Abstract
GH receptor (GHR) gene-disrupted mice (GHR−/−) have provided countless discoveries as to the numerous actions of GH. Many of these discoveries highlight the importance of GH in adipose tissue. For example GHR−/− mice are insulin sensitive yet obese with preferential enlargement of the sc adipose depot. GHR−/− mice also have elevated levels of leptin, resistin, and adiponectin, compared with controls leading some to suggest that GH may negatively regulate certain adipokines. To help clarify the role that GH exerts specifically on adipose tissue in vivo, we selectively disrupted GHR in adipose tissue to produce Fat GHR Knockout (FaGHRKO) mice. Surprisingly, FaGHRKOs shared only a few characteristics with global GHR−/− mice. Like the GHR−/− mice, FaGHRKO mice are obese with increased total body fat and increased adipocyte size. However, FaGHRKO mice have increases in all adipose depots with no improvements in measures of glucose homeostasis. Furthermore, resistin and adiponectin levels in FaGHRKO mice are similar to controls (or slightly decreased) unlike the increased levels found in GHR−/− mice, suggesting that GH does not regulate these adipokines directly in adipose tissue in vivo. Other features of FaGHRKO mice include decreased levels of adipsin, a near-normal GH/IGF-1 axis, and minimal changes to a large assortment of circulating factors that were measured such as IGF-binding proteins. In conclusion, specific removal of GHR in adipose tissue is sufficient to increase adipose tissue and decrease circulating adipsin. However, removal of GHR in adipose tissue alone is not sufficient to increase levels of resistin or adiponectin and does not alter glucose metabolism.
The GH/IGF-1 axis has important roles in growth, metabolism, and lifespan. Because GH signaling requires the binding of GH to its cognate receptor (R), disruption or deletion of either the GH or GHR gene ablates GH action. In this regard, GHR-disrupted or knockout mice (GHR−/− mice), which were generated in our laboratory approximately 15 yr ago, have produced many novel and clinically relevant discoveries that have played an important role in our understanding of GH function (1). GHR−/− mice are dwarf with low levels of IGF-1 and increased GH; thus, they are GH resistant (2). They are also extremely insulin sensitive (3), presumably due to the absence of the anti-insulin effects of GH. Remarkably, they exhibit up to a 50% increase in lifespan that cannot be further extended with calorie restriction (4, 5). The extended lifespan in GHR−/− mice is associated with lower morbidity and disease-related mortality, with almost half of the long-lived mice dying without obvious lethal pathological lesions as compared with 10% of their wild-type littermate controls (6). Importantly, the unique phenotype of GHR−/− mice has notable similarities with a population of Ecuadorian Laron Syndrome (LS) individuals (7). This population has a reduction in IGF-1 levels, an elevation in GH levels, and enhanced insulin sensitivity. Thus far, these individuals do not appear to experience life extension, but they are protected from diabetes and fatal neoplasms. The increased insulin sensitivity in LS individuals and GHR−/− mice is particularly interesting considering that both are obese, a characteristic not typically associated with improved glucose homeostasis or lifespan extension.
The adiposity of GHR−/− mice has been extensively studied. GHR−/− mice have a significantly higher percent body fat throughout their lifespan (8) with a disproportionate amount of fat deposition in the sc white adipose tissue (WAT) depot (8). In terms of adipokine expression, leptin levels (9) are elevated in GHR−/− mice, which is consistent with their increased obesity. Interestingly, adiponectin levels, which are usually negatively correlated with obesity, are elevated in GHR−/− mice (10). Adiponectin is an important adipokine with beneficial effects on inflammation and insulin sensitivity and is positively correlated with increased longevity in animals and humans (11–13). Furthermore, adiponectin has been reported to be negatively regulated by GH in multiple systems (14, 15). As stated above, GHR−/− mice are obese throughout life and have high adiponectin levels. This suggests that the lack of GH action, either directly via negative regulation by GH or indirectly through other physiological alterations in these mice, overrides the presence of obesity. Furthermore, the increased adiponectin may also contribute to the improved glucose metabolism of GHR−/− mice. Although less thoroughly studied, LS individuals have increased serum levels of adiponectin and leptin as well as notable enlargements in WAT (7, 16). Taken together, LS individuals and GHR−/− mice provide a means to better understand how adipose tissue mass can be enlarged without notable deleterious effects on health and lifespan. Because GHR signaling is disrupted in all tissues of LS patients and GHR−/− mice, it would be of value to disrupt GH signaling selectively in adipose tissue to better understand the impact of this tissue on whole-body metabolism and physiology.
Multiple groups have utilized the Cre-LoxP system to evaluate GHR disruption in various tissues and cell types. Liver-specific deletion of GHR results in no major changes in adiposity although these mice are reported to have marked insulin resistance and severe hepatic steatosis (17). Muscle-specific deletion of GHR has been reported by two groups with different results. Mavalli et al (18) report peripheral adiposity, insulin resistance, and glucose intolerance using the Mef-2c promoter/enhancer whereas mice generated using the muscle creatine kinase promoter/enhancer have reduced adiposity and overall improvement in glucose metabolism (19). Disruption of GHR in β-cells impairs insulin secretion that is exacerbated by a high-fat diet (20). Collectively, these results demonstrate that disruption of GHR in specific tissues can dramatically influence glucose homeostasis and adiposity. To date, no studies have assessed GHR gene disruption in adipose tissue. Because GH's action on adipose tissue plays an essential metabolic role in terms of whole-body physiology, we set out to generate and characterize adipose tissue GHR gene-disrupted mice. In this study, we used the Cre-LoxP system to disrupt the GHR−/− gene in adipose tissue to produce Fat GHR Knockout (FaGHRKO) mice. We hypothesized that disruption of the GHR in adipose tissue will 1) increase adiposity, 2) increase leptin and adiponectin levels, and 3) improve glucose homeostasis, all of which occur in global GHR−/− mice. Here, we report initial characterization of these mice including adiposity, adipokine levels, and glucose metabolic results as well as effects on morphometric, endocrine, and physiological parameters. We also discuss these results in comparison with previous reports characterizing the global GHR−/− mice to provide novel and more specific insight into the specific role of GH in adipose tissue.
Materials and Methods
FaGHRKO mouse production
The mouse strain carrying the conditional GHR floxed allele (GHRflox/flox) was generated by the Knockout Mouse Project (KOMP) as previously described (21). Adipose tissue-specific GHR−/− mice (FFCx) and floxed littermate controls (FFxx) were generated by breeding conditional GHRflox/flox mice to B6.Cg-Tg(Fabp4-cre)1Rev/J mice purchased from The Jackson Laboratory (Bar Harbor, Maine). B6.Cg-Tg(Fabp4-cre)1Rev/J mice have been crossed to C57BL/6 mice for nine generations at The Jackson Laboratory.
In the current study, 146 mice divided into two main cohorts were used. The first cohort of male and female FaGHRKO and littermate controls (63 total, n = 15–16 per group) were used for all measurements except body composition over time. The second cohort of FaGHRKO and littermate controls of both sexes (83 total, n = 16–25 per group) were used only to collect longitudinal body composition. Mice were housed three to four per cage and given ad libitum access to water and standard laboratory rodent chow (ProLab RMH 3000). The cages were maintained in a temperature- and humidity-controlled room and exposed to a 14-hour light, 10-hour dark cycle. All procedures were approved by the Ohio University Institutional Animal Care and Use Committee.
