Abstract
Acute myocardial infarction (MI), which involves the rupture of existing atheromatous plaque, remains highly unpredictable despite recent advances in the diagnosis and treatment of coronary artery disease. Accordingly, a biomarker that can predict an impending MI is desperately needed. Here, we characterize circulating endothelial cells (CECs) using the first automated and clinically feasible CEC 3-channel fluorescence microscopy assay in 50 consecutive patients with ST-elevation myocardial infarction (STEMI) and 44 consecutive healthy controls. CEC counts were significantly elevated in MI cases versus controls with median numbers of 19 and 4 cells/ml respectively (p = 1.1 × 10−10). A receiver-operating characteristic (ROC) curve analysis demonstrated an area under the ROC curve of 0.95, suggesting near dichotomization of MI cases versus controls. We observed no correlation between CECs and typical markers of myocardial necrosis (ρ=0.02, CK-MB; ρ=−0.03, troponin). Morphologic analysis of the microscopy images of CECs revealed a 2.5-fold increase (P<0.0001) in cellular area and 2-fold increase (P<0.0001) in nuclear area of MI CECs versus healthy control, age-matched CECs, as well as CECs obtained from patients with preexisting peripheral vascular disease. The distribution of CEC images containing from 2 up to 10 nuclei demonstrates that MI patients are the only group to contain more than 3 nuclei/image, indicating that multi-cellular and multi-nuclear clusters are specific for acute MI. These data indicate that CECs may serve as promising biomarkers for the prediction of atherosclerotic plaque rupture events.
INTRODUCTION
Acute myocardial infarction (MI) and ischemic stroke remain leading causes of death and disability worldwide. Each year, over 2.5 million individuals in the United States experience a new or recurrent heart attack or ischemic stroke.1 Currently, stable coronary artery disease (CAD) is readily diagnosed through functional stress testing and coronary angiography, while cardiovascular events such as MI and ischemic stroke involve atherosclerotic rupture and remain highly unpredictable. To exemplify this point, it is not at all unusual for an individual to have a normal stress test and days to weeks later develop a heart attack or die suddenly, with autopsy evidence of coronary artery plaque rupture as the proximate cause. In addition, previous studies have shown that up to 50% of individuals with MI lack the traditional risk factors for CAD such as hypertension, elevated LDL cholesterol, cigarette smoking and diabetes.1–5 Accordingly, there is a critical need for a non-invasive biomarker such as a protein, nucleic acid, or cellular based assay that can identify those individuals who are at the greatest risk for acute arterial disruptive events before they are clinically manifested.
Although the presence of elevated levels of circulating endothelial cells (CECs) have been associated with acute coronary syndromes, their enumeration has not been incorporated into clinical practice.6–8 The principal reasons for this include the lack of a practical or widely accepted methodology for quantifying CECs, disparate definitions in the literature for defining CECs, and a lack of in-depth scrutiny of these rare cells when isolated.9
Here, we address the above deficiencies by using the Veridex CellSearch System®, a commercially available rare cell isolation platform to characterize both the quantitative and qualitative features of CECs in patients with the highly restrictive MI phenotype known as ST-segment myocardial infarction (STEMI), which occurs secondary to acute arterial plaque rupture. Further, we sought to determine whether a population of cells isolated from patients with acute MI might show distinctive morphological, antigenic, and genetic signatures that are diagnostic for an acute plaque rupture events.
RESULTS
CEC levels are diagnostic of arterial injury in acute myocardial infarction
The median age for the STEMI patients was 58.5 years (range 39–80 years) with the median cardiac troponin and CK-MB values being 5.7 ng/ml and 27.9 ng/ml respectively. Quartiles and ranges for cardiac troponins and CK-MB values in all STEMI cases are shown in Table 1. Notably, the STEMI cases had CK-MB and troponin levels that were on average logarithmically higher than clinically accepted normal values (troponin < 0.1 ng/ml; CK-MB <3.0 ng/ml) and were well within the range expected for STEMI cases. Importantly, these values underscore the significant myocardial necrosis that typically accompanies this phenotype.
Table 1.
Phenotypic characteristics of STEMI and control patients. Median values are listed for CECs, Troponin, and CK-MB. Quartile values are listed in parenthesis. Normal intramyocardial enzyme levels are < 0.1ng/ml and < 3ng/ml for Troponin and CK-MB respectively.
| N | Age | %Male | CECs/ml | Troponin | CK-MB | |
|---|---|---|---|---|---|---|
| STEMI | 50 | 58.5 (39–80) | 82% | 19.4 (11.6, 51.1) | 5.2 (1.7, 17.8) | 26.9 (8.4, 81.9) |
| Controls | 44 | 30 (22–34) | 51% | 3.8 (2.3, 5.1) | - | - |
Our strategy to isolate enriched populations of CECs from blood was to use CD146-coated immunomagnetic beads, followed by immunostaining and imaging of individual cells by fluorescence microscopy.10 CECs were identified based on three criteria: positive staining for nuclei, positive staining for CD105 (an EC marker), and negative staining for CD45 (a leukocyte marker) (Supplemental Figure 1A and 1B). Fifty consecutive STEMI (82% male) patients were prospectively enrolled for cell enumeration. The median CEC (CD146+/CD105+/CD45−) count in STEMI patients was 19 cells/ml with upper and lower quartiles of 12 Cells/ml and 51 cells/ml (range 2.5 – 465 CECs/ml) respectively (Table 1, Fig 1). Interestingly, no correlation was observed between cell counts and initial intra-myocardial enzyme levels (ρ=0.02, CK-MB; ρ=−0.03, troponin) (Fig 2).
