Abstract
Background
Gastric cancers with microsatellite instabilities (MSI) have been reported to be associated with favorable prognosis. However, the significance of the effect of MSI on the clinicopathological features, as well as its association with mucin phenotype, remains unclear.
Methods
MSI status was assessed in 414 cases of gastric cancer using polymerase chain reaction analysis of five microsatellite loci, as recommended by National Cancer Institution criteria. The expression of mucins (MUC5AC, MUC6, MUC2, and CD10) was assessed.
Results
Out of 414 total cases of gastric cancer, 380 (91.7%), 11 (2.7%), and 23 (5.6%) were microsatellite stable (MSS), low-level MSI (MSI-L), and high-level MSI (MSI-H), respectively. Compared to MSS/MSI-L, MSI-H gastric cancers were associated with older age (p=0.010), tumor size (p=0.014), excavated gross (p=0.042), intestinal type (p=0.028), aggressive behaviors (increase of T stage [p=0.009]), perineural invasion [p=0.022], and lymphovascular emboli [p=0.027]). MSI-H gastric cancers were associated with tumor necrosis (p=0.041), tumor-infiltrating lymphocytes (≥2/high power field, p<0.001), expanding growth patterns (p=0.038), gastric predominant mucin phenotypes (p=0.028), and MUC6 expression (p=0.016). Tumor necrosis (≥10% of mass, p=0.031), tumor-infiltrating lymphocytes (p<0.001), intestinal type (p=0.014), and gastric mucin phenotypes (p=0.020) could represent independent features associated with MSI-H gastric cancers. MSI-H intestinal type gastric cancers had a tendency for poor prognosis in univariate analysis (p=0.054) but no association in Cox multivariate analysis (p=0.197).
Conclusions
Our data suggest that MSI-H gastric cancers exhibit distinct aggressive biologic behaviors and a gastric mucin phenotype. This contradicts previous reports that describe MSI-H gastric cancer as being associated with favorable prognosis.
Keywords: Stomach, Adenocarcinoma, Microsatellite instability, Mucin, Survival
Gastric cancer is one of the most prevalent malignancies and represents the second most common cause of cancer death worldwide.1 Microsatellite instability (MSI) is characterized by length mutations in tandem oligonucleotide repeats that are associated with defective DNA mismatch repair.2 In order to standardize MSI classification, a panel of microsatellite loci were established to test for MSI, including mononucleotide (BAT25 and BAT26) and dinucleotide (D2S123, D5S346, and D17S25) repeats (National Cancer Institute [NCI] Bethesda guidelines).3 Based on these NCI guidelines, MSI status is classified as high-level MSI (MSI-H), low-level MSI (MSI-L), or microsatellite stable (MSS). Gastric cancer with microsatellite instability (MSI-H) is reported to display distinct clinical and molecular features compared to MSS gastric cancer.4-7 MSI-H gastric cancer is associated with defective DNA mismatch repair (MMR) in cancer and inactivation of MMR genes (hMLH1 and hMSH2) by mutation or methylation.6
MSI-H colorectal cancers have been demonstrated to have better overall survival compared to MSS colorectal cancers.8 Histopathologic findings such as poor differentiation, mucinous histology, right-sided location, increased tumor-infiltrating lymphocytes (TILs), and prominent lymphoid aggregates at the advancing edge (Crohn's-like reaction) are also commonly used to predict MSI-H in colorectal cancers.9-11 In contrast to MSI-H colorectal cancers, the clinical significance of MSI status in gastric cancer remains controversial. Some investigators have reported that MSI-H gastric cancer is associated with distinct clinicopathological features (intestinal type, older age, distal tumor location, and lower incidence of lymph node metastasis) and better overall prognosis.4-7 However, other studies have reported no significant relationship between MSI status and clinical outcomes.12-14 Furthermore, there have been few reports on the utility of histopathological features to predict MSI status in gastric cancer, similar to colorectal cancers, as well the association of MSI with mucin phenotypes.15 Therefore, we investigated MSI status and its possible relationships with clinicopathological features, patient overall survival, mucin expression, and phenotypes in a large series of gastric cancers (414 gastric cancers in a high-risk population in Korea). We also analyzed the utility of histopathologic features to characterize MSI-H gastric cancer using logistic regression analysis.
MATERIALS AND METHODS
Clinicopathological and survival analyses in gastric cancer
We studied a cohort of 414 gastric cancer patients who underwent gastrectomy with lymph node dissection at Pusan National University Hospital (PNUH) between 2005 and 2007, which was same cohort previously published. The group comprised 288 males and 126 females with a mean age of 59.0 years (range, 42 to 75 years). Standard formalin-fixed and paraffin-embedded sections were obtained from the Department of Pathology, PNUH, and the National Biobank of Korea, PNUH. The Institutional Review Board of PNUH approved this study. None of the patients received preoperative radiotherapy and/or chemotherapy. We assessed the following clinicopathological factors according to the Korean Standardized Pathology Report for Gastric Cancer as well as the American Joint Committee on Cancer Staging Manual, 7th edition: site, gross type, tumor size, depth of invasion, histological classification (i.e., intestinal or diffuse), and lymphovascular invasion.16-18 To monitor clinical outcomes, patients were followed up from the date of surgery to the date of death or January 1, 2012. The follow-up period ranged from approximately 1-82 months (mean, 52.9 months). The cases lost to follow-up or death from any cause other than gastric cancer were designated censored data and were not included in our analysis of survival rates.
