Abstract
Although countless genomes have now been sequenced, the glycomes of the vast majority of eukaryotes still present a series of unmapped frontiers. However, strides are being made in a few groups of invertebrate and unicellular organisms as regards their N-glycans and N-glycosylation pathways. Thereby, the traditional classification of glycan structures inevitably approaches its boundaries. Indeed, the glycomes of these organisms are rich in surprises including a multitude of modifications of the core regions of N-glycans and unusual antennae. From the actually rather limited glycomic information we have, it is nevertheless obvious that the biotechnological, developmental and immunological relevance of these modifications, especially in insect cell lines, model organisms and parasites means that deciphering unusual glycomes is of more than just academic interest.
Keywords: N-linked oligosaccharides, protozoa, nematodes, trematodes, insects, molluscs
The traditional classification of N-linked oligosaccharides into oligomannosidic, complex and hybrid as, for instance, summarised by Kornfeld and Kornfeld (Kornfeld and Kornfeld 1985) in their classic review is based on the glycan structures found in mammals and other vertebrates. The extensively-studied complex N-glycans of vertebrates are exemplified by structures with N-acetylglucosamine (GlcNAc) residues on both the α1,3- and α1,6-linked mannose (Man) residues of the common trimannosylchitobiosyl core region; there is a large range of subsequent antennal modifications, particularly with galactose (Gal) and sialic acid residues, on such oligosaccharides. The term ‘hybrid’ was defined for those N-glycans displaying features of both the complex and oligomannosidic types; in this case, there are only GlcNAc residues linked to the α1,3-linked mannose (sometimes also, if bisected, to the β1,4-linked mannose), but not to the α1,6-linked mannose. The most basic hybrid glycan, with the composition Man5GlcNAc3 (Man5Gn), is also a key intermediate on the route to complex N-glycans. It is clear, from studies on mutant mice with defects in N-acetylglucosaminyltransferase I (GlcNAc-TI or GnTI), that the ability to produce hybrid and complex glycans is essential for mammalian development (Metzler, et al. 1994, Ioffe and Stanley 1994).
During the initial discovery process of what we now call ‘glycobiology’ there was an awareness that, in non-vertebrates, there exist glycans which cannot be assigned to the aforementioned classification. For instance, yeast (or perhaps more exactly Saccharomyces cerevisiae) produce extended structures consisting only of two core N-acetylglucosamine (GlcNAc) and polymannosidic extensions with, not just nine, but perhaps one hundred mannose residues (Herscovics and Orlean 1993); in addition, plants were known, as exemplified by the glycoprotein phytohaemagglutinin, to synthesise not just the ‘usual’ oligomannosidic oligosaccharides, but also ‘short’ structures containing β1,2-xylose (Xyl) and α1,3-fucose (Fuc) associated with the mannosylchitobiosyl core region (Sturm, et al. 1992). Around 1990, there was the first indication that insects also produced not only oligomannosidic N-glycans, but also ‘paucimannosidic’ structures with up to two core fucose residues on the reducing-terminal (innermost) GlcNAc - something which was initially greeted with scepticism (Staudacher, et al. 1992). The term ‘paucimannosidic glycan’, perhaps not yet familiar to the widest glycobiological audience, was introduced to describe those glycans, particularly found in plants and invertebrates with or without core modifications, but lacking antennal GlcNAc and possessing less than four mannose residues. However, in recent years, it has become apparent that the N-glycans of lower organisms (especially invertebrates and protists) cannot be easily classified. The designations ‘complex’, ‘hybrid’, ‘oligomannosidic’ and even ‘paucimannosidic’ are no longer adequate to summarise these structures and so terminologies such as ‘complex core modifications’, ‘truncated complex’ or ‘pseudohybrid’ have been coined to supplement the traditional terms. Indeed, rather complicated glycans are found in nematode species, unusual glycans are present in amoebae and partial mimics of mammalian glycans are expressed by parasites such as Schistosoma mansoni, Trichomonas vaginalis and Trypanosoma brucei. Here, therefore, we discuss neither the N-glycans of plants, yeasts and fungi nor the O-glycans and glycolipids of ‘lower’ animals, but summarise the knowledge about N-linked oligosaccharides of a range of protozoal and invertebrate species (see Figures 1 and 2) with a particular focus on parasitic and model organisms.
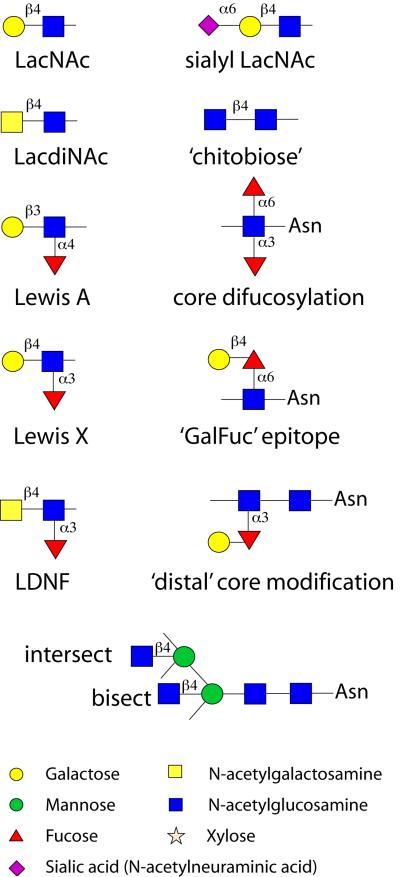
Figure 1. Structural elements in some N-glycans.
A selection of some epitopes of N-glycans are shown: LacNAc, LacdiNAc, sialyl LacNAc, chitobiose (strictly N,N’-diacetylchitobiose, as in the core region of N-glycans), Lewis A (present in plants and humans), Lewis X (Lex; the fucosylated form of ‘LacNAc’ present in, e.g., schistosomes and vertebrates), LDNF (fucosylated LacdiNAc; i.e., fucosylated GalNAcβ1,4GlcNAc), difucosylation of the reducing-terminal (i.e., proximal or innermost) GlcNAc of N-glycans in many invertebrates, the ‘GalFuc’ modification of the reducing-terminal GlcNAc, the modification of the distal (or second) core GlcNAc as found in some nematodes and the positions of the ‘intersecting’ and ‘bisecting’ GlcNAc residues of slime mould N-glycans. The depictions of monosaccharides are according to the nomenclature of the Consortium for Functional Glycomics: circles being hexoses, diamonds sialic acids, squares N-acetylhexosamines, stars pentoses and triangles deoxyhexoses; undefined monosaccharide isomers are uncoloured.
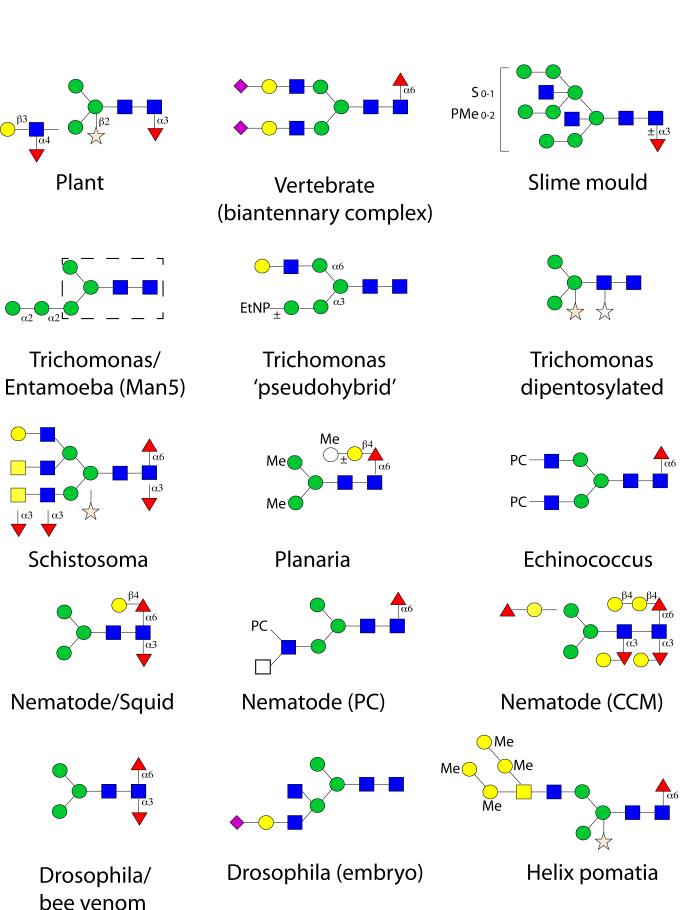
Figure 2. Examples of N-glycan structures from a selection of non-vertebrate eukaryotes.
In comparison to plants and vertebrates, examples of N-glycans from Dictyostelium discoideum (slime mould), Trichomonas vaginalis (protozoal parasite; the ‘biosynthetic’ Man5 structure being found also in Entamoeba histolytica, with the trimannosylchitobiosyl region being boxed with a dashed line), Schistosoma mansoni (trematode parasite), Dugesia japonica (planaria), Echinococcus granulosus (cestode parasite), Caenorhabditis elegans (nematode; the ‘GalFuc’ epitope being also found in some molluscs), Drosophila melanogaster (fruitfly; difucosylation also being found on bee venom glycoproteins) and Helix pomatia (mollusc) are shown. Incomplete lines indicate further structural possibilities. CCM core chitobiose modification, EtNP indicates ethanolamine phosphate, Me methyl, PC phosphorylcholine, PMe methylphosphate, S sulphate. Monosaccharides are depicted according to the nomenclature of the Consortium for Functional Glycomics (see Figure 1).
N-glycans of non-parasitic unicellular organisms
Other than yeasts, probably not so many species in this category have been glycomically examined, but some data on algae and one amoeba are reported in the literature. Many years ago the N-glycans of an algal pheromone, the sexuality-inducing glycoprotein of Volvox carteri, which actually forms multicellular colonies, were released using PNGase F from Flavobacterium and found to contain core β1,2-xylose on paucimannosidic glycans as in plants (Balshüsemann and Jaenicke, 1990); perhaps in retrospect, as many lower organisms and plants synthesise core α1,3-fucosylated glycans resistant to PNGase F and as Volvox possesses a potential α1,3-fucosyltransferase homologue, PNGase A from almonds, which can release such glycans, should have been used instead. Xylose is also present on the N-glycans of the microalga Porpyridium, but in this case is, e.g., present on the distal (second) core GlcNAc rather than on the core mannose (Levy-Ontman, et al. 2011).
