Abstract
This study explores the effect of continuous exposure to bright light on neuromelanin formation and dopamine neuron survival in the substantia nigra. Twenty-one days after birth, Sprague–Dawley albino rats were divided into groups and raised under different conditions of light exposure. At the end of the irradiation period, rats were sacrificed and assayed for neuromelanin formation and number of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra. The rats exposed to bright light for 20 days or 90 days showed a relatively greater number of neuromelanin-positive neurons. Surprisingly, TH-positive neurons decreased progressively in the substantia nigra reaching a significant 29% reduction after 90 days of continuous bright light exposure. This decrease was paralleled by a diminution of dopamine and its metabolite in the striatum. Remarkably, in preliminary analysis that accounted for population density, the age and race adjusted Parkinson's disease prevalence significantly correlated with average satellite-observed sky light pollution.
Parkinson's disease is a chronic, progressive, neurodegenerative disorder clinically characterized by motor symptoms such as tremor at rest, rigidity, bradykinesia, and postural instability. Motor disturbances are caused by the progressive loss of dopaminergic neurons in the substantia nigra. The substantia nigra derives its name, which means “black substance”, from the dark pigment “neuromelanin” found in many of its neurons. While its role in neurodegeneration is still a matter of debate, neuromelanin accumulates normally with age in human substantia nigra neurons1.
Neuromelanin is a product of dopamine auto-oxidation. When the amount of cytosolic dopamine exceeds the physiological concentration, dopamine can be metabolized via monoamine oxidase and aldehyde dehydrogenase to the 3,4-dihydroxyphenylacetic acid metabolite and hydrogen peroxide2, or it can be sequestered into the lysosomes3 where it can auto-oxidize to form neuromelanin4. Accordingly, increases in dopamine concentration by 3,4-dihydroxy-L-phenilalanine (L-DOPA) load in cultures of midbrain dopaminergic neurons or PC12 cells can enhance neuromelanin formation5.
Different parameters, such as oxygen and light exposure, can affect the oxidation of dopamine and induce the formation of neuromelanin. In vitro, oxygen can account for 40% of dopamine oxidation, and up to 20% can be attributed to light exposure6. The rationale to investigate the effect of light on neuromelanin formation in the substantia nigra arises from evidence that dopamine in complex with iron can absorb in the visible light spectrum. Barreto et al.7 have shown that in the presence of Fe(NO3)3 two broad bands of dopamine absorbance appear, with maxima at 437 and 740 nm. Although light with longer wavelengths penetrates into the brain more effectively8, shorter wavelength light can also penetrate into deep brain structures and regulate, for example, the seasonal cycle of reproduction in birds9. For instance, opsin 5, a non-retinal and non-pineal deep-brain opsin photoreceptor in the hypothalamus of quail, has a peak of excitation at 419 nm10. Penetration of light into the brain has also been demonstrated in larger animals, including sheep and dog11.
Based on the actions of toxins, post-mortem investigations and gene defects responsible for familial Parkinson's disease, there is now a general consensus about the mechanisms of cell death that contribute to neuronal loss in Parkinson's disease12. Amongst others, oxidative stress seems to play a major role in the pathogenesis of the disease, but the source of oxidative stress remains unclear.
Given the above-mentioned evidence, we hypothesised that light penetrating into the substantia nigra could be the source of oxidative stress. Light could oxidise dopamine and, in turn, induce the formation of neuromelanin, and cause dopamine neuron degeneration. To test this hypothesis, we exposed rats to a fluorescent lamp placed close to the cage for several weeks and examined the formation of neuromelanin in the substantia nigra and the detrimental effect of light on dopamine neurons. Furthermore, following the detailed work of David Marsden (1961)13, we compared the neuromelanization of the substantia nigra of several animals with their diurnal/nocturnal wildlife habitat as an index of the extent of light exposure. Finally, we analysed whether there is any correlation between the distribution maps of Parkinson's disease prevalence and sky light-pollution in the United States.
Results
Light induces neuromelanin formation in the substantia nigra
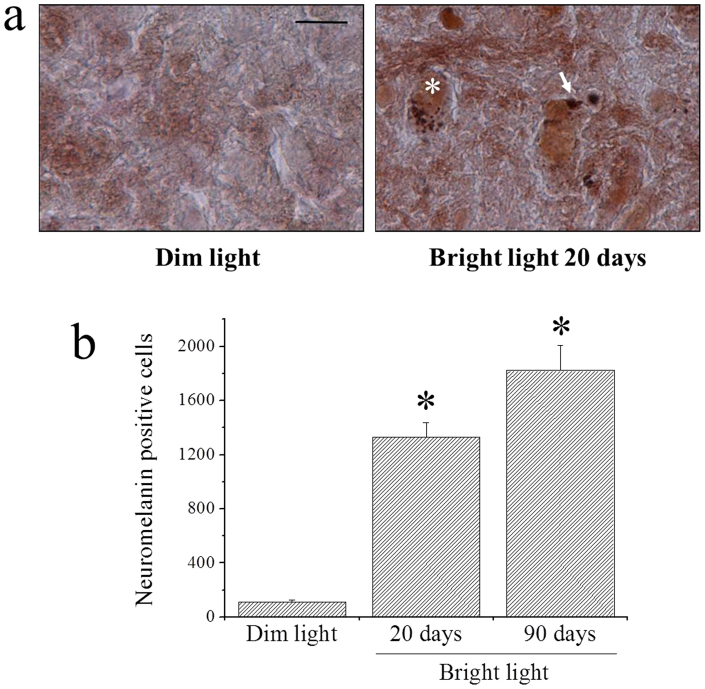
Sprague-Dawley albino rats were raised in a dark or dark/light environment (for lamp light spectrum, see Fig. 1) using different schedules and sacrificed after three months. To estimate the quantity of neuromelanin formed, we counted cells containing pigmented granules in the coronal brain section at the level of the ventral mesencephalon stained by Fontana-Masson. Sparse spots of neuromelanin staining could be found in some neurons of rats raised under dim light - dark cycle reaching a total of 113 ± 15 (s.e.m.) positive neurons throughout the substantia nigra (Fig. 2a left panel and b). In coronal sections from rats raised in dim light - dark cycle and then exposed for 20 days to continuous bright light, there was a clear increase in neuromelanin staining throughout the substantia nigra. In many neurons it was possible to observe several minute neuromelanin granules in the cytoplasm, while in others the granules were fewer (or unique) and larger, probably resulting from coalescence of smaller granules (Fig. 2a right panel). The total number of neuromelanin-positive neurons was 1,331 ± 107 (s.e.m.) (Fig. 2b). An even greater increase in neuromelanin-positive neurons was observed in sections taken from animals raised for three months under continuous bright light (1,823 ± 184 (s.e.m.); Fig. 2b).
