Abstract
The long non-coding RNA MALAT1, also known as MALAT-1 or NEAT2, is a highly conserved nuclear ncRNA and a predictive marker for metastasis development in lung cancer. To uncover its functional importance, we developed a MALAT1 knockout model in human lung tumor cells by genomically integrating RNA destabilizing elements using Zinc Finger Nucleases. The achieved 1000-fold MALAT1 silencing provides a unique loss-of-function model. Proposed mechanisms of action include regulation of splicing or gene expression. In lung cancer, MALAT1 does not alter alternative splicing but actively regulates gene expression including a set of metastasis-associated genes. Consequently, MALAT1-deficient cells are impaired in migration and form fewer tumor nodules in a mouse xenograft. Antisense oligonucleotides blocking MALAT1 prevent metastasis formation after tumor implantation. Thus, targeting MALAT1 with antisense oligonucleotides provides a potential therapeutic approach to prevent lung cancer metastasis with MALAT1 serving as both, predictive marker and therapeutic target. Lastly, regulating gene expression, but not alternative splicing is the critical function of MALAT1 in lung cancer metastasis. In summary, ten years after the discovery of the lncRNA MALAT1 as a biomarker for lung cancer metastasis, our loss-of-function model unravels the active function of MALAT1 as a regulator of gene expression governing hallmarks of lung cancer metastasis.
Keywords: non-coding RNA, MALAT1, lung cancer, metastasis, Zinc Finger Nucleases
Introduction
Activation of migration, invasion and metastasis is a crucial characteristic of malignancy as one of the hallmark capabilities of cancer (1). Metastasis is the major cause of cancer recurrence and tumor-related death (2–3). Most studies investigating metastasis mechanisms focused on protein-coding genes, although recent transcriptome-wide analyses have revealed an overwhelming amount of transcribed, but not translated genes (4–5). Hence, transcription generates many long non-coding RNAs, abbreviated as ncRNA or lncRNA, capable of influencing diverse cellular processes such as proliferation, cell cycle progression, apoptosis or cell growth (reviewed in: (6)). LncRNAs can apply diverse modes of action to regulate these processes and most lncRNAs studied so far regulate gene expression (7–12). Consequently, lncRNAs are deregulated in diverse human cancers and associated with disease progression (13–16). For example, the ncRNA HOTAIR (Hox Transcript Antisense RNA) is highly expressed in breast cancer and is a predictor for metastasis formation and associated with a poor prognosis (17).
One of the first cancer-associated lncRNAs discovered was MALAT1 (Metastasis-Associated Lung Adenocarcinoma Transcript 1) (18), also referred to as NEAT2 (Nuclear-Enriched Abundant Transcript 2). MALAT1 is extremely abundant in many human cell types and highly conserved over its full length (~8 kb) across mammalian species underscoring its functional importance. Its 3’-end can be modified by RNase P and RNase Z cleavage, which yields an additional tRNA-like ncRNA, the cytoplasmic mascRNA (19). The longer form of MALAT1 is retained in the nucleus and specifically localizes to nuclear speckles (20). These structures are regions enriched in pre-mRNA splicing factors and could serve as storage, assembly or modification sites (21). MALAT1 might regulate alternative splicing of a subset of pre-mRNAs by modulating serine / arginine splicing factor activity (22), which regulate tissue- or cell-type specific alternative splicing in a phosphorylation-dependent manner (23). However, splicing alterations were not found after Malat1 ablation in mice (24). In contrast, alternative functions for MALAT1 were recently identified (25): MALAT1 could interact with the demethylated form of CBX4 (Chromobox homolog 4), also referred to as Pc2 (Polycomb 2), a component of the Polycomb Repressive Complex 1 (PRC1). This interaction controls the re-localization of growth control genes between polycomb bodies and interchromatin granules, areas of silent or active gene expression, respectively. MALAT1 resides in these subnuclear structures and acts as an activator of gene expression potentially by mediating the assembly of coactivator complexes (25).
Given these two alternative proposed mechanisms of action for MALAT1, the exact function of MALAT1 is still unknown. Additionally, it remains to be elucidated whether the ubiquitously expressed MALAT1 has one universal function or whether its mechanisms of action might be tissue-specifically different.
MALAT1 was originally identified as a prognostic marker for metastasis and patient survival in non-small cell lung cancer (NSCLC), specifically in early stages of lung adenocarcinoma (18). In lung squamous cell carcinoma, high MALAT1 expression is also associated with poor prognosis. MALAT1 might impact growth and colony formation of NSCLC cells in vitro (26). Upon injection into nude mice, cells with moderately decreased MALAT1 expression show reduced tumor growth. Reduced MALAT1 levels impair cell motility in vitro (27).
A potential active role of MALAT1 in metastasis as well as its specific functions remain unknown. Thus, to clarify the function of MALAT1 at the cellular and molecular level - more precisely to determine its functional importance in metastasis and the regulation level affected by MALAT1 - we have developed a methodology to establish a comprehensive loss-of-function model for MALAT1 using Zinc Finger Nucleases (ZFNs) to stably integrate RNA destabilizing elements into the human genome (28). This approach resulted in a specific and more than 1000-fold silencing of MALAT1 and allowed specific and effective loss-of-function studies of this abundant ncRNA in human cancer cells.
Here, we report that loss of MALAT1 deregulates gene expression but not alternative splicing in lung cancer. MALAT1-deficient lung cancer cells are impaired in migration and form significantly fewer and smaller lung tumor nodules than their wild-type counterparts in a mouse xenograft assay. Beyond its value as a prognostic marker for metastasis development (18, 26), these findings uncover MALAT1 as an active player in lung cancer metastasis and establish gene regulation of metastasis-associated genes rather than alternative splicing as the critical MALAT1 mechanism affiliated with metastasis. Consequently, targeting MALAT1 expression in established human xenograft tumors with free-uptake antisense oligonucleotides (ASO) drastically reduces lung cancer metastasis formation in vivo and validates MALAT1 as a potential therapeutic target in lung cancer.
Materials and Methods
Cell culture
A549 lung adenocarcinoma cells were purchased from ATCC (CCL-185) in 2010 and cultivated at 37°C, 5% CO2 in DMEM + 10% fetal bovine serum (FBS); 0.2 mM Glutamine and antibiotics. A549 MALAT1 KO cells were generated as previously published (28). The EBC-1 lung squamous cell carcinoma line was obtained in 2010 from the Health Sciences Foundation, Japan, and maintained in RPMI-1640 media containing 10% FBS in a humidified incubator with 5% CO2 at 37°C. Cell lines were authenticated by ATCC or by the Health Science Foundation Japan via short tandem repeat (STR) DNA profiling. No further cell line authentication was performed, but the A549 WT and KO cells were tested for MALAT1 expression and mycoplasma negativity every six months. All cell lines were maintained in culture for a maximum of 20 passages (2 months).
