Abstract
Purpose
It has been hypothesized that vitamin D mediates the inverse relationship between sun exposure and non-Hodgkin lymphoma (NHL) risk reported in several recent studies. We evaluated the association of self-reported sun exposure at ages <13, 13–21, 22–40, and 41+ years and 19 single nucleotide polymorphisms (SNPs) from 4 candidate genes relevant to vitamin D metabolism (RXR, VDR, CYP24A1, CYP27B1) with NHL risk.
Methods
This analysis included 1,009 newly diagnosed NHL cases and 1,233 frequency-matched controls from an ongoing clinic-based study. Odds ratios (OR), 95 % confidence intervals (CI), and tests for trend were estimated using unconditional logistic regression.
Results
There was a significant decrease in NHL risk with increased sun exposure at ages 13–21 years (OR≥15 vs. ≤3 h/week = 0.68; 95 % CI, 0.43–1.08; ptrend = 0.0025), which attenuated for older ages at exposure. We observed significant main effect associations for 3 SNPs in VDR and 1 SNP in CYP24A1: rs886441 (ORper-allele = 0.82; 95 % CI, 0.70–0.96; p = 0.016), rs3819545 (ORper-allele = 1.24; 95 % CI, 1.10–1.40; p = 0.00043), and rs2239186 (ORper-allele = 1.22; 95 % CI, 1.05–1.41; p = 0.0095) for VDR and rs2762939 (ORper-allele = 0.85; 95 % CI, 0.75–0.98; p = 0.023) for CYP24A1. Moreover, the effect of sun exposure at age 13–21 years on overall NHL risk appears to be modified by germline variation in VDR (rs4516035; pinteraction = 0.0066). Exploratory analysis indicated potential heterogeneity of these associations by NHL subtype.
Conclusion
These results suggest that germline genetic variation in VDR, and therefore the vitamin D pathway, may mediate an association between early life sun exposure and NHL risk.
Keywords: Ultraviolet radiation, Vitamin D, VDR, Molecular epidemiology, Non-Hodgkin lymphoma
Introduction
The lifetime risk of developing non-Hodgkin lymphoma (NHL), the 6th most common cancer in the United States, is 1 in 44 for men and 1 in 52 for women [1]. Relatively few NHL risk factors have been established to date, and the etiology of a majority of NHL cases remains largely unexplained [2]. The remarkable rise in NHL incidence over the last 40 years suggests a major role for environmental factors in the etiology of this cancer. There is accumulating evidence from most [3–8] but not all [9–12] studies that increased sun exposure is associated with a decreased risk of NHL, and this was supported by a pooled analysis recently conducted by the InterLymph consortium [13].
While the underlying mechanism for an inverse association of sun exposure and NHL risk has not been established, a role for vitamin D has been hypothesized as the sun provides nearly 90 % of the vitamin D for most people [14]. There is currently limited or no support for an association between circulating vitamin D levels and NHL risk [15, 16]; however, such levels may be insufficient to capture early life or long-term vitamin D status. Genetic variation in the vitamin D metabolic pathway has been associated with cancer risk [17], and there is some preliminary evidence that VDR polymorphisms may be associated with NHL risk, at least in some subtypes [18–20].
Vitamin D obtained from sun exposure and dietary sources is first metabolized in the liver to 25-hydroxyvitamin D (25(OH)D) via the actions of a number of cytochrome p450 enzymes. It is then further hydroxylated via 1-α-hydroxylase (CYP27B1) to its active form, 1,25-dihydroxyvitamin D (1,25(OH)2D). The effects of 1,25(OH)2D are mediated through the vitamin D receptor (VDR), a nuclear hormone receptor which first heterodimerizes (usually with the retinoid X nuclear hormone receptor; RXR), and then directly binds DNA to modulate expression of hundreds of downstream gene targets [21]. Ligand-activated VDR upregulates expression of the CYP24 (CYP24A1) enzyme which catalyzes degradation of 1,25(OH2)D in a negative feedback loop [22]. While vitamin D is primarily known for its critical role in calcium homeostasis [23], there are broader biologic functions given that most cell types express both 1-α-hydroxylase and VDR [22, 24, 25]. Autocrine and paracrine effects of vitamin D activation and VDR binding include regulation of cell proliferation and induction of apoptosis and differentiation [22], functions biologically relevant to both cancer prevention and tumor progression.
Using a clinic-based case–control study, we evaluated whether sun exposure over the life course, germline genetic variation in vitamin D pathway genes, and the interaction of these factors provide insight into whether vitamin D is a potential underlying mechanism for the observed association between sun exposure and NHL risk.
Materials and methods
Study population
This study was reviewed and approved by the Human Subjects Institutional Review Board at the Mayo Clinic, and all participants provided written informed consent. Full details of this ongoing, clinic-based case–control study conducted at the Mayo Clinic in Rochester, Minnesota, have been previously reported [26, 27]. Briefly, patients included in this analysis were recruited from September 1, 2002 through February 29, 2008, within 9 months of their first lymphoma diagnosis; age 20 years or older at diagnosis; and residents of Minnesota, Iowa or Wisconsin at the time of diagnosis.
All cases were reviewed and histologically confirmed by a hematopathologist and classified according to the WHO criteria [28]. Of the 1,798 eligible cases, 1,236 (69 %) participated, 183 (10 %) refused, 39 (2 %) were lost to follow-up (i.e., we were unable to contact after multiple attempts), and 340 (19 %) did not complete all data collection within 12 months of diagnosis. Clinic-based controls were randomly selected from a dynamic population of patients seen for a prescheduled routine medical examination in the general medicine divisions of the Department of Medicine. Inclusion criteria included age 20 years or older at selection; resident of Minnesota, Iowa or Wisconsin; and no history of lymphoma, leukemia, or HIV infection. Controls were frequency-matched to cases by 5-year age group, gender, and geographic region. Of the 1,899 eligible subjects identified, 1,315 (69 %) participated, 548 (29 %) refused, and 36 (2 %) did not complete data collection within 12 months of selection. All participating subjects were asked to complete a self-administered risk-factor questionnaire and to provide a peripheral blood sample for genetic studies. For this specific analysis of sun exposure and genetic variation, we only included subjects who were successfully genotyped and reported their race as Caucasian.