Figure 1.
Enumeration of CECs in healthy controls and STEMI patients. (A) CEC quantification in 44 consecutive healthy control and 50 STEMI patients. The median number of CECs in healthy controls was 4 CECs/ml with upper and lower quartiles of 2 and 5 CECs/ml, respectively. The median number of CECs in STEMI patients was 19 with upper and lower quartiles of 12 and 51 CECs/ml (p = 1.1×10−10), respectively. (B) Area under the receiver operating characteristic curve (AUC) was equal to 0.95. The red point represents a classification threshold of 16.5 CECs/ml, which is associated with a sensitivity of 60% and specificity of 98%; blue equals a classification threshold of 9 CECs/ml, with an associated sensitivity of 90% and specificity of 93%; and green equals to a threshold of 4.3 CECs/ml with a 98% sensitivity and 64% specificity for correctly classifying an MI case or control.
Figure 2.
Correlation between CEC counts and typical markers of myocardial necrosis. (A) Using spearman’s rank correlation (rho) coefficient, CEC counts in MI patients were assessed for correlation to initial presenting serum cardiac troponin values. No evidence of correlation was seen (rho = 0.02, p = 0.9). (B) No correlation between CEC counts and initial CK-MB values were noted (rho = 0.03, p = 0.9).
Cell enumeration was done on blood samples from 44 consecutive, healthy controls. The median age of the healthy controls was 30 years (range 22 – 34). The median number of CECs/mL was 3.8 CECs/ml (range 0.75 –16.75) with upper and lower quartile thresholds being 2.25 and 5.06 respectively (Table 1, Fig 1). Importantly, the first 24 healthy controls enrolled were brought back for a repeat measurement at two months (Fig 3). Notably, the median numbers of CECs/ml were unchanged between the first and second visits with median values being 3.4 and 3.25 during the first and second visits respectively (P=0.5). These data suggest CEC levels remain stable over time in healthy individuals. Further, there was no correlation between CEC numbers and age (ρ=−0.12).
Figure 3.
Stability of CEC counts over time. Two repeat CEC measurements in 25 consecutive control individuals at visits separated by a 2-month time frame. Measurements performed on the same individual are connected with a gray line. The CEC counts measured at first visit ranged from 1 to 16.75 CECs/ml, with a median value of 3.38 CECs/ml. On the second visit, the CEC counts measured in the same 24 individuals ranged from 1 to 19.5 CECs/ml, with a median value equal to 3.25 CECs/ml. Using paired Wilcoxon signed rank test, we found no evidence of a difference between the CEC counts at the two time points.
The observed CEC counts were dramatically elevated in our STEMI population when compared to healthy controls (Fig 1, P=1.1×10−10). A receiver-operating characteristic (ROC) curve associated with a classifier based upon logistic regression demonstrated an area under the ROC curve of 0.95 (AUC of 1 would suggest perfect classification, AUC equal to 0.5 would indicate random selection) (Fig 1B). This classifier was able to correctly classify 86 (91.5%) of all instances (i.e. CEC/ml counts for 50 patients with STEMI and 44 healthy controls) as a MI case or healthy control with ten-fold cross-validation. For illustration, a value of 16.5 CECs/ml had a sensitivity of 60%, but 98% specificity in the diagnosis of STEMI (Fig 1B). Alternatively, at a classification threshold of 9 CECs/ml, the associated sensitivity is equal to 90%, while the specificity reduced slightly to 93%. In summary, CEC counts appear to be independent biomarkers of arterial injury in individuals with acute myocardial infarction.
CECs from MI patients are abnormally large and misshapen and often appear with multiple nuclei
In an attempt to further characterize CECs, we performed a detailed analysis of fluorescent images of isolated CECs from 8 STEMI cases and 10 healthy controls, with the anticipation that a morphologic signature of CECs could have incremental diagnostic value as compared to the assessment of CEC counts alone. STEMI cases and healthy controls with CEC numbers in the low, middle, and high range were selected for analysis (Supplemental Table 1). Additional control populations were also included for this portion of the study, so that any conclusions derived from the morphologic appearance of STEMI CECs could be placed in the context of real-world clinical populations. Specifically, 10 consecutive population controls that were age-matched to our STEMI patient group were selected. Additionally, 2 patients with documented peripheral arterial disease who had recently undergone an open vascular procedure were included as a vascular disease control group. Two patients with Non ST-segment elevation myocardial infarction (NSTEMI) were also included in order to assess any gross differences in CEC morphology that might distinguish the two MI phenotypes (STEMI vs. NSTEMI).
