Abstract
Fibroblast growth factors (FGFs) frequently fulfill prominent roles in the regulation of cell migration in various contexts. In Drosophila, the FGF8-like ligands Pyramus (Pyr) and Thisbe (Ths), which signal through their receptor Heartless (Htl), are known to regulate early mesodermal cell migration after gastrulation as well as glial cell migration during eye development. Herein, we show that Pyr and Ths also exert key roles during the long-distance migration of a specific sub-population of mesodermal cells that migrate from the caudal visceral mesoderm within stereotypic bilateral paths along the trunk visceral mesoderm toward the anterior. These cells constitute the founder myoblasts of the longitudinal midgut muscles. In a forward genetic screen for regulators of this morphogenetic process we identified loss of function alleles for pyr. We show that pyr and ths are expressed along the paths of migration in the trunk visceral mesoderm and endoderm and act largely redundantly to help guide the founder myoblasts reliably onto and along their substrate of migration. Ectopically-provided Pyr and Ths signals can efficiently re-rout the migrating cells, both in the presence and absence of endogenous signals. Our data indicate that the guidance functions of these FGFs must act in concert with other important attractive or adhesive activities of the trunk visceral mesoderm. Apart from their guidance functions, the Pyr and Ths signals play an obligatory role for the survival of the migrating cells. Without these signals, essentially all of these cells enter cell death and detach from the migration substrate during early migration. We present experiments that allowed us to dissect the roles of these FGFs as guidance cues versus trophic activities during the migration of the longitudinal visceral muscle founders.
Keywords: Cell migration, Cell survival, Fibroblast growth factor, Visceral mesoderm, Gut muscle development
Introduction
Signals by members of the fibroblast growth factor (FGF) family are known to play prominent roles in regulating cell migration in many different species and biological contexts (reviewed in Dorey and Amaya, 2010; Tulin and Stathopoulos, 2010a). However, in many cases it is difficult to separate the roles of FGFs in regulating migration from their functions in cell specification and proliferation during the same developmental events. Particularly in vertebrate species, an additional complication in functional studies of FGFs arises through the presence of multiple, closely related family members, which often make it difficult to dissect their distinct and often redundant roles in migration processes.
In Drosophila, there are only two FGF receptor tyrosine kinases, Breathless (Btl) and Heartless (Htl), and three FGF ligands, Branchless (Bnl), Pyramus (Pyr), and Thisbe (Ths). The Bnl/Btl ligand/receptor pair has a major role in the morphogenesis of the trachea during embryonic, larval, and pupal development (reviewed in Cabernard et al., 2004). By contrast, the ligands Pyr and Ths, which both belong to the FGF8/FGF17/FGF18 family, are dedicated to the receptor Htl and have important roles during early migration and cell specification in the embryonic and pupal mesoderm (Beiman et al., 1996; Gisselbrecht et al., 1996; Shishido et al., 1997; Gryzik and Müller, 2004; Stathopoulos et al., 2004; Dutta et al., 2005; Maqbool et al., 2006) as well as in glial cell migration and differentiation in the eye (Franzdottir et al., 2009).
During embryonic and larval morphogenesis of the tracheal tree, FGF signaling was shown to function reiteratively in a defined temporal sequence (reviewed in Ghabrial et al., 2003; Ghabrial and Krasnow, 2006). Initially, Bnl is expressed in six distinct groups of cells in the vicinity of the early tracheal sacs and acts as a chemotactic guidance cue for the Btl expressing tracheal progenitors to form the six primary tubes within each hemisegment. Subsequently, Bnl stimulates the sprouting of secondary branches via the induction of specific target genes at the tips of the primary branches. Finally, the stimulation of Bnl in oxygen-starved cells within different tissues in the larva again provides a chemoattractant for the outgrowth of new terminal branches, which ensures an evenly of oxygen to these tissues. During this step, only the leading cells respond to Bnl signals whereas the trailing cells follow in response to an unknown secondary signal from the leading cells (Ghabrial and Krasnow, 2006). During formation of a specialized tracheal structure, the thoracic air sac of the adult, Bnl expressed from a small group of cells in the disc epithelium induces cells from a nearby section of a tracheal branch to bud, proliferate, and migrate into the epithelial cell layer overlying the source of the Bnl signal to form the air sac (Sato and Kornberg, 2002; Cabernard and Affolter, 2005). A hallmark of cells responding to FGFs during both embryonic tracheal migrations and air sac morphogenesis are actin-based filopodial extensions oriented toward the source of the signals (Ribeiro et al., 2002; Sato and Kornberg, 2002).
In the mesoderm of Drosophila embryos after gastrulation, FGF signaling is required for the orderly spreading of the invaginated cells and the formation of a mesodermal monolayer underneath the ectoderm (Beiman et al., 1996; Gisselbrecht et al., 1996; Shishido et al., 1997). Similar to the situation in tracheal development, the receptor is expressed in the migrating cells whereas the ligands are expressed in the adjacent cells that form the substrate and target for the migration. During the early part of mesoderm migration the two ligands, Pyr and Ths, are co-expressed in the ventral-lateral ectoderm, whereas during later steps Pyr expression becomes restricted to the dorsal ectodermal cells that are eventually reached by migrating mesodermal cells (Gryzik and Müller, 2004; Stathopoulos et al., 2004; Dutta et al., 2005; Maqbool et al., 2006). Detailed analyses with both fixed tissues and live imaging revealed several distinct steps during the process of mesoderm spreading and migration (Schumacher et al., 2004; Wilson et al., 2005; Murray and Saint, 2007; McMahon et al., 2008; Supatto et al., 2009; McMahon et al., 2010; Clark et al., 2011). First, cells from the invaginated mesodermal tube closest to the ventral-most ectoderm extend filopodia toward these ectodermal cells and make contact with them. Then, in connection with an epithelial-to-mesenchymal transition (EMT), additional mesodermal cells are “zippering up” with the ectoderm, and the cells at the dorsal edge extend filopodia and migrate toward dorsal ectodermal cells. During this time, mesodermal cells from internal positions extend protrusions radially toward the ectoderm and intercalate with the spreading mesodermal cells already in contact with the ectoderm. The combination of these events leads to the formation of a mesodermal monolayer of cells that extends from the ventral midline toward the dorsal margin of the ectoderm. FGF signals coordinate this process by promoting the EMT, stimulating the formation of filopodial protrusions, and sustaining dorsal movements of the leading edge cells (Clark et al., 2011). These functions in changing the cellular morphologies and behavior are mediated through the adaptor proteins Dof and Shc downstream of the activated FGF receptor, but apparently not through Ras/MAPK (Wilson et al., 2005). They involve the small G-proteins Rac and Cdc42 as well as the RhoGEF Pebble. However, it is not known how these components fit into the FGF receptor pathway, or intersect with it in order to reorganize the actin cytoskeleton (van Impel et al., 2009; Clark et al., 2011).
It has been a matter of debate whether FGF signals are needed as long-range spatial attractants during this migration process or whether they act mostly locally in a more permissive fashion to promote migratory behaviors and ectodermal adhesion of mesodermal cells. The findings that mesoderm migration is rescued when endogenous Htl is replaced by constitutively-active versions of FGF receptors in the mesoderm, and efficiently occurs when spatial information in the ectoderm is drastically altered by genetic means, provided strong arguments for predominantly permissive functions (Frasch, 1995; Wilson and Leptin, 2000; Wilson et al., 2005). However, detailed investigations by live imaging with embryos lacking all Htl signaling or missing either the Pyr or the Ths signals provided some evidence that FGF-mediated chemotaxis does contribute to the coordinated sequence and robustness of events (Kadam et al., 2009; Klingseisen et al., 2009; McMahon et al., 2010; Clark et al., 2011). These latter studies indicated that the “zippering” process and radial intercalation are regulated rather locally by both Pyr and Ths, whereas the dorsal movement of leading edge cells is mostly triggered by Pyr being released from dorsal ectodermal cells at this stage as a longer-range chemo-attractant. Apart from the overlapping but ultimately diverging spatial distribution of the two ligands in the ectoderm, the partially-distinct roles of Pyr and Ths and the larger contribution of Pyr may be explained by differential processing of these ligands, which may lead to distinct biochemical properties. It has been proposed that the efficient intracellular clipping of the C-terminal domain renders the secreted FGF domain of Pyr more diffusible and allows it to act at a longer range as compared to Ths. By contrast, Ths partially retains the C-terminal domain in its secreted form, which appears to limit its potency and range of action (Tulin and Stathopoulos, 2010b). This would be also consistent with the finding that, normally, the induction of Even-skipped-expressing (Eve+) pericardial cells and muscle progenitors in the dorsal mesoderm after mesoderm migration almost exclusively depends on Pyr, which at this time is expressed in overlying dorsal ectodermal cells, whereas Ths expression occurs further away in ventral-lateral areas (Kadam et al., 2009; Klingseisen et al., 2009).
In our current study we dissect the role of FGF signaling in a later event of mesoderm migration, which provides additional clues to the roles of Pyr and Ths in cell migration and offers insights into new facets of their functions. The migrating cells under investigation are derived from the caudal visceral mesoderm (CVM) cells, which are specified by the bHLH transcription factor HLH54F at the very posterior end of the mesoderm during gastrulation (Georgias et al., 1997; Kusch and Reuter, 1999; Lee et al., 2005; Ismat et al., 2010). During the elongated germ band stage, these cells re-arrange into two bilateral clusters at posterior-internal positions of the mesoderm. Subsequently, cells start migrating out from these clusters, move onto the bands of cells of the trunk visceral mesoderm (TVM) on either side of the embryo, and undertake a long-distance migration along the trunk visceral mesoderm toward the anterior. Ultimately, the cells migrating furthest reach the anterior end of the trunk visceral mesoderm and the others are spread evenly along this tissue. Upon completion of their migration the CVM cells exert their roles as muscle founder cells of the longitudinal visceral muscles (LVM) (San Martin et al., 2001; Klapper et al., 2002). They undergo fusion with fusion-competent myoblasts residing in the trunk visceral mesoderm and then orchestrate morphogenesis and differentiation of the longitudinal gut muscles of the midgut. By contrast, the formation of circular midgut muscles is coordinated by muscle founder cells from the trunk visceral mesoderm (Englund et al., 2003; Lee et al., 2003).
In a forward genetic screen with EMS to identify genes required for caudal visceral mesoderm migration and longitudinal visceral muscle formation we have recovered mutations in pyr. As a previous report had also documented a requirement for htl (Mandal et al., 2004), these findings prompted us to examine the roles of FGF signals during this process in more detail. We show herein that FGF signals promote the ability of the trunk visceral mesoderm to serve as a substrate of migration and stimulate the survival of the migrating cells. In the total absence of all Htl signaling, pathfinding onto the trunk visceral mesoderm is partially disorganized, and after a short distance of migration along the trunk visceral mesoderm, the migrating cells detach and undergo cell death. When the cells are prevented from dying, their ability to migrate along the trunk visceral mesoderm is restored to a significant extent, but they still tend to veer off and migrate forward at a reduced rate. Pyr and Ths, which both are expressed in tissues along the tracks of migration, have partially redundant functions in sustaining this migration process. Importantly, forced ectopic release of Pyr or Ths signals from dorsal ectodermal cells either in the presence or absence of endogenous FGF signals during CVM migration efficiently redirects the path of migration of the CVM cells. These and other findings suggest that, normally, Pyr and Ths signals function as guidance signals to help biasing migration of the CVM cells toward their intended migration substrate, which is the trunk visceral mesoderm. This function can be overridden by strong ectopic signals. Once the CVM cells have reached this substrate, Pyr and Ths signals likely act at a rather close range and in a permissive and perhaps also chemotactic fashion by helping to increase adhesion to this migration substrate, “pulling back” cells that begin to veer off track, and promoting the survival of the migrating cells.
