Abstract
Background
Therapy-related myeloid neoplasms (t-MN) occur as late complications of cytotoxic therapy. This study reviewed clinical and cytogenetic characteristics of patients with t-MN at a single institution in Korea.
Methods
The study subjects included 39 consecutive patients diagnosed with t-MN. Each subject's clinical history of previous diseases, treatments, and laboratory data was reviewed, including cytogenetics. The primary diagnosis was hematologic malignancy in 14 patients and solid tumor in 25 patients.
Results
Therapy-related acute myeloid leukemia (t-AML, 66.7%) was found to be more common than therapy-related myelodysplastic syndrome (t-MDS). Primary hematologic malignancies that were commonly implicated included mature B-cell neoplasm and acute leukemia. Breast cancer was the most common primary solid tumor. The mean time interval from cytotoxic therapy initiation to t-MN detection was 49 months. Chromosomal aberrations were observed in 35 patients, and loss of chromosome 5, 7, or both accounted for 41% of all cases. Balanced rearrangements occurred in 13 patients; these patients showed shorter latency intervals (mean, 38 months) than patients with loss of chromosome 5 or 7 (mean, 61 months).
Conclusions
In this study, we determined the clinical and cytogenetic characteristics of Korean patients with t-MN. Although our results were generally consistent with those of previous reports, we found that t-MN resulting from de novo leukemia was common and that t-AML was more common than t-MDS at presentation. Multi-institutional studies involving a larger number of patients and additional parameters are required to investigate the epidemiology, genetic predisposition, and survival rate of t-MN in Korea.
Keywords: Therapy-related neoplasms, Myelodysplastic syndrome, Acute myeloid leukemia, Cytogenetics, Korea
INTRODUCTION
In 2008, the WHO introduced the diagnostic entity of therapy-related myeloid neoplasms (t-MN) to include therapy-related acute myeloid leukemia (t-AML), myelodysplastic syndromes (t-MDS), and myelodysplastic/myeloproliferative neoplasms (t-MDS/MPN) [1]. t-MN occurs as a late complication of cytotoxic chemotherapy (CT) and/or radiation therapy administered for a previous neoplastic or non-neoplastic disorder [2-5]. Recently, the incidence of t-MN was shown to be increasing worldwide, due to improved survival rates following treatment of primary malignancies [6].
The latency between primary diagnosis and therapy-related disease ranges from several months to several years and may depend on the cumulative dose or dose intensity preceding cytotoxic therapy as well as on exposure to specific agents [7, 8]. The clinical course is typically progressive and relatively resistant to conventional therapies used for de novo leukemia [5, 9, 10]. More than 90% of patients with t-MDS/t-AML have an abnormal karyotype, and cytogenetic abnormalities often correlate with the latent period between initial therapy and onset of the leukemic disorder, as wel as with previous cytotoxic therapy [4, 11, 12]. Previous studies have shown that t-MN associated with exposure to alkylating agents and/or radiation is associated with a long latency period, t-MDS on presentation, and unbalanced loss of genetic material, often involving chromosome 5, 7, or both. In contrast, most patients treated with topoisomerase 2 inhibitors showed t-MN development associated with a short latent period, t-AML on presentation, and balanced chromosomal translocations [3, 4, 12, 13].
Although the data and knowledge of t-MN are increasing, studies on t-MN have been limited in Korea. In this study, we have analyzed the clinical and cytogenetic characteristics of consecutive patients diagnosed with t-MN at a single institution in Korea.
METHODS
A total of 39 patients were diagnosed with t-MN (t-AML or t-MDS) between January 2005 and February 2011 at the Samsung Medical Center, a tertiary referral hospital in Seoul, Korea. In accordance with the WHO 2008 classification system, t-MN diagnosis was based on the patients' clinical history of antecedent treatment for primary malignancy as well as hematological findings [1]. Patients were classified as having t-MDS if the percentage of blasts in the marrow was less than 20%, whereas, t-AML was diagnosed if the percentage was 20% or greater. Conventional cytogenetic studies were performed on bone marrow aspirate samples using the standard G-banding techniques. Chromosomal abnormalities have been described by the International System for Human Cytogenetic Nomenclature [14].
We reviewed the medical records of study patients to characterize clinical and pathologic findings, including initial diagnosis with stage, age at diagnosis of t-MN, sex, primary cancer treatment, latency period, complete response (CR) duration, complete blood count (CBC) at diagnosis of t-MN, percentage of bone marrow blasts, and bone marrow cytogenetic findings. The latency interval was defined as the time period between the first cytotoxic therapy and first bone marrow examination exhibiting t-MN. CR duration was defined as the time period between CR of the primary cancer and the first bone marrow examination exhibiting t-MN.
Treatments administered to patients after t-MN development were individualized and variable, ranging from supportive care to intensive CT and/or bone marrow transplantation. CT agents were classified according to the mechanism of action and included alkylating agents, topoisomerase 2 inhibitors, antimetabolites, and antitubulin agents [1]. This study was approved by the Institutional Review Board of Samsung Medical Center.
