Abstract
A retropharyngeal abscess is a rare disease entity in young infants but can develop after nasopharyngeal viral infection. Group B Streptococcus and Staphylococcus aureus are the most common pathogens in young infants, however, Mycobacterium tuberculosis is very rare. We report the case of retropharyngeal abscess and coinfection with S. aureus and M. tuberculosis in a very young infant presenting with respiratory symptoms due to upper airway obstruction. Usually tuberculous retropharyngeal abscesses are caused by the direct invasion of the bacteria from the spine via anterior longitudinal ligament of the spine. However, in this case, no associated spinal disease was observed.
Keywords: Retropharyngeal abscess, Staphylococcus aureus, Mycobacterium tuberculosis
Introduction
Retropharyngeal abscess is a disease occurring secondary to acute infection of the pharynx1). In young infants, nasopharyngeal infection may be common, but secondary retropharyngeal abscess formation is rare2). In the event that a retropharyngeal abscess does develop, Group B Streptococcus and Staphylococcus aureus are common pathogens in these infants2,3). S. aureus is a very common source of superinfection after respiratory viral infection due to bacterial-viral interactions4). It is possible for tuberculous cervical lymphadenitis to extend into retropharyngeal space, as the lymphatics of the upper airway and oropharynx drain into this region. However, Mycobacterium tuberculosis is almost never detected from lymph nodes in this age group2,3,5,6). This case report details a 5-week-old boy with a retropharyngeal abscesses coinfected with both S. aureus and M. tuberculosis after rhinoviral infection.
Case Report
A 5-week-old boy was admitted to the hospital due to perioral cyanosis during crying, stridor, and refusal of feeds for three days. One week prior to admission, he developed rhinorrhea and sneezing, which continued to worsen after onset with the development of agitation and mild chest wall retractions. On admission, his blood pressure was 90/57 mmHg and pulse was 166 beats per minute. Although his mother perceived a tactile fever, he was afebrile. Though stridor was heard during inspiration and the respiratory rate was 60 breaths per minute, his oxygen saturation remained at 100% on room air. Due to his stridor, he was diagnosed with croup in addition to the common cold and was treated conservatively with budesonide and epinephrine nebulizer treatments. Initial laboratory testing displayed leukocytosis (white blood cell count 25,030/µL), thrombocytosis (platelet count 603,000/µL), and increased C-reactive protein (12.07 mg/dL, reference range <0.3 mg/dL). His electrolyte panel was within normal limits and chest radiograph did not show any lung abnormalities. Respiratory virus panel testing detected rhinovirus using multiplex polymerase chain reaction. As bacterial infection still could not be ruled out, ampicillin and gentamicin were administered. In spite of conservative treatment and administration of intravenous antibiotics, his stridor and head bobbing during respiration continued. Follow-up laboratory results revealed that his complete blood count was similar to the first results, but the C-reactive protein had decreased slightly (8.97 mg/dL). Because his symptoms and laboratory results had not improved as drastically as had been expected, we decided to pursue other causes of upper airway obstruction.