Quantitative real-time PCR
Whole frozen tissue was homogenized using the Precellys 24-Dual (Bertin Technologies, Montigny-le-Bretonneux, France). The homogenization conditions were optimized for each tissue. RNA was purified using Qiagen RNeasy Mini Kit (QIAGEN, Chatsworth, Calfornia), and the concentration and integrity of the mRNA was verified by the Thermo NanoDrop 2000c and Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, California), respectively. Qiagen QuantiTect Reverse Transcription Kit was used for cDNA synthesis. For real-time data collection, Qiagen QuantiTect SYBR Green PCR Kit was used with a BIO-RAD iCycler Thermal Cycler (Bio-Rad Laboratories, Inc, Hercules, California). Qbase Plus from Biogazelle (Zwijnaarde, Belgium) was used to analyze the qPCR results. Each tissue was tested with a pair of primers for the GHR gene as well as seven reference genes (EEF2, RPS3, B2M, ACTB, HPRT, EIF3F, and RPL38). For all other qPCR, the reactions and calculations were performed as previously described (22).
Western blot analysis of GHR
Frozen tissue samples were homogenized in lysis buffer (1% vol/vol Triton X-100, 150 mM NaCl, 10% vol/vol glycerol, 50 mM Tris-HCl [pH 8.0], 100 mM NaF, 2 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 10 μg/mL aprotinin) and proteins resolved by 8% SDS-PAGE. After electrophoresis, proteins were transferred to an Amersham Hybond-enhanced chemiluminescence (ECL) membrane (GE Healthcare, Pittsburgh, Pennsylvania), and the membrane incubated in Tris buffered saline (TBS) containing 2% gelatin and 0.05% Tween 20. The membrane was then rinsed briefly in TBS/Tween and incubated with rabbit anti-GHR primary antibody (AL47) in 1% gelatin-TBS/Tween overnight (23). The following day, the membranes were washed and incubated with secondary antibody for 2 h (NA934, ECL Antirabbit IgG HRP Linked, GE Healthcare, Piscataway, New Jersey) in 1% gelatin-TBS/Tween 20. The membrane was then washed and incubated for 5 minutes with ECL Plus Western Blotting Detection System (RPN2132, GE Healthcare) and exposed to Kodak Biomax XAR film (Eastman Kodak, Rochester, New York).
Body composition measurements
Body composition was measured in two cohorts of mice. In the first cohort, FaGHRKO and littermate controls (n = 15–16 per group per sex) were measured at 5 months of age before dissection and subsequent tissue analysis at 6 mo. In the second cohort, FaGHRKO and littermate controls of both sexes (n = 16–25 per group per sex) were measured over time starting at 2 months until 12 months of age. Body composition was measured using a Bruker Minispec NMR (Bruker Corp, The Woodlands, Texas) as previously described (8, 24).
Fasting blood glucose, glucose tolerance test (GTT), and insulin tolerance test (ITT) measurements
Fasting blood glucose was determined at 5 months of age using OneTouch Ultra test strips and glucometers (Lifescan, Inc, Milpitas, California). Blood samples were obtained by cutting approximately 1 mm from the tip of the tail and collecting the first drop of blood. Fasting blood glucose measurements occurred starting at 9:00 am after a 12-hour overnight fast. GTTs were performed at 5 months and 1 week of age. Mice were fasted for 12 hours before commencement of the experiment at 9:00 am. Each mouse received an ip injection of 10% glucose at a dose of 1 g/kg body weight. Blood glucose measurements were monitored before the glucose injection and at 15, 30, 45, 60, and 90 minuntes after injection. ITTs were performed at 5 months and 2 weeks of age in a fed state at approximately 3:00 pm. Recombinant human insulin (Humulin-R; Eli Lilly & Co, Indianapolis, Indiana) was prepared by diluting Humulin-R (100 U/ml) to 0.075 U/mL in sterile 0.9% NaCl. Each mouse received an ip injection of the 0.075 U/ml insulin solution at a dose of 0.75U/kg body weight. Blood glucose measurements were performed before the insulin injection and at 15, 30, 45, and 60 minutes after injection.
Serum measurements
Serum measurements were performed at 6 mo of age. Serum was collected starting at approximately 9:00 am after a 12-h fast. IGF-1 levels (total IGF-1) were measured using IGF-1 (mouse, rat) ELISA kits (Catalog no. 22-IG1MS-E01; ALPCO Diagnostics, Salem, New Hampshire). High molecular weight (HMW) and total adiponectin levels were measured using ELISA kits (47-ADPMS-E01) from ALPCO Diagnostics (Salem, New Hampshire). Insulin, c-peptide, leptin, resistin, and gastric inhibitory polypeptide were measured using a Mouse Metabolic Panel (catalog no. MMHMAG-44K; Millipore Corp., Billerica, Massachusetts). IGF binding proteins-1, -2, -3, -5, -6, and -7 were measured using the Mouse IGF Binding Protein MAGNETIC Bead Panel (catalog no. MIGFBPMAG-43; Millipore Corp.). Adipsin, AGP, α-2-macroglobulin, C-reactive protein (CRP), and haptoglobin were measured using the MILLIPLEX MAP Mouse Acute Phase Magnetic Bead Panel 2 (catalog no. MAP2MAG-76K; Millipore Corp.). Soluble receptors (sCD30, sgp130, sIL-1RI, sIL-1RII, sIL-2Ra, sIL-4R, sIL6R, sTNFRI, sTNFRII, soluble vascular endothelial growth factor receptor (sVEGFR)1, sVEGFR2, sVEGFR3) were measured using the MILLIPLEX MAP Mouse Soluble Cytokine Receptor Panel (catalog no. MSCR-42K, Millipore Corp.). Lipocalin-2 and pentraxin-3 were measured using the MILLIPLEX MAP Mouse Acute Phase Magnetic Bead Panel-1 (catalog no. MAP1MAG-76K; Millipore Corp.). All MILLIPEX kits were analyzed using a Milliplex 200 Analyzer (Millipore Corp.). All of the above procedures were performed according to the manufacturer's instructions.
Tissue collection, liver, and WAT analysis
All tissue was collected from 6-mo-old mice. Mice were killed starting at 9:00 am following a 12-hour overnight fast. Mice were first placed in a CO2 chamber until unconscious, after which blood was quickly collected from the orbital sinus. After blood collection, the mice were killed by cervical dislocation. Kidney, heart, lung, spleen, brain, skeletal muscle (gastrocnemius, soleus, and quadriceps), and interscapular BAT were collected, weighed, and flash frozen in liquid nitrogen and stored at −80ºC.
Liver tissue was collected and weighed, and a portion was flash frozen in liquid nitrogen and stored at −80ºC until processing for determining triglyceride content, while another portion was fixed in 10% formalin, embedded in paraffin, and processed for histology. For determining triglyceride content, liver tissues were thawed and used for extraction and measurement of triacylglycerol levels as described previously (25).
Subcutaneous, retroperitoneal, mesenteric, and perigonadal WAT were collected and weighed. A portion of WAT was flash frozen in liquid nitrogen and stored at −80ºC. For subcutaneous and perigonadal WAT, a portion of the sample was processed for histology by fixing in 10% formalin and embedding in paraffin. Adipocyte cell size and number were determined as previously described (26).
Statistical analysis
All values are given as means ± SEMs. Statistics were performed using SPSS version 14.0 (Chicago, Illinois). The two-tailed unpaired Student's t test was used to assess the significance of difference between two sets of data. Differences were considered to be statistically significant when P < .05.
