Abstract
The view that immunoglobulins function largely by potentiating neutralization, cytotoxicity or phagocytosis is being replaced by a new synthesis whereby antibodies participate in all aspects of the immune response, from protecting the host at the earliest time of encounter with a microbe to later challenges. Perhaps the most transformative concept is that immunoglobulins manifest emergent properties, from their structure and function as individual molecules to their interactions with microbial targets and the host immune system. Given that emergent properties are neither reducible to first principles nor predictable, there is a need for new conceptual approaches for understanding antibody function and mechanisms of antibody immunity.
In this perspective, we will first describe the historical underpinnings of longstanding concepts of how antibodies protect against microbes and then introduce newly identified mechanisms of antibody action, which have shown that the reach of antibody-mediated immunity (AMI) is far greater than would be predicted from classical mechanisms of antibody action. These novel mechanisms call for a new synthesis for understanding AMI to pathogens.
Historical concepts of AMI to pathogens
The discovery of AMI by von Bering and Kitasato in 1891 galvanized studies of immunity to microbial diseases and led to the first effective antimicrobial agent, serum therapy. AMI was a foundation of immunology, and since 1900, no fewer than eight Nobel prizes have been awarded for discoveries related to immunoglobulin structure and function or applications to medicine. However, in the final decades of the 20th century, the study of AMI entered the doldrums, as there was a sense that the major problems in the field had been solved. Other forces also diminished interest in AMI. The human immunodeficiency virus pandemic and rise of infectious diseases in immunocompromised patients highlighted the role of cellular immunity in host defense, as did the identification of the T cell antigen receptor and other molecules that regulate T cell responsiveness. Studies of T cells were also more in tune with the molecular reductionist approaches favored in the late 20th century. Linear T cell epitopes allowed elegant experiments that were either difficult or impossible to do with the three-dimensional epitopes recognized by immunoglobulins. Furthermore, theories that placed immunoglobulins at the center of complex regulatory networks fell out of favor. For example, Jerne’s idiotypic network1 was abandoned as different explanations for immune regulation, such as by cytokines, signal transduction cascades and cellular differentiation, were increasingly sought2. Paradoxically, the shift away from studies of AMI occurred at a time of great technological advances, including hybridoma technology, molecular tools for immunoglobulin expression, and the development of antibody therapies for cancer, infectious diseases and rheumatological conditions.
The great immunological catastrophe
Perhaps nothing has hindered immunological thought more than the debate on the relative importance of humoral and cellular immunity. This intellectual struggle initiated by Ehrlich and Metchnikoff and their disciples in the late 19th century had tremendous influence on immunological paradigms3 and greatly influenced research in the 20th century. The problem was that the methodologies available could not establish a role for AMI to many microbes. For most of 20th century, the efficacy of AMI was established by one of the following criteria: the demonstration that the transfer of immune serum confers resistance to the microbe, a correlation between a specific antibody and resistance to a microbe, or susceptibility to a microbe in organisms with antibody deficiency. If one or more of those criteria was met, then AMI was considered important for protection against the causative microbe, but those criteria failed to establish the efficacy of AMI to many microbes. For example, a role for AMI to tuberculosis could not be consistently demonstrated4. Thus, antibodies were long believed to have no role in resistance to Mycobacterium tuberculosis. Although this premise was indirectly supported by the importance of cellular immunity in resistance to tuberculosis, it was perpetuated by the erroneous conclusion that intracellular microbes were outside the reach of AMI because antibodies do not enter cells5. Furthermore, the conclusion that AMI has no role in protection against M. tuberculosis was logically flawed, as it is not possible to draw a positive inference from negative data. In fact, these conclusions were erroneous, as several independent groups have now established that the passive administration of defined monoclonal antibodies (mAbs) can alter the course of experimental M. tuberculosis infection to the benefit of the host6–9. In addition, a role for B cells in protection against M. tuberculosis has also been established10,11.
The ability of defined mAbs to mediate protection against M. tuberculosis and other pathogens for which the aforementioned criteria have provided negative data has now been established12. Thus, the difficulty in establishing a role for AMI was due to a failure of the available preparations to mediate protection. Furthermore, a lack of the understanding that ‘antibody’ comprises a heterogeneous mixture of molecules with different specificities and functions made it impossible to discriminate antibodies that confer a benefit from those that do not. Additionally, the possibility that antibodies could contribute to natural resistance to disease and/or enhance innate immunity was not considered. Pertinent to the last observation, immunoglobulin M (IgM) can enhance granuloma formation in the experimental infection of mice with mycobacteria or Cryptococcus neoformans, a fungal facultative intracellular pathogen13,14.
The view that intracellular pathogens are outside the reach of AMI fails to recognize that antibodies can contribute to host defense in many ways apart from promoting microbial clearance5. The prevailing notion that AMI can work only on the outside of a host cell or at the surface of a microbe by enhancing phagocytosis or neutralizing a toxin or virus was catastrophic in that it constrained vaccine and immunological research for decades. This is exemplified by the identification of antibodies that are highly protective against Streptococcus pneumoniae in mice but do not promote opsonic killing of pneumococcus by phagocytes in vitro15–17. Thus, the longstanding practice of dividing research on the immune response to a microbe based on microbial type and whether it is intracellular or extracellular precluded important avenues of investigation and caused researchers to miss the opportunity to assemble an integrated model of AMI to microbes based on host-microbe interaction. The intracellular-extracellular divide also precluded investigation of the importance of cellular immunity in protection against so-called extracellular microbes. For example, protection against S. pneumoniae was believed to be exclusively dependent on AMI, but subsequent evidence has shown a critical role for innate and cellular immunity in this17–20.
The old synthesis: antibodies as connector molecules
In their function as promoters of opsonization, complement activation and antibody-dependent cellular cytotoxicity, specific antibodies were held to mediate a connection between a microbial structure and a host component, such as receptor for the crystallizable fragment of an immunoglobulin (FcR) or receptors for complement (Box 1). The specific binding of antibodies to viral and toxin particles was held to mediate neutralization via antibody-antigen complexes that interfere with viral infectivity or toxin-mediated toxicity. By the 1960s, advances in protein structure analysis showed that immunoglobulins consist of two domains, one for binding antigen and the other for mediating effector functions that depend on components of the immune system, such as FcR and complement. These antibody functions solidified the view that antibody molecules function in a passive mode, serving to connect their cognate antigens to the host’s immune system.
