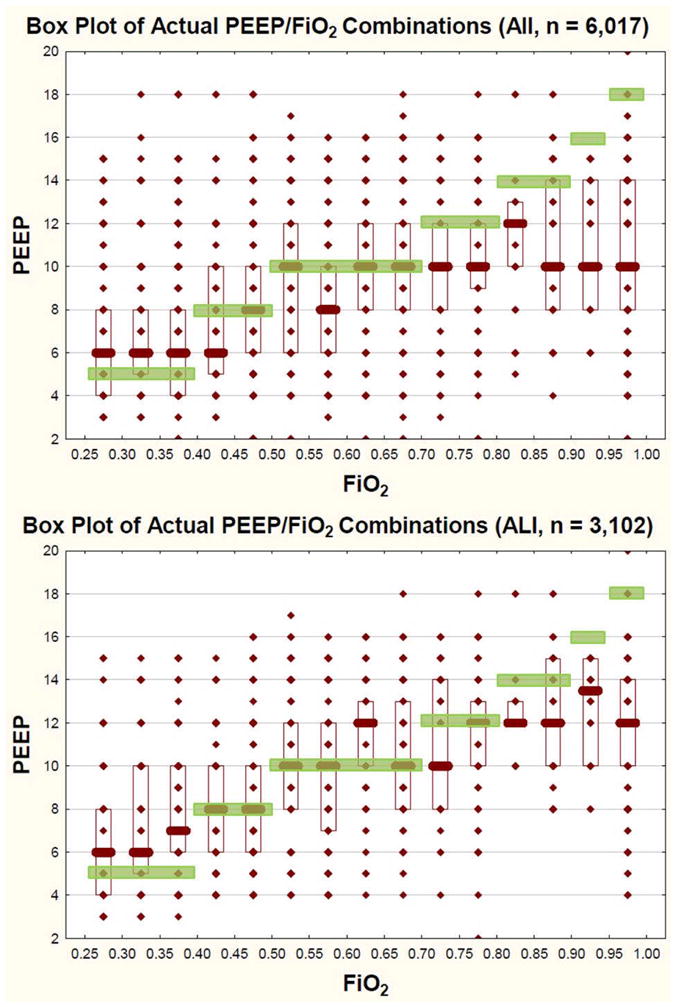
Fig 2 (a, b): PEEP/FiO2 titration tables from clinical care for all patients (a, top) and for patients with bilateral pulmonary infiltrates (ALI Cohort, b, bottom). The y axis represents actual PEEP values as a function of actual FiO2 used (x-axis). The superimposed green boxes represent the pediatric mechanical ventilation protocol target combinations of FiO2/PEEP. For all patients, there is variability in FiO2/PEEP combinations clinicians choose. In general, clinicians use less PEEP than the protocol would recommend, particularly when FiO2 climbs above 0.7. For the ALI cohort (b, bottom), clinicians may be more likely to increase PEEP, although they still use less PEEP than the protocol would recommend, particularly when FiO2 climbs above 0.8. Actual value (diamond), Median (Bar), IQR (Box), Protocol Target (Green Boxes).
Table 5.
The left hand side of the table summarizes the recommendations based on the blood gas, PIP and VR from the pediatric mechanical ventilator protocol. The right hand side describes the changes in PIP or VR made by clinicians in actual practice in response to a blood gas. The middle column (blue shaded cells) represents the number of observations per cell in the ventilation table. The columns to the right of that display the number and percentage (%) of observations in each cell for which clinicians made no change to VR or PIP, changed VR or PIP in the same direction as the protocol recommendation, change VR or PIP opposite the protocol recommendation, or made a combination of increasing one parameter (VR or PIP) and decreasing the other (VR or PIP).