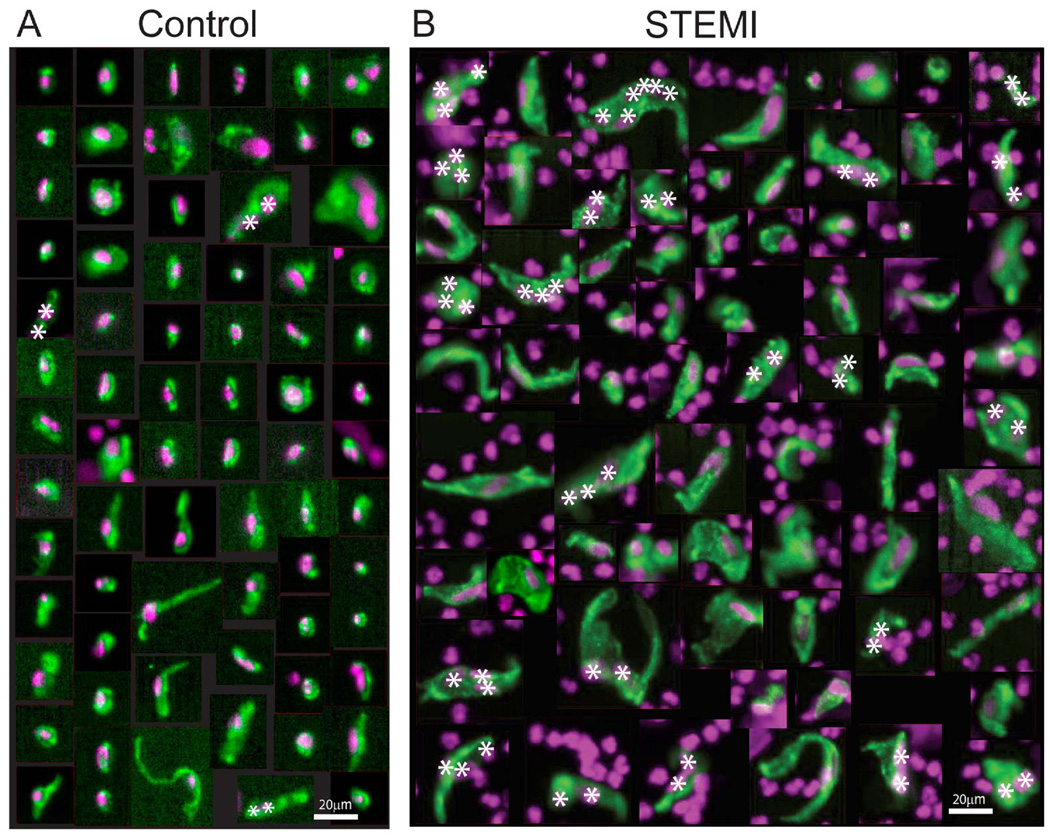
Visual inspection of individual fluorescent CEC images revealed the morphologies of CECs isolated from MI patients are strikingly different than those from controls. Figure 4 illustrates individual CECs isolated from healthy and age-matched controls, compared to CECs isolated from a representative STEMI patient (CEC03040). CECs from controls are relatively small, tend to be elongated in shape, and occasionally have two nuclei associated with one cell body, which may represent a cluster of two attached cells, or one cell with two nuclei (Fig. 4A, asterisks). In contrast, CECs from STEMI patients are more heterogeneous in appearance, with many cells that are obviously larger and grossly misshapen (Fig. 4B). The nuclei of CECs from the STEMI patient also tend to be larger and aberrantly shaped as compared to nuclei of CECs from control individuals (Fig. 4B, also see Fig. 5D). Many of the largest CEC images from STEMI patients often contain several nuclei associated with one continuous CD105-stained cell body region, thus appearing as multi-nuclear clusters with up to 10 nuclei (Fig. 4B) (also see below, Fig. 6). Since cell-cell boundaries are not clearly demarcated in these fluorescence images, it is not possible to determine rigorously whether these are multi-cellular clusters, or alternatively, multi-nuclear individual cells.
Figure 4.
Fluorescence microscopy images of individual CECs isolated from (A) age-matched controls, and (B) a representative STEMI patient (CEC03040). CECs are labeled with CD105 (green) and DAPI (purple) as markers for their cellular and nuclear compartments, respectively. CECs are identified in these images based on co-localization of CD105 and DAPI staining, resulting in a green cell body overlaying a purple nucleus. Purple nuclei not co-localized with green CD105 staining are contaminating white blood cells also present in the field of view (based on positive staining for CD45, not shown). CECs from the STEMI patient are heterogeneous in size and shape and many of them are much larger than CECs from controls, with multiple nuclei. Image magnification is constant for all images. All CEC images from 3 age-matched control individuals are shown, and 69 CEC images were randomly selected for presentation from STEMI patient CEC03040, who had 58 CECs/ml of blood, in the mid range of CECs/ml for the MI patients in this study. Bars, 20µm. Asterisks, Multiple nuclei associated with one CD105-stained cell body.
Figure 5.
CEC size analysis for control (age-matched and healthy), vascular and MI (STEMI and NSTEMI) patients.. (A) Cellular areas (µm2) for cells from each group (mean ± SD). P-values: 0.0133 (healthy vs. age-matched); <0.0001 (age-matched vs. MI); 0.003 (vascular vs. MI). (B) Nuclear areas (µm2) for cells from each group (mean ± SD). P-values: 0.0069 (healthy vs. age- matched); <0.0001 (aged matched vs. MI); 0.0059 (vascular vs. MI). (C) Ratios between the cellular area and the nuclear area for cells from each patient group (mean ± SD). P-values: 0.4848 (healthy vs. age-matched); <0.0001 (age-matched vs. MI); 0.0021 (vascular vs. MI). Numbers in each bar for A–C represent the number of patients analyzed from each group; all CECs from each control individual and 50–100 CECs randomly selected from each MI patient were analyzed. Image magnification is constant for all images. Bars, 5µm. (D) Representative images of CECs from control (age-matched and healthy) and MI (STEMI and NSTEMI) patients, illustrating typical CECs (i.e., mean area sizes), and the range of variation in CEC area sizes from these groups. CECs were identified with CD105 (green) and DAPI (purple) staining as markers for cellular and nuclear compartments respectively
Figure 6.