Material and methods
Fly stocks and genetics
The following mutant Drosophila strains were used: Df(3R)e-D7 tin-re28 as bap null mutant (Azpiazu and Frasch, 1993), binR22 (Zaffran et al., 2001), hltAB42 (Gisselbrecht et al., 1996), Alk1 (Englund et al., 2003), jeb-c1 (Weiss et al., 2001), Df(2R)pyr36 and Df(2R)ths238 (Kadam et al., 2009), pyr18 and ths759 (Klingseisen et al., 2009), Df(2R)BSC25 (Stathopoulos et al., 2004), Df(2R)BSC259 (S. Christensen and K. Cook, Bloomington Stock Center). Mutants were balanced over CyO or SM6b balancer chromosomes carrying either twi-GAL4 UAS-EGFP or eve-lacZ for the selection of homozygotes. For tissue specific expression the following UAS and GAL4 lines were crossed at 28°C: 2xPEtwi-GAL4 (Baker and Schubiger, 1996), UAS-slp1 (Lee and Frasch, 2000), SG24 twi-GAL4 (Greig and Akam, 1993), 5053A-GAL4 (Bloomington Stock Center, (Inaki et al., 2010), pnrMD237-GAL4 (Calleja et al., 1996; Heitzler et al., 1996; Fromental-Ramain et al., 2008), bap3-GAL4 (Lee et al., 2003), 48Y-GAL4 (Martin-Bermudo et al., 1999), UAS-EGFP, UAS-p35, UAS-htl, UAS-htlDN and UAS-htlAct (Bloomington Stock Center), UAS-pyr and UAS-ths (Kadam et al., 2009). For the rescue experiments we crossed UAS and GAL4 constructs on either X or 3rd chromosomes into the desired mutant backgrounds. An insertion of HLH54Fb-lacZ on chromosome 3 (Ismat et al., 2010) was crossed into the desired genetic backgrounds for LVM analysis. Reporter constructs driving expression of GFP or RFP under the control of the HLH54Fb enhancer, or RFP under the control of the bap3 enhancer, were crossed into wild type, Df(2R)BSC25 and pyrS0439 mutant backgrounds to label the LVM and its progenitors for live imaging.
Isolation of EMS mutants
We performed an EMS mutagenesis screen using a RFP/GFP reporter line, which expresses RFP driven by the LVM-specific enhancer of HLH54F and a somatic muscle enhancer of org-1, as well as GFP driven by a cardioblast-specific enhancer of tinman (Yin et al., 1997; Ismat et al., 2010; Schaub et al., 2012). Reporter males were treated with EMS and crossed with females of the balancer stock y w; hs-hid-2, Sp/CyO, twi≫EGFP. Balanced EMS lines were obtained by crossing single F1 males with the same balancer stock and heat-shocking the crosses. After dechorionation, embryos from F2 or F3 generation flies mounted in 16% glycerol were screened for abnormalities in muscle development.
Sequencing was done from PCR products amplified from genomic DNA isolated from homozygous pyrS0439 and pyrS3547 embryos, as identified by the absence of the GFP-labeled balancer. All mutations were verified by sequencing DNA extracted from pyrS0439 and pyrS3547 adult escapers obtained in trans with the deletion pyr18. DNA from the unmutagenized RFP/GFP reporter strain served as a reference.
Staining procedures
Immunostaining of embryos using diaminobenzidine (DAB) and fluorescent stainings for proteins and RNA were carried out essentially as previously described (Knirr et al., 1999; Reim and Frasch, 2005). The following antibodies were used: monoclonal mouse anti-FasIII (7G10, 1:20, Developmental Studies Hybridoma Bank, Univ. of Iowa), rabbit anti-β-gal (1:1500, Promega), rabbit anti-Eve (Frasch et al., 1987), rabbit anti-Mef2 (1:1000, from H.T. Nguyen, Erlangen University, Germany), rabbit and mouse 3E6 anti-GFP (1:2000 and 1:200, respectively; Invitrogen Molecular Probes), rat anti-Org-1 (Schaub et al., 2012). Full length cDNA clones of HLH54F (Ismat et al., 2010), pyramus and thisbe (Stathopoulos et al., 2004) were used to generate digoxigenin-labeled antisense RNA probes. For analysis of the larval visceral musculature third instar larval guts were dissected in phosphate-buffered saline (PBS), fixed in 3.7% formaldehyde for 45 minutes, washed three times with PBS + 0.1% Tween 20 and stained for F-actin using Alexa Fluor 488-conjugated phalloidin (30 minutes, 1: 500, Molecular Probes). Images of DAB stainings were obtained using Nomarski optics and images of fluorescent stainings using laser scanning microscopy with Zeiss LSM 510 META and Leica SP5 II confocal microscope systems.
Live imaging
Embryos were dechorionated, aligned on an agar block and transferred to a cover slip with a line of glue. The embryos were covered with halocarbone oil and attached to a LUMOXTM membrane slide (Greiner bio-one). Time-lapse imaging was performed on a Leica SP5 II confocal system equipped with a hybrid GaAsP Detector (HyD) using a DPSS 561 laser with 10-15 mW output for RFP exitation at 561 nm and detection at 570-700 nm and a argon laser with ∼5mW output for GFP exitation at 488 nm and detection at 500-550 nm. Acquisition was done over a time course of about 3-6 hours with the following settings: HC PL APO 20×/0.70 objective (with glycerol), pinhole 1.48-1.65 AU, scan speed 200 Hz, line accumulation 6-8, resolution 1024 × 400-800 pixel, Z-stack of about 16-20 sections with a step size of 3-4μm, and time intervals of 2-4 minutes per stack. For higher magnification scans a HCX PL APO 63×/1.30 objective (Movies 2-4), a step size of 1μm, a pinhole of 1 AU and twofold line averaging were used. Movies were generated using Leica Application Suite Advanced Fluorescence (LAS-AF) 2.4.1 and ImageJ 1.4 software.
Results
Survival and proper guidance of longitudinal visceral muscle founders depends on the trunk visceral mesoderm
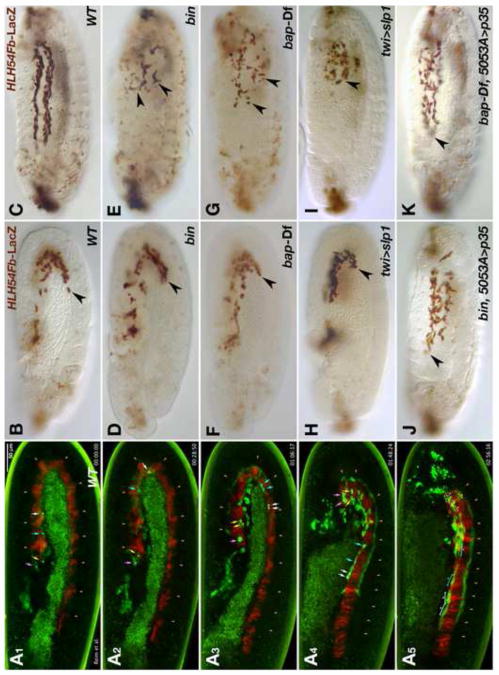
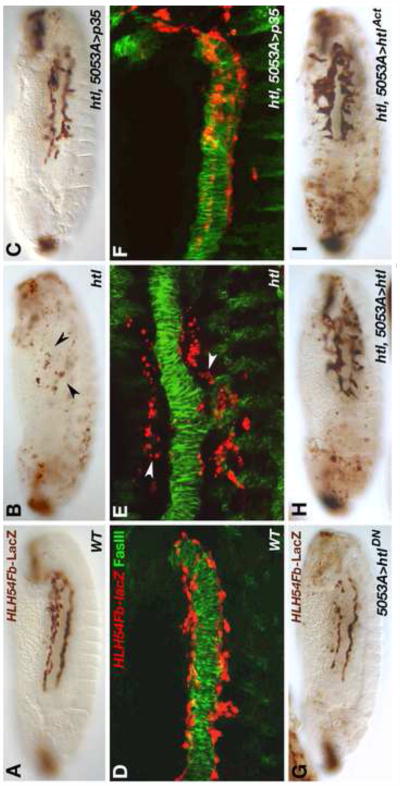
After the split of the caudal visceral mesoderm (CVM) into two bilateral clusters, the founder myoblasts of the longitudinal visceral muscles (LVM) migrate onto the trunk visceral mesoderm (TVM) on either side of the embryo at the onset and during germ band retraction (Kusch and Reuter, 1999; Lee et al., 2005) (note that a portion of CVM cells remain in posterior positions, therefore we refer to the migrating cells herein as LVM progenitor or founder cells). Subsequently these cells migrate in tight contact with the TVM toward the anterior, which largely occurs in two streams, one at the dorsal and another at the ventral margin of the linear band of TVM cells. Live imaging of embryos with GFP-marked CVM descendants (using HLH54Fb-GFP) and RFP-marked TVM (using bap3-RFP) allowed us to examine this migration process in more detail (Fig. 1A1-A5; Movie 1). The LVM progenitors near the migration front migrate fastest relative to the TVM, with a speed of ∼2.6 μm/min or 11-15 min per segment until they reach the U-turn of the TVM (Fig. 1A1, A2, white and turquoise arrows). Thereafter, migration slows down in connection with a mitosis event (Fig. 1A3), after which the cells continue migrating with a speed of 20-30 min per segment (Fig. A4). During the last phase, the LVM founder cells perform myoblast fusions and the cells complete the migration as syncytia (Fig. 1A5) (Movie 1). In contrast to the cells near the migration front, those at the lagging end migrate relatively short distances and remain largely stationary once they have reached their positions within posterior segments of the TVM (Fig. 1A1-A5, Movie 1; yellow and pink arrows). The cells in intermediate positions migrate with speeds that vary between these two extremes (Movie 1). The close association of migrating LVM founders with the TVM points toward a role of the TVM either as an adhesion substrate or possibly as a source of chemotactic molecules guiding LVM founder cell migration. To address these possibilities we followed migration of LVM founders labeled by HLH54Fb-LacZ reporter expression in wild type embryos (Fig. 1B, C) and in mutants in which TVM formation is abolished (Ismat et al., 2010). Two transcription factor encoding genes, bagpipe (bap) and biniou (bin) have been shown to be essential for TVM formation (Azpiazu and Frasch, 1993; Zaffran et al., 2001). In embryos carrying homozygous loss-of-function mutations in either bin or bap, LVM founder cell migration initiates similarly to the wild type situation (Fig. 1D, F), but the tracks become progressively irregular during germ band retraction (stage 12). By the end of germ band retraction none of the cells have reached the anterior of the trunk. Instead, we detect a reduced number of HLH54Fb-LacZ-labeled cells dispersed in the posterior half of the embryo (Fig. 1E, G). Most of the LacZ-staining appears in round and small cells or vesicles that eventually disappear altogether (Fig. 1E, G, and data not shown). Similar observations were made in embryos in which TVM specification was eliminated by expressing Sloppy-paired in the entire mesoderm, which causes the repression of bap, bin, and all other known TVM markers (Fig. 1H, I) (Lee and Frasch, 2000). The observed phenotypes indicate that in the absence of a proper TVM substrate LVM founders cease migrating shortly after the stage when normally they would have reached the TVM, and then undergo cell death. Hence, the termination of LVM cell migration could be an indirect consequence of apoptosis due to the lack of TVM-derived survival factors. Alternatively, apoptosis of LVM cells could be triggered by their inability to migrate as a result of missing guidance cues from the TVM. To distinguish between these possibilities we blocked cell death in the LVM founders of bin and bap null mutants by forcing the expression of the caspase inhibitor p35 in this migratory cell population. Under these conditions LVM founders regained much of their ability to migrate (Fig. 1J, K). However, their tracks were less regular, the migrating cells occupied a broader lateral area without being subdivided into a dorsal and a ventral stream, and fewer cells reached the very anterior end. These observations suggest that the TVM is indeed critical for the survival of the migrating LVM founders, but in addition cues from the TVM must be present to guide the LVM cells along stereotypic paths.
Fig. 1. Normal migration of longitudinal visceral muscle founders in the wild type and disrupted migration and survival in the absence of trunk visceral mesoderm.