Box 1. Classical and nonclassical antibody functions.
Antibody functions known in the 1970s, before there was the great shift in immunology away from studies of basic antibody function, are considered ‘classical’. Hence, classical antibody functions are historically defined mechanisms of antibody action; these functions include toxin neutralization, viral neutralization, complement fixation, antibody-dependent cellular cytotoxicity and opsonization. Toxin neutralization, viral neutralization, complement fixation and opsonization were each known by the first decades of the 20th century. In contrast, nonclassical antibody functions do not involve these mechanisms and have been discovered relatively recently. These functions include direct antimicrobial activity; alteration of microbial signal transduction; and immunomodulation and modulation of microbial physiology.
Insights into AMI originate from few model systems
Much of what is known about AMI has been provided by detailed studies of only a few model systems. The availability of animal models of S. pneumoniae– and toxin-mediated diseases, such as tetanus, in the early years of the 20th century provided key insights into antibody function21. Subsequently, classical studies of viruses in newly established cell culture systems provided key insights into antibody-mediated viral neutralization. In recent years, the development of new experimental models has led to new insights from previously unlikely sources, such as in vitro and animal models of the fungus C. neoformans. The latter is an encapsulated microbe for which, for most of the 20th century, AMI was not thought to have any role in host defense. As many of the arguments developed here originated with observations made of C. neoformans, we include a brief description of the microbe (Box 2). However, so far AMI has been extensively explored to only a relatively small number of microbes and toxins. Thus, caution should be exercised in making generalizations, as the extent to which observations made with one microbe can be extrapolated to others is unknown.
Box 2. The Cryptococcus neoformans system.
C. neoformans is a fungal pathogen that is a relatively frequent cause of life-threatening meningoencephalitis, mainly in people with impaired immunity. This microbe is a facultative intracellular pathogen that is unusual in that it not only replicates inside macrophages but is capable of nonlytic exocytosis. For most of the 20th century, AMI was held to be unimportant for host resistance to cryptococcal disease72. That view gained considerable support in the 1980s and early 1990s, when there was a staggering increase in cases of cryptococcosis in people infected with human immunodeficiency virus who had profound T cell deficiency. Thus, immunity to C. neoformans was considered to be mediated by cellular immunity. However, the development of mAbs to C. neoformans capsular polysaccharide opened new avenues of investigation that led to the discovery that, contrary to longstanding dogma, defined antibodies to defined C. neoformans determinants are able to mediate protection against experimental cryptococcosis in mice73–76. Antibodies to C. neoformans mediate protection by promoting phagocytosis and enhancing host cellular immunity. Further study of these mechanisms have identified new and unexpected aspects of antibody structure-function relationships, including the following: in normal mice, IgG1 and IgG2 are protective, whereas IgG3 is nonprotective; an excess of antibody or antigen can undermine antibody protection by a prozone-like effect; the C domain (subclass) can alter the specificity of an otherwise identical antibody (matched by V region); and defined binding of antibodies to cryptococcal cells can alter the gene expression of the cells, resulting in greater susceptibility to drugs77.
New antibody functions
AMI is correctly considered an outcome of the adaptive immune response, but there is ample evidence that naturally occurring antibodies, in particular IgM, are critical for the early response to many microbes, which suggests that they are actually part of the innate immune system. Naturally occurring IgM, derived from B-1 cells in mice and from memory B cells and/or B1-like cells in humans, binds conserved microbial polysaccharide determinants, thus providing a first line of pathogen defense22–24. Consistent with that role, naturally occurring IgM is essential for resistance to fungal, bacterial and viral diseases in mouse models14,25–30. Although naturally occurring IgM is of relatively low affinity, it has high avidity and is a potent activator of complement. In fact, the requirement for immunoglobulins at the earliest stages of infection blurs the line between adaptive and innate immunity, which makes it difficult to define each as an independent arm of the immune system.
The ability of immunoglobulins to modulate inflammation has been recognized for decades. Immunological phenomena, such as immediate hypersensitivity and the Arthus reaction, are examples of proinflammatory antibody effects that result in host damage. However, proinflammatory activity can also be beneficial. For example, inflammatory mediators such as chemokines and cytokines enhance microbial clearance, which is crucial to host defense. Similarly, antibody-mediated complement activation, a proinflammatory event, is also important in the clearance of viruses, bacteria and fungi. However, antibody is also able to dampen the inflammatory response. For example, intravenous administration of polyclonal immunoglobulin has numerous applications in clinical medicine as an anti-inflammatory agent. The anti-inflammatory activity of this therapy has been linked to sialation in the immunoglobulin glycan motif31, through a T helper type 2–related mechanism32. Evidence that stimulation of the FcR by IgG has adjuvant as well as immunomodulatory properties suggests that evolving antibody responses can alter themselves in the setting of infection33. Another type of AMI-based immunomodulation could stem from idiotype– anti-idiotype interactions. Such interactions, put forth many decades ago1, were believed to regulate the amount of specific antibody. An example of this phenomenon has been revealed by a study showing that idiotype–anti-idiotype binding is responsible for loss of the binding of antibody specific to the Staphylococcus aureus capsular polysaccharide to S aureus after immunization with capsular polysaccharide34,35.
Studies of C. neoformans have revealed how the same antibody can be proinflammatory or anti-inflammatory depending on the host and the state of infection. The administration of IgG1 after the onset of cryptococcal infection can result in rapid death associated with cardiovascular collapse resulting from release of the proinflammatory mediator platelet-activating factor36. However, the administration of IgG1 before intrapulmonary challenge dampens proinflammatory responses, resulting in improved granuloma formation and fungal clearance37. Hence, immunoglobulins can have pro- or anti-inflammatory properties depending on interactions between antibodies and host receptors. In this context, the data available suggest that the proinflammatory activity of IgM is related to its ability to activate complement, whereas the function of IgG is more complex, in that it is able to be pro- or anti-inflammatory depending on the amount of antibody, the FcR engaged and the IgG subtype.