RESULTS
1. Primary diagnosis and treatment history
The clinical characteristics of the 39 Korean patients with t-MN examined in this study are summarized in Tables 1 and 2. There were 19 male and 20 female patients with a mean age of 46 yr (range, 2-79 yr). Of them, 31 patients were adults and 8 were pediatric patients; 59% were aged older than 50 yr.
Table 1.
Clinical and cytogenetic features of therapy-related myeloid neoplasms from primary hematologic malignancies
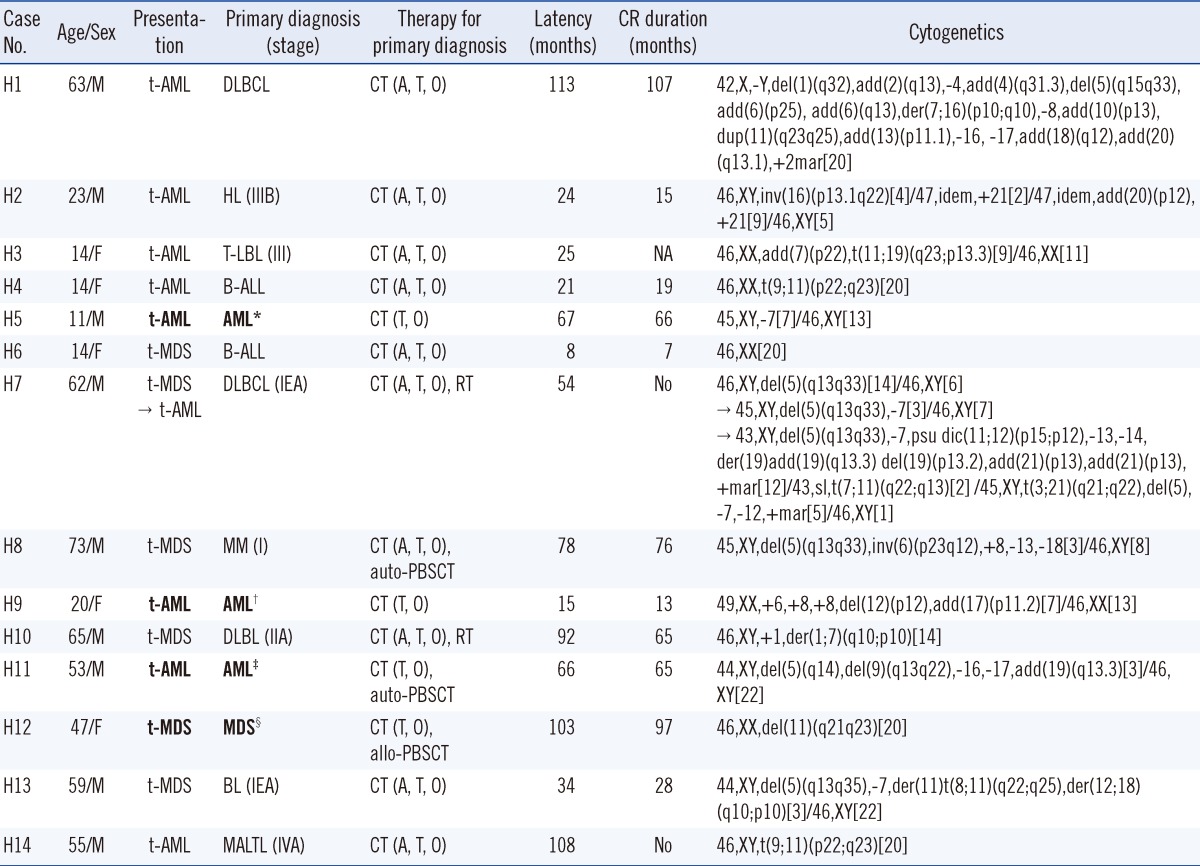
Bold text indicates t-MNs following treatment of de novo AML or MDS. Primary cytogenetics when diagnosed with de novo AML or MDS: *45,X,-Y,der(8)t(5;8)(q23;q21.3),del(21)(q22); †46,XX,t(15;17)(q22;q21)[14]/46,XX[6]; ‡45,X,-Y,t(8;21)(q22;q22)[14]/46,XY[6]; §47,XX,+8[16]/46,XX[4].
Abbreviations: CR, complete response; F, female; M, male; t-AML, therapy-related acute myeloid leukemia; t-MDS, therapy-related myelodysplastic syndrome; DLBCL, diffuse large B-cell lymphoma; HL, Hodgkin lymphoma; T-LBL, T lymphoblastic lymphoma; B-ALL, B lymphoblastic leukemia; AML, acute myeloid leukemia; MM, multiple myeloma; MDS, myelodysplastic syndrome; BL, Burkitt's lymphoma; MALTL, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue; CT, chemotherapy; A, alkylating agent; T, DNA topoisomerase II inhibitor; O, other chemotherapy; RT, radiotherapy; CBT, cord blood transplantation; auto-PBSCT, autologous peripheral blood stem cell transplantation; allo-PBSCT, allogeneic peripheral blood stem cell transplantation; NA, not available.
Table 2.
