Abstract
Context:
High bone mass (HBM), detected in 0.2% of dual-energy x-ray absorptiometry (DXA) scans, is characterized by raised body mass index, the basis for which is unclear.
Objective:
To investigate why body mass index is elevated in individuals with HBM, we characterized body composition and examined whether differences could be explained by bone phenotypes, eg, bone mass and/or bone turnover.
Design, Setting, and Participants:
We conducted a case-control study of 153 cases with unexplained HBM recruited from 4 UK centers by screening 219 088 DXA scans. A total of 138 first-degree relatives (of whom 51 had HBM) and 39 spouses were also recruited. Unaffected individuals served as controls.
Main Outcome Measures:
We measured fat mass, by DXA, and bone turnover markers.
Results:
Among women, fat mass was inversely related to age in controls (P = .01), but not in HBM cases (P = .96) in whom mean fat mass was 8.9 [95% CI 4.7, 13.0] kg higher compared with controls (fully adjusted mean difference, P < .001). Increased fat mass in male HBM cases was less marked (gender interaction P = .03). Compared with controls, lean mass was also increased in female HBM cases (by 3.3 [1.2, 5.4] kg; P < .002); however, lean mass increases were less marked than fat mass increases, resulting in 4.5% lower percentage lean mass in HBM cases (P < .001). Osteocalcin was also lower in female HBM cases compared with controls (by 2.8 [0.1, 5.5] μg/L; P = .04). Differences in fat mass were fully attenuated after hip bone mineral density (BMD) adjustment (P = .52) but unchanged after adjustment for bone turnover (P < .001), whereas the greater hip BMD in female HBM cases was minimally attenuated by fat mass adjustment (P < .001).
Conclusions:
HBM is characterized by a marked increase in fat mass in females, statistically explained by their greater BMD, but not by markers of bone turnover.
Population-based studies have identified a positive association between bone mineral density (BMD) and obesity (1–3), strongest in women, particularly those postmenopausal, and sedentary individuals (1, 4). Although increased skeletal loading by weight bearing may contribute, obesity is also positively related to BMD at non-weight-bearing sites, suggesting the role of systemic factors. Several obesity-related endocrine factors could theoretically stimulate bone formation, including bone-active hormones secreted by pancreatic β-cells and adipocytes (5). However, causal inference from observational epidemiological studies of BMD and obesity is difficult; ie, observed relationships may instead reflect a positive influence of BMD on fat deposition. Mouse models have suggested that bone turnover directly influences insulin sensitivity and adiposity via a relay involving osteocalcin (an osteoblast-specific protein) and adiponectin (produced by white adipose tissue). Reduced bone turnover, resulting in decreased osteocalcin, has been associated with lower adiponectin, impaired insulin sensitivity, and increased fat deposition due to reduced energy expenditure (6). Similar relationships between circulating osteocalcin and adiponectin, plus adiposity measures and insulin sensitivity, are reported in some human populations (7, 8). Changes in osteocalcin, induced by antiresorptive osteoporosis treatments, affect metabolic indices and body weight (9), although the effects of anabolic (recombinant human PTH) treatments on glucose homeostasis are inconsistent (9, 10).
Population-based genetic approaches can inform causal pathways and can examine relationships between obesity and bone mass; however, results have been conflicting. First, in adolescents from the Avon Longitudinal Study of Parents and Children (ALSPAC), a Mendelian randomization study exploiting established genetic markers of obesity supported a causal influence of obesity on BMD; the 2 body mass index (BMI)-related genotypes examined gave the same predictive effect of fat mass (FM) on BMD, providing evidence against pleiotropy (11). Second, within an extended family, individuals with extremely elevated bone mass resulting from an activating LRP5 (low-density lipoprotein receptor-related protein-5) mutation had increased FM and reduced bone turnover (12), consistent with a causal influence of reduced osteocalcin on fat accumulation. However, LRP5 has not been linked with obesity in the wider population (13).
Further understanding of the fat-bone relationship can be achieved by investigating a rare and extreme population of high bone mass (HBM) individuals. We previously observed that HBM is a sporadic finding of generalized raised BMD on dual-energy x-ray absorptiometry (DXA) scanning, with a prevalence of .2% among a UK DXA-scanned population (14). Having screened 335 115 DXA scans across 13 UK National Health Service (NHS) centers for HBM, we observed associated clinical characteristics suggestive of a mild skeletal dysplasia, concluding that most cases are likely to harbor as yet unidentified mutations/polymorphisms increasing BMD (14). Based upon comparison with a contemporaneous control population (comprising unaffected first-degree relatives and spouses), HBM cases had substantially elevated BMI, suggesting that this extreme HBM population can be utilized to examine fat-bone relationships.
We sought to gain further insights into causal pathways between fat and bone, exploring relationships between BMI and bone mass in HBM individuals, using total body (TB) DXA scanning to characterize body composition. DXA provides a simple, quick, and low-radiation method of measuring 3 tissue compartments (fat, mineral, and lean mass [fat/mineral-free]) with high precision (15). Specifically, we aimed to: 1) establish whether FM is elevated in HBM individuals once potential confounders, eg, physical activity (PA), are considered; 2) explore differences in age- and gender-specific associations; and 3) investigate whether FM changes could be explained by bone phenotypes including BMD and/or bone turnover.
Subjects and Methods
Participant recruitment
The HBM study is a UK-based multicentered observational study of adults with unexplained HBM. At 4 of our larger study centers, 788 cases of unexplained HBM were identified by screening NHS DXA databases (n = 219 088). Full details of DXA database screening and participant recruitment have previously been reported (14). In brief, HBM was defined as: 1) both L1 Z-score of ≥ +3.2 and total hip Z-score of ≥ +1.2; or 2) both total hip Z-score ≥ +3.2 and L1 Z-score of ≥ +1.2. The first lumbar vertebra (L1) was used because, in contrast to lower lumbar levels, it was not found to be associated with the presence of lumbar spine osteoarthritis assessed on DXA images (14). Cases with significant osteoarthritis and/or other causes of raised BMD were excluded (eg, surgical metalwork, Paget's disease, metastases), as were those with established diagnoses of osteopetrosis. A threshold of +3.2 was in keeping with the only published precedent for identifying HBM previously described using DXA (16), and most appropriately differentiated generalized HBM from artifact (14). Z-score rather than T-score was used to limit age bias. Index cases were asked to pass on study invitations to their first-degree relatives and spouses or partners. Relatives/spouses with HBM were in turn asked to pass on study invitations to their first-degree relatives and spouses. First-degree relatives and spouses were recruited, in whom HBM status was defined as summed L1 Z plus total hip Z-score of ≥ +3.2. Family-based controls comprised unaffected relatives and spouses. All participants were clinically assessed by one doctor using a standardized structured history and examination questionnaire, after which TB DXA scans and (nonfasted) phlebotomy were performed. Routine weight and height measurements were recorded.
Subsequently, current and lifetime PA was measured by a short (10-min) postal questionnaire (with prepaid reply envelope, sent up to 3 times) which included: 1) the short last 7-d self-administered International PA Questionnaire (IPAQ version 2002, http://www.ipaq.ki.se/ipaq.htm (17, 18); and 2) a historical PA questionnaire (19–21). A total of 86.5% of participants responded and completed the PA questionnaire; those who did not respond had similar anthropometric characteristics to those who did (data not shown).
Recruitment ran from September 2008 until April 2010. Written informed consent was collected for all, in line with the Declaration of Helsinki (22). Participants were excluded if they were aged <18 years, pregnant, or unable to provide written informed consent for any reason. This study was approved by the Bath Multicenter Research Ethics Committee (REC) and at each NHS Local REC.
