Abstract
The reproductive ability of female tephritids can be limited and prevented by denying access to host plants and restricting the dietary precursors of vitellogenesis. The mechanisms underlying the delayed egg production in each case are initiated by different physiological processes that are anticipated to have dissimilar effects on lifespan and reproductive ability later in life. The egg laying abilities of laboratory reared females of the Mediterranean fruit fly (Ceratitis capitata Wiedmann) and melon fly (Bactrocera cucurbitae Coquillett) from Hawaii are delayed or suppressed by limiting access to host fruits and dietary protein. In each case, this is expected to prevent the loss of lifespan associated with reproduction until protein or hosts are introduced. Two trends are observed in each species: Firstly, access to protein at eclosion leads to a greater probability of survival and higher reproductive ability than if it is delayed, and secondly, that delayed host access reduces lifetime reproductive ability without improving life expectancy. When host access and protein availability are delayed, the rate of reproductive senescence is reduced in the medfly, whereas the rate of reproductive senescence is generally increased in the melon fly. Overall, delaying reproduction lowers the fitness of females by constraining their fecundity for the remainder of the lifespan without extending the lifespan.
Keywords: Cost of reproduction, delayed reproduction, dietary restriction, medfly, melon fly, resource allocation, senescence, Tephritidae
Introduction
Reproduction and longevity are mutually dependent in sexually reproducing organisms, with an increase in the energy expended for reproduction resulting in a subsequent decrease in longevity (Partridge & Andrews, 1985). The nutritional conditions in an environment influence the trade-off between current reproductive effort and lifespan because of competition for nutrients between somatic maintenance and gamete production (Kirkwood & Rose, 1991). Poor dietary conditions, in which all of the dietary requirements of an organism cannot be met, lead to reduced or arrested reproductive effort, thereby increasing the allocation of resources to somatic upkeep and survival until conditions improve and reproduction can resume (Weithoff, 2007; Carey et al., 2008). In addition to nutritional conditions, reproductive trade-offs in many species of insects can be influenced by the availability of suitable oviposition sites, such as host organisms (Carey et al., 1986; Rosenheim et al., 2000). Without the correct host or oviposition substrate, mature eggs must be retained in the ovaries or deposited into unsuitable environments that may lead to reduced offspring survival (Wang & Horng, 2004). Life history trade-offs are expected to prevent egg maturation during periods of host deprivation, and allow the organism to avoid the loss of life expectancy due to egg production, which is a cost of reproduction, and to survive until the appropriate hosts become available. However, any period of suspended reproduction may result in a loss of fecundity, because physiological changes related to ageing lead to a decline and loss of reproductive ability, known as reproductive senescence (Austad, 2010; Tatar, 2010). Consequently, the trade-off between survival and reproduction is also influenced by the timing and duration of poor reproductive conditions, in which maximizing survival during unfavorable reproductive conditions may decrease the overall fecundity of an organism because of senescence unless the reproductive ability can be extended to advanced ages (Bonduriansky et al., 2008).
In anautogenous insects, poor nutritional conditions may limit access to the dietary precursors, such as protein, that are required for ovarian development (Webster & Stoffolano, 1978; Aluja et al., 2001) and vitellogenesis (Raikhel & Dhadialla, 1992), directly preventing females from maturing eggs. Also, the absence or rarity of hosts limits the abundance of correct oviposition substrates, leading to matured eggs being retained in the ovaries until a critical mass is reached (Bell & Bohm, 1975). Oostatic hormone is then produced to prevent further egg production until the retained eggs can be oviposited (Kelly et al., 1986; Borovsky, 1988), or the eggs are deposited into environments unsuitable for offspring development and/or survival (Wang & Horng, 2004). Hypotheses concerning life history trade-offs can be tested by either delaying or preventing reproduction through these two mechanisms of limiting access to either dietary requirements or oviposition sites. The loss of lifespan due to egg production is expected to exceed the lifespan benefit provided by access to dietary protein, whereas a diet lacking protein will allow females to avoid the cost of reproduction (Carey et al., 1998). Also, when egg production is delayed the cost of reproduction is expected to be alleviated early in life, increasing the lifespan and allowing increased egg production at later ages (Carey et al., 1986, 1998) so long as the rate of reproductive senescence is reduced.
The influence of the two delay mechanisms on survival and reproduction are tested in two species of Tephritidae: the Mediterranean fruit fly (Ceratitis capitata Wiedmann), commonly known as the medfly, and the melon fly (Bactrocera cucurbitae Coquillett). Both dietary conditions and host availability influence egg production directly in tephritids, and the medfly and the melon fly are used because of their differing life history characteristics but similar ecologies. Laboratory reared medflies are relatively short lived (average longevity of ~40 days), with a short preoviposition period (~5 days) and an oviposition period that continues until around age 35 days (Vargas & Carey, 1989), whereas laboratory reared melon flies are longer lived (average longevity of ~110 days), with a longer preoviposition period (~7 days) and oviposition period (>90 days) (Vargas et al., 1984; Carey et al., 1988). Overall, the melon fly lays fewer eggs over a longer lifespan than the medfly (Carey et al., 1988; Vargas et al., 1997). Both species are phytophagous and have relatively broad host ranges. However, the medfly is more of a generalist, utilizing more than 300 hosts across a wide range of plant families (Liquido et al., 1991). The melon fly has a host range of over 80 different plant species in a variety of families, but is more specialized than the medfly because of its preference for host species in the family Cucurbitaceae (Dhillon et al., 2005). Specific studies using the medfly as a model testing life history trade-offs focus on delaying and preventing reproduction through dietary restriction (Müller et al., 1997; Carey et al., 1998, 2005; Davies et al., 2005), irradiation (Chapman et al., 1998), and host deprivation (Carey et al. 1986). The general findings suggest that egg laying reduces life expectancy (Carey et al., 1986; Chapman et al., 1998) and that preventing egg maturation through dietary restriction extends the lifespan of medflies in some cases (Carey et al., 1998, 2005), but not in others (Muller et al., 1997; Davies et al., 2005). Adult female melon flies have not been experimentally subjected to delayed host or protein access in studies testing life history trade-offs, and neither species has been subjected to both mechanisms of delayed reproduction in a single study. Any similar species-specific responses to the delay mechanisms will strengthen the conclusions regarding the underlying physiological processes, whereas differences will demonstrate that life history traits can constrain the expression of trade-offs between survival and reproduction.