The cellular and molecular basis for pro- and anti-inflammatory effects of IgG has been elucidated with the discovery of stimulating and inhibitory FcRs38,39. Whether a pro- or anti-inflammatory effect is helpful or harmful is most probably a complex property that includes the microbe, the amount of antibody, the type of antibody and the inflammatory and immunological state of the host (Fig. 1). The damage-response framework of microbial pathogenesis provides an intellectual construct for understanding how pro- and anti-inflammatory antibodies can be helpful or harmful to individual hosts depending on the amount of damage that stems from host-microbe interactions, whereby damage can result from insufficient or excessive inflammation40–42. Relevant to this point, but also illustrative of the complexity of AMI, protective IgG1 mAbs to S. pneumoniae capsular polysaccharide show a requirement for different host FcRs and phagocytes17. An opsonic mAb that promotes the killing of S. pneumoniae by phagocytes in vitro requires neutrophils and the inhibitory receptor FcγRIIB to mediate protection against pneumococcal pneumonia in mice, whereas a nonopsonic mAb does not require neutrophils and requires FcγR but not FcγRIIB.
Figure 1.
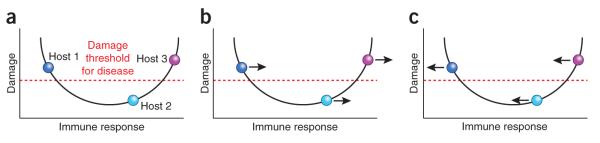
Effect of pro- and anti-inflammatory antibodies on three hosts that differ in their immune response to infection, as viewed in the context of the damage-response framework42. (a) Positions of three infected hosts in the immune response: hosts 1 and 3 manifest disease at the two extremes of the immune response, whereas host 2 remains asymptomatic by mounting an immune response that is sufficiently strong to control infection while avoiding immune-mediated host damage. (b) The effect of a proinflammatory antibody on the three hosts in a. In this situation, the immune response of host 1 is shifted to a more optimal position and the antibody is protective, whereas for hosts 2 and 3 it has the potential to promote disease by enhancing inflammation. (c) The effect of an anti-inflammatory antibody on the three hosts in a. In this situation, host 3 benefits, whereas the same antibody can be detrimental to host 1 and ‘indifferent’ to host 2.
In the past decade, much evidence has emerged showing that antibodies can also function as direct effector molecules against bacteria, fungi and parasites. Perhaps the best example of a directly bactericidal antibody is one that binds outer surface protein B of Borrelia burgdorferi43. The ability of antibodies to this protein to directly kill B. burgdorferi resides in their variable (V) region and seems to be the result of antibody-mediated disruption of the outer membrane, which results in the formation of membrane blebs as a prelude to cell lysis44. Fungi were historically considered impervious to AMI, but several mAbs that mediate direct antifungal activity have now been described. Anti-idiotypic antibodies to the fungal product known as ‘killer toxin’ mediate direct antifungal activity as well as activity against bacteria by mimicking toxin activity45. A mAb that binds to a Candida albicans surface protein mediates three antifungal activities: direct fungal killing, inhibition of filamentation and inhibition of tissue adhesion46. The candidacidal effects of this mAb result from blockade of iron uptake46. Antibodies to fungal cell-wall components, including β-glucans47 and melanin48, mediate direct antifungal effects on all fungi species that express the relevant determinant, presumably by interfering with cell-wall metabolism. Catalytic antibodies that produce ozone have been reported to have antibacterial activities49, and a mAb to S. pneumoniae capsular polysaccharide can enhance bacterial killing (or ‘fratricide’) in cultures rendered competent for bacterial transformation50.
AMI can trigger signal-transduction cascades by the engagement of FcRs. In another twist in microbicidial activity, IgE immunocomplexes reportedly trigger the intracellular killing of Toxoplasma gondii by engagement of FcεRII-CD23 receptors, which induces antiprotozoal cell signaling51. The ability of specific antibodies to mediate direct antimicrobial effects by different mechanisms involving FcRs underscores the proposal that AMI can mediate independent, regulatory effects. This regulatory ability holds great promise for the rational design of antibody-based therapies and vaccines, with the caveat that the outcome of antibody-mediated regulation of the inflammatory response is not fixed but is a function of host-microbe interactions. Another aspect of antibody-mediated regulation stems from the ability of antibody binding to microbial antigens on the cell surface to mediate changes in microbial signal transduction. For example, the binding of antibodies to viral antigens expressed on the cell surface can result in directional changes in the movement of these antigens to one pole in a phenomenon known as ‘capping’52. Capping is associated with the interference of viral spread and, interestingly, resembles the immunoreceptor capping that leads to tyrosine phosphorylation and the formation of lipid rafts containing viral glycoproteins53.
A new frontier in AMI and its interactions with microbes was opened by the demonstration that the binding of antibodies to the capsules of C. neoformans and S. pneumoniae can modulate gene expression. For C. neoformans, such antibody binding causes no obvious harm to the fungal cell54, but for S. pneumoniae, binding of antibody enhances quorum sensing and the expression of genes encoding molecules involved in transformation competence and fratricide50. Such observations demonstrate that another outcome of the binding of antibodies to microbial surface structures can be to affect microbial physiology, biology and communication. For C. neoformans, protective and nonprotective mAbs induce different types of changes in gene expression, with protective antibodies activating lipid-metabolic pathways that make the fungal cell more susceptible to antifungal drugs54. For S. pneumoniae, some protective mAbs enhance the frequency of pneumococcal transformation, whereas others do not, with a distinguishing characteristic of those that enhance transformation being the ability to promote bacterial aggregation and induce quorum sensing50. Protective mAbs that do not enhance transformation are opsonic antibodies that promote opsonophagocytosis and the killing of S. pneumoniae by effector cells. The precedent that AMI can modulate microbial physiology could have far-reaching implications, given that immunoglobulins are present in body compartments inhabited by commensal flora, and raises the possibility that another function of AMI could be to serve as a system for communication with the microbial flora in the body.
The uniqueness of humoral immunity
In contrast to other molecular components of the immune system, each antibody is structurally different, and such differences can ‘translate’ into functional differences. Immunoglobulin structure and function are defined by the gene elements used to generate the V region, somatic mutations to such genes, post-translation modifications to the expressed protein and the association of such V regions with constant (C) domains. There is now convincing evidence that the C domain can affect certain aspects of V-region function, such as affinity and specificity, possibly as a result of structural constraints resulting from isotype-related polymorphisms in the C heavy-chain region 1 domain55. Consequently, each combination of V region and C region has the potential to generate new molecules with new functions even when the V-region sequences are identical. Hence, in contrast to other products of the immune system such as complement proteins, defensins and cytokines, antibodies have different structures and functions, so their function cannot be generalized or easily predicted.
