Abstract
The National Lung Screening Trial (NLST) has provided compelling evidence of the efficacy of lung cancer screening using low-dose helical computed tomography (LDCT) to reduce lung cancer mortality. The NLST randomized 53,454 older current or former heavy smokers to receive LDCT or chest radiography (CXR) for three annual screens. Participants were observed for a median of 6.5 years for outcomes. Vital status was available in more than 95% of participants. LDCT was positive in 24.2% of screens, compared with 6.9% of CXRs; more than 95% of all positive LDCT screens were not associated with lung cancer. LDCT detected more than twice the number of early-stage lung cancers and resulted in a stage shift from advanced to early-stage disease. Complications of LDCT screening were minimal. Lung cancer–specific mortality was reduced by 20% relative to CXR; all-cause mortality was reduced by 6.7%. The major harms of LDCT are radiation exposure, high false-positive rates, and the potential for overdiagnosis. This review discusses the risks and benefits of LDCT screening as well as an approach to LDCT implementation that incorporates systematic screening practice with smoking cessation programs and offers opportunities for better determination of appropriate risk cohorts for screening and for better diagnostic prediction of lung cancer in the setting of screen-detected nodules. The challenges of implementation are considered for screening programs, for primary care clinicians, and across socioeconomic strata. Considerations for future research to complement imaging-based screening to reduce the burden of lung cancer are discussed.
INTRODUCTION
An effective test for early lung cancer detection has been an elusive goal for decades, despite the reality that lung cancer is the leading cause of cancer death worldwide. Efforts to address this void have been hampered by social, medical, and economic realities. Among these are the fact that lung cancer, a predominantly smoking-related disease, is often considered to be self-inflicted and remedied by smoking abstinence, although more than half of those who develop lung cancer are former smokers. High mortality rates among lung cancer victims have compromised advocacy efforts. Finally, federal funding for lung cancer research is dwarfed by funding for other major cancers, despite the dominance of lung cancer as the major contributor to cancer-related mortality.1,2
Early randomized screening trials that assessed combinations of chest radiography (CXR) and sputum cytology were inconclusive in showing a mortality benefit from screening.3–8 It has only been within the last decade with technologic advances in computed tomography (CT) that imaging-based screening became the focus of intense investigation. Multidetector helical CT enables the entire lung to be imaged as a single volume within one breath hold. Because of the inherently high contrast between aerated lung and soft tissue, low radiation dose preserves the detection of focal lung lesions despite higher image noise.
The results of several single-arm studies have provided valuable information on the performance characteristics of low-dose helical CT (LDCT). Studies have varied by cohort characteristics, numbers of screening rounds, interpretation criteria for a positive screen, and whether LDCT was performed alone or in conjunction with CXR.9–16 These differences partially account for significant differences in screen positivity rates, ranging from 5.1% to 51.4%. Nonetheless, these initial investigations indicate that LDCT screening increases the detection of lung nodules as well as early-stage lung cancers relative to CXR and historical epidemiologic rates and that the size of detected lesions on LDCT is generally smaller than with CXR. None of the studies was designed to address the effects of LDCT screening on lung cancer mortality.
NATIONAL LUNG SCREENING TRIAL DESIGN AND FINDINGS
It was against this backdrop that the National Lung Screening Trial (NLST) was designed. The NLST combined a contract administered by the Division of Cancer Prevention, National Cancer Institute with a grant administered by the American College of Radiology Imaging Network, funded by the Division of Cancer Treatment and Diagnosis, National Cancer Institute.17 Across 33 sites, the NLST enrolled 53,454 current or former smokers based on eligibility criteria of age 55 to 74 years and current or previous smoking history of a minimum of 30 pack-years (product of packs of cigarettes smoked daily and years of smoking). Former smokers had to have quit within the preceding 15 years. Relative to the US population that would have been eligible for the trial based on age and smoking status, NLST participants had comparable sex proportions and smoking intensity as measured by median pack-years of smoking. However, the NLST cohort tended to be younger, better educated, and more frequently former smokers than the comparable US eligible population, which made them slightly healthier overall (Table 1).18
Table 1.
Comparison of NLST Cohort to the Eligible US Population18
| Demographic or Characteristic | % of Population |
|
|---|---|---|
| NLST Population (N = 53,454) | Eligible US Population | |
| Male | 59.0 | 58.5 |
| Smoking | ||
| Median pack years | 48.0 | 47.0 |
| Former smoker | 51.8 | 42.9 |
| Age, years | ||
| 55-59 | 42.8 | 35.2 |
| 60-64 | 30.6 | 29.3 |
| 65-69 | 17.8 | 20.8 |
| 70-74 | 8.8 | 14.7 |
| Education | ||
| ≥ College | 31.5 | 14.4 |
| < High school | 6.1 | 21.3 |
| Race/ethnicity | ||
| Black | 4.4 | 5.5 |
| Hispanic | 1.7 | 2.4 |
Abbreviation: NLST, National Lung Screening Trial.
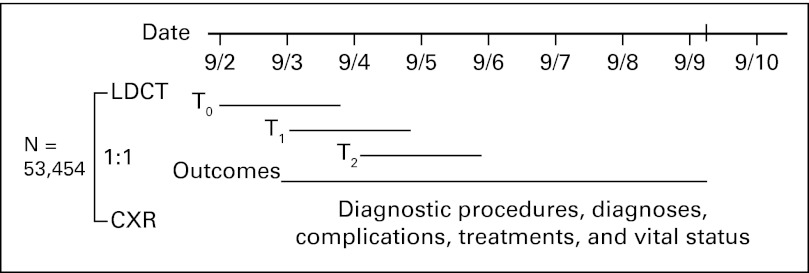
Participants were randomly assigned to receive either LDCT or CXR annually for three screens. Follow-up continued through December 31, 2009, for a median of 6.5 years. Diagnostic procedures, diagnoses, treatments, and outcomes were collected by manual abstraction of medical records on participants with positive screens and those with lung cancer diagnoses (Fig 1). Vital status was ascertained at least annually with confirmation by death certificates or query of the National Death Index.19
Fig 1.