| Cell | pH | PIP | VR | Recommendation | N = 2,719 | No Change | In Line | Opposite | Combination | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | ||||||
| 1 | > 7.45 | ≤ 28 | All | ↓PIP 2; ↓VR by 20% | 317 | 143 | 45.1 | 158 | 49.8 | 19 | 6 | 3 | 0.9 |
| 2 | 7.30–7.45 | ≤ 28 | All | ↓PIP 2 | 1021 | 651 | 63.8 | 223 | 21.8 | 155 | 15.2 | 8 | 0.8 |
| 3 | 7.15 – 7.29 | ≤ 28 | < 25 | ↑PIP 2; ↑VR by 20% | 126 | 57 | 45.2 | 61 | 48.4 | 13 | 10.3 | 5 | 4 |
| 4 | 7.15 – 7.29 | ≤ 28 | ≥ 25 | ↑PIP 4 | 62 | 23 | 37.1 | 30 | 48.4 | 13 | 21.0 | 4 | 6.5 |
| 5 | < 7.15 | ≤ 28 | < 25 | ↑PIP 4; ↑VR by 20% | 13 | 3 | 23.1 | 10 | 76.9 | 1 | 7.7 | 1 | 7.7 |
| 6 | < 7.15 | ≤ 28 | ≥ 25 | ↑PIP 4 | 5 | 3 | 60 | 2 | 40 | 0 | 0 | 0 | 0 |
| 7 | > 7.45 | 29–35 | All | ↓PIP 2 | 201 | 101 | 50.2 | 85 | 42.3 | 18 | 9 | 3 | 1.5 |
| 8 | 7.30–7.45 | 29–35 | All | ↓PIP 2 | 473 | 288 | 60.9 | 127 | 26.9 | 67 | 14.2 | 9 | 1.9 |
| 9 | 7.15 – 7.29 | 29–35 | < 25 | No Δ PIP; ↑VR | 84 | 38 | 45.2 | 35 | 41.7 | 15 | 17.9 | 4 | 4.3 |
| 10* | 7.15 – 7.29 | 29–35 | ≥ 25 | No Δ PIP | 82 | 44* | 53.7 | 27 | 32.9 | 14 | 17.1 | 3 | 3.7 |
| 11 | < 7.15 | 29–35 | < 25 | ↑PIP 2; ↑VR by 20% | 9 | 2 | 22.2 | 6 | 66.7 | 1 | 11.1 | 0 | 0 |
| 12 | < 7.15 | 29–35 | ≥ 25 | ↑PIP 2 | 13 | 5 | 38.5 | 5 | 38.4 | 4 | 30.8 | 1 | 7.7 |
| 13 | > 7.45 | > 35 | All | ↓PIP 4 | 47 | 17 | 36.2 | 28 | 59.6 | 2 | 4.3 | 0 | 0 |
| 14 | 7.30–7.45 | > 35 | All | ↓PIP 2 | 146 | 78 | 53.4 | 55 | 37.7 | 15 | 10.3 | 2 | 1.4 |
| 15** | 7.15 – 7.29 | > 35 | < 25 | ↓PIP 2; ↑VR by 20% | 37 | 18 | 48.7 | 8 | 21.6 | 13 | 35.1 | 2** | 5.4 |
| 16 | 7.15 – 7.29 | > 35 | ≥ 25 | ↓PIP 2 | 56 | 25 | 44.6 | 22 | 39.3 | 15 | 26.7 | 6 | 10.7 |
| 17 | < 7.15 | > 35 | < 25 | ↑VR by 20% | 6 | 3 | 50 | 2 | 33.3 | 1 | 16.7 | 0 | 0 |
| 18* | < 7.15 | > 35 | ≥ 25 | No Δ PIP | 21 | 10* | 47.6 | 6 | 28.5 | 8 | 38.1 | 3 | 14.3 |
In these two cells the protocol would have recommended no change to PIP or VR—for these cells in line means VR or PIP was increased, Opposite means PIP or VR was decreased.
In this cell the protocol recommended increasing the VR and decreasing the PIP—for this cell in line means VR or PIP was increased, opposite means PIP or VR was decreased.