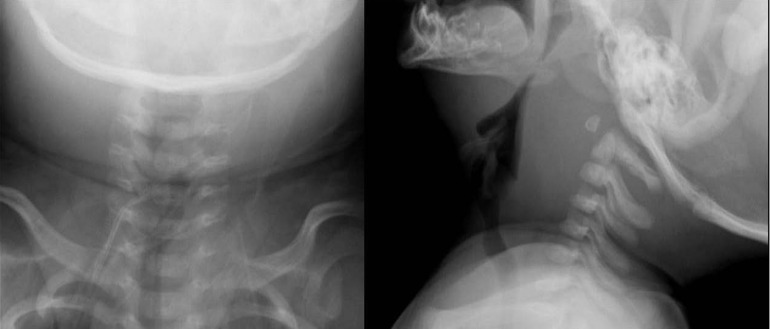
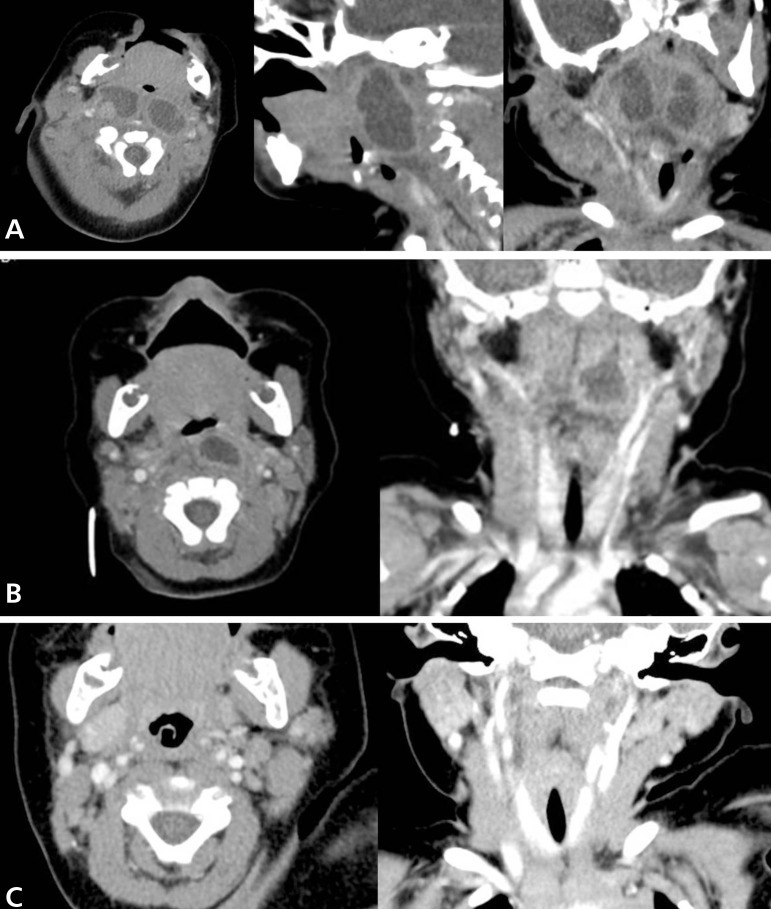
On hospital day 3, he had anteroposterior and lateral neck radiographs. The films showed a mass-like bulging lesion in the retropharyngeal area, complicated by severe narrowing of pharyngeal airway (Fig. 1). Multi-septated retropharyngeal abscesses and multifocal cervical lymphadenopathy with central necrosis were seen on computed tomography (CT) of the neck (Fig. 2A).
Fig. 1.
Neck radiographs showing retropharyngeal space widening with severe pharyngeal airway narrowing. The width of the prevertebral soft tissue is 3 times the width of the vertebral body.
Fig. 2.
(A) Initial computed tomography (CT) scan of the neck indicating multiseptated retropharyngeal abscesses and multiple enlarged cervical lymph nodes (transverse, sagittal, and coronal views, respectively). (B) Follow-up CT scan of the neck indicating a remnant retropharyngeal abscess after 2-week intravenous antibiotic administration (transverse and coronal views, respectively). (C) Final CT scan of the neck indicating the absence of residual abscess 6 months after antituberculous treatment (transverse and coronal views, respectively).
After making the diagnosing of a retropharyngeal abscess, screening tests for immunodeficiency were performed in light of this being such an uncommon diagnosis in this age range. Absolute neutrophil count, absolute lymphocyte count, lymphocyte subset analysis, respiratory burst assay, CH50, and immunoglobulin (Ig) levels were within normal limits with the exception of an increased IgE (29.3 IU/mL). At follow-up, the IgE level had normalized.
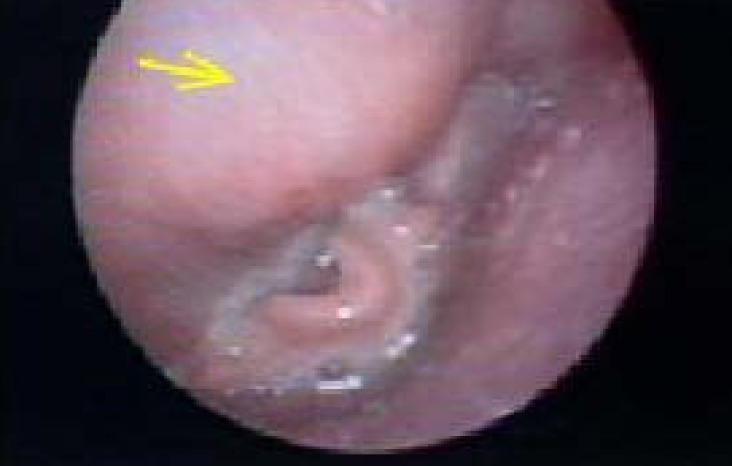
One day after the CT scan, incision and drainage of the right cervical lymph nodes, needle aspiration, and tissue biopsy from the bilateral retropharyngeal spaces were performed by otolaryngologists under general anesthesia. Gram stain and cultures for bacteria and tuberculosis were sent. Despite group B Streptococcus and S. aureus being the most common pathogens in retropharyngeal abscesses, there was no improvement after ampicillin administration. Intravenous antibiotics were changed empirically to vancomycin and imipenem to cover methicillin-resistant S. aureus, gram-negative bacteria, and anaerobes. Ultimately, methicillin-sensitive S. aureus was isolated from the exudate five days after the procedure. Tissue biopsy showed nonspecific chronic inflammation. After these results, cefazolin was administered for an additional two weeks. While he was receiving antibiotic therapy, his symptoms mostly improved except for the hoarseness and weakly audible stridor. Follow-up laboratory tests also normalized. To assess vocal cord injury after ventilator care, direct laryngoscopic examination was done by an otolaryngologist. The vocal cords were observed to move symmetrically, but there was a slight protrusion of a mass-like lesion on the posterior pharyngeal wall (Fig. 3). Follow-up CT scan showed that the abscesses, especially in the left pharyngeal space, still remained (Fig. 2B). Surgery was performed to remove the abscess causing the mass-effect.