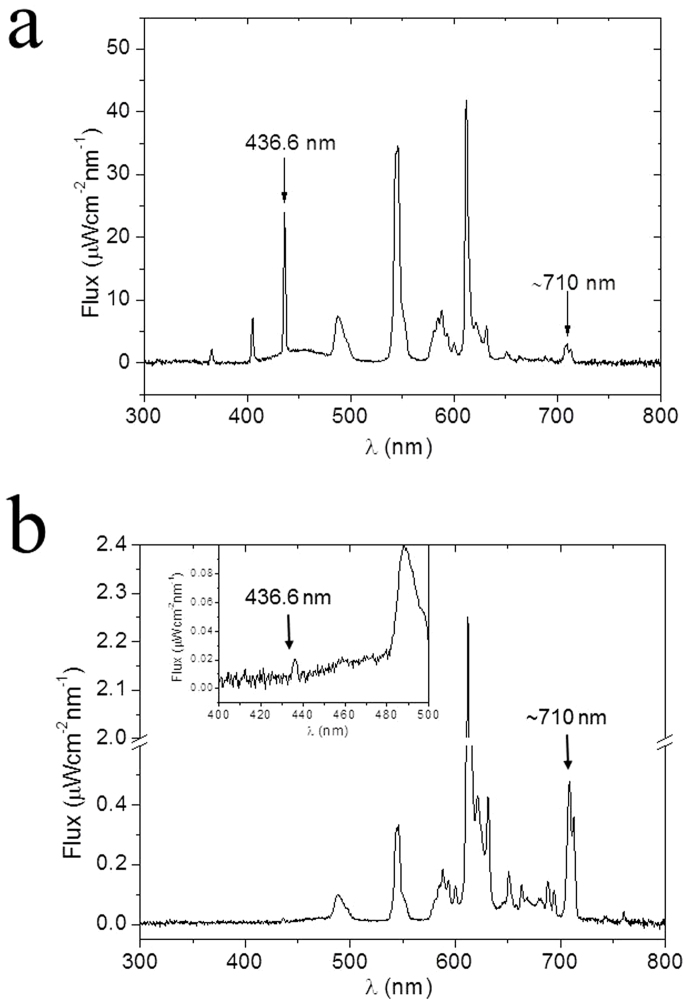
Figure 1. Light spectrum of the fluorescent lamp used to illuminate the animals.
(a) Light spectrum measured in the cages where the animals were housed and (b) light spectrum of the same lamp measured from the inside of the rat scalp and skull. The inset in panel (b) is a magnification of the spectrum between 400 and 500 nm to show the pick at 436.6 nm.
Figure 2. Fontana-Masson staining of neuromelanin granules in the substantia nigra and stereological counting of neuromelanin-positive neurons.
(a) Representative sections from rats raised in dim light – dark cycle (left panel) and rats raised in dim light – dark cycle for 70 days and than for 20 days under bright light (right panel). The asterisk on the right panel shows a cell with several tiny granules, while the arrow shows a large granule probably deriving from coalescence of tiny granules. Scale bar: 25 μm. (b) Stereological counting of neuromelanin-positive neurons in the substantia nigra of rats raised in dim light – dark cycle, and rats raised for 20 days or 3 months under bright light. *Significantly different from rats raised in dim light – dark cycle (F7,7,7 = 51.42870, p = 0.00001, using one way ANOVA plus Scheffe's F test). The three subscript digits adjacent to the F value represent the numbers of rats used for the analysis, sequentially: rats raised in dim light – dark cycle, rats raised for 20 days under bright light and rats raised for 3 months under bright light.
Light induces reduction of tyrosine hydroxylase (TH)-positive neurons in the substantia nigra
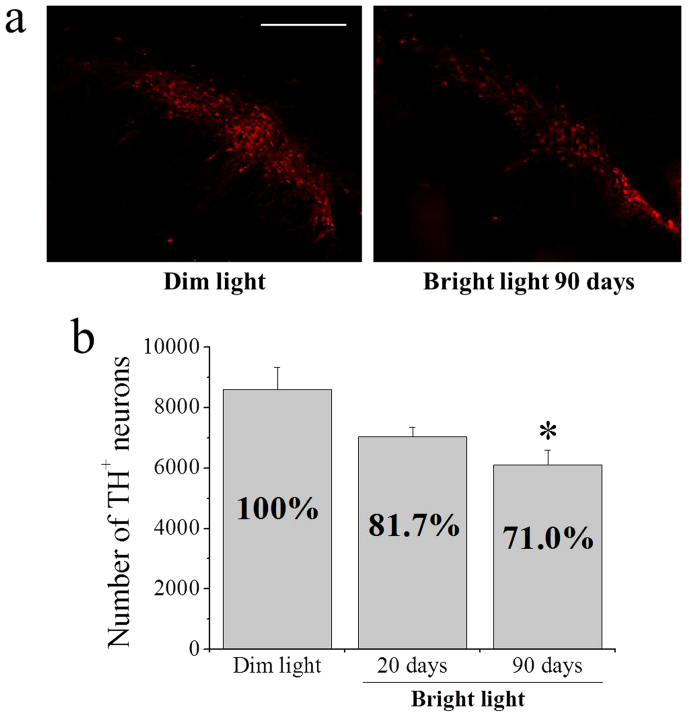
Exposure of animals to bright light caused a reduction of TH-positive neurons in the substantia nigra. Fig. 3a shows coronal sections of ventral mesencephalon from an animal raised in dim light – dark cycle (left panel) and an animal raised under bright light for three months (right panel) showing a consistent loss of dopamine neurons (~40%). Stereological counting of the left substantia nigra in animals raised under dim light - dark cycle produced a value of 8,605 ± 722 (s.e.m.) TH-positive neurons (Fig. 3b). In animals raised in dim light - dark cycle and then exposed to bright light for 20 days, there was a 18.3% decrease in TH-positive neurons compared to control animals, but the difference did not reach significance. In contrast, rats exposed for three months to bright light showed a significant loss of TH-positive neurons (29%), which averaged 6,112 ± 483 (s.e.m.) (Fig. 3b).
Figure 3. TH immunohistochemistry and stereological counting of TH-positive neurons in ventral mesencephalon.