Clinical and cytogenetic features of therapy-related myeloid neoplasms from primary solid tumors
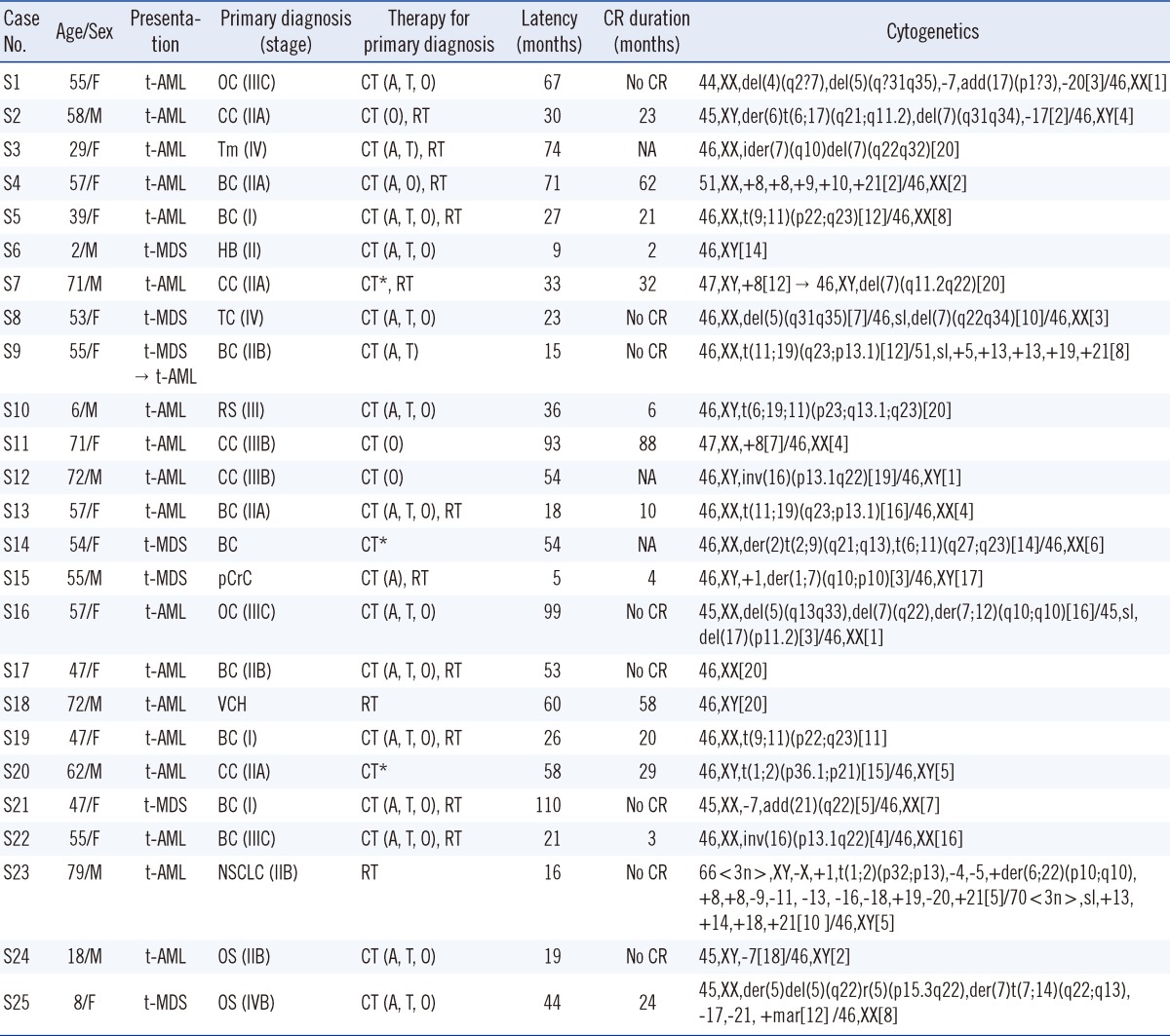
*Data regarding chemotherapy were incomplete for 3 patients.
Abbreviations: CR, complete response; F, female; M, male; t-AML, therapy-related acute myeloid leukemia; t-MDS, therapy-related myelodysplastic syndrome; OC, ovarian cancer; CC, colon cancer; Tm, thymoma; BC, breast cancer; HB, hepatoblastoma; TC, thymic cancer; RS, rhabdomyosarcoma; pCrC, posterior cricoid cancer; VCH, vocal cord hyperkeratosis with atypia; NSCLC, non-small cell lung cancer; OS, osteosarcoma; CT, chemotherapy; A, alkylating agent; T, DNA topoisomerase 2 inhibitor; O, other chemotherapy; RT, radiotherapy; NA, not available.