Unsolved problems in AMI
The discovery that antibody can protect mice against the intracellular pathogens M. tuberculosis, Listeria monocytogenes56,57, C. neoformans and Histoplasma capsulatum58 indicates that the historical inability to demonstrate a protective role for AMI to these microbes was not a limitation of antibody function but was a consequence of suboptimal antibody responses and inadequate experimental models. The question of why some microbes elicit ineffective natural antibody responses has many possible answers. In the case of C. neoformans, the immune response seems to be focused on epitopes that elicit nonprotective antibodies and/or the production of antibodies of isotypes that do not mediate beneficial effects. As for the latter, the most effective mouse IgG subclass to C. neoformans polysaccharide is IgG2a, but IgG2a is almost never elicited by natural infection or immunization. In fact, the most common IgG subclass elicited is IgG3, which is usually not protective or disease enhancing. In the case of H. capsulatum, antibodies that are protective bind antigens that do not elicit antibodies during the course of infection, possibly because they are only weakly immunogenic58. L. monocytogenes provides yet another example of this, as high concentrations of antibodies to listeriolysin O are protective, but natural infection does not elicit sufficiently high titers56,57.
The immunoglobulins present in mucosal secretions have long been assumed to serve a protective function, but this has been difficult to prove, particularly for IgA and IgM, the isotypes that predominate in the mucosa. In fact, classical mechanisms of antibody function probably do not operate in saliva or in vaginal or intestinal contents, as these do not contain cells or complement in the absence of inflammation. The finding that certain antibodies can directly modulate microbial function raises the possibility that the host-associated microbial flora can be regulated by antibody. Such a notion may be difficult to prove unambiguously with the experimental systems available at present. Nonetheless, the aforementioned findings obtained with C. neoformans and S. pneumoniae suggest that fuller understanding of mechanisms by which the host microbiota is established and maintained in health and disease could require studies of antibody function.
The older view of an antibody as a bifunctional molecule with two independent domains, with the V region serving as the determinant of antigen specificity and the C domain serving as the determinant of effector function, is no longer tenable, as C domains affect affinity and specificity. The ability of V and C regions to act together in antibody binding stems from structural constraints imposed by C domains on V-region structure and has been documented for three sets of mAbs that differ in isotype while sharing identical V-region sequences59,60. The biological importance of C domain–mediated effects on V-region structure is immense. Certain antigens, such as polysaccharides and viruses, tend to elicit responses restricted to the V region and C domain (isotype), yet isotype switching of a cryptococcal polysaccharide–binding mAb results in the appearance of new mAb reactivities, including reactivity to a variety of self antigens61, which raises the possibility that switching to certain isotypes contributes to autoimmunity. However, not all VC combinations are permissive for C-domain effects on the V region, as a set of IgG mAbs to anthrax toxin manifests identical specificity and affinity despite their different subclasses62. This indicates that the ability of the C domain to affect the V region is limited to certain VC combinations, which suggests the need for comparative studies of VC combinations to identify those that are able to transmit structural information to the V region from the C domain. In addition, it is possible that certain somatic mutations prevent or facilitate the transfer of structural information from the C domain to V regions. In this context, somatic mutation has been associated with changes in the flexibility of V-region structures63; V regions can show considerable flexibility in their ability to produce antibodies to different antigens64; and V regions can show ‘pre-encoded’ antigen specificities65. Furthermore, the fact that the C domain can affect V-region structure raises the possibility that some variation of the now discarded former adopted-fit hypothesis is viable, whereby antigen binding results in structural changes to the V region that are communicated to the C domain, which affects function. If resuscitated, this concept of antibody function, which was essentially abandoned in the late 1970s, could provide new insights into how the binding of antibody to an antigen activates the immunoglobulin molecule to carry out its effector functions.
Immunoglobulins have long been known to mediate their effects through other components of the immune system, such as phagocytes, complement or cells capable of antibody-dependent cellular cytotoxicity (Box 1). However, it is now apparent that immunoglobulin function is also dependent on cells that do not express FcRs. For example, the efficacy of antibodies to C. neoformans is critically dependent on cellular immunity, such that antibody-mediated protection is abrogated in mice deficient in CD4+ T cells or certain cytokines, such as interferon-γ.
It has been reported that combinations of immunoglobulins manifest emergent properties (Box 3) in their protective efficacy in toxin neutralization; two mAbs to S. aureus enterotoxin have been shown to be nonprotective when tested individually and protective when tested in combination66. Similarly, antibody protection against meningococcus requires the binding of two mAbs at nonoverlapping epitopes to trigger complement activation67.
Box 3. The concept of emergence.
Emergent properties are properties that cannot be accounted for by their individual components alone78–80. Examples of emergent properties are consciousness, surface tension, viscosity and the movements of fish schools. Immune responses manifest all the elements of emergent properties, such as novelty and irreducibility to individual parts. The immune response to a pathogen or vaccine is the result of numerous cellular and humoral components that can lead to a new state for the host (immunity or disease). Although this new state could be associated with a particular component of the immune system, such as the production of antibody or the toxic shock resulting from the activation of T cells by a superantigen, the wholeness of the response cannot be reduced to individual components that work in combination to produce unique properties. For example, phagocytosis is not reducible to antibodies and cells alone; it is emergent because it requires the presence of a microbe. The characteristic of novelty inherent in emergent properties indicates that their unpredictability is irreducible to the constituent elements. Notably, unpredictability does not necessarily mean that the inexplicable phenomenon of phagocytosis can be explained once certain facets of the antigen-antibody interaction, antibody-FcR and ingesting capacity of cells are understood. Given that immune responses have emergent properties, a deterministic approach to immunology may not be possible, even if all components of the immune system are fully understood. Instead, a probabilistic approach may be necessary and more rewarding. Probabilistic approaches are successfully used in weather prediction and the yearly development of vaccines against influenza.