National Lung Screening Trial design. The trial was launched in August 2002. Over 20 months, 53,454 individuals were randomly assigned to receive either low-dose computed tomography (LDCT) or chest x-ray (CXR) for three annual screens. Outcomes through December 31, 2009, were collected on participants, an average of 6.5 years.
LDCT screening tests were considered positive and potentially related to lung cancer if they revealed at least one noncalcified nodule ≥ 4 mm in longest diameter (or other abnormality suspicious for lung cancer), and CXR screens were positive if they revealed any noncalcified nodule or mass. A recommendation for some form of additional follow-up was made for all positive screens; diagnostic guidelines for positive screens based on nodule size and consistency were developed trial-wide, but were not mandated and could be used at the discretion of the interpreting radiologist (Appendix Fig A1, online only). Overall, 24.2% of CT screens and 6.9% of CXR screens were positive.19 Participants who received all three screens had a 39% rate of screen positivity. Screen positivity rates decreased at the T2 screen, largely because indeterminate nodules that were stable over all three screens could be considered negative at the discretion of the radiologist (Table 2). The results of diagnostic follow-up have been previously reported; however, complications of diagnostic follow-up were low overall and were very low in the participants with positive screens in whom no lung cancer was diagnosed.19
Table 2.
Screening Results and Screen-Detected Lung Cancers by Screening Arm and Round19
| Screening Round | Total Population Screened (No.) |
Positive Screens |
Screen-Detected Lung Cancers |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LDCT Arm |
CXR Arm |
LDCT Arm |
CXR Arm |
|||||||
| LDCT Arm | CXR Arm | No. | % | No. | % | No. | % Positive Screens | No. | % Positive Screens | |
| T0 | 26,309 | 26,035 | 7,191 | 27.3 | 2,387 | 9.2 | 270 | 3.8 | 136 | 5.7 |
| T1 | 24,715 | 24,089 | 6,901 | 27.9 | 1,482 | 6.2 | 168 | 2.4 | 65 | 4.4 |
| T2 | 24,102 | 23,346 | 4,054 | 16.8 | 1,174 | 5 | 211 | 5.2 | 78 | 6.6 |
| Total | 75,126 | 73,470 | 18,146 | 24.2 | 5,043 | 6.9 | 649 | 3.6 | 279 | 5.5 |
NOTE. Excludes inadequate screens (LDCT, n = 7; CXR, n = 26).
Abbreviations: CXR, chest radiograph; LDCT, low-dose computed tomography.
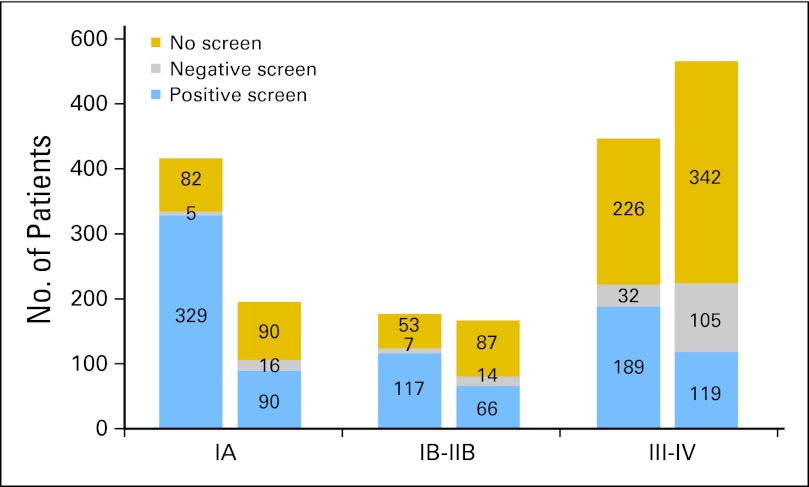
Lung cancer stage through the entire period of observation was analyzed and included lung cancers occurring in the setting of a positive screen, lung cancers after a negative screen, and lung cancers diagnosed with no screening (Fig 2). Ninety percent of lung cancer diagnoses in the setting of no screen occurred in the postscreening surveillance period. LDCT detected more lung cancers than CXR. Among lung cancers of known stage, stage IA cancers were more than two-fold greater in the LDCT arm, most of which were screen detected. There were also fewer stage III and IV lung cancers in the LDCT arm compared with the CXR arm. Finally, in both arms, the majority of lung cancers observed after negative screens were advanced, supporting a more aggressive biology of these lesions.
Fig 2.
Stage of lung cancers in the two screening arms based on result of screening. Lung cancers are shown as early-stage IA, intermediate-stage IB to IIB, and late-stage III to IV. In each arm, the stages are displayed by screen result, in which lung cancers were diagnosed after a positive screen, after a negative screen, or in participants who received no screen. Ninety percent of lung cancer diagnoses occurring with no screen occurred in the postscreening surveillance period. Lung cancers of unknown stage (n = 32) are excluded.