Analysis of numbers of CECs with multiple nuclei from control, random age-matched population sample, vascular, and MI (STEMI and NSTEMI) patients. (A) Images of CECs with multiple nuclei from the STEMI patient group stained with CD105 (green) and DAPI (purple, white asterisks) to identify the cellular and the nuclear compartments, respectively. The number of nuclei per CD105-stained cell body is specified in the upper right of each image. Image magnification is constant for all images. Bar, 15µm. (B) Percent of CEC images with multiple nuclei (two or more nuclei/image) in each group (mean ± SD). The numbers in each bar represent the number of individuals analyzed. (C) Distribution of number of nuclei/image for the different groups (mean ± SD). Bars indicate Healthy (white) and Age-matched (grey) controls, Vascular (red) and MI (black) patients. P-values: 0.2295 (healthy vs age-matched); 0.0008 (age-matched vs MI); 0.0231 (vascular vs MI).
To substantiate the qualitative differences apparent by visual inspection of CEC images from control and MI patients (Fig. 4), we developed a custom image analysis software method to measure cellular and nuclear areas for individual images of CECs (Supplemental Fig. 1C). CEC morphologies in 50–100 randomly selected images were analyzed for each MI patient, and compared to all CEC images from control patients. This revealed that the mean cellular area of CECs from MI patients was ~270 µm2, more than 2.5-fold greater than the mean ~100 µm2 cellular area of CECs from either healthy or age-matched controls (P<0.0001) (Fig. 5A). Similarly, the mean area of CEC nuclei from MI patients was ~65 µm2, significantly larger than the 30 to 40 µm2 area for CEC nuclei from healthy and age-matched controls (P<0.0001) (Fig. 5B). In contrast, the morphological parameters for CECs from patients undergoing vascular surgery for non-cardiac related conditions were not significantly different from controls (Fig. 5A and B). Finally, the ratio of cellular/nuclear area was ~1.5 fold greater for CECs from MI patients as compared to CECs from any of the control groups, with a significance level at P<0.0001. Representative examples of the ranges of cellular and nuclear size variations for CEC images of single cells from the control and MI patients analyzed are shown in Figure 5D.
Interestingly, while the cellular and nuclear areas of CECs from age-matched controls tended to be somewhat larger than those of CECs from healthy, younger controls (Fig. 5A and B), the cellular/nuclear area ratios were not different between these two control groups (Fig. 5C). Thus, the cellular and nuclear areas increased proportionately for CECs from age-matched compared to healthy controls, whereas the cellular area increased disproportionately to the nuclear area for CECs from MI patients. Therefore, the increased cellular/nuclear area ratio is a morphological abnormality specific to MI patients, since it is not observed for either age-matched or vascular control patients in comparison to healthy controls. In conclusion, our qualitative visual observations (Fig. 4) and our quantitative image analysis (Fig. 5) demonstrate that CECs from MI patients are significantly larger, with larger mean cell sizes and nuclei, and a greater ratio of cellular to nuclear area as compared to controls. A comparison between the STEMI and NSTEMI CECs analyzed for the cellular and nuclear areas as well as the cellular/nuclear area ratios did not show any significant difference between the two groups (Supplemental Fig 2).
Next, we analyzed the images to determine the percent of CECs present as single cells with one nucleus, or as cells with 2 or more nuclei, for CECs from both MI patients and controls. Examples of individual CEC images with multiple nuclei (from MI patients) are illustrated in Figure 6A. Quantitative analysis revealed that on average, ~25% of the CEC images from MI patients contained 2 or more nuclei, compared to 5–10% of CEC images from the healthy or age-matched controls, which is a several-fold increase in the numbers of nuclei per image (P = 0.0008) (Fig. 6B). While the percent of CEC images with multiple nuclei also appeared to be increased in the age-matched control group with respect to the healthy control or vascular group, this difference was not statistically significant due to the variability in this parameter in these two groups (P = 0.22) (Fig. 6B). The distribution of CEC images containing from 2 up to 10 nuclei demonstrates that MI patients are the only group in this study to contain more than 3 nuclei/image (Fig. 6C). In contrast, the vast majority of CEC images from healthy, age-matched or vascular patients contained only 1 or 2 nuclei (Fig. 6C). Interestingly, 10–15% of CEC images in both control and MI patients contain 2 nuclei, which may represent individual bi-nuclear cells (a characteristic feature of mature endothelium). However, inspection of individual CEC images from MI patients with multiple (> 2) nuclei reveals a multi-lobular morphology in many cases, suggesting that at least some of these images are due to groups of several CECs attached to one another. A previous study also observed images of CECs isolated from MI patients showing multiple nuclei, which could constitute multi-cellular clusters.8 In summary, we conclude that CECs from MI patients exhibit distinctive and quantifiable morphological features (increased size and multi-nuclearity parameters), which can provide an additional biomarker of arterial injury in individuals with acute myocardial infarction.