Antibody efficacy is concentration dependent, such that protection occurs only when the ‘correct’ amount of antibody is present and is present in the ‘correct’ ratio with antigen. Although lack of antibody efficacy due to insufficient immunoglobulin is instinctively understandable, the abrogation of antibody efficacy at very high concentrations seems counterintuitive. The phenomenon of in vivo effects of the prozone (a zone of relatively high antibody concentrations in which no reaction occurs) has been known since the 1930s, when it was repeatedly documented during the development of serum therapy against S. pneumoniae, but the mechanism of action remained elusive until relatively recently. Studies of C. neoformans have established three mechanisms by which a high specific antibody concentration can ‘translate’ into loss of antibody efficacy68,69. First, the inflammatory response differs at low, medium and high concentrations of antibody, presumably due to effects mediated by FcR crosslinking. Second, high concentrations of antibody on the capsule surface can interfere with oxidative killing. Third, deposition of complement on the capsule surface differs depending on antibody concentration. The occurrence of prozone phenomena in vivo has now been demonstrated in many systems, including viral neutralization, bacterial phagocytosis, toxin neutralization and tumor-cell killing. This phenomenon raises the disturbing possibility that highly immunogenic vaccines could fail if they elicit too much antibody. Furthermore, the dependence of antibody efficacy on antibody concentration raises the fascinating question of how the immune system regulates the amount of antibody to ensure there is enough for protection while avoiding the detrimental effects of too much. This problem is highlighted by the fact that viral, bacterial and fungal infections each challenge the immune system with very different molar amounts of microbial antigen. The mechanism by which the immune system senses the amount of antigen and mounts an antibody response commensurate with protection while avoiding prozone phenomena remains unsolved, although such information seems critical for better vaccine design. Furthermore, the fact that the antibody concentration and the antigen load each change during the course of infection and immune response introduces additional complexity into such regulatory mechanisms (Box 4).
Box 4. A conundrum in antibody response and efficacy.
One of the most perplexing aspects of AMI is that the most effective antibodies are made late in the immune response, often after the host has contained the infection. For example, historically, the diagnosis of many infectious diseases has been made by serology during the convalescent period, when the patient has already recovered or is improving. Furthermore, passive antibodies are most effective when given before infection; the administration of antibodies after infection is often ineffective in modifying the course of disease. In contrast, naturally occurring antibodies have now been shown to be critical for the control of viral, bacterial, and fungal infections. Such observations raise fundamental questions about the function of AMI early and late in the immune response to infection and after subsequent encounter with the same microbe in the setting of immunological memory. The inefficacy of passive antibodies after infection has been attributed to the inability of antibodies to control rapid microbial growth in early infection81, whereas the role of antibodies in the late immune response has been proposed to involve the regulation of cellular immunity82. It is conceivable that AMI differs early and later in the immune response, such that it is proinflammatory and anti-inflammatory during the initial and final stages of infection, respectively.
Toward a new synthesis
There is evidence that a single mAb can be protective, nonprotective and even disease enhancing depending on the host, the microbial inoculum and the amount of antibody present in the host68–70. Consequently, the protective efficacy of an immunoglobulin cannot be assigned on the basis of its molecular structure alone, as knowledge of the nature of the microbe, the state of the microbe, the state of the host immune system and antibody interactions with other immunoglobulins and/or host immune components is needed to understand its efficacy. Hence, antibody function is a complex activity that depends on host components and all aspects of antibody structure and function, each of which manifests emergent properties (Box 3). The unpredictability of antibody function from first principles has profound implications because it precludes the ability to predict a priori the outcome of antibody-microbe interaction in a given host at a given time. We have analyzed the phenomenon of microbial virulence given its emergent properties and conclude that microbial pathogenesis is unlikely to become a predictive science because of the inherent unpredictability associated with the phenomena of emergence71. Here we have extended that concept to AMI and have considered its consequences.
The main consequences of the emergence noted above are new and unpredictable outcomes, characteristics that suggest that definite prediction of the function of antibody molecules in a given host with any degree of certainty might not be possible. Nevertheless, it is known that it is possible to generate highly effective vaccines that elicit antibodies that mediate protection against toxins, viruses and certain bacteria. It is an unequivocal fact that some of the most effective vaccines available today mediate protection in a manner that is proportional to the induced antibody response and that the success of AMI in vivo can be correlated with antimicrobial activity in vitro. Most of these vaccines were developed empirically without consideration of the many complex issues raised here. Success with toxoid and conjugate vaccines indicates that it is relatively easy to elicit protective antibody responses to certain microbes and/or microbial components. Such precedents allow the optimistic view that although AMI exhibits emergent properties, it will be possible to harness antibody function to the benefit of the host. However, we note that microbes for which vaccines are not available at present are those for which classical mechanisms of antibody function have failed to identify a role for AMI in protection against disease, such as fungi, malaria and tuberculosis, despite tremendous efforts using both empiric and rational approaches for vaccine design. So far, there has been little or no success in generating vaccines against microbes thought to be controlled largely through cell-mediated immunity, possibly because in these cases, antibody function is dependent on mechanisms other than microbial clearance and/or on enhancing host immune mechanisms. For such microbes, it is possible that the inability to generate effective vaccines reflects the dual complexity of AMI and the response, whereby solving one is insufficient for the development of a vaccine that can either prevent infection or the subsequent development of disease.