Perhaps the most studies on N-glycans of a non-parasitic, non-yeast unicellular organism have been performed on Dictyostelium discoideum - which is indeed a part-time multicellular organism (also known as either a cellular slime mould or social amoeba) due to its ability to form aggregates upon starvation and produce fruiting bodies. Although the overall carbohydrate composition in D. discoideum is similar to that of animals except for the absence of sialic acid (West, et al. 2005), the N-glycans of this species are a good example of ‘complicated’ and unusual elaborations of typical oligomannosidic structures. The major neutral N-glycan in the amoebae has both ‘intersecting’ and ‘bisecting’ N-acetylglucosamine residues (see Figure 1) and core α1,3-fucose (Schiller, et al. 2009); furthermore, charged glycans carrying sulphate and methylphosphate residues were reported first in the early eighties (Freeze, et al. 1980, Freeze, et al. 1983a) and their presence has been verified by mass spectrometry (Gabel, et al. 1984, Feasley, et al. 2010). While the presence of core xylose on slime mould N-glycans is not substantiated by the latest data, core α1,6-fucosylation has been recently detected by mass spectrometry on a single glycoprotein (Nakagawa, et al. 2011).
It has become clear that the genetic basis for the glycosylation pathways of Dictyostelium shows many parallels to animal and plant pathways; indeed, Dictyostelium N-glycans are assembled via the common eukaryotic pathway using the standard eukaryotic precursor molecule Glc3Man9GlcNAc2 whose biosynthesis is catalysed by the action of the fourteen various alg gene products (Ivatt, et al. 1984, Samuelson, et al. 2005). However, the processing of the N-glycans is not dependent on GlcNAc-TI which in multicellular organisms is prerequisite for modifications such as addition of core fucose or bisecting GlcNAc. In the genome of D. discoideum putative glycosyltransferase and glycohydrolase genes could be identified and their number compared with homologous genes in its relative D. purpureum (West, et al. 2005, Sucgang, et al. 2010). The prediction includes α1,3/4-fucosyltransferases from the CAZy family GT10 (not less than 10 homologues), β-GlcNAc transferases and one gene encoding a putative GlcNAc-P-transferase. No glycosyltransferase involved in N-glycan biosynthesis has been characterized to date in recombinant form; however, a number of relevant transferase activities in crude extracts has been detected, e.g., intersecting and bisecting GlcNAc-transferases (Sharkey and Kornfeld 1991a), core α1,3-fucosyltransferase (Schiller, et al. 2009), the GlcNAc-phosphotransferase (Couso, et al. 1986) and the S-adenosylmethionine-dependent methyltransferase which modifies the Man-6-phosphate residues (Freeze and Wolgast 1986, Freeze, et al. 1992). Mutants defective in putative GlcNAc transferases and in phosphorylation of the N-glycans (specifically in the GlcNAc-P transferase) were recently identified (Pang, et al. 2007, Qian, et al. 2010), whereas defects in two enzymes of early N-glycan processing (a mannosyltransferase and a glucosidase) have been defined in earlier work (Freeze, et al. 1983b, Freeze, et al. 1989).
A fascinating feature of D. discoideum is the shift in the N-glycome observed during development (Ivatt, et al. 1981, Ivatt, et al. 1984, Sharkey and Kornfeld 1991b): whereas N-glycans released from vegetative cells were partly resistant to the endoglycosidase Endo H, during aggregation and culmination they were sensitive to this treatment and smaller in size. Furthermore, the degree of modification with sulphate and/or phosphate decreased dramatically during late tip formation. Recent mass spectrometric studies confirm these trends and show a shift from Man8GlcNAc4Fuc1 as the major neutral N-glycan to Man5GlcNAc2Fuc1 (Schiller, et al. 2009). Glycomic differences between the pre-spore and pre-stalk cells (i.e., those cells destined to later form the spore and stalk of the fruiting bodies) have also been observed (Riley, et al. 1993).
N-glycans of parasitic unicellular organisms
A primary finding regarding N-glycosylation in obligate protist parasites is the loss of genes involved in the formation of the N-glycan precursor. The dolichol-linked oligosaccharides of these organisms range in size from Man9GlcNAc2 in Trypanosoma cruzi to Man5GlcNAc2 in Entamoeba and Trichomonas. Most extreme are the examples of Plasmodium and Giardia with just GlcNAc1-2 as precursor or Theileria which apparently, even though eukaryotic, lacks an N-glycosylation capacity entirely. The ‘defects’ in precursor formation are due to a lack of a variable number of alg genes (Samuelson, et al. 2005). Those parasites synthesising at least Man5GlcNAc2 tend to also possess an ER glycan-dependent quality control mechanism involving glucosylation of nascent glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase (Banerjee, et al. 2007); thereby, the presence of glucose on the final N-glycan structures is due to post-transfer glucosylation and not to Dol-P-Glc-dependent modifications of the precursor.
Compatible with the secondary loss of the alg-3, alg-5, alg-6, alg-8, alg-9, alg-10 and alg-12 genes during evolution, the causative agent of amoebic dysentery Entamoeba histolytica has the unprocessed ‘biosynthetic’ form of Man5GlcNAc2, containing two α1,2-mannose residues, as the most abundant glycan detectable (see Figure 2). In addition, some processing occurs to yield, e.g., glycans such as Glc1Gal1Man3GlcNAc2 or Gal2Man4GlcNAc2. Neither incorporation of deoxyhexoses nor additional GlcNAc residues could be detected (Magnelli, et al. 2008). Similarly in several strains of Trichomonas vaginalis, a widespread sexually-transmitted parasite, the major glycan detectable is also Man5GlcNAc2 (see Figure 2); however, much additional variation was also observed. The C1 strain is capable of the attachment of one or two pentose residues to its N-glycans, one of which is most likely xylose attached to the core mannose as in plants; another pentosylation site is the second GlcNAc of the core, as also described (see above) in a microalga. Additionally, some strains exhibited also modifications by N-acetyllactosamine and/or phosphorylethanolamine moieties, whereas the hybrid-like structures in this organism are a ‘mirror-image’ of those in multicellular organisms and so we have proposed the term ‘pseudohybrid’ (see Figure 2) for such GlcNAc-modified N-glycans (Paschinger, et al. 2012a). Recently, we have studied the N-glycans of the opportunistic amoebal parasite Acanthamoeba; although pentosylation as in T. vaginalis has been detected, hexosylation of core fucose is present and the biosynthesis is based on a typical Glc3Man9GlcNAc2 precursor (Schiller et al, unpublished data).
Toxoplasma gondii is primarily a feline parasite, but can be passed to humans; it is incapable of synthesising glycans with more than five mannose and three glucose residues. However, the complication is that it can scavenge glucosylated dolichol-linked N-glycans from the cells in which it resides. Indeed, the N-glycans detected are dependent on the cells in which the parasite is cultivated. T. gondii grown in normal mammalian cells possess oligomannose glycans ranging in size from Man3GlcNAc2 to Man9GlcNAc2; however, when grown in a cell line deficient in Dol-P-Man synthase and so lacking glycans with the final four mannose residues, the parasite exhibited N-glycans, in part glucosylated, no different from its own endogenously-produced forms (Garenaux, et al. 2008).
The largest precursor synthesised in the trypanosomatids is Dol-P-P-Man9GlcNAc2; however, generally, the range of glycans reported in the literature is limited. For instance, in Trypanosoma cruzi (causing Chagas disease in South America), after transient glucosylation, Man6-9GlcNAc2 structures are present on proteins (Parodi, et al. 1983); also some galactose and sialic acid residues have been found in some studies (Couto, et al. 1990). Crithidia fasciculata, an insect parasite, synthesises unglucosylated Dol-P-P-Man7GlcNAc2 glycan precursors and Man7GlcNAc2 is also the most abundant detected glycan on proteins; a second glycan of the composition Hex7GlcNAc2 was shown to contain galactofuranose (Parodi, et al. 1981, Mendelzon 1986). Leishmania mexicana transfers unglucosylated Man6GlcNAc2 to proteins (Parodi, et al. 1984) and Man4-6GlcNAc2, as well as Glc1Man6GlcNAc2, are present on the Gp63 protease of both L. mexicana and L. major (Olafson, et al. 1990, Funk, et al. 1997). In the non-human parasite L. tarentolae, which infects a gecko but is also a potential expression system for recombinant proteins, an unsialylated biantennary, β-1,4-galactosylated, core α-1,6-fucosylated glycan has been detected (Breitling, et al. 2002).
The situation in Trypanosoma brucei, which causes African sleeping sickness, is more complicated as there are two pools of precursor (Bangs, et al. 1988); it seems that bloodstream-form T. brucei can transfer both Man9GlcNAc2 and Man5GlcNAc2 to the variant surface glycoprotein (VSG) in a site-specific manner (Jones, et al. 2005). Indeed both the blood borne and the procyclic form of the parasite express two paralogous oligosaccharyltransferases (TbSTT3A and TbSTT3B) with different specificity (Izquierdo, et al. 2009). The resulting glycosylation patterns differ for VSG types I, II and III in a site- and protein-specific manner; the structures include typical oligomannose-types, such as Man7-8GlcNAc2, paucimannosidic glycans with the compositions Man3-4GlcNAc2 and ‘hybrid’ and biantennary complex types, some of which are modified with terminal α1,3-linked galactose residues; particularly striking are glycans with sometimes highly extended and branched poly N-acetyllactosamine chains (Zamze, et al. 1990, Zamze, et al. 1991, Mehlert, et al. 2002, Atrih, et al. 2005, Mehlert, et al. 2010). In an alg3 null mutant strain lacking the sixth ER mannosyltransferase, mannosidase inhibition results in the presence of some pseudohybrid and glucosylated glycans (Manthri, et al. 2008) akin to those found in T. vaginalis.
In contrast, confirming the existence of N-glycosylation in the malaria parasite Plasmodium falciparum has proven a difficult task (Davidson and Gowda 2001). Indeed it is known that P. falciparum is missing all of the ALG glycosyltransferases except ALG7 (UDP-N-acetyl-glucosamine-1-phosphotransferase), ALG13 (the second GlcNAc transferase) and the STT3 oligosaccharyltransferase catalytic subunit (Samuelson, et al. 2005). Both P. falciparum and Giardia lamblia are capable of synthesizing Dol-P-P-GlcNAc2 and transferring this to proteins (Bushkin, et al. 2010, Ratner, et al. 2008). Earlier reports of larger structures in these species are probably to be explained by contamination with glycans derived from the host or the medium.