(A) Still images of wild type embryo from Movie 1 at five different time points (A1: 0 min; A2: 29 min, A3: 1 h 6 min, A4: 1 h 48 min, A5: 2 h 56 min). The embryo carries HLH54F-GFP, which marks the CVM and migrating LVM founders (green), as well as bap3-RFP, which marks the trunk visceral mesoderm (red; parasegmental borders marked by arrow heads). Four migrating LVM progenitors and their daughter cells are marked by color-coded arrows and the syncytia formed from these by brackets. (B – K) Embryos carrying HLH54Fb-lacZ are stained with anti-β-galactosidase (β-gal) antibodies at stage 11 (B, D, F, H• and late stage 13 (C, E, G, I-K). Shown are lateral views with the anterior on the left. (B) Wild type (WT) stage 11 control embryo. LacZ-positive LVM founder cells have left the posterior tip of the extended germ band and migrate around the U-turn toward the anterior on either side of the embryo. (C) At the end of stage 13 the leading cells from the migration paths along the dorsal and ventral edges of the TVM have reached the anterior of the trunk. (D, E) show bin22 and (F, G) bap null (Df(3R)e-D7, tin-re28) mutant embryos at corresponding stages. Early migration of the CVM is largely unaffected in these mutants. At stage 13, the most anterior cells have failed to migrate beyond the point reached already during stage 11 (arrow heads) and are scattered within in the posterior third of the embryo. They are also reduced in number, and many of the remaining LacZ-positive cells feature abnormal cell shapes and have shrunken in size. Similar defects are observed upon ectopic activation of slp1 via twi-GAL4 (H, I), which blocks TVM formation. (J, K) If cell death is prevented by expression of the caspase inhibitor p35 within LVM founders, the cells survive and regain their ability to migrate, albeit less orderly, into the anterior trunk in bin or bap mutant backgrounds.
EMS mutagenesis uncovers a function for the FGF gene pyramus in LVM formation
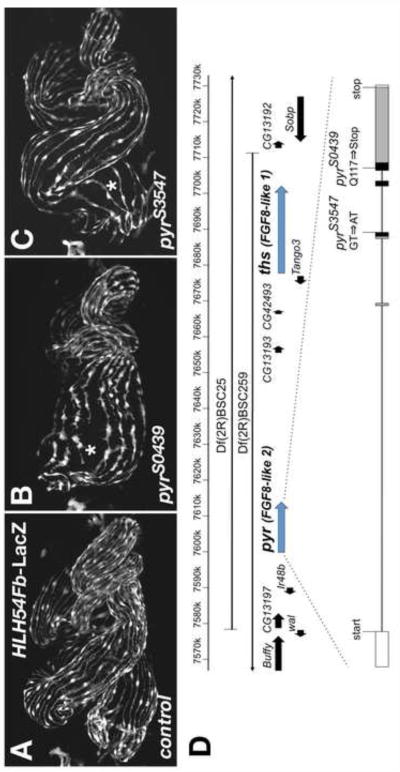
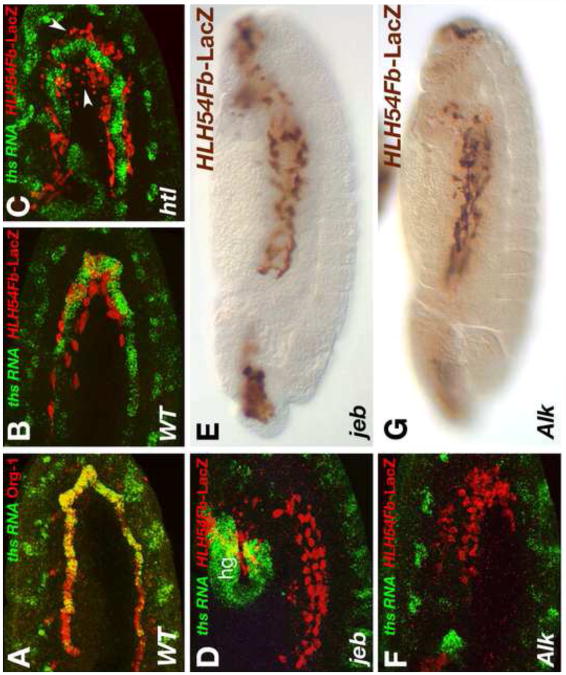
To identify genes involved in the formation of longitudinal visceral muscles and the migration of their progenitor cells we performed a forward mutagenesis screen with EMS (ethyl methane sulfate) on the second chromosome. The mutagenized line carried HLH54Fb-RFP as a specific live marker for the LVM (among other mesodermal RFP and GFP markers present; see Materials & Methods) (Ismat et al., 2010). Among a number of lines with disrupted LVM fibers there were two, S0439 and S3547, which featured a relatively mild reduction of the number of LMV fibers around the midgut. This was particularly noticeable in the anterior portion of the midgut, which featured a wider spacing of LVM fibers and, likely as a result, a frequent absence or reduction of the first midgut constriction (Fig. 2A, B, C). These lines were found to be allelic based on the lethality of the transheterozygous combination and were mapped to region 48C1-4 on chromosome 2R using deficiencies. Both the lethality and the described phenotype were reproduced in transheterozygous combinations with deficiencies uncovering this region. All non-complementing deficiencies tested also delete the FGF8-like genes pyramus (pyr) and thisbe (ths) (Fig. 2D). Because the FGF receptor Heartless (Htl) is known to play a critical role in migrating LVM founders (Mandal et al., 2004), and the mutants also showed heart phenotypes (data not shown) we tested whether they carried mutations in pyr or ths. Further complementation tests with small deletions of (or within) these two FGF genes previously generated by others (Kadam et al., 2009; Klingseisen et al., 2009) identified pyr as the most likely gene affected in both lines. Both EMS alleles were found to be lethal or semi-lethal over the pyr deletions Df(2R)pyr36 and pyr18, respectively. In contrast, deletions that partially or entirely remove ths but not pyr, Df(2R)ths238 and ths759, showed full complementation.
Fig. 2. Disrupted formation of longitudinal visceral muscles in homozygous pyr EMS mutants.
(A) HLH54Fb-lacZ wild type embryo with anti-β-gal staining to visualize longitudinal visceral muscle fibers spaced evenly around the midgut all along its length at stage 17. (B) Homozygous pyrS0439 EMS mutant embryo carrying HLH54Fb-lacZ and stained as in (A), which shows a partial depletion of longitudinal visceral muscle fibers in the anterior portion of the midgut (asterisk). (C) Similar defects are seen in an embryo homozygous for the pyrS3547 allele. Note the wider LVM fiber spacing as compared to (A). In addition, gut looping abnormalities connected to an incomplete anterior midgut constriction are seen to variable degrees in both alleles. (D) Map of the genomic region containing pyr and ths. Shown are gene models contained near the areas of overlap of the deficiencies used to eliminate both FGF8-like genes. The gene structure of pyr and positions of the mapped pyr mutations are depicted below. Several other amino acid sequence variations compared to the published sequence were found in both the mutagenized and unmutagenized strains: A shortened poly-asparagine stretch at position 260 (from 10 to 4 N′s), Q316K, A400V, A599V, and M614V. Exons are boxed and shaded in their coding areas. Black shading indicates the region corresponding to the FGF domain (Pfam HMM consensus).
Sequencing of exons of the pyr locus uncovered a unique point mutation in each of the EMS lines, confirming that we isolated the first known pyr EMS alleles. In pyrS0439 the CAG codon encoding a glutamine residue at position 117 within the FGF domain is converted into a premature stop codon (TAG). In pyrS3547 the splice donor site following exon 3 is mutated from GT to AT and the unspliced transcript is predicted to encode a protein that is truncated even earlier within the FGF domain (due to an intronic stop codon after the addition of the amino acid sequence TYHKYYSNI following Y60) (Fig. 2D). Other sequence variations were present in both mutants and in the unmutagenized control strain (Fig. 2D, legend). The truncated Pyr proteins are very likely non-functional as in either case a significant portion of the FGF domain is missing.
The FGF8-like genes pyramus and thisbe both contribute to LVM formation
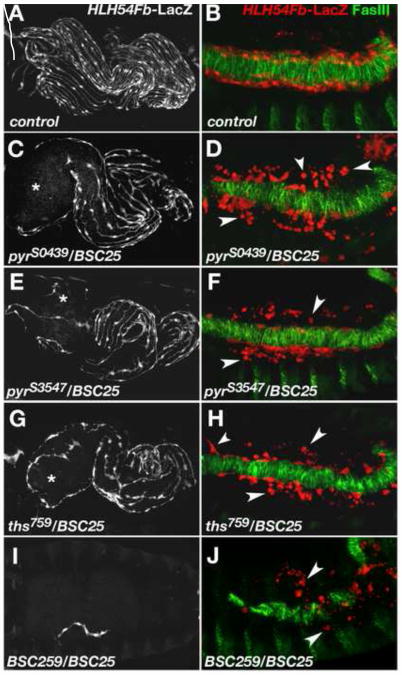
To avoid possible effects of second site mutations on the mutated chromosomes we analyzed the embryonic LVM phenotypes of the EMS alleles in trans to Df(2R)BSC25. In this combination the LVM defects with both pyrS0439 and pyrS3547 were stronger than in the homozygous EMS mutants (Fig. 3C, E, compare with A and Fig. 2B, C). We detect larger areas devoid of LVM fibers, in particular around the anterior midgut. The observed enhancement of the phenotype with Df(2R)BSC25 might indicate a small residual activity of the mutated pyr loci, but several observations support the idea that it rather is caused by the additional removal of one copy of the potentially redundant ths gene (see Fig. 2D). First, if we compare the phenotypes caused by pyrS0439 and pyrS3547 to each other, no significant differences are found (Fig. 3C-F and data not shown). Second, the LVM phenotypes caused by pyr18, a deletion that removes the entire pyr transcript (Klingseisen et al., 2009) are not stronger than that seen with the EMS alleles (in fact they appear weaker; data not shown; see discussion).
Fig. 3. Abnormal longitudinal visceral muscle development in mutants with reduced FGF ligand activity.
Anti-β-gal staining of HLH54Fb-lacZ embryos to visualize longitudinal visceral muscle fibers at stage 17 (A, C, E, G, I) and of migrating LVM progenitors at the end of germ band retraction (B, D, F, H, J). In the latter panels the TVM is marked by anti-fasciclin III (FasIII) staining in green. (A, B) Wild type. (C) Embryo carrying the EMS-induced mutation pyrS0439 in trans to the pyr- and ths-deleting deficiency Df(2R)BSC25 with a strong depletion of LVM fibers especially around the anterior midgut (asterisk). (D) Late stage 12 embryo of the same genotype as in (C). Numerous LVM founders have lost contact with the TVM and many of them appear as small rounded dots (arrow heads). (E, F) pyrS3547/Df(2R)BSC25 mutant embryo showing essentially the same phenotype as embryos in (C, D). Very similar defects are also observed in ths759/Df(2R)BSC25 mutants (G, H). (I) Removal of pyr and ths using overlapping deficiencies Df(2R)BSC25 and Df(2R)BSC259 (“FGF8 null” genotype) leads to a complete loss of the LVM with rare occurrence of individual LVM fibers. (J) In a FGF8 null mutant, the number of LVM founders at late stage 12 is greatly diminished and almost all cells have lost contact with the TVM.
When we examined the pyr/Df(2R)BSC25 mutant embryos at earlier stages of LVM development, we found a phenotype reminiscent of the one observed in embryos lacking the TVM (see above, Fig. 1). LVM founders are present at normal numbers and start migrating rather normally during stage 11 (Movie 3; compare to WT, Movie 2). However, during late stage 12 and stage 13, when the founders normally spread out along the entire TVM, in pyr mutant embryos many of the founders lose contact with the TVM (Fig. 3D, F, compare with B; Movie 3). During stage 13, we observe a large number of rounded and shrunken cells located at some distance from the TVM, indicative of dying cells. It should be noted that in this and the following experiments we selected embryos for analysis that had formed a complete band of TVM cells on the side monitored. Embryos with interruptions in the TVM, which occur in a portion of embryos with compromised FGF/Htl signaling as a result of uneven spreading of the early mesoderm, were omitted in the analysis in order to avoid indirect effects on LVM founder cell migration.