(a) Representative sections from rats raised in dim light – dark cycle (left panel) and rats raised in bright light for 3 months (right panel). Scale bar: 250 μm. (b) Stereological counting of TH-positive neurons in the substantia nigra of rats raised in dim light – dark cycle, and rats raised for 20 days or 3 months under bright light. *Significantly different from animals raised in dim light – dark cycle (F10,8,8 = 5.01318, p = 0.01559, using one way ANOVA plus Scheffe's F test).
Biochemical assays of neurotransmitters
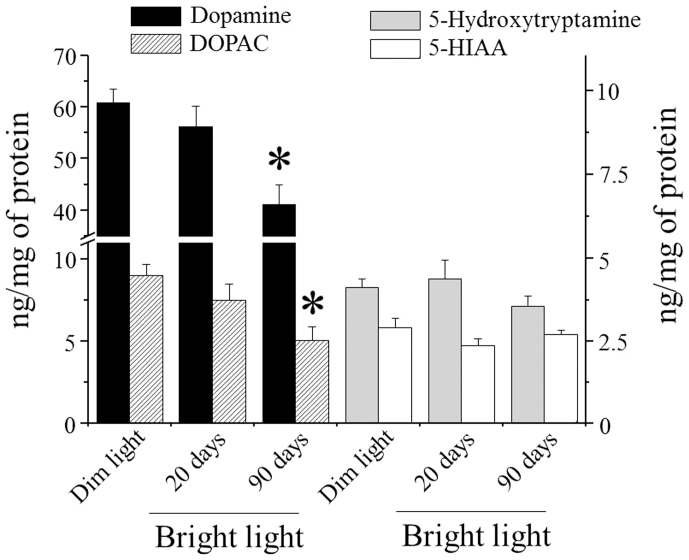
Striatal dopamine content, measured in animals raised under dim light - dark cycle, was 60.9 ± 2.52 (s.e.m.) ng/mg of protein, while 3,4-dihydroxyphenylacetic acid (DOPAC) was 9.03 ± 0.64 (s.e.m.) ng/mg of protein (Fig. 4). Rats raised under dim light - dark cycle and then exposed to bright light for 20 days showed a modest and non-significant reduction of dopamine and DOPAC in the striatum (Fig. 4). In contrast, animals continuously exposed to bright light for 3 months showed a significant decrease in dopamine (33%) and DOPAC (44%) in the striatum compared to control animals raised in dim light – dark cycle (Fig. 4). As an internal control, we measured the levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid (5-HIAA) in the same animals in which dopamine and its metabolites were analyzed. The striatal content of 5-hydroxytryptamine and 5-HIAA in rats raised in dim light – dark cycle was 4.13 ± 0.26 (s.e.m.) and 2.93 ± 0.26 (s.e.m.) ng/mg of protein, respectively. Neither rats treated for 20 days nor rats treated for 3 months with bright light showed a significant change in 5-hydroxytryptamine or 5-HIAA content (Fig. 4).
Figure 4. Levels of dopamine, DOPAC, 5-hydroxytryptamine and 5-HIAA in rats raised in dim light–dark cycle and rats raised for 20 days or 3 months under bright light.
The scale indicating dopamine and DOPAC levels is on the left y-axis while 5-hydroxytryptamine and 5-HIAA levels refer to the right y-axis. *Significantly different from animals raised in dim light – dark cycle (dopamine: F10,8,8 = 9.01461, p = 0.00129; DOPAC: F10,8,8 = 6.42800, p = 0.00606, using one way ANOVA plus Scheffe's F test).
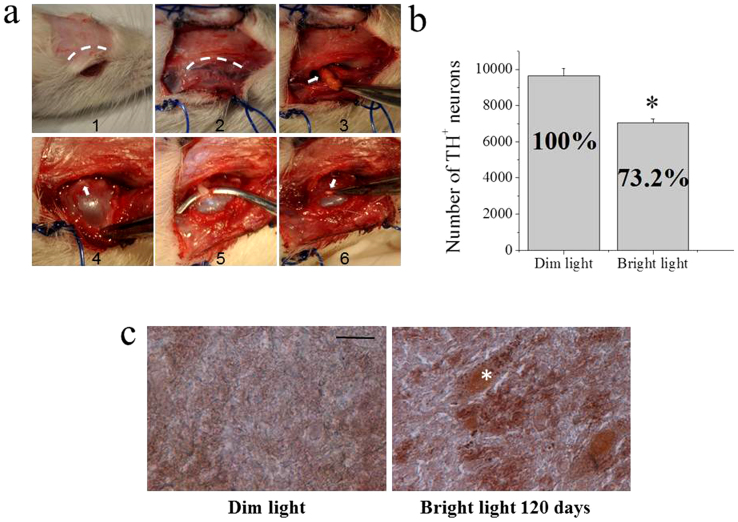
Light induces neuromelanin formation and reduction of TH-positive neurons in the substantia nigra of rats with bilateral transection of the optic nerve
Sixteen rats underwent bilateral transection of the optic nerve when they were three-months old (Fig. 5a). Half of them were exposed to dim light - dark cycle and the other half to bright light for four months. Animals exposed to bright light showed a significant loss of TH-positive neurons in the substantia nigra. Stereological counting of the left substantia nigra in animals raised under dim light - dark cycle produced a value of 9,650 ± 213 (s.e.m.) TH-positive neurons, while rats exposed to bright light showed a significant loss of TH-positive neurons (26.8%), which averaged 7,063 ± 305 (s.e.m.) (Fig. 5b). Furthermore, even though we did not make a systematic count, rats exposed to bright light showed a clear increase in neuromelanin staining with respect to rats raised under dim – light dark cycle (Fig. 5c).
Figure 5. TH immunohistochemistry and stereological counting of TH-positive neurons in ventral mesencephalon of the bilateral optic nerve transected rats.
(a) Surgical transection of the optic nerve. In panel 1 and 2 the curvilinear dashed lines indicate the incision of the scalp and the incision along the orbital rim to expose the retro-orbital tissue, respectively. Excess of retro-orbital fat (indicated by the arrow in panel 3) was removed and the orbit pulled forward to expose the optic nerve (indicated by the arrow in panel 4). The optic nerve was hooked with a surgical instrument (panel 5) and then cut. The arrow in panel 6 indicates the stump of the transected optic nerve. (b) Stereological counting of TH-positive neurons in the substantia nigra of rats raised in dim light – dark cycle, and rats raised under bright light for 4 months. *Significantly different from animals raised in dim light – dark cycle (t8,8 = 5.87463, p = 0.00004, using two-samples t-Test). (c) Fontana-Masson staining of neuromelanin granules in the substantia nigra. Representative sections from rats raised in dim light – dark cycle (left panel) and rats raised under bright light (right panel). The asterisk on the right panel shows a cell with numerous tiny neuromelanin granules.