N-glycans of platyhelminths
Our knowledge of platyhelminth (flatworm) N-glycosylation is focussed primarily on the parasitic trematodes Schistosoma mansoni and S. japonica. During the life-cycle of the parasite, some shifts in the N-glycosylation pattern occur and the glycosylation of some specific glycoproteins have also been investigated. It would appear that a real ‘mix’ of plant- and animal-type core modifications are present, in that xylosylation* of the core β-mannose as well as α1,3- and α1,6-fucosylation of the reducing GlcNAc can occur in various combinations on glycoproteins derived from eggs, cercariae, miracidia, adults or their secretions with core α1,3-fucose being apparently absent from adults and cercariae (Khoo, et al. 1997a, Khoo, et al. 2001, Wuhrer, et al. 2006a, Wuhrer, et al. 2006b, Hokke, et al. 2007, Jang-Lee, et al. 2007, Meevissen, et al. 2010, Meevissen, et al. 2011). Furthermore, up to three antennae have been identified on S. mansoni N-glycans; these antennae can consist of LacNAc (Galβ1,4GlcNAc) and LacdiNAc (GalNAcβ1,4GlcNAc) units (sometimes repeats) which may be decorated with fucose residues to result in, e.g., Lex, LDNF (see Figure 1) and difucosyl epitopes (Srivatsan, et al. 1992, Khoo, et al. 2001, Wuhrer, et al. 2006a, Jang-Lee, et al. 2007, Meevissen, et al. 2010, Meevissen, et al. 2011). Although fucosyl- and xylosyltransferase activities have been found in schistosome extracts (DeBose-Boyd, et al. 1996, Faveeuw, et al. 2003, Paschinger, et al. 2005), these have not yet been correlated with the relevant homologues in the schistosome genome but variations in their transcript levels have been found (Fitzpatrick, et al. 2009).
Amongst the cestodes, the N-glycans may be less complex than those of the schistosomes: core α1,6-fucosylation has been proven in three studies on Echinococcus glycoproteins (Khoo, et al. 1997b, Hülsmeier, et al. 2010, Paschinger, et al. 2012b); possible antennal modifications include galactose or phosphorylcholine – the latter accounting for the immunogenicity of the protein antigen Ag5. In another tapeworm, Taenia crassiceps, core fucosylation and terminal galactose is also a feature, but antennal fucose was also found (Lee, et al. 2005).
Not all flatworms are parasitic and the planaria have gained a status as a model for pluripotency; due to the stem cells present throughout the animal, regeneration of any amputated tissue is possible. The N-glycans of one species, Dugesia japonica, have been studied by two groups – the major N-glycan is a ‘processed’ Man5GlcNAc2 structure with all three non-reducing terminal mannoses being methylated (Natsuka, et al. 2011, Paschinger, et al. 2011); however, a small proportion of glycans is also core α1,6-fucosylated and the fucose residue is further modified by galactose and even a further methylhexose residue (Paschinger, et al. 2011).
N-glycans of nematodes
As recently summarised (Paschinger, et al. 2008), the simple roundworm Caenorhabditis elegans synthesises a wide range of N-glycans: with the oligomannosidic, paucimannosidic, fucosylated, ‘truncated complex’ and phosphorylcholine-modified types being supplemented by those with so-called ‘core chitobiose modifications’. However, C. elegans is a non-parasitic model organism with a large number of parasitic relatives; unfortunately, information about the actual covalent structures of the N-glycans is available for only a limited number of species: Haemonchus contortus, Ostertagia ostertagi, Dictyocaulus viviparus, Parelaphostrongylus tenuis, Ascaris suum, Onchocera volvulus, Acanthocheilonema viteae and Trichinella spiralis (Haslam, et al. 1996, Meyvis, et al. 2008, Haslam, et al. 2000, Duffy, et al. 2006, Pöltl, et al. 2007, Haslam, et al. 1999, Haslam, et al. 1997, Reason, et al. 1994).
There are two interesting features which C. elegans seems to share with its parasitic relatives, the presence of multiple fucoses (Haslam, et al. 2002, Paschinger, et al. 2004, Zhu, et al. 2004) with at least three of them bound to the chitobiose core of the N-glycans (Hanneman, et al. 2006, Struwe and Reinhold 2012) and the modifications of the N-glycan antennae with phosphorylcholine bound to the N-acetylglucosamine residues (Haslam, et al. 2002, Paschinger, et al. 2006) (see Figure 2). C. elegans shares the former feature with the sheep parasite H. contortus (Haslam, et al. 1996); this includes the presence of core α1,3-fucose, which is an epitope for anti-horseradish peroxidase (anti-HRP) as well as for IgE from H. contortus infected sheep (van Die, et al. 1999). On the other hand, N-glycans carrying the phosphorylcholine epitope have been found not only in filarial nematodes such as A. viteae and O. volvulus (Haslam, et al. 1997, Haslam, et al. 1999) but also in parasites with larvae migrating through different tissues in animal hosts, such as in T. spiralis and A. suum (Morelle, et al. 2000b, Pöltl, et al. 2007). This modification is of especial interest as phosphorylcholine is associated with immunomodulation by nematode parasites (Harnett and Harnett 2001)
A particularly unusual feature of a portion of C. elegans N-glycans is the capping of core fucose with galactose (Hanneman, et al. 2006, Gutternigg, et al. 2007a, Takeuchi, et al. 2008); to date, there is no report in the literature that these ‘GalFuc’ epitopes are also present in nematode parasites (although our ongoing work indeed indicates their occurrence in at least two parasitic species). The presence of galactose residues, on up to all three fucoses associated with the chitobiosyl core, confers a definite complexity to these glycans, which are recognised by both worm and fungal galectins (Takeuchi, et al. 2008, Butschi, et al. 2010); in terms of the evolutionary context, it is as if the galactose was perhaps first associated with the core and only later ‘migrated’ to the antennal positions familiar in vertebrate glycans.
Despite the various α1,3-fucosyltransferase homologues in C. elegans and their Lewis-type activity in vitro (Nguyen, et al. 2007), there is no sign that these are generating Lewis-type antennal modifications in vivo; the only nematode so far shown to actually possess Lex is D. viviparus (Haslam, et al. 2000). Indeed, the underlying (unfucosylated) LacNAc motif is not a general feature of nematode N-glycans and reports on the occurrence of related LacdiNAc epitope in nematode N-glycans are scarce, but this feature is found in T. spiralis (Morelle, et al. 2000a, Morelle, et al. 2000b) and Dirofilaria immitis (Kang, et al. 1993). Chito-oligomers (GlcNAcn), which were only detected after hydrofluoric acid treatment, are a feature of filarial worms (Haslam, et al. 1999). In all these species, multiantennary N-glycans are present; based on homologies and actual activity assays, C. elegans possesses GlcNAc-TI, -TII and –TV genes required for the synthesis of triantennary N-glycans (Chen, et al. 2002, Warren, et al. 2002). Otherwise, the core fucosyltransferases FUT-1 and FUT-8 and the ‘capping’ galactosyltransferase GALT-1 have demonstrated N-glycan-modifying activity correlating with N-glycan structures (Paschinger, et al. 2004, Paschinger, et al. 2005, Titz, et al. 2009). Other enzymes required for galactose, fucose or phosphorylcholine modifications of N-glycans in C. elegans are yet to be identified; certainly, surprises as to the specificities of such enzymes may well occur – the GlcNAc-TI-independence of the core α1,3-fucosyltransferase FUT-1 (see Figure 3 for a biosynthetic scheme) was unexpected and a possible indication of convergent evolution regarding the formation of anti-HRP epitopes in different species (as mentioned above, core α1,3-fucose is an epitope for anti-HRP).
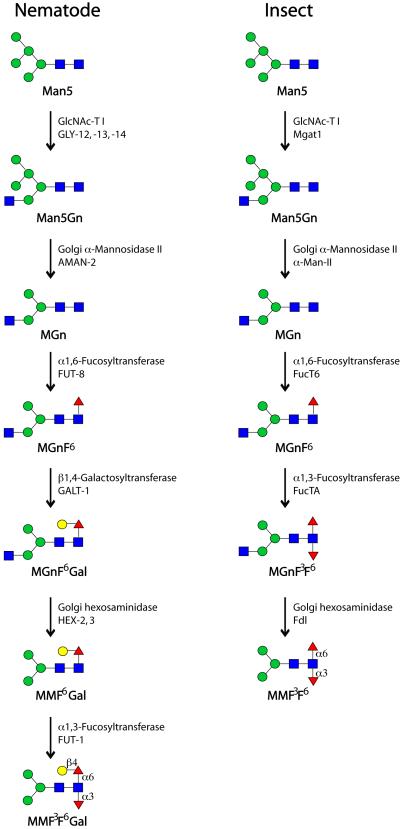
Figure 3. Biosynthetic routes for N-glycan core modifications in nematodes and insects.
Although similar, the routes to core modification of N-glycans in nematodes and insects are subtly different, due to the different specificity of the core α1,3-fucosyltransferase and the presence of the GalFuc epitope; the pathways shown begin with the ‘processed’ form of Man5, which results from the action of ER glucosidases and ER/Golgi class I mannosidases after transfer to protein. The specificities of the enzymes involved have been defined in a number of studies; both descriptive and official protein names are used. Monosaccharides are depicted according to the nomenclature of the Consortium for Functional Glycomics (see Figure 1).
N-glycans of molluscs
The N-glycans of a few species of molluscs, including slugs, snails, limpet, octopus and squid, have been studied over the years; in some cases mollusc extracts were examined, in others, specific proteins such as rhodopsins or haemocyanins. In some species, such as gastropods (Gutternigg, et al. 2004, Gutternigg, et al. 2007b), core β1,2-xylose and α1,6-fucose is present as well as methylation of terminal mannose residues and a low degree of core α1,3-fucosylation (see also the review by Staudacher in this issue) are a feature, whereas in others, e.g., squid and octopus rhodopsins (Zhang, et al. 1997, Takahashi, et al. 2004), the same ‘GalFuc’ motif (Galβ1,4Fuc on the reducing terminal GlcNAc; see Figure 1) as in planaria and nematodes has been found; in the squid, as is sometimes the case in C. elegans, the GalFuc motif is in the context of difucosylation of the core GlcNAc. In the snail Biomphalaria glabrata, the intermediate host of Schistosoma mansoni, over 100 N-glycan structures have been isolated from haemolymph proteins, including biantennary glycans with core xylose, core α1,6-fucose, methylmannose and fucosylated LacdiNAc (e.g., Fucα1,3GalNAcβ1,4GlcNAc) motifs; other than methylation, these features are shared with the trematode and cross-react with anti-schistosome antibodies (Lehr, et al. 2007).
