Abstract
This study sought to examine whole brain and regional gray matter (GM) phenotypes across the schizophrenia (SZ)–bipolar disorder psychosis dimension using voxel-based morphometry (VBM 8.0 with DARTEL segmentation/normalization) and semi-automated regional parcellation, FreeSurfer (FS 4.3.1/64 bit). 3T T1 MPRAGE images were acquired from 19 volunteers with schizophrenia (SZ), 16 with schizoaffective disorder (SAD), 17 with psychotic bipolar I disorder (BD-P) and 10 healthy controls (HC). Contrasted with HC, SZ showed extensive cortical GM reductions, most pronounced in fronto-temporal regions; SAD had GM reductions overlapping with SZ, albeit less extensive; and BD-P demonstrated no GM differences from HC. Within the psychosis dimension, BD-P showed larger volumes in fronto-temporal and other cortical/subcortical regions compared with SZ, whereas SAD showed intermediate GM volumes. The two volumetric methodologies, VBM and FS, revealed highly overlapping results for cortical GM, but partially divergent results for subcortical volumes (basal ganglia, amygdala). Overall, these findings suggest that individuals across the psychosis dimension show both overlapping and unique GM phenotypes: decreased GM, predominantly in fronto-temporal regions, is characteristic of SZ but not of psychotic BD-P, whereas SAD display GM deficits overlapping with SZ, albeit less extensive.
Keywords: Psychosis, Schizophrenia, Bipolar disorder, Voxel-based morphometry, FreeSurfer
1. Introduction
More than a century after Emil Kraepelin subdivided insanity into dementia praecox and manic depressive psychosis, the categorization of psychotic illnesses remains controversial. Recent large-scale genetic studies, as well as studies of intermediate phenotypes of psychosis, challenge a dichotomous conceptualization of psychosis and suggest that the two major psychotic illnesses, schizophrenia (SZ) and bipolar I disorder, display both shared and unique symptom dimensions, neurophysiological markers, and genetic underpinnings (O’Donnell et al., 2004; Sanchez-Morla et al., 2008; Purcell et al., 2009; Smith et al., 2009). Traditional manual region-of-interest morphometry, automated whole brain voxel-based morphometry (VBM) (Ashburner and Friston, 2000; Ashburner and Friston, 2005), as well as semi-automated regional brain segmentation and parcellation via FreeSurfer (FS) (Fischl et al., 1999; Dale et al., 1999; Fischl et al., 2004; Desikan et al., 2006) have been applied to address neuroanatomical correlatives of categorical and dimensional psychopathology in these disorders.
In schizophrenia, enlarged lateral ventricles and robust gray matter (GM) reductions in numerous regions, including but not limited to fronto-temporal cortices, hippocampus, cingulate, insula, thalamus, and cerebellum, have been consistently reported (see Woodruff et al., 1995; Nelson et al., 1998; Wright et al., 2000; Konick and Friedman, 2001; Honea et al., 2005; Baiano et al., 2007; Meda et al., 2008; Arnone et al., 2008; Ellison-Wright et al., 2008; Keshavan et al., 2008; Ellison-Wright and Bullmore, 2010; Yu et al., 2010 for reviews and meta-analyses). In bipolar disorder, findings have varied widely, ranging from normal to both increased and decreased GM volume/density in fronto-temporal and cingulate cortex, amygdala, hippocampus, and thalamus (Altshuler et al., 1995; Videbech,1997; McDonald et al., 2004; Kempton et al., 2008; Yu et al. 2010; Hallahan et al., 2011). A few studies that specifically focused on psychotic bipolar disorder have also produced inconsistent results: while Strasser et al. (2005) found similar alterations in ventricular and hippocampal volumes in bipolar and schizophrenia psychoses, others reported distinct GM phenotypes with characteristically intact cortical GM in bipolar psychosis (Hirayasu et al., 1999; McDonald et al., 2005).
The volumetric studies that directly compared GM in individuals across the schizophrenia–bipolar disorder boundary are few; nevertheless, they have provided evidence for both overlapping and unique structural characteristics. Larger hippocampal (Kempton et al., 2008) and amygdala (Arnone et al., 2009) volumes have been reported in bipolar individuals compared to those with schizophrenia. Several VBM analyses have suggested that GM reductions are selectively associated with schizophrenia not bipolar disorder (Harvey et al., 1994; Zipursky et al., 1997; Pearlson et al., 1997; Altshuler et al., 2000; Hirayasu et al., 2001; McDonald et al., 2005; Farrow et al., 2005), whereas others found partially overlapping GM reductions in the two disorders, nevertheless more pronounced in schizophrenia (Friedman et al., 1999; McIntosh et al., 2004; Janssen et al., 2008; Ellison-Wright and Bullmore, 2010; Yu et al., 2010). Recent FS analyses have confirmed observations of shared (enlarged lateral ventricles, decreased bilateral hippocampi and left thalamus in both schizophrenia and bipolar disorder) and unique (widespread cortical thinning and enlarged right putamen in schizophrenia, and variable observations in cortical thickness in bipolar disorder) regional phenotypes (Lyoo et al., 2006; Rimol et al., 2010; Hartberg et al., 2011).
Considerable debate surrounds the conceptualization of schizoaffective disorder (SAD) as a distinct diagnostic entity. Some suggest that a dimensional approach to schizoaffective disorder that emphasizes the psychosis and mood symptoms phenotypes within this categorical diagnosis offers a more useful framework for the studies of underlying disease neurobiology (see Abrams et al., 2008, for review). In the field of imaging, individuals with schizoaffective disorder are routinely clustered with the schizophrenia samples (Cannon et al., 2002; Prasad et al., 2004; Buchanan et al., 2004). The few studies that have focused on schizoaffective disorder alone (all based on small samples) have found diminished cerebral volume (Getz et al., 2002), increased sulcal cerebro-spinal fluid volume (Cannon et al., 1998), reduced cortical GM with most deficits found in fronto-temporal regions (Cannon et al. 1998), smaller hippocampal volumes (van Erp et al., 2004; Radonic et al., 2011) and larger volumes of striatum and globus pallidus (Getz et al., 2002). Overall, these findings point at a considerable overlap in cortical and subcortical alterations in schizoaffective disorder and schizophrenia, although similar neuroanatomical characteristics in schizoaffective and bipolar patients have also been reported (Getz et al., 2002). In addition, Smith et al. (2011) have recently reported thalamic surface deformities in medial and lateral regions unique to schizoaffective disorder as compared to schizophrenia, although the overall volume of the thalamus in schizoaffective patients did not differ from that in controls.
Taken together, this literature suggests that individuals within the schizophrenia–bipolar disorder dimension may have both unique (e.g., GM volume reductions throughout fronto-temporal regions in schizophrenia and schizoaffective disorder in contrast to largely normal GM in bipolar disorder) and overlapping (e.g., decreased volume of hippocampi in all three psychoses) volumetric phenotypes, with less known specifically about schizoaffective disorder and the psychotic variant of bipolar disorder.
The question of whether the structural brain alterations that are consistently observed in psychotic individuals are related to primary disease pathophysiology or reflect disease-associated factors (e.g., effect of psychotropic agents, co-morbid substance use) remains debatable. Typical structural alterations have been observed in individuals at high risk for psychosis and the psychosis prodrom (Keshavan et al., 2005; Steen et al., 2006; Kuroki et al., 2006; Vita et al., 2006; Sun et al., 2009) in first break schizophrenia (Kasai et al., 2003; Ho et al., 2003; DeLisi et al., 2004; Whitworth et al., 2005; Steen et al., 2006; Vita et al., 2006; Schultz et al., 2010; Gutierrez-Galve et al., 2010), and in medication- naïve individuals with schizophrenia (Keshavan et al., 2005; Steen et al., 2006; Kuroki et al., 2006), suggesting that neither psychosis duration nor chronic treatment is entirely responsible for the typical GM changes.
However, evidence suggests that treatment with psychotropic medications undoubtedly has an effect on brain structure. Use of typical antipsychotics (AP) has been associated with increased GM density/volume of basal ganglia and decreased GM in fronto-temporal cortex (Keshavan et al., 1994; Gaser et al., 1999; Wilke et al., 2001; Kubicki et al., 2002; Lieberman et al., 2005; Molina et al., 2007; Crespo-Facorro et al., 2008; Smieskova et al., 2009; Ho et al., 2011). The data on atypical AP are less consistent, with some studies reporting frontal GM reductions, and caudate/putamen volume increases, although less severe, compared to those found with typical AP (Molina et al., 2007; Ho et al., 2011); whereas others find minimal to no effect of atypicals on cortical GM and basal ganglia (Lieberman et al., 2005; Scherk and Falkai,2006; Smieskova et al., 2009), or even a reversal of the basal ganglia enlargement after switching from typical to atypical AP (Scherk and Falkai, 2006; Smieskova et al., 2009) or discontinuation of AP (Boonstra et al., 2011). Decreased white matter, but not GM, volumes have been associated with AP use in bipolar disorder (Jones et al., 2009). Longer duration of AP treatment has been associated with more pronounced cortical and subcortical alterations (Ho et al., 2011), although some suggest that the volume changes accompanying typical AP use can be detected as early as after 12 weeks (Scherk and Falkai, 2006).
Furthermore, there is accumulating evidence that lithium and other mood stabilizers may increase amygdala volume (Usher et al., 2009) and GM density in diffuse cortical regions (Kempton et al., 2008; Langan and McDonald, 2009) with greatest effect seen in prefrontal, cingulate, and paralimbic cortices (Bearden et al., 2007; Moore et al., 2009). Since the majority of individuals with schizophrenia and bipolar psychoses are chronically treated with a mixture of psychotropic agents, disentangling ‘primary’ disease phenotypes from medication effects poses a significant difficulty and has been acknowledged in the recent reports (Navari and Dazzan, 2009; Ho et al., 2011).
