Abstract
We report a case of CTX-M-55-type extended-spectrum β-lactamase (ESBL)-producing Shigella sonnei infection in a 27-year-old Korean woman who had traveled to China. The patient was admitted to the hospital due to abdominal pain, watery diarrhea, and fever (39.3℃). S. sonnei was isolated from her stool specimens, and the pathogen was found to be resistant to cefotaxime due to CTX-M-55-type ESBL. Insertion sequence (IS)Ecp1 was found upstream of the blaCTX-M-55 gene. The blaCTX-M-55 gene was transferred from the S. sonnei isolate to an Escherichia coli J53 recipient by conjugation. Pulsed-field gel electrophoresis and Southern blotting revealed that the blaCTX-M-55 gene was located on a plasmid of approximately 130 kb.
Keywords: CTX-M-55, Shigella sonnei, ESBL
INTRODUCTION
Shigellosis is very rare in Korea, but people who travel to certain countries may acquire the infection. The bacteria are excreted through feces and are transmitted by ingestion of contaminated food and water [1]. To reduce the severity of the clinical course of illness and the duration of fecal excretion of bacteria, the WHO recommends ciprofloxacin as the first-line treatment for shigellosis [2, 3]. Ampicillin and trimethoprim-sulfamethoxazole are no longer sufficiently effective due to increases in the bacterial resistance to these drugs [4]. Because of concerns regarding the adverse effects of ciprofloxacin in children and an increase in the resistance to ciprofloxacin, third generation cephalosporins are frequently used as alternative treatments [2, 4]. However, Shigella species producing CTX-M-type β-lactamase are frequently being reported in some parts of the world [1, 5-7]. CTX-M-type β-lactamase is one of the most common extended-spectrum β-lactamases (ESBL), and it confers resistance to all β-lactams except cephamycins and carbapenems [8]. The spread of CTX-M type ESBL-producing Shigella species has become of concern worldwide because the increasing circulation of this resistant strain further narrows the choice of effective antibiotics. In Korea, only CTX-M-14-producing Shigella sonnei have been reported [9-11].