Scratch / Wound Healing Assay
The CytoSelect™ 24-well Wound Healing Assay (Cell Biolabs, San Diego, CA) was used to analyze migration of A549 WT and MALAT1 KO cells. The assay was done according to the manufacturer’s recommendations using 2.0×105 cells per well. Image acquisition of wound fields was done after removal of inserts (0 h) and wound closure documentation was done after 24 h and 48 h with a phase-contrast microscope (Leica DM IL; Leica Microsystems, Wetzlar, Germany) equipped with a digital camera (Leica DFC300FX). Image analysis was performed with Adobe Photoshop CS3 software.
A549 xenograft lung cancer mouse model
A549 have previously been used to establish lung tumor nodules in mice (29). Two months after intravenous injection into NOD-SCID mice, mice were sacrificed and the lungs were isolated, fixed and tumor nodule numbers and areas were analyzed in a blinded fashion.
EBC-1 xenograft assay
Exponentially growing EBC-1 cells were collected by trypsin-EDTA and washed once with phosphate-buffered saline (PBS). The cell pellet was suspended in PBS and ten million cells were implanted by subcutaneous injection into BALB/c nude mice. Two weeks after implantation, the mice were randomly divided into two treatment groups. The first treatment group was injected with 250 mg/kg/week of MALAT1 ASO, administered subcutaneously for 5 weeks. The second treatment group was injected with 250 mg/kg/week of control oligonucleotide. In week 7, the subcutaneous tumor was surgically removed and the wound closed with a 4-0 suture. Mice were euthanized in week 12 after the start of ASO treatment. Lung tissues were collected and processed for further analysis. The tumor multiplicity was counted using a light microscope.
Additional details on Materials & Methods can be found in the Supplements.
Results
Efficient silencing of the highly abundant MALAT1 in human lung cancer cells
To enable this study, we recently developed a new method for highly effective and specific silencing of protein-coding as well as non-protein-coding genes (28). The functional knockout of MALAT1 was achieved by Zinc Finger Nuclease (ZFN)-mediated site-specific integration of RNA destabilizing elements into the genome of human cancer cells. Stable knockout (KO) clones showed on average 1000-fold less MALAT1 expression than their wild-type (WT) counterparts (28, 30). To exclude clone-specific effects, we selected a panel of six different cell lines derived from A549 lung cancer cells to analyze their phenotype. The loss of MALAT1 expression was previously validated (30). The expression level of MALAT1 was comparable in all control wild-type cells and not affected by clonal selection (A549 vs. WT and GFP) (30).
For the validation of our data and to prove that the obtained results were neither clone-specific nor dependent on the integration site within the MALAT1 gene, we have generated a second ZFN targeting MALAT1 downstream of the first ZFN (Supplementary Fig. S2A). With this ZFN-2, we created two additional KO clones (KO4, KO5) that also lost MALAT1 expression (Supplementary Fig. S2B).
Alternative splicing is not affected by loss of MALAT1
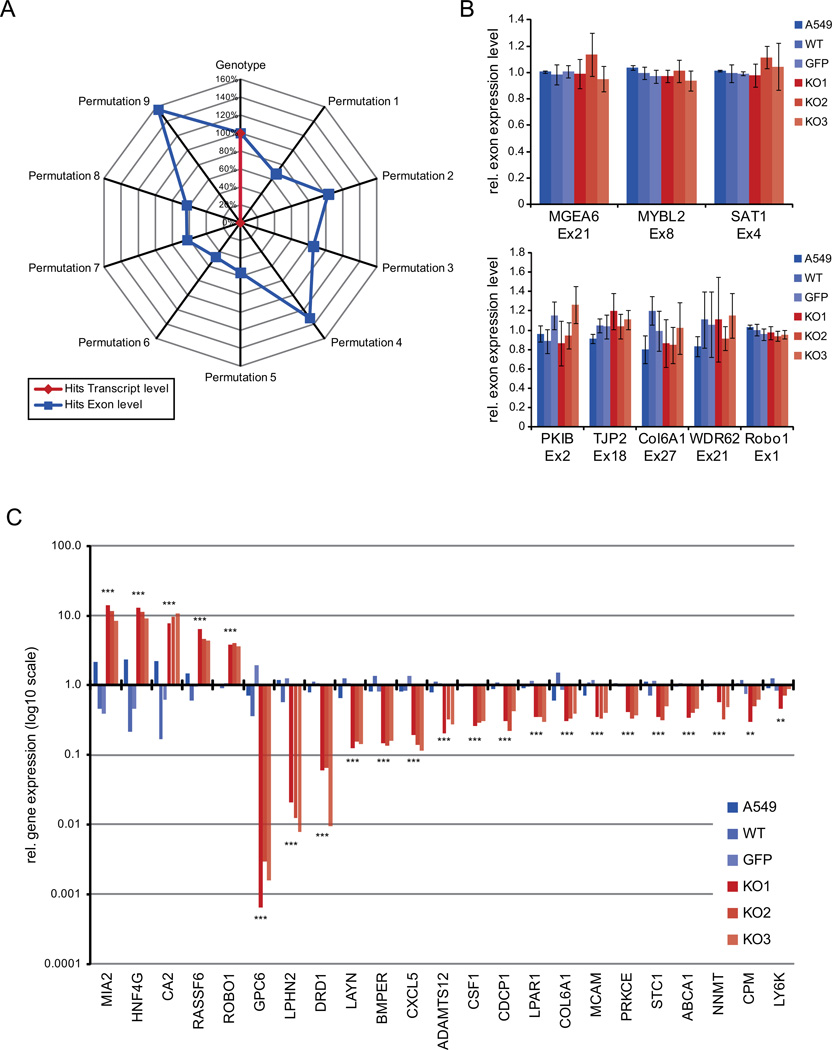
MALAT1 had been previously linked to two different molecular mechanisms: regulating alternative splicing (22) and regulating gene expression (25). Therefore, we started to analyze its effect on gene expression and alternative splicing in our quantitative loss-of-function system. We investigated both possible mechanisms using microarrays to analyze gene expression at the exon and the transcript level in all six cell lines (A549, WT, GFP, KO1-3). Statistical analysis of the exon array data revealed that MALAT1 appeared to regulate both, gene expression and splicing resulting in 459 significantly regulated transcripts (adj. p<0.05, Supplementary Table S1) and 215 / 3025 significantly regulated exons (splice index >1.0 / >0.5). To determine the specificity of the observed regulations, we performed dataset permutations. First, the six cell lines were grouped according to their genotype annotation comparing the three MALAT1 WT samples with the three MALAT1 KO samples. This analysis based on the genotypes yielded both, significant hits at the exon and the transcript level (Fig. 1A). Additionally, the six samples were divided randomly into two groups in all nine possible permutations. Surprisingly, all nine different sample permutations gave rise to significant hits at the exon level - two permutations even with larger numbers of regulated exons than the correct sample grouping. In stark contrast, none of the nine sample permutations gave rise to any significantly regulated transcript (Fig. 1A). This indicated that the differences at the transcript level were highly significant and specifically associated with the loss of MALAT1 while the detected differences at the exon level were not specific. The permutation analysis was repeated with groups containing equal numbers of WT and KO datasets (32 permutations of the technical replicates) yielding the same result (Supplementary Fig. S1A). Hence, MALAT1 specifically regulated gene expression but not alternative splicing in lung cancer cells.