Amongst the various haemocyanins examined, the one from keyhole limpet (Megathura crenulata; KLH) also features glycans cross-reactive with anti-schistosome antibodies. Fucosylated LacdiNAc, core xylose, galactosylated ‘GalFuc’ (i.e., Galβ1,4Galβ1,4Fucα1,6) and Galβ1,6Man motifs have been detected on KLH glycans by mass spectrometry (Kurokawa, et al. 2002, Wuhrer, et al. 2004, Geyer, et al. 2005). In other species, the glycans present on the haemocyanins vary from the ‘less exciting’ hybrid and Man5GlcNAc2 structures in Panulirus interruptus (Van Kuik, et al. 1986a) through to oligosaccharides with methylated mannose in Hippopus hippopus (Puanglarp, et al. 1995), methylated Lewis-like motifs in Haliotis tuberculata (Velkova, et al. 2011), disubstituted antennal fucose in Rapana thomasiana (Gielens, et al. 2005) and sulphated mannose, methylated GlcNAc and methylated galactose in Rapana venosa (Dolashka-Angelova, et al. 2003). Methylated galactose and core xylose have been found on the haemocyanins from Lymnaea stagnalis and Helix pomatia, with peripheral blood group H (Fucα1,2Galβ) in the former and core α1,6-fucose in the latter (van Kuik, et al. 1985, van Kuik, et al. 1986b, Van Kuik, et al. 1987, Lommerse, et al. 1997). Thereby, it is obvious that molluscs have a wide capacity to modify the basic biantennary N-glycan structure with many species-specific peculiarities; it is more than likely that many more types of modification remain to be discovered.
N-glycans of insects
The N-glycosylation capacity of insects (Rendić, et al. 2008) is of interest for both academic and biotechnological reasons with, on the one hand, the fruit fly Drosophila melanogaster as an important model organism and, on the other, the various insect cell lines used to produce recombinant proteins; also, the immunogenicity of insect venom glycoproteins is another factor. The first studies on the N-glycans of insects indicated the presence of oligomannosidic glycans and also of a core α1,6-fucosylated paucimannosidic structure (MMF6; see Figure 3 for related glycans) (Butters and Hughes 1981, Williams, et al. 1991). However, it was also obvious that an, until then unknown, modification was also present in insects: difucosylation of the core reducing-terminal GlcNAc – i.e., its modification by both α1,3- and α1,6-fucose. Core difucosylation (see Figure 1) was first observed on bee venom phospholipase A2 (Kubelka, et al. 1993), but also, e.g., on bee and wasp venom hyaluronidase (Kubelka, et al. 1995, Kolarich, et al. 2005) and glycoproteins from D. melanogaster adults and neuronal cells as well as on the pheromone DUP99B (Fabini, et al. 2001, Saudan, et al. 2002, Rendić, et al. 2006). Also recombinant glycoproteins produced in Trichoplusia ni (High Five) cells (Ailor, et al. 2000, Palmberger, et al. 2011) can be core difucosylated. Thereby, core α1,3-fucose, and not xylose, is responsible for the cross-reactivity of insect glycoproteins towards antisera recognising plant glycans, including anti-HRP.
In general, insect N-glycans are not normally possessing extended antennae. However, there are exceptions, such as the fucosylated LacdiNAc (LDNF; see Figure 1) found on a proportion of bee venom phospholipase (Kubelka, et al. 1993), Galβ1,3GlcNAc modifications of royal jelly glycoproteins (Kimura, et al. 2003) and sialyl-LacNAc (see Figure 1) on some Drosophila embryonal glycans (Aoki, et al. 2007); amongst these examples are even triantennary forms. In a locust apolipophorin, the rather unusual modification by aminoethylphosphonate was also proposed (Hård, et al. 1993). Thus, insects do possess the ability to initiate the processing of glycans in a ‘complex’ manner (Geisler and Jarvis 2012), even though pauci- and oligomannosidic forms dominate the spectra of those insect samples analysed to date. It may seem a paradox that even the biosynthesis of paucimannosidic glycans requires the prior action of GlcNAc-TI; however, due to the hexosaminidase activity encoded by the fused lobes gene in their secretory pathways (Léonard, et al. 2006), removal of the GlcNAc first transferred by GlcNAc-TI is an integral part of N-glycan processing not just in insects but in many invertebrates (Figure 3); thereby, the action of the fused lobes enzyme (named on the basis of the morphology of the brain in the corresponding Drosophila mutant) results in a lack of antennal elongation. Nevertheless, GlcNAc-TI activity generates the necessary ‘GO’ signal for core fucosylation and Golgi mannosidase II (Schachter 2009). However, a major interest in the exploitation of insect cells as expression systems is indeed to circumvent the removal of this ‘GO’ signal by the fused lobes hexosaminidase (Fdl) by, e.g., overexpressing mammalian glycosyltransferases which cap GlcNAc residues (Aumiller, et al. 2012).
N-glycans of ascidians
Ascidians or sea squirts are chordates and so are considered to be evolutionarily close to vertebrates. Despite the potential phylogenetic interest, only one study regarding their N-glycans has been published. Specifically, a glycan in the neural tissue of Ciona intestinalis has been described as co-eluting with plant glycans containing xylose and core α1,3-fucose; otherwise, oligomannosidic and fucosylated triantennary glycans were detected in other tissues of this organism (Yagi, et al. 2008).
Conclusion
The large diversity in glycan structures and the incredible glycogenomic potential of so-called lower organisms, whether unicellular or multicellular, are obvious. However, although the N-glycans of a wide range of ‘simple’ organisms have been studied over the years, this work has not been tackled systematically. This means there are many holes in our knowledge. Nevertheless, there are some trends to consider: one is the frequent lack of charged modifications of their N-glycans (in particular, sialic acid); however, there are exceptions (e.g., sulphation in slime moulds or phosphorylcholine in nematodes) and methodological constraints may lead to an underestimation of their occurrence. Another is the presence of unusual modifications of the core region; but a more general point to consider is the modification of N-linked oligosaccharides by the classical GlcNAc-TI. This is quite probably a hallmark of multicellular organisms – it is not quite clear how this enzyme evolved, but it is probable that even in unicellular organisms (such as trypanosomatids) which also synthesise biantennary glycans, the transfer of GlcNAc to the N-glycan in the Golgi does not take place via the same mechanism as in vertebrates and it appears that homologues of the ‘multicellular’ GlcNAc-TI are absent from these species. Indeed, it may well be that in unicellular parasites first the α1,6-mannose is modified before the α1,3-arm; at least, in T. vaginalis, there is probably only transfer of GlcNAc to the α1,6-arm to form pseudohybrid glycans.
This is just one example where it becomes obvious that the classical division of N-glycan types does not hold up when considering non-vertebrate species. It is even difficult to consider what the term ‘complex’ means when presented with some of the glycan structures – such as those carrying the ‘core chitobiose modifications’ in nematodes. Traditionally, ‘complex’ glycosylation refers to N-glycans with GlcNAc residues modifying both the α1,3- and α1,6-linked mannose residues of the conserved pentasaccharide core. However, a glycan lacking such residues, but possessing three capped fucose substitutions of the chitobiosyl region is also structurally complex (never mind its biosynthesis) – or should we just say it’s ‘complicated’? It is also obvious that previous attempts to ‘name’ glycans based on their terminal sugars (e.g., the Schachter nomenclature featuring names such as ‘GnGnF’ or ‘GalGal’ (Schachter 1986)) also cannot deal with the structures observed in lower organisms. We end up with referring to glycans by their mass or their composition or by referring to diagrams featuring squares, triangles and circles whose meanings are not even accepted by all, never mind understood by non-specialist; we also do not have so many simple and/or abbreviated names for the epitopes in lower organisms unless they are, such as Lewis-type glycans, shared with those in mammals. Currently, it seems that the flood of glycomic information from non-vertebrates has exhausted the normal human desire to name objects; it should certainly not mean that mammalian-centric researchers should ignore the nature of these unusual glycans or that we oversimplify or overgeneralise because we lack ‘nutshell’ summaries.
Both Rudolf and Hildegard Geyer have made substantial contributions to our knowledge about the glycosylation of non-vertebrate species: not just about their N-glycans (especially, as cited above, a number of studies on glycans cross-reacting with anti-schistosome antibodies) but also in the realm of glycolipids, a topic which is not addressed here. Their unique knowledge in glycan analysis has aided many glycobiologists, including ourselves; there would be indeed be a continued need for this internationally-respected expertise (apparently and unfortunately not a future focus in Gießen) as even two scientific lifetimes are insufficient for an exploration of the glyco-universe. What is certain is that their work has partially paved the way for others to explore further galaxies of glycomes.
Acknowledgements
Work in our laboratory related to the topic of this review has been and is primarily funded by the Austrian Fonds zur Förderung der wissenschaftlichen Forschung (grants to I.B.H.W. [P13810, P15475, P17681, P18447, P19615, P20565 and P23922] and K.P. [P21946]).
Footnotes
Xylose (derived from the Greek ξυλος, wood) is a monosaccharide normally considered to be a major component of plant N-glycans and polysaccharides; however, in animals, this monosaccharide is present in proteoglycans (as the ‘core’ linkage to protein of chondroitin and heparan sulphates) and in some O-glucose-based glycans present on vertebrate EGF domains as well as in the N-glycans of some trematodes and molluscs.