This study sought to examine GM phenotypes across the psychosis dimension in individuals with schizophrenia (SZ), schizoaffective disorder (SAD), and psychotic bipolar I disorder (BD-P) to test for a common psychosis phenotype. We hypothesized that (1) contrasted with HC, SZ will show GM reductions in numerous cortical and subcortical regions with most pronounced changes in fronto-temporal cortices, whereas BD-P will have largely normal GM volumes; and SAD will show cortical and subcortical GM changes intermediate between those in SZ and BD-P; and (2) within the psychosis dimension, SZ, SAD, and BD-P will show step-wise changes in GM from overall smaller volumes in SZ to larger volumes in BD-P. To test these hypotheses, we concomitantly used two volumetric methodologies: VBM and FS. VBM is an automated, highly repeatable approach to morphometry, and is often used to provide whole brain GM characterization (Kennedy et al., 2009), while automated parcellation and measurement of anatomically defined regions, such as those available with FS, provide high anatomical validity. For this reason, in order to explicitly characterize GM phenotypes across the SZ/BD-P boundary, we applied two analytic approaches: VBM, in order to define whole brain GM alterations, and FS, in order to verify regional cortical and subcortical GM phenotypes.
2. Methods
2.1. Subjects
The study included individuals who met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994) criteria for schizophrenia (SZ, n=19), schizoaffective disorder (SAD, n=16) or bipolar disorder, type I, with lifetime history of psychotic symptoms (BD-P, n=17), and 10 HC. All volunteers were recruited concurrently through advertising, and by referrals from community mental health centers and from the UT Southwestern Medical Center out-patient psychiatric clinics. Individuals with a history of major neurological or decompensated medical illness, mental retardation, traumatic brain injury, substance abuse within the last month or substance dependence within the last 3 months were excluded. The study was approved by the institutional review board of the UT Southwestern Medical Center and was consistent with standards for the ethical conduct of human research. All volunteers provided written informed consent after the study procedures had been fully explained.
Demographic and clinical characteristics of the study sample are presented in Table 1. All volunteers were English-speaking, had normal IQ estimates, and were matched for age and education. The groups did not differ in any of the socio-demographic or psychiatric history characteristics, except for the duration of lifetime psychosis where SAD reported longer history of psychosis compared to either SZ (P=0.01) or BD-P (P=0.006). The psychosis volunteers included in this study were clinically stable medicated out-patients with active psychosis and/or mood symptoms that varied in severity from remission/euthymic state to mild symptoms. In addition to the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 1996) used to determine the psychosis diagnoses, the Brief Psychiatric Rating Scale (Overall and Gorham, 1962) as a measure of active symptomatic severity and the Global Assessment of Functioning (DSM-IV Axis V) ratings were collected for the psychosis groups. No between group differences were obtained either in active symptom severity or in general level of functioning. Most subjects in the psychosis groups reported a chronic medication use history, and were being treated with a variety of psychotropic agents while participating in this study; only two subjects were off psychotropic medications at the time of the imaging acquisition.
Table 1.
Socio-demographic and clinical characteristics of the study groups.
| SZ(n=19) | SAD(n=16) | BD-P(n=17) | HC(n=10) | Test statistic | P value | |
|---|---|---|---|---|---|---|
| Socio-demographic characteristics | ||||||
| Age, years; Mean (S.D.) | 39.89(10.66) | 45.12(10.14) | 38.24(7.28) | 43.9(9.86) | F(3, 55) = 2.22 | 0.1 |
| Gender/male; n (%) | 10(52.63) | 9(56.25) | 8(47.05) | 4(40) | χ2(3) = 0.76 | 0.86 |
| Left-handed; n (%) | 4(21.05) | 1(6.25) | 2(11.76) | 1(10) | χ2(3) = 1.77 | 0.62 |
| Education, years; Mean (S.D.) | 13.84(2.14) | 15.43(2.63) | 14.05(2.05) | 13.3(0.95) | F(3, 55) = 2.63 | 0.12 |
| Race; n (%) | χ2(3) = 2.69 | 0.44 | ||||
| Caucasian | 13(68.42 | 13(81.25) | 14(82.35) | 9(90.00) | ||
| African-American | 6(31.58) | 2(12.5) | 1(5.88) | 1(10.00) | ||
| Other | 0(0.00) | 1(6.25) | 2(11.76) | 0(0.00) | ||
| Clinical characteristics, Mean (S.D.) | ||||||
| Psychosis duration, years | 15.74(8.99) | 24.81(8.48) | 14.76(9.07) | - | F(2, 49) = 6.46 | 0.003a |
| Age of the first hospitalization, years | 27.53(7.37) | 26.86(11.18) | 27.73(6.25) | - | F(2, 39) = 0.04 | 0.96 |
| Total number of hospitalizations | 5.47(8.07) | 9.13(11.14) | 2.29(2.75) | - | χ2(2) = 3.53 | 0.17 |
| BPRS, total score | 46.63(11.88) | 48.06(8.50) | 44.82(8.89) | - | F (2, 49) = 0.44 | 0.64 |
| GAF | 56.44(14.19) | 53.13(10.83) | 57.07(11.30) | - | F(2, 47) = 0.43 | 0.65 |
| WASI IQ | 107.32 (11.70) | 107.00(16.25) | 108.76 (9.46) | 114.70(6.88) | F(3, 58) = 1.03 | 0.39 |
| Concomitant medications, n (%) | ||||||
| Off medications | 1(5.26) | 0(0.00) | 1(5.88) | 10(100) | ||
| Typical antipsychotics | 5(26.31) | 2(12.5) | 0(0.00) | 0(0.00) | ||
| Atypical antipsychotics | 12(63.16) | 13(81.25) | 9(52.94) | 0(0.00) | ||
| Antidepressants | 12(63.16) | 5(31.25) | 9(52.94) | 0(0.00) | ||
| Mood stabilizers | 1(5.26) | 5(31.25) | 13(76.47) | 0(0.00) | ||
| Other | 5(26.31) | 6(37.5) | 7(41.18) | 0(0.00) | ||
| Combined medications | 13(68.42) | 12(75.0) | 14(82.35) | 0(0.00) | ||
SZ—individuals with schizophrenia, SAD—individuals with schizoaffective disorder, BD-P—individuals with psychotic bipolar I disorder, HC—healthy controls, BPRS—Brief Psychiatric Rating Scale, GAF —Global Assessment of Functioning scale, WASI IQ—Wechsler Abbreviated Scale of Intelligence IQ, S.D.—standard deviation.
Psychosis duration, post hoc Tukey HSD: SAD vs. SZ, P=0.01; SAD vs. BD-P, P=0.006.
2.2. MRI acquisition, voxel-based morphometry and FreeSurfer procedures
Structural magnetic resonance imaging was performed on a 3 T Siemens Magnetom Trio scanner. T1-weighted MPRAGE sequences were acquired using the following parameters: TR 2300 ms/TE 2.95 ms/TI 900 ms/BW 240 Hz/9° flip/total time 9:23 min, yielding 160 sagittal slices with a slice thickness of 1.2 mm with 1×1×1.2 mm3 voxel resolution. All structural images were processed by a single individual (EII, VBM, or AF, FS) blind to the volunteers’ diagnoses.
VBM analysis was conducted in Statistical Parametric Mapping (SPM8) software on MATLAB with the following processing steps: reorientation, high-dimensional DARTEL segmentation/normalization (Ashburner, 2007), and smoothing. T1-weighted images were set to match the standard T1 template [Montreal Neurological Institute (MNI) space] for Anterior Commissure–Posterior Commissure alignment and were segmented into GM, white matter, and cerebro-spinal fluid compartments using non-linear DARTEL normalization which utilizes a high-dimensional warping process and increases registration accuracy between the individual images, resulting in improved localization and increased sensitivity in analysis. Furthermore, DARTEL allows precise correction for individual brain size (Ashburner, 2007). Segmented images were modulated or scaled by the amount of warping to maintain the total amount of GM volume. Modulated GM images were then smoothed with a 12-mm isotropic Gaussian kernel and were selected for further statistical analyses. All images are displayed in neurological convention. Reported brain regions were identified using the Group ICA for fMRI Gift toolbox, version 1.3i (Calhoun et al., 2001) and checked against the standardized anatomical brain atlas (Duvernoy, 1999).
FS analysis was conducted using FreeSurfer 4.3.1, 64-bit version (Massachusetts General Hospital, http://surfer.nmr.mgh.harvard.edu/). FS processing included motion correction of volumetric T1-weighted images, removal of nonbrain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated transformation, segmentation of the subcortical white matter and deep GM volumetric structures (Fischl et al., 2002, 2004), intensity normalization, tessellation of the GM/white matter boundary, automated topology correction (Fischl et al., 2001; Segonne et al., 2007), and surface deformation following intensity gradients to optimally place the GM/white matter and GM/cerebro-spinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999). Image outputs for each stage of FS analysis were visually inspected and edited by an experienced imaging analyst (AF).
2.3. Statistical analyses
A one-way analysis of variance (ANOVA) with a subsequent post hoc Tukey HSD test and Yates corrected chi-square test were used as appropriate for demographic and clinical variables. For the VBM analyses, the processed GM images were analyzed using SPM8 parametric mapping for group comparisons using ‘full factorial’ function: analysis of covariance (ANCOVA), adjusted for age (using F contrast), with subsequent pairwise comparisons (using t contrast). Absolute threshold masking was set at 0.1. To further reduce the occurrence of GM and white matter difference map, a standard GM template within VBM8 was first binarized at a suitable threshold (0.4) where the majority of GM voxel intensities were included within the cut-off, then registered to the t-map, and finally applied as a mask on the resulting t-map. The VBM outcomes for the psychosis groups vs. HC contrast are reported at P<0.05, false discovery rate (FDR) corrected threshold. In the resulting t-maps only clusters with 100 or more contiguous voxels are reported. Since no GM volume differences between the psychosis groups (SZ vs. SAD vs. BD-P) remained significant after FDR correction, uncorrected outcomes for this comparison are reported at P<0.005. This less stringent threshold balanced with rather high voxel cluster size of 100 provided the most informative illustration of between-group differences within the psychosis dimension. Corresponding peak coordinates for each region of volume change based on the Gift outputs (Calhoun et al., 2001) are presented in Talairach space.
Analysis of covariance (ANCOVA) was used for the FS outcomes (automatically derived cortical and subcortical volumes) with individual volumes as dependent variables, group as an independent variable, and age and intracranial volume as covariates, similar to (Bhojraj et al., 2009). Bonferroni correction for regional comparisons within each lobe/subcortical structure was applied, similar to the procedure of Bhojraj et al., (2009) (detailed list of the regions provided in Table 3); both corrected and uncorrected probability values are reported.
Table 3.