Figure 1. MALAT1 specifically regulates gene expression but not splicing.
The impact of MALAT1 on genome-wide gene expression and alternative splicing was analyzed by Exon Microarrays. Three WT (A549, WT, GFP) and three KO (KO1-3) lines were analyzed in biological duplicates.
A) Spider web chart for the specificity of gene regulation (red) or alternative splicing (blue) alterations by loss of MALAT1. The exon array analysis revealed 459 significantly regulated transcripts and 3025 differentially spliced exons. To assess the specificity of the uncovered regulations, we permutated the dataset and repeated the analysis: first, the six cell lines were grouped into two groups according to their genotype (WT versus KO). Then, the six samples were randomly divided into two groups in all nine possible permutations. The genotype-based yielded both, significant hits at the exon and the transcript level (each set as 100%). At the exon level, all nine random sample permutations gave rise to significant hits. In stark contrast, none of the nine sample permutations gave rise to any significantly regulated transcripts indicating the specificity of the gene regulation.
B) Analysis of alternative splicing. MALAT1-dependent, alternatively spliced exons were analyzed by qRT-PCR (n=3; mean ±SEM). Alternative splicing alterations were detected neither for previously identified cassette exons (22) (upper panel) nor for cassette exons from our own exon array analysis (lower panel).
C) Validation of differentially expressed genes after loss of MALAT1. MALAT1 KO cells showed a significant deregulation of 459 genes (Supplementary Table S1). The differential expression of 23 metastasis-associated target genes was confirmed using qRT-PCR. All validation experiments were conducted in three independent RNA panels. Given is the relative expression of genes normalized to the mean of the WT cells. PPIA (Cyclophilin A) served as reference gene.
To expand this analysis, we tested the alternative splicing pattern of genes whose splicing pattern had previously been shown to be affected by knockdown of MALAT1 (22). However, none of the exons tested was differentially spliced upon complete loss of MALAT1 in the A549 lung cancer model (Fig. 1B, upper panel). In addition, splicing analysis by qRT-PCR of a set of candidate cassette exons regulated in the exon array screen in three independent replicates did not verify any differential splicing (Fig. 1B, lower panel). Thus, neither the previously published nor the new candidate exons from our microarray screen were reproducibly altered upon loss of MALAT1 indicating that MALAT1 does not impact alternative splicing in this system.
Additionally, neither the expression nor the phosphorylation of selected splicing factors were changed between MALAT1-expressing and MALAT1-deficient cells (Supplementary Fig. S1B) further corroborating that splicing remains unaffected after loss of MALAT1 in lung cancer cells.
MALAT1 regulates gene expression including metastasis-associated transcripts
Next, we focused on the deregulated genes at the transcript level whose expression levels were altered after MALAT1 depletion. An in-depth literature search revealed that several of the MALAT1 target genes contribute to or are associated with the metastatic potential of cancer cells (Supplementary Table S2). We were able to validate the differential expression of all 23 of these metastasis-associated target genes via qRT-PCR (Fig. 1C). Notably, these differential gene expression patterns were verified in additional KO clones generated with an independent ZFN (Supplementary Fig. S2D). Expression levels were highly consistent within each group (WT or KO), but differed strongly between the two genotypes. For example, MIA2 (Melanoma inhibitory activity 2), a negative regulator of tumor growth and invasion (31), or ROBO1 (Roundabout 1), an inhibitor of glioma migration and invasion (32–33), were increased in all KO cells compared to all WT cells. In turn, GPC6 (Glypican 6), a promoter of breast cancer metastasis (34), LPHN2 (Latrophilin 2), an important factor for the Epithelial-to-Mesenchmyal-Transition (EMT) of endothelial cells in the atrioventricular canal of the heart and involved in cancer cell invasion (35–36), CDCP1 (Cub domain containing protein 1), a promoter of lung adenocarcinoma metastasis in a mouse model (37), and ABCA1 (ATP-binding cassette, sub-family A, member 1), an important factor for prostate cancer cell migration and EMT (38–39), were strongly reduced by loss of MALAT1. The expression of several other MALAT1 target genes was associated with metastasis (e.g. DRD1, COL6A1, STC1) or they represented critical regulators of metastasis formation (e.g. GPC6, MCAM, PRKCE).
Notably, no gene ontology term “metastasis” exists - most likely due to the complexity of the underlying multistep process. However, a gene ontology analysis of the deregulated genes revealed a significant enrichment (all: p<10−7) of the biological processes “cell communication”, signal transduction” and “cell adhesion”, which are all related to the metastatic cascade, but also making it possible that loss of MALAT1 has an impact on other cellular processes than metastasis (Supplementary Fig. S1C).
Taken together, our microarray analysis revealed that MALAT1 might act as a regulator of gene expression inducing among others a set of genes previously associated with metastasis, i.e. suppressing the expression of metastasis inhibitors and activating the expression of metastasis promoters. These could act in concert determining the metastatic capacity to human A549 lung cancer cells (Supplementary Table S2) leading to the hypothesis that MALAT1 itself could function as a pro-metastatic factor.
