There has been increasing interest in recent years in the prevalence of low 25-hydroxy (OH) vitamin D concentrations in different human populations [1, 2]. There are 2 forms of vitamin D: cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Vitamin D3 is manufactured through the photoisomerization of 7-dehydrocholesterol to pre-vitamin D3, which then undergoes a temperature-dependent rearrangement to form vitamin D3. Vitamin D2 is a plant sterol found in foods, such as fatty fish, cod-liver oil, and eggs. Vitamin D is converted to 25-OH vitamin D, the major circulating form of vitamin D, and then to 1, 25-diOH vitamin D, the active form of vitamin D, by enzymes in the liver and kidney. 25-OH vitamin D assays should be able to measure both the D2 and D3 forms of 25-OH vitamin D as both forms are biologically active after 1-α-hydroxylation. For the majority of patients, total 25-OH vitamin D measurement is suitable for the diagnosis and monitoring of vitamin D deficiency. Quantification of 25-OH vitamin D2 and 25-OH vitamin D3 fractions may be useful in monitoring patients without clinical improvement despite treatment where inadequate dosing, non-adherence, or malabsorption are suspected [3].
High rates of low 25-OH vitamin D concentration have been described in most epidemiological studies, including those from tropical regions with high numbers of sunlight hours per year. However, changes in choice of measurand (25-OH vitamin D2, 25-OH vitamin D3, or total 25-OH vitamin D), analytical methodology, and the cutoffs defining deficiency/insufficiency over time mean that published findings soon become obsolete and require reassessment [4-6]. In 2011, Roche Diagnostics replaced their 25-OH vitamin D3 assay with a total 25-OH vitamin D assay. According to the manufacturer, this new assay shows 98% and 81% cross-reactivity with 25-OH vitamin D3 and 25-OH vitamin D2, respectively. We have previously shown low levels of 25-OH vitamin D3 in Asians using the Roche Diagnostics 25-OH vitamin D3 assay (Mannheim, Germany), particularly in Malays and Indians [7]. However, concerns have been raised that underestimation of 25-OH vitamin D3 concentrations with the Roche 25-OH vitamin D3 assay has led to over-reporting of vitamin D deficiency [8]. The new Roche total 25-OH vitamin D assay has been reported as fulfilling present analytical performance requirements with close agreement to other well-established methods for 25-OH vitamin D analysis, such as liquid chromatography-tandem mass spectrometry and high-performance liquid chromatography, making it fit for routine assessment of vitamin D status [9]. This study was designed to assess whether the same pattern of low total 25-OH vitamin D concentrations is seen in the same Asian population using the new Roche total 25-OH vitamin D assay using the 2010 Institute of Medicine guidelines for 25-OH vitamin D interpretation [10].
Total 25-OH vitamin D concentrations were measured on 240 (40 male and 40 female specimens from each race: Chinese, Malay, and Indian) anonymised left-over fasting venous serum samples from apparently healthy ambulatory outpatients undergoing health screening. These were not the same samples or individuals used in the previous 25-OH vitamin D3 study. Singapore is 1.4° north of the equator and has no seasons as such, with a daily difference of less than 30 min of sunlight hours over the year. The time of year at which samples were collected is thus unlikely to be a significant determinant of the pattern of 25-OH vitamin D concentrations observed. However, to replicate the collection conditions of the earlier study, samples were collected over similar months (April to July) as those in the 25-OH vitamin D3 study (February to July). This work was part of routine reference interval work during evaluation of a new assay and did not require institutional review board approval. The tests were performed on the Roche Cobas e601 immunoassay analyzer using the Roche Elecsys total 25-OH Vitamin D assay (Roche Diagnostics). Intra-batch imprecision ranged from 2.2% (175 nmol/L) to 6.8% (20 nmol/L), while inter-batch imprecision ranged from 3.4% (175 nmol/L) to 13% (20 nmol/L). Samples were collected over 1 month, stored at -20℃ until analysis and, after thawing and mixing, were randomly allocated to 3 analytical runs over 3 consecutive days. Statistical analysis was performed using Analyse-It for Microsoft Excel (Analyse-It Software Ltd, Leeds, UK) and SPSS v17 (SPSS Inc., Chicago, IL, USA). A P value <0.05 was considered statistically significant.