DXA measurements
DXA scans were performed using either GE Lunar Prodigy DXA (software version 13.2; GE Healthcare, Madison, Wisconsin) in Birmingham, Cambridge, and Hull, or Hologic Discovery/W DXA (software version Apex 3.0; Hologic Inc., Bedford, Massachusetts) in Sheffield. All scans were acquired and analyzed according to each manufacturer's standard scanning and positioning protocols. TB bone mineral content (BMC) and BMD, FM, and lean mass (LM) were measured, together with L1 and total hip BMD. The specific fat phenotype was measured using android, gynoid, peripheral (arms and legs), and trunk (TB—peripheral and head) regions of interest. All DXA images were reviewed for quality control purposes. TB scans with metallic artifacts were graded; this grade was added to all bone parameter regression models (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). Participants were centered for scanning; incomplete capture of soft tissue edges can introduce systematic bias because mass is lost as body size increases. Eighty-nine (27%) scans had evidence of incomplete capture. Hence, weighed weight (concurrently measured using scales) and calculated DXA weight were compared. Correlation (r2 = .984) and agreement (Bland-Altman rank correlation coefficient r = −.044, Pitman's test of difference in variance P = .426 [Ref. 23]) demonstrated no evidence of systematic bias. Hence, further adjustment of analyses was not judged necessary.
Known differences in calibration exist between Hologic and GE for all scan types (24, 25). For lumbar spine and hip scans, systematic bias was limited by converting all measures to standardized BMD (sBMD) (26, 27). For TB, systematic differences were limited using newly derived cross-calibration equations for all bone and soft tissue regions of interest (25, 28) (Supplemental Table 2). To ensure that no systematic bias was introduced by new formulae, sensitivity analyses were performed excluding Hologic DXA data. Lastly, because only 330 (59.5%) of 555 subjects in the original multicenter study population (14) had TB DXA scans performed, the principal characteristics of individuals who received a TB DXA scan were compared to those who did not. No differences were observed in weight, height, BMI, sex, age, or ethnicity (data not shown).
Bone turnover markers and adiponectin
Two nonfasted EDTA samples were collected, and serum was separated and frozen within 4 hours to −80°C. Bone formation (procollagen type 1 amino-terminal propeptide [P1NP], total osteocalcin) and resorption (β-C-telopeptides of type I collagen [βCTX]) markers were measured. All had inter- and intraassay coefficients of variation <6.0% across the assay working ranges. Electrochemiluminescence immunoassays (Roche Diagnostics, Lewes, United Kingdom) were used to measure plasma concentrations of P1NP, osteocalcin, and βCTX (detection limits, 4.0, .6, and .01 μg/L, respectively). Total and HMW-adiponectin was measured using assays developed in house as previously reported with inter- and intraassay coefficients of variation < 10% across the working range .5–30.0 mg/L (29, 30). Supplemental Table 3 provides reference ranges.
Statistical methods
Descriptive statistics are presented as mean (95% confidence interval [CI]) for continuous data and count (percentage) for categorical data. Analyses comparing 2 continuous variables are presented as β coefficients and 95% CI for standardized outcomes. Linear regression was used to analyze continuous variables, using random effects models to allow for the lack of statistical independence due to within-family clustering of environmental factors and shared genotypes. Age, gender, height, and menopausal status in women were considered a priori confounders of the associations between HBM status and all DXA and bone turnover parameters. Further potential confounders considered in sequential regression model adjustments included: smoking status, alcohol consumption, estrogen replacement therapy, history of malignancy (a proxy for current/previous aromatase inhibitor use for breast cancer and antiandrogens for prostate cancer as prior medication use was not available), hypothyroidism, PA, and glucocorticoid use (current/previous/never). Due to fat/bone effects of T4 and glucocorticoids, sensitivity analyses excluded those with current/previous steroid use or hypothyroidism. Key results were gender-stratified to assess interactions. To explore the bone-fat relationship, further adjustments were made for variables potentially lying on a hypothesized causal pathway (31). Data were managed using Microsoft Access (data entry checks; error rate <.12%) and analyzed using Stata release 11 statistical software (StataCorp, College Station, Texas).
Results
Participant characteristics
In total, 204 HBM cases (153 index cases, 49 affected relatives, 2 affected spouses) and 126 family controls (89 unaffected relatives, 37 unaffected spouses) were assessed. HBM cases (age range, 26–87 y) were older than family controls (19–88 y), were more commonly female and postmenopausal, and had used estrogen replacement (Table 1). Although weight was similar between HBM cases and controls, HBM cases were shorter, and their BMI was higher. HBM cases were more likely to report a history of cancer, steroid use, and hypothyroidism (Table 1). Diabetes mellitus was no more commonly reported among HBM cases than controls (20 [9.8%] vs 10 [7.9%], respectively; P = .6). Self-reported current and historical PA levels were also similar. Controls, who were more commonly male, reported heavier alcohol intake than cases; however, smoking status was similar. Only 2 HBM cases were not of white European origin.
Table 1.
Clinical Characteristics of HBM Cases Compared With Family Controls
| HBM Cases (n = 204) | Controls (n = 126) | P Value | |
|---|---|---|---|
| Age, y | 61.4 (13.9) | 55.3 (16.1) | <.001 |
| Height, cm | 166.6 (9.1) | 171.7 (10.5) | <.001 |
| Weight, kg | 85.6 (17.5) | 84.6 (17.5) | .604 |
| BMI, kg/m2 | 30.8 (5.8) | 28.8 (5.0) | <.001 |
| Female | 155 (76.0) | 57 (45.2) | <.001 |
| Postmenopausal | 128 (82.6) | 30 (52.6) | <.001 |
| Estrogen replacement use (ever)a | 78 (53.1) | 10 (19.6) | <.001 |
| Previous fractureb | 78 (38.2) | 62 (49.2) | .051 |
| Malignancy (ever)c | 31 (15.2) | 7 (5.6) | .010 |
| Steroid use (ever)d | 50 (24.5) | 20 (15.9) | .064 |
| Hypothyroidisme | 27 (13.2) | 2 (1.6) | .002 |
| Self-reported alcohol consumption | .003 | ||
| None | 57 (27.9) | 23 (18.3) | |
| Occasional | 23 (11.3) | 10 (7.9) | |
| Regular | 104 (51.0) | 62 (49.2) | |
| Heavy | 20 (9.8) | 31 (24.6) | |
| Self-reported smoking status | .187 | ||
| Never | 84 (41.2) | 65 (51.6) | |
| Ex-smoker | 96 (47.1) | 48 (38.1) | |
| Current | 23 (11.3) | 13 (10.3) | |
| Current PA (IPAQ) (n = 297) | .645 | ||
| Low | 30 (14.7) | 14 (11.1) | |
| Moderate | 72 (35.3) | 42 (33.3) | |
| High | 84 (41.2) | 55 (43.7) | |
| Historical PA score (n = 295)f | .258 | ||
| Very low (0–4) | 22 (10.8) | 13 (10.3) | |
| Low (5–7) | 35 (17.2) | 28 (22.2) | |
| Moderate (8–10) | 37 (18.1) | 28 (22.2) | |
| High (11–14) | 46 (22.5) | 17 (13.5) | |
| Very high (15–24) | 44 (21.6) | 25 (19.8) |
Data are expressed as number (percent), except for age, height, weight, and BMI, which are expressed as mean (SD). Unadjusted P values from regression models accounting for within-family clustering BMI were calculated as weight (kilograms)/height (meters2). Among both cases and family controls, there was 1 report of hyperthyroidism and 2 of polycystic ovary syndrome.
Postmenopausal estrogen replacement therapy.
From any mechanism.
Previous/current (HBM cases reported: 11 breast, 10 skin, 2 colon, 3 cervix, 2 renal, 2 endometrial, 1 each ovary, prostate, cerebral, thyroid, leukemia. Controls reported: 3 skin, 2 breast, 1 prostate, 1 myeloma. Of 31 HBM cases with history of cancer, 25 were index cases and 6 (11.8%) were relative/control cases.