Materials and Methods
Laboratory strain female medflies and melon flies were reared at the USDA ARS Pacific Basin Agricultural Research Center in Hilo, Hawaii following procedures described by Vargas (1989). Both strains originated from Oahu, Hawaii with the medfly strain estimated to be 414 generations old and the melon fly to be 370 generations old at the time of the experiments. Wild flies from the island of Hawaii have been periodically introduced into the laboratory colonies of both species to maintain genetic diversity. Upon eclosion, adult female flies were housed in 1 L group cages containing an average of 16 conspecific females under laboratory conditions (22 ± 3 °C and 60–80% R.H., under an LD 12 : 12 h photocycle). Five group cages were assigned to each control and treatment, resulting in a total of 45 group cages for each species. Since male insemination is not necessary for egg production in tephritids (Chapman et al., 1998; Davies et al., 2005), virgin females were used to avoid a potential mortality risk that is associated with copulation in the medfly and other female insects (Yanagi & Miyatake, 2003; Blanckenhorn et al., 2002; Davies et al., 2005; Kuijper et al., 2006). Egg production was monitored daily for the first 60 days of the lifespan, and daily survival was measured for 90 days. Unfortunately, the egg laying rates could not be monitored for the entire lifespan of the melon fly and survival could not be measured past age 90 days due to time and logistical constraints.
Host effects on lifespan and reproduction
The effect of egg production on lifespan was tested by comparing the life expectancies of females with different levels of host availability. Females were provided ad libitum access to a “full” diet comprised of a 3:1 mixture of sugar and yeast hydrolysate. For the appropriate treatments, an oviposition site was provided consisting of a 4.5 cm wide cylindrical plug cut from field collected papaya, Carica papaya, placed in a vial with the skin of the fruit exposed to stimulate oviposition. The plug of papaya would remain in the cage for 24 h, after which it would be removed and replaced with a new plug. Papaya was used since it stimulates oviposition under laboratory conditions for both species of tephritid (Vargas & Chang, 1991). Colour-break papaya was collected from cultivated trees and ripened in the laboratory to minimize the potential for field contamination by wild tephritid eggs (Liquido et al., 1989). The cost of egg laying was determined by comparing the life expectancy of females that were provided with the full diet, but no oviposition sites (n = 75 medfly; n = 83 melon fly), to that of females provided full diet and access to new oviposition sites daily until age 60 days (n = 75 medfly; n = 70 melon fly). Two treatments tested the effect of delayed host access on lifespan and reproductive ability by denying access to the papaya until either age 7 days (n = 82 medfly; n = 76 melon fly) or 14 days (n = 79 medfly; n = 73 melon fly). Following the deprivation period, host access was provided daily until age 60 days in both delay treatments. The eggs deposited into each day’s plug of papaya were counted. Any eggs that were deposited onto the cages were also counted, removed daily and included in estimates of gross fecundity. The egg laying ability of females in each treatment was estimated as the average number of eggs laid per female in each cage, and was recorded daily from eclosion until age 60 days. Gross fecundity was estimated as the average number of eggs laid per female during the entire experimental period.
Dietary effects on lifespan
For this experiment the effect of delaying full diet on the life expectancy and survival of females was tested in the absence of host access. The females in the three treatments were provided a sugar only ad libitum solid diet at eclosion. In the first treatment full diet was delayed for 7 days (n = 80 medfly; n = 84 melon fly), and in the second treatment full diet was delayed for 14 days (n = 83 medfly; n = 81 melon fly). After each deprivation period the females were provided ad libitum full diet until death. In the third treatment the females were only provided sugar diet for their entire lifespans (n = 74 medfly; n = 83 melon fly).
Dietary effects on egg laying
A third experiment was aimed at assessing the interaction of full diet deprivation and host availability. Females in the two treatments were provided the sugar only diet at eclosion with no host access. Females in the first treatment had host access and full diet delayed until age 7 days (n = 85 medfly; n = 73 melon fly), while the females in the second treatment experienced a 14 day delay to both full diet and host access (n = 89 medfly; n = 77 melon fly). The selection of 7 and 14 day deprivation periods to host fruit and full diet was based on the preoviposition periods of both species (Vargas et al., 1997). No treatment involved constant access to host combined with delayed access to a full diet, since host feeding may have provided the nutrition for egg production (Hendrichs & Hendrichs, 1990).
Statistical analyses
Life table analysis was performed on all the lifespan data to calculate life expectancy and age specific probability of survival. Statistical analysis was performed with SAS® version 9.3 (www.SAS.com). For the lifespan analysis, an accelerated failure time (AFT) model with a log-normal distribution was fitted to the survival data of each species to estimate the effect of each treatment on the average lifespan of females relative to the control (full diet from eclosion with no host access). There were a total of 9 treatment groups (including controls) with 5 group cages per treatment, and an average of 16 females in each cage (varied slightly among cages). Each female fly was used as a sample, resulting in total sample size 722 for medfly and 703 for melon fly. The samples within the same cages were not necessarily independent, so the models PROC MIXED and PROC NLMIXED were used to add random effect to account for the dependency in each cage. Since the design of the two factors (diet and host) was not balanced, the regression analysis was performed with one dummy variable for each treatment. Lifespan was log-transformed and the likelihood function was adjusted for right-censored observations because some of the melon flies survived beyond age 90 days. F-tests were conducted to test the effects of diet and host, while t-tests were used to compare each individual treatment to the control and the other treatments.