Fig. 3.
Direct laryngoscopic examination indicating a protruding mass on the posterior pharyngeal wall.
Afterward, pansensitive M. tuberculosis was cultured from the pus of the first procedure using the automated BACTEC MGIT 960 system (BD, Franklin Lakes, NJ, USA). The patient had a history of Bacillus Calmette-Guerin (BCG) vaccination but we couldn't find any BCG scar on his deltoid. Mantoux testing of the patient and all acid-fast bacilli staining from the exudate, cerebrospinal fluid (CSF), and morning gastric aspirates were negative. Mantoux test and QuantiFERON-TB test were checked in the mother to determine the possibility of congenital tuberculosis infection and were negative. The antituberculosis drugs isoniazid, pyrazinamide, rifampin, and ethambutol were started. On hospital day 29, the infant was discharged without any respiratory symptoms. After three weeks, no mycobacteria were cultured from the exudate of the second procedure, CSF, or gastric aspirates. Ethambutol was discontinued one month after initiation of treatment because of difficulty to evaluate opthalmologic problems. After finishing 6 months antituberculosis therapy (2 months of pyrazinamide and 6 months of isoniazide and rifampin), he had no residual lesion on the neck CT scan (Fig. 2C).
Discussion
Upper respiratory symptoms such as rhinorrhea and sneezing are common in the neonate. Unusual presenting symptoms such as stridor and cyanosis, along with poor response to appropriate treatment, should prompt an expanded differential diagnosis including congenital malformations such as laryngomalacia, subglottic stenosis, and vocal cord paralysis7). In this case, neck radiographs demonstrating retropharyngeal space widening narrowed the differential diagnosis, and a diagnosis of retropharyngeal abscess and cervical lymphadenopathy ultimately were made using CT scanning.
Rhinovirus is a major pathogen causing the common cold and leading to other respiratory diseases such as sinusitis, otitis media, pneumonia, and bacterial infection of the oropharynx8). Localized infection of the oropharynx can result in extension to regional lymph nodes. Bacteria of one infected node can spread to other nodes, as the lymph nodes in the deep neck space are in communication with one other9). This situation is not common, among immunocompetent young infants. Complicating matters is that two species of bacteria were obtained from the exudate of the first procedure. Although S. aureus is one of the common pathogens in retropharyngeal abscess formation in this age group2,3), M. tuberculosis is very rare.
The most common mechanism of neonatal tuberculosis transmission is postnatal inhalation of infected droplets from an adult with pulmonary tuberculosis infection10). Infants are high risk for progression to active disease after initial infection11,12). Their clinical presentations are usually endothoracic in nature, including hilar or paratracheal lymphadenopathy, lobar infiltrations, atelectasis, and miliary tuberculosis10,12). Cervical lymphadenopathy with normal chest radiograph is a rare presentation in this population. In our case, congenital tuberculosis was excluded by performing Mantoux and QuantiFERON-TB test in the mother. His grandmother, one of his close caregivers, had a history of pulmonary tuberculosis treatment 20 years ago, which suggests the possibility of postnatal transmission. But no diagnostic studies for tuberculosis were performed on her. Our case had normal chest radiographs and unusual sites of lymphadenitis. CT scans were also negative for active lung lesions and hilar lymphadenopathy. Tuberculous retropharyngeal abscess formation is a very rare presentation of infection with M. tuberculosis6,13). Most reported cases are associated with spinal tuberculosis, also known as Pott's disease6,14); however, this was not the case in this infant. In those cases, abscesses are the result of direct invasion through the anterior longitudinal ligament of the spine6). Tuberculosis of the middle ear can develop as a primary focus by direct extension from the upper respiratory tract via Eustachian tube in neonates or infants15,16). But we didn't evaluate it because the baby presented no otorrhea and intact tympanic membrane on physical examination. Additionally, M. tuberculosis is a difficult pathogen to isolate. In this case, it was only cultured from one sample of the aspirated exudate.