Table 1 shows the distribution of 25-OH vitamin D concentrations for the Chinese, Malay and Indian female and male groups. There were no cases of 25-OH vitamin D concentration >125 nmol/L. The median age was 36 yr (range: 19-71). The median 25-OH vitamin D concentration for Chinese males was significantly higher than that for Chinese females (60.0 vs. 49.5 nmol/L, P=0.02). Malay males had similarly higher 25-OH vitamin D concentrations than females (median 52.5 vs. 37.8 nmol/L, P<0.01), while there was no significant difference between Indian males and females (42.0 vs. 34.0 nmol/L, P=0.18). The Institute of Medicine classifies 25-OH vitamin D results in four categories: at risk of deficiency (<30 nmol/L), at risk of inadequacy (30-50 nmol/L), sufficiency (50-125 nmol/L) and possible harm (>125 nmol/L) [10]. The prevalence (and 95% confidence limits) of deficiency or inadequacy (<50 nmol/L) for the various groups was: Chinese male 30% (15.8-44.2), Chinese female 52.5% (37-68), Malay male 47.5% (32-63), Malay female 77.5% (64.6-90.4), Indian male 75% (61.6-88.4), Indian female 85% (73.9-96.1).
Table 1.
Distribution of 25-OH vitamin D concentrations in Chinese, Malay and Indian female and male groups, %* (N of cases)
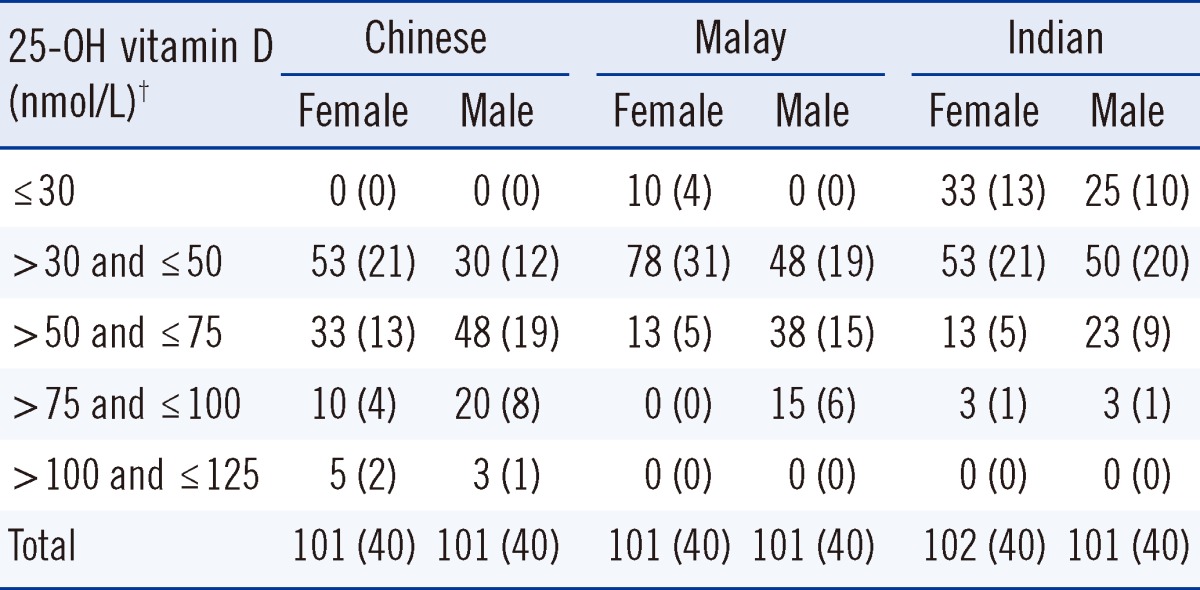
*Due to rounding of percentages to whole numbers, totals may exceed 100%. †There were no cases of 25-OH vitamin D concentration >125 nmol/L. The median age was 36 yr (range: 19-71). The median 25-OH vitamin D concentration for Chinese males was significantly higher than for Chinese females (60.0 vs. 49.5 nmol/L, P =0.02). Malay males had similarly higher 25-OH vitamin D concentrations than females (median 52.5 vs. 37.8 nmol/L, P <0.01) while there was no significant difference between Indian males and females (42.0 vs. 34.0 nmol/L, P =0.18).
Abbreviation: 25-OH vitamin D, 25-hydroxy vitamin D.
Multivariate linear regression with adjustment for age showed the change (95% confidence intervals, P value) in 25-OH vitamin D concentration attributable to sex and ethnicity to be: female (vs. male) -14.3 nmol/L (-9.5 to -19.0, P<0.01); Indian (vs. Chinese) -24.2 nmol/L (-30.5 to -18.0, P<0.01); Malay (vs. Chinese) -12.5 nmol/L (-17.0 to -8.0, P<0.01); Malay (vs. Indian) +11.8 nmol/L (5.5 to 18.3, P<0.01). Logistic regression analysis to predict vitamin D deficiency or inadequacy (25-OH vitamin D<50 nmol/L) controlling for age gave the following significant odds ratios (95% confidence intervals): female (vs. male): 5.0 (2.5 to 9.9); Malay (vs. Chinese) 3.4 (1.8 to 6.5); Indian (vs. Chinese) 9.0 (3.5 to 23.0), Indian (vs. Malay) 2.6 (1.1 to 6.5). Age was not a significant predictor of 25-OH vitamin D concentration or vitamin D deficiency/inadequacy.