The effect of age and delayed full diet and host access on the trajectory of egg laying rates was tested with piece-wise linear models. In the reproduction analysis, only 5 of treatment/control groups were compared (Figs. 3), with 5 group cages per treatment. For each cage there was a repeated measurement of the daily number eggs laid per female for 60 days. Therefore, the total sample size for each species is 1500. The repeated measurements over days were not independent (longitudinal data), so a random effect was added to account for the within cage dependence. The overall effect of each treatment variable on egg laying ability and the interaction of each with age were determined through F-tests, and two tailed t-tests determined the effects of the treatments on the age-specific egg laying compared to the reproductive control (full diet and host access from eclosion).
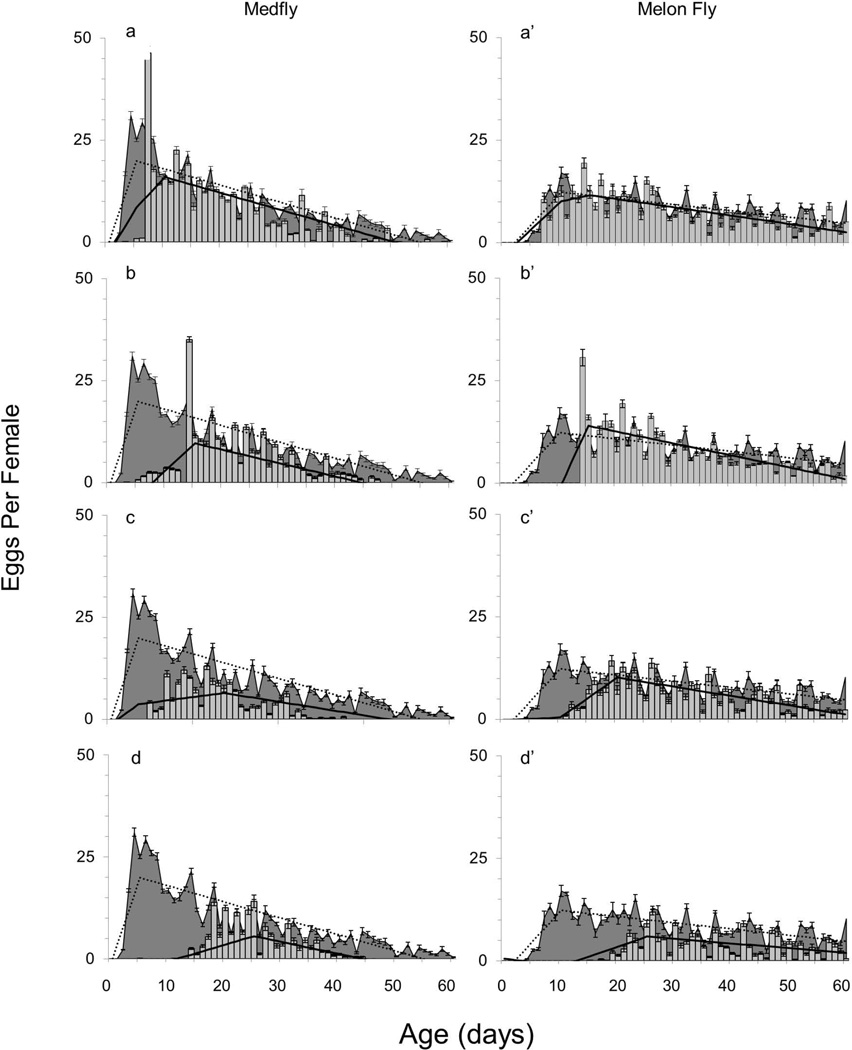
Fig. 3.
Average age-specific egg production for laboratory reared female medfly and melon fly from Hilo, Hawaii across deprivation treatments (grey bars) compared to the control (solid-filled background). Solid dark lines represent the estimated slopes from the piece-wise linear model describing the oviposition rates for the treatment, while the dashed line represents the slopes from the piece-wise linear model for the control. Graphs labelled “a” and “a’” represent the 7 day delay of host access when full diet was provided, while “b” and “b’” represent the 14 day delay of host access when full diet was provided in the medfly and melon fly respectively. Graphs labelled “c” and “c’” represent the treatments that experienced the 7 day delay of full diet and host access, while “d” and “d’” are the 14 day delay to full diet and host access in the medfly and the melon fly, respectively.