Regional cortical and subcortical volume differences between the psychosis groups and healthy controls, and across the psychosis dimension based on FreeSurfer analyses.
| Region of interest | Volume (mm3), Mean ± S.D. | ANCOVA | |||
|---|---|---|---|---|---|
| Psychosis groups vs. healthy controls contrast | |||||
| SZ | SAD | BD-P | HC | ||
| Frontal | SZ<HC | ||||
| Left inferior frontal gyrus (pars triangularis) | 3058 ± 649 | 3576 ± 710 | 3489 ± 864 | 3830 ± 779 | F (1,25)=9.74, P=0.005* |
| Left precentral gyrus | 12755 ± 1587 | 13439 ± 1799 | 13013 ± 1658 | 13598 ± 1779 | F (1,25) = 10.02, P=0.004n |
| Right precentral gyrus | 12730 ± 1697 | 13760 ± 1874 | 1334 ± 2099 | 13849 ± 1572 | F (1,25)=10.23, P=0.004* |
| Right superior frontal gyrus | 20304 ± 3226 | 21449 ± 3024 | 20846 ± 3259 | 21731 ± 2705 | F (1,25) = 9.42, P=0.005* |
| Right paracentral lobule | 3727 ± 599 | 4151 ± 853 | 3993 ± 603 | 4110 ± 455 | F (1,25) = 10.02, P=0.004n |
| Temporal | |||||
| Left middle temporal gyrus | 10783 ± 1902 | 11694 ± 1774 | 10751 ± 2248 | 11368 ± 846 | F (1,25)=5.85, P=0.023 |
| Left inferior temporal gyrus | 10088 ± 1697 | 11217 ± 1981 | 10713 ± 2549 | 10734 ± 1621 | F (1,25)=7.79, P=0.01 |
| Right middle temporal gyrus | 11384 ± 1666 | 12160 ± 1919 | 11952 ± 2545 | 12393 ± 1119 | F(1,25) = 7.83, P=0.01 |
| Parietal | |||||
| Left precuneus | 9112 ± 1556 | 10039 ± 1332 | 10181 ± 2100 | 9700 ± 1087 | F(1,25) = 6.23, P=0.02 |
| Left inferior parietal gyrus | 12895 ± 2356 | 13793 ± 1780 | 12911 ± 2311 | 14187 ± 2636 | F (1,25)=5.64, P=0.026 |
| Limbic (right hippocampus) | 3916 ± 489 | 3815 ± 428 | 3975 ± 644 | 4091 ± 439 | F (1,25)=4.63, P=0.041 |
| Right thalamus | 6765 ± 793 | 6672 ± 852 | 6854 ± 1208 | 6799 ± 714 | F (1,25)=6.36, P=0.02 |
| Right thalamus | 6765 ± 793 | 6672 ± 852 | 6854 ± 1208 | 6799 ± 714 | SZ > HC |
| Basal ganglia (left globus pallidus) | 1808 ± 244 | 1634 ± 271 | 1691 ± 249 | 1569 ± 245 | F (1,25) = 5.58, P=0.03 |
| Limbic | SAD < HC | ||||
| Left hippocampus | 4049 ± 496 | 3885 ± 439 | 4096 ± 610 | 4073 ± 324 | F (1,22)=4.91, P=0.037 |
| Right hippocampus | 3916 ± 489 | 3815 ± 428 | 3975 ± 644 | 4091 ± 439 | F (1,22)=6.63, P=0.017* |
| Basal ganglia (right caudate) | 3651 ± 618 | 3538 ± 399 | 3666 ± 532 | 3694 ± 410 | F(1,22) = 5.24, P=0.032 |
| Right thalamus | 6765 ± 793 | 6672 ± 852 | 6854 ± 1208 | 6799 ± 714 | F(1,22) = 5.33, P=0.031 |
| Psychosis dimension contrast | |||||
| SZ | SAD | BD-P | ||
|---|---|---|---|---|
| Frontal | SZ < BD-P | |||
| Left inferior frontal gyrus (pars triangularis) | 3058 ± 649 | 3576 ± 710 | 3489 ± 864 | F(l,32)=4.59, P=0.039 |
| Left inferior frontal gyrus (pars opercularis) | 4494 ± 869 | 4992 ± 722 | 5063 ± 1040 | F (1,32)=6.07, P=0.019 |
| Right superior frontal gyrus (paracentral lobule) | 3727 ± 599 | 4151 ± 853 | 3993 ± 603 | F (1,32)=4.02, P=0.05 |
| Temporal | ||||
| Left parahippocampal gyrus | 2381 ± 356 | 2604 ± 447 | 2635 ± 575 | F(1,32) = 7.07, P=0.012 |
| Cingulate gyrus | ||||
| Left isthmus/central cingulate | 2271 ± 391 | 2513 ± 460 | 2580 ± 636 | F(1,32) = 5.72, P=0.023 |
| Parietal | ||||
| Left superior parietal gyrus | 12878 ± 1805 | 13123 ± 1577 | 13766 ± 2503 | F (1,32)=4.63, P=0.039 |
| Left precuneus | 9112 ± 1556 | 10038 ± 1332 | 10180 ± 2100 | F(1,32) = 11.72, P=0.002* |
| Right precuneus | 9165 ± 1267 | 9566 ± 1195 | 9820 ± 1859 | F (1,32)=6.04, P=0.02 |
| Occipital | ||||
| Left lingual gyrus | 7478 ± 1175 | 7791 ± 1517 | 8330 ± 1928 | F(1,32) = 7.93, P=0.008* |
| Right lingual gyrus | 6794 ± 1222 | 6993 ± 1251 | 7434 ± 1549 | F(1,32) = 5.66, P=0.023 |
| Right pericalcarine gyrus | 2249 ± 554 | 2401 ± 491 | 2647 ± 561 | F(1,32) = 6.11, P=0.019 |
| Limbic (Right amygdala) | 1728 ± 379 | 1790 ± 279 | 1850 ± 220 | F (1,32)=4.25, P=0.047 |
| Left cerebellar cortex | 52590 ± 6119 | 51376 ± 5193 | 49602 ± 6947 | F (1,32)=5.28, P=0.028 |
| Temporal | SAD > SZ | |||
| Left inferior temporal gyrus | 10088 ± 1697 | 11217 ± 1981 | 10713 ± 2549 | F (1,31) = 9.93, P=0.004n |
| Cingulate gyrus | ||||
| Right rostral anterior cingulate | 1784 ± 427 | 2073 ± 319 | 1839 ± 509 | F(1,31) = 5.35, P=0.028 |
| Limbic | SAD < BD-P | |||
| Left hippocampus | 4049 ± 496 | 3885 ± 439 | 4096 ± 610 | F (1,29) = 9.64, P=0.004n |
| Right hippocampus | 3916 ± 489 | 3814 ± 428 | 3975 ± 644 | F(1,29) = 6.76, P=0.014* |
| Left amygdala | 1572 ± 265 | 1565 ± 155 | 1646 ± 322 | F (1,29) = 5.49, P=0.026 |
| Right thalamus | 6519 ± 669 | 6389 ± 745 | 6751 ± 1190 | F (1,29) = 6.2, P=0.002 |
| Left thalamus | 6765 ± 793 | 6672 ± 852 | 6854 ± 1208 | F(1,29) = 11.5, P=0.019 |
| Basal ganglia | SZ > BD-P | |||
| Left globus pallidus | 1808 ± 244 | 1634 ± 271 | 691 ± 249 | F(1,32) = 5.81, P=0.02* |
| Right globus pallidu | 1760 ± 261 | 1592 ± 317 | 1631 ± 241 | F(1,32) = 5.07, P=0.031 |
| SZ > SAD | ||||
| Left thalamus | 6765 ± 793 | 6389 ± 745 | 6854 ± 1208 | F(1,29) = 6.20, P=0.019 |
| Cingulate gyrus | SAD > BD-P | |||
| Left caudal anterior cingulate | 1957 ± 417 | 2139 ± 538 | 1811 ± 428 | F(1,29) = 5.89, P=0.022 |
SZ—individuals with schizophrenia, SAD—individuals with schizoaffective disorder, BD-P—individuals with psychotic bipolar I disorder, HC—healthy controls, S.D.—standard deviation.
ANCOVA (analyses of covariance) were adjusted for age and intracranial volume. Only statistically significant between group differences are reported. Regions that remained significant after Bonferroni correction marked with asterisk. Bonferroni correction within each lobe/subcortical regions were applied: (1) frontal lobe included 11 regions (superior frontal gyrus, rostral and caudal middle frontal gyrus, pars triangularis, pars opercularis, pars orbitalis, medial and lateral orbito-frontal gyrus, frontal pole, precentral gyrus, and paracentral lobule), corrected P=0.005; (2) temporal lobe included 8 regions (superior, medial, inferior, transverse, fusiform, parahippocampal gyri, entorhinal cortex, and temporal pole), corrected P = 0.006; (3) cingulate gyrus included 4 regions (rostral and caudal anterior cingulate, isthmus and posterior cingulate), corrected P=0.01; parietal lobe included 5 regions (superior, inferior, precentral and supramarginal gyri, and precuneus), corrected P=0.01; occipital lobe included 4 regions (cuneus, lingual, pericalcarine, and lateral occipital gyri), corrected P=0.01; limbic system included 3 regions (hippocampus, amygdala, and thalamus), corrected P=0.02; basal ganglia included 3 regions (caudate, putamen, globus pallidus), corrected P=0.02.
Given a well-known effect of age on brain structure (see Reuter-Lorenz and Park, 2010 for review), both VBM and FS analyses were adjusted for age. In addition, FS analysis was adjusted for total intracranial volume, which was derived from FS parcellation and subsequently included as a covariate in ANCOVA. VBM analysis was adjusted for individual brain volume. Since VBM/DARTEL normalization incorporates an automatic adjustment for individual brain volume, total intracranial volume was not added as a covariate at the level of VBM group analyses. Volumetric measures, age and intracranial volume were normally distributed [Shapiro–Wilk’s test, P>0.1]. The groups did not differ either in age [group mean ± standard deviation, years: SZ, 39.89±10.66; SAD, 45.12±10.14; BD-P, 38.24±7.28; HC, 43.9±9.86] or intracranial volume [group mean ± standard deviation, mm3: SZ, 1,470,876±161,799; SAD, 1,515,886±170380; BD-P, 1,449,657±229610; HC, 1,431,969±98,123].