Cell motility depends on MALAT1 in vitro
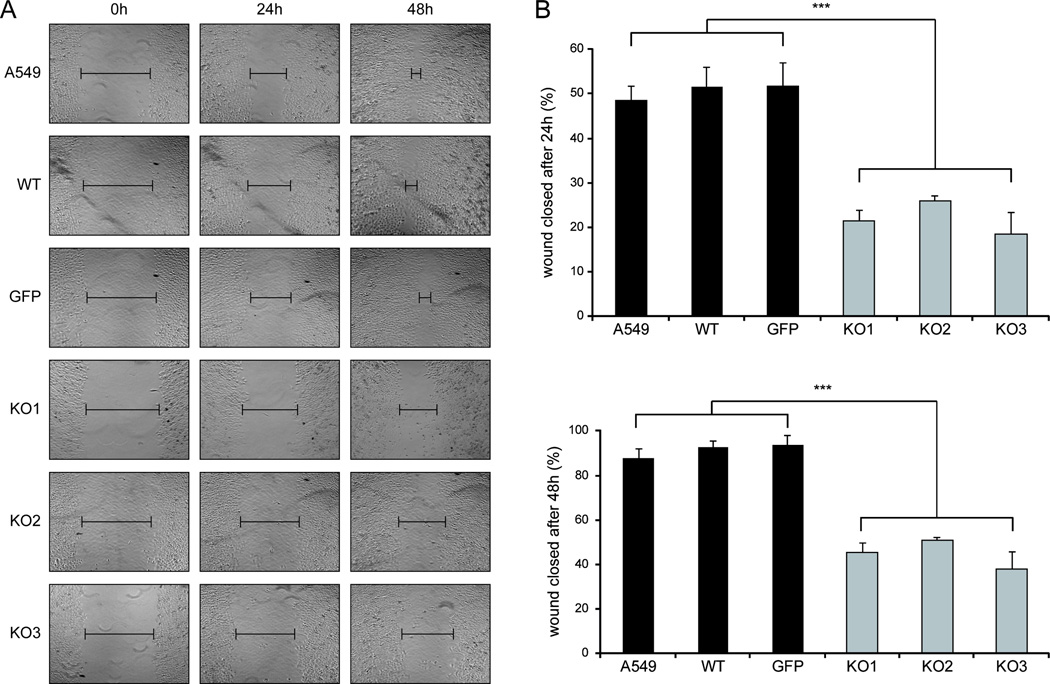
Given the impact of MALAT1 depletion on the expression of motility-relevant genes, we performed scratch assays with the six MALAT1 WT and KO cell lines to study this phenotype in vitro (Fig. 2A). Already after 24 h, the MALAT1 KO cells showed a significant reduction of cell motility (Fig. 2B). After 48 h, the WT cells showed an almost complete closure of the gap, whereas KO cells reduced the gap by only ~50% (Fig. 2A,B). Loss of MALAT1 in the additional clones generated with an independent ZFN also caused impaired migration validating these results (Supplementary Fig. S3). Of note, we could not detect differences in A549 cell proliferation in the KO clones KO1-KO3 (30) or KO4-KO5 (Supplementary Fig. S2C) further associating MALAT1 not with tumor growth but a metastatic phenotype.
Figure 2. MALAT1 is important for cell migration in vitro.
Scratch / wound healing assays were performed with MALAT1 WT and KO A549 cells. Shown are representative figures from three independent experiments. A) Wound fields were observed directly after removal of inserts (0h) and cell migration was followed for 24 h and 48 h. An obvious impact of MALAT1 loss on cell migration was detected at both time points.
B) Statistical analysis of wound closure. Gap size at 0 h was set to 100 percent and percentage of closed wound was calculated after 24 h and 48 h after image analysis. WT clones efficiently migrated into the gap while KO clones uniformly displayed a significantly impaired wound closure (p≤0.001).
MALAT1 is critical for important steps of the metastatic cascade in human lung cancer in vivo
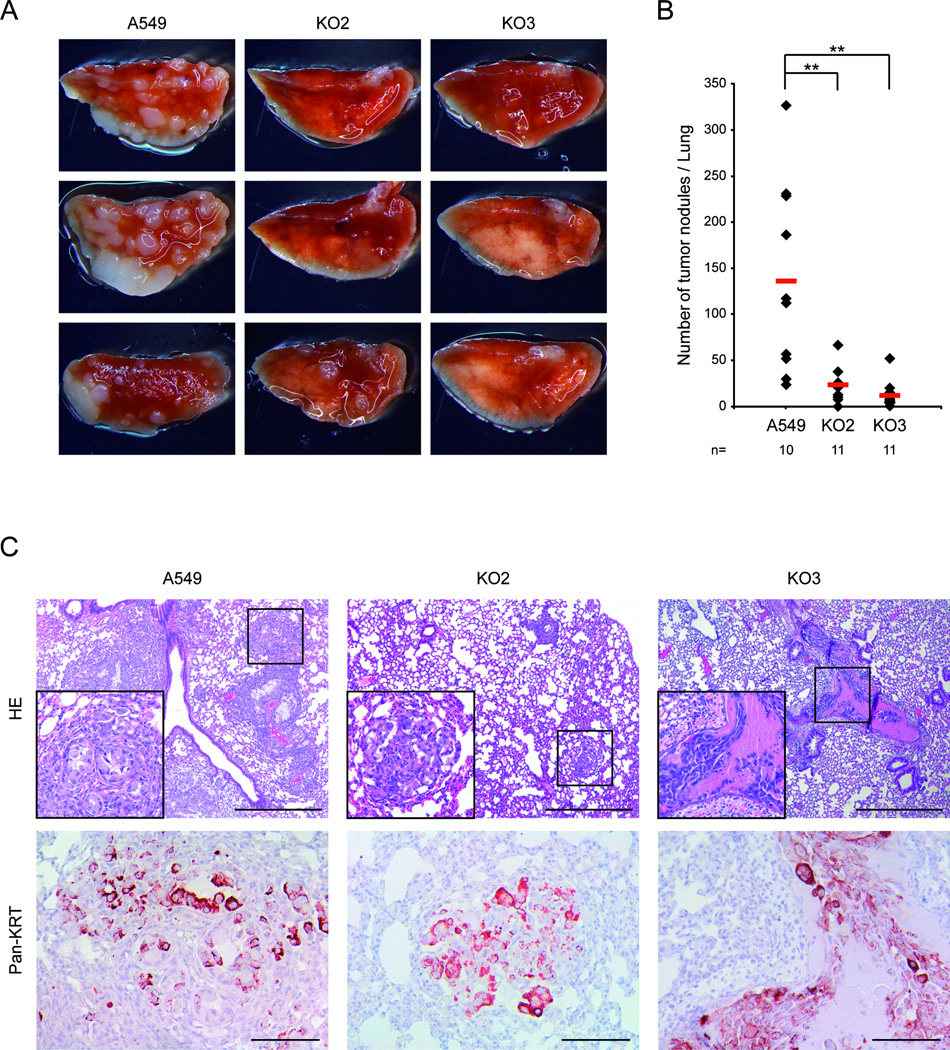
MALAT1 was originally discovered as a prognostic marker for lung cancer metastasis (18). Although the migratory capacity might impact the metastatic potential of a cancer cell, the complex metastatic phenotype has not been analyzed before in vivo and a functional link between MALAT1 and cancer metastasis has not been established so far. Therefore, we performed a mouse xenograft assay by injecting either A549 MALAT1 WT cells or the two KO cell lines with the lowest MALAT1 expression (KO2, KO3) into the tail vein of nude mice and analyzed the formation of lung tumor nodules after two months. While this assay did not recapitulate all steps of the complex metastatic cascade, it mimics the important steps of extravasation and tumor nodule formation in the lung. The results showed uniformly that MALAT1 was required for effective tumor nodule formation in vivo and thus played an active role in the metastatic process (Fig. 3A). A549 WT cells were able to establish numerous tumor nodules in the lung with an average number of 136 per lung (Fig. 3B). In contrast, two independent cell lines lacking MALAT1 (KO2 and KO3) showed a significantly reduced number of metastases (24 (83% reduction) and 12 (91% reduction), respectively).