One sobering fact is that most of what is known about AMI has been obtained from in-depth studies of relatively few microbes, and it is not clear to what extent generalizations are possible to other microbes or to other antibody targets, such as cancer cells. However, in the second decade of the 21st century, it is increasingly apparent that AMI is far from understood and that its emergent properties pose tremendous challenges to predictability and integration with other components of the immune system. In the past, the approach to AMI was largely deterministic, such that antibodies present in the setting of an infectious disease were considered either protective or nonprotective. However, the finding that the effects of antibodies can differ depending on the immune status of the host, as well as increasing evidence that the outcome of antibody binding and function is highly complex, suggest the need for a new synthesis to guide investigations of AMI. Although deterministic approaches that link AMI to a specific amount of antibody or an antibody characteristic, such as isotype or effector function, might allow the prediction of antibody efficacy for some microbes and/or toxins, it is clear that such parameters are not sufficient for the prediction of whether and which antibodies will mediate protection against many microbes and/or infectious diseases. In fact, the phenomenon of emergence suggests that many aspects of AMI will not be predictable, even with a full understanding of antibody action at the molecular or structural level. This suggests that perhaps deterministic views of antibody function might be abandoned for certain pathogens and toxins, and alternatives should be considered. One alternative is to construct a probabilistic framework based on estimates of the likelihood of a certain outcome given certain conditions. Clearly such an approach would require the integration of numerous observations into mathematical models that predict probabilities of various outcomes that include protection, nonprotection and disease enhancement in a given host. At this time there is insufficient information for the construction of such models, but just thinking about the problem suggests the need for different types of experimental data. For example, predicting the protective efficacy of an antibody in a host could require information on the effect of antibody on the inflammatory response and better knowledge of the evolution of the inflammatory response to the microbe. If indeed the best approach to this complex problem is to generate a probabilistic synthesis, then this synthesis should be fueled by revisiting correlates of antibody protection with the view that antibody function can be understood only in the context of microbial and host characteristics and the outcome of host-microbe interactions. Although such a synthesis could not be generalized to all hosts or all microbes, it would provide new parameters for future modeling efforts and new paradigms for the development of vaccines against microbes for which vaccines are not available at present.
ACKNOWLEDGMENTS
We thank M.D. Scharff for critical reading of this manuscript and suggestions. Supported by the US National Institutes of Health (AI45459, AI44374, AI033774 and HL059842) and the Northeastern Biodefense Center (5U54AI057158-08 – Lipkin to A.D.).
Footnotes
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Jerne NK. Towards a network theory of the immune system. Ann. Immunol. (Paris) 1974;125C:373–389. [PubMed] [Google Scholar]
- 2.Hébert J, Bernier D, Boutin Y, Jobin M, Mourad W. Generation of anti-idiotypic and anti-anti-idiotypic monoclonal antibodies in the same fusion. Support of Jerne’s Network Theory. J. Immunol. 1990;144:4256–4261. [PubMed] [Google Scholar]
- 3.Silverstein AM. History of immunology. Cellular versus humoral immunity: determinants and consequences of an epic 19th century battle. Cell. Immunol. 1979;48:208–221. doi: 10.1016/0008-8749(79)90113-8. [DOI] [PubMed] [Google Scholar]
- 4.Glatman-Freedman A, Casadevall A. Serum therapy for tuberculosis revisited: a reappraisal of the role of antibody-mediated immunity against Mycobacterium tuberculosis. Clin. Microbiol. Rev. 1998;11:514–532. doi: 10.1128/cmr.11.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect. Immun. 2003;71:4225–4228. doi: 10.1128/IAI.71.8.4225-4228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teitelbaum R, et al. A monoclonal antibody recognizing a surface antigen of Mycobacterium tuberculosis enhances host survival. Proc. Natl. Acad. Sci. USA. 1998;95:15688–15693. doi: 10.1073/pnas.95.26.15688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamasur B, et al. A mycobacterial lipoarabinomannan specific monoclonal antibody and its F(ab’) fragment prolong survival of mice infected with Mycobacterium tuberculosis. Clin. Exp. Immunol. 2004;138:30–38. doi: 10.1111/j.1365-2249.2004.02593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams A, et al. Passive protection with immunoglobulin A antibodies against tuberculous early infection of the lungs. Immunology. 2004;111:328–333. doi: 10.1111/j.1365-2567.2004.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pethe K, et al. The heparin-binding haemagglutinin of M. tuberculosis is required for extrapulmonary dissemination. Nature. 2001;412:190–194. doi: 10.1038/35084083. [DOI] [PubMed] [Google Scholar]
- 10.Maglione PJ, Xu J, Chan J. B cells moderate inflammatory progression and enhance bacterial containment upon pulmonary challenge with Mycobacterium tuberculosis. J. Immunol. 2007;178:7222–7234. doi: 10.4049/jimmunol.178.11.7222. [DOI] [PubMed] [Google Scholar]
- 11.Maglione PJ, Xu J, Casadevall A, Chan J. Fcγ receptors regulate immune activation and susceptibility during Mycobacterium tuberculosis infection. J. Immunol. 2008;180:3329–3338. doi: 10.4049/jimmunol.180.5.3329. [DOI] [PubMed] [Google Scholar]
- 12.Casadevall A, Pirofski LA. A reappraisal of humoral immunity based on mechanisms of antibody-mediated protection against intracellular pathogens. Adv. Immunol. 2006;91:1–44. doi: 10.1016/S0065-2776(06)91001-3. [DOI] [PubMed] [Google Scholar]