We report the isolation of CTX-M-55-producing S. sonnei from a Korean woman who was admitted to the hospital due to abdominal pain, watery diarrhea, and fever (39.3℃) immediately after having traveled to China.
CASE REPORT
A 27-yr-old woman was admitted to the hospital with watery diarrhea, abdominal pain, and fever (39.3℃) on the day of her return from a 4-day trip to China. Laboratory investigation showed a peripheral blood leukocyte count of 8.2×109/L with 80% polymorphonuclear cells and a C-reactive protein level of 107 mg/L. Stool samples were inoculated on MacConkey and SS agar (BD Diagnostics Systems, Sparks, MD, USA) and incubated at 37℃ for 20 hr, and colorless medium-sized colonies developed. The bacterium was identified as S. sonnei using the Vitek2 GN ID card (bioMérieux, Marcy l'Etoile, France); a positive slide agglutination test with a Shigella serogroup D antiserum (Joong Kyeom Co., Anshan, Korea) confirmed the identification.
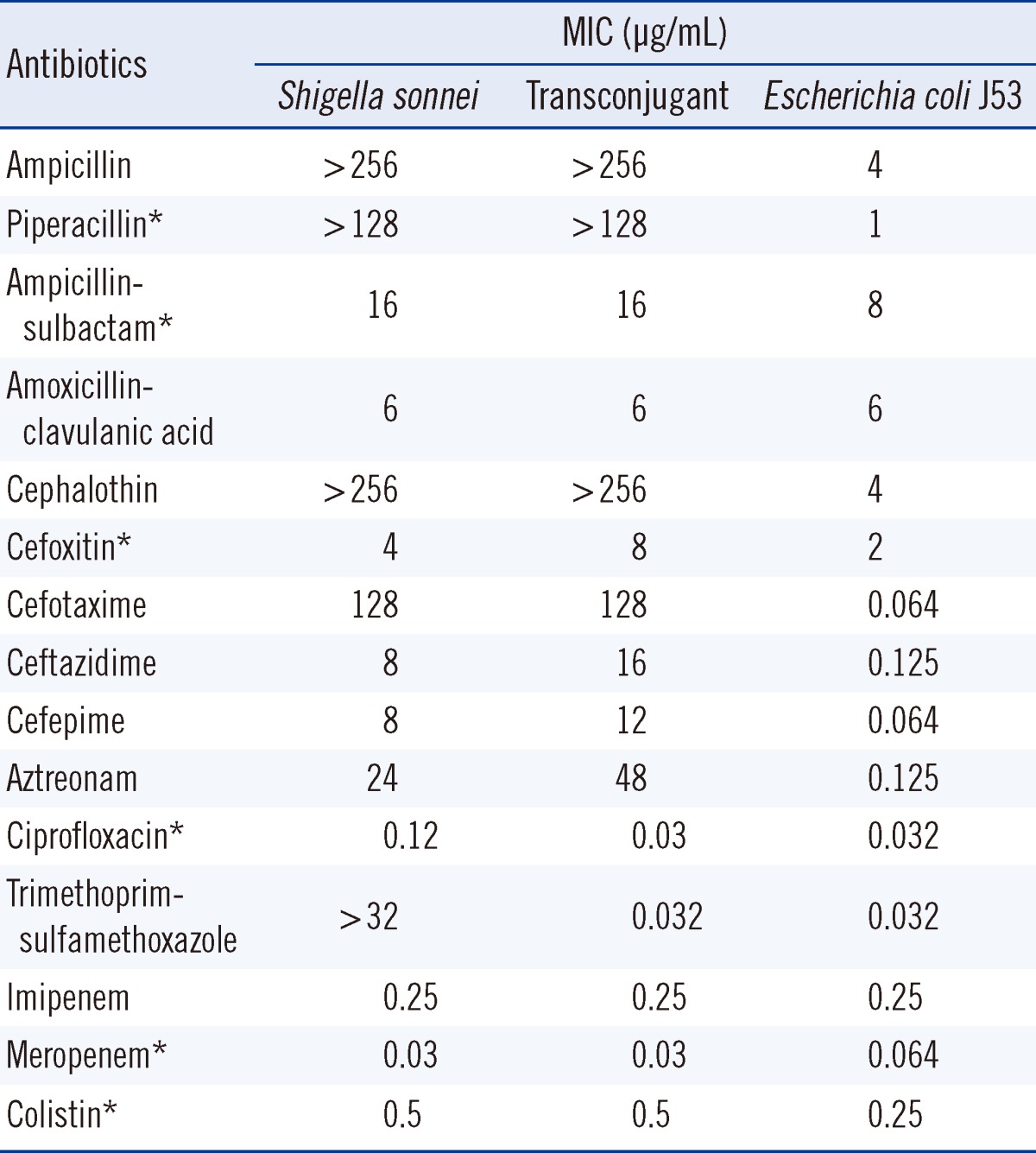
The pathogen's antimicrobial susceptibility was initially determined by the VITEK 2 AST N131 card (bioMérieux, Marcy l'Etoile, France). ESBL production was confirmed by the double disk synergy test using cefotaxime (30 µg), ceftazidime (30 µg) and amoxicillin-clavulanic acid (20/10 µg) disks (BD Diagnostics Systems) [12]. Minimum inhibitory concentrations (MICs) were determined by the CLSI agar dilution method or by the Etest (AB bioMérieux, Solna, Sweden) (Table 1). The isolate was resistant to cephalothin, aztreonam, and cefotaxime, but susceptible to cefoxitin and intermediate to ceftazidime. The patient was treated with oral ciprofloxacin, after which her symptoms began to improve. She was discharged 4 days after admission.
Table 1.
Antimicrobial susceptibility of the clinical isolate, transconjugant, and Escherichia coli J53 recipient determined by the Etest or the agar dilution method
*MIC was determined by the agar dilution method.
Abbreviation: MIC, minimum inhibitory concentration.