Lung cancer–specific mortality rates were 247 per 100,000 person-years and 309 per 100,000 person-years in the LDCT and CXR arms, respectively. This resulted in a 20% relative reduction in lung cancer mortality in the LDCT arm (95% CI, 6.8% to 26.7%) and an absolute risk reduction of lung cancer death by four per 1,000 individuals screened. Finally, there was a 6.7% reduction in all-cause mortality (95% CI, 1.2% to 13.6%) in the LDCT arm relative to CT. This is the first randomized screening trial to have shown improvements in both disease-specific and all-cause mortality, which indicates that screening resulted in no deleterious downstream effects that contributed to death and, importantly, that the reduced lung cancer mortality observed with LDCT did not result in deaths from competing causes such as cardiovascular disease. Given these data, an estimated 320 individuals needed to be screened to save one life from lung cancer.19 This compares favorably with screening mammography, in which some estimates suggest that 465 to 601 women must be screened to save one life.20,21
Overall, the NLST demonstrated the following: more lung cancers were detected with LDCT than with CXR; a stage shift was observed with LDCT, such that the absolute number of advanced-stage cancers was decreased relative to CXR; there was a 20% relative mortality reduction with LDCT compared with CXR, amounting to an absolute risk reduction of four individuals per 1,000 screened; there were few significant complications from LDCT screening; and a 6.7% reduction in all-cause mortality was observed with LDCT. The results of a formal cost-effectiveness evaluation of LDCT relative to CXR in the NLST are pending, but preliminary results based on available data suggest that LDCT screening could be cost effective if implemented in a fashion similar to the NLST.22
SCREENING HARMS
The benefits of screening must be reconciled with its potential harms, which are primarily related to radiation-induced carcinogenesis, high false-positive rates, and the potential for overdiagnosis. LDCT screening exposes individuals to excess radiation, not only at the time of screening, but also in the course of downstream follow-up and likely at consecutive intervals given that screening would be performed on a periodic basis. Although individual risk may be acceptable, the large number of individuals who might be exposed could translate into measurable population increases in radiation-induced cancers.21 This seems to be particularly true for LDCT screening and lung cancer risk; unlike cancers in other solid organs, in which radiation risks are highest at younger age, excess risk of radiation-induced lung cancer is highest in middle age, peaking at roughly age 55 years.23,24
Among the challenges of LDCT screening is effectively communicating radiation risk in ways that are understandable to both referring physicians and potential screenees. Effective dose is used to estimate participant dose that can be compared with other medical radiation procedures and depends heavily on the acquisition parameters used to acquire the scans as well as where the radiation dose is being absorbed. Conservative estimates of effective dose from the NLST based on representative imaging protocols for average-size participants were 1.6 mSv for men and 2.1 mSv for women, with sex differences primarily a result of breast dose in women.25 This can be compared with estimates of annual population radiation dose from all sources, averaging 3 mSv at sea level.
Whereas effective dose enables comparisons across different types of medical radiation, radiation risk of carcinogenesis is based on organ-specific doses and individual organ susceptibility to radiation. Using slightly higher estimates of dose than were reported in the NLST, Brenner24 used dose, sex, age, and smoking status to calculate excess relative risks of lung cancer among individuals who undergo annual LDCT screening from age 50 to 75 years. In smoking females, such annual LDCT screening conferred an excess radiation-related risk of 0.85% (95% CI, 0.28% to 2.2%) for developing lung cancer, in addition to the population-based expected risk of 17%, amounting to a 5% increase in risk. In male smokers, the estimated radiation-related risk was 0.23% (95% CI, 0.06% to 0.63%) in addition to the expected risk of 16%,24 a 1.5% increase in risk. These risks likely overestimate radiation risk because of the contemporary use of lower exposures and new image reconstruction algorithms that require less radiation to generate diagnostic images. Finally, the 20% relative mortality reduction observed with LDCT screening in high-risk populations offsets this risk.
A second major harm of LDCT in the NLST was a high screen positivity rate, particularly a high false-positive rate. The performance characteristics of a screening test are influenced by the risk profile (likelihood of the index disease) of the screening cohort. Although the NLST targeted high-risk individuals based on older age and significant smoking history (defined by pack-years of smoking), a number of other variables influence risk of lung cancer among ever-smokers. These variables include underlying chronic obstructive lung disease, occupational exposure to asbestos or other carcinogen, history of lung cancer in a first-degree relative, and prior history of lung (or other smoking-related) cancer. Combinations of these features have been proposed to determine eligibility in screening settings to increase the proportion of screen cohorts who will develop lung cancer.26,27
Screening test performance is also influenced by the interpretation criteria. The interpretation algorithm used in the NLST was dichotomous. LDCT screens were positive in the context of an indeterminate nodule of a minimum of 4 mm in diameter or other abnormality suspicious for lung cancer. Using this algorithm, 24.2% of all CT screens were positive; of positive LDCT screens, only 3.6% were associated with a lung cancer diagnosis trial-wide, representing a positive predictive value of less than 4%. Although there were few medical complications associated with the downstream diagnostic evaluation for these positive screens, there would be substantial gains in cost-effectiveness by improving the performance characteristics of LDCT screening.19
The Dutch–Belgian randomized lung cancer screening trial (Nederlands-Leuvens Longkanker Screenings Onderzoek [NELSON]) has used a two-step interpretation strategy based on nodule size to classify screens as positive or negative.28 Although the results of NELSON are not yet available, the investigators have reported the performance characteristics of their interpretation strategy. At prevalence screens, nodules less than 50 μL (5 mm in diameter) were negative, nodules greater than 500 μL (10 mm in diameter) were considered positive and underwent definitive evaluation, and nodules ranging from 5 to 10 mm in diameter were classified as indeterminate and reassessed with LDCT at 3 months.28 Nodule growth based on volumetric analysis at the follow-up scan was used to determine whether the scan was classified as negative or positive. Using a comparable strategy at incidence screening, the prevalence and incidence screens achieved sensitivities of 94.6% and 96.4%, specificities of 98.3% and 99.0%, positive predictive values of 35.7% and 42.2%, and negative predictive values of 99.9% and 99.9%, respectively.28 Medical resource utilization was comparable between the NLST and NELSON trials for subcentimeter nodules, because most NLST participants with subcentimeter nodules were also evaluated by follow-up imaging. However, beyond substantially improving the positive predictive value of a positive screen, the two-step process used by the NELSON provides interpretations that are much more representative of the true risk of lung cancer in screenees. This has implications for both the individual and the primary physicians who assist with the management of screened individuals. Finally, the NELSON appropriately conveys the notion that lung cancer screening is a process over time rather than a single examination.