Comparison of substantia nigra neuromelanization in several animals with their exposure to sunlight
In order to study whether environmental light could directly influence the formation of neuromelanin in the substantia nigra, we re-examined the pivotal work published in 1961 by David Marsden13, entitled “Pigmentation in the nucleus substantiae nigrae of mammals”, in the context of sunlight exposure. Marsden examined the brains of forty-nine mammals in order to determine which of these animals contained pigment granules in the substantia nigra. Owing to the precise description of the animals in the study, we were able to classify them on the basis of their exposure to sunlight (Table 1). We scored the extent of light exposure of animals as follows: (1) low (diurnal animals living in cavities, and nocturnal activities); (2) medium (animals in dim-light habitat, e.g. woodland, and nocturnal activities); and (3) high (diurnal animals living in open fields, e.g. savannas, desert). Humans (Homo sapiens) were classified as having medium exposure (score 2), because, except for some categories of workers like farmers and fishermen that spend a large part of their life outside, the majority of the general population spends most of the daytime indoors and is mostly exposed to artificial light, whose intensity is less than that of sunlight.
Table 1. Comparison between light exposure and substantia nigra pigmentation in mammals. Based on David Marsden work back in 1961 (ref. 13), forty-nine mammals were divided in pigmented and non pigmented animals. Afterward, we scored light exposure grade extent as follows: (1) low (diurnal animals living in cavities, and nocturnal activities), (2) medium (animals in dim-light habitat (e.g., woodland), and nocturnal activities), and (3) high (diurnal animals living in open fields, e.g., savannas, desert). The Wilcoxon – Mann – Whitney U test for non parametric data showed that pigmented animals were significantly more exposed to light than nonpigmented animals (p = 0.0008, one tailed test).
| Pigmented animals | Non pigmented animals | ||
|---|---|---|---|
| Artiodactyla: | Artiodactyla: | ||
| sheep (Ovis aries) | 2 | hog deer (Cervus porcinus) | 2 |
| musk deer (Moschus moschiferus) | 1 | pig (Sus scrofa) | 2 |
| Carnivora: | yellow-backed duiker (Cephalophus sylvicultrix), | 1 | |
| dog (Canis canis) | 3 | Carnivora: | |
| fox (Vulpes vulpes) | 2 | otter (Lutra lutra) | 1 |
| European badger (Meles meles) | 2 | seal (Phoca phoca) | 1 |
| sand-pig badger (Arctonyx collaris) | 1 | Chiroptera: | |
| Canadian skunk (Mephitis mephitis) | 1 | Indian fruit bat (Pteropus giganteus) | 1 |
| Indian civet (Viverra malaccensis) | 1 | Insectivora: | |
| African genet (Genetta pardina) | 1 | hedgehog (Erinaceus species) | 1 |
| domestic cat (Felis catus) | 3 | Lagomorpha: | |
| pampas cat (Felis dendrailurus) | 2 | rabbit (Oryctolagus cuniculus) | 2 |
| Marsupialia: | Marsupialia: | ||
| tree kangaroo (Dendrolagus species) | 2 | rufous rat kangaroo (Aepyprymnus rufescens) | 1 |
| common ring-tail (Pseudocheirus lanuginosus) | 2 | fat-tailed sminthopsis (Sminthopsis crassicaudata) | 1 |
| Perissodactyla: | Tasmanian barred-bandicoot (Perameles gunni) | 1 | |
| horse (Equus equus) | 3 | southern short-nosed bandicoot (Isoodon obesulus) | 1 |
| Primates: | Tasmanian ring-tail (Pseudocheirus convolutor) | 1 | |
| man (Homo sapiens) | 2 | Monotremata: | |
| chimpanzee (Pan satyrus) | 2 | Australian spiny ant-eater (Tachyglossus aculeatus) | 1 |
| gibbon (Hylobates muelleri) | 2 | Rodentia: | |
| white-collared mangabey (Cercocebus torquatus) | 1 | mouse (Mus musculus) | 1 |
| patas monkey (Cercopithecus patas) | 3 | rat (Rattus norvegicus) | 1 |
| macaque monkey (Macaca rhesus) | 2 | guinea-pig (Cavia porcellus) | 1 |
| black and white colobus monkey (Colobus species) | 2 | ||
| baboon (Papio species) | 2 | ||
| weeper capuchin monkey (Cebus capucinus) | 2 | ||
| cebus monkey (Cebus species) | 2 | ||
| humboldt's woolly monkey (Lagothrix humboldtii) | 2 | ||
| lion marmoset (Leontocebus rosalia) | 2 | ||
| black-tailed marmoset (Callithrix argentata) | 2 | ||
| black-fronted lemur (Lemur nigrifrons) | 1 | ||
| brown lemur (Lemur fulvus) | 3 | ||
| Malayan tree shrew (Tupaia belangeri) | 2 | ||
| Rodentia: | |||
| grey squirrel (Sciurus carolinensis) | 1 | ||
| Xenarthra: | |||
| six-banded armadillo (Euphractus sexcinctus) | 2 |
Applying the Wilcoxon-Mann-Whitney U test for non-parametric data we found that there was a good correlation between light exposure and nigral pigmentation, with pigmented animals being significantly more exposed to light than non-pigmented ones (p = 0.0008, one-tailed test; Table 1).
Correlation between Parkinson's disease prevalence and sky light-pollution in the United States
As mentioned above, the majority of the general population spends most of the daytime indoors, at home and work. If light is an environmental factor in the pathogenesis of Parkinson's disease, we believe it is more appropriate to consider artificial light rather than sunlight in epidemiological comparisons. Sky light-pollution, measured by satellites, is an index of exposure to artificial light.