PIP = Peak Inspiratory Pressure cmH2O; VR = Ventilator Rate (bpm)
To examine potential applicability of the pediatric mechanical ventilation protocol, we assessed percent agreement between clinical care changes in PEEP and FiO2 after an ABG and those that would have been recommended by the protocol (Table 3). We also calculated the concordance of actual clinical care with the protocol recommendation for each of the 18 ventilation bins (Tables 4, 5).
Table 3.
Clinical care changes to FiO2 and PEEP which clinicians made in response to an arterial blood gas (Clinician) compared to the changes that would have been recommended by the pediatric mechanical ventilation protocol (Protocol). Changes to FiO2 and PEEP are grouped by PaO2. Data are reported as the number and percentage of observations within each PaO2 range in which clinician choices for FiO2 and PEEP were less than, in line, or greater than the protocol’s recommendations.
| Entire Cohort | High PaO2 (>68 mmHg) | Mid PaO2 (55–68 mmHg) | Low PaO2 (<55 mmHg) |
|---|---|---|---|
| N observations 2,719 (100%) | 2,108 (77.5%) | 429 (15.8%) | 182 (6.7%) |
| PaO2 Median (IQR) | 104 (83,136) | 62 (59,66) | 47 (40,51) |
| FiO2 Changes | |||
| Clinician FiO2 = Protocol FiO2 | 631 (29.9%) | 128 (29.8%) | 87 (47.8%) |
| Clinician FiO2 < Protocol FiO2 | 407 (19.3%) | 138 (32.2%) | 38 (20.9%) |
| Clinician FiO2 > Protocol FiO2 | 1,070 (50.8%) | 163 (38%) | 57 (31.3%) |
| PEEP Changes | |||
| Clinician PEEP = Protocol PEEP | 1,237 (58.7%) | 164 (38.2%) | 44 (24.2%) |
| Clinician PEEP < Protocol PEEP | 90 (4.3%) | 182 (42.4%) | 122 (67%) |
| Clinician PEEP > Protocol PEEP | 781 (37%) | 83 (19.3%) | 16 (8.8%) |
All patients met at least 3 of 4 criteria for Acute Lung Injury (ALI). We performed subgroup analyses on patients with bilateral pulmonary infiltrates (ALI cohort, Table ESM 1a, Table 4b, Figure 2b), thus meeting all 4 ALI criteria. We also performed subgroup analyses on patients free from specific co-morbidities that may affect ventilator management (non co-morbid cohort, Table ESM 1b, Table ESM 2). The specific co-morbidities excluded were diseases that may elevate airway resistance and make physicians reluctant to increase PEEP (asthma, bronchiolitis); patients at risk for pulmonary hypertension, where higher PaO2 and pH may be preferred (history of congenital heart disease or secondary pulmonary hypertension); or those with diagnoses that predispose to intracranial hypertension (head trauma, hydrocephalus, intracranial tumor). We believe the non co-morbid cohort consists of patients more likely to be managed with a lung protective strategy. We compared the percent agreement between actual clinical care and computer protocol recommendations for these subgroups to the entire cohort using Yates corrected Chi-Squared Tests to determine if the subgroups differed from the overall group.
Results
We analyzed 6,017 ventilator settings from 461 patients, and were able to associate ventilator settings with 2,719 ABG values from 402 patients (Figure 1 and Table 1). The median P/F ratio was 140. Overall mortality was 24.4%; the median value of 28-day ventilator free days was 15.4 (Table 1). The median interval between ventilator changes was ~4 hours, with a PEEP of 8 cm H20, and tidal volume of 7.4 ml/kg (Table 2). Peak inspiratory pressure was ≤ 35 cm H20 in 90% of observations, and 98% were ≤ 40 cm H2O. Median VR was 20 breaths/min (bpm) and 75% of values were < 25 bpm (Table 2).
Fig 1.