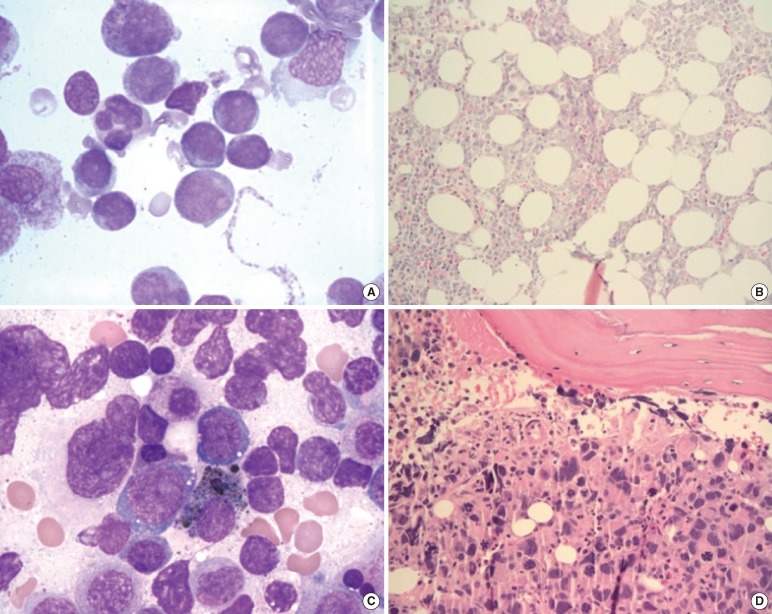
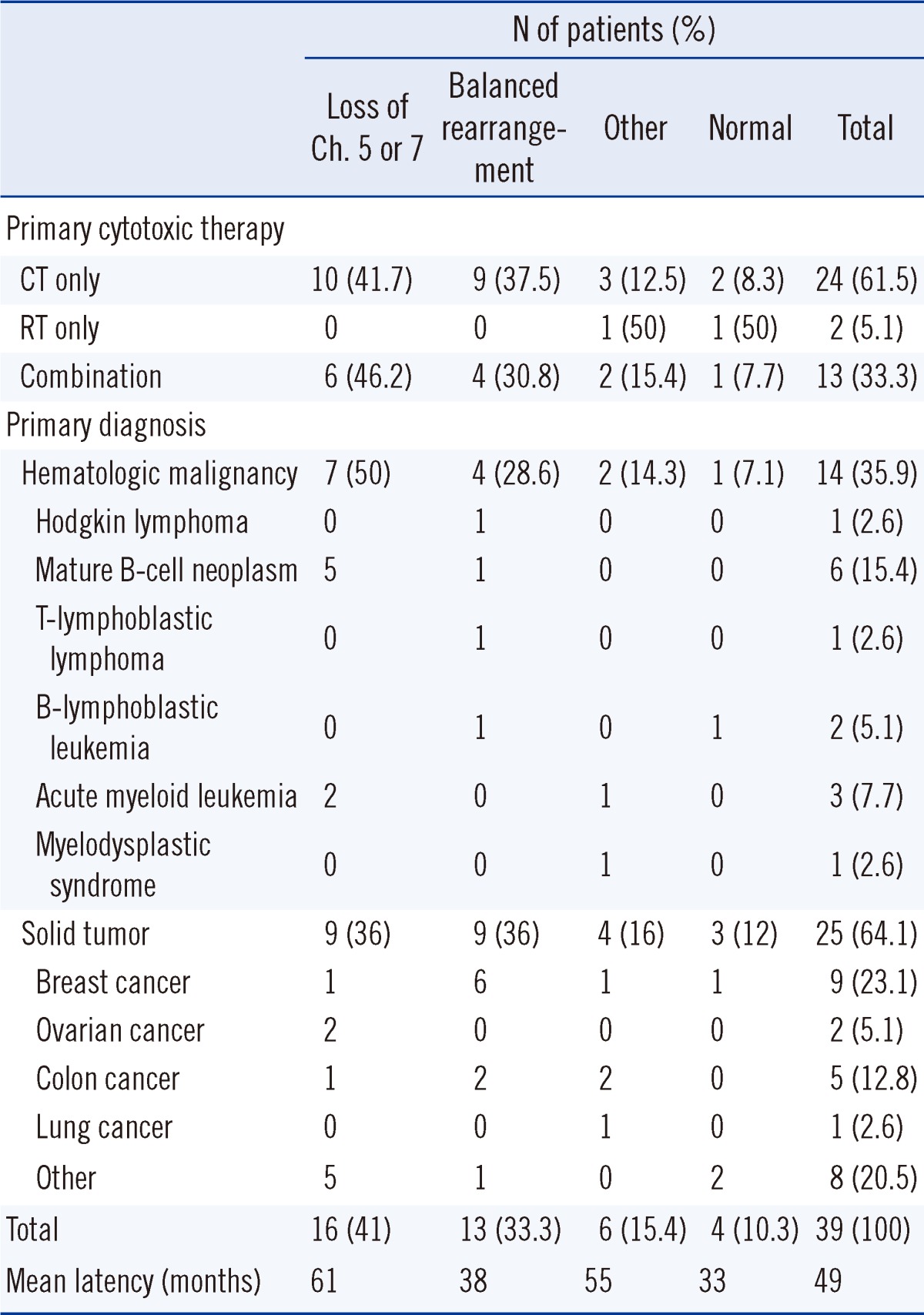
The primary diagnosis and primary cytotoxic therapy methods are summarized in Table 3. Fourteen (35.9%) patients showed primary hematologic malignancy, with mature B-cell neoplasm as the most common type (6 patients [15.4%]). Five patients had acute leukemia (AML, 3 patients [7.7%]; ALL, 2 patients [5.1%]) as the primary malignancy. Diagnosis of t-MN from de novo AML or MDS was based on clonal change, loss of the original clone, and presence of a new clone as well as on cytogenetic and molecular studies showing morphological alteration (Fig. 1) [15]. Table 1 summarizes the cytogenetic alterations of 4 patients with primary AML or MDS. Twenty-five (64.1%) patients had a solid tumor, with breast cancer being the most common type (9 patients, 23.1%).