Previous (20 controls, 38 HBM) or current (no controls, 12 HBM cases), includes eye drops, intraarticular steroid injections, and oral steroids, eg, for asthma, polymyalgia rheumatica, ulcerative colitis.
Currently taking T4 replacement. Overall 51 (25%) HBM cases had ≥1 of the following: hyperthyroidism, hypothyroidism, celiac disease, inflammatory bowel diseases, psoriasis, rheumatoid arthritis, vitiligo (42 were index cases), as did 5 (4%) controls. Autoimmune diseases are a common indication for DXA scanning, and therefore index cases would be expected to have a higher prevalence of such disease.
Constructed using best available evidence, grading PA between 0 (no PA) and 24 (very high PA) (17–19).
Comparison of metabolic phenotypes between HBM cases and controls: unadjusted analyses
As expected, L1 and total hip sBMD, TB BMC, and TB BMD were higher in HBM cases compared to controls (Table 2). No evidence was detected to support a disproportionate increase in skull BMD in HBM or a preferential increase in lower-to-upper limb BMD (ie, no weight-bearing effect), between HBM cases and controls (data not shown). Although TB %LM was 6.5% ([95% CI 4.6, 8.4]; P < .001) greater in controls in unadjusted analyses, TB %FM was 6.2% ([4.2, 8.2]; P < .001) greater in HBM cases. Osteocalcin levels were 9.4% lower in HBM cases compared with controls (mean difference, 1.8 [0.2, 3.4] μg/L; P = .03), whereas P1NP, βCTX, and adiponectin were no different. When stratified by gender, the unadjusted difference in TB FM between HBM cases and controls was 2.8-fold greater in women (P = .02 for gender interaction) (Table 3). Among men, HBM cases had lower osteocalcin and βCTX than controls.
Table 2.
Unadjusted DXA-Measured Body Composition and Bone Turnover Markers in HBM Cases Compared With Family Controls
| HBM, Mean (SD) | Control, Mean (SD) | Mean difference (95% CI) | P Value | |
|---|---|---|---|---|
| Spine and hip DXA (n = 330) | ||||
| L1 sBMD, g/cm2 | 1.40 (0.16) | 1.07 (0.16) | 0.33 (0.29, 0.36) | <.001 |
| Total hip sBMD, g/cm2 | 1.25 (0.18) | 0.99 (0.14) | 0.25 (0.21, 0.28) | <.001 |
| TB DXA (n = 330) | ||||
| BMD,a g/cm2 | 1.36 (0.13) | 1.24 (0.12) | 0.12 (0.09, 0.15) | <.001 |
| BMC, kga | 3.48 (0.69) | 3.16 (0.65) | 0.32 (0.17, 0.46) | <.001 |
| LM, kg | 47.0 (10.3) | 51.4 (11.4) | −4.34 (−6.72, −1.97) | <.001 |
| FM, kg | 35.6 (12.6) | 29.8 (11.3) | 5.83 (3.35, 8.32) | <.001 |
| % bone massa | 4.0 (0.7) | 3.7 (0.6) | 0.3 (0.2, 0.4) | <.001 |
| % LM | 55.3 (8.9) | 61.8 (8.7) | −6.5 (−8.4, −4.6) | <.001 |
| % FM | 40.7 (9.3) | 34.5 (9.1) | 6.2 (4.2, 8.2) | <.001 |
| FM phenotype (n = 330) | ||||
| Android, kg | 3.45 (1.40) | 3.01 (1.26) | 0.44 (0.16, 0.71) | .002 |
| Gynoid, kg | 5.70 (1.85) | 4.98 (1.82) | 0.72 (0.33, 1.10) | <.001 |
| Android:gynoid ratio | 0.60 (0.18) | 0.59 (0.20) | 0.00 (−0.04, 0.04) | .906 |
| Trunk, kg | 19.5 (6.74) | 16.7 (6.23) | 2.84 (1.51, 4.17) | <.001 |
| Trunk:peripheral ratiob | 1.18 (0.59) | 1.28 (0.62) | −0.11 (−0.21, 0.00) | .046 |
| Bone turnover markers and adiponectin (n = 326) | ||||
| P1NP, μg/L | 36.1 (17.9) | 38.1 (18.8) | −1.96 (−5.90, 1.98) | .329 |
| Osteocalcin (total), μg/L | 17.5 (7.20) | 19.3 (7.48) | −1.81 (−3.42, −0.19) | .028 |
| βCTX, μg/L | 0.21 (0.13) | 0.22 (0.13) | −0.02 (−0.04, 0.01) | .281 |
| Adiponectin (total), μg/L | 6.61 (3.79) | 6.55 (3.56) | 0.06 (−0.77, 0.89) | .893 |
Mean (SD) L1 Z-scores were 3.9 (1.3) and 0.5 (1.2), and total hip Z-scores were 3.0 (1.2) and 0.6 (0.9) in HBM cases and controls, respectively. Similar proportions of HBM cases and controls were scanned on Lunar Prodigy DXA scanners, 175 (86%) and 112 (90%), respectively (P = 0.4).
Adjusted for metallic artifact.
Peripheral = (right + left arms) + (right + left legs).
Table 3.
Unadjusted DXA Measured Body Composition and Bone Turnover Markers in HBM Cases Compared With Family Controls, Stratified by Gender
| Women (n = 212) |
Men (n = 118) |
|||||||
|---|---|---|---|---|---|---|---|---|
| HBM, Mean (95% CI) | Control, Mean (95% CI) | Mean difference (95% CI) | P Value | HBM, Mean (95% CI) | Control, Mean (95% CI) | Mean Difference (95% CI) | P Value | |
| Spine and hip DXA | ||||||||
| L1 sBMD, g/cm2 | 1.37 (1.35, 1.39) | 1.04 (1.00, 1.08) | 0.33 (0.29, 0.38) | <.001 | 1.49 (1.45, 1.54) | 1.11 (1.07, 1.15) | 0.38 (0.32, 0.44) | <.001 |
| Total hip sBMD, g/cm2 | 1.22 (1.20, 1.24) | 0.94 (0.90, 0.97) | 0.28 (0.24, 0.32) | <.001 | 1.34 (1.28, 1.39) | 1.05 (1.00, 1.10) | 0.29 (0.23, 0.35) | <.001 |
| TB DXA | ||||||||
| BMD,a g/cm2 | 1.31 (1.29, 1.33) | 1.15 (1.13, 1.18) | 0.16 (0.12, 0.19) | <.001 | 1.43 (1.39, 1.46) | 1.27 (1.24, 1.31) | 0.15 (0.11, 0.19) | <.001 |
| BMC, kga | 3.18 (3.11, 3.26) | 2.60 (2.48, 2.72) | 0.59 (0.46, 0.72) | <.001 | 4.04 (3.85, 4.23) | 3.55 (3.36, 3.73) | 0.49 (0.30, 0.69) | <.001 |