In order to examine effect of medication on GM phenotypes, individuals across the psychosis groups independent of their diagnoses who were on (n=39) and off (n=11) AP, and on (n=16) and off (n=36) mood stabilizers while active in this study were contrasted with respect to whole brain (derived from VBM) and regional (derived from FS) GM volumes. Multiple regression analysis in SPM8 was used to examine correlations between GM volumes and lifetime duration of psychosis.
3. Results
3.1. Gray matter phenotypes in psychosis vs. healthy controls
3.1.1. Whole brain gray matter volume differences in psychosis vs. healthy controls (voxel-based morphometry)
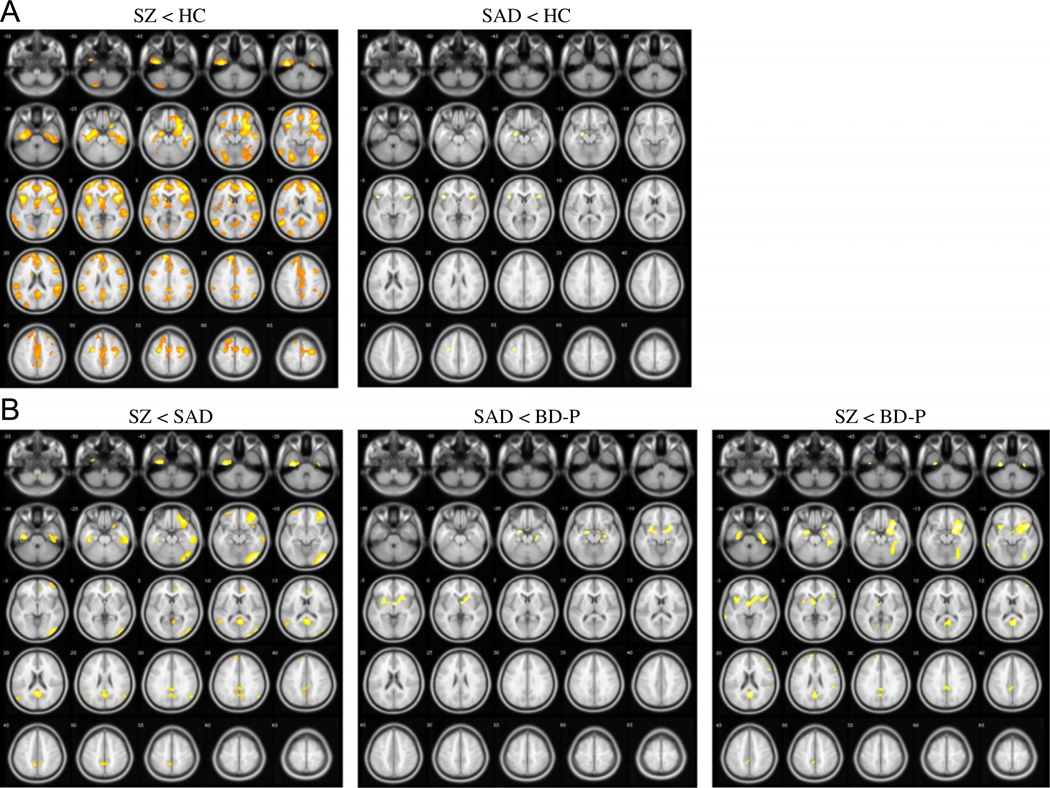
Whole brain GM differences between the psychosis groups and HC (P<0.05, FDR corrected) are shown in Table 2 and Fig. 1(A). An overall ANCOVA showed a main effect of group [F(3, 57)=7,75, P<0.05, FDR corrected]. Subsequent pairwise comparisons between the psychosis groups and HC revealed overlapping GM reductions in SZ and SAD in frontal, temporal and insular cortices. SZ showed many additional regions of decreased GM throughout neocortex, most prominent in fronto-temporal, cingulate, and parietal cortices, as well as in basal ganglia, thalamus, and cerebellum. In contrast, BD-P showed no differences in cortical and subcortical GM volumes compared to HC. No increases in GM volume were found in any of the psychosis groups, compared to HC.
Table 2.
Regions of gray matter volume difference between the psychosis groups and healthy controls, and across the psychosis dimension based on voxel-based morphometry analyses.
| Group comparison | Brain region | k (voxels), right/left | Pseudo-t (x, y, z), S right/left |
|---|---|---|---|
| Psychosis groups vs. healthy controls contrast [P=0.05, FDR corrected, k=100] | |||
| SZ < HCM | Frontal | ||
| Inferior frontal gyrus | 5422/1600 | 5.7 (43, 16, −5)/4.9 (−40, 16, −3) | |
| Middle frontal gyrus | 4978/2637 | 4.5 (45, 44, 7)/4.4.(−27, −2, 46) | |
| Superior frontal gyrus | 1689/3348 | 3.6 (30, 57, −5)/4.1 (−28, 54, 0) | |
| Medial frontal gyrus | 2193/2548 | 3.4(4,43, 14)/3.5(−6, 59, 19) | |
| Precental gyrus | 2015/237 | 4.4 (43, 19, 7)/3.6 (−30, −8, 52) | |
| Paracentral lobule | 296/207 | 3.3 (0, −15, 44)/3.1 (−3, −18, 45) | |
| Temporal | |||
| Superior temporal gyrus | 3644/1067 | 4.3 (40, 10, −12)/3.8 (−49, −56, 17) | |
| Middle temporal gyrus | 1689/1926 | 3.9 (53, −12, −16)/3.6 (−64, −45, 4) | |
| Parahippocampal gyrus | 1452/1363 | 3.8(27,5, −17)/4.3(−36, −17, −24) | |
| Fusiform gyrus | 1067/415 | 3.5 (46, −33, −19)/4.1 (−39, −14, −23) | |
| Inferior temporal gyrus | 504/770 | 4.2(56, −15, −18)/4.4(−37, −20, −28) | |
| Uncus | 237/593 | 4.1 (25,8, −19)/4.7(−36, −16, −28) | |
| Insula | 1926/1511 | 5.3 (40, 16, −2)143 (−37, 16, −1) | |
| Cingulate gyrus | |||
| Cingulate gyrus (BA 23, 24, 31, 32) | 2281/1244 | 3.3 (10, −33, 35)/3.1 (−3, −42, 28) | |
| Anterior cingulate (BA 10, 24, 25, 32, 33) | 1126/919 | 3.6(13, 44, 5)/3.3(−1,48, 4) | |
| Posterior cingulate (BA 23, 29, 30, 31) | 711/385 | 3.4 (6, −51, 21)/3.0 (−3, −53, 22) | |
| Parietal | |||
| Precuneus | 1067/444 | 3.3 (12, −50, 40)/2.9 (−4, −52, 33) | |
| Inferior parietal lobule | 919/900 | 3.9 (56, −34, 22)/3.9 (−55, −34, 25) | |
| Supramarginal gyrus | 444/89 | 3.3 (56, −58, 32)/2.6 (−55, −39, 31) | |
| Postcentral gyrus | 119/148 | 3.6 (34, −20, 45)/3.1 (−55, −28, 19) | |
| Occipital | |||
| Middle occipital gyrus | 1304/770 | 4.4 (48, −77, −4)/3.3 (−53, −60, −5) | |
| Inferior occipital gyrus | 622/207 | 4.7 (45, −80, −4)/2.8 (−30, −82, −6) | |
| Lingual gyrus | 356/1067 | 3.0 (27, −60, −6)/3.9 (−25, −72, −5) | |
| Cuneus | 119/385 | 2.9 (3, −64, 31)/2.8 (−27, −86, 22) | |
| Basal ganglia | |||
| Caudate | 148/533 | 3.5 (4, 7, −4)/3.9 (−4, 12, −1) | |
| Claustrum | 148/144 | 3.1 (28, 16, 3)/3.8 (−34, 7, −2) | |
| Lentiform nucleus | −/119 | −/2.7 (−30, −1, 1) | |
| Thalamus | 119/296 | 2.5 (3, −12, 1)/3.4 (−9, −4, 7) | |
| Cerebellum | |||
| Declive | 119/− | 2.6(24, −66, −11)/− | |
| Pyramis | −/178 | −/2.6(−31, −83, −34) | |
| Inferior semi-lunar lobule | −/296 | −/2.8 (−19, −83, −37) | |
| SAD < HC | |||
| Frontal | |||
| Inferior frontal gyrus | 296/385 | 4.5 (42, 22, 6)/5.2 (−42, 19, −1) | |
| Middle frontal gyrus | −/296 | −/4.9(−27, −3,49) | |
| Temporal | |||
| Parahippocampal gyrus | −/267 | −/4.6(−21, −9, −13) | |
| Insula | 207/200 | 4.2 (39, 17, −l)/4.8 (−39, 19, 2) | |
| Psychosis dimension contrast [P=0.005, uncorrected, k=100] | |||
| SZ < BD-P | |||
| Frontal | |||
| Inferior frontal gyrus | 1570/119 | 4.2 (37, 14, −13)/3.1 (−40, 16, −6) | |
| Superior frontal gyrus | −/119 | −/3.0(−9, 62, 22) | |
| Middle frontal gyrus | 119/− | 3.0 (39, 55, 12)/− | |
| Temporal | |||
| Parahippocampal gyrus | 919/444 | 3.8 (24, 5, −15)/3.4 (− 22, − 7, −17) | |
| Fusiform gyrus | 563/− | 3.4(39, −34, −17)/− | |
| Superior temporal gyrus | 326/− | 3.9(40, 11, −13)/− | |
| Uncus | 148/296 | 3.8 (24, 6, −19)/3.6 (−24, −4, −20) | |
| Middle temporal gyrus | −/207 | −/3.3 (−67, −39, −3) | |
| Insula | 119/148 | 3.1 (40, 11, −4)/3.1 (−33, 13, −4) | |
| Cingulate gyrus | |||
| Posterior cingulate (BA 23, 29, 30, 31) | 830/267 | 3.8 (6, −53, 19)/3.4 (−3, −53, 25) | |
| Posterior cingulate (BA 31) | 356/385 | 3.3 (1, −51, 27)/3.2 (−1, −55,28) | |
| Parietal | |||
| Precuneus | 237/237 | 3.2 (3, −60, 18)/3.1 (−4, −59, 21) | |
| Inferior parietla lobule | 119/− | 3.1 (50, −34, 22)/− | |
| Basal ganglia | |||
| Lentiform nucleus | 267/ | 3.3 (15, 13, −6)/− | |
| Caudate | −/119 | −/3.3(−6,4, −1) | |
| Cerebellum | |||
| Declive | 237/ | 3.0(31, −53, −11)/− | |
| Culmen | 119/ | 2.9(39, −39, −21)/− | |
| SZ < SAD | |||
| Frontal | |||
| Middle frontal gyrus | 1244/119 | 3.8(42,53, −13)/3.2 (−34, 53, −14) | |
| Inferior frontal gyrus | 919/− | 3.7 (27,31, −19)/− | |
| Superior frontal gyrus | 474/503 | 3.5 (34, 49, −15)/3.3 (9, 57, 32) | |
| Paracentral lobule | −/119 | −/3.2(−4, −44,53) | |
| Temporal | |||
| Fusiform gyrus | 800/237 | 3.6(56, −32, −19)/4.1 (−37, −17, −26) | |
| Inferior temporal gyrus | 593/207 | 3.7 (53, −30, −16)/3.7 (−40, −17, −28) | |
| Middle temporal gyrus | 474/356 | 3.3 (48, −78, 14)/3.0 (−52, −67, 23) | |