Table 3.
Primary diagnosis, primary cytotoxic therapy, and clinical presentation of 39 patients with therapy-related myeloid neoplasms
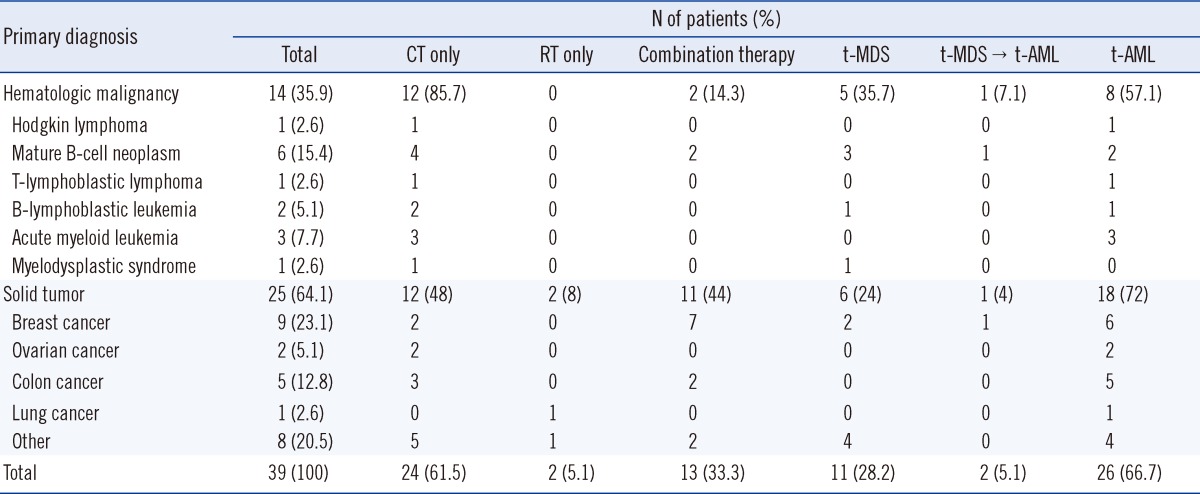
Abbreviations: CT, chemotherapy; RT, radiotherapy; t-AML, therapy-related acute myeloid leukemia; t-MDS, therapy-related myelodysplastic syndrome.
Fig. 1.
Morphology of the neoplastic cells of AML (A, B) and of subsequent t-AML (C, D) in patient H11. (A) Bone marrow aspirate smear (Wright-Giemsa stain, ×1,000) demonstrating myeloblast morphology. (B) Bone marrow biopsy section (H&E, ×200) demonstrating a uniform blast infiltrate. (C) Bone marrow aspirate smear (Wright-Giemsa, ×1,000) showing dysplastic megakaryocytes and increased blasts. (D) Biopsy section (H&E, ×200) showing prominent dysplastic megakaryocytes.
Abbreviation: H&E, hematoxylin and eosin stain.
Primary cytotoxic therapy consisted of CT, radiotherapy (RT), or both. A total of 24 patients (61.5%) received only CT, while 2 (5.1%) received only RT. Thirteen (33.3%) patients were treated using both methods, either concurrently (1 patient), sequentially (9 patients), or separated by several years (2 patients).
2. Clinical presentation
Table 3 summarizes clinical presentations according to the primary diagnosis and primary cytotoxic therapy. Thirteen (33.3%) patients were first diagnosed during the t-MDS phase of disease, and 2 of these cases later progressed to t-AML. A total of 26 (66.7%) patients had overt t-AML at presentation; for 15 of these patients, data were available regarding regular CBC for more than 1 yr before t-AML diagnosis. Cytopenia such as anemia, neutropenia, or moderate thrombocytopenia preceded t-AML by more than 3 months in 8 of the 15 patients, while persistent cytopenia was observed in 5 patients for 6 months and in 2 patients for more than 1 yr before t-AML diagnosis.
In total, 8 of 14 patients (57.1%) with primary hematologic malignancies and 18 of 25 patients (72%) with primary solid tumors had t-AML at presentation. Further, 6 patients with primary hematologic malignancies and 7 patients with solid tumors had t-MDS at presentation.