Figure 3. MALAT1 is a critical factor for lung cancer homing and nodule formation in vivo.
A549 lung cancer cells or MALAT1 KO cells (KO2 and KO3) were analyzed for the metastatic potential in a mouse xenograft assay recapitulating major steps of metastasis development. 10–11 animals were analyzed per cell line.
A) Lung cancer tumor nodule formation. After tail vein injection of MALAT1 WT or KO cells, formation of lung tumor nodules was analyzed. The lungs were resected, fixed and the total number of tumors and the tumor area were determined. The tumor burden was drastically reduced in MALAT1 KO cells (KO2 and KO3) compared to wild-type control cells. Representative pictures from the left lobe are shown.
B) Statistical analysis. On average, 136 distinct nodules were counted in mice injected with A549 WT cells, whereas only 24 and 12 metastases were found in KO cells (t-test: p=0.007 and p=0.004, respectively).
C) Histology of lung tumor nodules. HE staining (upper panel) shows an extensive growth of MALAT1 WT tumor cells, which destroyed the normal alveolar structure of the lung (left). MALAT1 KO cells either developed micrometastases (magnified inlet, mid) or tumor cells resided inside blood vessels (right). Staining of a consecutive section against human pan-cytokeratin (clone KL-1) proves the human origin of tumor cells that invaded into the mouse lung tissue (magnified inlets, lower panel). Scale bar = 200 µm for 4 × magnification and 50 µm for 20 × magnification.
For mice injected with WT cells, lungs stained with Hematoxylin-Eosin (HE) showed an extensive and diffuse growth of tumor cells, which destroyed the normal alveolar structure of the lung (Fig. 3C, left panel). Lungs of mice injected with A549 MALAT1 KO cells either developed very small lung tumor nodules or tumor cells resided inside blood vessels (Fig. 3C, mid and right panel). Magnified inlets of representative tumor nodules are shown. Staining of consecutive lung sections with an antibody detecting human pan-cytokeratin confirmed the human origin of cancer cells invading mouse lung tissue (Fig. 3C, lower panels).
Thus, MALAT1 appears as an important regulator of the metastatic cascade of lung cancer in vivo.
ASOs can effectively target MALAT1 in vivo
Cancer metastasis is the major cause of cancer recurrence and tumor-related death. After establishing a critical role for MALAT1 in this process, we hypothesized that MALAT1 might be a potential therapeutic target. Therefore, we developed an approach to target MALAT1 effectively in vivo relying on the administration of free-uptake antisense oligonucleotides (ASOs), which were designed to potently target both human and mouse MALAT1. These second generation ASOs are effective at low nanomolar concentrations and are efficiently taken up by the cells, avoiding any toxic effects of lipofection. First, we analyzed the effectiveness of this approach in vitro and treated human EBC-1 lung cancer cells with increasing amounts of MALAT1-ASOs. These knocked down MALAT1 up to 20-fold (Supplementary Fig. S4A).
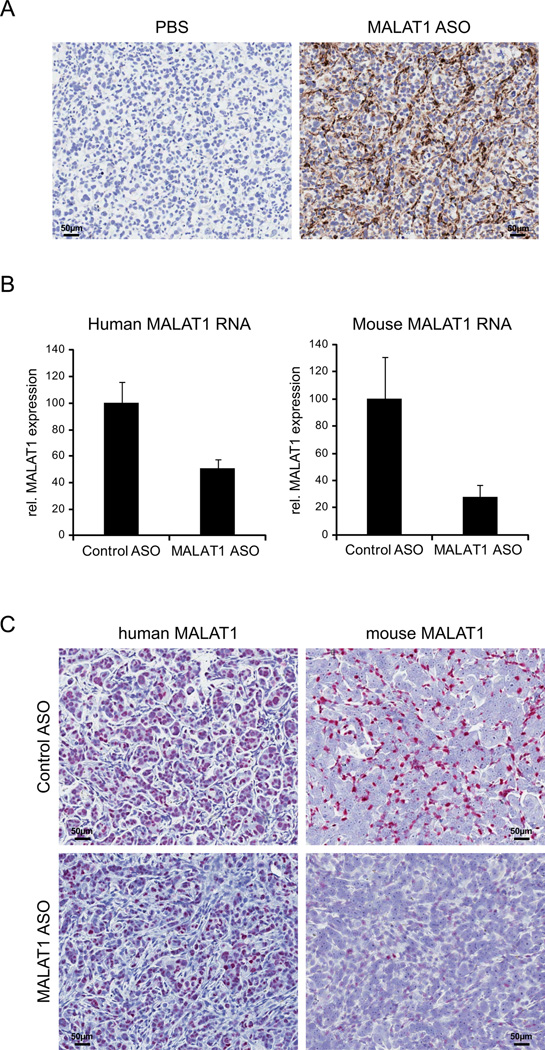
Next, we injected human NSCLC EBC-1 cells into mice and applied ASOs by s.c. injection at 50 mg/kg, five times a week for five weeks. We could observe an accumulation of MALAT1 ASO in both tumor and tumor-associated stromal cells (Fig. 4A). In these tissues, ASOs effectively reduced both human and mouse MALAT1 expression compared to control ASO (Fig. 4B) which was also validated in tumor and surrounding stromal cells by the ‘ViewRNA’ ISH method using species-specific probes (Fig. 4C). Thus, ASOs knocked down MALAT1 effectively in vitro and in vivo.
Figure 4. Downregulation of MALAT1 in EBC-1 lung cancer cells and tumor stroma.
Mice bearing human NSCLC EBC-1 cells were treated with ASOs by s.c. injection at 50 mg/kg, five times a week for five weeks.
A) Accumulation of MALAT1 ASO in both tumor and tumor-associated stromal cells was demonstrated by IHC using an antibody specific for ASOs.
B) Human and mouse MALAT1 RNA levels in EBC-1 tumor were measured by qRT-PCR using species-specific probe/primer sets and verified the downregulation of MALAT1 in human tumor and murine normal tissue after ASO application in vivo in the animal model.
C) Reduction in MALAT1 RNA levels in both tumor and its surrounding stromal cells was visualized by the ‘ViewRNA’ ISH method using species-specific probes.