Sun exposure assessment
Participants self-reported their sun exposure history during the following age periods: birth-12, 13–21, 22–40, and 41 years or older. Participants were asked how much midday (10 a.m. to 2 p.m.) sun exposure, on average, they had during each of these age periods, and responses were captured in the following categories: practically none (3 h or less than a week), little (4–7 h per week), moderate (8–14 h per week), or extensive (15 or more hours per week). For the same time periods, participants also reported how frequently they wore sunscreen or protective clothing when in the bright sun for more than 15 min. Response choices were never, rarely (less than 20 % of the time), most times (20–80 % of the time), or usually (more than 80 % of the time). Sun exposure and sunscreen questions were not season-specific, and thus, patients were reporting an annual average of exposure to each. The questionnaire also collected data to derive the following variables, which were evaluated for potential confounding of the main effect association between sun exposure and NHL risk: education; family history of lymphoma and other cancers; body mass index (2 years prior to diagnosis and at age 18); smoking history; alcohol consumption; physical activity level 2 years prior to enrollment (quartiles of MET-minutes/week); and participation in strenuous activity at age 18 (yes/no).
Gene and SNP selection
Candidate SNPs from VDR (n = 5), CYP24A1 (n = 4), CYP27B1 (n = 4), and RXRA (n = 2), RXRB (n = 2) were selected for genotyping based on prior report of potential relevance to cancer risk in the literature [17–19, 29, 30]. Several additional tagging SNPs for VDR were also available from a prior genotyping project [26]. VDR-tagging SNPs were selected from 5 kb upstream and downstream of each gene with Caucasian minor allele frequency (MAF) ≥0.05 and pairwise r2 threshold of 0.8.
Genotyping
DNA was extracted from blood samples using a standard procedure (Gentra Inc., Minneapolis, MN). Genotyping was conducted as part of a larger study using a custom Illumina GoldenGate [31] 1536 SNP OPA. Of the 1536 SNPs genotyped, 1,459 passed all quality controls (including no clustering issues and call rates >95 %), and of the 3,565 unique samples, 3,377 had a call rate >90 %. The concordance rate across 299,300 duplicate genotypes within this project was 99.96 %. Further comparison of 201,509 duplicate genotypes with a previous genotyping project was 99.6 %. Two CYP27B1 SNPs (rs10877012 and rs3782130) could not be designed for the OPA, and one VDR SNP (rs1544410, BsmI) did not pass quality control. For this analysis, 80 cases with HL were excluded, leaving 1,009 cases and 1,233 controls with data on 19 SNPs.
Statistical analysis
The association between sun exposure and NHL risk was evaluated separately within each age period. Odds ratios (OR), 95 % confidence intervals (CI), and tests for trend were estimated using unconditional logistic regression adjusted for age and gender; the other design variable of geographic region was not associated with risk and therefore was not included in the basic models. OR estimates and 95 % CI were first estimated separately for each level of sun exposure, with the lowest exposure level (<3 h per week) as the reference. Additionally, tests for trend were performed assuming an ordinal relationship between the categorical levels of sun exposure as captured on the questionnaire. Cases and controls were compared with regard to distribution of demographic, clinical, and other variables for evaluation as potential confounding factors (t tests for continuous variables, χ2 for categorical variables), including geographic residence (based on distance from Rochester, MN, and urban/rural status); history of NHL, skin cancer, or other cancers (yes/no); smoking history (never, former, current); alcohol consumption (never, former, current); BMI 2 years prior to enrollment (WHO categories) and at age 18 (gender-specific quartiles); and education (<high school, high school graduate, some college, college, graduate/professional school). Variables for which we had an a priori concern for confounding due to a link to reported sun exposure and vitamin D production as a result of sun exposure were assessed individually in the age period–specific logistic regression models, including sunscreen use (never; rarely/less than 20 %; most times/20–80 %; and usually/more than 80 %); physical activity level 2 years prior to enrollment (quartiles of MET-minutes/week) and regular strenuous physical activity at age 18 (yes/no); and season of consent (Fall/September–November, Winter/December–February, Spring/March–May, Summer/June–August). In addition, factors differentially distributed (p < 0.05) between the cases and controls were also assessed individually in the age period–specific logistic regression models, as appropriate. For all factors considered, only those factors that changed the OR estimate for the sun exposure variable by greater than 10 % were retained in the final model.
We also evaluated the association between age period–specific sun exposure and SNPs of interest with NHL risk by major NHL subtype (DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; and CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma). To generate the estimated association between each SNP of interest and NHL subtype, we used polytomous logistic regression to simultaneously calculate ORs and 95 % CIs for each of these three most common NHL subtypes relative to controls [32].
Allele frequencies from cases and controls were estimated using observed genotype frequencies. The frequencies in the controls were compared to genotype frequencies expected under Hardy–Weinberg equilibrium (HWE) using a Pearson goodness-of-fit test or Fisher’s exact test (MAF < 0.05). In this analysis, 4 of the 19 evaluated SNPs had a HWE p < 0.05 (rs886441, rs1536475, rs7975232, and rs2744537); since no genotype-calling errors were identified and cluster plots appeared reasonable, these SNPs were not excluded from analysis. We previously found no evidence of population stratification in our data [26].
Individual SNPs were examined using unconditional logistic regression to estimate odds ratios (ORs) and corresponding 95 % confidence intervals (CIs) separately for heterozygotes and minor allele homozygotes, using homozygotes for the major allele as the reference. ORs and corresponding 95 % CIs were also estimated per copy of minor allele for each SNP, and a ptrend was calculated assuming an ordinal (log-additive) genotypic relationship. All SNP association logistic regression models were adjusted for age, gender, and family history of lymphoma.