Results
Host effects on lifespan
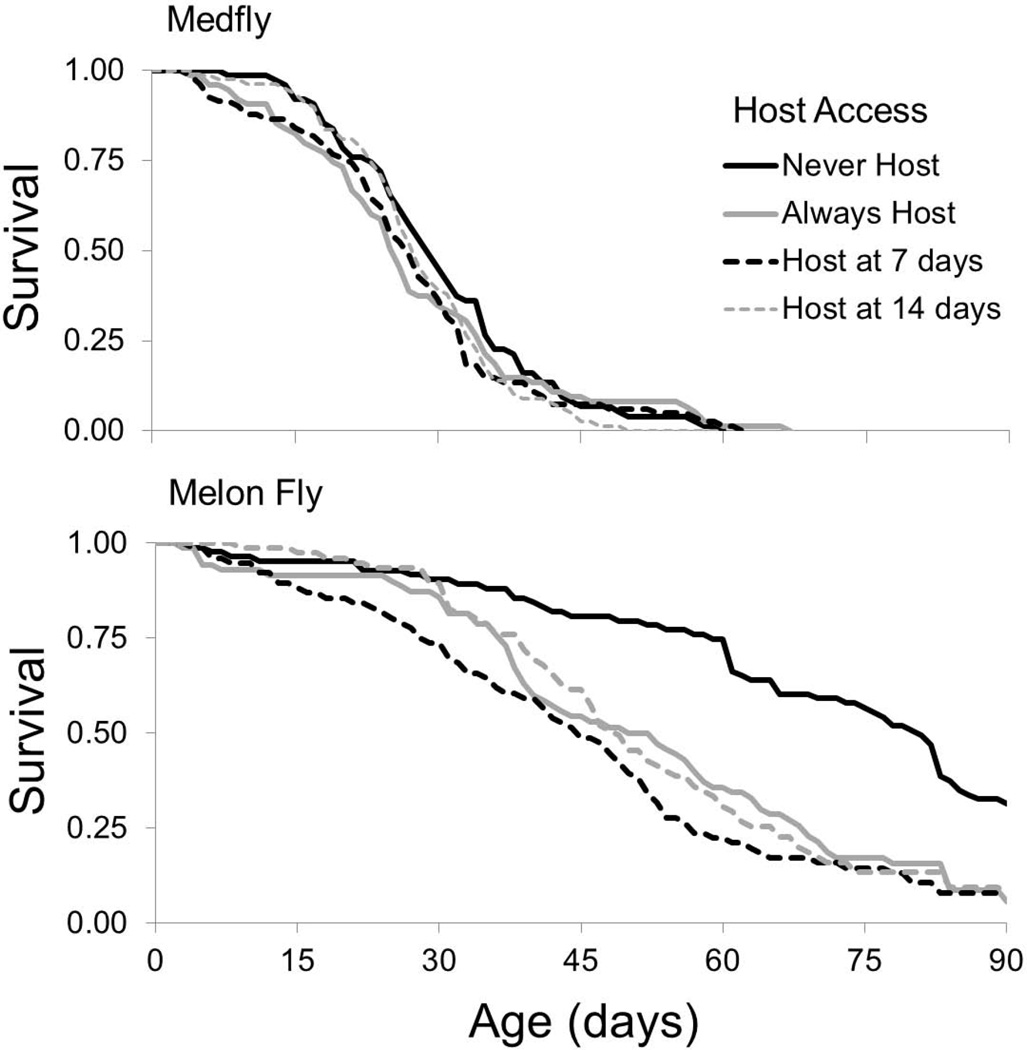
The average life expectancies of both species are presented in Table 1. The total treatment effect of host access on the life expectancy of the medfly was not statistically significant (F3, 677 = 1.19, P = 0.314), with the control and host access treatments showing similar age specific survival rates (Fig. 1). Conversely, host access significantly reduced life expectancy in the melon fly (F3,658 = 5.34, P = 0.0012), with individuals in the control having an improved probability of survival at all ages compared to those provided host access (Fig. 1). The life expectancy of the melon fly was also reduced when host access was delayed (F2, 658 = 7.32, P = 0.0008), but the duration of the delay did not influence life expectancy (F1, 658 = 1.73, P = 0.19).
Table 1.
Life expectancy (e0) in days, and standard error (± SE), of laboratory reared female medfly and melon fly from Hilo, Hawaii when host access begins at age 0, 7 and 14 days, with full diet provided, compared to females that never receive access to hosts (control). Different letters denote life expectancies that are significantly different across treatments within each species.
| Host Access at | Medfly e0 | Melon fly e0 |
|---|---|---|
| 0 | 24.18 ±1.13 a | 44.36 ±1.17 a |
| 7 | 23.98 ±1.13 a | 39.65 ±1.17 a |
| 14 | 26.59 ±1.13 a | 49.85 ±1.17 a |
| Control | 29.47 ±1.09 a | 76.18 ±1.12 b |
Fig. 1.
Effect of host access (never or always) and timing (7 and 14 day delay) on the proportion of laboratory reared female medfly and melon fly from Hilo, Hawaii surviving at each age class.
Dietary effects on lifespan
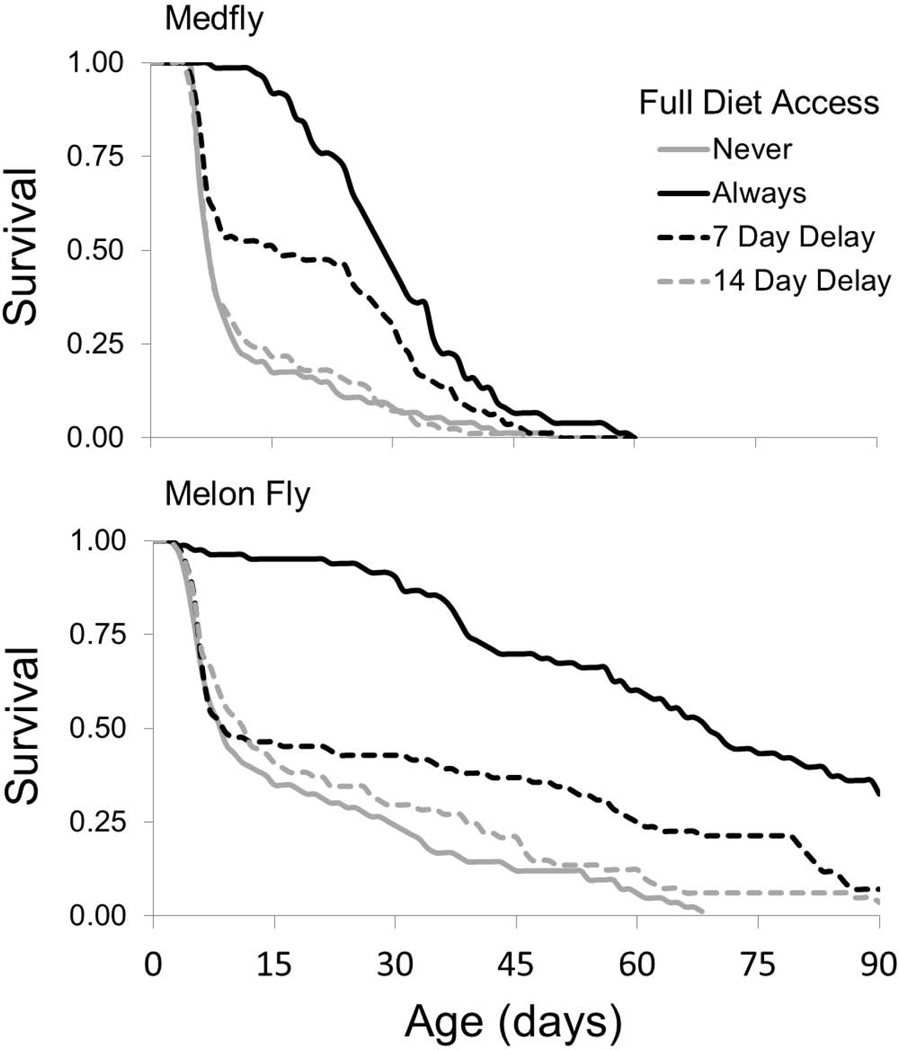
Life expectancies for female medfly and melon fly fed only the sugar diet were significantly reduced compared to females with constant access to full diet (t = −8.94, P < 0.0001; and t = −10.76, P < 0.0001, respectively) (Table 2, Fig. 2). The 7 day delay of full diet resulted in the medfly receiving sugar diet for 23.5% of their expected lifespan compared to only 9.2% of the lifespan in the melon fly, while the 14 day delay resulted in the medfly having access to sugar only diet for 47% of the lifespan compared to only 18.4% in the melon fly. Delayed access to full diet reduced the life expectancy of the medfly and melon fly (F2, 677 = 39.66, P < 0.0001; and F2, 658 = 52.61, P < 0.0001, respectively). The life expectancy of the medfly was dependent on the duration of the full diet deprivation period (F1,677 = 12.85, P = 0.0004), so that the 7 day delay decreased life expectancy by 48.4% compared to the control (t = −5.29, P < 0.0001), and the 14 day delay reduced life expectancy by 66.8% (t = −8.86, P < 0.0001). Conversely, the loss of life expectancy due to delayed full diet in the melon fly was not affected by the duration of the delay (F1, 658= 1.65, P = 0.20). The probability of survival for both species improved following the introduction of full diet at age 7 day relative to the females provided only sugar diet, while there was no improvement in age specific survival following the 14 day delay (Fig. 2). The reduction in life expectancy caused by delaying both full diet and host access was not different from delaying only full diet in the absence of host access in both the medfly and the melon fly (F2,677 = 1.69, P = 0.185; and F2,658 = 1.55, P = 0.21, respectively) (Table 2).
Table 2.