| LM, kg | 42.8 (41.7, 44.0) | 42.0 (40.2, 43.8) | 0.83 (−1.05, 2.70) | .389 | 59.3 (56.9, 61.6) | 59.5 (57.4, 61.7) | −0.26 (−3.19, 2.67) | .862 |
| FM, kg | 37.6 (35.4, 39.8) | 32.5 (29.0, 35.9) | 5.17 (1.54, 8.80) | .005b | 28.5 (25.9, 31.1) | 26.6 (24.4, 28.9) | 1.86 (−1.48, 5.20) | .274b |
| % bone massa | 3.9 (3.8, 4.1) | 3.5 (3.3, 3.7) | 0.4 (0.2, 0.6) | <.001 | 4.5 (4.3, 4.7) | 4.0 (3.8, 4.2) | 0.5 (0.3, 0.7) | <.001 |
| % LM | 52.1 (50.9, 53.4) | 55.9 (54.0, 57.8) | −3.8 (−5.7, −1.8) | <.001 | 65.1 (63.2, 67.1) | 67.0 (65.3, 68.8) | −1.9 (−4.3, 0.5) | .124 |
| % FM | 44.0 (42.7, 45.3) | 40.6 (38.6, 42.7) | 3.3 (1.3, 5.4) | <.001 | 30.4 (28.4, 32.4) | 29.0 (27.3, 30.8) | 1.4 (−1.1, 3.9) | .284 |
| FM phenotype | ||||||||
| Android, kg | 3.49 (3.25, 3.74) | 2.84 (2.45, 3.24) | 0.65 (0.24, 1.06) | <.001 | 3.23 (2.89, 3.56) | 2.98 (2.70, 3.27) | 0.24 (−0.18, 0.67) | .258 |
| Gynoid, kg | 6.10 (5.78, 6.42) | 5.72 (5.21, 6.22) | 0.38 (−0.14, 0.91) | <.001 | 4.35 (3.94, 4.77) | 4.18 (3.81, 4.55) | 0.18 (−0.33, 0.69) | .495 |
| Android:gynoid ratio | 0.55 (0.53, 0.58) | 0.47 (0.42, 0.51) | 0.09 (0.04, 0.13) | <.001b | 0.72 (0.67, 0.77) | 0.70 (0.66, 0.74) | 0.02 (−0.05, 0.08) | .605b |
| Trunk, kg | 20.0 (18.9, 21.2) | 16.4 (14.6, 18.3) | 3.60 (1.68, 5.53) | <.001 | 17.5 (15.8, 19.1) | 16.2 (14.8, 17.7) | 1.22 (−0.85, 3.30) | .249 |
| Trunk:peripheral ratiob | 1.10 (1.02, 1.19) | 0.94 (0.83, 1.05) | 0.17 (0.07, 0.26) | <.001b | 1.47 (1.28, 1.65) | 1.54 (1.36, 1.72) | −0.07 (−0.25, 0.10) | .405b |
| Bone turnover markers and adiponectina | ||||||||
| P1NP, μg/L | 37.1 (34.0, 40.1) | 38.3 (33.2, 43.4) | −1.24 (−6.84, 4.37) | .665 | 33.7 (28.2, 39.1) | 37.6 (32.5, 42.6) | −3.88 (−10.1, 2.32) | .220 |
| Osteocalcin (total), μg/L | 18.0 (16.8, 19.2) | 19.6 (17.6, 21.6) | −1.58 (−3.90, 0.75) | .184 | 16.4 (14.3, 18.4) | 19.4 (17.5, 21.2) | −3.01 (−5.34, −0.68) | .011 |
| βCTX, μg/L | 0.22 (0.19, 0.24) | 0.22 (0.18, 0.26) | −0.01 (−0.05, 0.04) | .783 | 0.17 (0.14, 0.20) | 0.22 (0.19, 0.25) | −0.05 (−0.09, −0.01) | .017 |
| Adiponectin (total), μg/L | 7.06 (6.42, 7.70) | 7.81 (6.75, 8.88) | −0.75 (−1.99, 0.49) | .237 | 5.15 (4.40, 5.89) | 5.54 (4.92, 6.16) | −0.39 (−1.36, 0.58) | .432 |
n = 209 women and 107 men.
Gender interaction, P < .05.
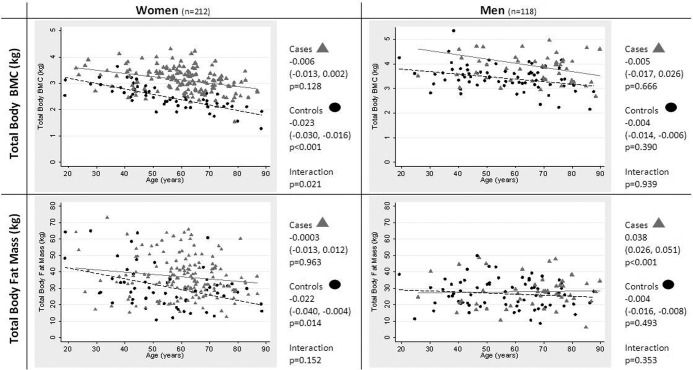
We examined relationships between age and TB BMC/FM stratified by gender (after minimal adjustment for height only). In women, whereas TB BMC was substantially greater among HBM cases compared to controls at all ages, this difference increased with advancing age, reflecting an inverse relationship between age and TB BMC in controls, which was only weakly evident in HBM cases (P = .02 for age interaction) (Figure 1). Among females, TB FM was similar in HBM cases and controls at younger ages, but with increasing years it became progressively greater among HBM cases compared to controls, reflecting an inverse association between age and TB FM in controls (β −.02 [−.004, −.04]; P = .01) not seen among HBM cases (−.0003 [−.01, .01]; P = .96). However, evidence supporting this age interaction was weak (P = .15). By contrast in men, no evidence was detected to support an association between age and TB BMC/FM, with the exception of a positive relationship between age and TB FM in HBM cases (which only emerged after taking into account confounding by height).
Figure 1.
Differences in TB BMC and FM with increasing age in HBM cases and controls, stratified by gender. Gray triangles, HBM cases; black circles, controls. B coefficients are standardized and adjusted for height only with 95% CI shown.
Comparison of metabolic phenotypes between HBM cases and controls: adjusted analyses
Adjustment for confounding factors (age, gender, height, alcohol consumption, smoking status, malignancy, steroid use, PA, hypothyroidism, and menopausal status and estrogen replacement use in women) strengthened conclusions from unadjusted analyses, with the following exceptions: 1) among women, although LM was now substantially higher in HBM cases compared to controls (mean difference, 3.3 [1.2, 5.4] kg; P = .002), the TB %LM remained lower in HBM cases than controls (mean difference, 4.5 [2.3, 6.8]%; P < .001); and 2) now in both men and women weak trends were seen toward lower P1NP, osteocalcin, βCTX, and adiponectin concentrations in HBM cases compared with controls, which was strongest for osteocalcin concentrations among females (mean difference, .04 [.01, .09] μg/L; P = .04) (Table 4). Adjustment strengthened FM differences, with mean %FM 4.2 (1.8, 6.6)% higher in female HBM cases compared to controls (P < .001). Increases in FM seen in women reflected a proportionately greater increase in central obesity as indicated by greater android:gynoid and trunk:peripheral fat ratios.
Table 4.