In exploratory analyses, we assessed the interaction of the main effects of sun exposure and SNPs; the criteria for inclusion in this analysis was ptrend < 0.15 for both sun exposure and SNP genotype in univariate main effects analysis and was based on a desire to include previously published associations [19] in the interaction model that were marginally significant in our dataset while keeping the number of interaction tests to a reasonable number. Interaction p values for each sun/SNP combination were calculated based upon a likelihood ratio test comparing logistic regression models with and without an interaction term. SNP genotypes were collapsed to a minor allele carrier framework, and an ordinal (log-additive) sun exposure relationship was assumed; subjects who were in the lowest category of sun exposure and homozygous major allele for genotype were the reference group. All interaction models were adjusted for age, sex, and family history of NHL.
To assess the robustness of our results in the setting of multiple hypothesis testing, we include an interpretation of our results in the context of an adjusted significance threshold. We used the Bonferroni method of adjustment by dividing the standard p < 0.05 threshold for significance by the number of hypotheses tested (29 total; 4 main effect sun exposure tests, 19 main effect SNP tests, 6 tests of interaction). For this analysis, our Bonferroni-adjusted threshold for statistical significance in the context of multiple hypothesis tests was p < 0.002. Analyses were implemented using SAS (SAS Institute, Cary, NC, Version 8, 1999), Plink (http://pngu.mgh.harvard.edu/purcell/plink/), and R software systems. All p values were 2-sided.
Results
Participant characteristics
There were 1,009 cases and 1,233 controls from the Mayo case–control study, which were eligible for inclusion in this analysis; their demographic and clinical characteristics are summarized in Table 1. The median age was 63 years for cases and for controls, and there was a greater proportion of male participants in both groups (60 and 55 %, respectively). More than 50 % of the controls were enrolled during the spring or summer months as compared to 45 % of the cases. As expected, a greater proportion of the cases had a family history of NHL than controls (14 versus 7 %). The most common NHL subtypes were CLL/SLL (n = 343; 34 %), FL (n = 245; 24 %), and DLBCL (n = 178; 18 %).
Table 1.
Patient characteristics, Mayo case–control study, 9/2002–2/2008
| Cases
|
Controls
|
|||
|---|---|---|---|---|
| n = 1,009 | %a | n = 1,233 | %a | |
| Gender | ||||
| Male | 601 | 59.6 | 673 | 54.6 |
| Female | 408 | 40.4 | 560 | 45.4 |
| Age | ||||
| ≤40 | 46 | 4.6 | 90 | 7.3 |
| 41–50 | 142 | 14.1 | 170 | 13.8 |
| 51–60 | 218 | 21.6 | 256 | 20.8 |
| 61–70 | 320 | 31.7 | 361 | 29.3 |
| 71+ | 283 | 28.0 | 356 | 28.9 |
| Medical history | ||||
| History of skin cancer | 72 | 8.6 | 93 | 8.7 |
| No history of skin cancer | 770 | 91.5 | 978 | 91.3 |
| Missingb | 167 | 162 | ||
| History of other cancer | 178 | 21.2 | 262 | 24.5 |
| No history of other cancer | 662 | 78.8 | 809 | 75.5 |
| Missingb | 169 | 162 | ||
| Family history of NHL | 118 | 14.4 | 77 | 7.4 |
| No family history of NHL | 702 | 85.6 | 964 | 92.6 |
| Missingb | 189 | 192 | ||
| Smoking history | ||||
| Current | 43 | 5.1 | 67 | 6.3 |
| Former | 358 | 42.6 | 423 | 39.5 |
| Never | 440 | 52.3 | 580 | 54.2 |
| Missingb | 168 | 163 | ||
| Alcohol use | ||||
| Current | 622 | 74.0 | 814 | 66.0 |
| Former | 130 | 15.5 | 157 | 14.7 |
| Never | 89 | 10.6 | 95 | 8.9 |
| Missingb | 168 | 167 | ||
| BMI | ||||
| < 25.0 | 256 | 30.9 | 329 | 31.5 |
| 25.0–29.9 | 333 | 40.2 | 447 | 42.8 |
| ≥30.0 | 240 | 29.0 | 269 | 25.7 |
| Missingb | 180 | 188 | ||
| Season of consent | ||||
| Spring | 218 | 21.6 | 384 | 31.1 |
| Summer | 234 | 23.2 | 284 | 23.0 |
| Fall | 288 | 28.5 | 281 | 22.8 |
| Winter | 269 | 26.7 | 284 | 23.0 |
| Histology | ||||
| SLL/CLL | 343 | 34.0 | ||
| FL | 245 | 24.3 | ||
| DLBCL | 178 | 17.6 | ||
Percent of non-missing total
Missing total not included in the percent distribution calculations
Sun exposure main effects analysis
The OR estimates for the association between sun exposure at various age periods and NHL risk, both overall and for the main NHL subtypes, are summarized in Table 2. Overall, we observed the strongest inverse association between the frequency of sun exposure at ages 13–21 years and overall NHL risk (OR≥15 vs. ≤3 h/week = 0.68; 95 % CI, 0.43–1.08; ptrend = 0.0025), although this association did not meet the Bonferroni-adjusted threshold for significance (p < 0.002). All models were adjusted for age, gender, and family history of lymphoma. Further adjustment for geographic residence, season of consent, sun-screen use, or physical activity (2 years prior to consent or at age 18) did not meaningfully affect the associations in Table 2; therefore, these factors did not remain in the final models presented. The association of sun exposure with overall NHL risk attenuated for sun exposure at older ages. We examined the distribution of self-reported sun exposure by season and found no evidence that reported average exposure during any time period was biased by the season in which the participants were enrolled (data not shown). Furthermore, similar associations were observed between sun exposure at ages 13–21 among patients recruited in the fall/winter (ordinal OR = 0.80; 95 % CI, 0.68–0.95; ptrend = 0.011) versus spring/summer (ordinal OR = 0.86; 95 % CI, 0.72–1.03; ptrend = 0.098). In exploratory analyses of NHL subtypes, the inverse association of early life sun exposure at ages 13–21 years was observed for CLL/SLL (OR≥15 vs.≤3 h/week = 0.73; 95 % CI, 0.36–1.50; ptrend = 0.013) and DLBCL (OR≥15 vs. ≤3 h/week = 0.75; 95 % CI, 0.33–1.74; ptrend = 0.45), while there was no association with FL.