Because of the potential redundancy of pyr and ths, we also investigated LVM development in mutants that lack a functional ths gene, or that contain neither ths nor pyr. In homozygous ths759 mutants and, more prominently, in transheterozygous ths759/Df(2R)BSC25 embryos we see defects during LVM founder migration and reductions of LVM fibers at the end of embryogenesis that are similar to the corresponding genotypes with the pyr EMS alleles (Fig. 3G, H; Fig. S1). The observed migration defects connected with cell death in pyr and ths mutants explain the lower density of longitudinal muscle fibers seen in late stage mutant embryos. Heterozygous Df(2R)BSC25/+ embryos displayed reduced anterior LVM densities akin to those of homozygous pyr and ths mutants (Fig. S1). These observations suggest that the phenotype is dosage sensitive because taking out two functional copies of either pyr or ths can cause similar defects as deleting one copy of each.
If all copies of both FGF8-like genes, pyr and ths, are eliminated by overlapping deficiencies (Df(2R)BSC25/Df(2R)BSC259, hereafter designated as FGF8 null genotype), no longitudinal gut musculature is formed (Fig. 3I). In this genetic background, the number of LVM founders is already somewhat diminished during stage 11, practically all of them lose contact with the TVM by late stage 12, and the vast majority eventually undergoes cell death and become fragmented (Fig. 3J, Movie 4). The few progenitors that occasionally survive can produce a small number of syncytial fibers (Fig. 3I).
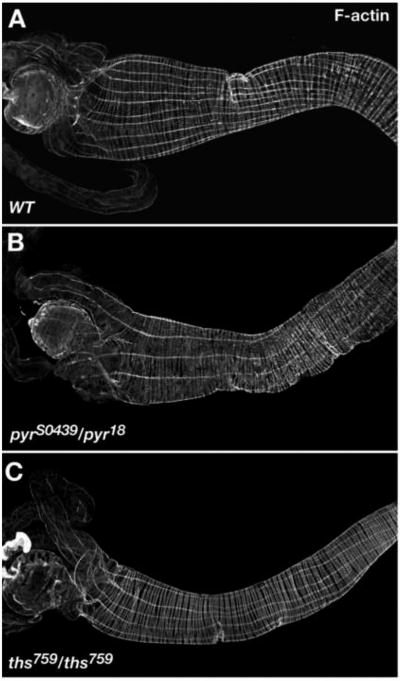
To further characterize the LVM phenotypes obtained with pyr and ths single mutants we also wanted to examine larval guts, which are easier to analyze because they are less convoluted. As homozygous ths759 and transheterozygous pyrS0439/pyr18 mutants are only semi-lethal, we were able to analyze the LVM of guts of 3rd instar larval escapers in these mutant backgrounds (pyr and ths transheterozygous escapers with Df(2R)BSC25 were not observed, except for the combination pyr18/Df(2R)BSC25). In phalloidin-stained wild type gut preparations 9-11 parallel LVM fibers are usually seen per half side (Fig. 4A). By contrast, guts from pyrS0439/pyr18 and homozygous ths759 larvae contained only 5-8 LVM fibers per half side (Fig. 4B, C).
Fig. 4. Larval gut muscle phenotypes of pyr and ths mutants.
Guts of 3rd instar larvae stained tor F-actin in visceral muscles using fluorescently labeled phalloidin. Shown are anterior portions with the proventriculus and gastric caecae to the left. (A) Wild type. (B) Gut from pyrS0439/pyr18 larva showing a reduction in the number of LVM fibers as compared to (A). (C) Gut from homozygous ths759 mutant larva also showing a reduction of LVM fibers.
Altogether the phenotypic data from FGF8-like mutants show that pyr and ths are partially redundant, but both genes are needed for optimal migration and survival of LVM founders and, therefore, for normal longitudinal gut muscle development. The simultaneous absence of both signals leads to a total detachment of the LVM founders from the substrate during early-to-mid migration and their complete loss due to cell death.
The FGF receptor Heartless supports LVM formation by regulating substrate adherence and survival of LVM cells
The FGFs Pyr and Ths are ligands of the receptor Htl, which is expressed in migrating LVM founders and is essential for LVM development (Mandal et al., 2004). Because the exact role of Htl in promoting LVM development has remained unclear, we reinvestigated the function of Htl in LVM founders in more detail using available mutants and inducible functional variants. In htl loss-of-function mutants HLH54Fb-lacZ-labeled LVM founders fail to keep contact with the TVM, disperse in the posterior trunk and eventually undergo cell death just as observed in FGF ligand mutants (compare Fig. 5B, E to Fig. 5A, D and Fig. 3D, F, H, J). Interestingly, both survival and long-distance migration toward the anterior are largely restored in htl mutants if cell death of LVM founders is blocked by CVM-specific (5053A-GAL4-driven) expression of the pan-caspase inhibitor p35 (Fig, 5C, F). Thus, htl appears to be mainly required to prevent cell death and is not essential for migration as such. However, the irregular path of htl mutant LVM cells rescued by UAS-p35, their less efficient migration toward the anterior, and the presence of cells veering off toward dorsal and ventral directions make it clear that FGF signaling has important functions in the guidance of LVM founder cell migration.
Fig. 5. Requirements for the FGF receptor Htl in migrating longitudinal visceral muscle founders.
Stage 13 embryos carrying HLH54Fb-lacZ stained with anti-β-gal and DAB (A-C, G-I•• and higher magnification views of anti-β-gal/anti-FasIII fluorescent double stainings (D-F). (A, D) Wild type embryos with normal migration of LVM founder cells, which are spindle-shaped and closely associated with the dorsal and ventral edges of the TVM. (B, E) In htl loss-of-function mutants LVM founders do not migrate into the anterior and acquire small, rounded shapes characteristic of dying cells (arrow heads). (C, F) Upon inhibition of apoptosis in LVM founders via 5053A-GAL4 driven expression of p35, a majority of LVM cells is attached to the TVM at any given time and the cells are able to migrate almost as far as in the wild type. (G) LVM founder-specific expression of a dominant-negative form of htl using 5053A-GAL4. The number of LVM cells is reduced, there are scattered cell fragments, and migration is incomplete. (H, I) htl mutants in which htl function is specifically restored in LVM founders using 5053A-GAL4 in combination with GAL4-inducible transgenes encoding either a wild type (H) or a constitutively active form (I) of Htl. In both cases significant rescue of LVM cell survival and migration is observed (compare to B). In comparison with the wild type (A), the distances of anterior migration are reduced and many cells take abnormal directions. Especially the expression of ligand-independent receptors causes cells to form clusters with aberrant extensions in vertical directions (I).
Next we tested whether htl function is specifically required within the LVM founders to promote their survival and migration. When we expressed a (weakly) dominant-negative form of the receptor (htlDN) in these cells via 5053A-GAL4, we detected a clear reduction of LVM cells and a presence of shrunken cells off the migration substrate (Fig. 5G). Furthermore, forced LVM-specific expression of either wild type htl or a constitutively active form of Htl (UAS-htlAct) in a htl mutant background with the same driver was sufficient for cell survival and improved long-range migration (Fig. 5H, I). Notably, LVM cells rescued by UAS-htl and even more so, UAS-htlAct, tend to acquire abnormal cell shapes, adhere to positions more distant from the TVM both dorsally and ventrally, and form clusters. Thus, while fully rescuing cell survival, excessive levels of signaling seem to interfere with efficient and directed migration, which contributes to the absence of LVM cells from the most anterior trunk segments in these embryos (Fig. 5H, I).
Our analysis of htl mutants clearly demonstrates that the FGF receptor Htl is required autonomously within LVM founders. The data also suggest that Htl activation is mainly required for cell survival, thus playing predominantly a permissive rather than instructive role during the migration of LVM founders along the anterior-posterior axis. The rescue data also imply that the levels of Htl activation must be tightly regulated both spatially and quantitatively to ensure completely normal migration behavior.
Multiple tissues may act as sources of FGF ligands for Htl in LVM founder cells
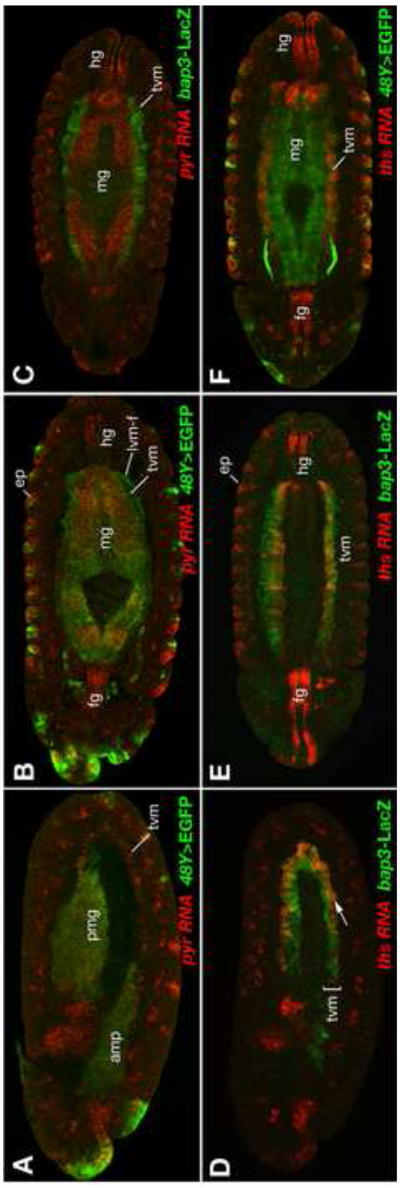
We have shown so far that the absence of the TVM, the inability of LVM founders to receive FGF signals, and the combined absence of Pyr and Ths signals all cause very similar effects on the migration and survival of the LVM founders. The simplest way to explain these similarities is that the TVM serves as the critical source of FGF signals to activate the Htl receptor in LVM cells migrating close to this source of signals. To investigate this possibility, we analyzed the expression and function of pyr and ths in the tissues along the path of LVM founder cell migration in more detail. First, we performed in situ hybridizations with pyr and ths probes in double labeling experiments with markers for the TVM (bap3-lacZ) and the endoderm (48Y-GAL4/UAS-EGFP). The stainings for pyr revealed that the ligand encoded by this gene is expressed in the TVM as well as in the endoderm (both the anterior and posterior midgut primordia) at early stages of LVM migration (stage 11, Fig. 6A). Thereafter, pyr expression ceases in the TVM but persists in the endoderm (Fig. 6B, C). As the LVM founders mostly migrate along the dorsal and ventral margin of the TVM they would also be in close vicinity to Pyr-expressing endodermal cells during stages 12 and 13. This endodermally-derived Pyr seems to be responsible for the observed formation of large cellular protrusions of the LVM founders that extend away from the TVM toward the endoderm during mid-migration in the wild type, but not in pyr mutants (Movies 2, 3).
Fig. 6. Expression of pyr and ths during LVM founder cell migration and gut formation.
Expression of pyr and ths RNA (red, as indicated) detected by in situ hybridization in embryos carrying either 48Y-GAL4/UAS-EGFP (A, B, F) or bap3-lacZ (C, D, E). 48Y-GAL4 drives GFP expression (green, anti-GFP) mainly in the endoderm, at low levels in the migrating LVM founder cells (lvm-f) but not in the trunk visceral mesoderm (tvm), and in some epidermal areas (ep). bap3-lacZ (green, anti-β-gal) is expressed exclusively in the trunk visceral mesoderm (tvm). (A) Lateral view of late stage 11 embryo showing pyr expression in the GFP-labeled primordia of the anterior and posterior midgut (amg, pmg) and in the row of TVM founder cells (tvm). (B, C) Ventral views of inner sections of stage 13 embryos demonstrating pyr co-expression with EGFP in the forming midgut endoderm (mg), but not in the TVM. (D) Lateral view of stage 11 embryo showing ths expression in the GFP-labeled primordia of the TVM. Within the TVM, only the ventrally located founder cells express ths (arrow). (E, F) Ventral views of inner sections of stage 13 embryos demonstrating ths co-expression with LacZ in the TVM, but not in the midgut (mg). Both genes, pyr and ths, show expression in the foregut (fg) and hindgut (hg) and in the epidermis (ep).