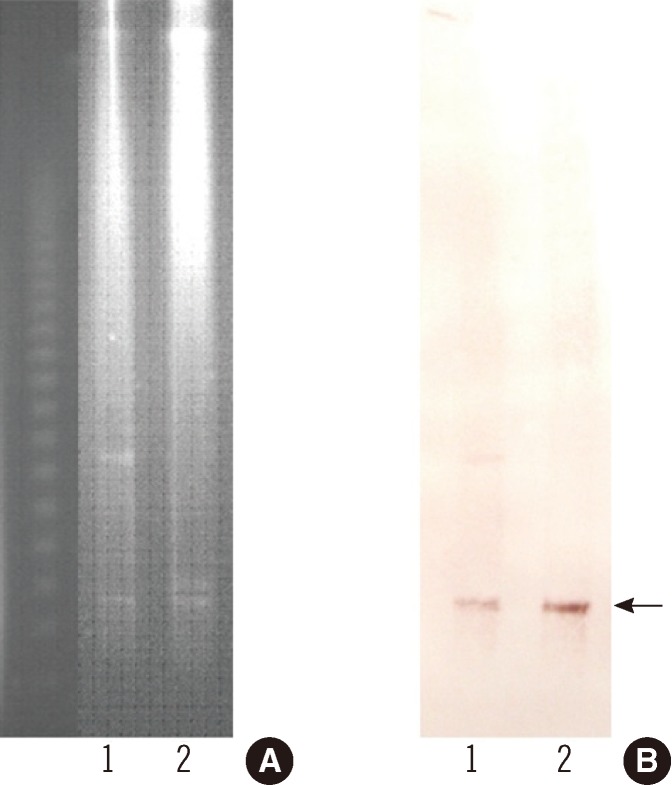
To determine the genetic characteristics of the ESBL, PCR and sequencing were carried out as described previously [13]. These investigations identified the ESBL as being encoded by blaCTX-M-55, which is identical to blaCTX-M-57 [14]. The blaCTX-M-55 gene and surrounding regions were investigated by PCR and sequencing [12], and insertion sequence (IS)Ecp1 was found to be upstream of blaCTX-M-55. The blaCTX-M-55 gene was successfully transferred from the S. sonnei isolate to an Eecherichia coli J53 (azideR) recipient by plate mating. MacConkey agar containing cefotaxime (2 µg/mL) and sodium azide (100 µg/mL) was used as the selective media. The β-lactam resistance patterns of the S. sonnei isolate and the transconjugant were similar, but the MICs of ceftazidime and aztreonam for the transconjugant were increased 2-fold, from 8 µg/mL and 24 µg/mL to 16 µg/mL and 48 µg/mL, respectively (Table 1). To estimate the size of the plasmid harboring blaCTX-M-55, genomic DNA from S. sonnei isolate and its transconjugant were digested with S1 nuclease and separated by pulsed-field gel electrophoresis [15]. Southern blotting was performed on the agarose gels by in-gel hybridization with a blaCTX-M-55 probe labeled with digoxigenin (Roche Diagnostics, Indianapolis, IN, USA) [15]. This experiment showed that blaCTX-M-55 was located on a plasmid of approximately 130 kb in each of the strains (Fig. 1). Replicon typing of the plasmid by PCR was unsuccessful [16].
Fig. 1.
Pulsed-field gel electrophoresis of the S1 nuclease-digested genomic DNA (A) and Southern blot hybridization with the blaCTX-M-55 probe (B). The arrow indicates plasmids in the Shigella sonnei isolate (lane 1) and the transconjugant (lane 2).
DISCUSSION
CTX-M β-lactamases are plasmid-mediated ESBLs that are typically found to have high hydrolytic activity against cefotaxime [17]. During the last 2 decades, CTX-M β-lactamases have rapidly spread though the world and 133 different CTX-M β-lactamases are now recognized [14]. CTX-M-55 was first reported in ESBL-producing E. coli and Klebsiella pneumoniae in Thailand during 2004 and 2005. That study reported 7 CTX-M-55-producing isolates (6 E. coli and 1 K. pneumoniae) [18]. Thereafter, CTX-M-55-producing Enterobacteriaceae isolates have been reported in both humans and animals in several countries [1, 19-23]. Interestingly, most of these studies have been conducted in China, which suggests that CTX-M-55-producing Enterobacteriaceae, including S. sonnei, are widely disseminated throughout China [1]. CTX-M-55 differs from CTX-M-15 only by a single amino acid substitution (valine for alanine) at position 80 (Ala80Val). Therefore, CTX-M-55 is expected to have similar hydrolytic activity as CTX-M-15 and to exhibit increased catalytic efficiency against ceftazidime as well as cefotaxime [24]. Previous studies have reported that CTX-M-55-producing isolates generally show high resistance to ceftazidime (MIC range from 32 to > 256 µg/mL) [13, 18, 25, 26]. However, 2 of 3 CTX-M-55-producing S. sonnei isolates from China were susceptible to ceftazidime [1]. Our isolate also had a relatively low MIC for ceftazidime (8 µg/mL), which was classified as intermediate sensitivity according to the CLSI breakpoints [27].
The blaCTX-M genes can be transferred to other bacteria more readily than other plasmid-mediated bla genes because ISEcp1, which is frequently found upstream of the blaCTX-M genes, has an important role in the mobilization and expression of blaCTX-M genes [23, 28]. In our isolate, the blaCTX-M-55 gene was flanked by ISEcp1 and both were located on the plasmid.