DISCUSSION
The lack of normal endothelial integrity is thought to be the unifying principle for susceptibility to atherosclerotic plaque rupture as well as other arterial, non-atherosclerotic vessel wall rupture or fissure events.11–13 Recent autopsy data demonstrating the presence of multiple healed nonlethal plaque ruptures in the coronary arteries of sudden cardiac death (SCD) victims suggests that ongoing arterial injury is actively transpiring during the time leading up to an acute arterial catastrophe.14, 15 Accordingly, the proposed framework of endothelial cell disruption from plaque rupture sites and their detection in the peripheral circulation in patients with MI and stroke is based on well-established biologic principles. In the present study, we have demonstrated the ability to identify, enumerate, and quantitatively characterize the morphology of circulating endothelial cells from patients with acute MI using an automated rare cell imaging system in combination with fluorescence image computational analysis. Our method is tantamount to a fluid-phase biopsy of the coronary artery that would otherwise be impossible to obtain in a living person, and therefore, provides a unique window into the disease from the precise cells that are being shed due to injury of inflamed arterial segments.
Notably, the main advance of this paper is the identification of distinct morphologic characteristics of CECs from patients with a myocardial infarction event. This was made possible by the use of a validated and automated technology in the form of a 3-channel fluorescent assay. The dysmorphic features of the CECs from individuals with MI, which have not been previously described, provide support that abnormal endothelial biology is likely a critical contributor to acute plaque rupture events, which is pathognomonic for heart attack and ischemic stroke.
To date, CEC levels have varied greatly in the published literature. This variability stems primarily from the use of highly divergent CEC isolation methods and variable immunophenotypic definitions of CECs.9, 16 In order to overcome these deficiencies, we have employed the CellTracks® image analyzer system for this study, which is the only CEC isolation methodology that has been validated by a protocol using the National Committee for Clinical Laboratory Standards guidelines.10 The CD146 enrichment step, followed by the multi-marker identification strategy of positive and negative selection criteria that we used, minimizes the analytical variability that frequently accompanies the processing of large sample volumes. Moreover, the use of validated, standardized criteria for signal intensity and automated image analysis allows for better reproducibility of results by reducing the inter-operator variability.10 Finally, all blood was collected from acute MI cases prior to diagnostic angiography and angioplasty, thus preventing contamination of MI derived CECs with cells released from the arterial endothelium due to mechanical disruption by catheters and coronary stents used in the treatment of coronary artery obstructions. This fact and the sole inclusion of patients with ST-segment elevation myocardial infarction – a phenotype that denotes an underlying acute arterial plaque rupture – lends further support that CECs present in patients with acute MI are cells that have been sloughed from injured arterial segments.
Our study shows that median CEC counts in MI patients are over 400% higher than in those found in healthy controls. Moreover, we have also demonstrated that CEC levels tend to remain stable over time in healthy individuals. On its own, the ROC curve analysis demonstrates that CEC counts provide an accurate means for classifying individuals as a case or control. Particularly noteworthy was the absolute lack of correlation between CEC counts and typical markers of ischemia in our MI population and the lack of correlation between CEC counts and age in our controls. Together, these findings bolster existing data that suggest elevated levels of CECs are independent predictive biomarkers of arterial injury in a variety of atherothrombotic conditions including unstable angina (a precursor syndrome to MI, typically leading to MI in 1–2 weeks if left untreated), non ST-segment elevation myocardial infarction (NSTEMI), and ischemic stroke.6–8 This is an especially important point, since the objective of this work is to identify a means of tagging an active plaque rupture event before myocardial necrosis has ensued. There are very refined methods for detecting myocardial necrosis with ultra-sensitive troponins, and excellent means of diagnosing stable, obstructive coronary atherosclerotic plaque via stress testing and coronary angiography. But to date a biomarker for imminent plaque rupture has not been identified.
Beyond simple CEC count data, we observed that MI CECs show a specific set of measurable differences in morphological parameters, namely, cell and nuclear areas, ratio of cellular/nuclear area, and association with multiple nuclei, when compared to various control groups. We took stringent measures to be sure this finding was authentic. First, we developed a customized image analysis Matlab program written specifically for this purpose in order to quantify and qualify all visual observations, thereby removing any bias that may be introduced by a qualitative or manual assessment of cellular characteristics (Supp Fig 1C). Second, we randomly selected CECs from 10 separate MI cases that included individuals with CEC ranges from low to high numbers in order to get a global estimate of all pertinent cellular features across the MI patient population (Supplemental Table 1). Third, we obtained additional control populations as we hypothesized that CEC morphology in healthy controls might be different than from that of an older population sample or those with existing vascular disease.
Accordingly, we found no significant differences in cellular and nuclear sizes, ratios, or presence of multiple nuclei between any of the various control groups (Figs. 5–6, see legends). However, in the age-matched control group only, there was a tendency to increased cell and both nuclear size and number compared with the healthy (younger) controls. Although this did not reach statistical significance, it may be that some individuals with undiagnosed arterial disease or impending cardiac events were included in this older population based sample. In addition, we found no statistically significant differences between STEMI and NSTEMI CECs. This suggests that individuals with disparate presentations of acute coronary syndromes share similar CEC phenotypes indicating a common underlying pathologic link.