Adjusted DXA-Measured Body Composition and Bone Turnover Markers in HBM Cases Compared With Family Controls, Stratified by Gender
| Women (n = 212) |
Men (n = 118) |
|||||||
|---|---|---|---|---|---|---|---|---|
| HBM, Mean (95%CI) | Control, Mean (95% CI) | Mean Difference (95% CI) | P Value | HBM, Mean (95% CI) | Control, Mean (95% CI) | Mean Difference (95% CI) | P Value | |
| Spine and hip DXA | ||||||||
| L1 sBMD, g/cm2 | 1.40 (1.32, 1.49) | 1.05 (0.96, 1.13) | 0.36 (0.31, 0.41) | <.001 | 1.61 (1.45, 1.77) | 1.19 (1.03, 1.35) | 0.42 (0.34, 0.49) | <.001 |
| Total hip sBMD, g/cm2 | 1.16 (1.08, 1.24) | 0.87 (0.79, 0.95) | 0.29 (0.24, 0.34) | <.001d | 1.39 (1.21, 1.57) | 1.03 (0.85, 1.20) | 0.37 (0.29, 0.44) | <.001d |
| TB DXA | ||||||||
| BMD,a g/cm2 | 1.35 (1.29, 1.42) | 1.18 (1.12, 1.24) | 0.18 (0.14, 0.21) | <.001 | 1.42 (1.30, 1.55) | 1.22 (1.10, 1.35) | 0.20 (0.15, 0.25) | <.001 |
| BMC, kga | 3.33 (3.10, 3.55) | 2.67 (2.46, 2.89) | 0.65 (0.53, 0.78) | <.001 | 3.82 (3.25, 4.38) | 3.02 (2.46, 3.58) | 0.80 (0.57, 1.03) | <.001 |
| LM, kg | 48.1 (44.5, 51.8) | 44.8 (41.3, 48.3) | 3.31 (1.22, 5.39) | .002 | 55.0 (48.5, 61.5) | 52.7 (46.3, 59.2) | 2.22 (−0.44, 4.89) | .102 |
| FM, kg | 42.3 (35.0, 49.6) | 33.4 (26.5, 40.4) | 8.88 (4.73, 13.0) | <.001d | 29.7 (20.5, 38.9) | 26.9 (17.8, 36.0) | 2.77 (−1.30, 6.85) | .183d |
| % bone massa | 3.7 (3.3, 4.2) | 3.4 (3.0, 3.9) | 0.3 (0.01, 0.5) | .039 | 4.4 (3.8, 5.0) | 3.8 (3.2, 4.4) | 0.6 (0.3, 0.8) | <.001 |
| % LM | 52.6 (48.6, 56.5) | 57.1 (53.3, 60.9) | −4.5 (−6.8, −2.3) | <.001 | 62.4 (55.5, 69.4) | 64.3 (57.4, 71.3) | −1.9 (−4.9, 1.1) | .216 |
| % FM | 43.6 (39.4, 47.8) | 39.4 (35.4, 43.4) | 4.2 (1.8, 6.6) | <.001 | 33.3 (26.1, 40.4) | 32.0 (24.9, 39.0) | 1.3 (−1.8, 4.4) | .417 |
| FM phenotype | ||||||||
| Android, kg | 4.07 (3.23, 4.90) | 3.07 (2.27, 3.87) | 1.00 (0.53, 1.47) | <.001d | 3.33 (2.22, 4.45) | 2.99 (1.88, 4.09) | 0.35 (−0.13, 0.83) | .154d |
| Gynoid, kg | 6.50 (5.44, 7.56) | 5.81 (4.79, 6.83) | 0.69 (0.09, 1.29) | .025 | 4.67 (3.24, 6.09) | 4.14 (2.73, 5.56) | 0.52 (−0.08, 1.12) | .087 |
| Android:gynoid ratio | 0.58 (0.49, 0.67) | 0.46 (0.37, 0.54) | 0.12 (0.07, 0.17) | <.001d | 0.68 (0.52, 0.83) | 0.70 (0.54, 0.85) | −0.02 (−0.09, 0.04) | .477d |
| Trunk, kg | 22.6 (18.8, 26.4) | 17.3 (13.6, 20.9) | 5.31 (3.16, 7.47) | <.001d | 18.4 (12.9, 24.0) | 16.5 (11.0, 22.1) | 1.89 (−0.56, 4.34) | .131d |
| Trunk:peripheral ratiob | 1.11 (0.88, 1.35) | 0.91 (0.69, 1.13) | 0.20 (0.08, 0.33) | .001d | 1.35 (0.79, 1.91) | 1.37 (0.80, 1.93) | −0.02 (−0.22, 0.19) | .867d |
| Bone turnover markers and adiponectinc | ||||||||
| P1NP, μg/L | 25.3 (13.7, 37.0) | 30.3 (19.0, 41.6) | −5.01 (−11.8, 1.73) | .145 | 42.3 (22.1, 62.5) | 46.5 (26.3, 66.7) | −4.21 (−12.3, 3.85) | .306 |
| Osteocalcin (total), μg/L | 14.0 (9.34, 18.7) | 16.8 (12.3, 21.4) | −2.79 (−5.51, −0.07) | .044 | 19.5 (12.5, 26.5) | 21.8 (14.8, 28.8) | −2.33 (−5.10, 0.45) | .101 |
| βCTX, μg/L | 0.15 (0.06, 0.23) | 0.19 (0.11, 0.27) | −0.04 (−0.09, 0.01) | .093 | 0.28 (0.16, 0.40) | 0.33 (0.21, 0.45) | −0.05 (−0.10, 0.01) | .079 |
| Adiponectin (total), μg/L | 5.55 (3.36, 7.75) | 6.60 (4.47, 8.74) | −1.05 (−2.32, 0.22) | .106 | 4.29 (1.77, 6.81) | 4.60 (2.11, 7.09) | −0.31 (−1.35, 0.73) | .559 |
Adjusted for metallic artifact.
Peripheral = (right + left arms) + (right + left legs).
n = 209 women and 107 men. Adjusted for age, gender, height, alcohol consumption, smoking status, malignancy and steroid use, current and historical PA, hypothyroidism, and menopausal status and estrogen replacement use in women (continuous variables centered for adjustment).
Gender interaction, P < .05.
Using our regression model adjusted for all confounders, we sequentially added further factors to investigate potential causal pathways between fat and bone (Table 5). If an association between exposure and outcome is attenuated by inclusion of a factor in the regression model, this implies that the factor may lie on the causal pathway (31). Differences in TB FM observed between HBM cases and controls were fully attenuated by conditioning on total hip sBMD and partially attenuated by conditioning on TB LM and TB BMD, whereas adjustment for L1 sBMD, bone turnover markers, or adiponectin had little or no impact. Differences in LM between HBM cases and controls were fully attenuated by adjustment for TB FM. In contrast, the differences in BMD, measured at any site, were only minimally attenuated by conditioning on TB FM, adiponectin, and/or bone turnover markers.
Table 5.