Effect on the life expectancy (days ± SE) of delaying full diet for 7 and 14 days of laboratory reared female medfly and melon fly from Hilo, Hawaii when hosts are never provided (Host Absent) or delayed for 7 or 14 days (Host Present), compared to females that have constant access to full diet (0 day delay duration) while hosts are absent or present (also presented in Table 1 as the Control and Host Access at 0 days, respectively). Different letters denote life expectancies that are significantly different across treatments within each species.*
| Full Diet Provided at |
Medfly |
Melon fly |
||
|---|---|---|---|---|
| Host Absent | Host Present | Host Absent | Host Present | |
| 0 days | 29.47 ±1.09 a | 24.18 ±1.13 a | 76.18 ±1.14 a | 44.36 ±1.17 b |
| 7 days | 15.21 ±1.13 b | 12.14 ±1.13 b | 18.54 ±1.16 c | 17.57 ±1.17 c |
| 14 days | 9.78 ±1.13 c | 9.76 ±1.13 c | 14.96 ±1.16 c | 20.10 ±1.16 c |
The effect of feeding on sugar only diet for the entire lifespan for the medfly and melon fly resulted in a life expectancy of 9.51 ±1.13 days and 12.11 ±1.16 days respectively.
Fig. 2.
Effect of the timing of protein provision on the proportion of laboratory reared female medfly and melon fly from Hilo, Hawaii surviving at each age. “Never” refers to females that were fed only sugar diet for the entire lifespan. The sugar diet was also provided during the delay periods before full diet was introduced. The survival curve for the treatment “Always” is the same as the treatment “Never Host” in Fig. 1, and is included here for comparison.
Reproduction
The gross fecundity of female medfly and melon fly in the reproductive control (host access and full diet provided daily from eclosion) are presented in Table 3. Oviposition rates were affected by age in both the medfly and the melon fly (F2, 1486 = 228.04, P < 0.0001; and F2, 1486 = 48.34, P < 0.0001, respectively) (Fig. 3), and the age specific reproductive ability was described by a two piece linear function in both species. The number of eggs laid per day by medfly females increased by an estimated 4.13 eggs per day (t = 11.95, P < 0.0001) until the change point at age 5 d, then decreased with a slope of −0.40 eggs per day until age 60 d (t = −12.61, P < 0.0001) (Fig. 3). The number of eggs laid by the melon fly increased by an estimated 1.51 eggs per day (t = 9.75, P < 0.0001) until the change point at age 10 d, then decreased with a slope of −0.15 eggs per day until age 60 d (t = −9.77, P < 0.0001) (Fig. 3). Oviposition onto the surface of the cage was recorded only in female medfly, especially those that never had access to hosts, but the gross fecundity of these females was lower than that of the other treatments (Table 3).
Table 3.
Average gross fecundity (total eggs per female) and the standard error (±SE) of laboratory reared female medfly and melon fly from Hilo, Hawaii measured from age 0 to 60 days, for females in the reproductive control (No Delay), when hosts and full diet are provided for the entire experimental period, and for the 7 and 14 day delays to only host access, while full diet is always provided (Only Host), and while both host access and full diet are delayed (Diet and Host). Different letters denote that the average gross fecundity is significantly different across treatments within each species.*
| Delay Treatment | Period | Medfly | Melon fly |
|---|---|---|---|
| No Delay (Control) | - | 534.2 ±41.8 a | 465.1 ±33.6 a |
| Only Host | 7 d | 375.6 ±51.5 b | 404.4 ±36.6 b |
| 14 d | 254.6 ±30.2 c | 371.8 ±31.9 c | |
| Diet and Host | 7 d | 160.3 ±32.8 d | 281.3 ±35.8 d |
| 14 d | 151.7±27.3 e | 170.0 ±26.5 e |
Medfly females that never had access to host, but were provided full diet, dumped eggs on cage with gross fecundity rates for 0–60 days of 136.2 (± 21.2) eggs.