A final potential harm of LDCT screening is overdiagnosis, meaning the diagnosis of a cancer that would not go on to cause symptoms or death. Overdiagnosis in lung cancer may result from one of following two scenarios: the cancer is so biologically indolent that it will not result in the death of the individual, or the cancer is treated or progresses sufficiently slowly that the individual dies of competing conditions such as cardiovascular or respiratory disease. Overdiagnosis results from the biologically heterogeneous behavior of cancer and is increasingly a consideration in screening programs in which cancers are diagnosed before signs or symptoms of disease. Autopsy studies have provided compelling evidence of overdiagnosis by observing clinically silent cancers of lung, prostate, and thyroid in individuals who have died of other causes.29–32 Estimates from randomized trials also suggest that a proportion of screen-detected cancers represent overdiagnosis. Estimates are based on the observation that invariably the screened arm detects more cancers than the control arm as a result of lead time. In the absence of overdiagnosis, once screening concludes, cancer diagnoses should catch up in the control arm. Persistent excess cancers in the screening arm provide compelling evidence of overdiagnosis and have been observed in a number of trials.33
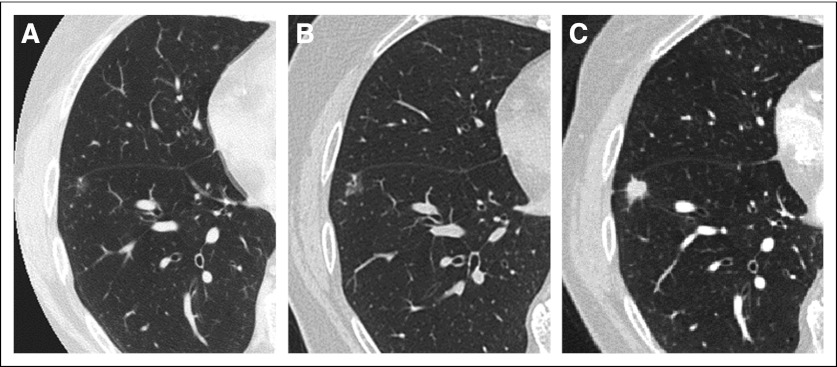
Complicating the notion of overdiagnosis in lung cancer is the anecdotal observation of an indolent lung lesion followed over several years, only to observe its transition to an aggressive phenotype34 (Fig 3). The logic in observing a ground-glass nodule over time to assess for morphologic changes relies on the predictability of a relatively linear growth model. However, in some instances, these lesions persist for years without significant evolution and then become aggressive with invasive or metastatic potential. This behavior may result from the acquisition of one critical molecular abnormality that changes the biology of cancer from indolent to aggressive. Unfortunately, there are no current mechanisms by which to distinguish the timing of this transformation.
Fig 3.
Evolution of a ground-glass nodule to invasive adenocarcinoma. (A) Initial axial high-resolution computed tomography (CT) shows a 6-mm ground-glass nodule in the right lower lobe. (B) Follow-up high-resolution CT after 2.5 years shows a slight increase in size of the ground-glass nodule. (C) Final high-resolution CT at 8 years from initial detection demonstrates evolution of the nodule to an 8-mm solid nodule. At biopsy, this was an invasive adenocarcinoma.
FROM EFFICACY TO EFFECTIVENESS
Establishing the evidence base for the benefits of LDCT screening over risks in a randomized controlled trial was a necessary but preliminary first step toward implementation. On the basis of the NLST, some medical organizations, including the National Comprehensive Cancer Network, published guidelines for LDCT screening, and several other medical organizations have followed suit.26,35 Indeed, a few of the major insurance providers now pay for LDCT screening in high-risk individuals. Nonetheless, it is estimated to take an average of 17 years for only 14% of new scientific discoveries to enter mainstream practice.36 The widespread implementation of LDCT screening for lung cancer will impose significant new challenges, which can be considered to involve the following three major categories of stakeholders in lung cancer screening: the transdisciplinary teams of imagers and clinicians who will offer comprehensive programs of lung cancer surveillance and prevention; the primary care physicians whose patients would most benefit from screening; and the at-risk population itself.
Transdisciplinary Screening Programs
The implementation of lung cancer screening requires considerable infrastructure support and organization. The ultimate goals are to improve the diagnosis and management of early-stage lung cancer when it is amenable to curative resection and to reduce lung cancer mortality. These goals are best served by coupling LDCT screening with proactive programs of smoking cessation and durable abstinence. The screening program is most robust when transdisciplinary, to include the imagers, thoracic surgeons, pulmonologists, and pathologists who will manage indeterminate nodules. The screening program must also be tightly interleaved with institutional lung cancer treatment programs.
For the imaging community, LDCT screening will require substantial modifications to workflow as well as infrastructure: multidetector CT scanners that can acquire high-quality images at minimum radiation dose; physicists and staff who can certify equipment and perform studies according to a consistent standard; experienced radiologists who will use standardized interpretation, communication, and diagnostic practices; and ongoing programs of image and clinical quality assurance. The programs must be able to track individual patients and their screening results and to easily recall individuals with positive screens for follow-up exams. There is growing evidence that computer-assisted diagnosis and nodule characterization software enables quantitative, more reproducible image interpretation. The introduction of computer-assisted diagnosis systems will necessarily alter historical workflow patterns and will only be practical if initial image analysis is performed by skilled staff or technologists who then transmit results with the original images to the radiologist for final interpretation.
