Abstract
A case of peripheral primitive neuroectodermal tumor of the small bowel mesentery with osseous component is reported. A 23-year-old man was admitted to our hospital because of acute severe abdominal pain. Abdominal computed tomography revealed a large solid and cystic, oval shaped mass, measuring 11.0×6.0 cm in the pelvic cavity. Histologically the resected lesion consisted of sheets of undifferentiated small round cells forming Homer-Wright rosettes and perivascular pseudorosettes, and showed areas of osteoid and bone formation. Immunohistochemical studies revealed that tumor cells expressed positivity against CD99 (MIC2), CD57, neuron-specific enolase, and vimentin. Fluorescence in situ hybridization study revealed Ewing sarcoma breakpoint region 1 (EWSR1) gene rearrangement on chromosome 22q12. To the authors' knowledge this is the first documentation of a peripheral neuroectodermal tumor with osteoid and bone formation of the small bowel mesentery.
Keywords: Neuroectodermal tumor, primitive, peripheral; Intestine, small; Osteogenesis; Metaplasia; EWSR1
Peripheral primitive neuroectodermal tumor (pPNET) is a rare, small, round cell malignancy that appears to develop from neuroectodermal cells1 in children and young adults. Also, now included in the pPNET-Ewing's sarcoma family are extra-osseous Ewing's sarcoma, peripheral neuroepithelioma, Askin's tumor of the thoracopulmonary wall, and peripheral neuroblastoma.2 Only four cases of pPNET arising from small bowel mesentery have been reported.3-6 It is known that tumors of neural crest origin show bidirectional or multidirectional differentiation.7-9 To the best of our knowledge, there has been no report of pPNET showing osteoid and bone production. A case which presented with severe acute abdominal pain due to a ruptured tumor is reported herein. This pPNET case developed from the small bowel mesentery with osteoid and bone formation. One year after surgery, recurrent tumor was resected, and osteogenesis persisted.
CASE REPORT
A 23-year-old man was referred to Inha University Hospital due to severe abdominal pain. Computed tomography revealed an ovoid solid and cystic tumor in the pelvic cavity, measuring 11.0×6.0 cm. Emergency surgery was performed, and a large ruptured mass was found in the jejunal mesentery, 1 cm from the ligament of Treitz. The tumor involved the jejunal wall, and another mass the size of a thumb was detected within the porta hepatis. Disseminated miliary nodules were present in the greater omentum, the right colonic gutter, and the pelvic peritoneum. Segmental resection of the small intestine and omentectomy were performed. Postoperative laboratory examination revealed normal levels of serum lactate dehydrogenase, cancer antigen 125, and carcinoembryonic antigen, and postoperative positron emission tomography computed tomography was normal. After operation, chemotherapy (vincristine, ifosfamide, etoposide, and doxorubicin) was performed. One year after the operation, a 10 cm, locally recurrent mesenteric mass was resected.
Macroscopically, a tumor measuring 12.0×8.0×7.5 cm was located in the mesentery of jejunum. Rupture was seen in the surface of tumor. The tumor showed a whitish-pink solid cut surface with foci of hemorrhage, extensive necrosis, and myxoid change. The jejunal wall was involved directly by the tumor and contained focal mucosal ulceration. The cut surface was white-gray and dense (Fig. 1).
Fig. 1.
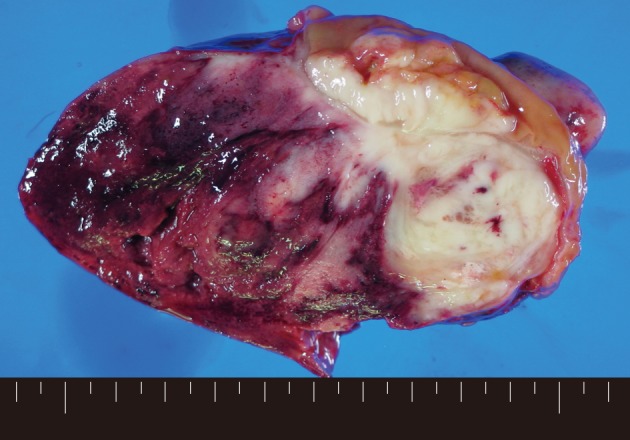
Grossly, the resected tumor, measuring 12.0×8.0×7.5 cm, is located in the mesentery of the jejunum. The cut surface of tumor demonstrates a whitish-pink solid appearance with foci of hemorrhage, extensive necrosis, and myxoid features. The border is irregular, and the tumor directly invades the wall of jejunum with focal ulceration.