The specific morphological features of CECs from MI patients, including their larger sizes and presence of multiple nuclei, may reflect their site of origin and provide clues to pathological processes leading to arterial injury and sloughing of the endothelium. A mature endothelial origin for CD146+/CD105+/CD45− cells is presumptive, but supported by several lines of evidence presented here; their capture using anti-CD146, their expression of CD105, and importantly their lack of CD45 expression. This phenotype can be shared by bone marrow derived mesenchymal cells; however, the lack of CD45 expression positivity makes activated T-cells and mesenchymal hematopoetic precursors unlikely candidates for CECs. In addition, we show that the CD146+/CD105+/CD45− cells captured also express CD31 (Supplemental Figure 3A), a marker found on vascular endothelium but not on bone marrow derived mesenchymal cells. The morphology of CECs is also inconsistent with the scant cytoplasm, spindle shape, and smaller size typical of mesenchymal and endothelial progenitor cells. Gene expression analysis of CEC enriched cells (Supplemental Figures 4A and 4B) show elevation of the endothelial specific markers endothelin and von Willebrand Factor (vWF) in STEMI patients over that of non-MI controls and peripheral blood mononuclear cells. Finally, CECs were also CD34 (Supplemental Figure 3B) and CD146 positive, consistent with an endothelial origin but not consistent with their being activated T-Cells, bone marrow derived mesenchymal cells (data not shown), or endothelial progenitor cells.
The CECs in MI patients may derive from older, and thus larger cells in the arterial endothelium, with injured cells released singly or as multi-cellular groups or sheets from injured regions during plaque rupture. CEC clusters in MI patients may also be due to abnormal aggregation of injured, disturbed cells during their passage through the circulation. By contrast, the smaller, less clustered (and fewer) CECs in control individuals may reflect infrequent release of younger and thus smaller ECs from regions of remodeling or proliferating vasculature in which ECs might normally lose their attachments. Future immunostaining of CECs from MI patients and controls with markers for endothelial activation and injury, cell junctions and cell-substrate attachments, as well as cell cycle and signaling pathways, may be helpful to uncover molecular and cellular mechanisms of arterial injury in patients with MI. Our automated strategy for enrichment, immunostaining, fluorescence imaging and computational analysis of CEC morphology lays the groundwork for more in-depth molecular, cellular and ultrastructural assessments of CECs from a variety of clinical conditions.
In summary, by using an automated, cell isolation and imaging platform in samples from patients with acute MI, we show there is a clear excess of CECs and that these cells have discrete antigenic and morphological signatures. These distinctive cell characteristics may be useful in developing a more refined biomarker for arterial injury as compared with CEC counts alone. Systematic application of these CEC biomarkers to prospective studies assessing the predictive potential of CECs in those at high risk for arterial injury syndromes will be needed for further validation. In addition, future studies are necessary to investigate the possible correlation between the altered cellular morphology of MI CECs and a unique genomic signature of these cells. Our findings may ultimately support the development of an assay to help predict imminent risk of a heart attack.
MATERIALS AND METHODS
Patient selection and specimen collection
Between January 2010 and February 2011, patients presenting to the emergency room with STEMI at 4 regional medical centers in San Diego County had blood drawn for CEC characterization. All STEMI blood samples were obtained in the cardiac catheterization laboratory via an arterial sheath and prior to catheter insertion for diagnostic coronary angiography or intervention. All patients met criteria for STEMI including ST-segment elevation of at least 0.2 mV in 2 contiguous precordial leads or 0.1 mV in contiguous limb leads. Positive markers of myocardial ischemia (CK-MB or troponin) and angiographic evidence of obstructive coronary artery disease were also required. Initial cardiac troponin and CK-MB values were obtained and recorded. Subsequent CK-MB and troponin values were not recorded.
Healthy controls were recruited from the normal blood donor program at The Scripps Research Institute for the purpose of comparing CEC levels and morphology to that obtained from STEMI patients. All healthy controls were between the ages of 18 and 35 and were deemed free of any chronic disorders via self-report. Blood for CEC ascertainment in healthy and random age-matched samples were obtained via venipuncture. Randomly selected samples of age-matched controls that via self-report denied the presence of any acute illnesses or symptoms were included in the CEC morphology analysis portion of the study. Smoking status, obesity, family history of cardiovascular disease, or acute febrile illness was not used as exclusionary criteria. Individuals with existing vascular disease who recently underwent an open endarterectomy procedure along with patients with non ST-segment elevation myocardial infarction (NSTEMI) were also recruited for the sole purpose of morphologic characterization of their CECs. Criteria for MI in the NSTEMI population included symptoms consistent with MI, serologic evidence of myocardial necrosis – as determined by elevated levels of cardiac troponins and CK-MB, and angiographic evidence of obstructive coronary artery disease.
All blood from cases and controls were collected in CellSave™ tubes containing a mild cellular fixative known to stabilize CEC levels. Subsequently, samples were kept at room temperature and shipped via courier to a central lab for processing within 48 hours of collection. Institutional review board approval was obtained from all recruiting sites, and all patients gave informed consent.