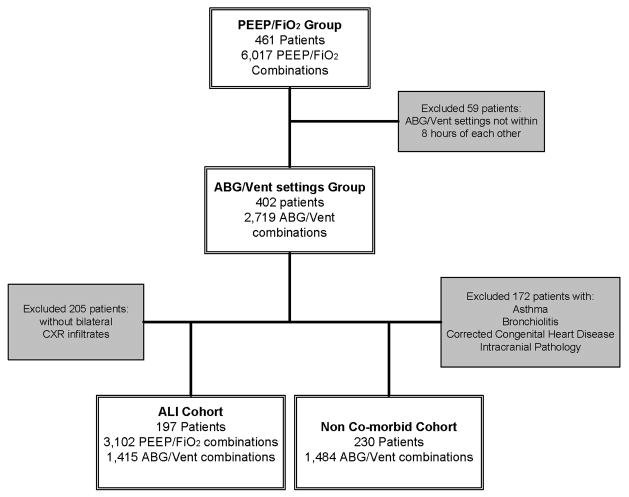
Flow diagram detailing the number of patients and observations of ABG and ventilator settings for the entire cohort of children, as well as the two subgroups of children with bilateral pulmonary infiltrates on chest X-ray (ALI Cohort) and those without co-morbidities that may affect ventilator management (Non co-morbid Cohort).
Table 1.
Demographics and Outcomes of included Patients.
| Variable | Count (%) or Median (IQR) N=402 |
|---|---|
| Gender (Male) | 227 (56.5) |
| Age (yrs) | 4.5 (1.0,12) |
| Weight (kg) | 16 (9,36) |
| Race | |
| White | 70 (17.4) |
| Latino | 194 (48.3) |
| Black | 48 (11.9) |
| Other | 90 (22.4) |
| Primary Diagnosis | |
| Pulmonary | 138 (34.3) |
| Cardiovascular | 41 (10.2) |
| Shock/Sepsis | 45 (11.2) |
| Neurologic | 46 (11.5) |
| Metabolic/Renal | 20 (5.0) |
| Other | 27 (6.7) |
| GI | 56 (13.9) |
| Ortho/Trauma | 29 (7.2) |
| Any Diagnosis | |
| Chronic Lung Disease | 34 (8.5) |
| Asthma or Bronchiolitis | 29 (7.2) |
| Congenital Heart Disease (corrected or non-cyanotic) | 59 (14.7) |
| Pulmonary HTN (acquired) | 18 (4.5) |
| Risk for intracranial HTN | 69 (17.2) |
| Immune compromised | 95 (23.6) |
| Pneumonia | 85 (21.1) |
| Shock/Sepsis | 109 (27.1) |
| Bilateral Pulmonary Infiltrates | 197 (49) |
| P/F Ratio | 140 (85,192) |
| Inotropes/Vasopressors | 184 (45.8) |
| Mortality (Died) | 98 (24.4) |
| 28 Day Vent Free Days | 15.4 (0,23.3) |
| Length of Stay (days) | 10 (5,17) |
Table 2.
Descriptive statistics from the 2,719 ventilator settings and blood gas pairs, generated from the 402 children.
| Variable | Median (IQR) N=2,719 |
|---|---|
| Time Between Ventilator Settings (hrs) | 4.01 (3.17,4.83) |
| pH | 7.38 (7.32,7.44) |
| PaCO2 (mmHg) | 43 (37,50) |
| PaO2 (mmHg) | 91 (71,125) |
| Ventilator Rate (bpm) | 20 (16,25) |
| FiO2 | 0.45 (0.40,0.60) |
| PEEP (cm H20) | 8 (6,10) |
| PIP (cm H20) | 28 (24,32) |
| VT (ml/kg)a | 7.4 (5.9,9.2) |
Exhaled tidal volume measured at the ventilator with appropriate compensation for tubing compliance. This value was then divided by actual body weight to report tidal volume (VT ml/kg).
Nearly half of the patients had bilateral pulmonary infiltrates (meeting all four ALI criteria), accounting for 3,102 of the 6,017 PEEP/FiO2 combinations, and 1,415 of the 2,710 ABG/Vent setting combinations (Figure 1). These patients were analyzed as the ALI cohort.