Regression Models Investigating the Hypothesized Causal Relationships Between Body Composition Parameters in HBM Cases and Controls, Stratified by Gender
| Outcome/Additional Factor Adjustment | Women (n = 212) |
Men (n = 118) |
P valueb | ||
|---|---|---|---|---|---|
| SD Difference in Outcome Between HBM Cases and Controls (95% CI) | P value | SD Difference in Outcome Between HBM Cases and Controls (95% CI) | P value | ||
| TB FM | |||||
| Adjusted modela | 0.71 (0.38, 1.04) | <.001 | 0.22 (−0.10, 0.55) | .183 | .078 |
| + TB LM | 0.31 (0.06, 0.57) | .015 | 0.18 (−0.15,0.50) | .283 | .076 |
| + L1 sBMD | 0.58 (0.09, 1.06) | .019 | −0.13 (−0.64, 0.38) | .618 | .053 |
| + Total Hip sBMD | 0.15 (−0.30, 0.59) | .524 | −0.14 (−0.61, 0.33) | .552 | .026 |
| + TB BMDc | 0.32 (−0.10, 0.74) | .130 | −0.21 (−0.60, 0.18) | .297 | .052 |
| + Adiponectin | 0.61 (0.27, 0.94) | <.001 | 0.22 (−0.11, 0.55) | .195 | .146 |
| + P1NP, OC, βCTX | 0.69 (0.34, 1.03) | <.001 | 0.19 (−0.15, 0.53) | .273 | .084 |
| TB LM | |||||
| Adjusted modela | 0.30 (0.11, 0.49) | .002 | 0.20 (−0.04, 0.45) | .102 | .948 |
| + TB FM | 0.05 (−0.10, 0.19) | .536 | 0.15 (−0.09, 0.40) | .226 | .484 |
| + Adiponectin | 0.26 (0.03, 0.42) | .022 | 0.23 (−0.03, 0.48) | .078 | .819 |
| + P1NP, OC, βCTX | 0.29 (0.09, 0.49) | .004 | 0.24 (−0.03, 0.51) | .083 | .930 |
| + TB FM, adiponectin, P1NP, OC, βCTX | 0.05 (−0.11, 0.20) | .572 | 0.22 (−0.06, 0.50) | .117 | .435 |
| L1 sBMD | |||||
| Adjusted modela | 1.54 (1.33, 1.75) | <.001 | 1.79 (1.49, 2.10) | <.001 | .115 |
| + TB FM | 1.53 (1.30, 1.75) | <.001 | 1.76 (1.45, 2.06) | <.001 | .077 |
| + Adiponectin | 1.54 (1.32, 1.76) | <.001 | 1.81 (1.50, 2.12) | <.001 | .082 |
| + P1NP, OC, βCTX | 1.52 (1.30, 1.74) | <.001 | 1.80 (1.47, 2.12) | <.001 | .086 |
| + TB FM, adiponectin, P1NP, OC, βCTX | 1.52 (1.28, 1.76) | <.001 | 1.80 (1.47, 2.13) | <.001 | .061 |
| Total hip sBMD | |||||
| Adjusted modela | 1.42 (1.19, 1.64) | <.001 | 1.79 (1.42, 2.17) | <.001 | .098 |
| + TB FM | 1.27 (1.04, 1.51) | <.001 | 1.73 (1.35, 2.10) | <.001 | .035 |
| + Adiponectin | 1.34 (1.10, 1.57) | <.001 | 1.80 (1.42, 2.18) | <.001 | .052 |
| + P1NP, OC, βCTX | 1.38 (1.14, 1.62) | <.001 | 1.79 (1.40, 2.18) | <.001 | .072 |
| + TB FM, adiponectin, P1NP, OC, βCTX | 1.22 (0.98, 1.46) | <.001 | 1.68 (1.29, 2.06) | <.001 | .031 |
| TB BMDa | |||||
| Adjusted modela | 1.28 (1.02, 1.54) | <.001 | 1.47 (1.09, 1.86) | <.001 | .461 |
| + TB FM | 1.15 (0.89, 1.42) | <.001 | 1.38 (1.01, 1.75) | <.001 | .233 |
| + Adiponectin | 1.24 (0.97, 1.50) | <.001 | 1.49 (1.11, 1.88) | <.001 | .312 |
| + P1NP, OC, βCTX | 1.25 (0.99, 1.52) | <.001 | 1.45 (1.05, 1.85) | <.001 | .412 |
| + TB FM, adiponectin, P1NP, OC, βCTX | 1.14 (0.86, 1.41) | <.001 | 1.39 (0.99, 1.79) | <.001 | .216 |
SD difference in outcome between cases and controls using the model adjusted for all confounders ± additional factors potentially lying on a bone-fat causal pathway.
Confounders in adjusted model: age, gender, height, alcohol consumption, smoking status, malignancy, steroid use, current and historical PA, hypothyroidism, estrogen replacement use, and years post menopause in women (continuous variables centered for adjustment).
P value for gender interaction.
Adjusted for metallic artifact.
Sensitivity analyses
Sensitivity analyses were performed in males and females combined with our model adjusted for all confounders (Supplemental Table 4). First, excluding current/previous steroid use (n = 70) and hypothyroidism (n = 29) did not materially affect point estimates (Supplemental Tables 5 and 6). Second, when restricting to those scanned by Lunar DXA, although absolute DXA values were marginally lower, results were no different from those obtained after combining Lunar and Hologic data using standardization formulae (Supplemental Table 7). In menopause-stratified analyses, although premenopausal women were fewer, differences in FM observed between HBM cases and controls were more evident among postmenopausal women, in whom HBM cases had greater android, gynoid, and trunk fat. As expected, bone turnover markers were higher among postmenopausal than premenopausal women (Supplemental Table 8) (small sample size constrained full adjustment for PA). The higher prevalence of prior cancer was investigated by restricting analyses to relatives and spouses only (ie, excluding index cases who may have been biased by DXA referral indication). Compared with 7 (5.6%) controls with a cancer history, 6 (11.8%) relative/spouse HBM cases reported a cancer history (P = .152); most (n = 25) reported that cancer occurred within the HBM index cases.
Discussion
We examined a population with unexplained extreme HBM, characterized by a mild skeletal dysplasia (14), to investigate the relationship between elevated BMD and BMI. We found that in HBM women FM is elevated, with a particular tendency toward central adiposity. HBM cases are thought to have a genetic predisposition to their raised BMD, suggesting a causal pathway whereby raised BMD leads to increased FM. Several observations support a primary bone phenotype with secondary fat effects (14). For example, although substantial, the observed FM elevation in HBM cases was less marked than their BMD increase (0.5 and 1.5 SD increases in TB FM and BMD in HBM cases compared to controls, respectively). Secondly, in regression analyses, FM differences between HBM cases and controls were attenuated by BMD adjustment; conversely, BMD differences were not attenuated by FM adjustment. Although absolute LM was greater among HBM cases than controls (by .25 SD), this was explained by observed FM differences; relative to bone and FM increases, female HBM cases had disproportionately reduced LM. Although a higher proportion of HBM cases had hypothyroidism and current/previous glucocorticoid use, the latter possibly reflecting the original indication for DXA scanning index cases, this did not explain their higher FM.
Our findings are consistent with those from an extended Danish family with extremely elevated bone mass, due to activating T253I LRP5 mutations, found to have elevated FM (11). However, normal BMIs have also been described in both M282V and G512T LRP5 HBM and sclerosteosis due to SOST mutations (32–36), possibly reflecting differences in gender, age, and small study numbers. Our findings supporting a primary bone phenotype with secondary FM changes conflict with our previous Mendelian randomization analysis in adolescents in ALSPAC, which suggested a causal influence of obesity on BMD (11). One explanation is a bidirectional causal pathway between fat and bone, with the effects of bone on fat prevailing in individuals with extreme BMD elevations.
Alternatively, rather than a physiological relationship between bone and FM, genetic pleiotropy, whereby mutations affect both BMD and FM, could explain our findings as well as monogenic LRP5 HBM FM increases (11), given the role of wnt signaling pathways in adipogenesis (37). Although direct sequencing of exons 2, 3, and 4 has identified anabolic LRP5 mutations in < 2% of HBM cases (32), other HBM-causing genes, yet to be identified, may also contribute to adipogenesis. Of note, CLCN7 mutations causing autosomal dominant osteopetrosis (ADOII) seem a less likely pleiotropic cause, first, because raised BMI has not been a reported feature of ADOII (38, 39), and second, because previous radiological evaluation of all our HBM cases has not shown any characteristics of ADOII (14).
The clinical implications of elevated FM in HBM may relate to established complications of obesity, such as type II diabetes, particularly because central adiposity increases insulin resistance risk (40). Furthermore, disproportionately low LM in HBM may reflect sarcopenic obesity (41). Trunk fat, a proxy for visceral fat (42), has been associated with dyslipidemia and insulin resistance (43, 44); however, our study was not powered to detect metabolic and cardiovascular health outcomes. Further studies to directly evaluate insulin resistance are planned, as are those to assess muscle function.
Determining the direction of causality between bone and fat in HBM cases aids mechanistic understanding of bone-fat pathways. Currently, the genetic basis underlying the great majority of our HBM cases is unknown (32). Given phenotypic similarities between our HBM cases and those with established activating LRP5 mutations, one might expect enhanced osteoblast function and elevated bone formation markers in HBM when the phenotype is developing (eg, during puberty). Consistent with this view, recent peripheral quantitative computed tomography evaluation has shown greater tibial and radial cross-sectional area in HBM, which is likely to reflect enhanced periosteal apposition secondary to increased osteoblast activity (45). Yet LRP5 HBM case reports/series have reported low, normal, and high turnover (12, 32–34, 46, 47). In our study, bone turnover markers, including osteocalcin, tended to be lower among HBM cases (4 cases with specific LRP5 mutations had either normal or higher turnover). It may be that the same mechanisms initially responsible for gaining higher bone mass then lead to suppression of bone turnover once HBM is achieved, thereby ensuring that HBM is maintained in later life.