Table 2.
Odds ratios and 95 % CI for the association between self-reported sun exposure at different ages and risk of NHL, overall and by major subtype, Mayo case–control study, 2002–2008
| Variable, level | Controls (n = 1,233) | All NHL (n = 1,009)
|
DLBCL (n = 178)
|
FL (n = 245)
|
SLL/CLL (n = 343)
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | ORa | 95 % CI | Cases | ORa | 95 % CI | Cases | ORa | 95 % CI | Cases | ORa | 95 % CI | ||
| Sun exposure: birth—12 years old | |||||||||||||
| ≤3 h/week | 70 | 37 | 1 | Reference | 7 | 1 | Reference | 7 | 1 | Reference | 11 | 1 | Reference |
| 4–7 h/week | 171 | 101 | 1.09 | (0.68–1.75) | 9 | 0.53 | (0.19–1.49) | 24 | 1.42 | (0.58–3.45) | 38 | 1.33 | (0.64–2.78) |
| 8–14 h/week | 375 | 220 | 1.12 | (0.72–1.73) | 35 | 0.99 | (0.42–2.32) | 58 | 1.58 | (0.69–3.62) | 89 | 1.47 | (0.74–2.92) |
| ≥15 h/week | 228 | 110 | 0.88 | (0.55–1.40) | 27 | 1.23 | (0.51–2.96) | 29 | 1.32 | (0.55–3.17) | 29 | 0.72 | (0.34–1.54) |
| Ordinal OR | 0.94 | (0.83–1.07) | 1.24 | (0.94–1.64) | 1.04 | (0.83–1.30) | 0.87 | (0.72–1.05) | |||||
| ptrend | 0.36 | 0.13 | 0.71 | 0.15 | |||||||||
| Sun exposure: 13–21 years old | |||||||||||||
| ≤3 h/week | 58 | 38 | 1 | Reference | 8 | 1 | Reference | 6 | 1 | Reference | 11 | 1 | Reference |
| 4–7 h/week | 156 | 117 | 1.13 | (0.70–1.83) | 19 | 0.90 | (0.37–2.17) | 23 | 1.33 | (0.51–3.45) | 44 | 1.47 | (0.71–3.07) |
| 8–14 h/week | 420 | 250 | 0.87 | (0.56–1.36) | 37 | 0.66 | (0.29–1.51) | 77 | 1.77 | (0.73–4.27) | 88 | 0.98 | (0.49–1.97) |
| ≥15 h/week | 353 | 173 | 0.68 | (0.43–1.08) | 35 | 0.75 | (0.33–1.74) | 39 | 1.12 | (0.45–2.79) | 62 | 0.73 | (0.36–1.50) |
| Ordinal OR | 0.83 | (0.73–0.94) | 0.91 | (0.71–1.16) | 0.97 | (0.78–1.19) | 0.80 | (0.67–0.95) | |||||
| ptrend | 0.0025 | 0.45 | 0.74 | 0.013 | |||||||||
| Sun exposure: 22–40 years old | |||||||||||||
| ≤3 h/week | 140 | 87 | 1 | Reference | 18 | 1 | Reference | 16 | 1 | Reference | 29 | 1 | Reference |
| 4–7 h/week | 298 | 166 | 0.86 | (0.62–1.20) | 27 | 0.71 | (0.38–1.34) | 50 | 1.49 | (0.82–2.72) | 59 | 0.86 | (0.53–1.42) |
| 8–14 h/week | 345 | 233 | 1.05 | (0.70–1.44) | 34 | 0.78 | (0.42–1.43) | 58 | 1.59 | (0.88–2.88) | 90 | 1.11 | (0.69–1.78) |
| ≥15 h/week | 218 | 104 | 0.70 | (0.48–1.01) | 21 | 0.75 | (0.38–1.51) | 23 | 1.09 | (0.55–2.18) | 34 | 0.57 | (0.33–1.00) |
| Ordinal OR | 0.93 | (0.84–1.04) | 0.94 | (0.75–1.17) | 1.02 | (0.84–1.23) | 0.90 | (0.76–1.05) | |||||
| ptrend | 0.23 | 0.58 | 0.86 | 0.18 | |||||||||
| Sun exposure: ≥41 years old | |||||||||||||
| ≤3 h/week | 191 | 113 | 1 | Reference | 15 | 1 | Reference | 31 | 1 | Reference | 40 | 1 | Reference |
| 4–7 h/week | 285 | 168 | 0.99 | (0.73–1.34) | 28 | 1.28 | (0.67–2.47) | 44 | 0.98 | (0.60–1.62) | 66 | 1.06 | (0.68–1.65) |
| 8–14 h/week | 273 | 198 | 1.19 | (0.88–1.62) | 33 | 1.53 | (0.80–2.92) | 40 | 1.03 | (0.61–1.73) | 77 | 1.23 | (0.79–1.90) |
| ≥15 h/week | 176 | 81 | 0.75 | (0.52–1.09) | 14 | 1.04 | (0.47–2.27) | 19 | 0.82 | (0.44–1.55) | 27 | 0.64 | (0.37–1.10) |
| Ordinal OR | 0.96 | (0.86–1.07) | 1.05 | (0.83–1.31) | 0.96 | (0.79–1.16) | 0.92 | (0.79–1.08) | |||||
| ptrend | 0.49 | 0.70 | 0.65 | 0.30 | |||||||||
DLBCL diffuse large B-cell lymphoma; FL follicular lymphoma; SLL/CLL small lymphocytic lymphoma/chronic lymphocytic lymphoma
Models adjusted for age, gender, and family history of lymphoma
Vitamin D-related gene-variant main effects
Next, we evaluated SNPs from 5 candidate vitamin D-related genes (Table 3). For CYP24A1, only rs2762939 was significantly associated with NHL risk: OR = 0.85 per minor allele (G) copy (95 % CI, 0.75–0.98). For VDR, 3 of the 9 SNPs were significant. Minor alleles were associated with increased NHL risk for two of these VDR SNPs: rs3819545, OR = 1.24 per G allele copy (95 % CI, 1.10–1.40) and rs2239186, OR = 1.22 per G allele copy (95 % CI, 1.05–1.41). Minor alleles in the third significant VDR SNP were associated with decreased NHL risk: rs886441, OR = 0.82 per G allele copy (95 % CI, 0.70–0.96). Two additional SNPs (rs731236 and rs4516035) reached p < 0.15 and therefore were considered of interest in our subsequent analysis of interaction with early life sun effects. The pairwise correlation of these five SNPs was variable and not particularly strong (r2 between 0.0 and 0.40). Only two of these 5 genotyped VDR SNPs (rs3819545 and rs4516035) remained statistically significant when modeled in a single logistic regression model, suggesting that these two SNPs represent separate NHL risk signals (p = 0.0003 and p = 0.05, respectively).