A total of 172 of the 402 patients had one or more of the specified co-morbidities. The remaining 230 patients were analyzed as the non co-morbid cohort, with 1,484 ABG/Vent setting combinations (Figure 1).
Changes in FiO2 and PEEP
Clinicians changed FiO2 34% (2023/6017) of the time. The most common FIO2 step size was 0.05, followed by 0.1, for both decreases and increases in FiO2 (Figure ESM 1). Clinicians changed PEEP only 14% of the time (803/6017). The most common changes were an increase or decrease of 2 cm H20 (60% of all PEEP changes). There was considerable variability in the amount of FiO2 used across different levels of PEEP (Figure 2a). Overall, clinicians generally used higher FiO2 and less PEEP than the protocol would have recommended, particularly when FiO2 exceeded 0.7 (Figure 2a).
Comparisons between actual clinical care changes to ventilator settings and protocol recommendations for the same state are shown in Table 3. In the high PaO2 range (>68 mmHg, 77.5% of observations) and mid PaO2 range (55–68 mmHg, 15.8% of observations), clinicians generally used higher levels of FiO2 than the protocol would recommend. In the high PaO2 range, there were many situations when clinicians also did not decrease PEEP when the protocol would have recommended it. In the mid PaO2 range and low PaO2 range (< 55 mmHg, 6.7% of observations), clinicians commonly used lower levels of PEEP than the protocol would have recommended.
ALI Cohort
The findings of the subgroup analyses for the ALI cohort were similar to the entire group. There was still variability in the amount of FiO2 used across different levels of PEEP (Figure 2b). It appears graphically that clinicians were more likely to increase PEEP for patients with bilateral infiltrates on chest x-ray, although they still used less PEEP than the protocol would have recommended, particularly when FiO2 climbed above 0.8 (Figure 2b). Across all three oxygenation tables, the concordance between actual practice changes to FiO2 and the recommendations of the ventilator protocol for the ALI cohort were similar to the entire group (p>0.05, Table ESM 1a). Changes to PEEP were similar between groups for the mid and low PaO2 ranges (p>0.15, Table ESM 1a). There was less agreement in PEEP changes in the high PaO2 range (4% reduction in concordance, p=0.001, Table ESM 1a).
Non co-morbid Cohort
In the low and mid PaO2 ranges, the concordance between actual practice changes to FiO2 and PEEP and the recommendations of the ventilator protocol for the non co-morbid cohort were similar to the entire group (p>0.05, Table ESM 1b). In the high PaO2 range, the subgroup had slightly higher agreement between actual practice and protocol recommendations for FiO2 changes (3.4% increase in concordance, p=0.05, Table ESM 1b) and slightly less agreement for PEEP changes (5% decrease in concordance, p=0.008, Table ESM 1b).
Changes in PIP and Ventilator Rate
We used the structure of the PC ventilation table from the protocol to categorize the changes that clinicians made to VR and PIP in response to a blood gas (Table 4a). There was a median of 59 (IQR 13,146) observations per cell. Clinician response variability existed within each cell, with clinicians most commonly making no change to either PIP or VR (median 45% IQR 37.1, 53.4) (Table 4a, Table 5). This was even true when the PIP was > 35 cm H2O and the pH was > 7.45 (36%), or the PIP was > 35 cm H2O and the pH was between 7.30–7.45 (53%). Excluding three cells where the protocol would recommend no change to PIP or VR, or had a combination recommendation (decrease PIP and increase VR), clinicians made changes similar to protocol recommendations a median 42% (IQR 37.7%,49.8%) of the time, and opposite the protocol’s recommendation a median of 11.1 % (IQR 7.7%,17.9%) of the time.
ALI Cohort
For the 1,415 ABG/Vent setting combinations in the ALI cohort seven cells had fewer than 10 observations, and were excluded from analysis. The responses in each of included cells were nearly identical to the responses for the entire cohort (Table 4b).