| Parahippocampal gyrus | −/296 | −/4.2 (−34, −20, −25) | |
| Uncus | 119/267 | 3.3 (36, −14, −27)/4.7 (34, −17, −28) | |
| Superiore temporal gyrus | 237/237 | 3.4 (50, −57, 28)/3.2 (−46, −50, 12) | |
| Cingulate gyrus | |||
| Posterior cingulate (BA 23, 29, 30, 31) | 859/385 | 3.9 (3, −52, 15)/3.7 (−4, −51, 25) | |
| Posterior cingulate (BA 31, 32) | 474/593 | 3.5 (4, −56, 26)/3.5 (−1, −51, 27) | |
| Anterior cingulate | 385/− | 3.0 (9, 35, 9)/− | |
| Parietal | |||
| Precuneus | 770/415 | 3.3 (6, −59, 18)/3.4 (−7, −44, 51) | |
| Occipital | |||
| Middle occipital gyrus | 1541/− | 4.5(43, −78, −9)/− | |
| Inferior occipital gyrus | 800/− | 4.8(36, −88, −7)1− | |
| Lingual gyrus | 356/− | 3.4(30, −76, −10)/− | |
| SAD < BD-P | |||
| Temporal | |||
| Parahippocampal gyrus | 267/356 | 3.0(30, −15, −13)/3.8 (−19, −4, −15) | |
| Basal ganglia | |||
| Lentiform nucleus | 356/207 | 3.9(15, 10, −2)/3.0(−25, 1, −6) | |
| SAD > BD-P | |||
| Frontal | |||
| Middle frontal gyrus | 119/− | 3.0 (27, 39, 44)/− | |
| Middle occipital gyrus | 237/− | 3.2 (50, −68, −10)/− | |
| Cerebellum (Tonsil) | 148/− | 3.0(27, −53, −40)/− | |
SZ—individuals with schizophrenia, SAD—individuals with schizoaffective disorder, BD-P—individuals with psychotic bipolar I disorder, HC—healthy controls, BA—Brodmann area, FDR—false discovery rate correction for multiple comparisons.
Fig. 1.
Whole brain gray matter phenotypes (A) between the psychosis groups and healthy controls and (B) across the psychosis dimension derived from the voxel-based morphometry analyses. SZ—individuals with schizophrenia, SAD—individuals with schizoaffective disorder, BD-P—individuals with psychotic bipolar I disorder, HC—healthy controls, FDR—false discovery rate correction for multiple comparisons. (A) Gray matter volume differences between the psychosis groups and healthy controls (P<0.05, FDR corrected, k=100). (B) Gray matter volume differences across the psychosis dimension (P<0.001, uncorrected, k=100).
3.1.2. Regional gray matter volume differences in psychosis vs. healthy controls (FreeSurfer)
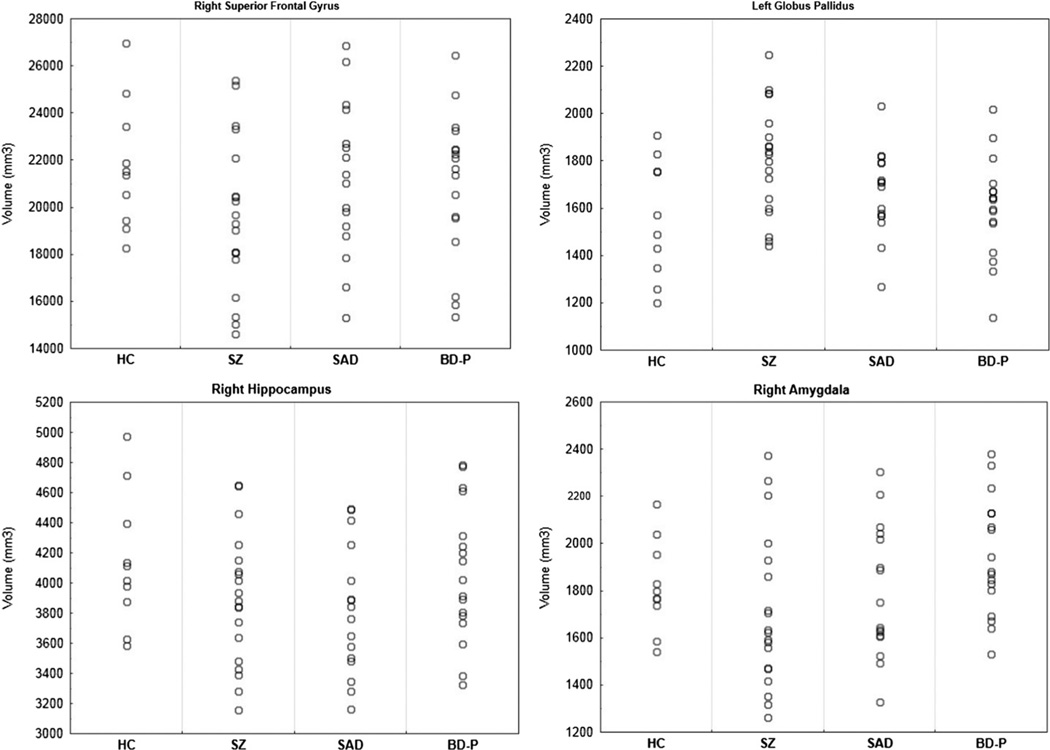
Regional cortical and subcortical volume differences between the psychosis groups and HC are shown in Table 3. Individual volumes distribution in the selected regions of interest (right superior frontal gyrus, left globus pallidus, right hippocampus, and right amygdala) are shown in Fig. 2. Consistent with the VBM results, FS analyses showed decreased volumes in several frontal and temporal regions bilaterally, the left parietal cortex and the right thalamus in SZ compared to HC. The differences in frontal regions remained significant after Bonferroni correction, although the changes in temporal and parietal cortex and thalamus did not survive multiple comparisons correction. In addition, SZ showed a larger volume of the left globus pallidus compared to HC. SAD had smaller volumes in bilateral hippocampi (significant in the right hippocampus after Bonferroni correction), the right caudate and thalamus (non-significant after Bonferroni correction), compared to HC. No differences in cortical or subcortical volumes emerged between BD-P and HC.
Fig. 2.
Individual volumes of selected regional outcomes derived from FreeSurfer analyses. SZ—individuals with schizophrenia, SAD—individuals with schizoaffective disorder, BD-P—individuals with psychotic bipolar I disorder, HC—healthy controls, FDR—false discovery rate correction for multiple comparisons.
3.2. Gray matter phenotypes across diagnoses in the psychosis dimension
3.2.1. Whole brain gray matter volume differences within the psychosis dimension (voxel-based morphometry)
Whole brain GM volume differences between the psychosis groups are shown in Table 2 and Fig. 1(B). An overall ANCOVA showed a main effect of group [F(2, 48)=5,93, P<0.005, uncorrected], although it was not significant after FDR correction. Similarly, no GM differences survived FDR correction when the individual psychosis groups were compared. The less stringent threshold (P<0.005, uncorrected) revealed a gradual pattern of GM changes (BD-P>SAD>SZ) where BD-P had larger volumes than SZ, and SAD showed intermediate volumes with less robust differences as compared to either SZ or BD-P. Specifically, SZ showed smaller GM volumes in bilateral fronto-temporal, insular, posterior cingulate, and parietal cortical regions, as well as in basal ganglia, and the right cerebellum, compared to BD-P. SAD had larger GM volumes in bilateral fronto-temporal, cingulate, parietal, and occipital cortices compared to SZ, and smaller volumes in bilateral parahippocampal gyri and basal ganglia compared to BD-P. In addition, SAD showed a few isolated clusters of larger GM in the right middle frontal, middle occipital and cerebellar regions compared to BD-P.
3.2.2. Regional gray matter volume differences within the psychosis dimension (FreeSurfer)
Regional cortical and subcortical volume differences between the psychosis groups are shown in Table 3. Consistent with the VBM results, FS analyses detected smaller volumes in fronto-temporal, cingulate, parietal, occipital, and cerebellar regions in SZ compared to BD-P. However, only differences in the left precuneus and lingual gyrus remained significant after Bonferroni correction. SAD showed larger GM volumes in the left inferior temporal and the right anterior cingulate gyri, compared to SZ; smaller hippocampal volumes bilaterally (significant after Bonferroni correction) compared to BD-P; and smaller volumes of thalamus compared to both BD-P and SZ. In addition, FS depicted enlarged volumes of amygdala in BD-P compared to both SZ and SAD, as well as an increased volume of the globus pallidus (the left-side difference significant after Bonferroni correction) in SZ compared to BD-P.
3.3. Associations between gray matter volume and medication, and lifetime psychosis duration
No differences in whole brain GM or regional cortical/subcortical volumes were obtained between SZ, SAD, and BD-P who were on- and off-AP or on-and off-mood stabilizer medications while active in this study. No correlations between GM cortical/ subcortical volumes and lifetime duration of psychosis were found in any of the psychosis groups.