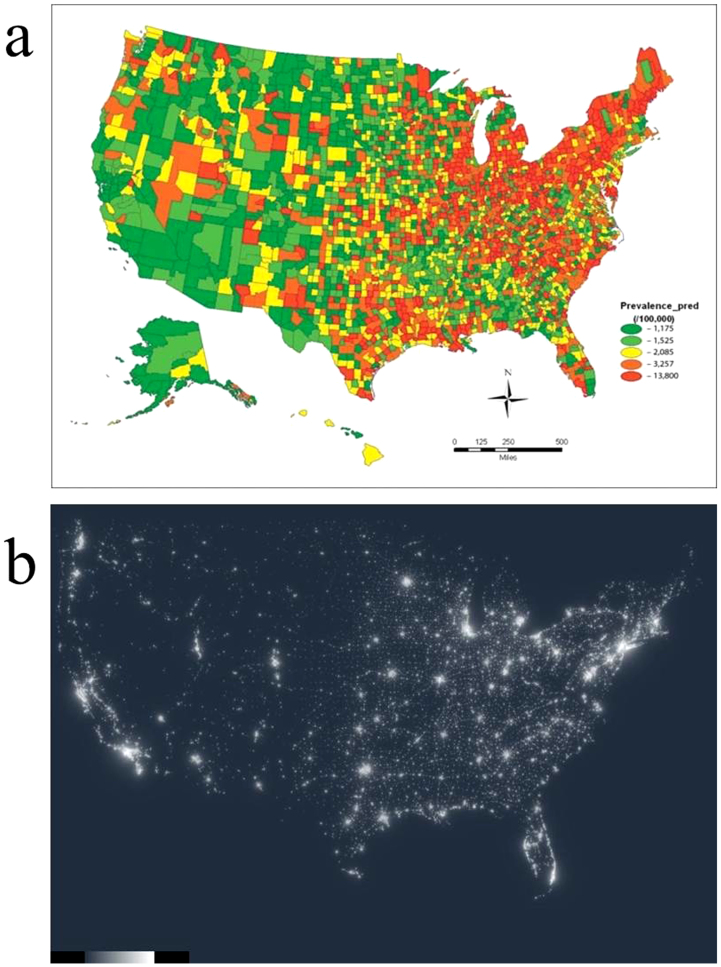
In a large population-based examination of Parkinson's disease spatial epidemiology by Willis et al. (2010)14, clustering of Parkinson's disease in the urban areas of the midwest and northeast portions of the United States was found. In this context, the co-authors Alison W. Willis and Brad A. Racette examined the spatial correlation between age- and race-adjusted prevalence of Parkinson's disease and sky light-pollution in the United States as measured by the Earth Observation Group (http://www.ngdc.noaa.gov/dmsp/dmsp.html). In a correlation analysis that accounted for population density, the age- and race-adjusted Parkinson's disease prevalence significantly correlated with average satellite-observed sky light-pollution (correlation coefficient = 0.13, p = 2.588E-13 (Fig. 6).
Figure 6. Similarity between Parkinson's disease prevalence and sky light pollution in U.S.A.
The prevalence of Parkinson's disease in U.S.A. (a) was reproduced from Willis et al.14 (with permission from S. Karger AG, Medical and Scientific Publishers, Allschwilerstrasse 10, 4009 Basel, Switzerland).The sky light pollution map of the U.S.A. (b) was obtained with permission from the Earth Observation Group (National Oceanic and Atmospheric Administration (NOAA) and the Department of Commerce, web site: http://www.ngdc.noaa.gov/dmsp/dmsp.html).
Discussion
In this manuscript we provided evidence of an increase in neuromelanin formation in rat substantia nigra after continuous light exposure. Twenty days of bright light exposure were sufficient to induce a relatively greater number of neuromelanin-positive neurons. The number of neuromelanin-positive neurons also increased with the time of exposure to bright light, indicating a positive correlation between the formation of neuromelanin and the time of irradiation. This is further supported by the fact that animals raised in dim light for three months show even fewer neuromelanin-positive neurons. In contrast with primates, rodents normally have a very low number of neuromelanin granules in the substantia nigra15, and the number of neuromelanin-containing neurons increase with age16.
That light has a role in neuromelanin formation can be deduced by reconsidering the pivotal work published in 1961 by David Marsden13 in the context of sunlight exposure. Marsden examined the brains of forty-nine mammals and determined which of these animals contained pigment granules in the substantia nigra. We classified and scored the same animals on the basis of their exposure to light and, though with some exceptions, we found that there was a good correlation between light exposure and nigral pigmentation, with animals living preferentially in the dark and leaving the nest at night showing no pigmentation.
Unexpectedly, in our study we also observed a significant decrease in TH-positive neurons in the substantia nigra after continuous bright light exposure that was paralleled by a significant reduction in dopamine and DOPAC in the striatum. As an internal control, in the same animals, 5-hydroxytryptamine and 5-HIAA did not diminish, indicating that the effect of light was specific to the dopaminergic neurons.
As stated in the introduction, neuromelanin is produced by auto-oxidation of dopamine, and its increase reflects an increase in dopamine oxidation. Dopamine oxidation in the presence of molecular oxygen leads to the formation of several cytotoxic molecules, including superoxide anions, hydroxyl radicals, and reactive quinones17. Reactive oxygen species derived from the oxidation of dopamine can damage cellular components, such as lipids, proteins, and DNA18, and can result in potentially deleterious effects on the cell19. Potentially, the reduction in TH-positive neurons observed in our experiments could be due to the increase in dopamine oxidation induced by continuous bright light exposure and induction of apoptosis by reactive oxygen species.
It is interesting to note that dopamine in complex with Fe3+ shows two bands of absorbance7, with maxima at 437 and 740 nm. Zecca20 and coworkers have shown that conversion of dopamine to neuromelanin is prompted by iron because the chelator desferrioxamine is able to block neuromelanin synthesis. As a matter of fact, iron is most abundant in areas that are rich in dopaminergic neurons, namely the globus pallidus, putamen, and substantia nigra of the basal ganglia21,22. The fluorescent lighting spectrum of the lamp we used to irradiate the animals shows peaks in the same range of the absorbance of dopamine in complex with Fe3+, for example, a peak at 436.6 nm and several minor peaks around 710 nm. At the end of the light exposure period in a rat of ~400 g, the percentage of energy that crossed the scalp and the skull was about ~0.1% for the peak at 436.6 nm and ~15% for the minor peaks around 710 nm. Together, these data would suggest that external light passes through the head of the animal, reaches the substantia nigra, hits dopamine in complex with Fe3+, and triggers its auto-oxidation.
In contrast with our results, Shaw et al.23 found that near-infrared light treatment neuroprotects dopaminergic cells in the substantia nigra pars compacta and the zona incerta of the hypothalamus from degeneration in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. In their protocol, the time of light exposure was very short: four 90-second exposure cycles, spaced over 30 hours. This is a completely different paradigm from the one we used in our experiments to induce neuromelanin formation and reduction in TH-positive neurons, but it indicates that the effect of light on dopamine neuron is strictly dependent on the wavelength used and the time of exposure.
A crucial point in our work was to understand whether the reduction in TH-positive neurons produced by continuous exposure to light was induced by a direct effect of light on the substantia nigra or whether it was indirectly produced by the stress caused by retina activation (i.e. the complete alteration of the circadian rhythm). Continuous bright light profoundly alters the circadian cycle of the animals and their hormonal homeostasis. For example, Abilio et al.24 have clearly shown that continuous exposure to light increases dopaminergic function as a consequence of light-induced stress and increase in corticosterone levels. Beside corticosterone, other hormones could be altered by continuous light exposure. For instance, melatonin secretion is suppressed by light and alteration of its level could influence dopamine content in the striatum25. In summary, the increase in dopamine turnover elicited from continuous bright light exposure could facilitate dopamine oxidation with the formation of neuromelanin and reduction of TH-positive neurons.