Results
FaGHRKO mice
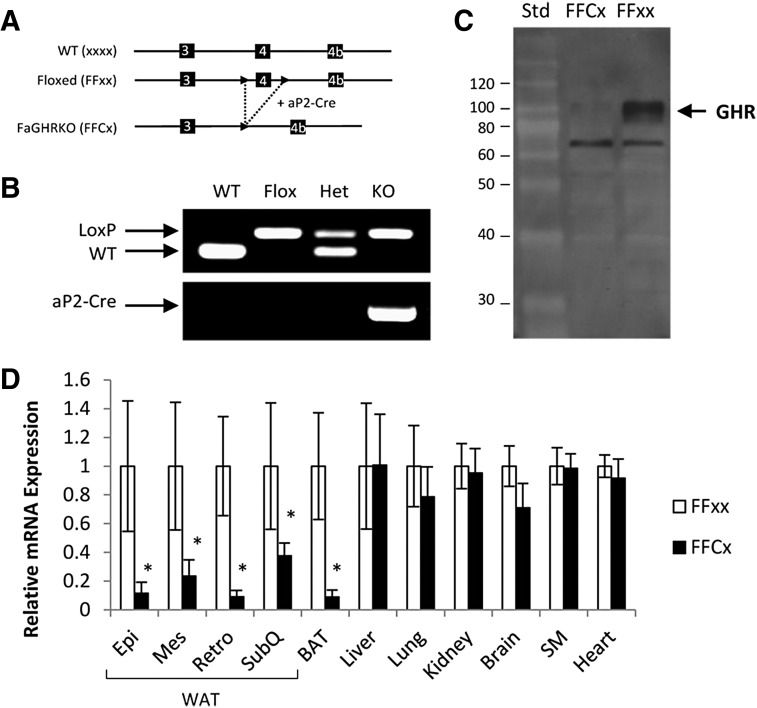
FaGHRKO mice (FFCx) and floxed littermate controls (FFxx) were generated by breeding conditional floxed GHRflox/flox mice to B6.Cg-Tg(Fabp4-cre)1Rev/J mice (Figure 1, A and B). Absence of GHR protein in WAT of FaGHRKO mice was shown by Western blot analysis (Figure 1C). Adipose tissue-specific deletion of GHR was quantified using qPCR in FaGHRKO mice (FFCx) and floxed littermate controls (FFxx) (Figure 1D). In the FaGHRKO mice, GHR mRNA expression is significantly decreased 89%, 77%, 85%, 60%, and 92% in epididymal, mesenteric, retroperitoneal, and sc WAT, and in brown adipose tissue (BAT), respectively, vs controls. No change in GHR gene expression was observed for liver, lung, kidney, brain, skeletal muscle, or heart.
Figure 1.
Generation and characterization of FaGHRKO mice. A, FaGHRKO mice were generated by crossing mice with a “floxed” exon 4 of the GHR to transgenic mice that express Cre recombinase under the control of the aP2 promoter/enhancer (aP2-Cre). Solid arrowheads depict the LoxP sites. B, PCR analysis detected the presence of the LoxP sites and the aP2-Cre transgene. C, Western blot analysis of GHR protein from WAT in FaGHRKO (FFCx) vs controls (FFxx). D, GHR mRNA expression level in various tissues from FaGHRKO mice (n = 6; black bars) compared with controls (n = 6; white bars). GHR expression was significantly decreased in both WAT and BAT. No changes to GHR gene expression were observed for liver, lung, kidney, brain, skeletal muscle (SM), or heart. Epi, epididymal; Mes, mesenteric; Retro, retroperitoneal; Std, standard; SubQ, subcutaneous.
Body weight and body composition
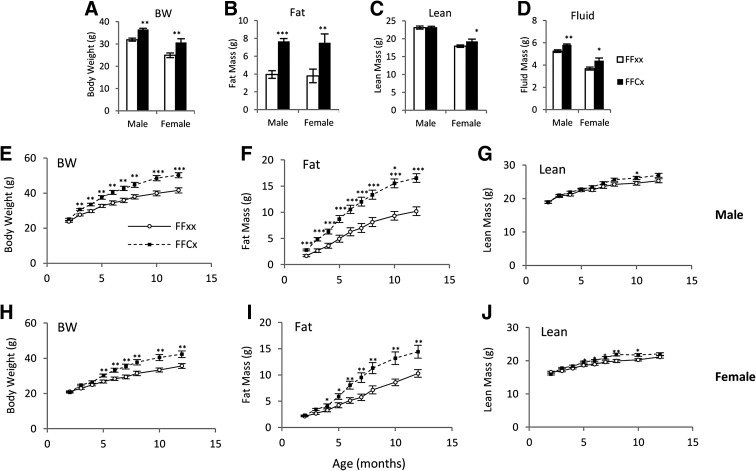
Five-month mean body weights of FaGHRKO mice that were also used for dissection were significantly greater than littermate controls with 14% and 23% increases for male and female, respectively (Figure 2A). Body composition analysis showed a 96% increase in total body fat mass in FaGHRKO mice compared with controls (Figure 2B). Total lean body mass was significantly increased in female (8%) but not male FaGHRKO mice compared with controls (Figure 2C). Additionally, total body fluid was significantly increased in both sexes of FaGHRKO mice (Figure 2D).
Figure 2.
GHR deletion in adipose tissue increases total body fat mass. Two cohorts of mice were studied, including 63 mice (n = 15–16 animals per group) that were used to measure body composition at 6 months of age before a 6-month dissection (A–D) and 83 mice (n = 16–25 animals per group) that were used to measure body composition over time (E–J). For the 63 mice used for dissections, body weight (A), fat mass (B), lean mass (C), and fluid mass (D) are shown for FaGHRKO (FFCx; black bars) and controls (FFxx; white bars) in both males and females. Body weight (E and H), fat mass (F and I), and lean mass (G and J) are shown for males and females over time. FaGHRKO (FFCx; black boxes) and controls (FFxx; white circles) are indicated. Values in A–J are represented as mean ± SEM (n = 15–25 per group). *P < .05; **P < .01; ***P < .001, FaGHRKO (FFCx) vs control (FFxx). BW, body weight.
Body composition measurements over time showed that the increase in body weight became significant for FaGHRKO males by 3 mo of age and for FaGHRKO females by 5 months of age (Figure 2, E and H). This difference continued to increase with age. Fat mass was significantly increased at 2 months in male FaGHRKO mice (Figure 2F). Although exhibiting a similar trend, female FaGHRKO mice had a delayed increase in fat mass with a significant difference not seen until 4 months of age (Figure 2I). Lean mass for FaGHRKO males increased only at 10 mo of age (Figure 2G), whereas FaGHRKO females had a significant increase from 5–10 months of age (Figure 2J).
Adipose tissue depot mass and adipocyte size and number
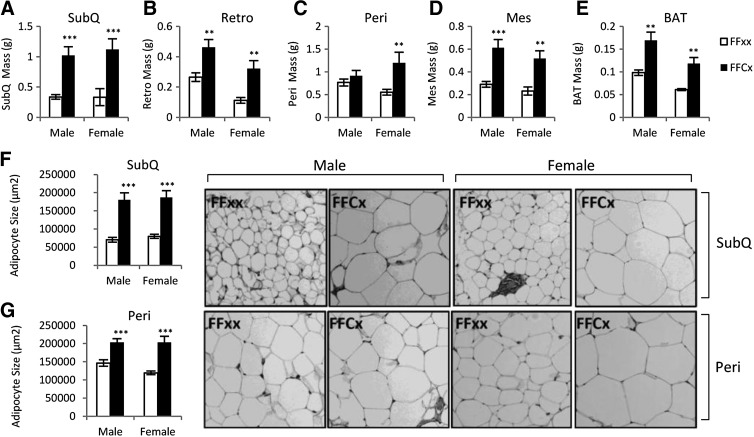
WAT mass was significantly increased in all four depots for female FaGHRKO mice compared with controls. In males, all depots but the perigonadal fat pad were increased (Figure 3). The sc depot in FaGHRKO mice was the most impacted by removal of the GHR because it showed the largest increase in males (204% increase) and females (237% increase). In male FaGHRKO mice, the retroperitoneal depot was increased by 74% and the mesenteric depot was increased by 111%, whereas the 20% increase in the perigonadal depot did not reach statistical significance. In female FaGHRKO mice, the retroperitoneal depot was increased by 188%, the mesenteric depot was increased by 124%, and the perigonadal depot was increased by 116%. The mass of BAT collected from FaGHRKO mice was significantly increased compared with littermate controls with 87% and 93% increases for male and female, respectively.