We assessed the following histopathological features by examination of standard hematoxylin and eosin stained slides: 1) extent of tumor necrosis, <10% vs. ≥10% of tumor mass, 2) proportion of extracellular mucin formation, <10% vs. ≥10% of tumor mass, 3) presence of Crohn's-like reaction (a minimum of three lymphoid aggregates), 4) TILs, <2/high power field (HPF) vs. ≥2/HPF, and 5) growth pattern of tumor at the advancing edge (expanding, infiltrative, or mixed).
Immunohistochemical staining for mucin phenotypes
Sections were dewaxed and rehydrated according to standard procedure and washed with phosphate buffered saline (PBS). For immunohistochemical staining, sections were heated twice in a 600-W microwave oven, for 5 minutes each, in 0.01 M citrate buffer (pH 6.0). Sections were immersed in 3% H2O2 to quench endogenous peroxidase activity, and unspecified binding was blocked in 5% normal goat serum (0.1% bovine serum albumin in PBS). Immunohistochemical staining was performed using the avidin-biotin peroxidase complex method with aminoethylcarbazole as a chromogen using the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer's instructions. Sections were counterstained with Mayer's hematoxylin solution. Immunohistochemical staining was carried out using monoclonal antibodies against the mucin antigens (Table 1).
Table 1.
Primary antibodies used in this study
MUC5AC and MUC6 reflect gastric phenotypes and are markers for gastric foveolar cells and antral/cardiac mucous glandular cells, respectively. MUC2 and CD10 exhibit the typical intestinal epithelial cell phenotype and are markers for goblet cells and the brush border of intestinal absorptive epithelial cells, respectively. Adenocarcinomas with at least 10% reactivity for each mucin were identified as positive. On the basis of the combination of positive staining for MUC5AC, MUC2, MUC6, and CD10, the cases were further subdivided into gastric mucin predominant (GC-GPs), intestinal mucin predominant (GC-IPs), and null phenotype, as described previously.19
Microsatellite analysis
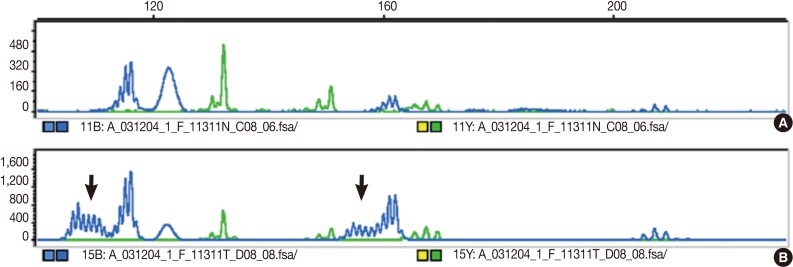
The DNA of cancerous tissue and the corresponding normal gastric mucosa were obtained from formalin-fixed and paraffin-embedded surgical blocks. The DNA was extracted by proteinase K digestion and the phenol-chloroform procedure. The extracted DNA was amplified by polymerase chain reaction (PCR) with fluorescent dye-labeled primers targeting five microsatellite loci: BAT25, BAT26, D5S346, D2S123, and D17S250, as recommended by the NCI guidelines.3 DNA was detected using a temperature-controlled DNA sequencer (PRISM 377, Perkin-Elmer Corp., Foster City, CA, USA), and fragment analyses were carried out with GeneScan software (Perkin-Elmer Corp.). MSI status was determined by size variation and the presence of additional bands in the PCR product from tumor DNA that were not observed in the DNA from normal tissue from the same patients (Fig. 1). In accordance with the NCI criteria,3 MSI-H was defined as instability in at least two of the five microsatellite loci; MSI-L as instability in only one locus; and MSS when none of the loci were shifted.
Fig. 1.
Microsatellite status is determined by size variation and the occurrence of additional bands (arrows) in the polymerase chain reaction product from tumor DNA (B) that are not observed in the analysis of DNA from normal tissue (A) from the same patients.
Statistical analysis
Clinicopathological features were analyzed by Student's t-test, the χ2 test, or Fisher's exact test to determine differences between MSI-H vs. MSS and MSI-L status. Histopathological factors associated with MSI-H gastric cancers were identified using the binary logistic regression analysis. Cumulative survival plots were obtained using the Kaplan-Meier method, and significance was compared using the log-rank test. Prognostic factors were identified using the Cox regression stepwise method (proportional hazard model) adjusted for the patient age, tumor site, depth of invasion, and lymph node metastasis. Statistical significance was set at p<0.05. Statistical calculations were performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
MSI-H gastric cancer was associated with increased aggressive behavior
Of the 414 gastric cancers examined, 380 (91.7%), 11 (2.7%), and 23 cases (5.6%) were MSS, MSI-L, and MSI-H, respectively, based on the NCI criteria (Table 2). Compared to MSS and MSI-L gastric cancers, MSI-H cancers were significantly associated with older age (p=0.010), increased tumor size (p=0.014), more excavated gross features (p=0.042), and intestinal histotypes (p=0.028). Furthermore, MSI-H gastric cancers exhibited more aggressive behaviors than MSS and MSI-L cancers, such as increased T stage (p=0.009), presence of perineural invasion (p=0.022), and lymphovascular tumor emboli (p=0.027) (Table 2). There was no significant relationship between MSI status and tumor location, gender, or lymph node metastasis.