Moreover, 15 (62.5%) of the CT-only patients, 2 (100%) of the RT-only patients, and 9 (69.2%) of the patients who had undergone combination therapy had overt t-AML at presentation. Nine (37.5%) of the CT-only patients and 4 (30.8%) of the patients who had undergone combination therapy had t-MDS at presentation. One of the t-MDS patients who had received CT-only and one who had received combination therapy progressed to t-AML.
The time interval from initiation of CT and/or radiation to detection of t-MN ranged from 5 to 113 months (mean, 49 months). The mean latency period for developing t-MN was 58 months for primary hematologic malignancies and 45 months for primary solid tumors. The mean latency period for developing t-AML (50 months) was similar to that for developing t-MDS (48 months). The latency period was 29 months for pediatric patients and 55 months for adult patients.
3. Cytogenetic aberrations
Table 4 summarizes the clonal cytogenetic abnormalities detected in the 39 patients with t-MN and their relationship with the primary disease and therapy. Four (10.3%) patients had no detectable abnormalities, and 35 (89.7%) patients had abnormal clones. Sixteen patients (41%) had a whole or partial loss of chromosomes 5 and/or 7, and 13 patients (33.3%) had balanced chromosomal translocations. The most common abnormalities were translocations involving 11q23 (9 patients, 23.1%), including 1 three-way translocation involving the MLL gene. Six different partner genes were observed, including 9p22 (4/9), 19p13.1 (2/9), 19q13.1 (1/9), 19p13.3 (1/9), 6q27 (1/9), and 6p23 (1/9). The second most common abnormality was inv(16) (3 patients, 7.7%). Deletion of the long arm of the chromosome, del(5q), was observed in 9 patients. Loss of all or part of the long arm of chromosome 7 was noted in 13 patients; 8 were del(7q) and 5 were monosomy 7. Monosomal karyotypes were detected in 10 (25.6%) patients.
Table 4.
Cytogenetic abnormalities and clinical characteristics of 39 patients with therapy-related myeloid neoplasms
Abbreviations: Ch, chromosome; CT, chemotherapy; RT, radiotherapy.
According to the primary disease, the frequencies of loss of chromosome 5 or 7 were 50% among patients with primary hematologic malignancies and 36% among those with solid tumors. In contrast, balanced translocation was more common among patients with a primary solid tumor (36%) than among patients with hematologic malignancies (28.6%). Of the 9 patients with t(11q23), 5 (55.6%) had primary breast cancer. A similar analysis based on primary cytotoxic therapy revealed a clonal loss of chromosome 5 or 7 in 10 (41.7%) patients treated with CT alone and in 6 (46.2%) patients treated with combination therapy. Balanced rearrangements were identified in 9 (37.5%) patients treated with CT alone and in 4 (30.8%) patients treated with combination therapy. Among 13 patients with t-MDS at presentation, 8 (61.5%) had a loss of chromosome 5 or 7 and 2 (15.4%) had balanced rearrangements. In contrast, of 26 patients with overt t-AML at presentation, balanced rearrangements occurred more frequently (11 patients, 42.3%) than loss of chromosome 5 or 7 (8 patients, 30.8%). Patients with balanced rearrangements had shorter latency intervals (mean, 38 months) than patients with loss of chromosome 5, 7, or both (mean, 61 months).
Two cases showed clonal evolution. One patient (S7 in Table 2) with colon cancer acquired t-AML with +8 and was in CR after treatment with CT (idarubicin and cytosine arabinoside). However, the patient developed t-MDS with a newly acquired chromosomal abnormality of del(7q) 19 months later. One male patient (H7 in Table 1) with previous diffuse large B-cell lymphoma (DLBCL), who had undergone combination therapy, was diagnosed with t-MDS in association with isolated del(5q); 14 months later, he had del(5q) with monosomy 7 in the same clone. Finally, the patient progressed to t-AML with a complex karyotype of del(5q), -7, and t(3;21) in the same clone.
DISCUSSION
Recently, t-MN has been recognized as one of the most serious challenges facing clinical oncology, due to improved survival rates following primary malignancies [6, 16]. Many studies have addressed various aspects of t-MN; however, this is the largest study to address the clinical and cytogenetic characteristics of the disease in Korea [17-24].
The previous WHO classification emphasized 2 different t-MNs: one that occurs after exposure to alkylating agents or radiation therapy (typically with abnormalities of chromosomes 5 or 7) and the other that occurs after exposure to topoisomerase 2 inhibitors (typically with balanced translocations involving 11q23 or 21q22) [4]. However, most patients who develop t-MNs are treated with both alkylating agents and topoisomerase 2 inhibitors; therefore, a division according to the type of therapy is usually not practical and not recommended in the revised classification [1]. In our study, only 4 patients received topoisomerase 2 inhibitors without alkylating agents, and only 2 patients received alkylating agents without topoisomerase 2 inhibitors; hence, it was not useful to compare these 2 groups in terms of latency or survival.
We found that recurring translocations or balanced rearrangements were significantly more common in patients who had previously undergone therapy to treat a solid tumor. In particular, 6 of 9 patients with primary breast cancer had recurring chromosomal rearrangements; 5 patients had t(11q23) and 1 had inv(16). Additionally, most patients with balanced translocations and other recurrent rearrangements were overt t-AML patients (11 of 13 patients, 84.6%) rather than t-MDS patients (2 of 13 patients, 15.4%). Smith et al. [4] also documented these patterns, which were subsequently confirmed by others [17, 25].