References
- Ailor E, Takahashi N, Tsukamoto Y, Masuda K, Rahman BA, Jarvis DL, Lee YC, Betenbaugh MJ. N-glycan patterns of human transferrin produced in Trichoplusia ni insect cells: effects of mammalian galactosyltransferase. Glycobiology. 2000;10:837–847. doi: 10.1093/glycob/10.8.837. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- Atrih A, Richardson JM, Prescott AR, Ferguson MA. Trypanosoma brucei glycoproteins contain novel giant poly-N-acetyllactosamine carbohydrate chains. J Biol Chem. 2005;280:865–871. doi: 10.1074/jbc.M411061200. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Mabashi-Asazuma H, Hillar A, Shi X, Jarvis DL. A new glycoengineered insect cell line with an inducibly mammalianized protein N-glycosylation pathway. Glycobiology. 2012;22:417–428. doi: 10.1093/glycob/cwr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshüsemann D, Jaenicke L. The oligosaccharides of the glycoprotein pheromone of Volvox carteri f. nagariensis Iyengar (Chlorophyceae) Eur J Biochem. 1990;192:231–237. doi: 10.1111/j.1432-1033.1990.tb19220.x. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Vishwanath P, Cui J, Kelleher DJ, Gilmore R, Robbins PW, Samuelson J. The evolution of N-glycan-dependent endoplasmic reticulum quality control factors for glycoprotein folding and degradation. Proc Natl Acad Sci U S A. 2007;104:11676–11681. doi: 10.1073/pnas.0704862104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs JD, Doering TL, Englund PT, Hart GW. Biosynthesis of a variant surface glycoprotein of Trypanosoma brucei. Processing of the glycolipid membrane anchor and N-linked oligosaccharides. J Biol Chem. 1988;263:17697–17705. [PubMed] [Google Scholar]
- Breitling R, Klingner S, Callewaert N, Pietrucha R, Geyer A, Ehrlich G, Hartung R, Muller A, Contreras R, Beverley SM, Alexandrov K. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Expr Purif. 2002;25:209–218. doi: 10.1016/s1046-5928(02)00001-3. [DOI] [PubMed] [Google Scholar]
- Bushkin GG, Ratner DM, Cui J, Banerjee S, Duraisingh MT, Jennings CV, Dvorin JD, Gubbels MJ, Robertson SD, Steffen M, O’Keefe BR, Robbins PW, Samuelson J. Suggestive evidence for Darwinian Selection against asparagine-linked glycans of Plasmodium falciparum and Toxoplasma gondii. Eukaryot Cell. 2010;9:228–241. doi: 10.1128/EC.00197-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butschi A, Titz A, Wälti M, Olieric V, Paschinger K, Nöbauer K, Guo X, Seeberger PH, Wilson IBH, Aebi M, Hengartner M, Künzler M. Caenorhabditis elegans N-glycan core β-galactoside confers sensitivity towards nematotoxic fungal galectin CGL2. PLOS Pathogens. 2010;6:e1000717. doi: 10.1371/journal.ppat.1000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters TD, Hughes RC. Isolation and characterisation of mosquito cell membrane glycoproteins. Biochim Biophys Acta. 1981;640:655–671. doi: 10.1016/0005-2736(81)90096-1. [DOI] [PubMed] [Google Scholar]
- Chen S, Tan J, Reinhold VN, Spence AM, Schachter H. UDP-N-acetylglucosamine:α-3-D-mannoside β-1,2-N-acetylglucosaminyltransferase I and UDP-N-acetylglucosamine:α-6-D-mannoside β-1,2-N-acetylglucosaminyltransferase II in Caenorhabditis elegans. Biochim Biophys Acta. 2002;1573:271–279. doi: 10.1016/s0304-4165(02)00393-8. [DOI] [PubMed] [Google Scholar]
- Couso R, Lang L, Roberts RM, Kornfeld S. Phosphorylation of the oligosaccharide of uteroferrin by UDP-GlcNAc:glycoprotein N-acetylglucosamine-1-phosphotransferases from rat liver, Acanthamoeba castellani, and Dictyostelium discoideum requires α1,2-linked mannose residues. J Biol Chem. 1986;261:6326–6331. [PubMed] [Google Scholar]
- Couto AS, Goncalves MF, Colli W, de Lederkremer RM. The N-linked carbohydrate chain of the 85-kilodalton glycoprotein from Trypanosoma cruzi trypomastigotes contains sialyl, fucosyl and galactosyl (α1-3)galactose units. Mol Biochem Parasitol. 1990;39:101–107. doi: 10.1016/0166-6851(90)90012-b. [DOI] [PubMed] [Google Scholar]
- Davidson EA, Gowda DC. Glycobiology of Plasmodium falciparum. Biochimie. 2001;83:601–604. doi: 10.1016/s0300-9084(01)01316-5. [DOI] [PubMed] [Google Scholar]
- DeBose-Boyd R, Nyame AK, Cummings RD. Schistosoma mansoni: Characterization of an α1-3 fucosyltransferase in adult parasites. Exp Parasitol. 1996;82:1–10. doi: 10.1006/expr.1996.0001. [DOI] [PubMed] [Google Scholar]
- Dolashka-Angelova P, Beck A, Dolashki A, Beltramini M, Stevanovic S, Salvato B, Voelter W. Characterization of the carbohydrate moieties of the functional unit RvH1-a of Rapana venosa haemocyanin using HPLC/electrospray ionization MS and glycosidase digestion. Biochem J. 2003;374:185–192. doi: 10.1042/BJ20030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy MS, Morris HR, Dell A, Appleton JA, Haslam SM. Protein glycosylation in Parelaphostrongylus tenuis - First description of the Galα1-3Gal sequence in a nematode. Glycobiology. 2006;16:854–862. doi: 10.1093/glycob/cwl001. [DOI] [PubMed] [Google Scholar]
- Fabini G, Freilinger A, Altmann F, Wilson IBH. Identification of core α1,3-fucosylated glycans and the requisite fucosyltransferase in Drosophila melanogaster. Potential basis of the neural anti-horseradish peroxidase epitope. J. Biol. Chem. 2001;276:28058–28067. doi: 10.1074/jbc.M100573200. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Mallevaey T, Paschinger K, Wilson IBH, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, Trottein F. Schistosome N-glycans containing core α3-fucose and core β2-xylose epitopes are strong inducers of Th2 responses in mice. Eur. J. Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- Feasley CL, Johnson JM, West CM, Chia CP. Glycopeptidome of a heavily N-glycosylated cell surface glycoprotein of Dictyostelium implicated in cell adhesion. J Proteome Res. 2010;9:3495–3510. doi: 10.1021/pr901195c. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick JM, Peak E, Perally S, Chalmers IW, Barrett J, Yoshino TP, Ivens AC, Hoffmann KF. Anti-schistosomal intervention targets identified by lifecycle transcriptomic analyses. PLoS Negl Trop Dis. 2009;3:e543. doi: 10.1371/journal.pntd.0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Miller AL, Kaplan A. Acid hydrolases from Dictyostelium discoideum contain phosphomannosyl recognition markers. J Biol Chem. 1980;255:11081–11084. [PubMed] [Google Scholar]
- Freeze HH, Yeh R, Miller AL, Kornfeld S. Structural analysis of the asparagine-linked oligosaccharides from three lysosomal enzymes of Dictyostelium discoideum. Evidence for an unusual acid-stable phosphodiester. J Biol Chem. 1983a;258:14874–14879. [PubMed] [Google Scholar]
- Freeze HH, Yeh R, Miller AL, Kornfeld S. The mod A mutant of Dictyostelium discoideum is missing the α1,3-glucosidase involved in asparagine-linked oligosaccharide processing. J Biol Chem. 1983b;258:14880–14884. [PubMed] [Google Scholar]
- Freeze HH, Wolgast D. Biosynthesis of methylphosphomannosyl residues in the oligosaccharides of Dictyostelium discoideum glycoproteins. Evidence that the methyl group is derived from methionine. J Biol Chem. 1986;261:135–141. [PubMed] [Google Scholar]
- Freeze HH, Willies L, Hamilton S, Koza-Taylor P. Two mutants of Dictyostelium discoideum that lack a sulfated carbohydrate antigenic determinant synthesize a truncated lipid-linked precursor of N-linked oligosaccharides. J Biol Chem. 1989;264:5653–5659. [PubMed] [Google Scholar]
- Freeze HH, Hindsgaul O, Ichikawa M. A novel pathway for phosphorylated oligosaccharide biosynthesis. Identification of an oligosaccharide-specific phosphate methyltransferase in Dictyostelium discoideum. J Biol Chem. 1992;267:4431–4439. [PubMed] [Google Scholar]
- Funk VA, Thomas-Oates JE, Kielland SL, Bates PA, Olafson RW. A unique, terminally glucosylated oligosaccharide is a common feature on Leishmania cell surfaces. Mol Biochem Parasitol. 1997;84:33–48. doi: 10.1016/s0166-6851(96)02780-6. [DOI] [PubMed] [Google Scholar]
- Gabel CA, Costello CE, Reinhold VN, Kurz L, Kornfeld S. Identification of methylphosphomannosyl residues as components of the high mannose oligosaccharides of Dictyostelium discoideum glycoproteins. J Biol Chem. 1984;259:13762–13769. [PubMed] [Google Scholar]
- Garenaux E, Shams-Eldin H, Chirat F, Bieker U, Schmidt J, Michalski JC, Cacan R, Guerardel Y, Schwarz RT. The dual origin of Toxoplasma gondii N-glycans. Biochemistry. 2008;47:12270–12276. doi: 10.1021/bi801090a. [DOI] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Substrate specificities and intracellular distributions of three N-glycan processing enzymes functioning at a key branch point in the insect N-glycosylation pathway. J Biol Chem. 2012;287:7084–7097. doi: 10.1074/jbc.M111.296814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer H, Wuhrer M, Resemann A, Geyer R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J Biol Chem. 2005;280:40731–40748. doi: 10.1074/jbc.M505985200. [DOI] [PubMed] [Google Scholar]
- Gielens C, Idakieva K, Van den Bergh V, Siddiqui NI, Parvanova K, Compernolle F. Mass spectral evidence for N-glycans with branching on fucose in a molluscan hemocyanin. Biochem Biophys Res Commun. 2005;331:562–570. doi: 10.1016/j.bbrc.2005.03.217. [DOI] [PubMed] [Google Scholar]