The Primary Care Clinic
Primary care providers will need to be convinced of the efficacy of lung cancer screening and that the benefits outweigh the risks. Provider education and their adoption of screening recommendations are influenced by a variety of factors, many of which derive from personal experience rather than the published evidence base, as well as their perceptions of blame.37–40 The primary care arena will necessarily require an understanding of which patients would best benefit from screening as well as the implications and management of indeterminate nodules. LDCT screening will almost certainly affect workflow in the primary setting, as clinicians incorporate into practice the education of patients about LDCT screening and implications of indeterminate nodules, as well as options for managing abnormal findings. Finally, the primary care clinician may be challenged to allocate already limited time and resources for lung cancer screening that could detract from other proven health maintenance activities. One justifiable concern with LDCT is the high screen positivity rate. Although positive screens decrease after the baseline exam, the screening programs themselves will need to assume mutual responsibility for communicating with patients and managing follow-up.
Community Engagement
Among the most challenging aspects of lung cancer screening implementation will be adoption by the community at risk. Psychological, cultural, economic, and geographic variables all play major roles in community adoption, more so than with other screening interventions. Specifically, lung cancer is a largely preventable disease. There is a prevailing stigma associated with smokers and patients with lung cancer, the subtext of which is that they are responsible for their condition. This cultivates a variety of responses, including fatalism and denial,41–46 attitudes that contribute to delays in presenting for medical evaluation when symptoms occur.47,48 Finally, there are significant, well-known differences in the understanding of smoking-related risks and lung cancer among different racial and sociodemographic groups. Individuals from disadvantaged backgrounds are more likely to have misperceptions about their individual risk of lung cancer, the benefits of surgical resection, and lung cancer mortality.49–53 Higher educational level, higher economic status, and white race are associated with greater understanding of the state of the science on smoking and lung cancer.54–56 These realities contribute to the epidemiologic observations that African Americans are more likely to present with advanced-stage disease, are less likely to consider surgery for resectable disease, and, among men, experience higher lung cancer mortality rates than their white counterparts.2,54
The diffusion of lung cancer screening across all socioeconomic strata will require a multipronged approach in which information strategies are used to educate across demographic divides. Community organizations, churches, community health care providers, and partnerships with academic institutions will be key to providing bidirectional dialogue. The medical community must understand cultural and class misperceptions of smoking and lung cancer, the basis of mistrust of the medical profession, and competing community interests that could prevent lung cancer awareness from gaining traction as a health priority. Similarly, these collaborations serve to educate the public about lung cancer, reinforce the causal role of tobacco in lung cancer and other related diseases, and understand the risks and benefits of LDCT screening. Failure to take directed steps to provide screening across all economic strata threatens to relegate lung cancer to a disease of underserved populations.
OPTIMIZING RISK PROFILES FOR SCREENING
Screening effectiveness is enhanced by identifying the optimal risk group most likely to harbor preclinical lung cancer. Between 80% and 90% of lung cancers occur in tobacco smokers, yet only 10% to 15% of chronic smokers develop lung cancer.57,58 Relative to smokers with normal lung function, those with chronic obstructive pulmonary disease (COPD) have up to a six-fold increased risk of lung cancer, making COPD by far the greatest known risk factor for lung cancer in ever-smokers.59,60 Although COPD and lung cancer have smoking exposure in common, several lines of evidence now support underlying shared genetic susceptibility that acts in concert with the shared risk of smoking-related genetic and epigenetic effects. Genome association studies have identified several heritable susceptibility or protective loci thought to impact both COPD and lung cancer development, including single nucleotide polymorphisms on loci 15q25 that regulate cholinergic nicotine receptors (CHRNA3/5)60–62; several haplotypes involved in the xenobiotic metabolism of tobacco lung carcinogens63–66; and genes involved in cell cycle control, apoptosis, airway inflammation, and repair.58,67,68
Recently, investigators have established that emphysema, as determined on LDCT, is associated with lung cancer independent of airflow obstruction on spirometry.69 A separate study observed that the highest frequency of lung cancer was in patients with both CT-based emphysema and moderate to severe spirometric airflow obstruction.70 Airflow obstruction in COPD derives from the variable presence of emphysema (destruction of airspaces distal to terminal bronchioles) and obstructive bronchiolitis as a result of small airways thickening and remodeling. Spirometry is the mainstay in the diagnosis of airflow obstruction but does not distinguish between these two pathologic entities or their relative contributions to airflow obstruction in a given individual. There is considerable opportunity to more comprehensively identify the highest risk population by better phenotyping smokers based on combinations of clinical and spirometric features.
Beyond evidence of smoking-related lung injury, nodule phenotypes captured in the screening exam can also be exploited to stratify individuals with indeterminate nodules who require more aggressive diagnostic evaluation. A number of quantitative nodule features have been identified using image analysis that have the potential to increase diagnostic discrimination between benign and malignant nodules.71,72 Ideally, these multidimensional phenotypes will be validated and combined with molecular biomarkers to construct better models of risk for lung cancer as well as models for the diagnostic management of screen-detected lung nodules.
CONCLUSION
We are at the cusp of implementing an entirely new paradigm into the management of lung cancer by screening high-risk individuals for preclinical, potentially curable disease. Screening becomes substantially more effective when combined with readily accessible programs of smoking cessation. Moreover, there is synergy in combining screening and smoking cessation by exploiting individualized lung cancer risk profiles to motivate smoking cessation. There are multiple remaining questions surrounding lung cancer screening implementation, among which include how to better define the optimal risk cohort for screening, the frequency of screening in cohorts at different levels of risk, methods to improve diagnostic discrimination between lung cancer and benign disease in patients with CT-detected nodules, and the roles of biomolecular markers in stratifying risk and in guiding the management of indeterminate nodules. How we choose to translate our current knowledge base to practice can have substantial, long-lasting beneficial effects that are entirely within our capacity to sustain if we so choose.