Table 3.
Odds ratios and 95 % CIs for associations between Vitamin D-related genotypes and risk of non-Hodgkin lymphoma, Mayo case–control study, 2002–2008
| Gene | rsid | Chromosome | Position | Type | W/V | MAFa | Cases | Controls | ORwv | ORvv | p valueb | ORordinal | p valuec |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CYP24A1 | rs927650 | 20 | 52772741 | Intron | G/A | 0.48 | 1,008 | 1,231 | 1.01 (0.83, 1.23) | 0.92 (0.72, 1.16) | 0.66 | 0.96 (0.85, 1.08) | 0.49 |
| rs2762939 | 20 | 52781251 | Intron | C/G | 0.27 | 1,001 | 1,227 | 0.83 (0.70, 1.00) | 0.76 (0.54, 1.08) | 0.070 | 0.85 (0.75, 0.98) | 0.023 | |
| rs2244719 | 20 | 52782858 | Intron | A/G | 0.46 | 1,004 | 1,228 | 1.11 (0.91, 1.34) | 1.11 (0.88, 1.41) | 0.55 | 1.06 (0.94, 1.19) | 0.34 | |
| rs2296241 | 20 | 52786219 | Synonymous | A/G | 0.49 | 1,008 | 1,233 | 0.97 (0.80, 1.19) | 0.88 (0.69, 1.12) | 0.55 | 0.94 (0.83, 1.06) | 0.31 | |
| CYP27B1 | rs4646537 | 12 | 58157281 | Intron | A/C | 0.03 | 1,009 | 1,232 | 1.20 (0.87, 1.67) | 2.38 (0.22, 26.4) | 0.43 | 1.22 (0.89, 1.68) | 0.21 |
| rs703842 | 12 | 58162739 | 5′upstream | A/G | 0.32 | 1,007 | 1,230 | 1.00 (0.84, 1.20) | 1.07 (0.79, 1.43) | 0.91 | 1.02 (0.90, 1.16) | 0.74 | |
| RXRA | rs1536475 | 9 | 137321156 | Intron | G/A | 0.18 | 991 | 1,217 | 0.92 (0.76, 1.10) | 0.76 (0.40, 1.43) | 0.49 | 0.91 (0.77, 1.07) | 0.25 |
| rs1805343 | 9 | 137328286 | Intron | A/G | 0.36 | 1,006 | 1,230 | 0.96 (0.80, 1.14) | 0.98 (0.75, 1.28) | 0.89 | 0.98 (0.87, 1.11) | 0.77 | |
| RXRB | rs2744537 | 6 | 33162215 | 3′UTR | C/A | 0.30 | 1,009 | 1,232 | 1.02 (0.86, 1.22) | 1.13 (0.85, 1.49) | 0.71 | 1.05 (0.93, 1.19) | 0.46 |
| rs2076310 | 6 | 33166034 | Intron | A/G | 0.21 | 1,009 | 1,232 | 1.18 (0.98, 1.41) | 0.95 (0.64, 1.40) | 0.17 | 1.08 (0.94, 1.25) | 0.28 | |
| VDR | rs731236 (TaqI) | 12 | 48238757 | Synonymous | A/G | 0.42 | 1,005 | 1,230 | 0.92 (0.77, 1.11) | 0.81 (0.63, 1.05) | 0.28 | 0.90 (0.80, 1.02) | 0.11 |
| rs7975232 (Apal) | 12 | 48238837 | Intron | A/C | 0.43 | 987 | 1,194 | 0.99 (0.82, 1.21) | 1.11 (0.88, 1.41) | 0.57 | 1.05 (0.93, 1.18) | 0.42 | |
| rs886441 | 12 | 48262964 | Intron | A/G | 0.19 | 1,008 | 1,232 | 0.78 (0.65, 0.93) | 0.90 (0.53, 1.52) | 0.024 | 0.82 (0.70, 0.96) | 0.016 | |
| rs3819545 | 12 | 48265006 | Intron | A/G | 0.36 | 1,008 | 1,232 | 1.13 (0.94, 1.36) | 1.63 (1.26, 2.10) | 0.0009 | 1.24 (1.10, 1.40) | 0.00043 | |
| rs2239186 | 12 | 48269410 | Intron | A/G | 0.19 | 1,002 | 1,217 | 1.20 (1.00, 1.43) | 1.55 (1.00, 2.39) | 0.033 | 1.22 (1.05, 1.41) | 0.0095 | |
| rs2228570d (Fokl) | 12 | 48272895 | Missense | G/A | 0.39 | 1,008 | 1,232 | 1.05 (0.87, 1.26) | 1.06 (0.82, 1.37) | 0.85 | 1.03 (0.91, 1.17) | 0.60 | |
| rs4334089 | 12 | 48286015 | Intron | G/A | 0.25 | 1,009 | 1,232 | 0.93 (0.78, 1.11) | 0.96 (0.68, 1.35) | 0.73 | 0.96 (0.84, 1.10) | 0.52 | |
| rs4516035 | 12 | 48299826 | 5′upstream | A/G | 0.43 | 1,006 | 1,231 | 1.14 (0.94, 1.38) | 1.24 (0.98, 1.58) | 0.17 | 1.12 (0.99, 1.26) | 0.064 | |
| rs11568820 (Cdx2) | 12 | 48302545 | 5′upstream | G/A | 0.19 | 1,009 | 1,233 | 0.94 (0.78, 1.13) | 0.89 (0.57, 1.38) | 0.72 | 0.94 (0.81, 1.09) | 0.42 |
Models adjusted for age, gender, and family history of lymphoma
MAF among controls
Wald p value for the general model
Wald p value for the ordinal effect
Previously rs10735810
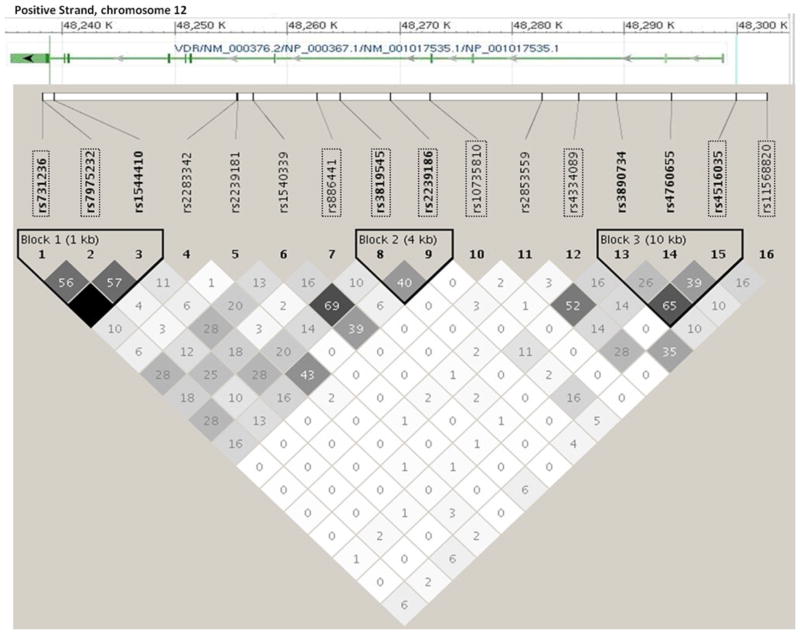
Of note, all of the SNPs that were significantly associated with NHL risk (at the conventional p < 0.05) were intronic. Furthermore, only rs3819545 in VDR met the Bonferroni-adjusted threshold for statistical significance in this analysis (p < 0.002). The relative position and linkage disequilibrium (based on HapMap CEPH population genotypes) of each of the genotyped VDR SNPs, together with the other VDR SNPs evaluated in relation to NHL risk in the literature, are illustrated in Fig. 1.
Fig. 1.