- Gutternigg M, Ahrer K, Grabher-Meier H, Burgmayr S, Staudacher E. Neutral N-glycans of the gastropod Arion lusitanicus. Eur J Biochem. 2004;271:1348–1356. doi: 10.1111/j.1432-1033.2004.04045.x. [DOI] [PubMed] [Google Scholar]
- Gutternigg M, Kretschmer-Lubich D, Paschinger K, Rendić D, Hader J, Geier P, Ranftl R, Jantsch V, Lochnit G, Wilson IBH. Biosynthesis of Truncated N-Linked Oligosaccharides Results from Non-orthologous Hexosaminidase-mediated Mechanisms in Nematodes, Plants, and Insects. J Biol Chem. 2007a;282:27825–27840. doi: 10.1074/jbc.M704235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutternigg M, Bürgmayr S, Pöltl G, Rudolf J, Staudacher E. Neutral N-glycan patterns of the gastropods Limax maximus, Cepaea hortensis, Planorbarius corneus, Arianta arbustorum and Achatina fulica. Glycoconj J. 2007b;24:475–489. doi: 10.1007/s10719-007-9040-5. [DOI] [PubMed] [Google Scholar]
- Hanneman AJ, Rosa JC, Ashline D, Reinhold V. Isomer and glycomer complexities of core GlcNAcs in Caenorhabditis elegans. Glycobiology. 2006;16:874–890. doi: 10.1093/glycob/cwl011. [DOI] [PubMed] [Google Scholar]
- Hård K, Van Doorn JM, Thomas-Oates JE, Kamerling JP, Van der Horst DJ. Structure of the Asn-linked oligosaccharides of apolipophorin III from the insect Locusta migratoria. Carbohydrate-linked 2-aminoethylphosphonate as a constituent of a glycoprotein. Biochemistry. 1993;32:766–775. doi: 10.1021/bi00054a005. [DOI] [PubMed] [Google Scholar]
- Harnett W, Harnett MM. Modulation of the host immune system by phosphorylcholine-containing glycoproteins secreted by parasitic filarial nematodes. Biochim Biophys Acta. 2001;1539:7–15. doi: 10.1016/s0167-4889(01)00101-x. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Coles GC, Munn EA, Smith TS, Smith HF, Morris HR, Dell A. Haemonchus contortus glycoproteins contain N-linked oligosaccharides with novel highly fucosylated core structures. J. Biol. Chem. 1996;271:30561–30570. doi: 10.1074/jbc.271.48.30561. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Khoo KH, Houston KM, Harnett W, Morris HR, Dell A. Characterisation of the phosphorylcholine-containing N-linked oligosaccharides in the excretory-secretory 62 kDa glycoprotein of Acanthocheilonema viteae. Mol Biochem Parasitol. 1997;85:53–66. doi: 10.1016/s0166-6851(96)02807-1. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Houston KM, Harnett W, Reason AJ, Morris HR, Dell A. Structural studies of N-glycans of filarial parasites. Conservation of phosphorylcholinesubstituted glycans among species and discovery of novel chito-oligomers. J Biol Chem. 1999;274:20953–20960. doi: 10.1074/jbc.274.30.20953. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Coles GC, Morris HR, Dell A. Structural characterisation of the N-glycans of Dictyocaulus viviparus: discovery of the Lewisx structure in a nematode. Glycobiology. 2000;10:223–229. doi: 10.1093/glycob/10.2.223. [DOI] [PubMed] [Google Scholar]
- Haslam SM, Gems D, Morris HR, Dell A. The glycomes of Caenorhabditis elegans and other model organisms. Biochem. Soc. Symp. 2002;69:117–134. [PubMed] [Google Scholar]
- Herscovics A, Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993;7:540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- Hokke CH, Deelder AM, Hoffmann KF, Wuhrer M. Glycomics-driven discoveries in schistosome research. Exp Parasitol. 2007;117:275–283. doi: 10.1016/j.exppara.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Hülsmeier AJ, Deplazes P, Naem S, Nonaka N, Hennet T, Kohler P. An Echinococcus multilocularis coproantigen is a surface glycoprotein with unique O-glycosylation. Glycobiology. 2010;20:127–135. doi: 10.1093/glycob/cwp155. [DOI] [PubMed] [Google Scholar]
- Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci USA. 1994;91:728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivatt RJ, Das OP, Henderson EJ, Robbins PW. Developmental regulation of glycoprotein biosynthesis in Dictyostelium. J Supramol Struct Cell Biochem. 1981;17:359–368. doi: 10.1002/jsscb.380170407. [DOI] [PubMed] [Google Scholar]
- Ivatt RL, Das OP, Henderson EJ, Robbins PW. Glycoprotein biosynthesis in Dictyostelium discoideum: developmental regulation of the protein-linked glycans. Cell. 1984;38:561–567. doi: 10.1016/0092-8674(84)90510-5. [DOI] [PubMed] [Google Scholar]
- Izquierdo L, Schulz BL, Rodrigues JA, Guther ML, Procter JB, Barton GJ, Aebi M, Ferguson MA. Distinct donor and acceptor specificities of Trypanosoma brucei oligosaccharyltransferases. EMBO J. 2009;28:2650–2661. doi: 10.1038/emboj.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang-Lee J, Curwen RS, Ashton PD, Tissot B, Mathieson W, Panico M, Dell A, Wilson RA, Haslam SM. Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics. 2007;6:1485–1499. doi: 10.1074/mcp.M700004-MCP200. [DOI] [PubMed] [Google Scholar]
- Jones DC, Mehlert A, Güther ML, Ferguson MA. Deletion of the glucosidase II gene in Trypanosoma brucei reveals novel N-glycosylation mechanisms in the biosynthesis of variant surface glycoprotein. J Biol Chem. 2005;280:35929–35942. doi: 10.1074/jbc.M509130200. [DOI] [PubMed] [Google Scholar]
- Kang S, Cummings RD, McCall JW. Characterization of the N-linked oligosaccharides in glycoproteins synthesized by microfilariae of Dirofilaria immitis. J Parasitol. 1993;79:815–828. [PubMed] [Google Scholar]
- Khoo K-H, Chatterjee D, Caulfield JP, Morris HR, Dell A. Structural mapping of the glycans from the egg glycoproteins of Schistosoma mansoni and Schistosoma japonicum: Identification of novel core structures and terminal sequences. Glycobiology. 1997a;7:663–677. doi: 10.1093/glycob/7.5.663. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Nieto A, Morris HR, Dell A. Structural characterisation of the N-glycans from Echinococcus granulosus hydatid cyst membrane and protoscholeces. Mol Biochem Parasitol. 1997b;86:237–248. doi: 10.1016/s0166-6851(97)00036-4. [DOI] [PubMed] [Google Scholar]
- Khoo K-H, Huang H-H, Lee K-M. Characteristic structural features of schistosome cercarial N-glycans: expression of Lewis X and core xylosylation. Glycobiology. 2001;11:149–163. doi: 10.1093/glycob/11.2.149. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Tsumura K, Kimura M, Okihara K, Sugimoto H, Yamada H. First evidence for occurrence of Galβ1-3GlcNAcβ1-4Man unit in N-glycans of insect glycoprotein: β1-3Gal and β1-4GlcNAc transferases are involved in N-glycan processing of royal jelly glycoproteins. Biosci Biotechnol Biochem. 2003;67:1852–1856. doi: 10.1271/bbb.67.1852. [DOI] [PubMed] [Google Scholar]
- Kolarich D, Leonard R, Hemmer W, Altmann F. The N-glycans of yellow jacket venom hyaluronidases and the protein sequence of its major isoform in Vespula vulgaris. FEBS J. 2005;272:5182–5190. doi: 10.1111/j.1742-4658.2005.04841.x. [DOI] [PubMed] [Google Scholar]
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kubelka V, Altmann F, Staudacher E, Tretter V, März L, Hård K, Kamerling JP, Vliegenthart JFG. Primary structures of the N-linked carbohydrate chains from honeybee venom phospholipase A2. Eur J Biochem. 1993;213:1193–1204. doi: 10.1111/j.1432-1033.1993.tb17870.x. [DOI] [PubMed] [Google Scholar]
- Kubelka V, Altmann F, März L. The asparagine-linked carbohydrate of honeybee venom hyaluronidase. Glycoconjugate J. 1995;12:77–83. doi: 10.1007/BF00731872. [DOI] [PubMed] [Google Scholar]
- Kurokawa T, Wuhrer M, Lochnit G, Geyer H, Markl J, Geyer R. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(β1-6)Man-motifs. Eur J Biochem. 2002;269:5459–5473. doi: 10.1046/j.1432-1033.2002.03244.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Dissanayake S, Panico M, Morris HR, Dell A, Haslam SM. Mass spectrometric characterisation of Taenia crassiceps metacestode N-glycans. Mol Biochem Parasitol. 2005;143:245–249. doi: 10.1016/j.molbiopara.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Lehr T, Geyer H, Maass K, Doenhoff MJ, Geyer R. Structural characterization of N-glycans from the freshwater snail Biomphalaria glabrata cross-reacting with Schistosoma mansoni glycoconjugates. Glycobiology. 2007;17:82–103. doi: 10.1093/glycob/cwl048. [DOI] [PubMed] [Google Scholar]
- Léonard R, Rendić D, Rabouille C, Wilson IBH, Préat T, Altmann F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J. Biol. Chem. 2006;281:4867–4875. doi: 10.1074/jbc.M511023200. [DOI] [PubMed] [Google Scholar]
- Levy-Ontman O, Malis Arad S, Harvey DJ, Parsons TB, Fairbanks A, Tekoah Y. Unique N-glycan moieties of the 66-KDa cell-wall glycoprotein from the red microalga Porpyridium sp. J Biol Chem. 2011;286:21340–21352. doi: 10.1074/jbc.M110.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommerse JPM, Thomas-Oates JE, Gielens C, Préaux G, Kamerling JP, Vliegenthart JFG. Primary structure of 21 novel monoantennary and diantennary N-linked carbohydrate chains from αD-hemocyanin of Helix pomatia. Eur. J. Biochem. 1997;249:195–222. doi: 10.1111/j.1432-1033.1997.00195.x. [DOI] [PubMed] [Google Scholar]