Previous studies on t-MN have shown that the number of patients with a history of treatment for a previous hematological malignancy was nearly equal to that for a previous solid tumor [4, 11]. Of primary hematologic malignancies, lymphoma (non-Hodgkin lymphoma and Hodgkin lymphoma) was the most common [3, 4]. In our study, 14 patients had a primary hematologic malignancy and 25 had a solid tumor. Notably, 4 patients developed t-MN after treatment for de novo AML/MDS, which has rarely been reported. This may be due to improvement of long-term disease-free survival after treatment for AML. In the present study, altered cytogenetic and/or molecular abnormalities were present in all patients with t-MN developing from de novo AML/MDS, and dysplastic changes were observed. One patient (H12 in Table 1) developed t-MN from AML with t(8;21) (q22;q22), which is well known to be associated with a good prognosis. He had received CT that included idarubicin and cytosine arabinoside, followed by autologous peripheral blood stem cell transplantation. This patient developed t-MN after 5 yr, despite the absence of factors associated with a poor prognosis, such as CD56 expression or the presence of the KIT mutation.
Several previous studies have investigated the clinical presentation of t-MN. These studies have consistently found that the majority (60-80%) of patients were diagnosed with t-MDS with or without subsequent progression to t-AML [2-4]. However, our data showed that t-AML (66.7%) was more common than t-MDS. This suggests the possibility of delayed diagnosis of t-MDS after progression to t-AML. Indeed, we observed that cytopenia preceded the diagnosis of t-AML in more than half of the 15 patients whose regular CBC data were available. Of these cases, persistent cytopenia was observed in 5 patients for 6 months before the diagnosis of t-AML, and in 2 patients for more than 1 yr.
Although our data do not demonstrate the survival rate of t-MN patients due to incomplete data regarding clinical outcome and a short follow-up period, it is well known that the survival of patients with t-MN is often poor despite prompt diagnosis and treatment [26-28]. Poor survival of patients with t-MN is associated with multiple competing risks, including persistence of primary malignant disease, significant organ dysfunction from previous therapies, prolonged immunocompromised status, and lack of uniform treatment [4]. Therefore, early diagnosis or prevention of this late complication of otherwise curative cancer therapies is critical, and close monitoring for signs of bone marrow dysfunction, such as progressive cytopenia, is necessary.
This study described the clinical and cytogenetic characteristics of Korean patients with t-MN. Although our results were generally consistent with those of previous reports, we found that t-MN from de novo leukemia was common and that t-AML was more common than t-MDS at presentation. Multi-institutional studies involving a larger number of patients and additional parameters are required to investigate the epidemiology, genetic predisposition, and survival rate of t-MN in Korea.
Acknowledgement
This work was supported by a grant #CRS111-03-2 from the Clinical Research Development Program at Samsung Medical Center, Seoul.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Swerdlow SH, Campo E, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press; 2008. p. 110. [Google Scholar]
- 2.Michels SD, McKenna RW, Arthur DC, Brunning RD. Therapy-related acute myeloid leukemia and myelodysplastic syndrome: a clinical and morphologic study of 65 cases. Blood. 1985;65:1364–1372. [PubMed] [Google Scholar]
- 3.Le Beau MM, Albain KS, Larson RA, Vardiman JW, Davis EM, Blough RR, et al. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986;4:325–345. doi: 10.1200/JCO.1986.4.3.325. [DOI] [PubMed] [Google Scholar]
- 4.Smith SM, Le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52. doi: 10.1182/blood-2002-11-3343. [DOI] [PubMed] [Google Scholar]
- 5.Rowley JD. International workshop on the relationship of prior therapy to balanced chromosome aberrations in therapy-related myelodysplastic syndromes and acute leukemia: overview report. Genes Chromosomes Cancer. 2002;33:331–345. doi: 10.1002/gcc.10040. [DOI] [PubMed] [Google Scholar]