Linkage disequilibrium plot of selected VDR SNPs evaluated in relation to NHL risk, based on the HapMap CEU subjects (NCBI build 37). SNPs outlined with a rectangle were genotyped and evaluated in the present study (Mayo Clinic case–control study of NHL, 2002–2008). The numbers and shading indicate r2 values; the darker shading indicates higher r2 values of correlation between SNPs. The gene diagram was modified from dbSNP genome build 37.1 (http://www.ncbi.nlm.nih.gov/projects/SNP/)
Similar associations (as assessed by the direction and magnitude of ordinal odds ratios obtained from polytomous logistic regression) were observed for the VDR SNP rs886441 with the subtypes of CLL/SLL, follicular lymphoma, and DLBCL. In contrast, there was some evidence that the associations between the remaining individual SNPs of interest differed among the three NHL subtypes (Table 4). With the exception of rs4516035 in VDR, which appears most strongly associated with DLBCL, it appeared that the SNPs in VDR and CYP24A1 were stronger among the indolent subtypes: VDR rs731236 was associated with FL (ORper-allele = 0.81; 95 % CI, 0.66–1.00; ptrend = 0.049); VDR rs3819545 was associated with FL (ORper-allele = 1.40; 95 % CI, 1.14–1.70; ptrend = 0.0011) and CLL/SLL (ORper-allele = 1.30; 95 % CI, 1.09–1.55; ptrend = 0.0031); VDR rs2239186 was associated with FL (ORper-allele = 1.41; 95 % CI, 1.12–1.78; ptrend = 0.0039) and CLL/SLL (ORper-allele = 1.32; 95 % CI, 1.07–1.63; ptrend = 0.0087); and CYP24A1 rs2762939 was associated with CLL/SLL (ORper-allele = 0.77; 95 % CI, 0.63–0.95; ptrend = 0.013). Caution is warranted in interpreting the subtype results due to small sample sizes, especially for SNPs with lower minor allele frequencies.
Table 4.
Odds ratios and 95 % CIs for association between vitamin D-related gene variants and non-Hodgkin lymphoma risk by subtype, Mayo case–control study, 2002–2008
| Gene | SNP ID | W/V | All NHL (n = 1,009)
|
DLBCL (n = 178)
|
FL (n = 245)
|
SLL/CLL (n = 343)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ordinal OR (95 % CI) | ptrend | Ordinal OR (95 % CI) | ptrend | Ordinal OR (95 % CI) | ptrend | Ordinal OR (95 % CI) | ptrend | |||
| VDR | rs731236 (TaqI) | A/G | 0.90 (0.80, 1.02) | 0.11 | 0.91 (0.72, 1.15) | 0.43 | 0.81 (0.66, 1.00) | 0.049 | 0.88 (0.74, 1.06) | 0.17 |
| VDR | rs886441 | A/G | 0.82 (0.70, 0.96) | 0.016 | 0.81 (0.59, 1.10) | 0.18 | 0.80 (0.61, 1.05) | 0.11 | 0.89 (0.71, 1.11) | 0.29 |
| VDR | rs3819545 | A/G | 1.24 (1.10, 1.40) | 0.00043 | 1.11 (0.88, 1.40) | 0.37 | 1.40 (1.14, 1.70) | 0.0011 | 1.30 (1.09, 1.55) | 0.0031 |
| VDR | rs2239186 | A/G | 1.22 (1.05, 1.41) | 0.0095 | 1.07 (0.81, 1.42) | 0.63 | 1.41 (1.12, 1.78) | 0.0039 | 1.32 (1.07, 1.63) | 0.0087 |
| VDR | rs4516035 | A/G | 1.12 (0.99, 1.26) | 0.064 | 1.26 (1.01, 1.57) | 0.041 | 0.99 (0.82, 1.21) | 0.95 | 1.05 (0.88, 1.24) | 0.60 |
| CYP24A1 | rs2762939 | C/G | 0.85 (0.75, 0.98) | 0.023 | 1.07 (0.84, 1.37) | 0.59 | 0.87 (0.69, 1.09) | 0.24 | 0.77 (0.63, 0.95) | 0.013 |
Models adjusted for age, gender, and family history of lymphoma
Exploratory analysis of the interaction between sun exposure and vitamin D-related SNPs
Analysis of the interaction between sun exposure at ages 13–21 years and genotype in the 6 VDR and CYP24A1 SNPs (based on a main effect p < 0.15) with NHL risk revealed that the observed inverse association of sun exposure in early life appeared to be limited to participants who were homozygous wildtype at VDR SNP rs4516035 (Table 5). When formally tested in a logistic regression with main effects, we observed a statistically significant interaction between sun exposure at ages 13–21 years and germline variation in the VDR at rs4516035 (pinteraction = 0.006). Analysis by subtype revealed that this interaction may be strongest among the FL subtype, although the magnitude of the OR estimates indicated lower NHL risk for homozygous wildtype at rs4516035 across all NHL subtypes evaluated.