Like pyr, ths is expressed in the TVM, as previous experiments had already indicated (Stathopoulos et al., 2004), but in contrast to pyr its expression persist in this tissue (Fig. 6D-F). During stages 11 – 12, ths expression is strongest in the posterior ∼2/3 of the TVM (Fig. 6D) and no expression is seen in the endoderm at any stage. Both genes are also expressed in similar patterns in the foregut and the hindgut, as well as in the epidermis as reported previously (Gryzik and Müller, 2004; Stathopoulos et al., 2004).
Closer inspection showed that ths and pyr are expressed specifically in the ventral cell rows of the TVM (Fig. 6A, D), and double stainings with antibodies against the T-box transcription factor Org-1 defined this ths (and pyr) expressing subset of TVM cells as the founder cells of the circular visceral muscles (Fig. 7A) (Lee et al., 2003; Schaub et al., 2012). As shown in Fig. 7B, the LVM founders normally migrate very close to these FGF-producing circular muscle founders of the TVM. By contrast, if the migrating cells are unable to receive FGF signals they detach from the TVM early in their migration (Fig. 7C). Because these observations implicated TVM-derived FGFs in promoting normal migration, we aimed to remove specifically this source of FGF signals. To achieve this, we examined LVM founder cell migration in mutants for jelly belly (jeb) and Anaplastic lymphoma kinase(Alk), which code for a ligand/receptor pair that is crucial for specifying the circular visceral muscle founder cells (Englund et al., 2003; Lee et al., 2003). As shown in Fig. 7D, F, ths expression is specifically missing in the TVM in embryos with these mutant backgrounds, and the same is expected for pyr. Nevertheless, LVM founder cell migration is normal during stage 11-12 in the absence of TVM-derived FGF signals and very few, if any, dying cells are seen (Fig. 7D, F). Moreover, during stage 13 the front of the migrating cells reaches the anterior end of the TVM in jeb and Alk mutants like in the wild type (Fig. 7E, G, compare to Fig. 1C). During late stage 13, the migration begins to become disordered and there is progressive cell death in jeb and Alk mutants. We ascribe this phenotype to the disappearance of the migration substrate, namely the TVM fusion-competent cells, from this late stage onwards as a result of their aberrant fusion with founder cells of the somatic mesoderm (Fig. 7E, G) (Lee et al., 2003).
Fig. 7. LVM founder migration without ths and pyr expression in the visceral mesoderm.
(A) Double staining of ths RNA(green) together with Org-1 protein (red) in a stage 11 embryo. At this stage Org-1 labels the founder cells of the circular TVM. Co-staining confirms specific expression of ths in these cells. (B-D, F) Stage 12 embryos carrying the LVM marker HLH54Fb-lacZ stained with anti-β-gal (red) and in situ hybridized for detection of ths RNA (green). (B) In the wild type, LVM founders (red) migrate close to the ths expressing TVM founders (green). (C) htl mutant in which LVM founders (red) begin to lose contact with the TVM. Some of the LacZ-labeled cells have become small and rounded (arrow heads). (D) Stage 12 jeb mutant embryo showing normal migration and survival of LVM founders in the absence of TVM-expressed ths. (hg: ths in hindgut anlage) (E) Late stage 13 jeb mutant embryo stained with anti-β-gal to detect migration of HLH54Fb-lacZ expressing LVM cells at stage 13. The distance of migration is almost normal. LVM tracks are slightly irregular (compare with control in Fig. 5A and with htl mutant in Fig. 5B). (F) Stage 12 Alk mutant embryo showing normal migration and survival of LVM founders in the absence of TVM-expressed ths and pyr. (G) Late stage 13 Alk mutant embryo stained with anti-β-gal and showing the same LVM migration behaviour as jeb mutant in (E).
Altogether, these observations demonstrate that FGF signals from the TVM are largely dispensible for the anterior migration and survival of the LVM founders. Presumably, Pyr signals from the endoderm can substitute for TVM-derived Pyr and Ths under these conditions. Conversely, in the presence of a normal TVM, the endoderm is largely dispensible for anterior LVM founder cell migration (Wolfstetter et al., 2009). These data led us to the conclusion that, contrary to our expectation, the exact tissue source for the FGF signals is not very critical during this process. However, it is likely that peak signals, either through Pyr + Ths from the TVM or through Pyr from the endoderm, must be provided along the projected path of migration. Obviously, both signals together are needed for full robustness of migration and cell survival.
Ectopic FGF signals can redirect migration of LVM founders and support their survival
Thus far, our data cannot distinguish unequivocally between either a mostly permissive function of FGF signals for substrate adhesion and survival of migrating LVM cells or a chemotactic function in their guidance along a specific path. Therefore, our next goal was to investigate whether ectopic FGF sources can affect the migration and survival of LVM founders, and in particular whether they can influence their choice of direction. In these experiments, we performed gain-of-function studies with inducible UAS-pyr and UAS-ths transgenes (Kadam et al., 2009) and various tissue-specific GAL4 drivers either in the presence or the absence of endogenous Pyr and Ths signals.
In wild type controls, early migration occurred strictly along separate bilateral paths onto and along the TVM (Fig. 8A, Movie 5). However, in the embryos lacking both FGF signals, the speed of early migration often differed between the right and left sides (Fig. 8B, Movie 6 embryo 1), and frequently a portion of cells occupied more medial positions (Fig. 8B). In the latter situation, cells from the right and the left sides can come into contact at the midline (Movie 6 embryo 2).
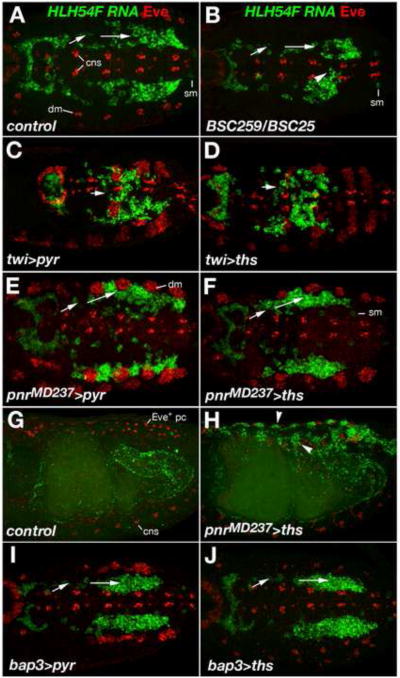
Fig. 8. Effects of Pyr and Ths on the direction of LVM founder migration.
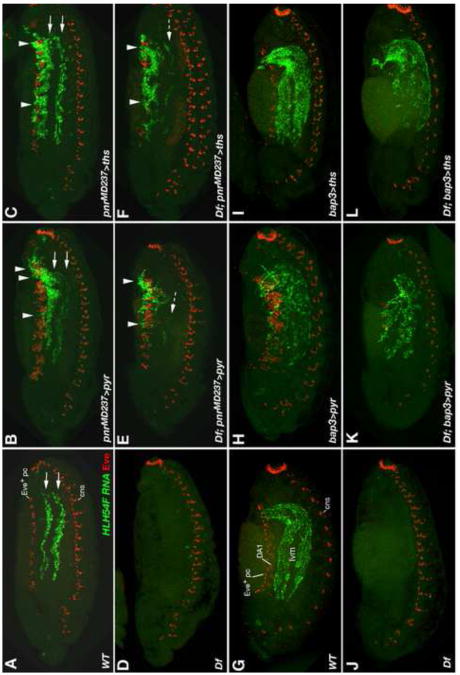
Wild type, mutant, and FGF over-expressing embryos were stained for HLH54F RNA (green) and Even-skipped (Eve) protein (red). In A-F, I, and J, dorsal views of germ band extended embryos are shown with caudal ends to the left and anterior directions toward the right. (A) Stage 11 wild type embryo; HLH54F expression (green) marks migrating LVM founders derived from the caudal visceral mesoderm and Eve (red) marks FGF-dependent precursors of specific pericardial and somatic muscle cells in the dorsal mesoderm (dm). Arrows indicate the lateral-anterior directions of LVM founder migration. HLH54F is also weakly expressed in one ventrolateral somatic muscle progenitor per segment (sm) and Eve also labels cells of the central nervous system (cns; in a lower focal plain than the visceral mesoderm but included in the Z-stack projections as a reference for the location of the ventral midline). (B) FGF8 null mutant stage 11 embryo. At this stage, overall migration is relatively normal but less symmetric, with some cells straying from the normal path and migrating toward the midline and/or the Z direction (arrow head). The number of migrating LVM founders is slightly reduced. There is no eve activation in the dorsal mesoderm. (C, D) Ectopic expression of either pyr (C) or ths (D) in the entire mesoderm via twi-GAL4 (SG24) prevents LVM founders from forming bilateral groups. Migration of individual cells appears to be undirected with only little net movement toward the anterior trunk. Mesodermal Eve clusters are enlarged. (E, F) Ectopic expression of either pyr (E) or ths (F) in the dorsal ectoderm via pnrMD237-GAL4 causes LVM founders to migrate further laterally as compared to cells of the same stage in normal embryos. LVM founders between the (enlarged) Eve clusters of the dorsal mesoderm extend processes toward the ectoderm. (G) Lateral view of stage 15 wild type embryo in which LVM fibers have aligned along the entire midgut. Eve expression is seen dorsally in Eve+ pericardial cells, weakly in somatic muscle DA1, and ventrally in the CNS. (H) Stage 15 embryo with pnrMD237-GAL4-driven ectopic expression of ths in the dorsal ectoderm. LVM fibers are seen along the entire midgut as in (G), but many LVM cells have been redirected toward dorsal areas underneath the ectoderm (arrow heads). (I, J) LVM founders migrate normally in stage 11 embryos when either pyr (I) or ths (J) are over-expressed in the TVM via bap3-GAL4 (compare to A).
When we employed the twist-GAL4 driver SG24 (Greig and Akam, 1993) to express either pyr or ths in the entire mesoderm, migration behaviors were strongly altered even in the presence of endogenous FGFs. Under both conditions the LVM founders started moving in the right direction (away from the caudal end), but in contrast to the wild type, they failed to separate into two bilateral streams, and also occupied areas around the midline (Fig. 8C, D, compare with A). The cells appeared to be oriented more randomly, and migration was much less directional as compared to the wild type. At later stages partial loss of LVM cells was observed (with a milder reduction upon pan-mesodermal ths expression). Notably, after germ band retraction all remaining LVM cells were still retained in the posterior third of the embryo, where they formed irregular aggregates of fibers (data not shown).
In addition to mesodermal sources, we also wanted to provide FGF signals from totally different directions and monitor how the migrating LVM founders respond to them. Therefore we employed a GAL4 enhancer trap in the pannier (pnr) locus, pnrMD237-GAL4 (Calleja et al., 1996; Heitzler et al., 1996; Fromental-Ramain et al., 2008), which reflects endogenous pnr expression in the dorsal ectoderm but lacks expression in the cardiogenic mesoderm (Supplemental Fig. S2). Notably, over-expression of either pyr or ths in the dorsal ectoderm causes a shift of most LVM founders away from the TVM toward a more lateral migration path (Fig. 8E, F). Many LVM founders were targeted toward areas between the expanded clusters of even-skipped-expressing pericardial and somatic muscle progenitors in the dorsal mesoderm, often surrounding them and sending extensions toward the ectoderm. Many of the cells guided laterally continued to migrate in anterior directions. In the case of ths over-expression, many cells reached the anterior trunk through this ectopic route, whereas others took their regular path (Fig. 8H, see also Fig. 9C). Eventually, LVM fibers were found both underneath the FGF-secreting dorsal ectoderm and around the midgut (Fig. 8H; compare to normal situation in Fig. 8G showing HLH54F expressing muscle fibers exclusively around the midgut). Forced dorsal ectodermal expression of pyr triggered similar ectopic migration, but long-range migration toward the anterior was partially inhibited and the LVM fibers clustered more densely near the posterior dorsal ectoderm (Fig. 9B and data not shown). Although not as severe, this “trapping” of LVM precursors is reminiscent of the posterior clustering observed upon pan-mesodermal pyr or ths expression. Because ectopic Eve clusters were less strongly expanded overall after over-expression of ths as compared to pyr (see also below), we assume that pyr provides higher signaling activity in our assays and that posterior clustering and concomitant inhibition of long-range migration is due to excessive FGF signaling.