- 6.Rund D, Krichevsky S, Bar-Cohen S, Goldschmidt N, Kedmi M, Malik E, et al. Therapy-related leukemia: clinical characteristics and analysis of new molecular risk factors in 96 adult patients. Leukemia. 2005;19:1919–1928. doi: 10.1038/sj.leu.2403947. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen-Bjergaard J. Insights into leukemogenesis from therapy-related leukemia. N Engl J Med. 2005;352:1591–1594. doi: 10.1056/NEJMe048336. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen-Bjergaard J, Specht L, Larsen SO, Ersbøll J, Struck J, Hansen MM, et al. Risk of therapy-related leukaemia and preleukaemia after Hodgkin's disease. Relation to age, cumulative dose of alkylating agents, and time from chemotherapy. Lancet. 1987;2:83–88. doi: 10.1016/s0140-6736(87)92744-9. [DOI] [PubMed] [Google Scholar]
- 9.Thirman MJ. Therapy-related myeloid leukemia. Hematol Oncol Clin North Am. 1996;10:293–320. doi: 10.1016/s0889-8588(05)70340-3. [DOI] [PubMed] [Google Scholar]
- 10.Singh ZN, Huo D, Anastasi J, Smith SM, Karrison T, Le Beau MM, et al. Therapy-related myelodysplastic syndrome: morphologic subclassification may not be clinically relevant. Am J Clin Pathol. 2007;127:197–205. doi: 10.1309/NQ3PMV4U8YV39JWJ. [DOI] [PubMed] [Google Scholar]
- 11.Mauritzson N, Albin M, Rylander L, Billstrom R, Ahlgren T, Mikoczy Z, et al. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976-1993 and on 5098 unselected cases reported in the literature 1974-2001. Leukemia. 2002;16:2366–2378. doi: 10.1038/sj.leu.2402713. [DOI] [PubMed] [Google Scholar]
- 12.Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 13.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 14.Shaffer LG, Slovak ML, et al., editors. ISCN 2009: An International System of Human Cytogenetic Nomenclature. Basel: Karger; 2009. [Google Scholar]
- 15.Arana-Yi C, Block AW, Sait SN, Ford LA, Barcos M, Baer MR. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following treatment of acute myeloid leukemia: possible role of cytarabine. Leuk Res. 2008;32:1043–1048. doi: 10.1016/j.leukres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Rund D, Ben-Yehuda D. Therapy-related leukemia and myelodysplasia: evolving concepts of pathogenesis and treatment. Hematology. 2004;9:179–187. doi: 10.1080/10245330410001701503. [DOI] [PubMed] [Google Scholar]
- 17.Shim H, Chi HS, Jang S, Seo EJ, Park CJ, Lee JH, et al. Therapy-related acute leukemia in breast cancer patients: twelve cases treated with a topoisomerase inhibitor. Korean J Hematol. 2010;45:177–182. doi: 10.5045/kjh.2010.45.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur M, Lee DS, Shin HY, Ahn HS, Kim BK, Cho HI. Four cases of therapy-related leukemia. J Korean Med Sci. 1999;14:327–329. doi: 10.3346/jkms.1999.14.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SY, Kim M, Lim J, Kim Y, Han K, Kim SY, et al. A case of therapy-related acute myeloid leukemia associated with inv(16) Korean J Lab Med. 2007;27:19–21. doi: 10.3343/kjlm.2007.27.1.19. [DOI] [PubMed] [Google Scholar]
- 20.Seo YI, Park R, Choi TY, Shin JW, Won JH, Park HS, et al. A case of therapy-related acute monocytic leukemia following low-dose of etoposide treatment for hemophagocytic lymphohistiocytosis. Korean J Lab Med. 2007;27:244–247. doi: 10.3343/kjlm.2007.27.4.244. [DOI] [PubMed] [Google Scholar]
- 21.Park TS, Cheong JW, Song J, Choi JR. Therapy-related myelodysplastic syndrome with der(17)t(12;17)(q13;p13) as a new recurrent cytogenetic abnormality after treatment for chronic lymphocytic leukemia. Leuk Res. 2009;33:1001–1004. doi: 10.1016/j.leukres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Jung CL, Kim HJ, Kim DH, Huh HJ, Song MJ, Kim SH. CKIT mutation in therapy-related acute myeloid leukemia with MLLT3/MLL chimeric transcript from t(9;11)(p22;q23) Ann Clin Lab Sci. 2011;41:193–196. [PubMed] [Google Scholar]
- 23.Kim YG, Cho SY, Park TS, Oh SH, Yoon HJ. Therapy-related myelodysplastic syndrome/acute myeloid leukemia with del(7)(q22) in a patient with de novo AML. Ann Clin Lab Sci. 2011;41:79–83. [PubMed] [Google Scholar]
- 24.Kwon A, Park JY, Kwon JH, Song HH, Shin KS, Lee YK, et al. A case of therapy-related myeloid neoplasm after successful treatment of acute promyelocytic leukemia. Lab Med Online. 2011;1:227–231. [Google Scholar]
- 25.Chandra P, Luthra R, Zuo Z, Yao H, Ravandi F, Reddy N, et al. Acute myeloid leukemia with t(9;11)(p21-22;q23): common properties of dysregulated ras pathway signaling and genomic progression characterize de novo and therapy-related cases. Am J Clin Pathol. 2010;133:686–693. doi: 10.1309/AJCPGII1TT4NYOGI. [DOI] [PubMed] [Google Scholar]
- 26.Larson RA. Therapy-related myeloid neoplasms. Haematologica. 2009;94:454–459. doi: 10.3324/haematol.2008.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18:120–125. doi: 10.1038/sj.leu.2403187. [DOI] [PubMed] [Google Scholar]
- 28.Kern W, Haferlach T, Schnittger S, Hiddemann W, Schoch C. Prognosis in therapy-related acute myeloid leukemia and impact of karyotype. J Clin Oncol. 2004;22:2510–2511. doi: 10.1200/JCO.2004.99.301. [DOI] [PubMed] [Google Scholar]