Delaying both host access and full diet reduced the gross fecundity of females more than delaying only host access in both the medfly and the melon fly (F8,1486 = 26.31, P < 0.0001; and F8,1486 = 29.58, P < 0.0001, respectively) (Table 3). Additionally, the gross fecundity of both species was reduced more by the 14 day delay period than the 7 day period if only the host was delayed (medfly: F4, 1486= 2.81, P = 0.024; melon fly: F4, 1486= 12.24, P < 0.0001) and if both the host and full diet were delayed (medfly: F4, 1486 = 24.37, P < 0.0001; melon fly: F4, 1486 = 11.41, P < 0.0001) (Table 3).
The change pattern of age specific oviposition rates were significantly different among the different delay types in the medfly (F12, 1486 = 37.89, P < 0.0001) and in the melon fly (F12, 1486 = 22.96, P < 0.0001) (Table 3, Fig. 3). When host access was delayed for 7 days, the shape of the age specific oviposition rates were modelled by a three-piece linear function, with an additional change point at age 10 days that was found to be significant for the medfly (t = 5.86, P < 0.0001). Following the new change point, oviposition rates declined with a slope of −0.40 eggs per day until age 60 d (Fig. 3a). By contrast, there was no significant change in the egg laying rates in the female melon fly when host access was delayed 7 days (Fig. 3a).
When host access was delayed for 14 days the oviposition rates were modelled as a three-piece linear function, with change points at age 5 d and 15 d for the medfly. After age 15 d the oviposition rate of female medfly was estimated to decrease by a slope of −0.33 eggs per day until age 60 d (t = −10.67, P < 0.0001) (Fig. 3b). When host access was delayed for 14 days the oviposition rates of the melon fly were modelled as a three-piece linear function with change points at age 10 d and age 15 d. After the newly defined change point the oviposition rate decreased with an estimated slope of −0.28 eggs per day (t = −10.83, P < 0.0001) (Fig. 3b’).
When both full diet and host access were delayed for 7 days the oviposition rates of the female medfly were modelled as three-piece linear function with the change points at age 5 d and age 15 d. Following the new change point, the oviposition rate declined with an estimated slope of −0.22 eggs per day until age 60 d (t = −3.93, P < 0.0001) (Fig. 3c). When both full diet and host access were delayed for 7 days the oviposition rates of melon fly females were modelled by a three-piece linear function with change points at age 10 d and age 20 d. Following age 20 d the oviposition rate declined with a slope of −0.23 eggs per day (t = −7.72, P < 0.0001) (Fig. 3c’).
When full diet and host access were delayed for 14 d the oviposition rates of female medfly were modelled as a three-piece linear function with change points at age 5 d and 15 d. After age 15 days, the oviposition rate decreased by a slope of −0.29 eggs per day (t =−8.38, P < 0.0001) (Fig. 3d). When full diet and host access were delayed for 14 days the oviposition rates of the female melon fly were modelled by a three-piece linear function with change points at age 10 d and age 25 d. After the newly defined change point the oviposition rate decrease by a slope of −0.11 eggs per day (t = −5.34, P < 0.0001) (Fig. 3d’).
Discussion
The life expectancy of the female melon fly is reduced by host access, a result consistent with the cost of egg production in other insects (Partridge et al., 1987; Yanagi & Miyatake, 2003), but delaying host access does not alleviate the loss of life expectancy. Conversely, and in contrast to earlier results for the medfly (Carey et al., 1986; Chapman et al., 1998), the lifespan of the medfly is not influenced by oviposition. Egg “dumping,” in which a female deposits eggs into an unsuitable substrate for larval hatch and development, partially explains the unobserved loss of lifespan due to host access, since females without an appropriate host still produced and deposit eggs (Prokopy et al., 1993; Wang & Horng, 2004). However, the gross fecundity of females that “dumped” eggs in the absence of hosts is considerably less than when females oviposited into hosts. Consequently, egg “dumping” cannot entirely explain the similar life expectancies of medfly females with and without host access. The energy required to retain matured eggs may also contribute to the reduced life expectancy of females that are not allowed to oviposit. This potential energy cost of egg retention can account for the discrepancy between the present findings and those of the previous investigations. Egg retention was avoided by Chapman et al. (1998) due to egg production being prohibited by sterilizing the ovaries of the medflies, and Carey et al. (1986) could not detect any effect of egg retention since every treatment involved some level of host access.
Sugar diet was expected to prevent the costs of egg production. However, the life expectancy of both species is substantially reduced by the sugar diet regardless of host access. Therefore, the detriment of sugar diet on life expectancy outweighs any lifespan benefit that may have resulted from preventing egg production. Despite experiencing poor dietary conditions for a greater proportion of their expected lifespan, the medfly shows more tolerance to the sugar diet than the melon fly. Specifically, the loss of lifespan resulting from sugar diet at eclosion is lessened for the medfly females when full diet is provided at age 7 days, while there is no improvement in lifespan for melon flies following the introduction of full diet. However, if full diet is delayed for 14 days it no longer prevents the loss of life expectancy associated with the sugar diet in the medfly.