To face this fundamental problem we prepared rats with a bilateral lesion of the optic nerve, and half of them were exposed to continuous bright light for four months, to ensure no confounding by surgery effects, and no stress caused by light activating the retina. Animals under continuous bright light showed a significant reduction of TH-positive neurons and a greater number of neuromelanin granules in the substantia nigra compared to animals exposed to dim light, suggesting that light causes its effect directly reaching this midbrain structure.
Extrapolating data from animals to humans is always very difficult and speculative, but some aspects of our findings that could be relevant to Parkinson's disease deserve comment. One would suspect that the amount of light that reaches the substantia nigra in the human brain—for the size of the head, the thickness of the skull and the deep brain localization of the substantia nigra—will be negligible. However, scientific data refutes that assumption. Using near-infrared techniques, in 1977 Jobsis26 showed that light photons can travel a 13.3 cm path from side-to-side of the human head. In 10 sec, he was able to register, from one side of the head, a higher than background (darkness) photon count emitted from a light source positioned at the contralateral side of the head. This observation suggests that a tiny amount of light can reach deep brain structures in human beings and this amount is proportional to the length of exposure and the brightness of the light source.
If natural environmental light plays any role in Parkinson's disease it would be expected that there should be a correlation between sunlight intensity in different parts of the world and prevalence of Parkinson's disease, such as the north-south gradient of disease seen in multiple sclerosis27,28. A geographical relationship between latitude and proportional mortality ratios for Parkinson's disease by state of birth in the U.S.A. was originally found by Kurtzke and Goldberg (1988)29 and Lux and Kurtzke (1987)30 with a north-to-south gradient. However, such a north-to-south gradient was not observed in the studies of Lilienfeld et al. (1990)31 and Betemps and Buncher (1993)27. The latter study found a geographical relationship between longitude and proportional mortality ratios for Parkinson's disease by state of birth with an east-to-west gradient. These differences could reflect changes in lifestyle of the population as the studies of Lilienfeld et al. (1990)31 and Betemps and Buncher (1993)27 consider geographic distribution of Parkinson's disease death rates in the same regions two decades later. Indeed, lifestyle has changed in the last decades and today the majority of the general population, with the exception of some categories of workers like farmers and fishermen, spends most of the daytime indoors, at home and work, therefore it would be more appropriate to consider exposure to artificial light rather than sunlight in a correlation study with Parkinson's disease prevalence. According to this, in a correlation analysis that accounted for population density, the age- and race-adjusted Parkinson's disease prevalence significantly correlated with average satellite-observed sky light-pollution.
Together, these observations suggest that artificial light rather than sunlight could play a role in Parkinson's disease, although future spatial epidemiological analyses using an incident Parkinson's disease cohort, detailed exposure modeling, and inclusion of other potential confounders are prudent to examine this association further.
It is worth noting that the spectral characteristics of artificial light, especially fluorescent light, are completely different from sunlight, as the sunlight spectrum is continuous while fluorescent light spectrum is discontinuous and shows several peaks. Light frequencies of the sunlight spectrum could protect rather than damage the dopamine neurons, as we have mentioned above for near-infrared light23. Given that, it would be worth identifying which frequency(s) of the fluorescent lamp used in our experiments is damaging the dopaminergic neurons, and to study whether other electromagnetic frequencies derived by other light sources, like computer and TV screens, are detrimental to dopamine neurons. Eventually, these findings could lead to safer light devices. Furthermore Parkinson's disease is currently considered a multifocal illness with progressive stages32. This plurifocality may be clearly in contrast with the specific lesion of substantia nigra induced by bright light, as suggested in this study. However the same bright light may hit all the cells carrying dopamine, or catecholamines in general, as a major constituent. Besides, the concept of plurifocal illness in Parkinson's disease is absolutely questionable since it is linked to the diagnostic purity and to the fact that the degenerative foci may be the consequence of a primary lesion.
Our findings are at an early stage and much more work will be required to establish the mechanism of light-induced neuromelanin formation and reduction of TH-positive neurons. Furthermore, we do not know yet whether the loss of TH-positive staining means dopaminergic cell death or simply the loss of the dopaminergic phenotype. Another issue to deal with would be to evaluate if the effect of continuous bright light could be reverted by returning the animals to a normal light setting. Finally, it would be important to study whether or not these findings are relevant to humans.
Methods
Animals
All experiments were performed in compliance with the Animal Care and Use Committee guidelines and approved by the Italian Ministry of Health (Italian Legislative Decree, Directive n.86/609/CEE, 27 January 1992 n.116). All efforts were made to minimize the number of animals used and their suffering. The data presented in this article were obtained from experiments carried out on Sprague–Dawley albino male rats. Animals born to pregnant females (brought from Charles Rivers), were housed with the mother until weaning (from birth to 21 days) in dim light (~5 lux) - dark cycle (12 h–12 h). After 21 days, they were separated from the mother and treated as described below.
Light exposure
Twenty-one days after birth, rats were placed in acrylic glass (Plexiglas) cages (two rats in each cage) with food available on the floor and water in plastic bottles. They were exposed to light or dark according to the following three protocols: a) dim light (~5 lux) - dark cycle (12 h–12 h) for three months; b) dim light (~5 lux) - dark cycle (12 h–12 h) for seventy days and then continuous bright light (~3,000 lux) for 20 days; c) continuous bright light (~3,000 lux) for three months.
Bilateral optic nerve transection
Sixteen animals underwent a bilateral surgical transection of the optic nerve. The surgical procedure was performed in three-months old rats for easier viewing of the surgical field. The animals were housed in dim light (~5 lux) - dark cycle (12 h–12 h) until surgery. The surgical approach is described and illustrated in Fig. 5. Both optic nerves were transected in the same surgical session and animals were allowed to recover for one week before light exposure. Eight animals were exposed to dim light (~5 lux) - dark cycle (12 h–12 h) and the other eight to continuous bright light (~3,000 lux) for four months.
Immunohistochemistry for tyrosine hydroxylase (TH)
At the end of the light exposure period, rats were anesthetized with 20% urethane and perfused transcardially with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (PBS), pH 7.4. Brains were then removed, placed for two days in 4% paraformaldehyde and cryo-protected by placing them sequentially in 15 and 30% sucrose (in PBS 1×) at 4°C until they sank. Tissues were then embedded in TISSUE TEK OCT® Compound and frozen in liquid nitrogen until cut.