- 13.Russo RT, Mariano M. B-1 cell protective role in murine primary Mycobacterium bovis bacillus Calmette-Guerin infection. Immunobiology. 2010;215:1005–1014. doi: 10.1016/j.imbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Subramaniam K, et al. IgM+ memory B cell expression predicts HIV-associated cryptococcosis status. J. Infect. Dis. 2009;200:244–251. doi: 10.1086/599318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns T, Abadi M, Pirofski LA. Modulation of the lung inflammatory response to serotype 8 pneumococcal infection by a human immunoglobulin m monoclonal antibody to serotype 8 capsular polysaccharide. Infect. Immun. 2005;73:4530–4538. doi: 10.1128/IAI.73.8.4530-4538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabrizio K, Groner A, Boes M, Pirofski LA. A human monoclonal immunoglobulin M reduces bacteremia and inflammation in a mouse model of systemic pneumococcal infection. Clin. Vaccine Immunol. 2007;14:382–390. doi: 10.1128/CVI.00374-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian H, Weber S, Thorkildson P, Kozel TR, Pirofski LA. Efficacy of opsonic and nonopsonic serotype 3 pneumococcal capsular polysaccharide-specific monoclonal antibodies against intranasal challenge with Streptococcus pneumoniae in mice. Infect. Immun. 2009;77:1502–1513. doi: 10.1128/IAI.01075-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman JR, Papamichail D, Yano M, Garcia-Suarez MM, Pirofski LA. Designed reduction of Streptococcus pneumoniae pathogenicity via synthetic changes in virulence factor codon-pair bias. J. Infect. Dis. 2011;203:1264–1273. doi: 10.1093/infdis/jir010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber SE, Tian H, Pirofski LA. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J. Immunol. 2011;186:432–442. doi: 10.4049/jimmunol.1001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeMessurier K, Hacker H, Tuomanen E, Redecke V. Inhibition of T cells provides protection against early invasive pneumococcal disease. Infect. Immun. 2010;78:5287–5294. doi: 10.1128/IAI.00431-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson DA, Musher DM, Jacobson JW, Verhoef J. A brief history of the pneumococcus in biomedical research: a panoply of scientific discovery. Clin. Infect. Dis. 1993;17:913–924. doi: 10.1093/clinids/17.5.913. [DOI] [PubMed] [Google Scholar]
- 22.Baumgarth N, et al. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc. Natl. Acad. Sci. USA. 1999;96:2250–2255. doi: 10.1073/pnas.96.5.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man. Immunol. Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 24.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajan B, Ramalingam T, Rajan TV. Critical role for IgM in host protection in experimental filarial infection. J. Immunol. 2005;175:1827–1833. doi: 10.4049/jimmunol.175.3.1827. [DOI] [PubMed] [Google Scholar]
- 26.Boes M, Prodeus AP, Schmidt T, Carroll MC, Chen J. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J. Exp. Med. 1998;188:2381–2386. doi: 10.1084/jem.188.12.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramaniam KS, et al. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J. Immunol. 2010;184:5755–5767. doi: 10.4049/jimmunol.0901638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jayasekera JP, Moseman EA, Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J. Virol. 2007;81:3487–3494. doi: 10.1128/JVI.02128-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diamond MS, et al. A critical role for induced IgM in the protection against West Nile virus infection. J. Exp. Med. 2003;198:1853–1862. doi: 10.1084/jem.20031223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kruetzmann S, et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 2003;197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nimmerjahn F, Ravetch JV. The antiinflammatory activity of IgG: the intravenous IgG paradox. J. Exp. Med. 2007;204:11–15. doi: 10.1084/jem.20061788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature. 2011;475:110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li F, Ravetch JV. Inhibitory Fcgamma receptor engagement drives adjuvant and anti-tumor activities of agonistic CD40 antibodies. Science. 2011;333:1030–1034. doi: 10.1126/science.1206954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skurnik D, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J. Clin. Invest. 2010;120:3220–3233. doi: 10.1172/JCI42748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirofski LA. Why antibodies disobey the Hippocratic Oath and end up doing harm: a new clue. J. Clin. Invest. 2010;120:3099–3102. doi: 10.1172/JCI44312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lendvai N, Qu X, Hsueh W, Casadevall A. Mechanism for the isotype dependence of antibody-mediated toxicity in Cryptococcus neoformans infected mice. J. Immunol. 2000;164:4367–4374. doi: 10.4049/jimmunol.164.8.4367. [DOI] [PubMed] [Google Scholar]
- 37.Feldmesser M, Mednick A, Casadevall A. Antibody-mediated protection in murine Cryptococcus neoformans infection is associated with subtle pleotrophic effects on the cytokine and leukocyte response. Infect. Immun. 2002;70:1571–1580. doi: 10.1128/IAI.70.3.1571-1580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunol. Rev. 2010;236:265–275. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 39.Nimmerjahn F, Ravetch JV. FcgammaRs in health and disease. Curr. Top. Microbiol. Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 40.Casadevall A, Pirofski L. Host-Pathogen Interactions: The basic concepts of microbial commensalisms, colonization, infection, and disease. Infect. Immun. 2000;68:6511–6518. doi: 10.1128/iai.68.12.6511-6518.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Casadevall A, Pirofski L. Host-pathogen interactions: redefining the basic concepts of virulence and pathogenicity. Infect. Immun. 1999;67:3703–3713. doi: 10.1128/iai.67.8.3703-3713.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Casadevall A, Pirofski L. The damage-response framework of microbial pathogenesis. Nat. Rev. Microbiol. 2003;1:17–24. doi: 10.1038/nrmicro732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Connolly SE, Thanassi DG, Benach JL. Generation of a complement-independent bactericidal IgM against a relapsing fever Borrelia. J. Immunol. 2004;172:1191–1197. doi: 10.4049/jimmunol.172.2.1191. [DOI] [PubMed] [Google Scholar]
- 44.LaRocca TJ, Katona LI, Thanassi DG, Benach JL. Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J. Immunol. 2008;180:6222–6228. doi: 10.4049/jimmunol.180.9.6222. [DOI] [PubMed] [Google Scholar]
- 45.Magliani W, et al. Protective antifungal yeast killer toxin-like antibodies. Curr. Mol. Med. 2005;5:443–452. doi: 10.2174/1566524054022558. [DOI] [PubMed] [Google Scholar]
- 46.Brena S, et al. Fungicidal monoclonal antibody C7 interferes with iron acquisition in Candida albicans. Antimicrob. Agents Chemother. 2011;55:3156–3163. doi: 10.1128/AAC.00892-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torosantucci A, et al. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alviano DS, et al. Melanin from Fonsecaea pedrosoi induces production of human antifungal antibodies and enhances the antimicrobial efficacy of phagocytes. Infect. Immun. 2004;72:229–237. doi: 10.1128/IAI.72.1.229-237.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wentworth P, Jr., et al. Evidence for antibody-catalyzed ozone formation in bacterial killing and inflammation. Science. 2002;298:2195–2199. doi: 10.1126/science.1077642. [DOI] [PubMed] [Google Scholar]