Table 2.
Clinicopathologic characteristics and MSI status in 414 gastric cancers
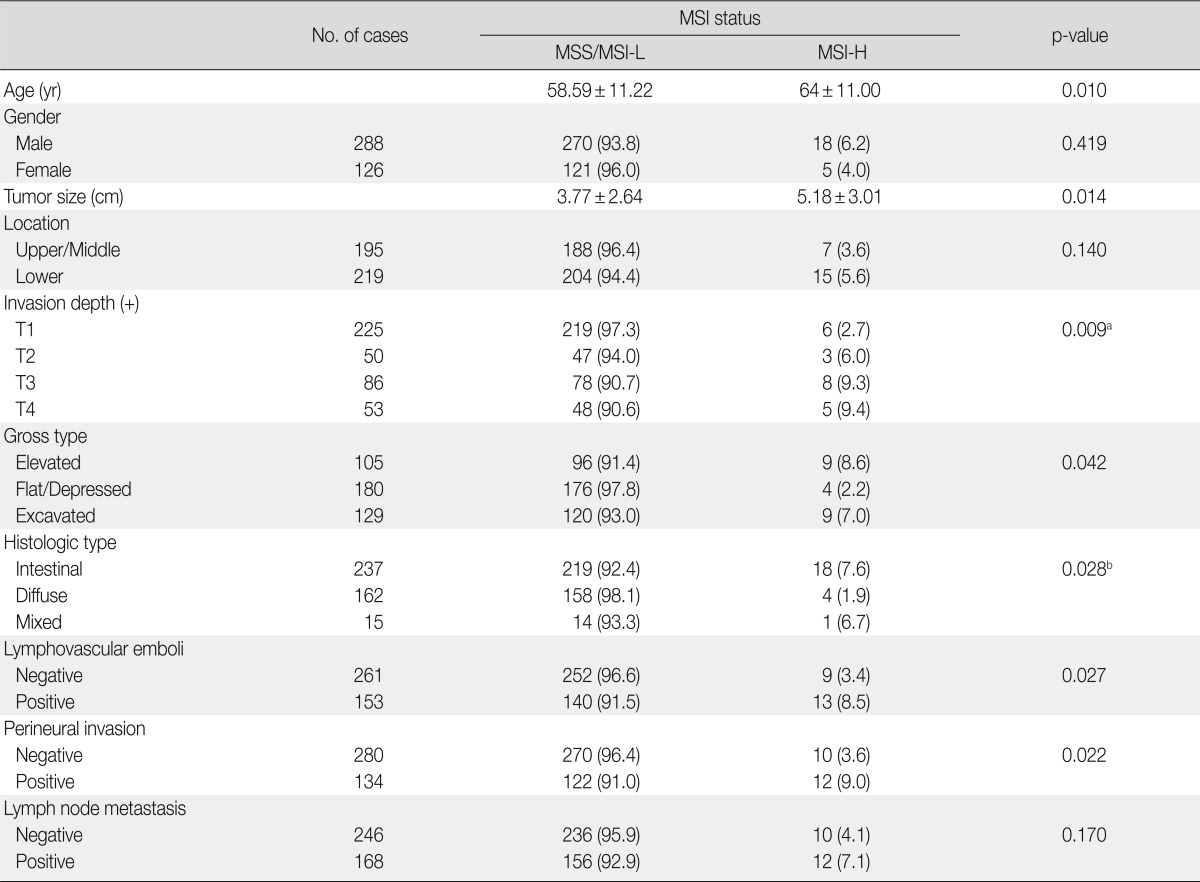
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI.
aBetween T1 vs. T2+T3+T4; bBetween intestinal type+mixed type vs. diffuse type.
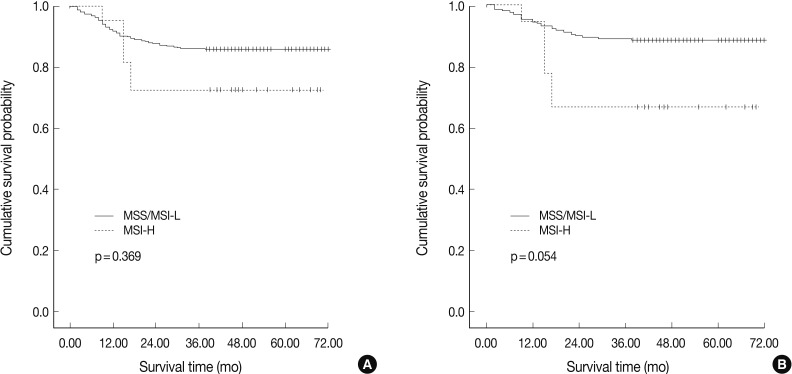
In agreement with our finding of a positive relationship between MSI-H and increased aggressiveness (increased depth of invasion, presence of lymphovascular tumor emboli, and perineural invasion), there was a tendency for MSI-H to associate with reduced overall survival in intestinal-type gastric cancer (p=0.054), but not in overall gastric cancer (p=0.369) (Fig. 2). MSI-H intestinal-type gastric cancers were associated with poorer survival compared to MSS/MSI-L intestinal-type gastric cancers (71.0±1.6 months vs. 58.6±7.2 months). However, MSI status was not identified as an independent prognostic factor after adjusting for tumor location, depth of invasion, and lymph node status in the Cox regression proportional hazard model (p=0.197) (Table 3).