4. Discussion
In this study we examined whole brain and regional GM phenotypes in individuals within the schizophrenia–bipolar disorder psychosis dimension. The analytic strategy employed two volumetric methodologies: VBM for the examination of global GM differences, and FS for precise characterization of regional cortical and subcortical GM phenotypes. Our findings suggest that individuals across the psychosis dimension show both unique (e.g., SZ as compared to BD-P) and overlapping (e.g., SZ as compared to SAD) GM characteristics. Compared to HC, SZ showed extensive and diffuse cortical GM volume reductions, most prominent in fronto-temporal cortices, as well as in several subcortical structures including thalamus and cerebellum; SAD showed GM reductions overlapping with SZ, albeit less extensive reductions, whereas BD-P had cortical and subcortical GM volumes not different from HC. Direct contrast of GM phenotypes within the psychosis dimension revealed a step-wise pattern of differences where BD-P had larger GM volumes in fronto-temporal, insular, cingulate, and parietal cortices, as well as basal ganglia and cerebellum, compared with SZ, whereas SAD showed intermediate volumes in these regions as compared with either SZ or BD-P.
In this study the individuals with the categorical DSM-IV diagnoses within the psychosis dimension (schizophrenia–schizoaffective disorder–psychotic bipolar I disorder) were directly compared in the same analyses. Overall, both cortical and subcortical GM structural phenotypes distinguished SZ and BD-P, whereas SAD appeared to have GM deficits overlapping with SZ, albeit less extensive deficits. The observations of GM reductions in SZ/SAD, and normal GM in BD-P, are consistent with previous reports that contrasted SZ and BD-P samples (Harvey et al., 1994; McDonald et al., 2005; Farrow et al., 2005). The differences in GM structural phenotype in SZ/SAD and BD-P can be interpreted to say that the GM reductions in SZ/SAD may be related to a primary disease pathophysiology that is not characteristic for BD-P. Although the exact mechanisms underlying GM reductions in schizophrenia are not understood, it is known from post-mortem examination that general neuronal loss is not present to any substantial extent in schizophrenia, excluding this as an explanation for decreased cortical volume observed with imaging. The same studies showed reductions in interstitial volume with increased neuronal cell packing, offering reductions in the dendritic branching as a more likely putative explanation (Selemon et al., 1995; Selemon and Goldman-Rakic, 1999). In addition, the possibility exists that GM volume preservation (or volume increase) in bipolar disorder may be secondary to an action of mood stabilizers and that, even if disease-associated loss of neocortical volume is present in BD-P, it is obscured by a volume-enchancing effect of mood stabilizers (Bearden et al., 2007; Moore et al., 2009). It is unlikely that the differential GM findings in SZ/SAD and BD-P are related to higher severity of illness in SZ/SAD compared to BD-P, given a careful psychiatric history characterization of the study sample and the absence of between-group differences in overall illness severity estimated by the lifetime number of hospitalizations and BPRS scores, as well as the absence of correlation between the duration of lifetime psychosis and GM outcomes.
Measurable alterations in the brain structure are first observed in individuals near the onset of psychosis, with some studies finding most pronounced changes in subcortical structures (e.g., hippocampus, basal ganglia, and thalamus) (Watson et al., 2012), as well as in fronto-temporal and fronto-parietal white matter (Whitford et al., 2007; Watson et al., 2012). These alterations are thought to progress rather rapidly during the initial years of psychosis (Whitford et al., 2007; Andreasen et al., 2011), and, although they may plateau during subsequent years (Andreasen et al., 2011), ultimately lead to a characteristic pattern of diffuse structural changes found in chronic SZ samples (Meisenzahl et al., 2008). Since individuals with psychosis are chronically exposed to various psychotropic treatments, as well as other disease-associated factors (e.g., substance use and increased medical comorbidities), disentangling the ‘primary’ GM changes from the ‘secondary’ disease-associated effects presents considerable difficulty. The reports from unmedicated early psychosis samples advocate for a ‘primary’ intrinsic effect of illness on GM) (Borgwardt et al., 2011; Fusar-Poli et al., 2011). At the same time, convincing evidence has cumulated to support an effect of psychotropic medications on GM structure (Gaser et al., 1999; Wilke et al., 2001; Kubicki et al., 2002; Lieberman et al., 2005; Crespo-Facorro et al., 2008; Jones et al., 2009; Ho et al., 2011). It is possible that GM volume deficits in SZ/SAD found in this study could be, at least partially, accounted for by an effect of a lifetime treatment with first- and second-generation AP (Lieberman et al., 2005; Molina et al., 2007; Crespo-Facorro et al., 2008), whereas a preservation of GM volumes in BD-P could be related to chronic treatment with mood stabilizers (Usher et al., 2009). Not surprisingly, we did not find significant differences in GM volume in the psychosis volunteers that were on and off AP or mood stabilizers while active in this study. Given that this sample comprises chronically ill individuals the vast majority of whom had years of treatment prior to entering this study, acute effects of AP and mood stabilizers on brain structure were likely obscured by the longitudinal effects of both disease and medication.
In this study, both volumetric methodologies, VBM and FS, produced highly overlapping results with regard to cortical GM volume, although VBM depicted GM changes in broader regions compared to FS. However, the two analyses showed somewhat divergent outcomes for subcortical volumes. The schematic summary of overlapping and divergent VBM and FS outputs are presented in Table 4. For example, FS depicted larger volume of amygdala in BD-P compared to either SZ or SAD, which was not found with VBM. In addition, FS showed an enlargement of globus pallidus in SZ compared to HC and BD-P, whereas VBM depicted decreased GM volumes in basal ganglia (in the lentiform nucleus, caudate and claustrum regions) in SZ as compared to either HC or BD-P. The finding of enlarged amygdala in BD-P is consistent with many previous reports (Altshuler et al., 1998; Velakoulis et al., 2006; Brambilla et al., 2008; Arnone et al., 2009; Kalmar et al., 2009; Usher et al., 2009), with some attributing it to primary disease pathophysiology, such as increased response to emotional stimuli (Brambilla et al., 2008; Kalmar et al., 2009), and others to lifetime treatment with mood stabilizers, particularly, lithium (Usher et al., 2009; Hallahan et al., 2011). The divergent findings in basal ganglia may reflect differential changes in various subregions within this large subcortical structure related to disease process and/or medications. Similar to our findings, a recent large meta-analysis in SZ/bipolar disorder GM phenotypes (Yu et al., 2010) reported mixed results with both reduced (in the caudate head) and increased (in the left lentiform nucleus, putamen, and globus pallidus) GM volumes in SZ and bipolar disorder. Earlier literature also provided variable observations, with some studies showing basal ganglia enlargement, likely associated with AP use (Gaser et al., 1999; Wilke et al., 2001; Kubicki et al., 2002; Ellison-Wright and Bullmore, 2010), and others reporting no difference in SZ vs. HC (Crespo-Facorro et al., 2007), especially prior to drug treatment (Chua et al., 2007; Leung et al., 2011). Furthermore, some studies provided evidence for basal ganglia functional dissociation, with the caudate head being responsible for feedback processing and linked to the dorsolateral prefrontal cortex, whereas the caudate body and tail mediated stimulus-category learning and were linked to the extrastriate and visual inferotemporal cortex (Seger and Cincotta, 2006). Therefore, there is a possibility that this functional dissociation may translate into differential structural morphology within the basal ganglia, although further work on the role of the caudate in psychosis is needed to support this observation. Alternately, these divergent observations in subcortical volumes derived from VBM and FS may be attributed to differences in volume estimation algorithms used in the two methodologies. While VBM utilizes the three-dimensional tissue classification method where each voxel is assigned a probability of belonging to a particular tissue class (GM, white matter, cerebro-spinal fluid) based on its intensity and prior probability maps (Ashburner and Friston, 2000), FS uses a Markov Random Fields approach where the probability of cortical/ subcortical labels at a given voxel is computed based on the tissue intensity, prior probabilities at each voxel, and spatial positioning of neighboring labels (Fischl et al., 2002). The latter approach may increase accuracy of voxel labeling, specifically, in subcortical structures known to have significantly heterogeneous tissue intensity properties (Fischl et al., 2002). In addition, subcortical structures may present a challenge for precise intersubject registration in VBM (Meisenzahl et al., 2008), making detection of differences in such structures problematic. Overall, both volumetric methodologies, VBM and FS, have proven to be successful in characterization of the psychosis GM phenotypes. However, they produced both overlapping (e.g., cortical GM) and divergent (e.g., subcortical structures) outcomes. VBM showed somewhat higher sensitivity to cortical GM alterations, whereas FS demonstrated more substantial sensitivity to subcortical volume changes. Therefore, both methodologies may be used in conjunction for precise characterization of brain structure phenotypes.
Table 4.
The schematic summary of neocortical and subcortical gray matter differences detected with the two volumetric methodologies: voxel-based morphometry and FreeSurfer.
| VBM | FS | |||||
|---|---|---|---|---|---|---|
| Psychosis groups vs. healthy controls |
||||||
| SZ < HC | SAD < HC | BD-P < HC | SZ < HC | SAD < HC | BD-P < HC | |
| Cortical regions | ||||||
| Frontal | ↓↓↓ | ↓ | - | ↓↓↓ | - | - |
| Temporal | ↓↓↓ | ↓ | - | ↓↓ | - | - |
| Insular | ↓↓↓ | ↓ | - | - | - | - |
| Cingulate | ↓↓↓ | - | - | - | - | - |
| Parietal | ↓↓ | - | - | ↓ | - | - |
| Occipital | ↓↓ | - | - | - | - | - |
| Subcortical regions | ||||||
| Hippocampus | - | - | - | ↓ | ↓↓ | - |
| Amygdala | - | - | - | - | - | - |
| Basal ganglia* | ↓↓* | - | - | ↑* | ↓ | - |
| Thalamus | ↓ | - | - | ↓ | ↓ | - |
| Cerebellum | ↓ | - | - | - | - | - |
|
Psychosis dimension |
||||||
| SZ < BD-P | SZ < SAD | SAD < BD-P | SZ < BD-P | SZ < SAD | SAD < BD-P | |
| Cortical regions | ||||||
| Frontal | ↓↓ | ↓↓ | ↑ | ↓↓ | - | - |
| Temporal | ↓↓↓ | ↓↓↓ | ↓ | ↓ | ↓ | - |
| Insular | ↓ | - | - | - | - | - |
| Cingulate | ↓↓ | ↓↓↓ | - | ↓ | ↓ | ↑ |
| Parietal | ↓ | ↓ | - | ↓↓ | - | ↑ |
| Occipital | - | ↓↓ | ↑ | ↓↓ | - | ↑ |
| Subcortical regions | ||||||
| Hippocampus | - | - | - | - | - | ↓↓ |
| Amygdala* | - | - | - | ↓* | - | ↓* |
| Basal ganglia* | ↓* | - | ↓↓ | ↑↑* | - | - |
| Thalamus | - | - | - | - | ↑ | ↓↓ |
| Cerebellum | ↓ | - | ↑ | ↓ | - | - |
VBM - Voxel-Based Morphometry, FS - FreeSurfer, SZ - individuals with schizophrenia, SAD - individuals with schizoaffective disorder, BD-P - individuals with psychotic bipolar I disorder, HC - healthy controls.