MALAT1 ASO inhibits EBC-1 tumor metastasis to the lung
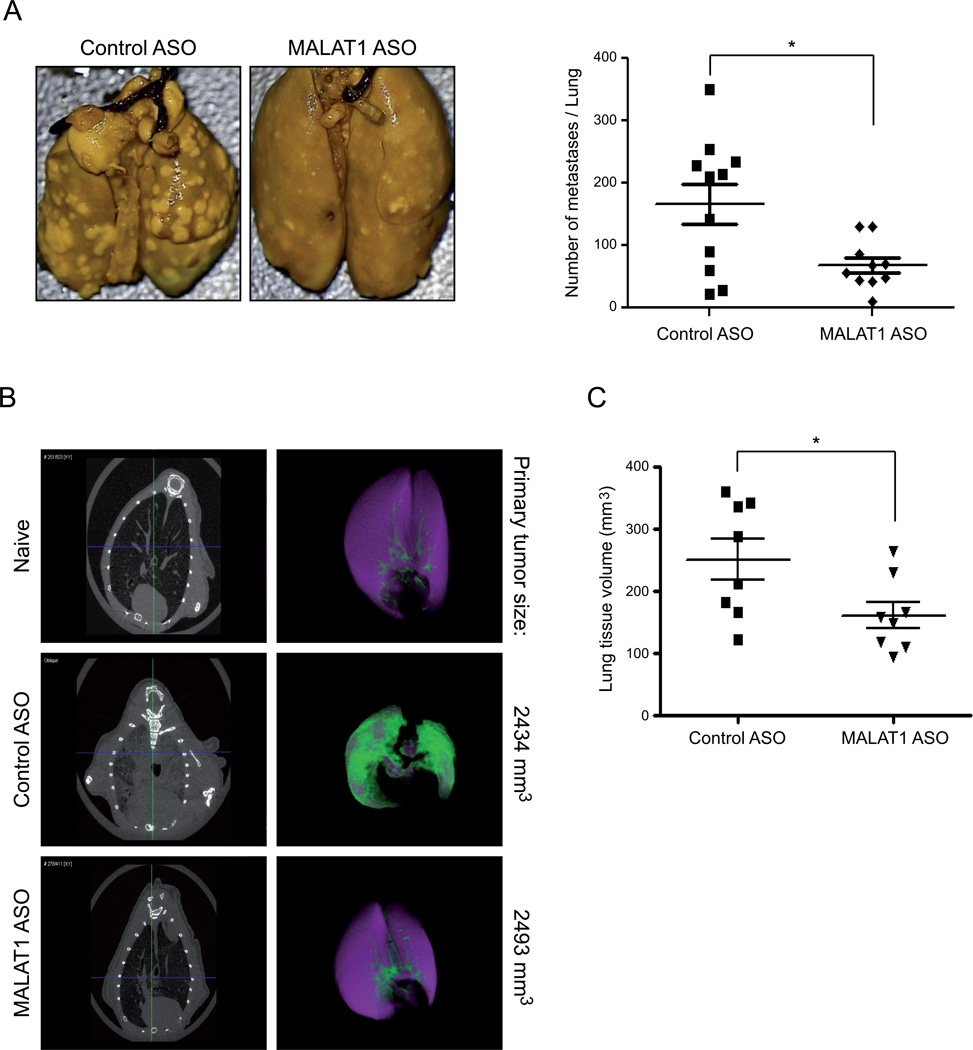
As our ASO approach proved to be effective in targeting MALAT1 in solid tumors, we took advantage of a well-established pulmonary metastatic model of human NSCLC (40) to analyze the effect of MALAT1 depletion on the spread of EBC-1 cells. In this assay, EBC-1 derived primary tumors were induced by subcutaneous injection of tumor cells and their ability to form distant tumor nodules in the lung was determined recapitulating all major steps of the metastastic cascade in vivo including invasion and intravasation not assayed in the A549 xenografts. While the depletion of MALAT1 in EBC-1 cells had no effect on cell proliferation in vitro (Supplementary Fig. S4B), the application of ASO had a minor impact on EBC-1 tumor growth in vivo (data not shown). To avoid any influence on tumor metastasis, EBC-1 tumors were controlled for their size and were surgically excised from their primary sites after five weeks of ASO treatment. Animals were kept for additional seven weeks without ASO treatment. At week 12, the lung tissues were collected and analyzed for tumor burden and histology. Indeed, significantly fewer and smaller tumor nodules were found in the lung in the MALAT1 ASO group compared to control ASO (Fig. 5A). A significant decrease in tumor volume in the MALAT1 ASO-treated group compared to the control ASO group was also revealed by microCT scanning for primary tumors of similar size. (Fig. 5B,C).
Figure 5. MALAT1 ASO inhibits the metastatic spread of EBC-1 tumors to the lung.
EBC-1-derived primary tumors were induced by flank injections into nude mice. The animals were then treated with MALAT1 ASO. After five weeks, tumors were surgically excised from their primary sites and the animals were kept for the following seven weeks without ASO treatment. At week 12, lung tissues were collected and analyzed for tumor burden and histology.
A) Animals treated with MALAT1 ASO had significantly fewer tumor nodules in the lung compared to control ASO-treated animals (p=0.038).
B-C) A significant decrease in tumor volume in the MALAT1 ASO-treated group compared to the control ASO group was demonstrated by microCT scanning (p=0.038). ‘Purple’ indicates airway volumes and ‘green’ represents lung tissue volumes including tumor mass.
Thus, the knockdown of MALAT1 in vivo was effective possibly providing a therapeutic option for metastasis prevention.
Discussion
Dysregulation of the highly conserved, nuclear long non-coding RNA MALAT1 has been linked to many cancer entities (for review: see (6)), but it was originally discovered as a marker for metastasis development in early stages of lung adenocarcinoma (18) and more recently in squamous cell carcinoma of the lung (26). However, its functional role in this process was only beginning to emerge by virtue of its link to cell migration (27).
At the functional level, our A549 knockout model corroborated the strong influence of MALAT1 on A549 cell migration as described previously (26–27) and migration of other malignant cells (41–43).
One hurdle in elucidating MALAT1 function was its high expression and its localization to the nucleus making it a less efficient target for conventional RNAi-based gene silencing. Previous loss-of-function studies using shRNAs or siRNAs decreased MALAT1 expression 2- to 4-fold (26–27) which left a high remaining concentration of the abundant MALAT1. Despite overlapping findings regarding cell migration, both studies differ for other phenotypes: While one study found an impact of MALAT1 on tumor cell growth and colony formation, the other study could not establish a function for MALAT1 in cellular proliferation, adhesion or anchorage-independent growth. This contradiction might be due to inadequate loss-of-function models using shRNAs or siRNAs moderately reducing MALAT1 and prone to different off-target effects. Our ZFN-based approach reduces MALAT1 expression more than 1000-fold and thus allows a quantitative loss-of-function analysis of its cellular and molecular impact. The treatment of cultured cells with ASOs also effectively silenced MALAT1 up to 20-fold (Supplementary Fig. S4A) and hence was more effective than standard RNAi-based approaches. Importantly, the A549 approach based on genetically modified cancer cell clones was complemented and validated by the use of highly potent ASOs in bulk cell culture and xenograft models independent of clonal selection.