Figure 3.
FaGHRKO mice have increased adipose tissue depot weight and increased adipocyte cell size. Adipose tissue was collected at 6 mo of age. Subcutaneous (A), perigonadal (B), retroperitoneal (C), and mesenteric (D) WAT depots as well as BAT (E) are shown for male and female FaGHRKO (FFCx; black bars) and controls (FFxx; white bars). Mean adipocyte cell size is shown for sc (F) and perigonadal (G) depots. Hematoxylin and eosin (H&E) staining of sc (top four images) and perigonadal (bottom four images) adipose tissue depots are shown. Values in A–G are represented as mean ± SEM (n = 15–16 per group). *P < .05; **P < .01; ***P < 0.001, FaGHRKO (FFCx) vs control (FFxx). Mes, mesenteric; Peri, perigonadal; Retro, retroperitoneal; SubQ, subcutaneous.
Subcutaneous and perigonadal WAT depots were analyzed for cell size and number. Adipocyte cell size was significantly increased in the sc depot (Figure 3F) in both male FaGHRKO (157% compared with controls) and female FaGHRKO mice (135% compared with controls). Adipocytes from the perigonadal depot (Figure 3G) were modestly increased in FaGHRKO mice. Adipocyte cell numbers did not differ in sc or perigonadal adipose depots (data not shown).
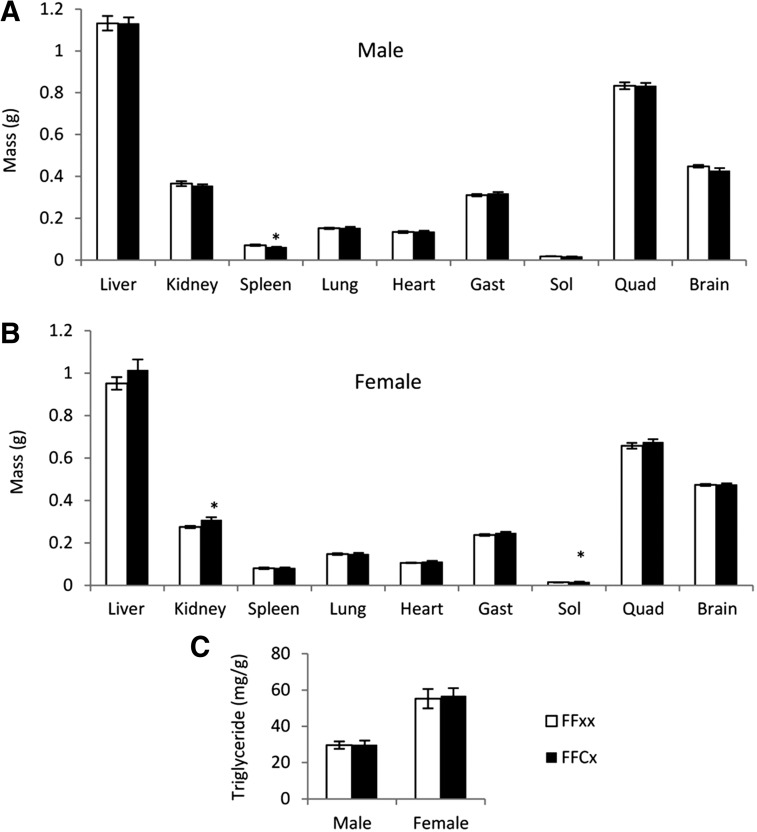
Organ weight and liver triglyceride content
No change was observed in the absolute weights of liver, heart, lung, brain, gastrocnemius muscle, and quadriceps between FaGHRKO vs control mice of males (Figure 4A) or females (Figure 4B). However, several sex-specific differences were observed in other organs. For example, kidneys and soleus muscle from FaGHRKO mice were significantly larger than controls in females but not in males, whereas the spleens from FaGHRKO mice were smaller than controls in males but normal in females. Liver triglyceride levels did not differ in FaGHRKO mice compared with controls (Figure 4C).
Figure 4.
Organ weights and liver tryglyceride content. A total of 63 mice were dissected at 6 mo of age. A and B, Liver, kidney, spleen, lung, heart, skeletal muscle (gastrocnemius, soleus, and quadriceps), and brain, are shown for FaGHRKO (FFCx; black bars) and controls (FFxx; white bars) for males (A) and females (B). C, Liver triglyceride is shown for FaGHRKO mice and controls for both sexes. Values in A–C are represented as mean ± SEM (n = 15–16 per group). *P < .05, FaGHRKO (FFCx) vs control (FFxx). Gast, gastrocnemius; Quad, quadriceps; Sol, soleus.
Adipokines
Mean values for circulating leptin were only significantly increased in female FaGHRKO mice (Table 1). Resistin levels were unchanged in FaGHRKO mice compared with controls. Mean values for total and HMW adiponectin were decreased slightly in both male and female FaGHRKO mice with only male total adiponectin values reaching statistical significance (P = .047). Circulating levels of adipsin were significantly decreased in both male and female FaGHRKO mice compared with controls.
Table 1.
Serum Adipokine Levels of FaGHRKO and Control Male and Female Mice
| Fat GHR−/− (Male) |
Fat GHR−/− (Female) |
|||||
|---|---|---|---|---|---|---|
| FFxx | FFCx | P Value | FFxx | FFCx | P Value | |
| Total adiponectin, n = 9–10 (pg/ml) | 22 302 ± 1227 | 18 782 ± 1106a | .047 | 43 736 ± 7135 | 39 642 ± 12 224 | .372 |
| HMW adiponectin, n = 9–10 (pg/ml) | 3923 ± 1412 | 3405 ± 864 | .335 | 11 214 ± 3429 | 10 922 ± 6816 | .905 |
| Leptin, n = 15–16 (pg/ml) | 3850 ± 736 | 5451 ± 835 | .160 | 3183 ± 739 | 6267 ± 1057a | .023 |
| Resistin, n = 15–16 (pg/ml) | 11 347 ± 857 | 13 318 ± 1575 | .272 | 11 427 ± 1216 | 11 779 ± 907 | .818 |
| Adipsin, n = 9–10 (pg/ml) | 1816 ± 88 | 1117 ± 100a | 8.E-05 | 2020 ± 187 | 1248 ± 153a | .005 |
Abbreviations: FFxx, controls; FFCx, FaGHRKO. Adipokine values of mice at 6 months of age. Values are represented as mean ± SEM (n = 9–16 per group).
Indicates significance with P values given to the right.