Non co-morbid Cohort
For the 1,484 ABG/Vent setting combinations from the non co-morbid cohort, 5 cells had fewer than 10 observations, and were excluded. The responses in each of the included cells were similar to the entire cohort (Table ESM 2).
Discussion
This analysis demonstrates that clinicians behave inconsistently in their decisions to change ventilator support for children with hypoxemic respiratory failure or ALI. During this period, the pediatric practitioners in our unit claimed to embrace the general tenets of lung protective pressure control ventilation; however changes in ventilator settings were variable for similar patient states. Most notably, clinicians did not decrease FIO2 when the PaO2 was in a high range. Clinicians used low levels of PEEP and high levels of FiO2 when PaO2 was in the low range. High peak pressures and ventilator rates were frequently not decreased, even when the pH was >7.45. This was also true for subgroups of patients with bilateral infiltrates, or without co-morbidities that may affect ventilator practice (i.e. increased airway resistance, pulmonary hypertension, and intracranial hypertension).
We used our pediatric mechanical ventilation protocol as a framework to examine variability in clinician decision-making. We evaluated the potential acceptability of the protocol by comparing actual changes to changes the protocol would have recommended given the same patient state. Because of the variability in change size (e.g. one provider weans PIP by 2 cm H20, another provider by 4 cm H20); we evaluated the direction but not size of change. If the protocol is actually representative of best available evidence (based on adult studies and expert review by pediatric intensivists), then times when clinician responses differed from protocol recommendations might represent missed opportunities to improve lung protective ventilation practices. We identified 38% of physician responses to ABGs as potential missed opportunities to reduce FiO2 (Table 3), and 46% as potential missed opportunities to decrease PIP or ventilator rate (Table 5). This assumes that physicians would have followed 100% of the computer protocol recommendations – something we cannot know.
This pediatric mechanical ventilation computer protocol has not been formally validated against clinically important outcomes such as ventilator free days or mortality. Its actual benefits are unknown, and prospective validation studies are needed.
However, prior to prospective studies of the protocol, it must first be acceptable to clinicians. From our current analysis, the behavior of clinicians was directly contradictory to the computer protocol’s recommendations on average 11% of the time (Table 5). We cannot be sure of the circumstances surrounding clinicians’ decisions from this retrospective data. Clinicians could have been responding to changes in non-invasive measurements of oxygenation or ventilation, as we used pulse oximetry and capnography routinely in our PICU. This information would not have been captured if no arterial blood gas measurements had been obtained. This low (11%) rate of direct contradiction suggests that the computer protocol is, in general, consistent with current practice and is likely acceptable to pediatric clinicians.
We may also need to refine certain protocol recommendations such as the modified ventilator rate stratification of 25 bpm. In practice, clinicians escalated ventilator rate above 25 bpm in nearly a quarter of the cases (Table 2). Perhaps the original adult cut off of 35 bpm is appropriate for pediatrics as well. This may also be the case for the proposed reduction of FiO2 step changes to 0.05, as physicians appear to make almost as many 0.1 changes. We plan to refine the protocol by analyzing “controversial” cells through directed scenarios. This will involve multiple clinicians from multiple institutions with the intent of making the protocol generalizable to other PICUs. This may help achieve a balance between adherence to the protocol’s recommendations, and the degree of lung protection recommended by the protocol.
Our analysis has limitations. We could not elucidate the reasons for clinician decisions. It is possible that clinicians used other data (e.g., end tidal CO2 or pulse oximetery) or factors surrounding the patient’s care (e.g., hemodynamics or sedation) in their ventilator decisions. Moreover, decision making during the 7-year study period could have evolved. However, there did not appear to be a significant effect of time when we compared data from 2000–2003 with data from later years (2004–2007; analysis not shown). Nonetheless, the clear trends and consistent findings from a large number of observations from well over 40 critical care physicians give strength to our conclusions.