The gross reproductive ability of both species is reduced by delaying just host access and by delaying access to both full diet and hosts. Delaying host fruit access shifts the age of peak oviposition rates, as estimated by the change point, to the approximate age of host introduction. A spike in egg laying coincides with the introduction of the host, demonstrating that eggs are matured and retained before the host is provided. However, the spike and shifted peak in egg laying ability do not allow the females to recover the missed reproductive ability that results from host deprivation. The reduction in lifetime reproduction that results from delayed full diet is due to two primary causes, 1) the physiological inability to produce eggs during the deprivation period and the delay of vitellogenesis until full diet is provided (Raikhel and Dhadialla 1992) or delayed ovarian maturation (Webster and Stoffolano 1978) and 2) the shortened lifespan. Also, the sugar diet may have reduced the number of oocytes in the ovaries of the females, as recorded in two other species of tephritid (Aluja et al., 2001). Medfly females are able to lay a few eggs on the day that the full diet and the hosts were introduced, suggesting they are able to use larval nutrient reserves (Rivero et al., 2001) to develop ovaries and retain a few eggs until a host fruit is available. Conversely, egg laying in the melon fly does not begin until several days after the introduction of the full diet.
The rate in which oviposition ability declines with age for the medfly is lessened following each full diet delay period, suggesting the rate of reproductive senescence is being slowed in response to the missed reproductive opportunity. However, despite this slow rate of reproductive senescence the medfly is still unable to recover the lost reproductive ability caused by the delay period. The melon fly exhibits an increased rate of reproductive senescence following all delay treatments except when both the full diet and host fruit were delayed for 14 days. However, this slow rate of reproductive senescence is likely due to oviposition rates being so reduced that age cannot lead to a further reduction.
Both species demonstrate a physiological requirement for a dietary protein to maximize fecundity and survival, and the protein must be acquired shortly after eclosion to avoid a mortality surge early in life caused by sugar diet (Müller et al., 1997). The life history characteristics of the medfly suggest that they are better adapted to delayed reproduction due to dietary restriction than the melon fly, as evident by the slowed rate of reproductive senescence following delayed access to full diet and their ability to mature some eggs from larval energy reserves when there is no protein available. However, the medfly does not appear to have adaptive life history characteristics to host deprivation, as suggested by the potential loss of life expectancy due to egg retention. The medfly has a broader host range than the melon fly, so in nature the medfly is not as likely to experience a long period of host scarcity since it can easily switch to other available hosts (Harris et al., 1993; Vargas et al., 1983). The melon fly, by contrast, has a narrower host range that may lead to periods that hosts may be unavailable. Therefore, the extended lifespan and reproductive period of the melon fly along with its ability to avoid the cost of egg production when hosts are unavailable suggests it is better adapted to periods of host deprivation than the medfly. Overall, these results demonstrate that delaying reproduction may lower the fitness of female by constraining the gross reproductive output for the remainder of the lifespan without substantially improving the life expectancy.
Acknowledgements
We thank S. Souder, R. Ijima, Y. Nakane, and A. Morice for logistical support and assistance in conducting experiments and P. Lower and F. Zalom for comments on earlier drafts and editorial assistance. This research was funded through the NIH/NIA PO1 program grant P01 AG022500-01 and P01 AG08761-10.
References
- Aluja M, Díaz-Fleischer F, Papaj DR, et al. Effects of age, diet, female density, and the host resource on egg load in Anastrepha ludens and Anastrepha obliqua (Diptera: Tephritidae) Journal of Insect Physiology. 2001;47:975–988. doi: 10.1016/s0022-1910(01)00072-5. [DOI] [PubMed] [Google Scholar]
- Austad SN. Animal models of reproductive aging: what can they tell us? Annals of the New York Academy of Sciences. 2010;1204:123–126. doi: 10.1111/j.1749-6632.2010.05609.x. [DOI] [PubMed] [Google Scholar]
- Bell WJ, Bohm MK. Oosorption in insects. Biological Reviews. 1975;50:373–396. doi: 10.1111/j.1469-185x.1975.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Blanckenhorn WU, Hosken DJ, Martin OY, et al. The costs of copulating in the dung fly Sepsis cynipsea. Behavioral Ecology. 2002;13:353–358. [Google Scholar]
- Bonduriansky R, Maklakov A, Zajitschek F, et al. Sexual selection, sexual conflict and the evolution of ageing and life span. Functional Ecology. 2008;22:443–453. [Google Scholar]
- Borovsky D. Oostatic hormone inhibits biosynthesis of midgut proteolytic enzymes and egg development in mosquitoes. Archives of Insect Biochemistry and Physiology. 1988;7:187–210. [Google Scholar]
- Carey JR, Harshman LG, Liedo P, et al. Longevity-fertility trade-offs in the tephritid fruit fly, Anastrepha ludens, across dietary-restriction gradients. Aging Cell. 2008;7:470–477. doi: 10.1111/j.1474-9726.2008.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Krainacker DA, Vargas RI. Life-history response of female Mediterranean fruit flies, Ceratitis capitata, to periods of host deprivation. Entomologia Experimentalis et Applicata. 1986;42:159–167. [Google Scholar]
- Carey JR, Liedo P, Müller H-G, et al. Stochastic dietary restriction using a Markov-chain feeding protocol elicits complex, life history response in medflies. Aging Cell. 2005;4:31–39. doi: 10.1111/j.1474-9728.2004.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Liedo P, Müller HG, et al. Dual modes of aging in Mediterranean fruit fly females. Science. 1998;281:996–998. doi: 10.1126/science.281.5379.996. [DOI] [PubMed] [Google Scholar]