Table 5.
Odds ratios and 95 % CIs for association of sun exposure at ages 13–21 and VDR rs4516035 genotype with risk of non-Hodgkin lymphoma, overall and by subtype, Mayo case–control study, 2002–2008
|
VDR rs4516035 genotype |
Sun exposure at 13–21
|
Ordinal sun OR (95 % CI)b | p interactionc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤3 h/week (reference)
|
4–7 h/week
|
8–14 h/week
|
≥15 h/week
|
|||||||
| Nco/Nca | OR (95 % CI) | Nco/Nca | OR (95 % CI) | Nco/Nca | OR (95 % CI) | Nco/Nca | OR (95 % CI) | |||
| All NHL (n = 576)a | ||||||||||
| AA | 19/14 | 1.0 (ref) | 43/43 | 1.49 (0.66, 3.38) | 142/73 | 0.70 (0.33, 1.49) | 117/40 | 0.42 (0.19, 0.93) | 0.66 (0.53, 0.83) | |
| AG + GG | 39/23 | 0.83 (0.35, 1.98) | 112/74 | 0.88 (0.41, 1.87) | 278/177 | 0.84 (0.41, 1.73) | 235/132 | 0.71 (0.34, 1.48) | 0.92 (0.79, 1.07) | 0.0063 |
| Diffuse large B-cell lymphoma (n = 99) | ||||||||||
| AA | 19/3 | 1.00 (ref) | 43/6 | 0.95 (0.21, 4.23) | 142/9 | 0.44 (0.11, 1.77) | 117/8 | 0.46 (0.11, 1.92) | 0.70 (0.45, 1.11) | |
| AG + GG | 39/5 | 0.81 (0.17, 3.77) | 112/13 | 0.77 (0.20, 2.98) | 278/28 | 0.68 (0.19, 2.48) | 235/27 | 0.78 (0.21, 2.86) | 1.01 (0.76, 1.34) | 0.23 |
| Follicular lymphoma (n = 144) | ||||||||||
| AA | 19/2 | 1.00 (ref) | 43/10 | 2.19 (0.43,11.03) | 142/28 | 1.95 (0.43, 8.92) | 117/8 | 0.70 (0.14, 3.58) | 0.80 (0.56, 1.16) | |
| AG + GG | 39/3 | 0.73 (0.11, 4.79) | 112/13 | 1.04 (0.22, 5.04) | 278/49 | 1.70 (0.38, 7.62) | 235/31 | 1.33 (0.29, 6.05) | 1.12 (0.86, 1.46) | 0.066 |
| Small lymphocytic lymphoma/chronic lymphocytic leukemia (n = 195) | ||||||||||
| AA | 19/5 | 1.00 (ref) | 43/12 | 1.30 (0.39, 4.30) | 142/24 | 0.64 (0.21, 1.94) | 117/16 | 0.43 (0.14, 1.37) | 0.65 (0.46, 0.92) | 0.33 |
| AG + GG | 39/6 | 0.66 (0.17, 2.49) | 112/31 | 1.08 (0.36, 3.20) | 278/60 | 0.79 (0.27, 2.25) | 235/41 | 0.57 (0.19, 1.67) | 0.83 (0.67, 1.04) | |
Sample size listed are the numbers of subjects with all data available and therefore included in this analysis of interaction
All models adjusted for age, gender, and family history of NHL
All interaction p values are based upon a likelihood ratio test and treat sun exposure as ordinal
Discussion
In this clinic-based case–control study, we observed nominally significant (p < 0.05) main effect associations of early life sun exposure and germline variation in VDR (3 SNPs) and CYP24A1 (1 SNP) with NHL risk. Most notably, we identified a previously unreported SNP 5′ upstream of the VDR promoter that appears to modify the association between early life sun exposure and NHL risk, lending support to the hypothesis that the vitamin D pathway over the lifespan may in part underlie the association of sun exposure with NHL risk.
Our sun exposure observations are broadly consistent with the current literature regarding the association between self-reported personal sun exposure and NHL risk [13], although two recently published cohort studies did not observe an association between sun exposure and NHL risk [10, 11], and a third reported increased NHL risk among women living in areas with high ambient UV radiation [12]. It has been hypothesized that differential BMI and physical activity levels among those who have high and low sun exposure could confound this association, although we found no evidence for this. Our assessment of early life physical activity was limited, so there is potential for residual confounding; however, there is relatively little evidence to support a strong association between physical activity and NHL [33]. Our findings also suggest, as others have previously [6, 7, 13], that early life sun exposure may be most relevant. The relative strength of previously observed associations of both early life sun exposure and, in particular, recreational sun exposure have led to proposed relevance of a chronic intermittent pattern of sun exposure in relation to NHL risk, though the biologic relevance of early life exposure requires further investigation.