Conclusions
In summary, although pediatric critical care practitioners have embraced lung protective ventilation, ventilator management is variable, with lost opportunities to minimize potentially injurious ventilator settings. A computer ventilator management protocol might prompt consistent ventilator changes to encourage lung protective decisions.
Supplementary Material
Acknowledgments
This work was partially supported by U10 HD050012-04 and U01 HD049934
Footnotes
Work performed at Children’s Hospital Los Angeles
References
- 1.Santschi M, Jouvet P, Leclerc F, Gauvin F, Newth CJ, Carroll C, Flori H, Tasker RC, Rimensberger P, Randolph A PALIVE Investigators, PALISI Network, ESPNIC. Acute lung injury in children: therapeutic practice and feasibility of international clinical trials. Pediatr Crit Care Med. 2010;11:681–689. doi: 10.1097/PCC.0b013e3181d904c0. [DOI] [PubMed] [Google Scholar]
- 2.ARDSnet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151:566–576. doi: 10.7326/0003-4819-151-8-200910200-00011. [DOI] [PubMed] [Google Scholar]
- 4.Villar J, Kacmarek RM, Perez-Mendez L, Aguirre-Jaime A. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine. 2006;34:1311–1318. doi: 10.1097/01.CCM.0000215598.84885.01. [DOI] [PubMed] [Google Scholar]
- 5.Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, Pronovost PJ, Needham DM, Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, Pronovost PJ, Needham DM. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Critical Care Medicine. 2008;36:1463–1468. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 6.Fan E, Needham DM, Stewart TE, Fan E, Needham DM, Stewart TE. Ventilatory management of acute lung injury and acute respiratory distress syndrome. JAMA. 2005;294:2889–2896. doi: 10.1001/jama.294.22.2889. [DOI] [PubMed] [Google Scholar]
- 7.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD, Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Critical Care Medicine. 2004;32:1289–1293. doi: 10.1097/01.ccm.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 8.Weinert CR, Gross CR, Marinelli WA, Weinert CR, Gross CR, Marinelli WA. Impact of randomized trial results on acute lung injury ventilator therapy in teaching hospitals. Am J Respir Crit Care Med. 2003;167:1304–1309. doi: 10.1164/rccm.200205-478OC. [DOI] [PubMed] [Google Scholar]
- 9.Curley MA, Hibberd PL, Fineman LD, Wypij D, Shih MC, Thompson JE, Grant MJ, Barr FE, Cvijanovich NZ, Sorce L, Luckett PM, Matthay MA, Arnold JH. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294:229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willson DF, Thomas NJ, Markovitz BP, Bauman LA, DiCarlo JV, Pon S, Jacobs BR, Jefferson LS, Conaway MR, Egan EA Pediatric Acute Lung Injury and Sepsis I. Effect of exogenous surfactant (calfactant) in pediatric acute lung injury: a randomized controlled trial. JAMA. 2005;293:470–476. doi: 10.1001/jama.293.4.470. [DOI] [PubMed] [Google Scholar]
- 11.Khemani RG, Newth CJL. The design of future pediatric mechanical ventilation trials for acute lung injury. Am J Respir Crit Care Med. 2010;182:1465–1474. doi: 10.1164/rccm.201004-0606CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khemani R, Sward K, Newth C. Adaptation of an Adult Based Mechanical Ventilation Protocol for Application in Pediatric ALI/ARDS. Am J Respir Crit Care Med. 2010;181:A3903. [Google Scholar]
- 13.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler AP, Wickersham N, Ancukiewicz M, Brower R, Thomspon T, GB Low Tidal Volume (VT) Ventilation Reduces Plasma Cytokines in Human Acute Lung Injury (ALI) Am J Respir Crit Care Med. 2000;161:A83. [Google Scholar]
- 15.Khemani RG, Conti D, Alonzo TA, Bart RD, Newth CJ. Effect of tidal volume in children with acute hypoxemic respiratory failure. Intensive Care Med. 2009;35:1428–1437. doi: 10.1007/s00134-009-1527-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.