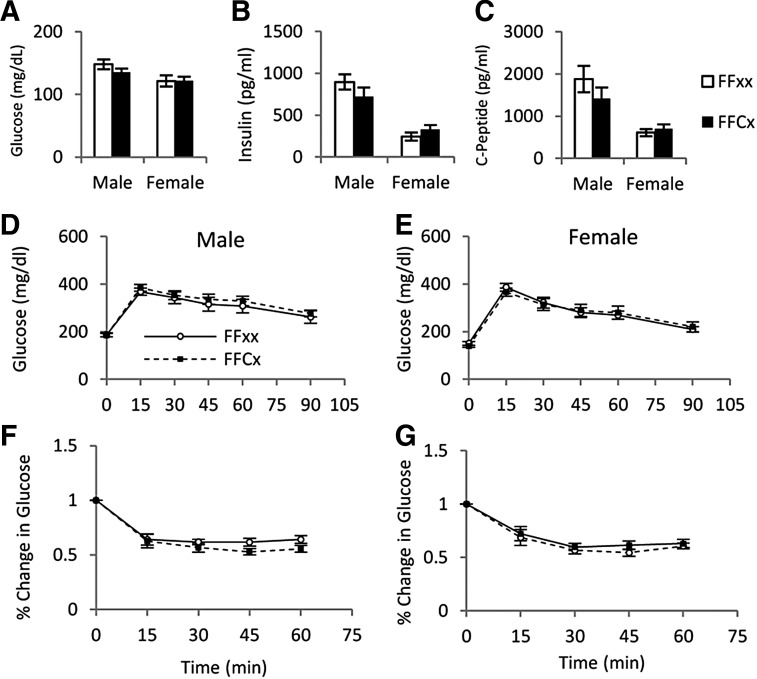
Glucose metabolism
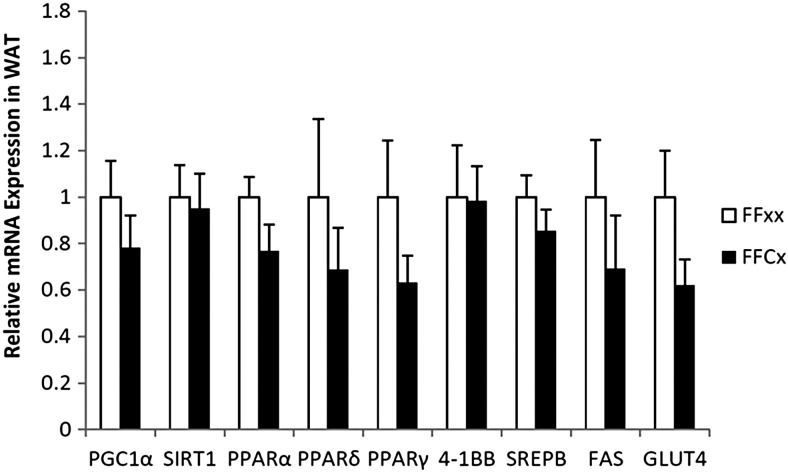
Fasting blood glucose, serum insulin, and C-peptide did not differ between FaGHRKO and controls (Figure 5). GTTs and ITTs also showed no significant differences between FaGHRKO mice and controls. No changes were seen in RNA levels encoding intracellular signaling molecules that are known to affect glucose metabolism and other processes in WAT from FaGHRKO mice including: peroxisome proliferator-activated receptor (PPAR)γ coactivator 1, sirtuin1 (SIRT1), PPARα, PPARδ, PPARγ, 4-1BB, sterol regulatory element binding protein (SREBP), fatty acid synthase (FAS), and glucose transporter (GLUT)4 (Figure 6).
Figure 5.
GHR deletion in adipose tissue does not alter glucose homeostasis. Fasting blood glucose (A), fasting serum insulin (B), and c-peptide (C) are shown for FaGHRKO (FFCx; black bars) and controls (FFxx; white bars). D and E, GTTs were performed at 5 months and 1 week of age after a 12-hour fast by ip injection of a 10% glucose solution at 0.01 ml/g body weight for FaGHRKO (FFCx; black boxes) and controls (FFxx; white circles) in males (D) and females (E). F and G, ITTs were performed in males (F) and females (G) at 5 months and 2 weeks of age in nonfasted mice via ip injection of 0.075 U/ml insulin solution at 0.01 ml/g body weight. Values in A–G are represented as mean ± SEM (n = 15–16 per group). *P < .05, FaGHRKO (FFCx) vs. control (FFxx).
Figure 6.
GHR gene disruption in adipose tissue does not alter RNA levels encoding intracellular signaling molecules that are known to affect glucose metabolism in WAT. Retroperitoneal WAT from female FaGHRKO mice was collected at 8 months of age. Relative mRNA levels of PGC1α, SIRT1, PPARα, PPARδ, PPARγ, 4-1BB, SREBP, FAS, and GLUT4, are shown for FaGHRKO (FFCx; black bars) and controls (FFxx; white bars). Values are presented relative to control values ± SEM (n = 6–7 per group). FaGHRKO (FFCx) vs control (FFxx).
GH, IGF-1, and IGF-binding proteins (IGFBPs)
GH levels were not significantly different in FaGHRKO mice relative to controls. Serum IGF-1 levels were elevated in FaGHRKO compared with controls for both males (22%) and females (8%); however, only in males did this reach statistical significance (Table 2). No significant changes were observed for any of the IGFBPs except for IGFBP-5, which was significantly increased (19%) in female FaGHRKO mice (P = .033) but did not quite reach statistical significance in males (P = .06).
Table 2.
IGF-1 and IGFBP Levels of FaGHRKO and Control Male and Female Mice
| Fat GHR−/− (Male) |
Fat GHR−/− (Female) |
|||||
|---|---|---|---|---|---|---|
| FFxx | FFCx | P Value | FFxx | FFCx | P Value | |
| GH and IGF-1 | ||||||
| GH, n = 9–10 (ng/ml) | 7.9 ± 3.2 | 12.1 ± 4.5 | .466 | 7.6 ± 1.6 | 7.9 ± 2.1 | .915 |
| IGF-1, n = 9–10 (ng/ml) | 503 ± 19 | 613 ± 30a | .006 | 655 ± 31 | 705 ± 32 | .275 |
| IGFBPs | ||||||
| IGFBP-1, n = 9–10 (pg/ml) | 7.4 ± 2.0 | 11 ± 1.7 | .147 | 22 ± 6.4 | 38 ± 13 | .285 |
| IGFBP-2, n = 9–10 (pg/ml) | 160 ± 8.5 | 172 ± 8.0 | .329 | 199 ± 10 | 204 ± 16 | .789 |
| IGFBP-3, n = 9–10 (pg/ml) | 189 ± 19 | 229 ± 14 | .109 | 248 ± 13 | 241 ± 11 | .711 |
| IGFBP-5, n = 9–10 (pg/ml) | 5.8 ± 0.3 | 7 ± 0.5 | .062 | 7 ± 0.4 | 9 ± 0.8a | .033 |
| IGFBP-6, n = 9–10 (pg/ml) | 133 ± 12 | 142 ± 11 | .586 | 155 ± 8.8 | 134 ± 14 | .228 |
| IGFBP-7, n = 9–10 (pg/ml) | 13 ± 1.0 | 13 ± 1.2 | .850 | 13 ± 1.4 | 15 ± 1.5 | .428 |
Abbreviations: FFxx, controls; FFCx, FaGHRKO. Circulating peptide values of male and female mice at 6 months of age. Values are represented as mean ± SEM (n = 9–10 per group).
Significance with P values given to the right.
Other blood parameters
Circulating levels of IL-6 were significantly elevated in female FaGHRKO compared with controls (P = .005), whereas no change was seen in males (Table 3). MCP-1 did not differ between FaGHRKO and controls for either sex. Circulating levels of acute phase peptides including lipocalin-2, pentraxin-3, AGP, CRP, α-2-macroglobulin, and haptoglobin did not differ between FaGHRKO and controls regardless of sex. A large number of soluble serum receptors including sgp130, sIL-1RI, sIL-1RII, sIL-2Ra, sIL6R, sTNFRI, sTNFRII, sVEGFR1, sVEGFR2, and sVEGFR3 were similar between FaGHRKO and controls regardless of sex. However, sCD30 (decreased in female FaGHRKO) and sIL-4R (increased in male FaGHRKO) differed between FaGHRKO and controls.