Acknowledgment
Presented in part at the American Society of Clinical Oncology (ASCO)/American Society for Therapeutic Radiology and Oncology/International Association for the Study of Lung Cancer/University of Chicago Multidisciplinary Symposium in Thoracic Oncology, Chicago, IL, December 9-11, 2011; the 2011 Radiological Society of North America 97th Scientific Assembly and Annual Meeting, Chicago, IL, November 27-December 2, 2011; the 47th Annual Meeting of ASCO, Chicago, IL, June 1-5, 2011; the US Department of Veteran Affairs, Los Angeles, CA, March 7, 2012; the 30th Annual Meeting of the Society of Thoracic Radiology, Huntington Beach, CA, March 11-14, 2012; the University of Texas Southwestern Medical Center Meeting, Dallas, TX, March 24, 2012; the 103rd Annual Meeting of the American Association for Cancer Research, Chicago, IL, March 31-April 4, 2012; the Vanderbilt-Ingram Cancer Center Retreat on Lung Cancer, Nashville, TN, May 8, 2012; the American Roentgen Ray Society Chest Conference, Redondo Beach, CA, October 5-6, 2012; the 48th Annual Meeting of the Society of Thoracic Surgeons, Fort Lauderdale, FL, January 28-29, 2012; the European Society of Thoracic Imaging 2012 Annual Meeting, London, United Kingdom, June 22-24, 2012; and the 13th International Lung Cancer Congress, Huntington Beach, CA, July 19-22, 2012.
Appendix
Fig A1.
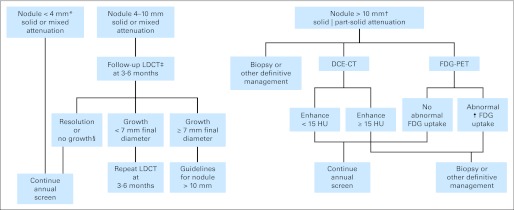
The original guidelines for the management of computed tomography (CT) –detected nodules in the National Lung Screening Trial. These guidelines were not mandated and could be used at the discretion of the interpreting radiologist. (*) Pure ground-glass nodules < 10 mm can be followed with low-dose helical CT (LDCT) at 6 to 12 months. At the final screen (T2), new nodules < 4 mm can be followed up at the discretion of the radiologist. (†) With lesions > 10 mm in diameter, institutional resources and preferences vary. (‡) The timing of repeat LDCT varies according to nodule size; larger nodules are followed up sooner than small nodules. (§) No growth is defined as < 15% increase in largest diameter or, with part-solid nodules, no increase in solid component. DCE-CT, dynamic contrast-enhanced computed tomography; FDG-PET, 18-fluorodeoxyglucose positron emission tomography; HU, Hounsfield unit.
Footnotes
Supported by the National Cancer Institute. The American College of Radiology Imaging Network component of the National Lung Screening Trial was funded through Grants No. U01-CA-80098 and U01-CA-79778 under a cooperative agreement with the Cancer Imaging Program, Division of Cancer Treatment and Diagnosis.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00047385
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Denise R. Aberle
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.National Institutes of Health: Estimates of funding for various research, condition, and disease categories (RCDC) http://www.report.nih.gov/categorical_spending.aspx.
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Melamed MR. Lung cancer screening results in the National Cancer Institute New York study. Cancer. 2000;89(suppl 11):2356–2362. doi: 10.1002/1097-0142(20001201)89:11+<2356::aid-cncr8>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- Tockman MS. Survival and mortality from lung cancer in a screened population: The Johns Hopkins Study. Chest. 1986;89(supp):324S–325S. [Google Scholar]
- 5.Fontana RS, Sanderson DR, Woolner LB, et al. Screening for lung cancer: A critique of the Mayo Lung Project. Cancer. 1991;67(suppl 4):1155–1164. doi: 10.1002/1097-0142(19910215)67:4+<1155::aid-cncr2820671509>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: Impact of extended follow-up. J Natl Cancer Inst. 2000;92:1308–1316. doi: 10.1093/jnci/92.16.1308. [DOI] [PubMed] [Google Scholar]
- 7.Kubík A, Polák J. Lung cancer detection: Results of a randomized prospective study in Czechoslovakia. Cancer. 1986;57:2427–2437. doi: 10.1002/1097-0142(19860615)57:12<2427::aid-cncr2820571230>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Kubík AK, Parkin DM, Zatloukal P. Czech study on lung cancer screening: Post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer. 2000;89(suppl 11):2363–2368. doi: 10.1002/1097-0142(20001201)89:11+<2363::aid-cncr9>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer. 2001;84:25–32. doi: 10.1054/bjoc.2000.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nawa T, Nakagawa T, Kusano S, et al. Lung cancer screening using low-dose spiral CT: Results of baseline and 1-year follow-up studies. Chest. 2002;122:15–20. doi: 10.1378/chest.122.1.15. [DOI] [PubMed] [Google Scholar]
- 11.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: Anti-lung cancer association project. J Clin Oncol. 2002;20:911–920. doi: 10.1200/JCO.2002.20.4.911. [DOI] [PubMed] [Google Scholar]
- 12.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early Lung Cancer Action Project: Overall design and findings from baseline screening. Lancet. 1999;354:99–105. doi: 10.1016/S0140-6736(99)06093-6. [DOI] [PubMed] [Google Scholar]
- 13.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early Lung Cancer Action Project: Initial findings on repeat screenings. Cancer. 2001;92:153–159. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med. 2002;165:508–513. doi: 10.1164/ajrccm.165.4.2107006. [DOI] [PubMed] [Google Scholar]
- 15.Swensen SJ, Jett JR, Hartman TE, et al. CT screening for lung cancer: Five-year prospective experience. Radiology. 2005;235:259–265. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 16.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low dose spiral CT: Prevalence in 817 asymptomatic smokers. Radiology. 2002;222:773–781. doi: 10.1148/radiol.2223010490. [DOI] [PubMed] [Google Scholar]
- 17.National Lung Screening Trial Research Team. Aberle DR, Berg CD, et al. The National Lung Screening Trial: Overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tabar L, Vitak B, Yen MF, et al. Number needed to screen: Lives saved over 20 years of follow-up in mammographic screening. J Med Screen. 2004;11:126–129. doi: 10.1258/0969141041732175. [DOI] [PubMed] [Google Scholar]
- 21.Richardson A. Screening and the number needed to treat. J Med Screen. 2001;8:125–127. doi: 10.1136/jms.8.3.125. [DOI] [PubMed] [Google Scholar]
- 22.Montes RP. The cost of CT screening: Year gained via lung cancer screening could cost $38,000. Cancer Lett. 2011;37:15–16. [Google Scholar]
- 23.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078–2086. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology. 2004;231:440–445. doi: 10.1148/radiol.2312030880. [DOI] [PubMed] [Google Scholar]
- 25.Larke FJ, Kruger RL, Cagnon CH, et al. Estimated radiation dose associated with low-dose chest CT of average-size participants in the National Lung Screening Trial. AJR Am J Roentgenol. 2011;197:1165–1169. doi: 10.2214/AJR.11.6533. [DOI] [PubMed] [Google Scholar]
- 26.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: lung cancer screening. Version 1.2012. http://www.nccn.org/professionals/physician_gls/pdf/lung_screening.pdf.