Microscopically, the entire wall of the jejunum contained tumor cells which also proliferated within the mesentery. The mucosa of the jejunum was involved by the tumor with surface erosion (Fig. 2A). Small round cells containing uniform vesicular, round or oval nuclei, scanty clear, or eosinophilic cytoplasms and indistinct cytoplasmic borders comprised the tumor in sheet or lobule formation (Fig. 2B). Often, intermingled spindle-shaped cells were found. Though some cells had a single prominent nucleolus, most cells had indistinct nucleoli. Well-formed Homer-Wright rosettes were frequently observed (Fig. 2B). Flexner-Wintersteiner rosettes did not exist, but perivascular pseudorosettes were occasionally seen. The tumor contained concentrated mild desmoplastic stromal changes. Mitotic figures were present in approximately 35 per 10 high-powered fields. Areas of production of osseous matrix with calcification and partly well-formed bony trabeculae were observed (Fig. 3A). The osteogenic area was not more than 1% of the tumor. Although cytologically malignant cells were admixed with this osteogenic area, benign metaplastic bone formation was suspected, because the atypical cells were present only in the peripheral portion of the osteoid (Fig. 3B) and positive for CD99. The osteogenic area, in contrast, was CD99 negative. The pathologic findings of the metastatic lesions of the omental mass were identical to those of the primary mesenteric mass. Cytoplasmic glycogen was undetected in the tumor cells when pretreated with and without diastase in periodic acid-Schiff reactions. No cytoplasmic reactivity was found with Grimelius and Masson-Fontana staining.
Fig. 2.

(A) The tumor focally invades the mucosa of jejunum, resulting in mucosal erosion. (B) The tumor is composed of sheets or lobules of small round cells containing uniform vesicular, round, or oval nuclei; scanty clear or eosinophilic cytoplasms; and indistinct cytoplasmic borders. Well-formed Homer-Wright rosettes are frequently observed.
Fig. 3.
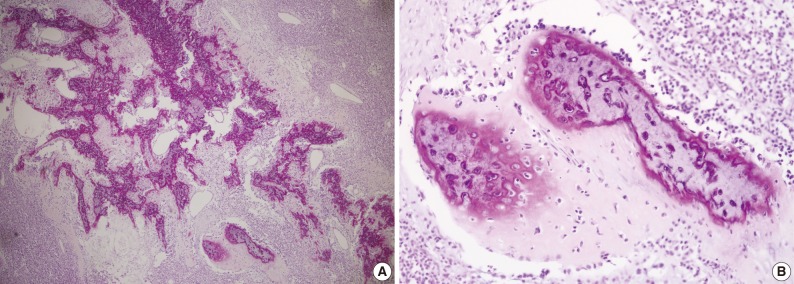
(A) Areas of osseous matrix with calcification and partly well-formed bony trabeculae are observed. (B) The periphery of osteoid and bone formation shows frankly malignant tumor cells, but the central portion of the bone reveals lacuna containing, benign-looking nuclei.
Positive CD99 (MIC2), CD57, and neuron-specific enolase identified many tumor cells immunohistochemically (Fig. 4), whereas positive vimentin and neurofilament results were also present in some cells. Synaptophysin, chromogranin, desmin, cytokeratin, S-100, CD117, and leukocyte common antigen were all absent in the tumor cells.
Fig. 4.
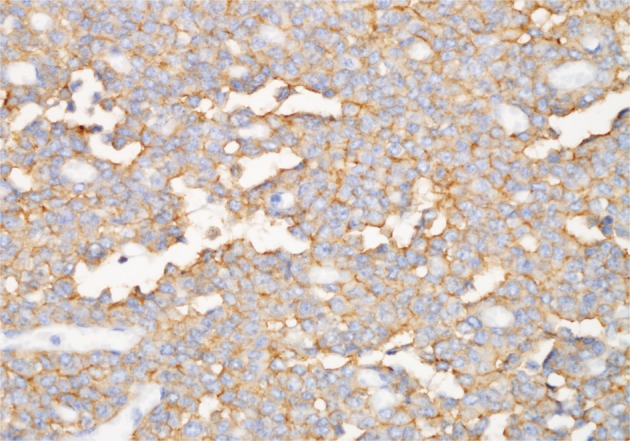
Most tumor cells are positive for CD99 (MIC2) (labeled streptavidin biotin).