The molecular mechanism of MALAT1 action is currently under debate: Previous studies identified MALAT1 as a regulator of alternative splicing of a subset of genes (22) while others suggested a mechanism of gene regulation (25). Our data strongly indicate that MALAT1 has no major impact on alternative splicing in lung cancer cells - matching most recent data in mouse models (24, 44), while we find the critical and specific function of MALAT1 in regulating the expression of several target genes. Our study identified a set of genes that might act in concert to promote lung cancer metastasis and whose expression depends on MALAT1. However, since only a subset of the deregulated genes have a proven connection to metastasis without a significant enrichment, MALAT1 could also regulate other important processes.
In contrast to previous studies in HeLa cells, our knockout strategy for MALAT1 did not lead to differential expression of growth-control genes in our microarray analysis or altered growth of A549 KO cells. This might indicate cell-type specific functions for MALAT1 and future studies will uncover the underlying principles, e.g. cell-type specific co-activator molecules recruited by MALAT1. This lack of regulation of major growth regulatory genes is also underscored by the lack of a growth or proliferation phenotype after loss of MALAT1 in mouse models (24, 30, 44).
Based on the gene regulation of several metastasis-associated genes and its impact on cell migration, we went one step further and hypothesized that MALAT1 could be an active player in the metastatic process since this in vivo phenotype had not been analyzed before. Therefore, we used two model systems recapitulating hallmark steps of the metastatic cascade in vivo: Intravenous A549 xenografts revealed the important role of MALAT1 in lung cancer cell extravasation and formation of new tumor nodules in the lung. The EBC-1 metastasis model mimicked the process of metastasis formation from a primary subcutaneous tumor. In summary, these data indicate for the first time that MALAT1 is not only a prognostic biomarker for metastasis development, but also a major player for disease progression by regulating a metastatic gene expression program.
The knockdown efficiency of the ASO approach in vivo (Fig. 4B) was significantly weaker than the knockdown efficiency in vitro (Supplementary Fig. S4A) or in our A549 knockout model (28). While the strong silencing in vitro was enabling unequivocal determination of the impact of MALAT1 on lung cancer cell proliferation or migration, the metastasis formation in vivo seems to be dependent on higher levels of MALAT1 and thus was already impaired at these comparatively weaker knockdown levels. On the one hand side, one could speculate that MALAT1 could impact multiple steps in the metastatic cascade and hence the sensitivity towards its knockdown was larger in vivo than in vitro. On the other hand, this result is encouraging for the development of MALAT1 knockdown strategies as an antimetastatic therapy approach since this effective level of downregulation could be achievable also in human tumors.
The metastatic cascade is a complex process and, as exemplified here for MALAT1, lncRNAs can significantly contribute to this malignant cancer phenotype. Hence, identification and functional analysis of other involved lncRNAs might add another layer of complexity and may even foster novel therapeutic options. In a mouse xenograft model, we established that the application of ASOs against MALAT1 proved to be effective in blocking lung cancer spreading. These data put MALAT1 forward as a valid therapeutic target to be further tested in vivo. Thus, MALAT1 is uncovered as a valuable prognostic marker, an important active player and a promising therapeutic target in lung cancer metastasis.
Supplementary Material
Acknowledgments
We thank Drs. Georg Stoecklin, Dirk Ostareck and Peter Angel for helpful discussions. We thank Tomi Bähr-Ivacevic and Vladimir Benes (EMBL) for Exon Array hybridization.
Grant Support
Our research is supported by the German Research Foundation (DFG Transregio TRR77, TP B03), the Marie Curie Program, the Helmholtz Society (VH-NG-504), the Virtual Helmholtz Institute for Resistance in Leukemia. T.G. is supported by a DKFZ PhD Fellowship. D.L.S. is supported by NCI 5P01CA013106-40. M.Z. is supported by the LOEWE Center for Cell and Gene Therapy Frankfurt [HMWK III L 4-518/17.004 (2010)], the LOEWE Initiative Oncogenic Signaling Frankfurt [HMWK III L 4-518/55.004 (2009)] and the Georg-Speyer-Haus.
Footnotes
Disclosure of Potential Conflict of Interest:
No potential conflicts of interest were disclosed. J.H., Y.K., G.H., A.R., A.R.M. are employees of ISIS Pharmaceuticals.