- 27.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: An individual risk prediction model for lung cancer. Br J Cancer. 2008;98:270–276. doi: 10.1038/sj.bjc.6604158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med. 2009;361:2221–2229. doi: 10.1056/NEJMoa0906085. [DOI] [PubMed] [Google Scholar]
- 29.McFarlane MJ, Feinstein AR, Wells CK. Clinical features of lung cancers discovered as a postmortem “surprise.”. Chest. 1986;90:520–523. doi: 10.1378/chest.90.4.520. [DOI] [PubMed] [Google Scholar]
- 30.Sakr WA, Grignon DJ, Haas GP, et al. Age and racial distribution of prostatic intraepithelial neoplasia. Eur Urol. 1996;30:138–144. doi: 10.1159/000474163. [DOI] [PubMed] [Google Scholar]
- 31.Stamatiou K, Alevizos A, Agapitos E, et al. Incidence of impalpable carcinoma of the prostate and of non-malignant and precarcinomatous lesions in Greek male population: An autopsy study. Prostate. 2006;66:1319–1328. doi: 10.1002/pros.20339. [DOI] [PubMed] [Google Scholar]
- 32.Harach HR, Franssila KO, Wasenius VM. Occult papillary carcinoma of the thyroid: A “normal” finding in Finland—A systematic autopsy study. Cancer. 1985;56:531–538. doi: 10.1002/1097-0142(19850801)56:3<531::aid-cncr2820560321>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102:605–613. doi: 10.1093/jnci/djq099. [DOI] [PubMed] [Google Scholar]
- 34.Takashima S, Maruyama Y, Hasegawa M, et al. CT findings and progression of small peripheral lung neoplasms having a replacement growth pattern. AJR Am J Roentgenol. 2003;180:817–826. doi: 10.2214/ajr.180.3.1800817. [DOI] [PubMed] [Google Scholar]
- 35.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: A systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westfall JM, Mold J, Fagnan L. Practice-based research: “Blue Highways” on the NIH roadmap. JAMA. 2007;297:403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 37.Henderson S, DeGroff A, Richards TB, et al. A qualitative analysis of lung cancer screening practices by primary care physicians. J Community Health. 2011;36:949–956. doi: 10.1007/s10900-011-9394-2. [DOI] [PubMed] [Google Scholar]
- 38.Klabunde CN, Marcus PM, Silvestri GA, et al. U.S. primary care physicians' lung cancer screening beliefs and recommendations. Am J Prev Med. 2010;39:411–420. doi: 10.1016/j.amepre.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wassenaar TR, Eickhoff JC, Jarzemsky DR, et al. Differences in primary care clinicians' approach to non-small cell lung cancer patients compared with breast cancer. J Thorac Oncol. 2007;2:722–728. doi: 10.1097/JTO.0b013e3180cc2599. [DOI] [PubMed] [Google Scholar]
- 40.Marlow LA, Waller J, Wardle J. Variation in blame attributions across different cancer types. Cancer Epidemiol Biomarkers Prev. 2010;19:1799–1805. doi: 10.1158/1055-9965.EPI-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stuber J, Galea S, Link BG. Smoking and the emergence of a stigmatized social status. Soc Sci Med. 2008;67:420–430. doi: 10.1016/j.socscimed.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keeley B, Wright L, Condit CM. Functions of health fatalism: Fatalistic talk as face saving, uncertainty management, stress relief and sense making. Sociol Health Illn. 2009;31:734–747. doi: 10.1111/j.1467-9566.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 43.Niederdeppe J, Levy AG. Fatalistic beliefs about cancer prevention and three prevention behaviors. Cancer Epidemiol Biomarkers Prev. 2007;16:998–1003. doi: 10.1158/1055-9965.EPI-06-0608. [DOI] [PubMed] [Google Scholar]
- 44.Silvestri GA, Nietert PJ, Zoller J, et al. Attitudes towards screening for lung cancer among smokers and their non-smoking counterparts. Thorax. 2007;62:126–130. doi: 10.1136/thx.2005.056036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dillard AJ, McCaul KD, Klein WM. Unrealistic optimism in smokers: Implications for smoking myth endorsement and self-protective motivation. J Health Commun. 2006;11(suppl 1):93–102. doi: 10.1080/10810730600637343. [DOI] [PubMed] [Google Scholar]
- 46.Salander P. Attributions of lung cancer: My own illness is hardly caused by smoking. Psychooncology. 2007;16:587–592. doi: 10.1002/pon.1121. [DOI] [PubMed] [Google Scholar]
- 47.Tod AM, Craven J, Allmark P. Diagnostic delay in lung cancer: A qualitative study. J Adv Nurs. 2008;61:336–343. doi: 10.1111/j.1365-2648.2007.04542.x. [DOI] [PubMed] [Google Scholar]
- 48.Tod AM, Joanne R. Overcoming delay in the diagnosis of lung cancer: A qualitative study. Nurs Stand. 2010;24:35–43. doi: 10.7748/ns2010.04.24.31.35.c7690. [DOI] [PubMed] [Google Scholar]
- 49.Rutten LF, Hesse BW, Moser RP, et al. Public perceptions of cancer prevention, screening, and survival: Comparison with state-of-science evidence for colon, skin, and lung cancer. J Cancer Educ. 2009;24:40–48. doi: 10.1080/08858190802664610. [DOI] [PubMed] [Google Scholar]