This study demonstrates a high prevalence of low 25-OH vitamin D concentration in Singapore. The odds ratios associated with gender and race are not significantly different from those shown previously with the Roche 25-OH vitamin D3 assay. Factors which may contribute to the variation between groups include differences in skin pigmentation, food, and clothing [11]. Increased skin pigment reduces the capacity of skin to synthesize vitamin D3 [12]. Differences in dressing styles between races and between sexes may also play a part in the observed variation. Asian women tend to have a negative attitude towards sunlight, and many take measures to avoid exposure whenever possible [13]. This may be reflected in their choice of clothing and a greater reluctance to full body sun exposure during summer and during outdoor sports compared to men [14]. The higher proportion of body fat in women compared to men may also contribute to the lower concentrations seen, through sequestration of vitamin D in body fat [2]. The ability of the Roche total 25-OH vitamin D assay to measure both the D2 and D3 forms overcomes any concerns in the earlier study regarding the underestimation of vitamin D concentrations in individuals taking vitamin D2 supplements. Limitations of this study include its small size, the lack of other laboratory and clinical data (e.g. serum calcium, parathyroid hormone, body weight), the lack of medical, dietary and medication history of participants (particularly vitamin D supplementation), and the selected nature of the population sampled. A larger epidemiological study collecting more clinical and drug information would be ideal to further characterize the differences in 25-OH vitamin D concentration between groups.
In conclusion, there are high rates of low total 25-OH vitamin D concentration in Singapore, with the prevalence of 25-OH vitamin D concentration<50 nmol/L ranging from 30 to 85% depending on sex and race. Lower 25-OH vitamin D concentrations are seen in Indians and Malays compared to Chinese individuals and in women compared to men. These findings suggest the presence of low 25-OH vitamin D concentrations in many South East Asians and demonstrate the importance of sex and race in identifying those most at risk.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Rosen CJ. Clinical practice. Vitamin D insufficiency. N Engl J Med. 2011;364:248–254. doi: 10.1056/NEJMcp1009570. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Kennel KA, Drake MT, Hurley DL. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc. 2010;85:752–757. doi: 10.4065/mcp.2010.0138. quiz 757-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 5.Cavalier E, Delanaye P, Chapelle JP, Souberbielle JC. Vitamin D: current status and perspectives. Clin Chem Lab Med. 2009;47:120–127. doi: 10.1515/CCLM.2009.036. [DOI] [PubMed] [Google Scholar]
- 6.Roth HJ, Schmidt-Gayk H, Weber H, Niederau C. Accuracy and clinical implications of seven 25-hydroxyvitamin D methods compared with liquid chromatography-tandem mass spectrometry as a reference. Ann Clin Biochem. 2008;45:153–159. doi: 10.1258/acb.2007.007091. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins RC. 25-OH vitamin D3 concentrations in Chinese, Malays, and Indians. Clin Chem. 2009;55:1749–1751. doi: 10.1373/clinchem.2009.129403. [DOI] [PubMed] [Google Scholar]
- 8.Connell AB, Jenkins N, Black M, Pasco JA, Kotowicz MA, Schneider HG. Overreporting of vitamin D deficiency with the Roche Elecsys Vitamin D3 (25-OH) method. Pathology. 2011;43:368–371. doi: 10.1097/PAT.0b013e328346431c. [DOI] [PubMed] [Google Scholar]
- 9.Emmen JM, Wielders JP, Boer AK, van den Ouweland JM, Vader HL. The new Roche Vitamin D Total assay: fit for its purpose? Clin Chem Lab Med. 2012;50:1969–1972. doi: 10.1515/cclm-2011-0085. [DOI] [PubMed] [Google Scholar]
- 10.Institute of Medicine of the National Academies. Dietary reference intakes for adequacy: calcium and vitamin D. Washington, DC: National Academy of Sciences; 2010. pp. 345–402. [Google Scholar]
- 11.Mithal A, Wahl D, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 12.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–76. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 13.Kung AW, Lee KK. Knowledge of vitamin D and perceptions and attitudes toward sunlight among Chinese middle-aged and elderly women: a population survey in Hong Kong. BMC Public Health. 2006;6:226. doi: 10.1186/1471-2458-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Goaziou MF, Contardo G, Dupraz C, Martin A, Laville M, Schott-Pethelaz AM. Risk factors for vitamin D deficiency in women aged 20-50 years consulting in general practice: a cross-sectional study. Eur J Gen Pract. 2011;17:146–152. doi: 10.3109/13814788.2011.560663. [DOI] [PubMed] [Google Scholar]