Using dual color break-apart Ewing's sarcoma probing, fluorescence in situ hybridization (FISH) was performed on sections of the tissues that were formalin-fixed and paraffin embedded (Vysis, Downers Grove, IL, USA) with a mixture of 2 FISH DNA probes. The first was a 500-kb probe, labeled in the spectrum orange, and flanking the 5' side of the Ewing sarcoma breakpoint region 1 (EWSR1) gene. The second probe, which flanked the 3' end of the EWSR1 gene, was a 1,100-kb probe, utilizing a spectrum green label. Introns 7 through 10, used as restrictions within the EWSR1 gene, were the known break points. FISH showed a split signal pattern (one green and one orange) in interphase nuclei which was indicative of a EWSR1 gene rearrangement (Fig. 5). A pPNET of the small bowel mesentery diagnosis was ascribed to the lesion, given these results.
Fig. 5.
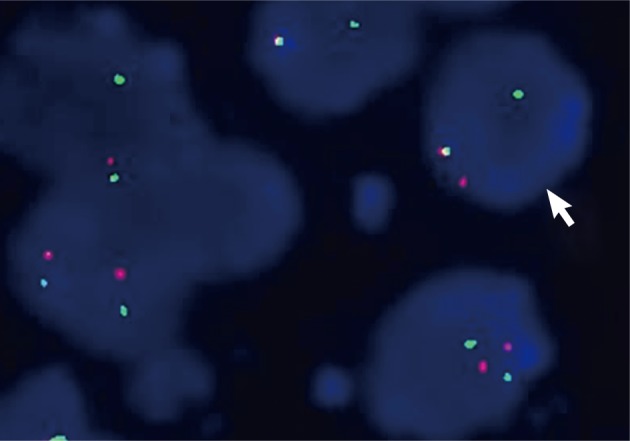
Ewing sarcoma breakpoint region 1 (EWSR1) fluorescence in situ hybridization (FISH) on interphase cells showing split-apart signals. Interphase nuclei with fused orange and green hybridization signals are interpreted as indicative of an intact (not rearranged) copy of the EWSR1 gene. A split signal pattern (one green and one orange) seen on interphase nuclei is interpreted as indicative of a EWSR1 gene rearrangement. This case has evidence of EWSR1 rearrangement by FISH.
The recurrent tumor resected one year after surgery, revealed similar histologic features: a typical small round cell tumor with rosette formation and metaplastic bone formation (Fig. 6). The bony islands were more mature than the primary tumor.
Fig. 6.
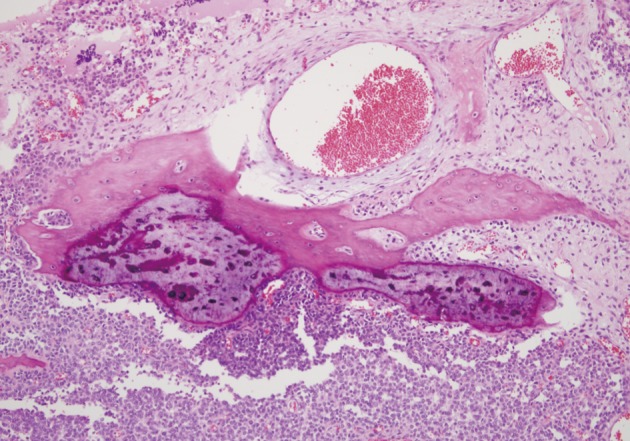
Recurrent tumor showing same morphology of tumor cells with primary tumor and more mature metaplatic bone.
DISCUSSION
The entire body is vulnerable to peripheral primitive neuroectodermal tumor invasion. The primary sites of pPNET are, indescending frequency, the chest wall, pelvis, retroperitoneum, abdomen, limb, and neck.10 In viscera, distinct cases of pPNET have been studied.3-6 However, in the English literature, only one case of pPNET of the mesentery was reported with perforation at presentation as was presented in our case study.4 pPNET prognosis is poor despite combined surgical, chemotherapeutic, and irradiation therapies. Only 25% of patients with tumors greater than 5 cm survive to 24 months according to Kushner et al.10 Histologically, Homer-Wright or Flexner-Wintersteiner rosettes and perivascular pseudorosettes may form from undifferentiated small round cells which constitute pPNET. Fibrosarcoma or malignant peripheral nerve sheath tumors, small cell undifferentiated carcinomas, and carcinoid tumors may resemble some areas within the lesions. It is known that tumors of neural crest origin can show bidirectional or multidirectional differentiation.7-9
Additionally, glial, ependymal, cartilaginous, and epithelial elements, though rare, have been found associated within pPNET.7-9 Hachitanda et al.11 reported a case of pPNET with epithelial and glial differentiation, and they suggested that the neoplastic neuroectodermal tissue can display a spectrum of differentiation. Although there has been no report of pPNET showing osteoid and bone production, it is thought that osteogenesis is a kind of differentiation of the tumor. Its prognostic implication is uncertain. Although several cases of bone and/or cartilage forming sarcomas have been reported in the literature,12-15 bone-forming pPNET has not.