Fig. 9. Rescue and re-routing of LVM founder migration in FGF8 null mutants by forced ectopic expression of pyr and ths.
Wild type and mutant embryos with and without GAL4-driven FGF expression were stained for HLH54F RNA (green) and Even-skipped (Eve) protein (red) as in Fig. 8. Shown are lateral views of either stage 13 (A-F) or stage 14 (G-L) embryos. (A) In the wild type LVM precursors spread across the entire trunk along a dorsal and a ventral track (on each side of the embryo). (B) pnrMD237-GAL4-driven ectopic expression of pyr in the dorsal ectoderm of an otherwise wild type embryo leads to clustering of LVM precursors near the dorsal ectoderm (arrow heads). Many cells still take the normal route of migration (arrows). Eve expression in the dorsal somatic/cardiogenic area is expanded. (C) pnrMD237-GAL4-driven ectopic expression of ths has similar effects as pyr, although more cells are able to migrate to the anterior underneath the dorsal ectoderm and Eve expansion is less severe. (D) FGF8 null mutant (Df = Df(2R)BSC25/Df(2R)BSC259) in which almost no LVM forms. Pericardial/dorsal somatic Eve precursors are also absent. (E, F) In the absence of an endogenous FGF8-like ligand source, pnrMD237-GAL4-driven ectopic expression of either pyr or ths allows survival and migration of LVM founders. Almost all LVM cells cluster near the dorsal ectoderm and very few cells take a near normal route of migration (dashed arrows). In FGF8 null mutants with forced ths expression (F) Eve precursors are rescued only in some segments, but more LVM cells are able to migrate to the anterior than in mutants with forced pyr expression (E). (G) Wild type stage 14 embryo with several rows of HLH54F-expressing syncytial LVM fibers being formed. Eve expression is seen dorsally in Eve+ pericardial cells, weakly in somatic muscle DA1, and ventrally in the CNS. (H, I) bap3-GAL4-driven expression of pyr (H) or ths (I) in the TVM has little or no effect on the migration of LVM precursors except for mild disruptions in the arrangement of LVM fibers upon pyr over-expression. TVM-derived Pyr, but not Ths, also causes a strong expansion of dorsal Eve expression. (J) Stage 14 FGF8 null mutant (Df(2R)BSC25/Df(2R)BSC259) without any LVM and dorsal Eve expression. (K, L) Forced expression of either pyr or ths from a bap3-GAL4 controlled transgene rescues survival and migration of LVM founders in Df(2R)BSC25/Df(2R)BSC259 embryos. LVM formation proceeds, with some arrangement defects seen when driving pyr (K) and relatively normally when driving ths (L). Specification of Eve progenitors in the dorsal mesoderm is rescued partially (with pyr; K) or not at all (with ths; L).
As a third tissue for FGF over-expression we chose the TVM, which is more similar to the endogenous signaling source (see above, Fig. 6). Expanding visceral pyr or ths expression from the TVM founders to all TVM cells using bap3-GAL4 (Lee et al., 2003) neither had any effects on the direction nor on the distance of LVM founder migration (Fig. 8I, J, see also Fig. 9H, I). Mild disruptions in the arrangement of LVM fibers were seen with ectopic pyr in the TVM at later stages of development (see Fig. 9H), which probably reflects local clustering as a result of moderate hyper-activation of the FGF pathway.
Next we asked whether Pyr and/or Ths are able to rescue any of the defects in the migration and survival of LVM founders of FGF8 null mutants if they are provided from ectopic sources. Since twist-GAL4-mediated activation of pyr and ths traps LVM founders in the posterior, pan-mesodermal pyr or ths expression is not able to rescue long-range LVM migration, although we did observe a significant increase in LVM cell survival as compared to regular FGF8 null mutant embryos (data not shown). LVM cell survival and long-range migration were both rescued when pyr or ths were expressed in the dorsal ectoderm via pnrMD237-GAL4 (Fig. 9E, F; compare with A, D). However, in the absence of endogenous Htl ligands almost all LVM cells took the ectopic path of migration underneath the dorsal ectoderm and many fewer cells migrated along the TVM and endoderm as compared to the analogous UAS/GAL4 experiments in the wild type background (Fig. 9E, F; compare with B, C). Nevertheless, some cells did migrate along the normal path and survive even though the FGF signals are exclusively coming from the dorsal ectoderm, particularly with Ths, which appears slightly less active than Pyr in redirecting migration (Fig. 9F, compare with E).
The above observations show that the survival function of FGFs can be provided from non-native sources and further confirm that the TVM has an inherent ability to guide LVM founder migration even in the absence of local FGF signals. Clearly, however, the migration works best if the FGF signals are provided by the substrate of migration. This was demonstrated by our rescue experiments with bap3-GAL4 mediated expression of pyr and ths in the TVM. Expression of either ths or pyr in the TVM of FGF8 null mutants allowed largely normal migration of the LVM founders (Fig. 9K, L, compare with J). Compared to the wild type or analogous UAS/GAL expression in wild type backgrounds, migration was somewhat retarded and, particularly for UAS-pyr, the migrating cells were arranged in a less orderly fashion (Fig. 9K, L; compare with G, H, I). The morphogenesis of the longitudinal visceral muscles proceeded relatively normally when ths was used for the rescue. With pyr there was also a significant degree of rescue of LVM formation, although less complete than with ths, possibly because of too much signaling activity, which caused the mild defects observed at earlier stages (data not shown; see Fig. 9H). Similar rescue data as with bap3-GAL4 were obtained by using the 48Y-GAL4 driver, which predominantly expresses in the endoderm and from stage 13 also weakly in the LVM itself (data not shown; see Fig. 6A, B, F).
Taken together, our mis-expression and rescue experiments demonstrate that the FGF ligands Pyr and Ths are capable of determining the path of LVM founder cell migration and of ensuring the survival of these cells at ectopic positions. We propose that these activities, revealed upon their ectopic expression, also reflect functions exerted by Pyr and Ths in the normal situation, when they are secreted from tissues along the proper path of LVM founder cell migration (see discussion).
Discussion
We have demonstrated herein that pyr and ths, which encode FGF8-like signals, play important, largely redundant, and dosage-sensitive roles in the migration of longitudinal visceral muscle founder (LVM) cells in Drosophila. The major role of the encoded FGFs is to guarantee the survival of the migrating cells. Therefore, without these signals very few of the migrating cells survive beyond mid-migration. Independently of this function, these FGFs also serve critical roles in the proper guidance of the migrating cells. These roles are evident from the imprecise migration behaviors of death-blocked cells that do not receive these FGF signals and, particularly, from their efficient mis-routing toward ectopic FGF signals. The guidance functions of Pyr and Ths must be integrated with those of other crucial guidance cues that help steer the migrating cells onto the bilateral bands of trunk visceral mesoderm and retain them on this substrate during their anterior migration.
Partial redundancy and dosage effects of Pyr and Ths
The significant degree of functional redundancy of pyr and ths during this process is obvious from comparisons of the phenotypes of homozygous pyr or ths mutants with those of double mutants or receptor mutants. Whereas single pyr or ths mutants display few dying LVM founder cells and a mild reduction in the density of longitudinal visceral muscles, particularly around anterior portions of the midgut, pyr ths double mutants or htl mutants display almost complete cell death of the LVM founders by mid-migration with no cells reaching the anterior portions of the midgut. As a result, hardly any longitudinal visceral muscles are formed. The phenotypes of single pyr or ths mutations in trans to an FGF8-null deficiency are very similar to each other and are markedly stronger than the homozygous single mutant phenotypes, showing that the reduction from two copies to one copy of functional FGF8-like genes leads to a critical drop of signaling inputs into the migrating LVM cells. The effects of reducing functional copies from four to two, as in homozygous pyr or ths mutants and in embryos heterozygous for a deficiency uncovering both FGF genes, are also quite similar.
A study published during the submission of this report, which overall comes to similar conclusions on the roles of FGFs in LVM migration as we do, differs from our proposed functional equivalence of Pyr and Ths in proposing vital synergistic interactions between the two ligands (Kadam et al., 2012). This view rests on ectopic expression data, in which co-expression of Pyr and Ths causes stalling of migration, whereas analogous expression of each individual ligand does not. However, in our experiments with different drivers (e.g., twi-GAL4 driving either ligand and pnr-GAL4 driving Pyr) we observe stalling effects also upon expression of individual ligands. Thus, we believe that these effects largely depend on the overall signal levels, which in turn rely on the particular drivers and FGF used (Pyr being more potent than Ths), rather than on qualitative differences caused by mutual synergies between the two ligands. Nevertheless, it is possible that combined Pyr and Ths synergize to provide higher signaling activities as compared to each FGF alone under the same conditions. The effects of forced expression of constitutively-active Htl receptors in LVM founders in otherwise wild type embryos likewise show that over-activation of the pathway disturbs normal migration, presumably because it disrupts the normal balance of the finely tuned signals coming from the cells along the normal path of migration.
Surprisingly, our pyr EMS point mutant alleles produce a stronger LVM phenotype and full lethality as compared to the semilethal pyr18 allele, although the latter carries a deletion of pyr coding and downstream sequences (in both cases analyzed in trans to a FGF8-null deficiency). We speculate that, in pyr18, enhancer sequences upstream of pyr have been brought closer to the ths promoter and are able to up-regulate ths, which ameliorates some of the defects caused by the loss of pyr.
FGF signals and cell survival
The survival-promoting activity of Pyr/Ths/Htl signals for migrating LVM founder cells is intriguing, as such a trophic activity is not evident during the regulation of early mesoderm migration and mesodermal cell specification. Pyr does have an effect on glial cell numbers during eye development, but it has not been determined whether this is due to a survival function of Pyr or to a role in regulating cell proliferation (Franzdottir et al., 2009). Notably, our findings are very reminiscent of data that implicated FGF signaling in germ cell migration in the mouse (Takeuchi et al., 2005). These authors showed that the migrating primordial germ cells express the FGF receptors FGFR1-IIIc and FGFR2-IIIb and found that FGFR2-IIIb is essential for the normal survival of the migrating germ cells whereas FGFR1-IIIc signals affect their motility and the formation of cellular processes. Due to the large diversity of murine FGFs, the relevant endogenous ligands could not be defined. In Drosophila, the survival functions are mediated by a single receptor, Htl, which binds both ligands, Pyr and Ths. The situation is also akin to the role of a different receptor tyrosine kinase (RTK) in Drosophila, PDGF/VEGF receptor (Pvr), which is required both for the survival and normal migration of embryonic hemocytes (Brückner et al., 2004). Cell death of receptor-inactivated hemocytes can be rescued by the apoptosis inhibitor p35, as is the case for the LVM founders. By contrast, these mechanisms differ from the ones that protect migrating Drosophila germ cells from dying, which require the Wunen lipid phosphate phosphatases, have no known requirement for RTK signals, and act through non-apoptotic cell death pathways that are not rescuable with p35 (Hanyu-Nakamura et al., 2004; Yamada et al., 2008; Renault et al., 2010). Moreover, in the absence of zygotic Wunen functions we did not detect any effects on migration and survival of the LVM founder cells (Jasmin Raufer & M.F., unpublished data).