Cryostat sections, 40 μm thick, were collected on gelatin-coated slides (one per slide). Slides were rinsed three time in PBS 1× (10 min each) and then placed in PBS 1× solution with 0.75% horse serum + 0.03% Triton for 20–30 min at room temperature. They were incubated with TH-specific primary rabbit polyclonal antibody (1:200, Millipore) overnight at 4°C and then rinsed three times in PBS 1× (10 min each).
Incubation with secondary Alexa Fluor 594 goat anti-rabbit IgG antibody (Invitrogen-Life Technologies) was performed at 37°C for 2 h, followed by three washes with PBS 1× (10 min each). After washing, coverslips were mounted using glycerol gelatin.
Fontana-Masson staining of neuromelanin
Cryostat sections (40 μm thick), prepared as described above, were placed in Fontana silver nitrate working solution and incubated in a 56°C oven for 2 h. Then, they were rinsed three times in distilled water (5 min each), toned in gold chloride working solution for 1 min, and rinsed three times in distilled water (5 min each). They were then placed in 5% sodium thiosulfate solution for 1 min, rinsed three times in distilled water (5 min each) and counterstained with nuclear fast red solution (dilution 1:10 from stock solution) for 2–5 min. Finally, they were rinsed thoroughly in distilled water twice (5 min each) and coverslips were mounted using glycerol gelatin.
Stereological counting of TH-positive neurons and neuromelanin-positive cells in the substantia nigra
Counting the number of TH-positive neurons in the substantia nigra was performed using Stereo Investigator software 8.0 (MicroBrightField, Colchester, VT, USA) and a Zeiss microscope. Every sixth section (sections prepared as described above) was taken throughout the entire substantia nigra, with a total of nine sections for each animal. In each section, the substantia nigra area was traced at 5× magnification and the count executed at 20× magnification. Sections directly adjacent to the one used for stereological counting of TH-positive neurons were used for counting neuromelanin-positive cells and processed as above. Counting was performed on the left substantia nigra.
Dopamine, 5-hydroxytryptamine, and metabolites assay
Rats were sacrificed by guillotine decapitation after ether anesthesia, their brains were removed and quickly chilled in ice-cold 0.9% NaCl, and the striatum was dissected, frozen in dry ice, and stored at −80°C until assay. The tissue samples were homogenized in 600 μl ice-cold 0.1 mol/l perchloric acid containing 10 pg/μl of dihydroxybenzylamine (DBA) as the internal standard; an aliquot of homogenate was assayed for protein. The homogenates were centrifuged and the levels of dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 5-hydroxytryptamine, and 5-hydroxyindoleacetic acid (5-HIAA) in the supernatant were determined by reverse-phase HPLC coupled with an electrochemical detector as described by Vaglini et al.33. Results are expressed as the mean ± SEM of eight animals per group.
Fluorescent lighting spectrum and penetration of light into the rat brain
Rats were exposed to Philips Master TL-D Secura 36W/840 1 SL, 120 cm length fluorescent tubes. The fluorescent tubes were placed just over and beneath the cages. The electromagnetic spectrum of this lamp, measured with a calibrated UV-VIS spectroradiometer (Ocean Optics, mod. USB2000-XR1) equipped with a 400 μm optical fiber and UV-VIS cosine corrector is presented in Fig. 1a. The total intensity of the light emitted by the lamp was ~3000 lux (~1 mWcm2).
In order to detect which component of the spectrum and to what extent the light could cross the rat scalp and skull, we positioned the recording probe just inside the skull using fresh postmortem tissue. The recorded light spectrum inside the skull is shown in Fig. 1b. As expected, the amount of light crossing the scalp and skull increases with longer wavelength34.
Statistical analysis
One way ANOVA plus Scheffe's F test was used for comparison of neuromelanin positive neurons, TH positive neurons and amine and metabolite levels between the different experimental groups. Wilcoxon-Mann-Whitney U test was used for the comparison between light exposure and substantia nigra pigmentation in mammals.
To match the two panels in Figure 5, we took the raw raster data from NASA (National Aeronautics and Space Administration), and performed a county level spatial join using ESRI (Environmental Systems Research Institute) ARC MAP version 10 geoprocessing software. The total recorded light emission was then calculated per county. The correlation analysis included the county level age and race adjusted Parkinson's disease prevalence among Americans with Medicare and the county level total light emission, adjusting for county total population.
Medicare is a government-mandated insurance program used by 98% of 65 years and older Americans. We searched all Medicare outpatient and physician claims data. Beneficiaries with International Statistical Classification of Diseases, Ninth Revision code 332.0 were extracted. Those who also had diagnostic claims for secondary Parkinsonism (code 332.1) or other degenerative diseases of the basal ganglia (code 333.0), regardless of the order of diagnoses, were excluded from further analysis. All calculations were performed on beneficiaries who qualified for Medicare by virtue of being 65 years of age or older. To calculate Parkinson disease prevalence, we performed age and sex standardization using the direct standardization method with all Medicare beneficiaries as the standard population.
Author Contributions
R.Mag. wrote the manuscript text and co-designed experiments, discussed analyses, interpretation, and presentation with G.U.C. and C.M. D.D.C. and L.L. prepared figure 1. S.R., C.R., M.Cap., G.A., M.Cal. and R.Mac. prepared figures 2, 3 and 5 and Table 1. C.V. and F.V. prepared figure 4. A.W.W. and B.A.R. prepared figure 6.
Acknowledgments
This study was supported by Ministero dell'Istruzione, dell'Università e della Ricerca (PRIN 20085PPEK7) and by Cariplo Foundation (Scientific Research in Biomedicine 2011). Stefania Romeo was supported by a fellowship from LIMPE (Lega Italiana per la Lotta Contro la Malattia di Parkinson le Sindromi Extrapiramidali e le Demenze). We thank Dr. Pierantonio Tetè, Zoologist of the University of L'Aquila, for assistance in compiling Table 1.