- 50.Finney Rutten LJ, Augustson EM, Moser RP, et al. Smoking knowledge and behavior in the United States: Sociodemographic, smoking status, and geographic patterns. Nicotine Tob Res. 2008;10:1559–1570. doi: 10.1080/14622200802325873. [DOI] [PubMed] [Google Scholar]
- 51.George M, Margolis ML. Race and lung cancer surgery: A qualitative analysis of relevant beliefs and management preferences. Oncol Nurs Forum. 2010;37:740–748. doi: 10.1188/10.ONF.740-748. [DOI] [PubMed] [Google Scholar]
- 52.Walsh MC, Trentham-Dietz A, Schroepfer TA, et al. Cancer information sources used by patients to inform and influence treatment decisions. J Health Commun. 2010;15:445–463. doi: 10.1080/10810731003753109. [DOI] [PubMed] [Google Scholar]
- 53.Viswanath K, Breen N, Meissner H, et al. Cancer knowledge and disparities in the information age. J Health Commun. 2006;11(suppl 1):1–17. doi: 10.1080/10810730600637426. [DOI] [PubMed] [Google Scholar]
- 54.Cykert S, Dilworth-Anderson P, Monroe MH, et al. Factors associated with decisions to undergo surgery among patients with newly diagnosed early-stage lung cancer. JAMA. 2010;303:2368–2376. doi: 10.1001/jama.2010.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reimer RA, Gerrard M, Gibbons FX. Racial disparities in smoking knowledge among current smokers: Data from the health information national trends surveys. Psychol Health. 2010;25:943–959. doi: 10.1080/08870440902935913. [DOI] [PubMed] [Google Scholar]
- 56.Lathan CS, Okechukwu C, Drake BF, et al. Racial differences in the perception of lung cancer: The 2005 Health Information National Trends Survey. Cancer. 2010;116:1981–1986. doi: 10.1002/cncr.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woloshin S, Schwartz LM, Welch HG. The risk of death by age, sex, and smoking status in the United States: Putting health risks in context. J Natl Cancer Inst. 2008;100:845–853. doi: 10.1093/jnci/djn124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Young RP, Hopkins RJ, Hay BA, et al. A gene-based risk score for lung cancer susceptibility in smokers and ex-smokers. Postgrad Med J. 2009;85:515–524. doi: 10.1136/pgmj.2008.077107. [DOI] [PubMed] [Google Scholar]
- 59.Punturieri A, Szabo E, Croxton TL, et al. Lung cancer and chronic obstructive pulmonary disease: Needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Young RP, Hopkins RJ, Christmas T, et al. COPD prevalence is increased in lung cancer, independent of age, sex and smoking history. Eur Respir J. 2009;34:380–386. doi: 10.1183/09031936.00144208. [DOI] [PubMed] [Google Scholar]
- 61.Brennan P, Hainaut P, Boffetta P. Genetics of lung-cancer susceptibility. Lancet Oncol. 2011;12:399–408. doi: 10.1016/S1470-2045(10)70126-1. [DOI] [PubMed] [Google Scholar]
- 62.Rooney C, Sethi T. The epithelial cell and lung cancer: The link between chronic obstructive pulmonary disease and lung cancer. Respiration. 2011;81:89–104. doi: 10.1159/000323946. [DOI] [PubMed] [Google Scholar]
- 63.Adcock IM, Caramori G, Barnes PJ. Chronic obstructive pulmonary disease and lung cancer: New molecular insights. Respiration. 2011;81:265–284. doi: 10.1159/000324601. [DOI] [PubMed] [Google Scholar]
- 64.Rotunno M, Yu K, Lubin JH, et al. Phase 1 metabolic genes and risk of lung cancer: Multiple polymorphisms and mRNA expression. PLoS One. 2009;4:e5652. doi: 10.1371/journal.pone.0005652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Calikoglu M, Tamer L, Ates Aras N, et al. The association between polymorphic genotypes of glutathione S-transferases and COPD in the Turkish population. Biochem Genet. 2006;44:307–319. doi: 10.1007/s10528-006-9031-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhang JY, Wang Y, Prakash C. Xenobiotic-metabolizing enzymes in human lung. Curr Drug Metab. 2006;7:939–948. doi: 10.2174/138920006779010575. [DOI] [PubMed] [Google Scholar]
- 67.Young RP, Hopkins RJ, Hay BA, et al. FAM13A locus in COPD is independently associated with lung cancer: Evidence of a molecular genetic link between COPD and lung cancer. Appl Clin Genet. 2011;4:1–10. doi: 10.2147/TACG.S15758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou X, Baron RM, Hardin M, et al. Identification of a chronic obstructive pulmonary disease genetic determinant that regulates HHIP. Hum Mol Genet. 2012;21:1325–1335. doi: 10.1093/hmg/ddr569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–1938. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 70.Wilson DO, Weissfeld JL, Balkan A, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Way T, Chan HP, Hadjiiski L, et al. Computed-aided diagnosis of lung nodules on CT scans: ROC study of its effect on radiologists' performance. Acad Radiol. 2010;17:323–332. doi: 10.1016/j.acra.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shah SK, McNitt-Gray MF, Rogers SR, et al. Computer-aided diagnosis of the solitary pulmonary nodule. Acad Radiol. 2005;12:570–575. doi: 10.1016/j.acra.2005.01.018. [DOI] [PubMed] [Google Scholar]