The regions with divergent VBM and FS findings are marked with an asterisk.
Several limitations to this study should be noted. The modest sample size, especially the limited number of HC, warrants cautious interpretation of these findings. The broad effects of race and gender on disease neuropathology were not considered in this analysis. In addition, within-group variability in volumetric characteristics could contribute to the lack of between-groups differences in SZ vs. SAD vs. BD-P contrast at corrected statistical threshold in VBM. Because the majority of subjects in this sample had a chronic treatment history with various psychotropic medications, here it was not possible to distinguish between primary disease effects and chronic medication effects on brain structure. Future longitudinal studies applying pre- and post-treatment imaging, studies in unmedicated psychosis individuals, as well as studies in biological relatives of probands with psychoses may provide better understanding of structural changes that reflect ‘primary’ markers of disease vs. an effect of confounding factors, such as medications.
In conclusion, this is the first study to directly compare GM phenotypes in individuals across the schizophrenia–bipolar disorder psychosis dimension using a combination of two volumetric approaches: global GM analysis (VBM) and regional analysis (FS). Our findings indicate that, parallel to clinical manifestations, where SZ, SAD, and BD-P demonstrate shared and unique symptom dimensions, they exhibit both overlapping and unique structural GM phenotypes: decreased GM, predominantly, in fronto-temporal regions, is characteristic of SZ, but not of psychotic, BD-P, whereas SAD display intermediate phenotype, with GM decreases partially overlapping but less robust than those seen in SZ. Future research examining brain structure and other phenotypes and their genetic underpinnings in larger family psychosis samples may shed further light on a dimensional definition of psychosis and aid in the development of a biologically based classification of psychosis which is treatment-relevant.
Acknowledgment
We would like to thank Richard Briggs, Ph.D., David Morris, Ph.D., Darwynn Cole, B.S., and Dorothy Denton, B.A., for assistance with data collection, analysis, and the manuscript preparation; all clinicians for patient referral and all patients who took part in this study.
Funding
This work was supported by National Institute of Mental Health (MH077851-01A1, and MH 78113, Bipolar & Schizophrenia Consortium for Parsing Endophenotypes). The NIMH had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the manuscript for publication.
References
- Abrams DJ, Rojas DC, Arciniegas DB. Is schizoaffective disorder a distinct categorical diagnosis? A critical review of the literature. Neuropsychiatric Disease Treatment. 2008;4:1089–1109. doi: 10.2147/ndt.s4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archive of General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Curran JG, Hauser P, Mintz J, Denicoff K, Post R. T2 hyperintensities in bipolar disorder: magnetic resonance imaging comparison and literature meta-analysis. American Journal of Psychiatry. 1995;152:1139–1144. doi: 10.1176/ajp.152.8.1139. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. Washington, DC: APA; 1994. [Google Scholar]
- Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biological Psychiatry. 2011;70:672–679. doi: 10.1016/j.biopsych.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Arnone D, McIntosh AM, Tan GM, Ebmeier KP. Meta-analysis of magnetic resonance imaging studies of the corpus callosum in schizophrenia. Schizophrenia Research. 2008;101:124–132. doi: 10.1016/j.schres.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuro-Image. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a metaanalysis of MRI studies. Schizophrenia Research. 2007;93:1–12. doi: 10.1016/j.schres.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dalwani M, Hayashi KM, Lee AD, Nicoletti M, Trakhtenbroit M, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Soares JC. Greater cortical gray matter density in lithium-treated patients with bipolar disorder. Biological Psychiatry. 2007;62:7–16. doi: 10.1016/j.biopsych.2006.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Francis AN, Rajarethinam R, Eack S, Kulkarni S, Prasad KM, Montrose DM, Dworakowski D, Diwadkar V, Keshavan MS. Verbal fluency deficits and altered lateralization of language brain areas in individuals genetically predisposed to schizophrenia. Schizophrenia Research. 2009;115:202–208. doi: 10.1016/j.schres.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra G, van Haren NE, Schnack HG, Cahn W, Burger H, Boersma M, de Kroon B, Grobbee DE, Hulshoff Pol HE, Kahn RS. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. Journal of Clinical Psychopharmacology. 2011;31:146–153. doi: 10.1097/JCP.0b013e31820e3f58. [DOI] [PubMed] [Google Scholar]
- Borgwardt S, McGuire P, Fusar-Poli P. Gray matters!—mapping the transition to psychosis. Schizophrenia Research. 2011;133:63–67. doi: 10.1016/j.schres.2011.08.021. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Hatch JP, Soares JC. Limbic changes identified by imaging in bipolar patients. Current Psychiatry Reports. 2008;10:505–509. doi: 10.1007/s11920-008-0080-8. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Francis A, Arango C, Miller K, Lefkowitz DM, McMahon RP, Barta PE, Pearlson GD. Morphometric assessment of the heteromodal association cortex in schizophrenia. American Journal of Psychiatry. 2004;161:322–331. doi: 10.1176/appi.ajp.161.2.322. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lonnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Rosso IM, Huttunen M, Lonnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Archives of General Psychiatry. 2002;59:35–41. doi: 10.1001/archpsyc.59.1.35. [DOI] [PubMed] [Google Scholar]
- Chua SE, Cheung C, Cheung V, Tsang JT, Chen EY, Wong JC, Cheung JP, Yip L, Tai KS, Suckling J, McAlonan GM. Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophrenia Research. 2007;89:12–21. doi: 10.1016/j.schres.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santianez R, Pelayo-Teran JM, Gonzalez-Blanch C, Perez-Iglesias R, Gutierrez A, de Lucas EM, Tordesillas D, Vazquez-Barquero JL. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophrenia Research. 2007;91:87–96. doi: 10.1016/j.schres.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Roiz-Santianez R, Perez-Iglesias R, Pelayo-Teran JM, Rodriguez-Sanchez JM, Tordesillas-Gutierrez D, Ramirez M, Martinez O, Gutierrez A, de Lucas EM, Vazquez-Barquero JL. Effect of antipsychotic drugs on brain morphometry. A randomized controlled one-year follow-up study of haloperidol, risperidone and olanzapine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:1936–1943. doi: 10.1016/j.pnpbp.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Maurizio AM, Relja M, Hoff AL. Cerebral ventricular change over the first 10 years after the onset of schizophrenia. Psychiatry Research. 2004;130:57–70. doi: 10.1016/j.pscychresns.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. Second, Completely Revised and Enlarged Edition. 2nd ed. Vienna, New York: Springer; 1999. The Human Brain: Surface, Blood Supply, and Three-Dimensional Sectional Anatomy. [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. American Journal of Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow TF, Whitford TJ, Williams LM, Gomes L, Harris AW. Diagnosis-related regional gray matter loss over two years in first episode schizophrenia and bipolar disorder. Biological Psychiatry. 2005;58:713–723. doi: 10.1016/j.biopsych.2005.04.033. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders/Patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute, Biometrics Research Department; 1996. [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der KA, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der KA, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Friedman L, Findling RL, Kenny JT, Swales TP, Stuve TA, Jesberger JA, Lewin JS, Schulz SC. An MRI study of adolescent patients with either schizophrenia or bipolar disorder as compared to healthy control subjects. Biological Psychiatry 46. 1999 doi: 10.1016/s0006-3223(98)00351-5. following 584. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM studies. Schizophrenia Bulletin; electronic publication 17 November. 2011 doi: 10.1093/schbul/sbr134. http://dx.doi.org/10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed]
- Gaser C, Volz HP, Kiebel S, Riehemann S, Sauer H. Detecting structural changes in whole brain based on nonlinear deformations-application to schizophrenia research. NeuroImage. 1999;10:107–113. doi: 10.1006/nimg.1999.0458. [DOI] [PubMed] [Google Scholar]
- Getz GE, DelBello MP, Fleck DE, Zimmerman ME, Schwiers ML, Strakowski SM. Neuroanatomic characterization of schizoaffective disorder using MRI: a pilot study. Schizophrenia Research. 2002;55:55–59. doi: 10.1016/s0920-9964(01)00210-9. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Galve L, Wheeler-Kingshott CA, Altmann DR, Price G, Chu EM, Leeson VC, Lobo A, Barker GJ, Barnes TR, Joyce EM, Ron MA. Changes in the frontotemporal cortex and cognitive correlates in first-episode psychosis. Biological Psychiatry. 2010;68:51–60. doi: 10.1016/j.biopsych.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppa T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biological Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Hartberg CB, Sundet K, Rimol LM, Haukvik UK, Lange EH, Nesvag R, Melle I, Andreassen OA, Agartz I. Subcortical brain volumes relate to neurocognition in schizophrenia and bipolar disorder and healthy controls. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:1122–1130. doi: 10.1016/j.pnpbp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Harvey I, Persaud R, Ron MA, Baker G, Murray RM. Volumetric MRI measurements in bipolars compared with schizophrenics and healthy controls. Psychological Medicine. 1994;24:689–699. doi: 10.1017/s0033291700027847. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cerebral Cortex. 2001;11:374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Shenton ME, Salisbury DF, Kwon JS, Wible CG, Fischer IA, Yurgelun-Todd D, Zarate C, Kikinis R, Jolesz FA, McCarley RW. Subgenual cingulate cortex volume in first-episode psychosis. American Journal of Psychiatry. 1999;156:1091–1093. doi: 10.1176/ajp.156.7.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Archives of General Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Archives of General Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Janssen J, Reig S, Parellada M, Moreno D, Graell M, Fraguas D, Zabala A, Garcia VV, Desco M, Arango C. Regional gray matter volume deficits in adolescents with first-episode psychosis. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1311–1320. doi: 10.1097/CHI.0b013e318184ff48. [DOI] [PubMed] [Google Scholar]