Identification of CECs by CellTracks® System
The CellTracks® system consists of an automated CellTracks® Autoprep sample preparation device, and a CellTracks Analyzer II® (CTA II) image analysis platform. The CellTracks system used the CellSearch endothelial cell kit to automate all sample enrichment and staining steps as described previously.10 Briefly, CECs in whole blood were bound by anti-CD146 antibody conjugated magnetic nanoparticles, and enriched by repeated magnetic incubations and automated washings. CD146+ enriched cells were stained with fluorescent antibodies to CD105 and CD45, and the magnetically enriched and fluorescent antibody labeled cells placed into a MagNest® Cell Presentation Device. Supplemental Figure 1A shows the steps during sample and image analysis by the CTA II. The MagNest device consists of a disposable sample cartridge positioned between two permanent magnets in order to orient the magnetically labeled cells in a monolayer for fluorescent image analysis. The MagNest is placed in the CTA II, a four-color semi-automated fluorescent microscope. The analyzer then scans the entire cartridge surface collecting images for each of the four fluorescent colors. It records 180 images for each fluorescent channel (720 images per scan). The CTA II’s software automatically analyzes each frame and identifies those objects within the frame that based on their DAPI and CD105 fluorescence were possible candidate CECs. Candidate CECs are placed as a series of thumbnails in an image gallery for review and identification by a trained operator (Supplemental Fig 2A). The thumbnail images show, from right to left, an unused (FITC) channel, CD45-APC signal, DAPI stained nuclei, CD105-PE reactivity, and finally an overlay of the CD105-PE & DAPI staining. The FITC channel can be used to phenotype CECs with additional markers of interest. To be scored as a CEC a cell had to have a nucleus, express CD105, have the morphology of a cell, and be negative for CD45. Supplemental Fig 1B shows an example of 3 objects as presented to the reviewer by the CTA II software. The first two objects met the criteria and were scored as CECs by the operator (checked box). The third object was judged a leukocyte because it was CD45 positive (box not checked). The software automatically tabulated the checked boxes within each sample, and results were expressed as the number of CECs per 4 mL of blood.
CD146+/CD105+/CD45− cells were also reacted with antibody to CD31, or CD34, to characterize the cells and better determine their likely origin. The marker(s) CD31-FITC or CD34-FITC were added during sample preparation on the CellTracks AutoPrep system as described above. CD31-FITC and CD34-FITC were purchased from BD Biosciences and used at a final concentration of 1ug/ml/. CD146+/CD105+/CD45− cells expressing CD31 and CD34 will stain positive and appear in the thumbnail images in the corresponding FITC channel. The CD31+ and CD34+ CECs are shown in Supplemental Figure 3.
CEC morphology analysis
CEC image analysis was performed using a customized Matlab program (The Math Works; release 2009b or newer) written for this purpose. Data analysis was performed either using Matlab or GraphPad Prism (release 4.00). Fluorescence intensity measurements of fixed CECs from CellSearch (Veridex) were carried out in a four-step process (Supplemental Figure 1C) (1) Cropping and separation of single CEC color images into their individual channels (CD105 and DAPI), which were then saved as tifs. (2) Cell body (CD105) and nuclear (DAPI) segmentation. (3) Area calculation of the segmented images. (4) Single cell and nuclear area calculation. Two Gaussian transformations with s.d. σ1 and σ2 were calculated for the CD105 and DAPI channels of each CEC image. The background pixels were removed using unimodal thresholding17 and the final objects corresponding to CEC cell body and nuclei were determined as thresholded difference of two Gaussians images. The set of s.d. σ1 and σ2 used in the Gaussian transformations was chosen to generate the most accurate mask by visual inspection. The area of the CD105 channel for each CEC image was divided by the number of nuclei identified in the nuclear masks contained within the CD105 area. Data analysis was performed either using Matlab or GraphPad Prism (release 4.00). All statistical comparisons were performed using pairwise t-test (two tails) in GraphPad Prism (release 4.00).
CEC Gene Expression Analysis
Two 10 ml EDTA-containing vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) were used to collect blood from 12 Myocardial Infarction (MI) patients and 13 age-matched healthy donors. Circulating endothelial cells (CECs) were isolated using the CellSearch system with the CellSearch CEC Profile kit (Veridex LLC, Raritan, NJ). An aliquot of 1 ml Trizol reagent (Life Technologies, CA) was added to the isolated CECs and stored at −80°C until use. The total RNAs were isolated from CECs according to standard Trizol method provided by the manufacturer. The quantity and quality of RNA was examined by NanoDrop 1000 (NanoDrop, Wilmington, DE). 50 ng total RNA was first converted to labeled target cDNA using the Ovation RNA Amplification System V2 (NuGEN, San Carlos, CA). Subsequently, 3.75µg of the purified cDNA underwent a two-step fragmentation and labeling process using the Encore Biotin Module (NuGEN). Targets were hybridized to Affymetrix human U133 Plus 2.0 array following protocols as suggested by the supplier (Affymetrix, Santa Clara, CA). Following hybridization, arrays are washed and stained using standard Affymetrix procedures before scanning on the Affymetrix GeneChip Scanner and data extraction using Expression Console. Each probe set was considered a separate gene. Expression values for each gene were calculated using Robust Multi-array Analysis (RMA) method.