References
- Xuan Q. et al. Increase expression of α-synuclein in aged human brain associated with neuromelanin accumulation. J. Neural. Transm. 118, 1575–1583 (2011). [DOI] [PubMed] [Google Scholar]
- Elsworth J. D. & Roth R. H. Dopamine synthesis, uptake, metabolism, and receptors: relevance to gene therapy of Parkinson's disease. Exp. Neurol. 144, 4–9 (1997). [DOI] [PubMed] [Google Scholar]
- Sulzer D. & Zecca L. Intraneuronal dopamine-quinone synthesis: a review. Neurotox. Res. 1, 181–195 (2000). [DOI] [PubMed] [Google Scholar]
- Bisaglia M., Mamm S. & Bubacco L. Kinetic and structural analysis of the early oxidation products of dopamine: analysis of the interactions with α-synuclein. J. Biol. Chem. 282, 15597–15605 (2007). [DOI] [PubMed] [Google Scholar]
- Sulzer D. et al. Neuromelanin biosynthesis is driven by excess cytosolic catecholamines not accumulated by synaptic vesicles. Proc. Natl. Acad. Sci. USA. 97, 11869–11874 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rivera A. E. et al. Spectrophotometric study on the stability of dopamine and the determination of its acidity constants. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 59, 3193–3203 (2003). [DOI] [PubMed] [Google Scholar]
- Barreto W. J., Barreto S. R. G. & Silva W. P. A study of the aerobic reaction between dopamine and Fe(III) in the presence of S2O32−. Transition. Met. Chem. 34, 677–681 (2009). [Google Scholar]
- Smith M. Shedding light on the adult brain: a review of the clinical applications of near-infrared spectroscopy. Philos. Transact. A. Math. Phys. Eng. Sci. 369, 4452–4469 (2011). [DOI] [PubMed] [Google Scholar]
- Foster R. G. & Follett B. K. The involvement of a rhodopsin-like photopigment in the photoperiodic response of the Japanese quail. J. Comp. Physiol. A. Neuroethol. Sens. Neural. Behav. Physiol. 157, 519–528 (1985). [Google Scholar]
- Nakane Y. et al.. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc. Natl. Acad. Sci. USA. 107, 15264–15268 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Brunt E. E., Shepherd M. D., Wall J. R., Ganong W. F. & Clegg M. T. Penetration of light into the brain of mammals. Ann. N Y. Acad. Sci. 117, 217–224 (1964). [DOI] [PubMed] [Google Scholar]
- Schapira A. H. & Jenner P. Etiology and pathogenesis of Parkinson's disease. Mov. Disord. 26, 1049–1055 (2011). [DOI] [PubMed] [Google Scholar]
- Marsden C. D. Pigmentation in the nucleus substantiae nigrae of mammals. J. Anat. 95, 256–261 (1961). [PMC free article] [PubMed] [Google Scholar]
- Willis A. W., Evanoff B. A., Lian M., Criswell S. R. & Racette B. A. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 34, 143–151 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattei M., Levi A. C. & Fariello R. G. Neuromelanic pigment in substantia nigra neurons of rats and dogs. Neurosci. Lett. 72, 37–42 (1986). [DOI] [PubMed] [Google Scholar]
- Kim S. T., Choi J. H., Kim D. & Hwang O. Increases in TH immunoreactivity, neuromelanin and degeneration in the substantia nigra of middle aged mice. Neurosci. Lett. 396, 263–268 (2006). [DOI] [PubMed] [Google Scholar]
- Graham D. G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 14, 633–643 (1978). [PubMed] [Google Scholar]
- Lotharius J. & Brundin P. Pathogenesis of Parkinson's disease: dopamine, vesicles and alpha-synuclein. Nat. Rev. Neurosci. 3, 932–942 (2002). [DOI] [PubMed] [Google Scholar]
- LaVoie M. J. & Hastings T. G. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. J. Neurosci. 19, 1484–1491 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca L. et al. Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. J. Clin. Pathol: Mol. Pathol. 5, 414–418 (2001). [PMC free article] [PubMed] [Google Scholar]
- Dexter D. T., Sian J., Jenner P. & Marsden C. D. Implications of alterations in trace element levels in brain in Parkinson's disease and other neurological disorders affecting the basal ganglia. Adv. Neurol. 60, 273–281 (1993). [PubMed] [Google Scholar]
- Sian-Hülsmann J., Mandel S., Youdim M. B. & Riederer P. The relevance of iron in the pathogenesis of Parkinson's disease. J. Neurochem. 118, 939–957 (2011). [DOI] [PubMed] [Google Scholar]
- Shaw V. E. et al. Neuroprotection of midbrain dopaminergic cells in MPTP-treated mice after near-infrared light treatment. J. Comp. Neurol. 518, 25–40 (2010). [DOI] [PubMed] [Google Scholar]
- Abilio V. C., Freitas F. M., Dolnikoff M. S. & Castrucci A. M. L. Frussa-Filho, R. Effects of continuous exposure to light on behavioural dopaminergic supersensitivity. Biol. Psychiatry. 45, 1622–1629 (1999). [DOI] [PubMed] [Google Scholar]
- Khaldy H. et al. Circadian rhythms of dopamine and dihydroxyphenyl acetic acid in the mouse striatum: effects of pinealectomy and of melatonin treatment. Neuroendocrinology. 75, 201–208 (2002). [DOI] [PubMed] [Google Scholar]
- Jöbsis F. F. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 198, 1264–1267 (1977). [DOI] [PubMed] [Google Scholar]
- Betemps E. J. & Buncher C. R. Birthplace as a risk factor in motor neurone disease and Parkinson's disease. Int. J. Epidemiol. 22, 898–904 (1993). [DOI] [PubMed] [Google Scholar]
- Pugliatti M., Sotgiu S. & Rosati G. The worldwide prevalence of multiple sclerosis. Clin. Neurol. Neurosurg. 104, 182–191 (2002). [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. & Goldberg I. D. Parkinsonism death rates by race, sex, and geography. Neurology. 38, 1558–1561 (1988). [DOI] [PubMed] [Google Scholar]
- Lux W. E. & Kurtzke J. F. Is Parkinson's disease acquired? Evidence from a geographic comparison with multiple sclerosis. Neurology. 37, 467–471 (1987). [DOI] [PubMed] [Google Scholar]
- Lilienfeld D. E. et al. Parkinsonism death rates by race, sex and geography: a 1980s update. Neuroepidemiology. 9, 243–247 (1990). [DOI] [PubMed] [Google Scholar]
- Braak H. et al. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol. Aging. 24, 197–211 (2003). [DOI] [PubMed] [Google Scholar]
- Vaglini F., Fascetti F., Fornai F., Maggio R. & Corsini G. U. (+)MK-801 prevents the DDC-induced enhancement of MPTP toxicity in mice. Brain. Res. 668, 194–203 (1994). [DOI] [PubMed] [Google Scholar]
- Viggiani E., Ciesla M. & Russo O. L. The shielding power of the rat skull to visible light. Experientia. 26, 850–851 (1970). [DOI] [PubMed] [Google Scholar]