- Magnelli P, Cipollo JF, Ratner DM, Cui J, Kelleher D, Gilmore R, Costello CE, Robbins PW, Samuelson J. Unique Asn-linked Oligosaccharides of the Human Pathogen Entamoeba histolytica. J Biol Chem. 2008;283:18355–18364. doi: 10.1074/jbc.M800725200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthri S, Guther ML, Izquierdo L, Acosta-Serrano A, Ferguson MAJ. Deletion of the TbALG3 gene demonstrates site-specific N-glycosylation and N-glycan processing in Trypanosoma brucei. Glycobiology. 2008;18:367–383. doi: 10.1093/glycob/cwn014. [DOI] [PubMed] [Google Scholar]
- Meevissen MH, Wuhrer M, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Hokke CH. Structural characterization of glycans on omega-1, a major Schistosoma mansoni egg glycoprotein that drives Th2 responses. J Proteome Res. 2010;9:2630–2642. doi: 10.1021/pr100081c. [DOI] [PubMed] [Google Scholar]
- Meevissen MH, Balog CI, Koeleman CA, Doenhoff MJ, Schramm G, Haas H, Deelder AM, Wuhrer M, Hokke CH. Targeted glycoproteomic analysis reveals that kappa-5 is a major, uniquely glycosylated component of Schistosoma mansoni egg antigens. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.005710. M110 005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlert A, Bond CS, Ferguson MA. The glycoforms of a Trypanosoma brucei variant surface glycoprotein and molecular modeling of a glycosylated surface coat. Glycobiology. 2002;12:607–612. doi: 10.1093/glycob/cwf079. [DOI] [PubMed] [Google Scholar]
- Mehlert A, Sullivan L, Ferguson MAJ. Glycotyping of Trypanosoma brucei variant surface glycoprotein MITat1.8. Mol Biochem Parasitol. 2010;174:74–77. doi: 10.1016/j.molbiopara.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelzon DH, Parodi AJ. N-linked high mannose-type oligosaccharides in the protozoa Crithidia fasciculata and Crithidia harmosa contain galactofuranose residues. J. Biol. Chem. 1986;261:2129–2133. [PubMed] [Google Scholar]
- Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994;13:2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyvis Y, Callewaert N, Gevaert K, Timmerman E, Van Durme J, Schymkowitz J, Rousseau F, Vercruysse J, Claerebout E, Geldhof P. Hybrid N-glycans on the host protective activation-associated secreted proteins of Ostertagia ostertagi and their importance in immunogenicity. Mol Biochem Parasitol. 2008;161:67–71. doi: 10.1016/j.molbiopara.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Morelle W, Haslam SM, Morris HR, Dell A. Characterization of the N-linked glycans of adult Trichinella spiralis. Mol Biochem Parasitol. 2000a;109:171–177. doi: 10.1016/s0166-6851(00)00241-3. [DOI] [PubMed] [Google Scholar]
- Morelle W, Haslam SM, Olivier V, Appleton JA, Morris HR, Dell A. Phosphorylcholine-containing N-glycans of Trichinella spiralis: identification of multiantennary lacdiNAc structures. Glycobiology. 2000b;10:941–950. doi: 10.1093/glycob/10.9.941. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Tojo H, Fujii S. A glycan of ψ-factor from Dictyostelium discoideum contains a bisecting-GlcNAc, an intersecting-GlcNAc, and a core α-1,6-fucose. Biosci Biotechnol Biochem. 2011;75:1964–1970. doi: 10.1271/bbb.110369. [DOI] [PubMed] [Google Scholar]
- Natsuka S, Hirohata Y, Nakakita S, Sumiyoshi W, Hase S. Structural analysis of N-glycans of the planarian Dugesia japonica. FEBS J. 2011;278:452–460. doi: 10.1111/j.1742-4658.2010.07966.x. [DOI] [PubMed] [Google Scholar]
- Nguyen K, van Die I, Grundahl KM, Kawar ZS, Cummings RD. Molecular cloning and characterization of the Caenorhabditis elegans α1,3-fucosyltransferase family. Glycobiology. 2007;17:586–599. doi: 10.1093/glycob/cwm023. [DOI] [PubMed] [Google Scholar]
- Olafson RW, Thomas JR, Ferguson MAJ, Dwek RA, Chaudhuri M, Chang KP, Rademacher TW. Structures of the N-linked oligosaccharides of Gp63, the major surface glycoprotein, from Leishmania mexicana amazonensis. J Biol Chem. 1990;265:12240–12247. [PubMed] [Google Scholar]
- Palmberger D, Rendic D, Tauber P, Krammer F, Wilson IBH, Grabherr R. Insect cells for antibody production: evaluation of an efficient alternative. J Biotechnol. 2011;153:160–166. doi: 10.1016/j.jbiotec.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Pang TL, Wu CJ, Chen PA, Weng YL, Chen MY. Dictyostelium gnt15 encodes a protein with similarity to LARGE and plays an essential role in development. Biochem Biophys Res Commun. 2007;360:83–89. doi: 10.1016/j.bbrc.2007.06.016. [DOI] [PubMed] [Google Scholar]
- Parodi AJ, Quesada Allue LA, Cazzulo JJ. Pathway of protein glycosylation in the trypanosomatid Crithidia fasciculata. Proc Natl Acad Sci U S A. 1981;78:6201–6205. doi: 10.1073/pnas.78.10.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parodi AJ, Lederkremer GZ, Mendelzon DH. Protein glycosylation in Trypanosoma cruzi. The mechanism of glycosylation and structure of protein-bound oligosaccharides. J Biol Chem. 1983;258:5589–5595. [PubMed] [Google Scholar]
- Parodi AJ, Martin-Barrientos J, Engel JC. Glycoprotein assembly in Leishmania mexicana. Biochem Biophys Res Commun. 1984;118:1–7. doi: 10.1016/0006-291x(84)91058-1. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Rendić D, Lochnit G, Jantsch V, Wilson IBH. Molecular basis of anti-horseradish peroxidase staining in Caenorhabditis elegans. J. Biol. Chem. 2004;279:49588–49598. doi: 10.1074/jbc.M408978200. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Staudacher E, Stemmer U, Fabini G, Wilson IBH. Fucosyltransferase substrate specificity and the order of fucosylation in invertebrates. Glycobiology. 2005;15:463–474. doi: 10.1093/glycob/cwi028. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Hackl M, Gutternigg M, Kretschmer-Lubich D, Stemmer U, Jantsch V, Lochnit G, Wilson IBH. A Deletion in the Golgi α-Mannosidase II Gene of Caenorhabditis elegans Results in Unexpected Non-wild-type N-Glycan Structures. J Biol Chem. 2006;281:28265–28277. doi: 10.1074/jbc.M602878200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Gutternigg M, Rendić D, Wilson IBH. The N-glycosylation pattern of Caenorhabditis elegans. Carbohydr Res. 2008;343:2041–2049. doi: 10.1016/j.carres.2007.12.018. [DOI] [PubMed] [Google Scholar]
- Paschinger K, Razzazi-Fazeli E, Furukawa K, Wilson IBH. Presence of galactosylated core fucose on N-glycans in the planaria Dugesia japonica. J Mass Spectrom. 2011;46:561–567. doi: 10.1002/jms.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Hykollari A, Razzazi-Fazeli E, Greenwell P, Leitsch D, Walochnik J, Wilson IBH. The N-glycans of Trichomonas vaginalis contain variable core and antennal modifications. Glycobiology. 2012a;22:300–313. doi: 10.1093/glycob/cwr149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschinger K, Gonzalez-Sapienza GG, Wilson IBH. Mass spectrometric analysis of the immunodominant glycan epitope of Echinococcus granulosus antigen Ag5. Int J Parasitol. 2012b doi: 10.1016/j.ijpara.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pöltl G, Kerner D, Paschinger K, Wilson IBH. N-glycans of the porcine nematode parasite Ascaris suum are modified with phosphorylcholine and core fucose residues. FEBS J. 2007;274:714–726. doi: 10.1111/j.1742-4658.2006.05615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puanglarp N, Oxley D, Currie GJ, Bacic A, Craik DJ, Yellowlees D. Structure of the N-linked oligosaccharides from tridacnin, a lectin found in the haemolymph of the giant clam Hippopus hippopus. Eur J Biochem. 1995;232:873–880. [PubMed] [Google Scholar]
- Qian Y, West CM, Kornfeld S. UDP-GlcNAc:Glycoprotein N-acetylglucosamine-1-phosphotransferase mediates the initial step in the formation of the methylphosphomannosyl residues on the high mannose oligosaccharides of Dictyostelium discoideum glycoproteins. Biochem Biophys Res Commun. 2010;393:678–681. doi: 10.1016/j.bbrc.2010.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner DM, Cui J, Steffen M, Moore LL, Robbins PW, Samuelson J. Changes in the N-glycome, glycoproteins with Asn-linked glycans, of Giardia lamblia with differentiation from trophozoites to cysts. Eukaryot Cell. 2008;7:1930–1940. doi: 10.1128/EC.00268-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reason AJ, Ellis LA, Appleton JA, Wisnewski N, Grieve RB, McNeil M, Wassom DL, Morris HR, Dell A. Novel tyvelose-containing tri- and tetra-antennary N-glycans in the immunodominant antigens of the intracellular parasite Trichinella spiralis. Glycobiology. 1994;4:593–604. doi: 10.1093/glycob/4.5.593. [DOI] [PubMed] [Google Scholar]
- Rendić D, Linder A, Paschinger K, Borth N, Wilson IBH, Fabini G. Modulation of neural carbohydrate epitope expression in Drosophila melanogaster cells. J. Biol. Chem. 2006;281:3343–3353. doi: 10.1074/jbc.M508334200. [DOI] [PubMed] [Google Scholar]
- Rendić D, Wilson IBH, Paschinger K. The glycosylation capacity of insect cells. Croatica Chimica Acta. 2008;8:7–21. [Google Scholar]
- Riley GR, West CM, Henderson EJ. Cell differentiation in Dictyostelium discoideum controls assembly of protein-linked glycans. Glycobiology. 1993;3:165–177. doi: 10.1093/glycob/3.2.165. [DOI] [PubMed] [Google Scholar]