Rodent studies point toward a pathway of reduced bone turnover resulting in decreased osteocalcin, associated with lower adiponectin, impaired insulin sensitivity, and increased fat deposition due to reduced energy expenditure (6). We explored whether a similar mechanism might explain the elevated FM observed in our HBM cases. Bone turnover sampled at a single time point may not reflect accumulated exposure over the life course, which we are unable to measure. Nevertheless, on the basis of our results suggesting that 1) bone turnover tends to be suppressed once HBM is established, and 2) higher FM in HBM cases is age-dependent, our findings are consistent with an equivalent causal pathway between bone turnover and energy metabolism to that seen in rodents. However, conversely, FM differences between HBM cases and controls were not attenuated by adjustment for bone turnover. Differences in FM between HBM cases and controls were attenuated by adjustment for hip but not lumbar spine BMD, potentially reflecting a preferential cortical bone-fat relationship. Cortical bone influencing fat metabolism may reflect a cortical-derived factor, eg, sclerostin, which was recently found to be positively related to FM in postmenopausal women (48).
We observed large FM differences between HBM cases and controls in women but not men, particularly postmenopausal women, suggesting a possible interaction between BMD, FM, and sex steroid deficiency. This apparent sex interaction is hard to explain, particularly in light of recent evidence that osteocalcin acts as a testosterone regulator in men, which might be expected to exert secondary effects on body composition (49). However, we also observed a trend toward increasing FM differences in women according to HBM status with age. Hence, despite adjustment, it is difficult to determine whether the greater FM elevation of postmenopausal HBM cases simply reflects their greater age as opposed to duration of estrogen deficiency.
Limitations
A key limitation is our current lack of longitudinal data, limiting conclusions regarding directions of causality. Relative/spouse controls were considered to be appropriate controls because 1) they share common environmental factors with cases that would otherwise be difficult to measure and control for as confounding factors; 2) their inclusion aids future genetic studies because it permits the use of more powerful family-based analyses; and 3) they had appropriate BMD (Table 2). However, because controls were recruited from within families, they are likely to have been more similar to HBM cases than unrelated general population controls; hence, clustered analyses were performed to account for the lack of statistical independence due to within-family clustering of environmental factors and shared genotypes. Despite this, our reported differences may still underestimate the true magnitude of the HBM phenotype, than would HBM cases if compared with general population controls. Considering referral indications for clinical DXA services, our study design most likely accounts for differences between cases and controls in gender, postmenopausal status, estrogen replacement, steroid use, and prior history of malignancy. For example, index cases were more often 1) female, hence heterosexual partner controls were more often male; and 2) postmenopausal, and hence their children rather than their parents more often participated, explaining the age difference between cases and controls. Although fewer men were sampled, adjusted FM analyses still had 89.6% power (α = .05) to detect the observed difference between cases and controls. Although we collected data regarding a range of potential confounders, residual confounding cannot be excluded, particularly considering the differences in clinical characteristics between HBM cases and controls. Due to logistical limitations, βCTX was sampled without controlling for possible effects of diurnal variation or food ingestion; however, bias would be expected to be nondifferential.
Conclusions
To investigate causal pathways between bone and fat, we conducted a case-control study of body composition and bone turnover in a UK-based population of HBM cases in whom BMD is thought to be markedly increased as a result of a primary genetic cause. We found that, compared with controls, TB FM was on average 9 kg higher in female HBM cases, in whom FM remained constant with age, in contrast to an inverse association observed among controls. Differences in FM observed between HBM cases and controls were explained by adjustment for BMD, but not by bone turnover; the latter being modestly reduced in HBM. In contrast, the greater BMD of female HBM cases compared with controls was not attenuated by adjustment for FM. We conclude that HBM is associated with greater FM, reflecting either genetic pleiotropic effects or supporting, within the context of an extreme bone phenotype, a causal pathway between bone mass and FM that is independent of bone turnover. Further studies are justified to explore the mechanisms involved and to determine their impact on insulin resistance and other metabolic health outcomes.
Supplementary Material
Acknowledgments
We thank all our study participants and the staff at our collaborating centers, particularly at the Wellcome Trust Clinical Research Facility in Birmingham, Cambridge National Institute for Health Research (NIHR) Biomedical Research Centre, and Addenbrooke's Wellcome Trust Clinical Research Facility, including Dr. Ken Poole, NIHR Bone Biomedical Research Unit in Sheffield, and the Centre for Metabolic Bone Disease in Hull.
This study was supported by The Wellcome Trust and the National Institute for Health Research Clinical Research Network (portfolio no. 5163); supporting Comprehensive Local Research Networks included Birmingham and the Black Country, North and East Yorkshire and Northern Lincolnshire, South Yorkshire, West Anglia, and Western. C.L.G. was funded through a Wellcome Trust Clinical Research Training Fellowship (080280/Z/06/Z).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADOII
- Autosomal dominant osteopetrosis
- BMC
- bone mineral content
- BMD
- bone mineral density
- BMI
- body mass index
- CI
- confidence interval
- βCTX
- β-C-telopeptides of type I collagen
- DXA
- dual-energy x-ray absorptiometry
- FM
- fat mass
- HBM
- high bone mass
- IPAQ
- International Physical Activity Questionnaire
- L1
- first lumbar vertebra
- LM
- lean mass
- PA
- physical activity
- P1NP
- procollagen type 1 amino-terminal propeptide
- sBMD
- standardized BMD
- TB
- total body.