Table 3.
Circulating Cytokines, Acute Phase Proteins, and Soluble Receptors of FaGHRKO and Control Male and Female Mice
| Fat GHR−/− (Male) |
Fat GHR−/− (Female) |
|||||
|---|---|---|---|---|---|---|
| FFxx | FFCx | P Value | FFxx | FFCx | P Value | |
| Cytokines | ||||||
| IL-6, n = 15–16 (pg/ml) | 51 ± 13 | 58 ± 13 | .675 | 30 ± 4 | 56 ± 7a | .005 |
| MCP-1, n = 15–16 (pg/ml) | 112 ± 27 | 121 ± 27 | .819 | 402 ± 221 | 135 ± 21 | .247 |
| Acute phase 1 | ||||||
| Lipocalin-2, n = 9–10 (pg/ml) | 59 ± 4 | 106 ± 22 | .061 | 37 ± 2 | 49 ± 9 | .218 |
| Pentraxin-3, n = 9–10 (pg/ml) | 15 ± 1 | 21 ± 2a | .008 | 18 ± 2 | 18 ± 1 | .726 |
| Acute phase 2 | ||||||
| AGP, n = 9–10 (pg/ml) | 176 ± 14 | 223 ± 21 | .083 | 160 ± 9 | 214 ± 57 | .382 |
| α-2-Macroglobulin, n = 9–10 (ng/ml) | 2490 ± 117 | 2381 ± 105 | .499 | 2242 ± 98 | 2139 ± 201 | .662 |
| CRP, n = 9–10 (ng/ml) | 14 ± 1 | 17 ± 1 | .058 | 15 ± 1 | 16 ± 2 | .956 |
| Haptoglobin, n = 9–10 (ng/ml) | 32 ± 11 | 69 ± 27 | .232 | 12 ± 2 | 47 ± 37 | .383 |
| Soluble receptor | ||||||
| sCD30, n = 9–10 (pg/ml) | 65 ± 19 | 129 ± 69 | .383 | 145 ± 36 | 47 ± 17a | .029 |
| sgp130, n = 9–10 (pg/ml) | 653 ± 54 | 973 ± 295 | .326 | 944 ± 362 | 3142 ± 1777 | .266 |
| sIL-1RI, n = 9–10 (pg/ml) | 810 ± 94 | 1084 ± 205 | .248 | 415 ± 67 | 549 ± 78 | .214 |
| sIL-1RII, n = 9–10 (pg/ml) | 4601 ± 114 | 4445 ± 87 | .287 | 3581 ± 370 | 3193 ± 116 | .310 |
| sIL-2Ra, n = 9–10 (pg/ml) | 414 ± 34 | 368 ± 25 | .289 | 394 ± 59 | 345 ± 22 | .430 |
| sIL-4R, n = 9–10 (pg/ml) | 1385 ± 105 | 2305 ± 287a | .010 | 1734 ± 138 | 1872 ± 198 | .585 |
| sIL-6R, n = 9–10 (pg/ml) | 9010 ± 301 | 8628 ± 362 | .434 | 10 430 ± 956 | 9888 ± 367 | .608 |
| sTNFRI, n = 9–10 (pg/ml) | 1457 ± 85 | 1663 ± 114 | .174 | 1525 ± 146 | 1515 ± 87 | .954 |
| sTNFRII, n = 9–10 (pg/ml) | 3398 ± 382 | 4478 ± 533 | .125 | 2838 ± 396 | 3235 ± 395 | .489 |
| sVEGFR1, n = 9–10 (pg/ml) | 2739 ± 115 | 2826 ± 202 | .724 | 2187 ± 438 | 1940 ± 110 | .574 |
| sVEGFR2, n = 9–10 (ng/ml) | 29 ± 1 | 28 ± 1 | .635 | 30 ± 1 | 29 ± 2 | .768 |
| sVEGFR3, n = 9–10 (ng/ml) | 32 ± 1 | 31 ± 1 | .542 | 32 ± 1 | 32 ± 1 | .566 |
Abbreviations: FFxx, controls; FFCx, FaGHRKO. Circulating peptide values of male and female mice at 6 mo of age. Values are represented as mean ± SEM (n = 9–16 per group).
Significance with P values given to the right.
Discussion
The GHR−/− mouse was generated in our laboratory nearly 15 yr ago (2). These mice are dwarf with low IGF-1 and high GH levels. GHR−/− mice are obese with the sc WAT depot being preferentially increased (8). Despite the obese phenotype, the mice are insulin sensitive with very low levels of serum insulin (4). Interestingly, these mice are long-lived (4) with decreased rates of cancer (6). Another interesting finding is that GHR−/− mice have elevated levels of adiponectin despite being obese (10). In an effort to determine the tissues responsible for the above mentioned phenotypes, attempts are being made to disrupt the GHR gene in a tissue-specific manner. Others have already reported on muscle-, liver-, and pancreas-specific GHRKO mice (17–19). Here, we describe the fat GHR knockout (FaGHRKO) mouse.
To delete the GHR in WAT, GHRflox/flox mice were crossed with transgenic aP2-cre mice. This cre promoter/enhancer has been used by many for disruption of a variety of genes in adipose tissue (27–30). Whereas aP2 expression is induced in nonadipogenic tissues during early development, albeit in cells that are of an analogous cell lineage (31), studies that have analyzed adult tissues show a specific localization to WAT and BAT (29, 30). Our results support the expression of aP2 specifically in adult adipose tissue because the levels of GHR mRNA were reduced in four WAT depots and interscapular BAT but not significantly altered in any other tissue tested. It has been previously claimed that aP2 expression is induced in activated macrophages (32). However, more recent reports show that the efficiency of Cre recombination in macrophages is much less than that in adipocytes (27, 28). Because we cannot rule out macrophage expression or embryonic expression, these possibilities need to be taken into consideration when interpreting our results.
The body weights of male and female FaGHRKO mice were significantly increased. This weight gain was mainly attributed to a near doubling of fat mass. The increase in fat mass was expected because GH possesses lipolytic and antilipogenic effects on adipose tissue, and removal of this action should result in increased fat mass (33, 34). Moreover, GHR−/− mice have repeatedly been shown to be obese relative to littermate controls throughout life (8). This is also true for FaGHRKO mice up to 1 year of age; thus, our first hypothesis that FaGHRKO mice would have increased adiposity is supported. However, there are important differences in the adiposity of FaGHRKO mice relative to GHR−/− mice. In this study, all adipose depots (four WAT depots and interscapular BAT) analyzed with the exception of the perigonadal (epididymal) fat pad in males were significantly enlarged in the FaGHRKO mice compared with controls. The fact that perigonadal was the lone exception in males is not surprising because previous studies have shown this particular depot to be the least responsive to GH treatment (24). In contrast to FaGHRKO mice, our laboratory and others have shown that GHR−/− mice in a similar C57BL/6 genetic background have a preferential enlargement of the sc depot and occasionally the retroperitoneal depot with other fat pads being proportional to their dwarf size (8, 35). It has been proposed that the obesity in GHR−/− mice may represent a form of “healthy” obesity because of the preferential accumulation of excess of sc adipose tissue (10). Although the sc WAT mass did show the largest increase in mass compared with other depots, all fat pads (except for perigonadal in males) in the FaGHRKO mice were enlarged. The enlargement of most depots, as seen in FaGHRKO mice, may not provide the same benefit to lifespan and health as seen in the GHR−/− mice. Ongoing longevity studies will provide important information about the long-term outcome of this alternative fat deposition.