Statistical analysis of CEC counts
A two-sample test for the nonparametric Behrens-Fisher problem was used to see whether STEMI cases exhibited higher counts of CECs compared to controls. Spearman rank correlation was used to determine the linear relationship between the number of CECs and levels of MB, troponin, as well as age. We built a mathematical model using logistic regression with ten-fold cross-validation that classified patients into two groups, STEMI and controls, based on their observed CEC counts. This model generated in Weka software18 was used to assess the number of correctly classified instances and the area under the receiver-operating characteristic (ROC) curve. The ROC curve shown in Fig. 1B is based on a logistic regression model using all available data (i.e., without cross-validation), and was generated using the ROCR package of the R statistical computing environment.19 Area under the ROC curve (AUC) was calculated using somers2 function of the Hmisc package in the R statistical computing environment.20
Statistical analysis for Endothelin and vWF levels (Supplemental Figures 4A and 4B)
The difference between the two groups for both endothelin 1 and vWF is statistically different by both parametric and nonparametric testing. A Student’s t-Test was used with the variance of each group treated as unequal. The values in the Figure (Supplemental Figures 4A and 4B) are scaled to log base 2. An increase in one on this scale would represent a doubling of the magnitude of the difference between the two means on a natural scale.
Note that the difference between these two groups remains significant if we switch to a nonparametric test. The Mann-Whitney test p-value for endothelin is 5.34e-05 and for vWF it is 0.00123. Unlike the Student’s t-Test, a normal distribution is not assumed, and this p-value would not change due to any scaling of the data.
Supplementary Material
REFERENCES
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Hennekens CH. Increasing burden of cardiovascular disease: current knowledge and future directions for research on risk factors. Circulation. 1998;97:1095–1102. doi: 10.1161/01.cir.97.11.1095. [DOI] [PubMed] [Google Scholar]
- 3.Heller RF, Chinn S, Pedoe HD, Rose G. How well can we predict coronary heart disease? Findings in the United Kingdom Heart Disease Prevention Project. Br Med J (Clin Res Ed) 1984;288:1409–1411. doi: 10.1136/bmj.288.6428.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Futterman LG, Lemberg L. Fifty percent of patients with coronary artery disease do not have any of the conventional risk factors. Am J Crit Care. 1998;7:240–244. [PubMed] [Google Scholar]
- 5.Tavazzi L. Clinical epidemiology of acute myocardial infarction. Am Heart J. 1999;138:S48–S54. doi: 10.1016/s0002-8703(99)70320-0. [DOI] [PubMed] [Google Scholar]
- 6.Boos CJ, Soor SK, Kang D, Lip GY. Relationship between circulating endothelial cells and the predicted risk of cardiovascular events in acute coronary syndromes. Eur Heart J. 2007;28:1092–1101. doi: 10.1093/eurheartj/ehm070. [DOI] [PubMed] [Google Scholar]
- 7.Quilici J, Banzet N, Paule P, et al. Circulating endothelial cell count as a diagnostic marker for non-ST-elevation acute coronary syndromes. Circulation. 2004;110:1586–1591. doi: 10.1161/01.CIR.0000142295.85740.98. [DOI] [PubMed] [Google Scholar]
- 8.Mutin M, Canavy I, Blann A, Bory M, Sampol J, Dignat-George F. Direct evidence of endothelial injury in acute myocardial infarction and unstable angina by demonstration of circulating endothelial cells. Blood. 1999;93:2951–2958. [PubMed] [Google Scholar]
- 9.Woywodt A, Kirsch T, Haubitz M. Immunomagnetic isolation and FACS--competing techniques for the enumeration of circulating endothelial cells. Thromb Haemost. 2006;96:1–2. doi: 10.1160/TH06-06-0293. [DOI] [PubMed] [Google Scholar]
- 10.Rowand JL, Martin G, Doyle GV, et al. Endothelial cells in peripheral blood of healthy subjects and patients with metastatic carcinomas. Cytometry A. 2007;71:105–113. doi: 10.1002/cyto.a.20364. [DOI] [PubMed] [Google Scholar]
- 11.Sluimer JC, Kolodgie FD, Bijnens AP, et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. J Am Coll Cardiol. 2009;53:1517–1527. doi: 10.1016/j.jacc.2008.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 13.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 14.Kramer MC, Rittersma SZ, de Winter RJ, et al. Relationship of thrombus healing to underlying plaque morphology in sudden coronary death. J Am Coll Cardiol. 55:122–132. doi: 10.1016/j.jacc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Burke AP, Kolodgie FD, Farb A, et al. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103:934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 16.Boos CJ, Lip GY, Blann AD. Circulating endothelial cells in cardiovascular disease. J Am Coll Cardiol. 2006;48:1538–1547. doi: 10.1016/j.jacc.2006.02.078. [DOI] [PubMed] [Google Scholar]
- 17.Rosin P. Unimodal Thresholding. Pattern Recognition. 2001;34:2083. [Google Scholar]
- 18.Hall M, F E, Holmes G, Pfahringer B, Reutemann P, Witten I, Hall M, Frank E, Holmes G, Pfahringer B, Reutemann P, Witten I. WEKA Data Mining Software: An Update. SIGKDD Explorations. 2009;11 [Google Scholar]
- 19.Sing T, S O, Beerenwinkel N, Lengauer T. ROCR: visualizing classifier performance in R. Bioinformatics. 2005;21 doi: 10.1093/bioinformatics/bti623. [DOI] [PubMed] [Google Scholar]
- 20.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. 2009 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.