Most authors agree that a useful tool in diagnosing pPNET immunohistochemically is CD99 (MIC2), which recognizes a 30/32 kDa surface glycoprotein.16 This marker is found in more than 90% of pPNET cases. Yet, many tumors, such as malignant lymphoma, leukemia, gastrointestinal stromal tumor, and small cell carcinoma, may demonstrate CD99 expression.17-20 Regarding pediatric malignant lymphoma and leukemia of T-cell lineage, Riopel et al.17 reported that CD99 expression was not uncommon.
The most objective diagnostic tool for pPNET is now considered to be karyotypic analysis for t(11;22)(q24;q12) translocation.2,16 This translocation occurs in more than 87% of the pPNET-Ewing's sarcoma cases. The detection of EWS/FLI-1 chimeric mRNA originating from the t(11;22)(q24;q12) translocation of the pPNET-Ewing's sarcoma family, facilitated by reverse transcription-polymerase chain reactions, have been reported in recent studies.2
Other small round cell tumors, including malignant lymphoma, leukemia (granulocytic sarcoma), rhabdomyosarcoma, leiomyosarcoma, gastrointestinal stromal tumor, desmoplastic small round cell tumor, malignant mesothelioma, undifferentiated carcinoma, small cell carcinoma, and conventional neuroblastoma offer a differential diagnosis of the current lesion being discussed. Through histological, histochemical, immunohistochemical and molecular methods, the lesion was meticulously examined to maintain distinction. Immunohistochemical staining with desmin, smooth muscle actin, CD34, cytokeratin, leukocyte common antigen, CD117, and CD99 were used to exclude the diagnosis of other small round cell tumors and gastrointestinal stromal tumors. In addition, chromosomal rearrangements involving the EWSR1 gene on chromosome 22q12 was detected by FISH, which was a strong supportive finding for pPNET. Most of the mass at the primary site was found in the mesentery of the jejunum. Direct invasion of the jejunal wall was also present, yet despite the large size of the tumor (12.0×8.0 cm), the degree of involvement of the mucosa of the jejunum was relatively limited. A final diagnosis of pPNET of the jejunal mesentery was applied to this tumor.
In conclusion, we have reported the first case of pPNET with osteoid and bone formation, arising from the mesentery of the small bowel with rupture at onset. The rupture was considered to be caused by local ischemic change, necrosis, and massive hemorrhage. Intraabdominal pPNET may present with acute abdomen and may reveal osteoid and bone formation, which leads to a high degree of importance for both surgeons and pathologists to consider.
Acknowledgments
This work is supported by Inha Research Grant.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Dehner LP. Primitive neuroectodermal tumor and Ewing's sarcoma. Am J Surg Pathol. 1993;17:1–13. doi: 10.1097/00000478-199301000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Kawauchi S, Fukuda T, Miyamoto S, et al. Peripheral primitive neuroectodermal tumor of the ovary confirmed by CD99 immunostaining, karyotypic analysis, and RT-PCR for EWS/FLI-1 chimeric mRNA. Am J Surg Pathol. 1998;22:1417–1422. doi: 10.1097/00000478-199811000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Bala M, Maly A, Remo N, Gimmon Z, Almogy G. Peripheral primitive neuroectodermal tumor of bowel mesentery in adults. Isr Med Assoc J. 2006;8:515–516. [PubMed] [Google Scholar]
- 4.Horie Y, Kato M. Peripheral primitive neuroectodermal tumor of the small bowel mesentery: a case showing perforation at onset. Pathol Int. 2000;50:398–403. doi: 10.1046/j.1440-1827.2000.01045.x. [DOI] [PubMed] [Google Scholar]
- 5.Tokudome N, Tanaka K, Kai MH, Sueyoshi K, Matsukita S, Setoguchi T. Primitive neuroectodermal tumor of the transverse colonic mesentery defined by the presence of EWS-FLI1 chimeric mRNA in a Japanese woman. J Gastroenterol. 2002;37:543–549. doi: 10.1007/s005350200084. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanian B, Dinakarababu E, Molyneux AJ. Primary primitive neuroectodermal tumour of the small bowel mesentery: case report. Eur J Surg Oncol. 2002;28:197–198. doi: 10.1053/ejso.2001.1155. [DOI] [PubMed] [Google Scholar]