After much research into the matter, this appears to be the first case of neonatal retropharyngeal abscess coinfection with both S. aureus and M. tuberculosis. In the future, one must consider a variety of possible diagnoses if the baby demonstrates an unusual clinical presentation, as was the case in our patient. Because it is difficult to isolate, M. tuberculosis should be considered in any case in which infection with the bacteria is suspected and response to appropriate antibiotics is suboptimal.
References
- 1.Coulthard M, Isaacs D. Retropharyngeal abscess. Arch Dis Child. 1991;66:1227–1230. doi: 10.1136/adc.66.10.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bourgeois FT, Shannon MW. Retropharyngeal cellulitis in a 5-week-old infant. Pediatrics. 2002;109:E51. doi: 10.1542/peds.109.3.e51. [DOI] [PubMed] [Google Scholar]
- 3.Coticchia JM, Getnick GS, Yun RD, Arnold JE. Age-, site-, and time-specific differences in pediatric deep neck abscesses. Arch Otolaryngol Head Neck Surg. 2004;130:201–207. doi: 10.1001/archotol.130.2.201. [DOI] [PubMed] [Google Scholar]
- 4.Nichol KP, Cherry JD. Bacterial-viral interrelations in respiratory infections of children. N Engl J Med. 1967;277:667–672. doi: 10.1056/NEJM196709282771301. [DOI] [PubMed] [Google Scholar]
- 5.Craig FW, Schunk JE. Retropharyngeal abscess in children: clinical presentation, utility of imaging, and current management. Pediatrics. 2003;111(6 Pt 1):1394–1398. doi: 10.1542/peds.111.6.1394. [DOI] [PubMed] [Google Scholar]
- 6.Mathur NN, Bais AS. Tubercular retropharyngeal abscess in early childhood. Indian J Pediatr. 1997;64:898–901. doi: 10.1007/BF02725522. [DOI] [PubMed] [Google Scholar]
- 7.Daniel SJ. The upper airway: congenital malformations. Paediatr Respir Rev. 2006;7(Suppl 1):S260–S263. doi: 10.1016/j.prrv.2006.04.227. [DOI] [PubMed] [Google Scholar]
- 8.Peltola V, Waris M, Osterback R, Susi P, Hyypia T, Ruuskanen O. Clinical effects of rhinovirus infections. J Clin Virol. 2008;43:411–414. doi: 10.1016/j.jcv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Pappas DE, Hendley JO. Retropharyngeal abscess, lateral pharyngeal abscess, and peritonsillar cellulitis/abscess. In: Kliegman RM, Stanton BF, St. Geme JW, Schor NF, Behrman RE, editors. Nelson textbook of pediatrics. 19th ed. Philadelphia: Elsevier Saunders; 2011. pp. 1440–1442. [Google Scholar]
- 10.Moven SE. Postnatal bacterial infections. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Neonatal-perinatal medicine. 9th ed. Missouri: Elsevier; 2011. pp. 826–827. [Google Scholar]
- 11.Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B. Paediatric tuberculosis. Lancet Infect Dis. 2008;8:498–510. doi: 10.1016/S1473-3099(08)70182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaaf HS, Collins A, Bekker A, Davies PD. Tuberculosis at extremes of age. Respirology. 2010;15:747–763. doi: 10.1111/j.1440-1843.2010.01784.x. [DOI] [PubMed] [Google Scholar]
- 13.Carroll N, Bain RJ, Tseung MH, Edwards RH. Tuberculous retropharyngeal abscess producing respiratory obstruction. Thorax. 1989;44:599–600. doi: 10.1136/thx.44.7.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizumura K, Machino T, Sato Y, Ooki T, Hayashi K, Nakagawa Y, et al. Tuberculous retropharyngeal abscess associated with spinal tuberculosis well controlled by fine-needle aspiration and anti-tuberculous chemotherapy. Intern Med. 2010;49:1155–1158. doi: 10.2169/internalmedicine.49.3353. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen T, Townsend H, Stauffer W, Belani K, Kamat D. A case of tuberculous otitis media. Clin Pediatr (Phila) 2006;45:83–87. doi: 10.1177/000992280604500114. [DOI] [PubMed] [Google Scholar]
- 16.Kirsch CM, Wehner JH, Jensen WA, Kagawa FT, Campagna AC. Tuberculous otitis media. South Med J. 1995;88:363–366. doi: 10.1097/00007611-199503000-00024. [DOI] [PubMed] [Google Scholar]