Fig. 2.
Overall survival rates according to microsatellite status for all gastric cancers (A) and intestinal-type gastric cancers (B). High-level microsatellite instability (MSI-H) gastric cancer is associated with reduced survival compared to microsatellite stable (MSS)/low-level MSI (MSI-L) gastric cancers in intestinal-type gastric cancers.
Table 3.
Multivariate survival analysis with Cox regression model in intestinal type of gastric cancers
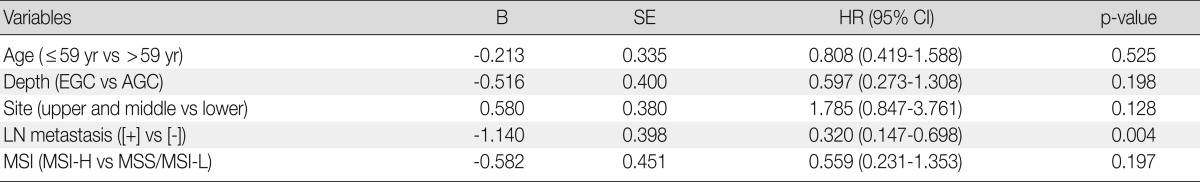
B, coefficient; SE, standard error; HR, hazard ratio; CI, confidence interval; EGC, early gastric cancer; AGC, advanced gastric cancer; LN, lymph node; MSI, microsatellite instability; MSI-H, high-level MSI; MSS, microsatellite stable; MSI-L, low-level MSI.
MSI-H gastric cancer was associated with TILs and gastric predominant mucin phenotypes
We examined the histopathological features that were associated with MSI and found that tumor necrosis (p=0.041), presence of TIL (≥2/HPF) (p<0.001), and expanding/mixed growth patterns (p=0.038) were associated with MSI-H gastric cancer compared to MSS/MSI-L gastric cancers (Table 4, Fig. 3). Crohn's-like lymphoid aggregates and extracellular mucin formation were not features of MSI-H gastric cancers.
Table 4.
Relationship between MSI status and histopathologic features in 414 gastric cancers
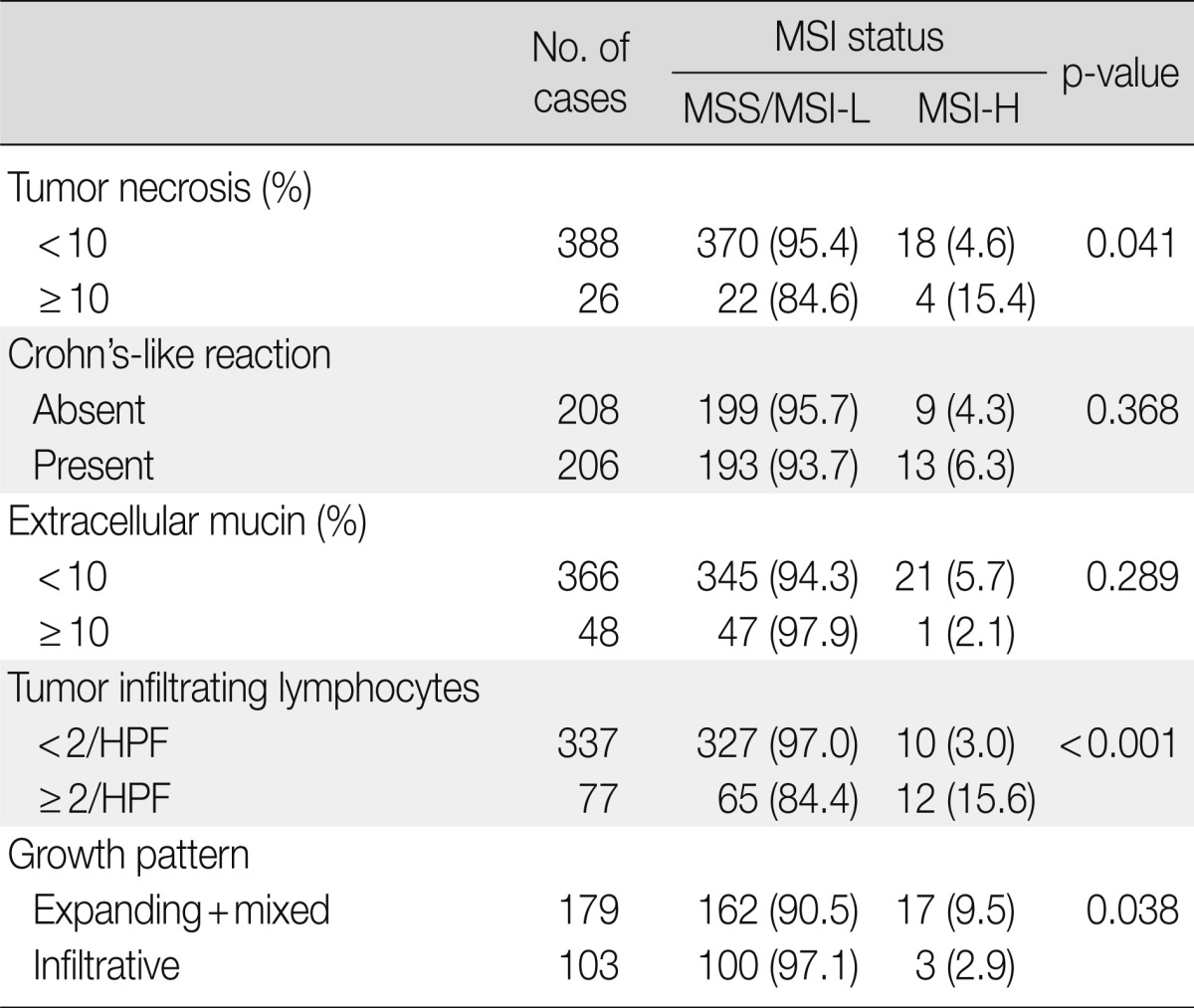
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI; HPF, high power filed.