- 50.Yano M, Gohil S, Coleman JR, Manix C, Pirofski LA. Antibodies to Streptococcus pneumoniae capsular polysaccharide enhance pneumococcal quorum sensing. mBio. 2011 Sep 13; doi: 10.1128/mBio.00176-11. doi:10.1128/mBio.00176-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vouldoukis I, et al. IgE mediates killing of intracellular Toxoplasma gondii by human macrophages through CD23-dependent, interleukin-10 sensitive pathway. PLoS ONE. 2011;6:e18289. doi: 10.1371/journal.pone.0018289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lampert PW, Joseph BS, Oldstone MB. Antibody-induced capping of measles virus antigens on plasma membrane studied by electron microscopy. J. Virol. 1975;15:1248–1255. doi: 10.1128/jvi.15.5.1248-1255.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desplanques AS, Nauwynck HJ, Tilleman K, Deforce D, Favoreel HW. Tyrosine phosphorylation and lipid raft association of pseudorabies virus glycoprotein E during antibody-mediated capping. Virology. 2007;362:60–66. doi: 10.1016/j.virol.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 54.McClelland EE, Nicola AM, Prados-Rosales R, Casadevall A. Ab binding alters gene expression in Cryptococcus neoformans and directly modulates fungal metabolism. J. Clin. Invest. 2010;120:1355–1361. doi: 10.1172/JCI38322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torres M, Casadevall A. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol. 2008;29:91–97. doi: 10.1016/j.it.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 56.Edelson BT, Cossart P, Unanue ER. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 1999;163:4087–4090. [PubMed] [Google Scholar]
- 57.Edelson BT, Unanue ER. Intracellular antibody neutralizes Listeria growth. Immunity. 2001;14:503–512. doi: 10.1016/s1074-7613(01)00139-x. [DOI] [PubMed] [Google Scholar]
- 58.Nosanchuk JD, Steenbergen JN, Shi L, Deepe GS, Jr., Casadevall A. Antibodies to a cell surface histone-like protein protect against Histoplasma capsulatum. J. Clin. Invest. 2003;112:1164–1175. doi: 10.1172/JCI19361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torres M, May R, Scharff MD, Casadevall A. Variable-region identical antibodies differing in isotype demonstrate differences in fine specificity and isotype. J. Immunol. 2005;174:2132–2142. doi: 10.4049/jimmunol.174.4.2132. [DOI] [PubMed] [Google Scholar]
- 60.Torosantucci A, et al. Protection by anti-β-glucan antibodies is associated with restricted β-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS ONE. 2009;4:e5392–1. doi: 10.1371/journal.pone.0005392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres M, Fernandez-Fuentes N, Fiser A, Casadevall A. The immunoglobulin heavy chain constant region affects kinetic and thermodynamic parameters of antibody variable region interactions with antigen. J. Biol. Chem. 2007;282:13917–13927. doi: 10.1074/jbc.M700661200. [DOI] [PubMed] [Google Scholar]
- 62.Abboud N, et al. A requirement for FcγR in antibody-mediated bacterial toxin neutralization. J. Exp. Med. 2010;207:2395–2405. doi: 10.1084/jem.20100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wong SE, Sellers BD, Jacobson MP. Effects of somatic mutations on CDR loop flexibility during affinity maturation. Proteins. 2011;79:821–829. doi: 10.1002/prot.22920. [DOI] [PubMed] [Google Scholar]
- 64.Wedemayer GJ, Patten PA, Wang LH, Schultz PG, Stevens RC. Structural insights into the evolution of an antibody combining site. Science. 1997;276:1665–1669. doi: 10.1126/science.276.5319.1665. [DOI] [PubMed] [Google Scholar]
- 65.Kirkham PM, Mortari F, Newton JA, Schroeder HW. Immunoglobulin VH clan and family identity predicts variable domain structure and may influence antigen binding. EMBO J. 1991;11:603–609. doi: 10.1002/j.1460-2075.1992.tb05092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varshney AK, et al. Generation, characterization and epitope mapping of neutralizing and protective monoclonal antibodies against staphylococcal enterotoxin B induced lethal shock. J. Biol. Chem. 2011;286:9737–9747. doi: 10.1074/jbc.M110.212407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beernink PT, et al. Fine antigenic specificity and cooperative bactericidal activity of monoclonal antibodies directed at the meningococcal vaccine candidate factor h-binding protein. Infect. Immun. 2008;76:4232–4240. doi: 10.1128/IAI.00367-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taborda CP, Casadevall A. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence and prozone-like effects in passive protection experiments. J. Immunol. 2001;66:2100–2107. doi: 10.4049/jimmunol.166.3.2100. [DOI] [PubMed] [Google Scholar]
- 69.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: ‘Prozone-like’ effects in passive immunization with Immunoglobulin G. J. Immunol. 2003;140:3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 70.Rivera J, Casadevall A. Mouse genetic background is a major determinant of isotype-related differences for antibody-mediated protective efficacy against Cryptococcus neoformans. J. Immunol. 2005;174:8017–8026. doi: 10.4049/jimmunol.174.12.8017. [DOI] [PubMed] [Google Scholar]
- 71.Casadevall A, Fang FC, Pirofski LA. Microbial virulence as an emergent property: consequences and opportunities. PLoS Pathog. 2011;7:e1002136. doi: 10.1371/journal.ppat.1002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Casadevall A. Antibody immunity and invasive fungal infections. Infect. Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect. Immun. 1987;55:749–752. doi: 10.1128/iai.55.3.749-752.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schlageter AM, Kozel TR. Opsonization of Cryptococcus neoformans by a family of isotype-switch variant antibodies specific for the capsular polysaccharide. Infect. Immun. 1990;58:1914–1918. doi: 10.1128/iai.58.6.1914-1918.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect. Immun. 1992;60:4534–4541. doi: 10.1128/iai.60.11.4534-4541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fleuridor R, Lees A, Pirofski L. A cryptococcal capsular polysaccharide mimotope prolongs the survival of mice with Cryptococcus neoformans infection. J. Immunol. 2001;166:1087–1096. doi: 10.4049/jimmunol.166.2.1087. [DOI] [PubMed] [Google Scholar]
- 77.Casadevall A, Pirofski L. Insights into mechanisms of antibody-mediated immunity from studies with Cryptococcus neoformans. Curr. Mol. Med. 2005;5:421–433. doi: 10.2174/1566524054022567. [DOI] [PubMed] [Google Scholar]
- 78.Ponge JF. Emergent properties from organisms to ecosystems: towards a realistic approach. Biol. Rev. Camb. Philos. Soc. 2005;80:403–411. doi: 10.1017/S146479310500672X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ablowitz R. The theory of emergence. Philos. Sci. 1939;6:1–16. [Google Scholar]
- 80.Baylis CA. The philosophic functions of emergence. Philos. Rev. 1929;38:372–384. [Google Scholar]
- 81.Robbins JB, Schneerson R, Szu SC. Perspective: Hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J. Infect. Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 82.Casadevall A, Pirofski LA. Antibody-mediated regulation of cellular immunity and the inflammatory response. Trends Immunol. 2003;24:474–478. doi: 10.1016/s1471-4906(03)00228-x. [DOI] [PubMed] [Google Scholar]