Tängdén et al. [29] reported that 24 of the 100 travelers, who had been studied, had recently been infected with ESBL-producing E. coli after a trip to a foreign country. Five of the 21 patients who completed a 6-month follow-up were found to be infected with ESBL-producing strains. A study analyzing the duration of colonization with ESBL-producing E. coli in patients with travelers' diarrhea found that 10 of the 41 patients carried ESBL-producing E. coli at the first follow-up (3-8 months), and 4 patients still carried ESBL-producing E. coli after 3 yr [30]. The results from these studies indicate that travel to a foreign country plays an important role in the acquisition of and infection with ESBL-producing Enterobacteriaceae. Furthermore, these ESBL-producing Enterobacteriaceae can disseminate through the population. Specifically, the dissemination of new antimicrobial resistance genes may lead to major healthcare issues. In order to prevent the inflow of ESBL-producing Enterobacteriaceae, it is important to monitor suspected carriers of ESBL-producing organisms.
In summary, we report a case of CTX-M-55-producing S. sonnei from a Korean patient who had just returned from China, suggesting that the infection was acquired during the travel.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Zhang W, Luo Y, Li J, Lin L, Ma Y, Hu C, et al. Wide dissemination of multidrug-resistant Shigella isolates in China. J Antimicrob Chemother. 2011;66:2527–2535. doi: 10.1093/jac/dkr341. [DOI] [PubMed] [Google Scholar]
- 2.Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst Rev. 2010:CD006784. doi: 10.1002/14651858.CD006784.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Livermore DM, Mushtaq S, Nguyen T, Warner M. Strategies to overcome extended-spectrum β-lactamases (ESBLs) and AmpC β-lactamases in shigellae. Int J Antimicrob Agents. 2011;37:405–409. doi: 10.1016/j.ijantimicag.2010.11.028. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya D, Purushottaman SA, Bhattacharjee H, Thamizhmani R, Sudharama SD, Manimunda SP, et al. Rapid emergence of third-generation cephalosporin resistance in Shigella sp. isolated in Andaman and Nicobar Islands, India. Microb Drug Resist. 2011;17:329–332. doi: 10.1089/mdr.2010.0084. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen NT, Ha V, Tran NV, Stabler R, Pham DT, Le TM, et al. The sudden dominance of blaCTX-M harbouring plasmids in Shigella spp. Circulating in Southern Vietnam. PLoS Negl Trop Dis. 2010;4:e702. doi: 10.1371/journal.pntd.0000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung TK, Chu YW, Tsang GK, Ngan JY, Hui IS, Kam KM. Emergence of CTX-M-type β-lactam resistance in Shigella spp. in Hong Kong. Int J Antimicrob Agents. 2005;25:350–352. doi: 10.1016/j.ijantimicag.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Andres P, Petroni A, Faccone D, Pasterán F, Melano R, Rapoport M, et al. Extended-spectrum β-lactamases in Shigella flexneri from Argentina: first report of TOHO-1 outside Japan. Int J Antimicrob Agents. 2005;25:501–507. doi: 10.1016/j.ijantimicag.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Rossolini GM, D'Andrea MM, Mugnaioli C. The spread of CTX-M-type extended-spectrum β-lactamases. Clin Microbiol Infect. 2008;14:33–41. doi: 10.1111/j.1469-0691.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 9.Hong SJ, Lee CH, Wang JH, Song W, Jung SH. Clinical characteristics of extended-spectrum β-lactamase producing Shigella sonnei infection outbreaked in Chungju Area. Korean J Lab Med. 2006;26:168–173. doi: 10.3343/kjlm.2006.26.3.168. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Kim J, Kang Y, Park Y, Lee B. Occurrence of extended-spectrum β-lactamases in members of the genus Shigella in the Republic of Korea. J Clin Microbiol. 2004;42:5264–5269. doi: 10.1128/JCM.42.11.5264-5269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pai H, Choi EH, Lee HJ, Hong JY, Jacoby GA. Identification of CTX-M-14 extended-spectrum β-lactamase in clinical isolates of Shigella sonnei, Escherichia coli, and Klebsiella pneumoniae in Korea. J Clin Microbiol. 2001;39:3747–3749. doi: 10.1128/JCM.39.10.3747-3749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SG, Jeong SH, Lee H, Kim CK, Lee Y, Koh E, et al. Spread of CTX-M-type extended-spectrum β-lactamases among bloodstream isolates of Escherichia coli and Klebsiella pneumoniae from a Korean hospital. Diagn Microbiol Infect Dis. 2009;63:76–80. doi: 10.1016/j.diagmicrobio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Park Y, Kang HK, Bae IK, Kim J, Kim JS, Uh Y, et al. Prevalence of the extended-spectrum β-lactamase and qnr genes in clinical isolates of Escherichia coli. Korean J Lab Med. 2009;29:218–223. doi: 10.3343/kjlm.2009.29.3.218. [DOI] [PubMed] [Google Scholar]
- 14.Jacoby G, Bush K. β-lactamase classification and amino acid sequences for TEM, SHV and OXA extended-spectrum and inhibitor resistant enzymes. [Updated on Aug 2012]. http://www.lahey.org/Studies/other.asp#table1.