The absence of the trunk visceral mesoderm (TVM), which serves as the major substrate of LVM founder cell migration, causes virtually the same cell death effects for the migrating cells as the total absence of FGF8/Htl signals. Because the genetic ablation of the circular gut muscle founder cells that are responsible for pyr and ths expression within the TVM does not cause any increases in LVM cell death during their anterior migration, it is not clear whether the severe effects upon loss of the TVM are solely due to a reduction of FGF8/Htl signaling levels. A priori, Pyr from the posterior midgut endoderm could still be expected to be able to support their survival in posterior embryonic regions, even though the endoderm does not migrate anteriorly without the TVM (Azpiazu and Frasch, 1993). However, in the absence of the TVM the contact between the endoderm and mesoderm is lost (Tepass and Hartenstein, 1994), while the LVM founders still prefer to migrate along the mesoderm (see Fig. 1J, K). So in this situation, the Pyr signals from the endoderm may be too far away from the LVM founders to be able to support their survival. Perhaps, the anti-apoptotic function of these FGFs requires direct cell-cell contacts between the signaling and signal-receiving cells, or contacts with extracellular matrix that was “loaded” with FGFs by underlying signaling cells. This explanation would also be compatible with our findings that LVM founders readily survive when re-routed along the dorsal ectoderm upon ectopic expression of Pyr or Ths in this tissue. Such a short-range function would also explain why Pyr and Ths are equally active in promoting cell survival, even though they exhibit clear differences in their range of action as for example shown with the induction of Eve+ mesodermal cells (see Fig. 9; (Tulin and Stathopoulos, 2010b). Clearly, the pro-survival activity does not require any directional cues because it can be provided solely by expressing constitutively-active Htl receptors within the LVM founders.
FGF-mediated guidance of migratory cells
The guidance function of Pyr and Ths is obvious from the aberrant migration behavior of the LVM founder cells in three different situations. First, in the absence of both signals the migration of the cells from the bilateral clusters of the caudal visceral mesoderm onto the bilateral bands of TVM cells is less orderly in that a portion of the cells occupies medial instead of purely lateral areas and some of them migrate toward the interior instead of the anterior of the embryo. We propose that, normally, the combined activities of Pyr from the posterior midgut endoderm and of both ligands from the TVM contribute to the fidelity of the migration of LVM founders along bilateral paths onto the TVM cells on either side. These spatial cues can be overridden by providing strong uniform Pyr or Ths signals via twi-Gal4, and as a result the cells disperse throughout the posterior germ band where they get “stuck”, presumably because of excessive signaling. The findings that in the total absence of any Htl-mediated signals most LVM founder cells still manage to reach the TVM, and that in pyr or ths single mutants early migration is largely unaffected, imply that, apart from Pyr and Ths, there must be other potent signals guiding the caudal visceral mesoderm (CVM) cells onto the TVM, which remain to be identified.
The second, most persuasive situation showing guidance activities of Pyr and Ths toward LVM cells is their re-routing when Pyr or Ths are expressed ectopically in the dorsal ectoderm. In this situation, high levels of these ectopic signals are able to compete very successfully both with the properly positioned endogenous FGF8 signals (in the experiments done in wild type backgrounds), and with the proposed second types of TVM-specific signals. Initially, the dorsal ectodermal cells releasing these ectopic signals are relatively close to the lateral margins of the bilateral CVM cell clusters (albeit with a mesodermal layer in between), so the ectopic signals do not have to act long-range to attract migrating CVM cells toward them. Probably for this reason, Ths, which appears to have a shorter range of action, is able to re-rout the CVM cells almost as efficiently as Pyr. Interestingly, the LVM founders migrate and spread out toward the anterior along the ectopic route almost as efficiently as they normally do on their native route. In principle, this could be due to a general anterior attractant that is present both in the TVM and the dorsal germ band. However, we think it is more likely a result of the tendency of the migrating cells to spread out along fields of FGF8-expressing cells, for example as a result of a balance between mutual repulsion and their attraction (or adhesion) to FGF8-expressing substrates. This interpretation would also explain the decreases in spreading observed upon forced expression on Pyr in the dorsal ectoderm (via pnr-Gal4), TVM (via bap-Gal4), and whole mesoderm (via twi-Gal4), where this balance may be shifted by excessive levels of signal toward adhesive forces to the substrate as compared to the normal situation in the TVM. (Kadam et al., 2012) reported similar re-routings, in their case along the ventral midline when using a sim-GAL4 driver. In contrast to our ectopic expression data with pnr-GAL4, sim-GAL4 was not able to misdirect migration in the presence of endogenous Pyr and Ths, which may be due to lower signaling levels, e.g. because of a weaker driver or a larger distance of the migrating cells from the signaling source. Hence, sim-GAL4-driven FGFs are insufficient to compete with the endogenous signals whereas pnr-GAL4-driven FGFs (in combination with the FGFs released endogenously from the dorsal ectoderm) are potent enough to do so.
The third indication for attractive activities of Pyr and Ths toward LVM founder cells comes from the aberrant migratory behavior of the cells when they are kept alive by p35 in the absence of any FGF8/Htl signals. In this situation, we see cells veering off from their normal migration substrate dorsally and ventrally. Normally, these cells would enter cell death after losing contact with the TVM, scatter, and become fragmented. However, if they are not allowed to die, most of the cells migrating off-track appear to be able to return temporarily and continue on the TVM before they detach again. This looser and more dynamic interaction, as compared to wild type, would explain their retarded anterior migration in this situation as well as the observation that only a portion of the cells are found off-track at any given time. Importantly, the fact that the death-blocked cells do follow, by and large, normal migration paths along the TVM in the absence of FGF8/Htl signals implies that, also during this later phase of migration, other potent adhesive or signaling activities must exist that can keep the LVM founder cells on the TVM. Apart from yet undefined cell surface molecules expressed in TVM cells, extracellular matrix (ECM) components are good candidates for such activities. Recently, it was reported that nidogen-containing ECM is deposited along the TVM in a pattern that mirrors the tracks of LVM founder cell migration (Urbano et al., 2011; Wolfstetter and Holz, 2012). Furthermore, laminin was found to be essential for efficient LVM founder cell migration along the TVM, most likely serving as a ligand for αPS1βPS integrin. αPS1 integrin is expressed in the migrating LVM cells and, together with βPS, is also needed for normal migration (Urbano et al., 2011). The tissue source of these ECM components, and whether they are deposited prior to or during LVM founder cell migration, is currently not known. In addition to its direct interaction with the LVM founders, the ECM along the TVM may also serve to accumulate the FGF ligands Pyr and Ths and present them to the Htl receptors on the migrating LVM cells. FGFs commonly interact with heparan-like glycosaminoglycans in the ECM and heparan sulfate proteoglycans are necessary for FGF signaling in Drosophila (Lin et al., 1999; reviewed in Powers et al., 2000). This scenario would provide an intimate functional integration of the adhesive and signaling activities of integrins with the FGF signals. Altogether, we propose that several different adhesion and signaling mechanisms are employed in parallel, some being partially redundant and others being essential individually, which collectively ensure an efficient and faithful migration of the LVM founder cells along the proper paths.
Chemotactic versus other modes of guidance by FGFs
Do Pyr and Ths function as classical chemoattractants in guiding LVM cells towards increasing signal concentrations during their migrations? At first glance, particularly the results of re-routing of cells towards ectopic expression sources reported herein and in Kadam et al. (2012) would seem to argue for such a mechanism. A long-range activity of these FGFs as chemoattractants was indicated by the partial rescue of anterior migration upon their forced expression via fkh-GAL4 in the salivary glands in the complete absence of endogenous signals (Kadam et al., 2012). However, as the cells failed to reach the salivary glands in this experiment, an alternative explanation could be that additional, posterior sources of these FGFs, which are provided by the fkh-GAL4 driver during earlier stages (A.I., unpublished data), allowed LVM founder cells to survive and use the proposed adhesion molecules for migrating along the TVM. Furthermore, the relatively normal anterior migration of LVM founders when, a) cell death is rescued in htl mutants, b) activated Htl is expressed in the migrating cells of htl mutants (this report), and c) pyr or ths are expressed within the migrating cells of pyr, ths double mutant embryos (Kadam et al., 2012) seem to argue against a major role of Pyr and Ths as chemotactic signals in this process. In these examples, FGF signals are either not active or not presented to the cells in a spatially-controlled manner. An alternative explanation, which we favor as being the predominant one, could be that Pyr and Ths largely act in a short-range fashion that does not require any graded distribution. In this model, the major role of Pyr and Ths (apart from their pro-survival functions) would be to promote adhesiveness of the migrating cells to substrates that contain proper adhesion molecules. Increased local FGF signals, normally present along the native migration path (e.g., in the ECM), would bias the equilibrium of attachment/detachment of migrating cells towards attachment and allow them to continue migrating along the TVM. In artificial situations, cell-intrinsic Htl activation in LVM founder cells without spatially-controlled external signals would provide similar effects on selective adhesion and migration. Likewise, ectopic signals from sources nearby would shift the balance of attachment/detachment toward these alternative substrates, especially if they provide higher signal levels than the native source and contain adhesion molecules that can be recognized by the migrating cells. This scenario is also compatible with the observations that excessive signals cause stalled migration, presumably because the balance is then shifted too much towards adhesion. This interpretation does not preclude a contribution of a chemoattractive mode of action of Pyr and Ths over intermediate distances, but if this mode exists we think it is less critical. Overall, we propose that the mode of action of Pyr and Ths in guiding LVM founder cells is similar to their mode of action during early mesoderm migration, which is also thought to involve mainly the promotion of local adhesion to the migration substrate (in this case, the ectoderm) and to a lesser degree, relatively short-range chemotactic activities that assist in the orderly migration towards dorsal positions.