- 7.Shuangshoti S. Primitive neuroectodermal (neuroepithelial) tumour of soft tissue of the neck in a child: demonstration of neuronal and neuroglial differentiation. Histopathology. 1986;10:651–658. doi: 10.1111/j.1365-2559.1986.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 8.Shinoda M, Tsutsumi Y, Hata J, Yokoyama S. Peripheral neuroepithelioma in childhood: immunohistochemical demonstration of epithelial differentiation. Arch Pathol Lab Med. 1988;112:1155–1158. [PubMed] [Google Scholar]
- 9.Karcioglu Z, Someren A, Mathes SJ. Ectomesenchymoma: a malignant tumor of migratory neural crest (ectomesenchyme) remnants showing ganglionic, schwannian, melanocytic and rhabdomyoblastic differentiation. Cancer. 1977;39:2486–2496. doi: 10.1002/1097-0142(197706)39:6<2486::aid-cncr2820390627>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 10.Kushner BH, Hajdu SI, Gulati SC, Erlandson RA, Exelby PR, Lieberman PH. Extracranial primitive neuroectodermal tumors: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 1991;67:1825–1829. doi: 10.1002/1097-0142(19910401)67:7<1825::aid-cncr2820670702>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Hachitanda Y, Tsuneyoshi M, Enjoji M, Nakagawara A, Ikeda K. Congenital primitive neuroectodermal tumor with epithelial and glial differentiation: an ultrastructural and immunohistochemical study. Arch Pathol Lab Med. 1990;114:101–105. [PubMed] [Google Scholar]
- 12.Song JS, Gardner JM, Tarrant WP, et al. Dedifferentiated liposarcoma with peculiar meningothelial-like whorling and metaplastic bone formation. Ann Diagn Pathol. 2009;13:278–284. doi: 10.1016/j.anndiagpath.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Macarenco RS, Erickson-Johnson M, Wang X, Jenkins RB, Nascimento AG, Oliveira AM. Cytogenetic and molecular cytogenetic findings in dedifferentiated liposarcoma with neural-like whorling pattern and metaplastic bone formation. Cancer Genet Cytogenet. 2007;172:147–150. doi: 10.1016/j.cancergencyto.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Weidner N. Atypical tumor of the mediastinum: epithelioid hemangioendothelioma containing metaplastic bone and osteoclastlike giant cells. Ultrastruct Pathol. 1991;15:481–488. doi: 10.3109/01913129109016253. [DOI] [PubMed] [Google Scholar]
- 15.Bhagavan BS, Dorfman HD. The significance of bone and cartilage formation in malignant fibrous histiocytoma of soft tissue. Cancer. 1982;49:480–488. doi: 10.1002/1097-0142(19820201)49:3<480::aid-cncr2820490315>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 16.Scotlandi K, Serra M, Manara MC, et al. Immunostaining of the p30/32MIC2 antigen and molecular detection of EWS rearrangements for the diagnosis of Ewing's sarcoma and peripheral neuroectodermal tumor. Hum Pathol. 1996;27:408–416. doi: 10.1016/s0046-8177(96)90115-x. [DOI] [PubMed] [Google Scholar]
- 17.Riopel M, Dickman PS, Link MP, Perlman EJ. MIC2 analysis in pediatric lymphomas and leukemias. Hum Pathol. 1994;25:396–399. doi: 10.1016/0046-8177(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 18.Menasce LP, Banerjee SS, Beckett E, Harris M. Extra-medullary myeloid tumour (granulocytic sarcoma) is often misdiagnosed: a study of 26 cases. Histopathology. 1999;34:391–398. doi: 10.1046/j.1365-2559.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 19.Lumadue JA, Askin FB, Perlman EJ. MIC2 analysis of small cell carcinoma. Am J Clin Pathol. 1994;102:692–694. doi: 10.1093/ajcp/102.5.692. [DOI] [PubMed] [Google Scholar]
- 20.Shidham VB, Chivukula M, Gupta D, Rao RN, Komorowski R. Immunohistochemical comparison of gastrointestinal stromal tumor and solitary fibrous tumor. Arch Pathol Lab Med. 2002;126:1189–1192. doi: 10.5858/2002-126-1189-ICOGST. [DOI] [PubMed] [Google Scholar]