- 15.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, et al. Characterization of a new metallo-β-lactamase gene, blaNDM-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–5054. doi: 10.1128/AAC.00774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods. 2005;63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Bush K, Jacoby GA. Updated functional classification of β-lactamases. Antimicrob Agents Chemother. 2010;54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiratisin P, Apisarnthanarak A, Saifon P, Laesripa C, Kitphati R, Mundy LM. The emergence of a novel ceftazidime-resistant CTX-M extended-spectrum β-lactamase, CTX-M-55, in both community-onset and hospital-acquired infections in Thailand. Diagn Microbiol Infect Dis. 2007;58:349–355. doi: 10.1016/j.diagmicrobio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Ma Y, Hu C, Jin S, Zhang Q, Ding H, et al. Dissemination of cefotaxime-M-producing Escherichia coli isolates in poultry farms, but not swine farms, in China. Foodborne Pathog Dis. 2010;7:1387–1392. doi: 10.1089/fpd.2010.0581. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y, Zeng Z, Chen S, Ma J, He L, Liu Y, et al. High prevalence of blaCTX-M extended-spectrum β-lactamase genes in Escherichia coli isolates from pets and emergence of CTX-M-64 in China. Clin Microbiol Infect. 2010;16:1475–1481. doi: 10.1111/j.1469-0691.2010.03127.x. [DOI] [PubMed] [Google Scholar]
- 21.Zheng H, Zeng Z, Chen S, Liu Y, Yao Q, Deng Y, et al. Prevalence and characterisation of CTX-M β-lactamases amongst Escherichia coli isolates from healthy food animals in China. Int J Antimicrob Agents. 2012;39:305–310. doi: 10.1016/j.ijantimicag.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Sjölund-Karlsson M, Howie R, Krueger A, Rickert R, Pecic G, Lupoli K, et al. CTX-M-producing non-Typhi Salmonella spp. isolated from humans, United States. Emerg Infect Dis. 2011;17:97–99. doi: 10.3201/eid1701.100511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamang MD, Nam HM, Jang GC, Kim SR, Chae MH, Jung SC, et al. Molecular characterization of extended-spectrum β-lactamase and plasmid mediated AmpC β-lactamase-producing Escherichia coli isolated from stray dogs from Korea. Antimicrob Agents Chemother. 2012;56:2705–2712. doi: 10.1128/AAC.05598-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poirel L, Gniadkowski M, Nordmann P. Biochemical analysis of the ceftazidime-hydrolysing extended-spectrum β-lactamase CTX-M-15 and of its structurally related β-lactamase CTX-M-3. J Antimicrob Chemother. 2002;50:1031–1034. doi: 10.1093/jac/dkf240. [DOI] [PubMed] [Google Scholar]
- 25.Yu F, Chen Q, Yu X, Li Q, Ding B, Yang L, et al. High prevalence of extended-spectrum β-lactamases among Salmonella enterica Typhimurium isolates from pediatric patients with diarrhea in China. PLoS One. 2011;6:e16801. doi: 10.1371/journal.pone.0016801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song W, Lee H, Lee K, Jeong SH, Bae IK, Kim JS, et al. CTX-M-14 and CTX-M-15 enzymes are the dominant type of extended-spectrum β-lactamase in clinical isolates of Escherichia coli from Korea. J Med Microbiol. 2009;58:261–266. doi: 10.1099/jmm.0.004507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Twenty-second Informational supplement, M100-S22. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 28.Eckert C, Gautier V, Arlet G. DNA sequence analysis of the genetic environment of various blaCTX-M genes. J Antimicrob Chemother. 2006;57:14–23. doi: 10.1093/jac/dki398. [DOI] [PubMed] [Google Scholar]
- 29.Tängdén T, Cars O, Melhus A, Löwdin E. Foreign travel is a major risk factor for colonization with Escherichia coli producing CTX-M-type extended-spectrum β-lactamases: a prospective study with Swedish volunteers. Antimicrob Agents Chemother. 2010;54:3564–3568. doi: 10.1128/AAC.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tham J, Walder M, Melander E, Odenholt I. Duration of colonization with extended-spectrum β-lactamase-producing Escherichia coli in patients with travellers' diarrhoea. Scand J Infect Dis. 2012;44:573–577. doi: 10.3109/00365548.2011.653582. [DOI] [PubMed] [Google Scholar]