Supplementary Material
EMS-induced mutations in the Drosophila FGF8-like gene pyramus were isolated
Pyr and Ths are key for proper cell migration of longitudinal gut muscle founders
Both FGF signals act as guidance cues during this migration event
The Pyr and Ths signals are essential for survival of these cells during migration
The trophic and guidance activities of Pyr and Ths toward the migrating cells are largely redundant
Acknowledgments
We thank Patrick Lo for participating in the early phases of the EMS screen, Andreas Acs and Jasmin Matuszak for technical assistance, the Bloomington Stock Center, Angela Stathopoulos, and H.-Arno Müller for fly stocks, and the Developmental Studies Hybridoma Bank (University of Iowa, USA) for antibodies. This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG) to I.R. and M.F. and from the NIH (DK59406, HD30832) to M.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azpiazu N, Frasch M. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev. 1993;7:1325–1340. doi: 10.1101/gad.7.7b.1325. [DOI] [PubMed] [Google Scholar]
- Baker R, Schubiger G. Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development. 1996;122:617–626. doi: 10.1242/dev.122.2.617. [DOI] [PubMed] [Google Scholar]
- Beiman M, Shilo B, Volk T. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- Brückner K, Kockel L, Duchek P, Luque CM, Rorth P, Perrimon N. The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell. 2004;7:73–84. doi: 10.1016/j.devcel.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Cabernard C, Neumann M, Affolter M. Cellular and molecular mechanisms involved in branching morphogenesis of the Drosophila tracheal system. J Appl Physiol. 2004;97:2347–2353. doi: 10.1152/japplphysiol.00435.2004. [DOI] [PubMed] [Google Scholar]
- Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- Clark IB, Muha V, Klingseisen A, Leptin M, Müller HA. Fibroblast growth factor signalling controls successive cell behaviours during mesoderm layer formation in Drosophila. Development. 2011;138:2705–2715. doi: 10.1242/dev.060277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorey K, Amaya E. FGF signalling: diverse roles during early vertebrate embryogenesis. Development. 2010;137:3731–3742. doi: 10.1242/dev.037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D, Shaw S, Maqbool T, Pandya H, Vijayraghavan K. Drosophila Heartless acts with Heartbroken/Dof in muscle founder differentiation. PLoS Biol. 2005;3:e337. doi: 10.1371/journal.pbio.0030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Loren CE, Grabbe C, Varshney GK, Deleuil F, Hallberg B, Palmer RH. Jeb signals through the Alk receptor tyrosine kinase to drive visceral muscle fusion. Nature. 2003;425:512–516. doi: 10.1038/nature01950. [DOI] [PubMed] [Google Scholar]
- Franzdottir SR, Engelen D, Yuva-Aydemir Y, Schmidt I, Aho A, Klambt C. Switch in FGF signalling initiates glial differentiation in the Drosophila eye. Nature. 2009;460:758–761. doi: 10.1038/nature08167. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Frasch M, Hoey T, Rushlow C, Doyle H, Levine M. Characterization and localization of the even-skipped protein of Drosophila. EMBO J. 1987;6:749–759. doi: 10.1002/j.1460-2075.1987.tb04817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental-Ramain C, Vanolst L, Delaporte C, Ramain P. pannier encodes two structurally related isoforms that are differentially expressed during Drosophila development and display distinct functions during thorax patterning. Mech Dev. 2008;125:43–57. doi: 10.1016/j.mod.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Georgias C, Wasser M, Hinz U. A basic-helix-loop-helix protein expressed in precursors of Drosophila longitudinal visceral muscles. Mech Dev. 1997;69:115–124. doi: 10.1016/s0925-4773(97)00169-x. [DOI] [PubMed] [Google Scholar]
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol. 2003;19:623–647. doi: 10.1146/annurev.cellbio.19.031403.160043. [DOI] [PubMed] [Google Scholar]
- Ghabrial AS, Krasnow MA. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature. 2006;441:746–749. doi: 10.1038/nature04829. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, Skeath J, Doe C, Michelson A. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev. 1996;10:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- Greig S, Akam M. Homeotic genes autonomously specify one aspect of pattern in the Drosophila mesoderm. Nature. 1993;362:630–632. doi: 10.1038/362630a0. [DOI] [PubMed] [Google Scholar]
- Gryzik T, Müller HA. FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr Biol. 2004;14:659–667. doi: 10.1016/j.cub.2004.03.058. [DOI] [PubMed] [Google Scholar]
- Hanyu-Nakamura K, Kobayashi S, Nakamura A. Germ cell-autonomous Wunen2 is required for germline development in Drosophilaembryos. Development. 2004;131:4545–4553. doi: 10.1242/dev.01321. [DOI] [PubMed] [Google Scholar]
- Heitzler P, Haenlin M, Ramain P, Calleja M, Simpson P. A genetic analysis of pannier, a gene necessary for viability of dorsal tissues and bristle positioning in Drosophila. Genetics. 1996;143:1271–1286. doi: 10.1093/genetics/143.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaki M, Shinza-Kameda M, Ismat A, Frasch M, Nose A. Drosophila Tey represses transcription of the repulsive cue Toll and generates neuromuscular target specificity. Development. 2010;137:2139–2146. doi: 10.1242/dev.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismat A, Schaub C, Reim I, Kirchner K, Schultheis D, Frasch M. HLH54F is required for the specification and migration of longitudinal gut muscle founders from the caudal mesoderm of Drosophila. Development. 2010;137:3107–3117. doi: 10.1242/dev.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, Ghosh S, Stathopoulos A. Synchronous and symmetric migration of Drosophila caudal visceral mesoderm cells requires dual input by two FGF ligands. Development. 2012;139:699–708. doi: 10.1242/dev.068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam S, McMahon A, Tzou P, Stathopoulos A. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development. 2009;136:739–747. doi: 10.1242/dev.027904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klapper R, Stute C, Schomaker O, Strasser T, Janning W, Renkawitz-Pohl R, Holz A. The formation of syncytia within the visceral musculature of the Drosophila midgut is dependent on duf, sns and mbc. Mech Dev. 2002;110:85–96. doi: 10.1016/s0925-4773(01)00567-6. [DOI] [PubMed] [Google Scholar]
- Klingseisen A, Clark IB, Gryzik T, Müller HA. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development. 2009;136:2393–2402. doi: 10.1242/dev.035451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knirr S, Azpiazu N, Frasch M. The role of the NK-homeobox gene slouch (S59) in somatic muscle patterning. Development. 1999;126:4525–4535. doi: 10.1242/dev.126.20.4525. [DOI] [PubMed] [Google Scholar]
- Kusch T, Reuter R. Functions for Drosophila brachyenteron and forkhead in mesoderm specification and cell signalling. Development. 1999;126:3991–4003. doi: 10.1242/dev.126.18.3991. [DOI] [PubMed] [Google Scholar]
- Lee HH, Zaffran S, Frasch M. The Development of the Visceral Musculature of Drosophila. In: Sink H, editor. Drosophila Muscle Development. Landes; Georgetown, TX: 2005. Vol. http://www.eurekah.com/abstract.php?chapid=2028&bookid=162&catid=20. [Google Scholar]
- Lee HH, Frasch M. Wingless effects mesoderm patterning and ectoderm segmentation events via induction of its downstream target sloppy paired. Development. 2000;127:5497–5508. doi: 10.1242/dev.127.24.5497. [DOI] [PubMed] [Google Scholar]
- Lee HH, Norris A, Weiss JB, Frasch M. Jelly belly protein activates the receptor tyrosine kinase Alk to specify visceral muscle pioneers. Nature. 2003;425:507–512. doi: 10.1038/nature01916. [DOI] [PubMed] [Google Scholar]
- Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- Mandal L, Dumstrei K, Hartenstein V. Role of FGFR signaling in the morphogenesis of the Drosophila visceral musculature. Dev Dyn. 2004;231:342–248. doi: 10.1002/dvdy.20088. [DOI] [PubMed] [Google Scholar]
- Maqbool T, Soler C, Jagla T, Daczewska M, Lodha N, Palliyil S, VijayRaghavan K, Jagla K. Shaping leg muscles in Drosophila: role of ladybird, a conserved regulator of appendicular myogenesis. PLoS ONE. 2006;1:e122. doi: 10.1371/journal.pone.0000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Alvarez-Garcia I, Brown NH. Migration of the Drosophila primordial midgut cells requires coordination of diverse PS integrin functions. Development. 1999;126:5161–5169. doi: 10.1242/dev.126.22.5161. [DOI] [PubMed] [Google Scholar]
- McMahon A, Reeves GT, Supatto W, Stathopoulos A. Mesoderm migration in Drosophila is a multi-step process requiring FGF signaling and integrin activity. Development. 2010;137:2167–2175. doi: 10.1242/dev.051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A, Supatto W, Fraser SE, Stathopoulos A. Dynamic analyses of Drosophila gastrulation provide insights into collective cell migration. Science. 2008;322:1546–1550. doi: 10.1126/science.1167094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray MJ, Saint R. Photoactivatable GFP resolves Drosophila mesoderm migration behaviour. Development. 2007;134:3975–3983. doi: 10.1242/dev.005389. [DOI] [PubMed] [Google Scholar]
- Powers CJ, McLeskey SW, Wellstein A. Fibroblast growth factors, their receptors and signaling. Endocr Relat Cancer. 2000;7:165–197. doi: 10.1677/erc.0.0070165. [DOI] [PubMed] [Google Scholar]
- Reim I, Frasch M. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development. 2005;132:4911–4925. doi: 10.1242/dev.02077. [DOI] [PubMed] [Google Scholar]
- Renault AD, Kunwar PS, Lehmann R. Lipid phosphate phosphatase activity regulates dispersal and bilateral sorting of embryonic germ cells in Drosophila. Development. 2010;137:1815–1823. doi: 10.1242/dev.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell. 2002;2:677–683. doi: 10.1016/s1534-5807(02)00171-5. [DOI] [PubMed] [Google Scholar]
- San Martin B, Ruiz-Gomez M, Landgraf M, Bate M. A distinct set of founders and fusion-competent myoblasts make visceral muscles in the Drosophila embryo. Development. 2001;128:3331–3338. doi: 10.1242/dev.128.17.3331. [DOI] [PubMed] [Google Scholar]
- Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the Drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- Schaub C, Nagaso H, Jin H, Frasch M. Org-1, the Drosophila ortholog of Tbx1, is a direct activator of known identity genes during muscle specification. Development. 2012;139:1001–1012. doi: 10.1242/dev.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher S, Gryzik T, Tannebaum S, Müller HA. The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development. 2004;131:2631–2640. doi: 10.1242/dev.01149. [DOI] [PubMed] [Google Scholar]
- Shishido E, Ono N, Kojima T, Saigo K. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development. 1997;124:2119–2128. doi: 10.1242/dev.124.11.2119. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev. 2004;18:687–699. doi: 10.1101/gad.1166404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supatto W, McMahon A, Fraser SE, Stathopoulos A. Quantitative imaging of collective cell migration during Drosophila gastrulation: multiphoton microscopy and computational analysis. Nat Protoc. 2009;4:1397–1412. doi: 10.1038/nprot.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi Y, Molyneaux K, Runyan C, Schaible K, Wylie C. The roles of FGF signaling in germ cell migration in the mouse. Development. 2005;132:5399–5409. doi: 10.1242/dev.02080. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. Epithelium formation in the Drosophila midgut depends on the interaction of endoderm and mesoderm. Development. 1994;120:579–590. doi: 10.1242/dev.120.3.579. [DOI] [PubMed] [Google Scholar]
- Tulin S, Stathopoulos A. Extending the family table: Insights from beyond vertebrates into the regulation of embryonic development by FGFs. Birth Defects Res C Embryo Today. 2010a;90:214–227. doi: 10.1002/bdrc.20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulin S, Stathopoulos A. Analysis of Thisbe and Pyramus functional domains reveals evidence for cleavage of Drosophila FGFs. BMC Dev Biol. 2010b;10:83. doi: 10.1186/1471-213X-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbano JM, Dominguez-Gimenez P, Estrada B, Martin-Bermudo MD. PS integrins and laminins: key regulators of cell migration during Drosophila embryogenesis. PLoS One. 2011;6:e23893. doi: 10.1371/journal.pone.0023893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Impel A, Schumacher S, Draga M, Herz HM, Grosshans J, Müller HA. Regulation of the Rac GTPase pathway by the multifunctional Rho GEF Pebble is essential for mesoderm migration in the Drosophila gastrula. Development. 2009;136:813–822. doi: 10.1242/dev.026203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JB, Suyama KL, Lee HH, Scott MP. Jelly belly: a Drosophila LDL receptor repeat-containing signal required for mesoderm migration and differentiation. Cell. 2001;107:387–398. doi: 10.1016/s0092-8674(01)00540-2. [DOI] [PubMed] [Google Scholar]
- Wilson R, Leptin M. Fibroblast growth factor receptor-dependent morphogenesis of the Drosophila mesoderm. Philos Trans R Soc Lond B Biol Sci. 2000;355:891–895. doi: 10.1098/rstb.2000.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Vogelsang E, Leptin M. FGF signalling and the mechanism of mesoderm spreading in Drosophila embryos. Development. 2005;132:491–501. doi: 10.1242/dev.01603. [DOI] [PubMed] [Google Scholar]
- Wolfstetter G, Holz A. The role of LamininB2 (LanB2) during mesoderm differentiation in Drosophila. Cell Mol Life Sci. 2012;69:267–282. doi: 10.1007/s00018-011-0652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfstetter G, Shirinian M, Stute C, Grabbe C, Hummel T, Baumgartner S, Palmer RH, Holz A. Fusion of circular and longitudinal muscles in Drosophila is independent of the endoderm but further visceral muscle differentiation requires a close contact between mesoderm and endoderm. Mech Dev. 2009;126:721–736. doi: 10.1016/j.mod.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Davis KD, Coffman CR. Programmed cell death of primordial germ cells in Drosophila is regulated by p53 and the Outsiders monocarboxylate transporter. Development. 2008;135:207–216. doi: 10.1242/dev.010389. [DOI] [PubMed] [Google Scholar]
- Yin Z, Xu XL, Frasch M. Regulation of the Twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development. 1997;124:4871–4982. doi: 10.1242/dev.124.24.4971. [DOI] [PubMed] [Google Scholar]
- Zaffran S, Küchler A, Lee HH, Frasch M. biniou (FoxF), a central component in a regulatory network controlling visceral mesoderm development and midgut morphogenesis in Drosophila. Genes Dev. 2001;15:2900–2915. doi: 10.1101/gad.917101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.