Fig. 3.
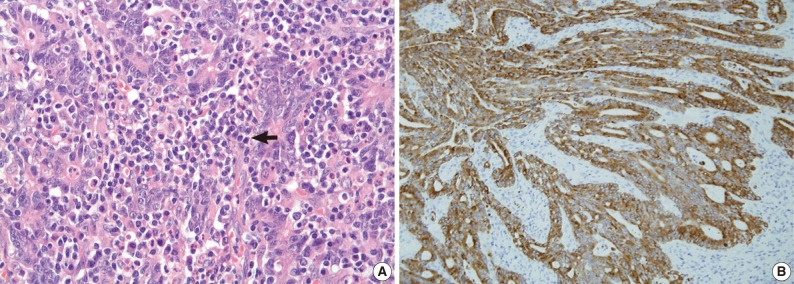
High-level microsatellite instability gastric cancer exhibits increased tumor-infiltrating lymphocytes (arrow) (A) and cytoplasmic positivity for MUC6 mucin expression (B).
Mucin expression analysis of the 414 gastric cancers determined that 35.5% (147/414), 67.4% (279/414), 44.9% (186/414), and 20.5% (85/414) were positive for MUC2, MUC5AC, MUC6, and CD10, respectively (Table 4). In contrast to MSS and MSI-L cancer, MSI-H gastric cancer was associated with MUC6 expression (p=0.024) (Fig. 3). There was no statistically significant difference in MUC5AC, MUC2, or CD10 expression between MSI-H and MSS/MSI-L gastric cancers. Based on their mucin expression patterns, gastric cancers were further subdivided into GC-GPs (248/414, 59.9%), GC-IPs (118/414, 28.5%), and null phenotypes (48/414, 11.6%). MSI-H gastric cancer demonstrated GC-GPs phenotypes compared to MSS and MSI-L gastric cancers (p=0.031) (Table 5). Using binary logistic regression analysis, we determined that tumor necrosis (≥10% of tumor mass) (p=0.031), TILs (≥2/HPF) (p<0.001), intestinal histologic type (p=0.014), and gastric predominant mucin phenotypes (p=0.020) could be considered independent features associated with MSI-H gastric cancers (Table 6).
Table 5.
Relationship between MSI status and mucin phenotypes and mucin expression in 414 gastric cancers
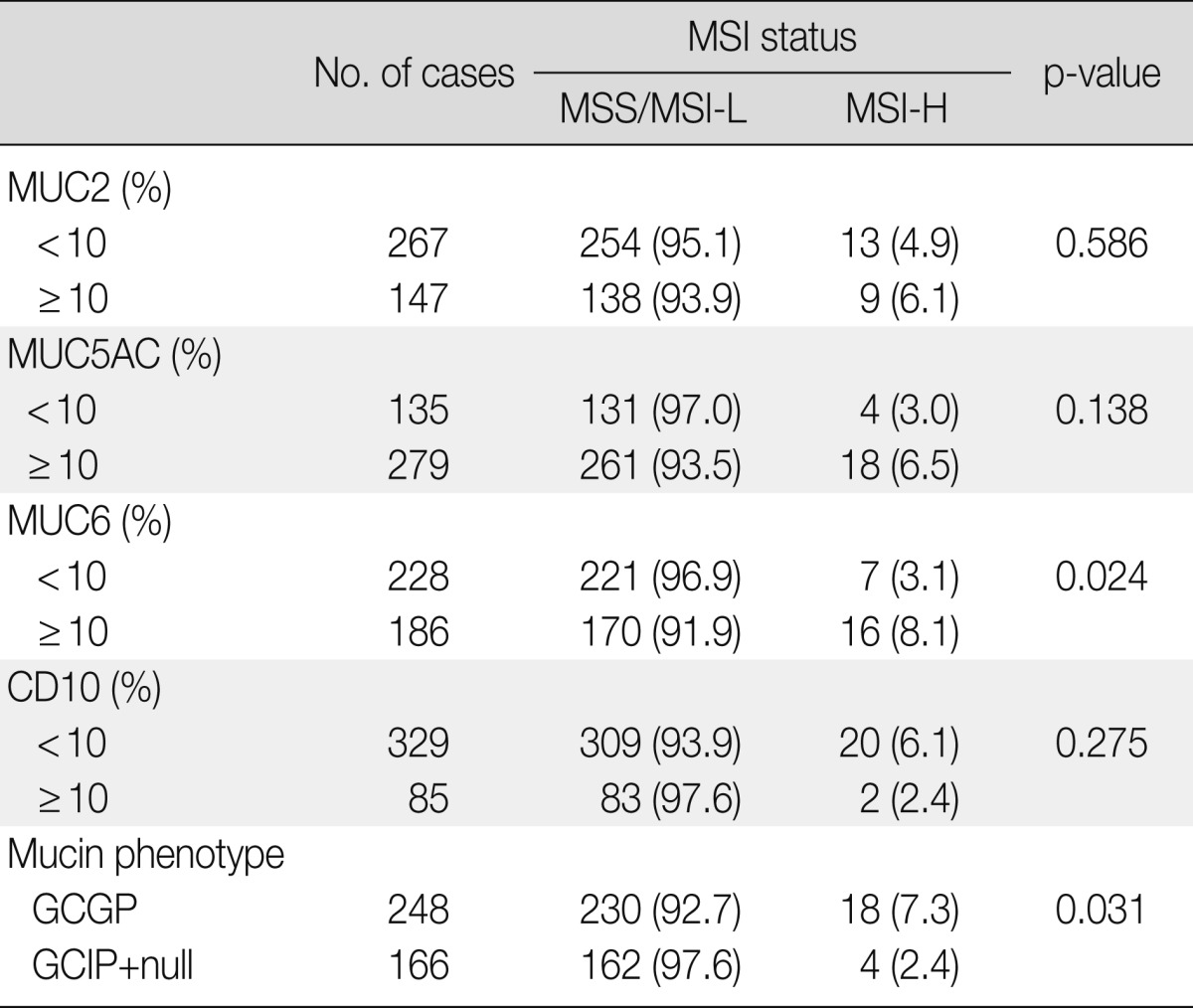
MSI, microsatellite instability; MSS, microsatellite stable; MSI-L, low-level MSI; MSI-H, high-level MSI; GCGP, gastric cancer with gastric mucin predominant type; GCIP, gastric cancer with intestinal mucin predominant type.
Table 6.
Histopathologic features associated with MSI-H gastric cancers
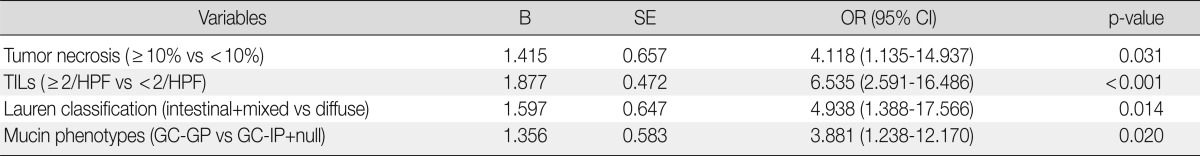
The clinicopathologic factors for MSI-H gastric cancers are analyzed by binary logistic regression analysis (backward, stepwise).
MSI-H, high-level microsatellite instability; B, coefficient; SE, standard error; OR, odds ratio; CI, confidence interval; TIL, tumor-infiltrating lymphocytes; HPF, high power field; GC-GP, gastric cancer with gastric mucin predominant type; GC-IP, gastric cancer with intestinal mucin predominant type.
DISCUSSION
In this study, MSI status in gastric cancers was significantly associated with tumor progression, lymphovascular and perineural invasion, and poor prognosis. Furthermore, we confirmed previous reports that stated that MSI-H gastric cancers are significantly associated with older age, increased tumor size, and intestinal histologic type. It was also revealed that tumor necrosis (≥10% of tumor mass), TILs (≥2/HPF) (p<0.001), intestinal histologic type (p=0.014), and gastric predominant mucin phenotypes (p=0.020) could represent independent features associated with MSI-H gastric cancers.