References
- 1. Reid IR, Plank LD, Evans MC. Fat mass is an important determinant of whole body bone density in premenopausal women but not in men. J Clin Endocrinol Metab. 1992;75:779–782 [DOI] [PubMed] [Google Scholar]
- 2. Khosla S, Atkinson EJ, Riggs BL, Melton LJ. Relationship between body composition and bone mass in women. J Bone Miner Res. 1996;11:857–863 [DOI] [PubMed] [Google Scholar]
- 3. Felson DT, Zhang Y, Hannan MT, Anderson JJ. Effects of weight and body mass index on bone mineral density in men and women: The Framingham study. J Bone Miner Res. 1993;8:567–573 [DOI] [PubMed] [Google Scholar]
- 4. Reid IR, Legge M, Stapleton JP, Evans MC, Grey AB. Regular exercise dissociates fat mass and bone density in premenopausal women. J Clin Endocrinol Metab. 1995;80:1764–1768 [DOI] [PubMed] [Google Scholar]
- 5. Reid I. Relationships between fat and bone. Osteoporos Int. 2008;19:595–606 [DOI] [PubMed] [Google Scholar]
- 6. Lee NK, Sowa H, Hinoi E, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kindblom JM, Ohlsson C, Ljunggren O, et al. Plasma osteocalcin is inversely related to fat mass and plasma glucose in elderly Swedish men. J Bone Miner Res. 2009;24:785–791 [DOI] [PubMed] [Google Scholar]
- 8. Wu N, Wang QP, Li H, Wu XP, Sun ZQ, Luo XH. Relationships between serum adiponectin, leptin concentrations and bone mineral density, and bone biochemical markers in Chinese women. Clin Chim Acta. 2010;411:771–775 [DOI] [PubMed] [Google Scholar]
- 9. Schafer AL, Sellmeyer DE, Schwartz AV, et al. Change in undercarboxylated osteocalcin is associated with changes in body weight, fat mass, and adiponectin: parathyroid hormone (1–84) or alendronate therapy in postmenopausal women with osteoporosis (the PaTH study). J Clin Endocrinol Metab. 2011;96:E1982–E1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anastasilakis AD, Efstathiadou Z, Plevraki E, et al. Effect of exogenous intermittent recombinant human PTH 1–34 administration and chronic endogenous parathyroid hormone excess on glucose homeostasis and insulin sensitivity. Horm Metab Res. 2008;40:702–707 [DOI] [PubMed] [Google Scholar]
- 11. Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res. 2009;24:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frost M, Andersen T, Gossiel F, et al. Levels of serotonin, sclerostin, bone turnover markers as well as bone density and microarchitecture in patients with high bone mass phenotype due to a mutation in Lrp5. J Bone Miner Res. 2011;26:1721–1728 [DOI] [PubMed] [Google Scholar]
- 13. Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gregson CL, Steel SA, O'Rourke KP, et al. ‘Sink or swim’: an evaluation of the clinical characteristics of individuals with high bone mass. Osteoporos Int. 2012;23:643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kiebzak GM, Leamy LJ, Pierson LM, Nord RH, Zhang ZY. Measurement precision of body composition variables using the lunar DPX-L densitometer. J Clin Densitom. 2000;3:35–41 [DOI] [PubMed] [Google Scholar]
- 16. Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hallal PC, Victora CG. Reliability and validity of the International Physical Activity Questionnaire (IPAQ). Med Sci Sports Exerc. 2004;36:556. [DOI] [PubMed] [Google Scholar]
- 18. Hagstromer M, Oja P, Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Public Health Nutr. 2006;9:755–762 [DOI] [PubMed] [Google Scholar]
- 19. Kriska AM, Sandler RB, Cauley JA, LaPorte RE, Hom DL, Pambianco G. The assessment of historical physical activity and its relation to adult bone parameters. Am J Epidemiol. 1988;127:1053–1063 [DOI] [PubMed] [Google Scholar]
- 20. Suleiman S, Nelson M. Validation in London of a physical activity questionnaire for use in a study of postmenopausal osteopaenia. J Epidemiol Community Health. 1997;51:365–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chasan-Taber L, Erickson JB, McBride JW, Nasca PC, Chasan-Taber S, Freedson PS. Reproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol. 2002;155:282–289 [DOI] [PubMed] [Google Scholar]
- 22. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. Seoul, Korea: 59th World Medical Association General Assembly; 2008 [Google Scholar]
- 23. Pitman E. A note on normal correlation. Biometrika. 1939;31:9–12 [Google Scholar]
- 24. Fan B, Lu Y, Genant H, Fuerst T, Shepherd J. Does standardized BMD still remove differences between Hologic and GE-Lunar state-of-the-art DXA systems? Osteoporos Int. 2010;21:1227–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shepherd JA, Fan B, Wu X, Wacker WK, Ergun D, Levine M. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. JBMR. 2012;10:2208–2016 [DOI] [PubMed] [Google Scholar]
- 26. Hanson J. Standardization of femur BMD. J Bone Miner Res. 1997;12:1316–1317 [DOI] [PubMed] [Google Scholar]
- 27. Hui SL, Gao S, Zhou XH, et al. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res. 1997;12:1463–1470 [DOI] [PubMed] [Google Scholar]
- 28. Shepherd JA, Fan B, Lu Y, et al. A multinational study to develop universal standardization of whole body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. J Bone Miner Res. 2012;27:2208–2216 [DOI] [PubMed] [Google Scholar]
- 29. Barber TM, Hazell M, Christodoulides C, et al. Serum levels of retinol-binding protein 4 and adiponectin in women with polycystic ovary syndrome: associations with visceral fat but no evidence for fat mass-independent effects on pathogenesis in this condition. J Clin Endocrinol Metab. 2008;93:2859–2865 [DOI] [PubMed] [Google Scholar]
- 30. Ebinuma H, Miyazaki O, Yago H, Hara K, Yamauchi T, Kadowaki T. A novel ELISA system for selective measurement of human adiponectin multimers by using proteases. Clin Chim Acta. 2006;372:47–53 [DOI] [PubMed] [Google Scholar]
- 31. Greenland S, Brumback B. An overview of relations among causal modelling methods. Int J Epidemiol. 2002;31:1030–1037 [DOI] [PubMed] [Google Scholar]
- 32. Johnson ML, Gong G, Kimberling W, Recker SM, Kimmel DB, Recker RB. Linkage of a gene causing high bone mass to human chromosome 11 (11q12–13). Am J Hum Genet. 1997;60:1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Balemans W, Devogelaer JP, Cleiren E, Piters E, Caussin E, Van Hul W. Novel LRP5 missense mutation in a patient with a high bone mass phenotype results in decreased DKK1-mediated inhibition of Wnt signaling. J Bone Miner Res. 2007;22:708–716 [DOI] [PubMed] [Google Scholar]
- 35. Hamersma H, Gardner J, Beighton P. The natural history of sclerosteosis. Clin Genet. 2003;63:192–197 [DOI] [PubMed] [Google Scholar]
- 36. Van Lierop A, Hamersma H, Hamdy N, Papapoulos S. Gene dose effect of a SOST mutation on circulating sclerostin and P1NP. Bone. 2011;48(supp 2):S85 (Abstract) [Google Scholar]
- 37. Prestwich TC, MacDougald OA. Wnt/B-catenin signaling in adipogenesis and metabolism. Curr Opin Cell Biol. 2007;19:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Waguespack SG, Hui SL, DiMeglio LA, Econs MJ. Autosomal dominant osteopetrosis: clinical severity and natural history of 94 subjects with a chloride channel 7 gene mutation. J Clin Endocrinol Metab. 2007;92:771–778 [DOI] [PubMed] [Google Scholar]
- 39. Benichou OD, Laredo JD, de Vernejoul MC. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): clinical and radiological manifestations in 42 patients. Bone. 2000;26:87–93 [DOI] [PubMed] [Google Scholar]
- 40. Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811 [DOI] [PubMed] [Google Scholar]
- 41. Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395 [DOI] [PubMed] [Google Scholar]
- 42. Sayers A, Timpson NJ, Sattar N, et al. Adiponectin and its association with bone mass accrual in childhood. J Bone Miner Res. 2010;25:2212–2220 [DOI] [PubMed] [Google Scholar]
- 43. Nelson TL, Bessesen DH, Marshall JA. Relationship of abdominal obesity measured by DXA and waist circumference with insulin sensitivity in Hispanic and non-Hispanic white individuals: the San Luis Valley Diabetes Study. Diabetes Metab Res Rev. 2008;24:33–40 [DOI] [PubMed] [Google Scholar]
- 44. Sun Q, van Dam RM, Spiegelman D, Heymsfield SB, Willett WC, Hu FB. Comparison of dual-energy x-ray absorptiometric and anthropometric measures of adiposity in relation to adiposity-related biologic factors. Am J Epidemiol. 2010;172:1442–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gregson CL, Sayers A, Lazar V, et al. The high bone mass phenotype is characterised by a combined cortical and trabecular bone phenotype: findings from a pQCT case-control study. Bone. 2013;51:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521 [DOI] [PubMed] [Google Scholar]
- 47. Whyte MP, Reinus WH, Mumm S. High-bone mass disease and LRP5. N Engl J Med. 2004;350:2096–2099 [DOI] [PubMed] [Google Scholar]
- 48. Urano T, Shiraki M, Ouchi Y, Inoue S. 2012 Association of circulating sclerostin levels with fat mass and metabolic disease-related markers in Japanese postmenopausal women. J Clin Endocrinol Metab. 97:E1473–E1477 [DOI] [PubMed] [Google Scholar]
- 49. Karsenty G. The mutual dependence between bone and gonads. J Endocrinol. 2012;213:107–114 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.