- Carey JR, Yang P, Foote D. Demographic analysis of insect reproductive levels, patterns and heterogeneity: case study of laboratory strains of three Hawaiian tephritids. Entomologia Experimentalis et Applicata. 1988;46:85–91. [Google Scholar]
- Chapman T, Miyatake T, Smith HK, et al. Interactions of mating, egg production and death rates in females of the Mediterranean fruit fly, Ceratitis capitata. Proceedings of the Royal Society of London Series B-Biological Sciences. 1998;265:1879–1894. doi: 10.1098/rspb.1998.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies S, Kattel R, Bhatia B, et al. The effect of diet, sex and mating status on longevity in Mediterranean fruit flies (Ceratitis capitata), Diptera : Tephritidae. Experimental Gerontology. 2005;40:784–792. doi: 10.1016/j.exger.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Dhillon MK, Singh R, Naresh JS, et al. The melon fruit fly, Bactrocera cucurbitae: a review of its biology and management. Journal of Insect Science. 2005;5 doi: 10.1093/jis/5.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EJ, Vargas RI, Gilmore JE. Seasonality in occurrence and distribution of Mediterranean fruit fly (Diptera: Tephritidae) in upland and lowland areas on Kauai, Hawaii. Environmental Entomology. 1993;22:404–410. [Google Scholar]
- Hendrichs J, Hendrichs MA. Mediterranean fruit fly (Diptera: Tephritidae) in nature: location and diel pattern of feeding and other activities on fruiting and nonfruiting hosts and nonhosts. Annals of the Entomological Society of America. 1990;83:632–641. [Google Scholar]
- Kelly TJ, Masler EP, Schwartz MB, et al. Inhibitory effects of oostatic hormone on ovarian maturation and ecdysteroid production in Diptera. Insect Biochemistry. 1986;16:273–279. [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1991;332:15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kuijper B, Stewart AD, Rice WR. The cost of mating rises nonlinearly with copulation frequency in a laboratory population of Drosophila melanogaster. Journal of Evolutionary Biology. 2006;19:1795–1802. doi: 10.1111/j.1420-9101.2006.01186.x. [DOI] [PubMed] [Google Scholar]
- Liquido NJ, Cunningham RT, Couey HM. Infestation rates of papaya by fruit-flies (Diptera, Tephritidae) in relation to the degree of fruit ripeness. Journal of Economic Entomology. 1989;82:213–219. [Google Scholar]
- Liquido NJ, Shinoda LA, Cunningham RT. Host plants of the Mediterranean fruit fly (Diptera: Tephritidae): an annotated world review. Miscellaneous Publications of Entomological Society of America. 1991;77:1–52. [Google Scholar]
- Müller HG, Wang JL, Capra WB, et al. Early mortality surge in protein-deprived females causes reversal of sex differential of life expectancy in Mediterranean fruit flies. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:2762–2765. doi: 10.1073/pnas.94.6.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L, Andrews R. The effect of reproductive activity on the longevity of male Drosophila melanogaster is not caused by an acceleration of aging. Journal of Insect Physiology. 1985;31:393–395. [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. Journal of Insect Physiology. 1987;33:745–749. [Google Scholar]
- Prokopy RJ, Averill AL, Green TA, et al. Does food shortage cause fruit flies (Diptera: Tephritidae) to dump eggs? Annals of the Entomological Society of America. 1993;86:362–365. [Google Scholar]
- Raikhel AS, Dhadialla TS. Accumulation of yolk proteins in insect oocytes. Annual Review of Entomology. 1992;37:217–251. doi: 10.1146/annurev.en.37.010192.001245. [DOI] [PubMed] [Google Scholar]
- Rivero A, Giron D, Casas J. Lifetime allocation of juvenile and adult nutritional resources to egg production in a holometabolous insect. Proceedings of the Royal Society of London. Series B: Biological Sciences. 2001;268:1231–1237. doi: 10.1098/rspb.2001.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheim JA, Heimpel GE, Mangel M. Egg maturation, egg resorption and the costliness of transient egg limitation in insects. Proceedings of the Royal Society of London Series B-Biological Sciences. 2000;267:1565–1573. doi: 10.1098/rspb.2000.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M. Reproductive aging in invertebrate genetic models. Annals of the New York Academy of Sciences. 2010;1204:149–155. doi: 10.1111/j.1749-6632.2010.05522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas RI. Mass Production of Tephritid Fruit Flies. In: Robinson AS, Hooper G, editors. World Crop Pests, Volume 3B, Fruit Flies, Their Biology, Natural Enemies and Control. Amsterdam: Elsevier Science Publishers B.V.; 1989. pp. 141–151. [Google Scholar]
- Vargas RI, Carey JR. Comparison of demographic parameters for wild and laboratory-adapted Mediterranean fruit-fly (Diptera, Tephritidae) Annals of the Entomological Society of America. 1989;82:55–59. [Google Scholar]
- Vargas RI, Chang HB. Evaluation of oviposition stimulants for mass-production of melon fly, Oriental fruit-fly, and Mediterranean fruit-fly (Diptera, Tephritidae) Journal of Economic Entomology. 1991;84:1695–1698. [Google Scholar]
- Vargas RI, Harris EJ, Nishida T. Distribution and seasonal occurrence of Ceratitis capitata (Wiedemann)(Diptera: Tephritidae) on the Island of Kauai in the Hawaiian Islands. Environmental Entomology. 1983;12:303–310. [Google Scholar]
- Vargas RI, Miyashita DH, Nishida T. Life history and demographic parameters of three laboratory reared tephritids (Diptera: Tephritidae) Annals of the Entomological Society of America. 1984;77:651–656. [Google Scholar]
- Vargas RI, Walsh WA, Kanehisa D, et al. Demography of four Hawaiian fruit flies (Diptera: Tephritidae) reared at five constant temperatures. Annals of the Entomological Society of America. 1997;90:162–168. [Google Scholar]
- Wang MH, Horng SB. Egg dumping and life history strategy of Callosobruchus maculatus. Physiological Entomology. 2004;29:26–31. [Google Scholar]
- Webster RP, Stoffolano JG. The influence of diet on the maturation of the reproductive system of the apple maggot, Rhagoletis pomonella. Annals of the Entomological Society of America. 1978;71:844–849. [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on life span and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
- Yanagi SI, Miyatake T. Costs of mating and egg production in female Callosobruchus chinensis. Journal of Insect Physiology. 2003;49:823–827. doi: 10.1016/S0022-1910(03)00119-7. [DOI] [PubMed] [Google Scholar]