It has been reported that MSI-H cancers are associated with defects involving DNA MMR genes such as hMLH1 and hMSH2 as a result of mutations or promoter methylation.20 MSI-H colorectal cancer was reported to have good survival rates, more favorable responses to chemotherapy, and is an indicator of hereditary non-polyposis colorectal carcinoma syndrome.8,21,22 The recent discovery of impaired epithelial-to-mesenchymal transition in MSI-H colorectal cancers provides a molecular mechanism supporting the favorable prognosis of MSI-H colorectal cancers.23 Based on the clinical significance of MSI in colorectal cancers, many attempts to identify histopathologic predictors of MSI status have been conducted.9-11 In contrast to colorectal cancer, the clinical significance and role of MSI in gastric cancer remain controversial (Table 7). It has been reported that MSI-H gastric cancer is associated with older age, increased tumor size, and intestinal histologic type, which is in accordance with our data. However, there are conflicting reports of the relationship between MSI status and gastric cancer aggressiveness and survival. The present study demonstrated that, compared to MSS and MSI-L gastric cancers, MSI-H cancers exhibit aggressive behaviors (increase of T stage, presence of perineural invasion, and lymphovascular tumor emboli). Furthermore, poor survival was demonstrated for MSI-H gastric cancer in intestinal-type gastric cancers, but not in diffuse or overall gastric cancers. In accordance with our data, Oki et al.24 reported increased lymphovascular tumor emboli, and Seo et al.12 reported increased lymphatic invasion (p=0.104) and lymph node metastasis (p=0.157) in MSI-H gastric cancer compared to MSS/MSI-L gastric cancers. Recently, a study comprising a large series of gastric cancer patients demonstrated clinical significance of MSI status.24,25 An et al.25 reported the largest series of Koreans (1,990 gastric cancer patients), showing no prognostic significance of the disease. They revealed that there was no significant difference of disease-free survival between MSS/MSI-L and MSI-H gastric cancers at each clinical stage of I, II, III, and IV. Also it was reported that there were no benefits of 5-fluorouracil (5-FU)-based adjuvant chemotherapy in MSI-H gastric cancer compared to better disease-free survival in the MSS/MSI-L gastric cancers.25 Oki et al.24 reported that MSI status has no significant effect on overall survival or response to 5-FU in gastric cancer. These conflicting reports render it difficult to reach a conclusion regarding the effect of MSI in gastric cancer and are in contrast to the consensus data that point to a favorable prognosis in MSI-H colorectal cancers. We speculate that the reasons for this difference might be due to the high heterogeneity of gastric cancer in terms of morphological, phenotypic, and molecular aspects.