Other than leptin, circulating adipokine levels in the FaGHRKO mice are distinct from what has previously been reported for GHR−/− mice. Leptin levels in male GHR−/− mice are consistently elevated although female mice are less thoroughly studied (12). Likewise, leptin levels are increased in female FaGHRKO mice, whereas the increase in males did not reach statistical significance. The elevation of leptin in both GHR−/− and female FaGHRKO mice is not surprising considering that these mice are obese, and this hormone has been shown to be consistently and positively correlated with an increase in fat mass. However, the physiological consequences of elevated leptin in these mice have not been thoroughly studied. Possible connections between elevated leptin and alterations in adaptive and innate immunity (36) as well as organ function and disease states (37) are worthy of further exploration. However, it should also be noted that obese states are typically associated with leptin resistance (38); thus, the increased leptin in these mice may not be accompanied by significant changes in leptin action at the cellular level.
Unlike leptin, adiponectin levels have been shown to decrease as fat mass increases (39). In contrast, GHR−/− mice are obese yet have elevated levels of adiponectin. This observation in GHR−/− mice suggests that GH may negatively regulate adiponectin. Cell culture studies have also shown this to be the case because GH treatment of differentiated 3T3-L1 adipocytes results in a decrease in adiponectin levels (40). Furthermore, studies on GH-transgenic and GH-deficient rodents suggest that GH suppresses adiponectin secretion (12). Based on the above observations, our second hypothesis was that disruption of the GHR in adipose tissue will increase adiponectin levels similar to what is seen in GHR−/− mice. Surprisingly, we found that FaGHRKO mice did not have elevated levels of adiponectin, but rather had no change in females or decreased levels in males. Thus, our second hypothesis did not hold true. This suggests that any negative regulatory activity of GH on adipose tissue, as observed in global GHR−/− mice, is dependent on the consequences of disrupting GHR in other tissues or that GHR is not important for regulating adiponectin secretion.
We also measured resistin and adipsin, two additional adipokines, in FaGHRKO mice. Resistin levels remained unchanged and adipsin levels were significantly decreased in both sexes. Recently, it has been shown that resistin is increased in the GHR−/− mice (41). Thus, it appears that, like adiponectin, circulating resistin also is differentially regulated when GHR is selectively disrupted from adipose tissue as opposed to global disruption. Adipsin levels have not been assessed in GHR−/− mice. Because adipsin is thought to function primarily in the alternative pathway of the complement system, it is possible that the FaGHRKO mice have alterations to immune function, which was not assessed in this study. However, recent evidence suggests that the complement system in adipose tissue may play an important role in fat storage and insulin sensitivity (42). Thus, further investigation into the effects of adipsin reduction in FaGHRKO and GHR−/− mice would also be of interest.
Various measures of glucose homeostasis did not differ between FaGHRKO mice and controls including fasting glucose, fasting insulin, glucose tolerance, and insulin tolerance. We expected adipose tissue to dispose of glucose more efficiently than control mice because the diabetogenic action of GH action was lacking in this tissue. However, it appears that removal of GHR in adipose tissue does not produce a measurable effect on whole-body readouts of glucose metabolism. Because the largest proportion of glucose disposal occurs in skeletal muscle (43), it is likely that adipose tissue's contribution to whole-body glucose disposal is negligible. Moreover, most other adipose tissue-specific mouse models show similar lack of effect on whole-body glucose metabolism. A partial exception can be seen with adipose-specific overexpression of GLUT4 in isolated adipocytes ex vivo, where a 2- to 3-fold increase in glucose disposal is reported; however, no difference in insulin-stimulated glucose disposal can be detected in vivo (44). When adipose tissue is selectively made insulin resistant by fat-specific removal of insulin receptor (seen in the FIRKO mouse), no changes in glucose or insulin tolerance occur at a young age (2 mo), but these parameters do change in older mice (10 mo); thus, it is also possible that we may see changes at more advanced ages (45). Alternatively, adiponectin is considered a potent insulin sensitizer, and high levels of this adipokine could be important for the increased insulin sensitivity in GHR−/− mice (12). However, adiponectin levels are not increased in FaGHRKO mice, which may partially explain why no improvements were seen in glucose homeostasis. Furthermore, the difference in glucose metabolism may also be partially affected by location of fat storage. As discussed earlier, GHR−/− mice have increased adiposity primarily due to increased sc adipose tissue (8). FaGHRKO mice have increases in all WAT depots including mesenteric, which is thought to have a negative effect on glucose homeostasis (10). Analysis of genes involved in glucose metabolism by quantitative real time RT-PCR in adipose tissue of FaGHRKO mice revealed similar results to that of whole-body analysis of glucose metabolism as no changes were seen. We hypothesized that the FaGHRKO mice would have improved glucose homeostasis; however, this was not the case.
Interestingly, FaGHRKO mice are quite different from global GHR−/− mice. We expect that the differences between the global GHR−/− and FaGHRKO mice with regard to nonadipose parameters (such as body size) are due to the fact that GHR is disrupted in all tissues in GHR−/− mice whereas FaGHRKO mice have normal levels of GHR in nonadipose tissues. In contrast, differences in adipose tissue parameters (such as adiponectin production and depot differences) are difficult to explain because the GHR is disrupted in adipose tissue in both mouse lines; however, we speculate that these differences are due to the action of GH in tissues other than adipose, and these other tissues, in turn, are able to influence adipose tissue physiology via endocrine/paracrine mechanisms.
Because this is the first description of the FaGHRKO mice, many additional studies will be performed. For example, analyzing gene expression and/or protein production in tissues such as adipose, liver, and muscle will be needed to investigate the potential of tissue cross talk in FaGHRKO vs GHR−/− mice. It also would be of interest to determine whether local IGF-1 expression is altered in various tissues of these mice because local IGF-1 may be decreased in tissues where GHR is absent. Additionally, we would like to quantify expression of various lipogenic/lypolytic enzymes (such as lipoprotein lipase, adrenergic receptors, lipid droplet proteins, and intracellular lipases) in adipose tissue depots of these mice. Because lipoprotein lipase has been shown previously to respond to GH differently in different adipose tissue depots (33), such studies may help explain the differences observed in the current study. Finally, we now have the capability to cross various tissue-specific GHRKO lines (such as muscle- and liver-specific GHRKO mice) with the FaGHRKO line in order to determine which tissue(s) require GHR disruption in concert with adipose to achieve a similar phenotype as global GHR−/− mice.
In conclusion, FaGHRKO mice share few characteristics with global GHR−/− mice. FaGHRKO mice are obese with increased total body fat, increased adipocyte cell size, and increased circulating leptin. However, unlike global GHR−/− mice, these mice show no improvements in measures of glucose homeostasis, have normal levels of resistin, and normal/decreased levels of adiponectin. Thus, it appears that the increases in adipokines seen in global GHR−/− mice are probably due to the removal of GH's action in all tissues and not a result of deletion of the GHR in adipose tissue alone.