- Jones LD, Payne ME, Messer DF, Beyer JL, MacFall JR, Krishnan KR, Taylor WD. Temporal lobe volume in bipolar disorder: relationship with diagnosis and antipsychotic medication use. Journal of Affective Disorders. 2009;114:50–57. doi: 10.1016/j.jad.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmar JH, Wang F, Chepenik LG, Womer FY, Jones MM, Pittman B, Shah MP, Martin A, Constable RT, Blumberg HP. Relation between amygdala structure and function in adolescents with bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:636–642. doi: 10.1097/CHI.0b013e31819f6fbc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:869–874. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Archives of General Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Acker JD, Raz N. Age-related differences in regional brain volumes: a comparison of optimized voxel-based morphometry to manual volumetry. Neurobiology of Aging. 2009;30:1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;19:344. doi: 10.1016/s0140-6736(94)90599-1. (8934), 1434. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Berger G, Zipursky RB, Wood SJ, Pantelis C. Neurobiology of early psychosis. British Journal of Psychiatry. 2005;48:s8–s18. doi: 10.1192/bjp.187.48.s8. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: what we know in 2008 Part 3: neurobiology. Schizophrenia Research. 2008;106:89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Konick LC, Friedman L. Meta-analysis of thalamic size in schizophrenia. Biological Psychiatry. 2001;49:28–38. doi: 10.1016/s0006-3223(00)00974-4. [DOI] [PubMed] [Google Scholar]
- Kubicki M, Shenton ME, Salisbury DF, Hirayasu Y, Kasai K, Kikinis R, Jolesz FA, McCarley RW. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. NeuroImage. 2002;17:1711–1719. doi: 10.1006/nimg.2002.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki N, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Ersner-Hershfield H, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. American Journal of Psychiatry. 2006;163:2103–2110. doi: 10.1176/appi.ajp.163.12.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan C, McDonald C. Neurobiological trait abnormalities in bipolar disorder. Molecular Psychiatry. 2009;14:833–846. doi: 10.1038/mp.2009.39. [DOI] [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophrenia Bulletin. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, Perkins D, Hamer RM, Gu H, Tohen M. Antipsychotic drug effects on brain morphology in first-episode psychosis. Archives of General Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disorder. 2006;8:65–74. doi: 10.1111/j.1399-5618.2006.00284.x. [DOI] [PubMed] [Google Scholar]
- McDonald C, Bullmore E, Sham P, Chitnis XA, Suckling J, MacCabe J, Walshe M, Murray RM. Regional volume deviations of brain structure in schizophrenia and psychotic bipolar disorder: computational morphometry study. The British Journal of Psychiatry. 2005;186:369–377. doi: 10.1192/bjp.186.5.369. [DOI] [PubMed] [Google Scholar]
- McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, Murray RM, Kennedy N. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biological Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Job DE, Moorhead TW, Harrison LK, Forrester K, Lawrie SM, Johnstone EC. Voxel-based morphometry of patients with schizophrenia or bipolar disorder and their unaffected relatives. Biological Psychiatry. 2004;56:544–552. doi: 10.1016/j.biopsych.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Meda SA, Giuliani NR, Calhoun VD, Jagannathan K, Schretlen DJ, Pulver A, Cascella N, Keshavan M, Kates W, Buchanan R, Sharma T, Pearlson GD. A large scale (N=400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophrenia Research. 2008;101:95–105. doi: 10.1016/j.schres.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jager M, Teipel SJ, Holzinger S, Frodl T, Preuss U, Schmitt G, Burgermeister B, Reiser M, Born C, Moller HJ. Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophrenia Research. 2008;104:44–60. doi: 10.1016/j.schres.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sanchez J, Pascau J, Desco M. Changes in cortical volume with olanzapine in chronic schizophrenia. Pharmacopsychiatry. 2007;40:135–139. doi: 10.1055/s-2007-981479. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Cortese BM, Glitz DA, Zajac-Benitez C, Quiroz JA, Uhde TW, Drevets WC, Manji HK. A longitudinal study of the effects of lithium treatment on prefrontal and subgenual prefrontal gray matter volume in treatment-responsive bipolar disorder patients. Journal of Clinical Psychiatry. 2009;70:699–705. doi: 10.4088/JCP.07m03745. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nelson MD, Saykin AJ, Flashman LA, Riordan HJ. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Archives of General Psychiatry. 1998;55:433–440. doi: 10.1001/archpsyc.55.5.433. [DOI] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. International Journal of Psychophysiology. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Pearlson GD, Barta PE, Powers RE, Menon RR, Richards SS, Aylward EH, Federman EB, Chase GA, Petty RG, Tien AY. Ziskind-Somerfeld Research Award 1996. Medial and superior temporal gyral volumes and cerebral asymmetry in schizophrenia versus bipolar disorder. Biological Psychiatry. 1997;41:1–14. doi: 10.1016/s0006-3223(96)00373-3. [DOI] [PubMed] [Google Scholar]
- Prasad KM, Rohm BR, Keshavan MS. Parahippocampal gyrus in first episode psychotic disorders: a structural magnetic resonance imaging study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28:651–658. doi: 10.1016/j.pnpbp.2004.01.017. [DOI] [PubMed] [Google Scholar]
- Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radonic E, Rados M, Kalember P, Bajs-Janovic M, Folnegovic-Smalc V, Henigsberg N. Comparison of hippocampal volumes in schizophrenia, schizoaffective and bipolar disorder. Collegium Antropologicum. 2011;35(Suppl 1):249–252. [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC. Human neuroscience and the aging mind: a new look at old problems. The Journal of Gerontology B Psychological Sciences and Social Sciences. 2010;65:405–415. doi: 10.1093/geronb/gbq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvag R, Fennema-Notestine C, Hagler DJ, Jr, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Sanchez-Morla EM, Garcia-Jimenez MA, Barabash A, Martinez-Vizcaino V, Mena J, Cabranes-Diaz JA, Baca-Baldomero E, Santos JL. P50 sensory gating deficit is a common marker of vulnerability to bipolar disorder and schizophrenia. Acta Psychiatrica Scandinavica. 2008;117:313–318. doi: 10.1111/j.1600-0447.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Current Opinion in Psychiatry. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlosser RG. Reduced cortical thickness in first episode schizophrenia. Schizophrenia Research. 2010;116:204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Seger CA, Cincotta CM. Dynamics of frontal, striatal, and hippocampal systems during rule learning. Cerebral Cortex. 2006;16:1546–1555. doi: 10.1093/cercor/bhj092. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Transactions on Medical Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biological Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Archives of General Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. The effects of antipsychotics on the brain: what have we learnt from structural imaging of schizophrenia?—a systematic review. Current Pharmaceutical Design. 2009;15:2535–2549. doi: 10.2174/138161209788957456. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Barch DM, Csernansky JG. Bridging the gap between schizophrenia and psychotic mood disorders: relating neurocognitive deficits to psychopathology. Schizophrenia Research. 2009;107:69–75. doi: 10.1016/j.schres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. Thalamic morphology in schizophrenia and schizoaffective disorder. Journal of Psychiatric Research. 2011;45:378–385. doi: 10.1016/j.jpsychires.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. The British Journal of Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biological Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, Yung A, McGorry PD, Wood SJ, van Erp TG, Thompson PM, Toga AW, Cannon TD, Pantelis C. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophrenia Research. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usher J, Menzel P, Schneider-Axmann T, Kemmer C, Reith W, Falkai P, Gruber O, Scherk H. Increased right amygdala volume in lithiumtreated patients with bipolar I disorder. Acta Psychiatrica Scandinavica. 2009 doi: 10.1111/j.1600-0447.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standertskjold-Nordenstam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Archives of General Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, Smith D, Brewer W, Proffitt T, Desmond P, Pantelis C. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of General Psychiatry. 2006;63:139–149. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Videbech P. MRI findings in patients with affective disorder: a meta-analysis. Acta Psychiatrica Scandinavica. 1997;96:157–168. doi: 10.1111/j.1600-0447.1997.tb10146.x. [DOI] [PubMed] [Google Scholar]
- Vita A, De Peri L, Silenzi C, Dieci M. Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophrenia Research. 2006;82:75–88. doi: 10.1016/j.schres.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Watson DR, Anderson JM, Bai F, Barrett SL, McGinnity TM, Mulholland CC, Rushe TM, Cooper SJ. A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behavioural Brain Research. 2012;227:91–99. doi: 10.1016/j.bbr.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Whitford TJ, Grieve SM, Farrow TF, Gomes L, Brennan J, Harris AW, Gordon E, Williams LM. Volumetric white matter abnormalities in first-episode schizophrenia: a longitudinal, tensor-based morphometry study. American Journal of Psychiatry. 2007;164:1082–1089. doi: 10.1176/ajp.2007.164.7.1082. [DOI] [PubMed] [Google Scholar]
- Whitworth AB, Kemmler G, Honeder M, Kremser C, Felber S, Hausmann A, Walch T, Wanko C, Weiss EM, Stuppaeck CH, Fleischhacker WW. Longitudinal volumetric MRI study in first- and multiple-episode male schizophrenia patients. Psychiatry Research: Neuroimaging. 2005;140:225–237. doi: 10.1016/j.pscychresns.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Wilke M, Kaufmann C, Grabner A, Putz B, Wetter TC, Auer DP. Gray matter-changes and correlates of disease severity in schizophrenia: a statistical parametric mapping study. NeuroImage. 2001;13:814–824. doi: 10.1006/nimg.2001.0751. [DOI] [PubMed] [Google Scholar]
- Woodruff PW, McManus IC, David AS. Meta-analysis of corpus callosum size in schizophrenia. Journal of Neurology, Neurosurgery, & Psychiatry. 1995;58:457–461. doi: 10.1136/jnnp.58.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Yu K, Cheung C, Leung M, Li Q, Chua S, McAlonan G. Are bipolar disorder and schizophrenia neuroanatomically distinct? an anatomical likelihood meta-analysis. Frontiers in Human Neuroscience. 2010;4:189. doi: 10.3389/fnhum.2010.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky RB, Seeman MV, Bury A, Langevin R, Wortzman G, Katz R. Deficits in gray matter volume are present in schizophrenia but not bipolar disorder. Schizophrenia Research. 1997;26:85–92. doi: 10.1016/s0920-9964(97)00042-x. [DOI] [PubMed] [Google Scholar]