Table 7.
Reproted datasets on the microsatellite instability and survival in gastric cancer
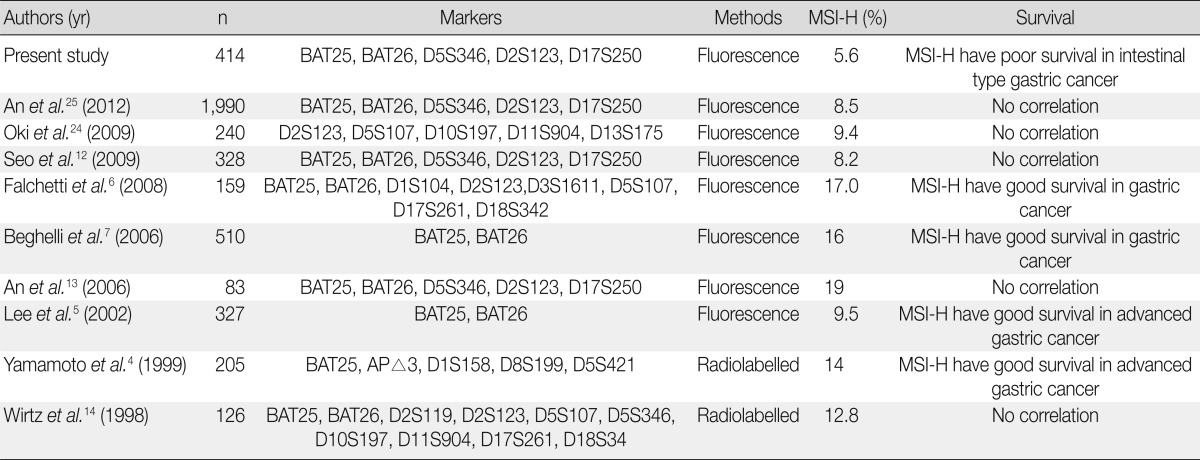
MSI-H, high-level microsatellite instability.
Gastric cancer is usually divided into two groups based on the tendency of gland formation: intestinal (differentiated) and diffuse (undifferentiated). It was reported that intestinal- and diffuse-type gastric cancers demonstrate different phenotypic and molecular features.26-28 In addition, there is the possibility of gastric cancer type being influenced by different types and numbers of microsatellite markers (2-10 markers), as well as ethnic differences (tendency of lower MSI-H gastric cancer prevalence in Asians [commonly <10% of all gastric cancer cases]) (Table 6). In accordance with previous studies,29,30 our study demonstrated that MUC6-positive or gastric mucin-phenotype gastric cancers were associated with MSI-H gastric cancer. Compared to previous reports that limited data to intestinal-type early gastric cancer30 or intestinal-type gastric cancer,29 we investigated a large series of all gastric cancer types (intestinal+diffuse types) and demonstrated the importance of MUC6 or gastric mucin phenotypes in view of MSI status in gastric cancer. Furthermore, we investigated histopathologic parameters associated with MSI status of gastric cancers. We found that tumor necrosis, expanding growth pattern, and TILs were associated with MSI-H gastric cancers. Unlike in colorectal cancers, poor differentiation (diffuse-type morphology), extracellular mucin, and prominent lymphoid aggregates at advancing edge (Crohn's-like reaction) were not associated with MSI status in gastric cancer.9-11 We assume that these differences are associated with different roles of MSI between gastric and colorectal cancers. Further analysis on a defined patient population will be required to achieve consensus on the utility of microsatellite repeats as predictive markers of prognosis and response to chemotherapy in gastric cancer.