Acknowledgments
This work was supported by the State of Ohio's Eminent Scholar Program that includes a gift from Milton and Lawrence Goll; by National Institutes of Health (NIH) Grants P01AG031736, AG032290, DK58259, and DK083729; by the AMVETS; by the Diabetes Institute at Ohio University; and by Polish Ministry of Science and Higher Education Grant NN401042638. The floxed GHR mouse strain used for this research project was generated by the trans-NIH KOMP and obtained from the KOMP Repository (www.komp.org). NIH grants to Velocigene at Regeneron, Inc (U01HG004085) and the CHORI-Sanger-UCDavis Consortium (U01HG004080) funded the generation of gene-targeted embryonic stem cells for 8500 genes in the KOMP Program and archived and distributed by the KOMP Repository at University of California Davis and Children's Hospital Oakland Research Institute (CHORI) (U42RR024244).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- CRP
- C-reactive protein
- ECL
- enhanced chemiluminescence
- FAS
- fatty acid synthase
- GHR
- GH receptor
- GHRKO
- GHR knockout
- GTT
- glucose tolerance test
- HMW
- high molecular weight
- IGFBP
- IGF-binding protein
- ITT
- insulin tolerance test
- KOMP
- Knockout Mouse Project
- LS
- Laron syndrome
- PPAR
- peroxisome proliferator-activated receptor
- sTNFR
- soluble TNF receptor
- sVEGFR
- soluble vascular endothelial growth factor receptor.
References
- 1. List EO, Sackmann-Sala L, Berryman DE, et al. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR−/−) mouse. Endocr Rev. 2011;32:356–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou Y, Xu BC, Maheshwari HG, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94:13215–13220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590 [DOI] [PubMed] [Google Scholar]
- 4. Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin and IGF-1 levels and increased lifespan. Endocrinology. 2003;144:3799–3810 [DOI] [PubMed] [Google Scholar]
- 5. Bonkowski MS, Dominici FP, Arum O, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS ONE. 2009;4:e4567. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6. Ikeno Y, Hubbard GB, Lee S, et al. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berryman DE, List EO, Palmer AJ, et al. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Egecioglu E, Bjursell M, Ljungberg A, et al. Growth hormone receptor deficiency results in blunted ghrelin feeding response, obesity, and hypolipidemia in mice. Am J Physiol Endocrinol Metab. 2006;290:E317–E325 [DOI] [PubMed] [Google Scholar]
- 10. Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kloting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 299:E506–E515 [DOI] [PubMed] [Google Scholar]
- 12. Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14:309–318 [DOI] [PubMed] [Google Scholar]
- 13. Bik W, Baranowska B. Adiponectin—a predictor of higher mortality in cardiovascular disease or a factor contributing to longer life? Neuro Endocrinol Lett. 2009;30:180–184 [PubMed] [Google Scholar]
- 14. Ibanez L, Lopez-Bermejo A, Diaz M, Jaramillo A, Marin S, de Zegher F. Growth hormone therapy in short children born small for gestational age: effects on abdominal fat partitioning and circulating follistatin and high-molecular-weight adiponectin. J Clin Endocrinol Metab. 2010;95:2234–2239 [DOI] [PubMed] [Google Scholar]
- 15. Nilsson L, Binart N, Bohlooly YM, et al. Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochem Biophys Res Commun. 2005;331:1120–1126 [DOI] [PubMed] [Google Scholar]
- 16. Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf). 2006;65:114–117 [DOI] [PubMed] [Google Scholar]
- 17. Fan Y, Menon RK, Cohen P, et al. Liver-specific deletion of the growth hormone receptor reveals essential role of GH signaling in hepatic lipid metabolism. J Biol Chem. 2009;284(30):19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mavalli MD, DiGirolamo DJ, Fan Y, et al. Distinct growth hormone receptor signaling modes regulate skeletal muscle development and insulin sensitivity in mice. J Clin Invest. 2010;120:4007–4020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vijayakumar A, Wu Y, Sun H, et al. Targeted loss of GHR signaling in mouse skeletal muscle protects against high-fat diet-induced metabolic deterioration. Diabetes. 2012;61:94–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu Y, Liu C, Sun H, et al. Growth hormone receptor regulates β cell hyperplasia and glucose-stimulated insulin secretion in obese mice. J Clin Invest. 2011;121:2422–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature. 2011;474:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, et al. Caloric restriction results in decreased expression of peroxisome proliferator-activated receptor superfamily in muscle of normal and long-lived growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2005;60:1238–1245 [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Guan R, Jiang J, et al. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem. 2001;276:24565–24573 [DOI] [PubMed] [Google Scholar]
- 24. List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52:1647–1655 [DOI] [PubMed] [Google Scholar]
- 25. Salmon DM, Flatt JP. Effect of dietary fat content on the incidence of obesity among ad libitum fed mice. Int J Obes. 1985;9:443–449 [PubMed] [Google Scholar]
- 26. Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring). 2010;18:1875–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wueest S, Rapold RA, Schumann DM, et al. Deletion of Fas in adipocytes relieves adipose tissue inflammation and hepatic manifestations of obesity in mice. J Clin Invest. 2010;120:191–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kumar A, Lawrence JC, Jr, Jung DY, et al. Fat cell-specific ablation of rictor in mice impairs insulin-regulated fat cell and whole-body glucose and lipid metabolism. Diabetes. 2010;59:1397–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He W, Barak Y, Hevener A, et al. Adipose-specific peroxisome proliferator-activated receptor γ knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA. 2003;100:15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abel ED, Peroni O, Kim JK, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733 [DOI] [PubMed] [Google Scholar]
- 31. Urs S, Harrington A, Liaw L, Small D. Selective expression of an aP2/fatty acid binding protein 4-Cre transgene in non-adipogenic tissues during embryonic development. Transgenic Res. 2006;15:647–653 [DOI] [PubMed] [Google Scholar]
- 32. Makowski L, Boord JB, Maeda K, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richelsen B, Pedersen SB, Borglum JD, Moller-Pedersen T, Jorgensen J, Jorgensen JO. Growth hormone treatment of obese women for 5 wk: effect on body composition and adipose tissue LPL activity. Am J Physiol. 1994;266:E211–E216 [DOI] [PubMed] [Google Scholar]
- 34. Etherton TD. Porcine growth hormone: a central metabolic hormone involved in the regulation of adipose tissue growth. Nutrition. 2001;17:789–792 [DOI] [PubMed] [Google Scholar]
- 35. Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–E413 [DOI] [PubMed] [Google Scholar]
- 36. Moraes-Vieira PM, Bassi EJ, Araujo RC, Camara NO. Leptin as a link between the immune system and kidney-related diseases: leading actor or just a coadjuvant? Obes Rev. 2012;13:733–743 [DOI] [PubMed] [Google Scholar]
- 37. Mantzoros CS, Magkos F, Brinkoetter M, et al. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567–E584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Moon HS, Matarese G, Brennan AM, et al. Efficacy of metreleptin in obese patients with type 2 diabetes: cellular and molecular pathways underlying leptin tolerance. Diabetes. 2011;60:1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arita Y, Kihara S, Ouchi N, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83 [DOI] [PubMed] [Google Scholar]
- 40. Wolfing B, Neumeier M, Buechler C, Aslanidis C, Scholmerich J, Schaffler A. Interfering effects of insulin, growth hormone and glucose on adipokine secretion. Exp Clin Endocrinol Diabetes. 2008;116:47–52 [DOI] [PubMed] [Google Scholar]
- 41. Vijeyta F. Effects of Growth Hormone on Circulating Resistin Levels in Mice. [masters' thesis] Ohio University, Athens, Ohio: 2012;1–141 [Google Scholar]
- 42. Schaffler A, Scholmerich J. Innate immunity and adipose tissue biology. Trends Immunol. 2010;31:228–235 [DOI] [PubMed] [Google Scholar]
- 43. DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5:177–269 [Google Scholar]
- 44. Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–E561 [DOI] [PubMed] [Google Scholar]
- 45. Bluher M, Michael MD, Peroni OD, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38 [DOI] [PubMed] [Google Scholar]