- Samuelson J, Banerjee S, Magnelli P, Cui J, Kelleher DJ, Gilmore R, Robbins PW. The diversity of dolichol-linked precursors to Asn-linked glycans likely results from secondary loss of sets of glycosyltransferases. Proc Natl Acad Sci U S A. 2005;102:1548–1553. doi: 10.1073/pnas.0409460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saudan P, Hauck K, Soller M, Choffat Y, Ottiger M, Spörri M, Ding Z, Hess D, Gehrig PM, Klauser S, Hunziker P, Kubli E. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur J Biochem. 2002;269:989–997. doi: 10.1046/j.0014-2956.2001.02733.x. [DOI] [PubMed] [Google Scholar]
- Schachter H. Biosynthetic controls that determine the branching and microheterogeneity of protein-bound oligosaccharides. Biochem Cell Biol. 1986;64:163–181. doi: 10.1139/o86-026. [DOI] [PubMed] [Google Scholar]
- Schachter H. Paucimannose N-glycans in Caenorhabditis elegans and Drosophila melanogaster. Carbohydr Res. 2009;344:1391–1396. doi: 10.1016/j.carres.2009.04.028. [DOI] [PubMed] [Google Scholar]
- Schiller B, Hykollari A, Voglmeir J, Pöltl G, Hummel K, Razzazi-Fazeli E, Geyer R, Wilson IBH. Development of Dictyostelium discoideum is associated with alteration of fucosylated N-glycan structures. Biochem J. 2009;423:41–52. doi: 10.1042/BJ20090786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey DJ, Kornfeld R. Developmental regulation of processing α-mannosidases and “intersecting” N-acetylglucosaminyltransferase in Dictyostelium discoideum. J Biol Chem. 1991a;266:18477–18484. [PubMed] [Google Scholar]
- Sharkey DJ, Kornfeld R. Developmental regulation of asparagine-linked oligosaccharide synthesis in Dictyostelium discoideum. J Biol Chem. 1991b;266:18485–18497. [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewis X antigen. J Biol Chem. 1992;267:20196–20203. [PubMed] [Google Scholar]
- Staudacher E, Altmann F, März L, Hård K, Kamerling JP, Vliegenthart JFG. α1-6(α1-3)-Difucosylation of the asparagine-bound N-acetylglucosamine in honeybee venom phospholipase A2. Glycoconjugate J. 1992;9:82–85. doi: 10.1007/BF00731703. [DOI] [PubMed] [Google Scholar]
- Struwe WB, Reinhold VN. The Conserved Oligomeric Golgi (COG) Complex is Required for Fucosylation of N-Glycans in C. elegans. Glycobiology. 2012 doi: 10.1093/glycob/cws053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm A, Bergwerff AA, Vliegenthart JFG. 1H-NMR structural determination of the N-linked carbohydrate chains on glycopeptides obtained from the bean lectin phytohemagglutinin. Eur J Biochem. 1992;204:313–316. doi: 10.1111/j.1432-1033.1992.tb16639.x. [DOI] [PubMed] [Google Scholar]
- Sucgang R, Kuo A, Tian X, Salerno W, Parikh A, Feasley CL, Dalin E, Tu H, Huang E, Barry K, Lindquist E, Shapiro H, Bruce D, Schmutz J, Salamov A, Fey P, Gaudet P, Anjard C, Babu MM, Basu S, Bushmanova Y, van der Wel H, Katoh-Kurasawa M, Dinh C, Coutinho PM, Saito T, Elias M, Schaap P, Kay RR, Henrissat B, Eichinger L, Rivero F, Putnam NH, West CM, Loomis WF, Chisholm RL, Shaulsky G, Strassmann JE, Queller DC, Kuspa A, Grigoriev IV. Comparative genomics of the social amoebae Dictyostelium discoideum and Dictyostelium purpureum. Genome Biol. 2010;12:R20. doi: 10.1186/gb-2011-12-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Masuda K, Hiraki K, Yoshihara K, Huang H-H, Khoo K-H, Kato K. N-glycan structures of squid rhodopsin. Existence of the α1-3 and α1-6 difucosylated innermost GlcNAc residue in a molluscan glycoprotein. Eur. J. Biochem. 2004;270:2627–2632. doi: 10.1046/j.1432-1033.2003.03636.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Hayama K, Hirabayashi J, Kasai K. Caenorhabditis elegans N-glycans containing a Gal-Fuc disaccharide unit linked to the innermost GlcNAc residue are recognized by C. elegans galectin LEC-6. Glycobiology. 2008;18:882–890. doi: 10.1093/glycob/cwn077. [DOI] [PubMed] [Google Scholar]
- Titz A, Butschi A, Henrissat B, Fan YY, Hennet T, Razzazi-Fazeli E, Hengartner MO, Wilson IBH, Künzler M, Aebi M. Molecular basis for galactosylation of core fucose residues in invertebrates: Identification of Caenorhabditis elegans N-glycan core α1,6-fucoside β1,4-galactosyltransferase GALT-1 as a member of a novel glycosyltransferase family. J Biol Chem. 2009;284:36223–36233. doi: 10.1074/jbc.M109.058354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Die I, Gomord V, Kooyman FNJ, van der Berg TK, Cummings RD, Vervelde L. Core α1→3-fucose is a common modifcation of N-glycans in parasitic helminths and constitutes an important epitope for IgE from Haemonchus contortus infected sheep. FEBS Lett. 1999;463:189–193. doi: 10.1016/s0014-5793(99)01508-2. [DOI] [PubMed] [Google Scholar]
- van Kuik JA, van Halbeek H, Kamerling J,P, Vliegenthart JFG. Primary structure of the low-molecular-weight carbohydrate chains of Helix pomatia α-hemocyanin. Xylose as a constituent of N-linked oligosaccharides in an animal glycoprotein. J Biol Chem. 1985;260:13984–13988. [PubMed] [Google Scholar]
- Van Kuik JA, Van Halbeek H, Kamerling JP, Vliegenthart JF. Primary structure of the neutral carbohydrate chains of hemocyanin from Panulirus interruptus. Eur J Biochem. 1986a;159:297–301. doi: 10.1111/j.1432-1033.1986.tb09867.x. [DOI] [PubMed] [Google Scholar]
- van Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JFG, Wood EJ. Primary structure of a low-molecular-mass N-linked oligosaccharide from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-mannose as a constituent of the xylose-containing core structure in an animal glycoprotein. Eur. J. Biochem. 1986b;160:621–625. doi: 10.1111/j.1432-1033.1986.tb10083.x. [DOI] [PubMed] [Google Scholar]
- Van Kuik JA, Sijbesma RP, Kamerling JP, Vliegenthart JF, Wood EJ. Primary structure determination of seven novel N-linked carbohydrate chains derived from hemocyanin of Lymnaea stagnalis. 3-O-methyl-D-galactose and N-acetyl-D-galactosamine as constituents of xylose-containing N-linked oligosaccharides in an animal glycoprotein. Eur J Biochem. 1987;169:399–411. doi: 10.1111/j.1432-1033.1987.tb13626.x. [DOI] [PubMed] [Google Scholar]
- Velkova L, Dolashka P, Lieb B, Dolashki A, Voelter W, Van Beeumen J, Devreese B. Glycan structures of the structural subunit (HtH1) of Haliotis tuberculata hemocyanin. Glycoconj J. 2011;28:385–395. doi: 10.1007/s10719-011-9337-2. [DOI] [PubMed] [Google Scholar]
- Warren CE, Krizius A, Roy PJ, Culotti JG, Dennis JW. The C. elegans gene, gly-2, can rescue the N-acetylglucosaminyltransferase V mutation of Lec4 cells. J. Biol. Chem. 2002;277:22829–22838. doi: 10.1074/jbc.M201390200. [DOI] [PubMed] [Google Scholar]
- West CM, van der Wel H, Coutinho PM, Henrissat B. Glycosyltransferase Genomics. In: Loomis WF, Kuspa A, editors. Dictyostelium Genomics. Horizon Bioscience; 2005. pp. 235–264. [Google Scholar]
- Williams PJ, Wormald MR, Dwek RA, Rademacher TW, Parker GF, Roberts DR. Characterisation of oligosaccharides from Drosophila melanogaster glycoproteins. Biochim Biophys Acta Gen Subj. 1991;1075:146–153. doi: 10.1016/0304-4165(91)90245-c. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Robijn ML, Koeleman CA, Balog CI, Geyer R, Deelder AM, Hokke CH. A novel Gal(β1-4)Gal(β1-4)Fuc(α1-6)-core modification attached to the proximal N-acetylglucosamine of keyhole limpet haemocyanin (KLH) N-glycans. Biochem J. 2004;378:625–632. doi: 10.1042/BJ20031380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Balog CI, Catalina MI, Jones FM, Schramm G, Haas H, Doenhoff MJ, Dunne DW, Deelder AM, Hokke CH. IPSE/alpha-1, a major secretory glycoprotein antigen from schistosome eggs, expresses the Lewis X motif on coredifucosylated N-glycans. FEBS J. 2006a;273:2276–2292. doi: 10.1111/j.1742-4658.2006.05242.x. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CA, Deelder AM, Hokke CH. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006b;273:347–361. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- Yagi H, Nakagawa M, Takahashi N, Kondo S, Matsubara M, Kato K. Neural complex-specific expression of xylosyl N-glycan in Ciona intestinalis. Glycobiology. 2008;18:145–151. doi: 10.1093/glycob/cwm128. [DOI] [PubMed] [Google Scholar]
- Zamze SE, Wooten EW, Ashford DA, Ferguson MAJ, Dwek RA, Rademacher TW. Characterisation of the asparagine-linked oligosaccharides from Trypanosoma brucei type-I variant surface glycoproteins. Eur J Biochem. 1990;187:657–663. doi: 10.1111/j.1432-1033.1990.tb15350.x. [DOI] [PubMed] [Google Scholar]
- Zamze SE, Ashford DA, Wooten EW, Rademacher TW, Dwek RA. Structural characterization of the asparagine-linked oligosaccharides from Trypanosoma brucei type II and type III variant surface glycoproteins. J Biol Chem. 1991;266:20244–20261. [PubMed] [Google Scholar]
- Zhang Y, Iwasa T, Tsuda M, Kobata A, Takasaki S. A novel monoantennary complex-type sugar chain found in octopus rhodopsin: occurrence of the Galβ1→4Fuc group linked to the proximal N-acetylglucosamine residue of the trimannosyl core. Glycobiology. 1997;7:1153–1158. doi: 10.1093/glycob/7.8.1153. [DOI] [PubMed] [Google Scholar]
- Zhu S, Hanneman A, Reinhold V, Spence A, Schachter H. Caenorhabditis elegans triple null mutant lacking UDP-N-acetyl-D-glucosamine:α-3-D-mannoside β1,2 N-acetylglucosaminyltransferase I. Biochem. J. 2004;382:995–1001. doi: 10.1042/BJ20040793. [DOI] [PMC free article] [PubMed] [Google Scholar]