Acknowledgments
This study was supported by a two-year research grant of Pusan National University. The biospecimens for this study were provided by the Pusan National University Hospital, a member of the National Biobank of Korea, which is supported by the Ministry of Health, Welfare and Family Affairs. All samples derived from the National Biobank of Korea were obtained with informed consent under Institutional Review Board-approved protocols.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Thibodeau SN, French AJ, Roche PC, et al. Altered expression of hMSH2 and hMLH1 in tumors with microsatellite instability and genetic alterations in mismatch repair genes. Cancer Res. 1996;56:4836–4840. [PubMed] [Google Scholar]
- 3.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 4.Yamamoto H, Perez-Piteira J, Yoshida T, et al. Gastric cancers of the microsatellite mutator phenotype display characteristic genetic and clinical features. Gastroenterology. 1999;116:1348–1357. doi: 10.1016/s0016-5085(99)70499-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS, Choi SI, Lee HK, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol. 2002;15:632–640. doi: 10.1038/modpathol.3880578. [DOI] [PubMed] [Google Scholar]
- 6.Falchetti M, Saieva C, Lupi R, et al. Gastric cancer with high-level microsatellite instability: target gene mutations, clinicopathologic features, and long-term survival. Hum Pathol. 2008;39:925–932. doi: 10.1016/j.humpath.2007.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Beghelli S, de Manzoni G, Barbi S, et al. Microsatellite instability in gastric cancer is associated with better prognosis in only stage II cancers. Surgery. 2006;139:347–356. doi: 10.1016/j.surg.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 8.Hemminki A, Mecklin JP, Järvinen H, Aaltonen LA, Joensuu H. Microsatellite instability is a favorable prognostic indicator in patients with colorectal cancer receiving chemotherapy. Gastroenterology. 2000;119:921–928. doi: 10.1053/gast.2000.18161. [DOI] [PubMed] [Google Scholar]
- 9.Alexander J, Watanabe T, Wu TT, Rashid A, Li S, Hamilton SR. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol. 2001;158:527–535. doi: 10.1016/S0002-9440(10)63994-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halvarsson B, Anderson H, Domanska K, Lindmark G, Nilbert M. Clinicopathologic factors identify sporadic mismatch repair-defective colon cancers. Am J Clin Pathol. 2008;129:238–244. doi: 10.1309/0PP5GDRTXUDVKAWJ. [DOI] [PubMed] [Google Scholar]
- 11.Greenson JK, Huang SC, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33:126–133. doi: 10.1097/PAS.0b013e31817ec2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo HM, Chang YS, Joo SH, et al. Clinicopathologic characteristics and outcomes of gastric cancers with the MSI-H phenotype. J Surg Oncol. 2009;99:143–147. doi: 10.1002/jso.21220. [DOI] [PubMed] [Google Scholar]
- 13.An C, Choi IS, Yao JC, et al. Prognostic significance of CpG island methylator phenotype and microsatellite instability in gastric carcinoma. Clin Cancer Res. 2005;11(2 Pt 1):656–663. [PubMed] [Google Scholar]
- 14.Wirtz HC, Müller W, Noguchi T, et al. Prognostic value and clinicopathological profile of microsatellite instability in gastric cancer. Clin Cancer Res. 1998;4:1749–1754. [PubMed] [Google Scholar]
- 15.Mizoshita T, Tsukamoto T, Cao X, et al. Microsatellite instability is linked to loss of hMLH1 expression in advanced gastric cancers: lack of a relationship with the histological type and phenotype. Gastric Cancer. 2005;8:164–172. doi: 10.1007/s10120-005-0331-x. [DOI] [PubMed] [Google Scholar]
- 16.Kim WH, Park CK, Kim YB, et al. A standardized pathology report for gastric cancer. Korean J Pathol. 2005;39:106–113. [Google Scholar]
- 17.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 18.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2009. [Google Scholar]
- 19.Tsukashita S, Kushima R, Bamba M, Sugihara H, Hattori T. MUC gene expression and histogenesis of adenocarcinoma of the stomach. Int J Cancer. 2001;94:166–170. doi: 10.1002/ijc.1460. [DOI] [PubMed] [Google Scholar]
- 20.Wu MS, Lee CW, Shun CT, et al. Distinct clinicopathologic and genetic profiles in sporadic gastric cancer with different mutator phenotypes. Genes Chromosomes Cancer. 2000;27:403–411. [PubMed] [Google Scholar]
- 21.Jass JR. Diagnosis of hereditary non-polyposis colorectal cancer. Histopathology. 1998;32:491–497. doi: 10.1046/j.1365-2559.1998.00442.x. [DOI] [PubMed] [Google Scholar]
- 22.Lynch HT, Smyrk T, Lynch JF. Overview of natural history, pathology, molecular genetics and management of HNPCC (Lynch syndrome) Int J Cancer. 1996;69:38–43. doi: 10.1002/(SICI)1097-0215(19960220)69:1<38::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 23.Pino MS, Kikuchi H, Zeng M, et al. Epithelial to mesenchymal transition is impaired in colon cancer cells with microsatellite instability. Gastroenterology. 2010;138:1406–1417. doi: 10.1053/j.gastro.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oki E, Kakeji Y, Zhao Y, et al. Chemosensitivity and survival in gastric cancer patients with microsatellite instability. Ann Surg Oncol. 2009;16:2510–2515. doi: 10.1245/s10434-009-0580-8. [DOI] [PubMed] [Google Scholar]
- 25.An JY, Kim H, Cheong JH, Hyung WJ, Kim H, Noh SH. Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection. Int J Cancer. 2012;131:505–511. doi: 10.1002/ijc.26399. [DOI] [PubMed] [Google Scholar]
- 26.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma: an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura K, Sugano H, Takagi K. Carcinoma of the stomach in incipient phase: its histogenesis and histological appearances. Gann. 1968;59:251–258. [PubMed] [Google Scholar]
- 28.Ming SC. Cellular and molecular pathology of gastric carcinoma and precursor lesions: a critical review. Gastric Cancer. 1998;1:31–50. doi: 10.1007/s101200050053. [DOI] [PubMed] [Google Scholar]
- 29.Yamazaki K, Tajima Y, Makino R, et al. Tumor differentiation phenotype in gastric differentiated-type tumors and its relation to tumor invasion and genetic alterations. World J Gastroenterol. 2006;12:3803–3809. doi: 10.3748/wjg.v12.i24.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin N, Kim HY, Kim WK, et al. Molecular biological characteristics of differentiated early gastric cancer on the basis of mucin expression. Korean J Pathol. 2011;45:69–78. [Google Scholar]