Author Contributions
T.G., A.R.M., D.L.S. and S.D. conceived and planned the experiments and analyzed and interpreted the results. T.G., M.H., M.E., J.H., Y.K., G.H., A.R., G.A., M.S., M.G., M.Z., A.R.M., S.D. performed and / or analyzed experiments. T.G and S.D. wrote the manuscript.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 3.Weigelt B, Peterse JL, van 't Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 4.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 5.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–1488. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 6.Gutschner T, Diederichs S. The Hallmarks of Cancer: A long non-coding RNA point of view. RNA Biol. 2012;9:703–719. doi: 10.4161/rna.20481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran M, Puig I, Pena C, Garcia JM, Alvarez AB, Pena R, et al. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng J, Bi C, Clark BS, Mady R, Shah P, Kohtz JD. The Evf-2 noncoding RNA is transcribed from the Dlx-5/6 ultraconserved region and functions as a Dlx-2 transcriptional coactivator. Genes Dev. 2006;20:1470–1484. doi: 10.1101/gad.1416106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martianov I, Ramadass A, Serra Barros A, Chow N, Akoulitchev A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature. 2007;445:666–670. doi: 10.1038/nature05519. [DOI] [PubMed] [Google Scholar]
- 10.Tsai MC, Manor O, Wan Y, Mosammaparast N, Wang JK, Lan F, et al. Long noncoding RNA as modular scaffold of histone modification complexes. Science. 2010;329:689–693. doi: 10.1126/science.1192002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang KC, Yang YW, Liu B, Sanyal A, Corces-Zimmerman R, Chen Y, et al. A long noncoding RNA maintains active chromatin to coordinate homeotic gene expression. Nature. 2011;472:120–124. doi: 10.1038/nature09819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faghihi MA, Modarresi F, Khalil AM, Wood DE, Sahagan BG, Morgan TE, et al. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matouk IJ, Abbasi I, Hochberg A, Galun E, Dweik H, Akkawi M. Highly upregulated in liver cancer noncoding RNA is overexpressed in hepatic colorectal metastasis. Eur J Gastroenterol Hepatol. 2009;21:688–692. doi: 10.1097/meg.0b013e328306a3a2. [DOI] [PubMed] [Google Scholar]
- 14.Panzitt K, Tschernatsch MM, Guelly C, Moustafa T, Stradner M, Strohmaier HM, et al. Characterization of HULC, a novel gene with striking up-regulation in hepatocellular carcinoma, as noncoding RNA. Gastroenterology. 2007;132:330–342. doi: 10.1053/j.gastro.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Prensner JR, Iyer MK, Balbin OA, Dhanasekaran SM, Cao Q, Brenner JC, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29:742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calin GA, Liu CG, Ferracin M, Hyslop T, Spizzo R, Sevignani C, et al. Ultraconserved regions encoding ncRNAs are altered in human leukemias and carcinomas. Cancer Cell. 2007;12:215–229. doi: 10.1016/j.ccr.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji P, Diederichs S, Wang W, Boing S, Metzger R, Schneider PM, et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 19.Wilusz JE, Freier SM, Spector DL. 3' end processing of a long nuclear-retained noncoding RNA yields a tRNA-like cytoplasmic RNA. Cell. 2008;135:919–932. doi: 10.1016/j.cell.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutchinson JN, Ensminger AW, Clemson CM, Lynch CR, Lawrence JB, Chess A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genomics. 2007;8:39. doi: 10.1186/1471-2164-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spector DL, Lamond AI. Nuclear speckles. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, Arun G, Mao YS, Lazar Z, Hung G, Bhattacharjee G, et al. The lncRNA Malat1 is dispensable for mouse development but its transcription plays a cis-regulatory role in the adult. Cell Rep. 2012;2:111–123. doi: 10.1016/j.celrep.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Lin C, Liu W, Zhang J, Ohgi KA, Grinstein JD, et al. ncRNA- and Pc2 methylation-dependent gene relocation between nuclear structures mediates gene activation programs. Cell. 2011;147:773–788. doi: 10.1016/j.cell.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt LH, Spieker T, Koschmieder S, Humberg J, Jungen D, Bulk E, et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J Thorac Oncol. 2011;6:1984–1992. doi: 10.1097/JTO.0b013e3182307eac. [DOI] [PubMed] [Google Scholar]
- 27.Tano K, Mizuno R, Okada T, Rakwal R, Shibato J, Masuo Y, et al. MALAT-1 enhances cell motility of lung adenocarcinoma cells by influencing the expression of motility-related genes. FEBS Lett. 2010;584:4575–4580. doi: 10.1016/j.febslet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Gutschner T, Baas M, Diederichs S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito I, Ji L, Tanaka F, Saito Y, Gopalan B, Branch CD, et al. Liposomal vector mediated delivery of the 3p FUS1 gene demonstrates potent antitumor activity against human lung cancer in vivo. Cancer Gene Ther. 2004;11:733–739. doi: 10.1038/sj.cgt.7700756. [DOI] [PubMed] [Google Scholar]
- 30.Eißmann M, Gutschner T, Hämmerle M, Günther S, Caudron-Herger M, Groß M, et al. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076–1087. doi: 10.4161/rna.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellerbrand C, Amann T, Schlegel J, Wild P, Bataille F, Spruss T, et al. The novel gene MIA2 acts as a tumour suppressor in hepatocellular carcinoma. Gut. 2008;57:243–251. doi: 10.1136/gut.2007.129544. [DOI] [PubMed] [Google Scholar]
- 32.Legg JA, Herbert JM, Clissold P, Bicknell R. Slits and Roundabouts in cancer, tumour angiogenesis and endothelial cell migration. Angiogenesis. 2008;11:13–21. doi: 10.1007/s10456-008-9100-x. [DOI] [PubMed] [Google Scholar]
- 33.Xu Y, Li WL, Fu L, Gu F, Ma YJ. Slit2/Robo1 signaling in glioma migration and invasion. Neurosci Bull. 2010;26:474–478. doi: 10.1007/s12264-010-0730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yiu GK, Kaunisto A, Chin YR, Toker A. NFAT promotes carcinoma invasive migration through glypican-6. Biochem J. 2011;440:157–166. doi: 10.1042/BJ20110530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshida D, Nomura R, Teramoto A. Regulation of cell invasion and signalling pathways in the pituitary adenoma cell line, HP-75, by reversion-inducing cysteine-rich protein with kazal motifs (RECK) J Neurooncol. 2008;89:141–150. doi: 10.1007/s11060-008-9606-5. [DOI] [PubMed] [Google Scholar]
- 36.Doyle SE, Scholz MJ, Greer KA, Hubbard AD, Darnell DK, Antin PB, et al. Latrophilin-2 is a novel component of the epithelial-mesenchymal transition within the atrioventricular canal of the embryonic chicken heart. Dev Dyn. 2006;235:3213–3221. doi: 10.1002/dvdy.20973. [DOI] [PubMed] [Google Scholar]
- 37.Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekine Y, Demosky SJ, Stonik JA, Furuya Y, Koike H, Suzuki K, et al. High-density lipoprotein induces proliferation and migration of human prostate androgen-independent cancer cells by an ABCA1-dependent mechanism. Mol Cancer Res. 2010;8:1284–1294. doi: 10.1158/1541-7786.MCR-10-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka E, Yamashita J, Hayashi N, Kato S, Kondo K, Ogawa M. A pulmonary metastatic model of human non-small cell lung carcinoma cells that produce a neutrophil elastase-like molecule in severe combined immunodeficiency mice. Chest. 2003;123:1248–1253. doi: 10.1378/chest.123.4.1248. [DOI] [PubMed] [Google Scholar]
- 41.Guo F, Li Y, Liu Y, Wang J, Li G. Inhibition of metastasis-associated lung adenocarcinoma transcript 1 in CaSki human cervical cancer cells suppresses cell proliferation and invasion. Acta Biochim Biophys Sin (Shanghai) 2010;42:224–229. doi: 10.1093/abbs/gmq008. [DOI] [PubMed] [Google Scholar]
- 42.Tseng JJ, Hsieh YT, Hsu SL, Chou MM. Metastasis associated lung adenocarcinoma transcript 1 is up-regulated in placenta previa increta/percreta and strongly associated with trophoblast-like cell invasion in vitro. Mol Hum Reprod. 2009;15:725–731. doi: 10.1093/molehr/gap071. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3' end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39:169–175. doi: 10.3892/ijo.2011.1007. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa S, Ip JY, Shioi G, Tripathi V, Zong X, Hirose T, et al. Malat1 is not an essential component of nuclear speckles in mice. RNA. 2012;18:1487–1499. doi: 10.1261/rna.033217.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.