A recent comprehensive review of the literature by an IOM panel concluded that there was limited support for an association between vitamin D status and cancer risk [34]. With the exception of the findings by Polesel et al. [35] and Lim et al. [36], the published estimates of the association of dietary vitamin D intake or serum 25(OH)D with NHL risk specifically were largely weak or null [3, 4, 19, 37–40]. Limitations in retrospective vitamin D insufficiency exposure assessment in epidemiologic research have been discussed in the literature [41] and could obscure a true association between vitamin D and NHL risk. While circulating 25(OH)D is the preferred biomarker for determining vitamin D sufficiency [42], the relevant etiologic period of exposure for vitamin D is unclear, and our ability to accurately and feasibly estimate remote vitamin D status retrospectively or prospectively is limited. Furthermore, variability in vitamin D assays, the effect of season on circulating levels, unknown stability of an individual’s vitamin D levels over time, the relative scarcity of dietary vitamin D sources, and potential reverse causality in a case–control setting limit our ability to drawn firm conclusions at this time from the literature. We therefore chose to evaluate germline variation in both the vitamin D receptor gene and along the vitamin D metabolism pathway as a proxy measure of ongoing vitamin D ‘exposure’ for this evaluation of vitamin D’s potential role with regard to NHL risk.
Three prior studies have evaluated various VDR SNPs in relation to NHL risk [18–20]. Purdue et al. [18] examined the relationship between three widely studied restriction fragment length polymorphisms (FokI, rs10735810; BsmI, rs1544410; and TaqI, rs731236) in the VDR within the New South Wales (NSW) case–control study and observed an association between variant alleles at the BSMI and TaqI sites with DLBCL risk and an association with FokI variants and T-cell lymphoma risk; no association with NHL risk overall was observed. In a similar analysis in the NCI-SEER NHL case–control study, no association was observed between either BsmI or TaqI SNPs and overall NHL, DLBCL, or FL risk [19]. Smedby and colleagues also failed to demonstrate an association of the TaqI, BsmI, or 7 additional VDR SNPs with NHL risk, overall or by major subtype, in the Scandinavian Lymphoma Etiology (SCALE) case–control study [20]. Consistent with these prior studies, we did not observe a significant association of either the TaqI or FokI SNPs with overall NHL. However, we did observe a significant association of three intronic VDR SNPs (rs886441, rs3819545, and rs2239186) with overall NHL risk, none of which have been previously evaluated. Two of these SNPs (rs3819545 and rs2239186) appeared to be in an LD block (Fig. 1), and when all three SNPs were modeled simultaneously in a logistic regression, only rs3819545 remained statistically significant. These three SNPs do not appear to be correlated with the SNPs evaluated in the prior NSW, SCALE, and NCI-SEER studies and thus may represent a new region in the VDR that is associated with NHL risk.
Most notably, our observations provide some evidence that vitamin D mediates the observed association between sun exposure and NHL risk. We report a significant interaction between rs4516035 and early life sun exposure, such that the decreased risk with increased sun exposure at ages 13–21 appears largely limited to those participants that were homozygous wildtype at this position in VDR. This SNP is upstream of the 5′ VDR promoter, and the functional relevance of variation at this position is unclear. The rs4516035 A allele has been associated with both increased susceptibility of melanoma [43] and more aggressive tumor location in the head, neck and trunk among melanoma patients [44]. However, our bioinformatics search did not find overlap of rs4516035, or any VDR SNP in LD with rs4516035 (r2 > 0.6), with known functional domains. This particular SNP has not been evaluated in the three prior studies evaluating the association between VDR variants and NHL risk. Statistically significant interaction between VDR SNP TaqI (rs731236) and sun exposure in relation to NHL risk was reported in both the SCALE [20] and NCI-SEER [19] studies, although the effect was limited to the T-cell lymphoma subset in the SCALE study [20]. The rs4516035 SNP, which was found to modify the sun relationship between early life sun exposure and NHL risk in the current study, does not appear to be in LD with the TaqI SNP at the 3′ end of the gene, and thus, we believe this is the first report of effect modification at this additional distinct VDR locus.
Taken in context with the current literature, this finding lends further support to the hypothesis that the vitamin D pathway may be biologically relevant to the association between sun and NHL risk. As stated previously, the effects of 1,25(OH)D are mediated through the VDR. Ligand-activated VDR directly binds DNA to modulate gene expression, and vitamin D receptor response elements have been identified in hundreds of downstream gene targets. Perhaps the most prominent non-calcemic vitamin D action is the control of cell proliferation (through upregulation of cell cycle progression inhibitors including p21 and p27) and a shift toward apoptosis (through upregulation of pro-apoptotic proteins such as bax and downregulation of anti-apoptotic proteins such as bcl-2) via both a direct effect of ligand-activated VDR on target genes and an indirect interaction with downstream transcription and cell signaling [21, 22, 45, 46]. Further research is necessary and warranted to validate this finding and to further understand the functional relevance of these VDR variants and the biology of this association. We do acknowledge that, particularly in the context of multiple testing, this observed interaction could be due to chance alone. Furthermore, functions of the VDR independent of 1(25)2D ligand binding have been reported [47] and may explain the observed interaction with sun exposure. As such, potential vitamin D-independent mechanisms should also be considered when interpreting these results and in planning future studies.
To our knowledge, this analysis is the first to evaluate the potential relevance of germline variation in the CYP24A1, CYP27B1, and RXRA and RXRB genes with NHL risk. We found no evidence to support a role for either CYP27B1 or RXR genes in relation to NHL risk, but we did observe a decreased risk of NHL among participants with the variant G allele at the CYP24A1 SNP rs2762939 (per-allele OR = 0.85; 95 % CI, 0.75–0.98). The CYP24A1 gene encodes the protein that degrades activated 1,25(OH)2D in a negative feedback loop [22]; vitamin D sensitivity and resulting systematic hypercalcemia with CYP24A1 loss of function mutations have been recently established [48]. While we did not observe any evidence that the early life sun exposure association is modified by variation in the CYP24A1 gene, this association may support a role for vitamin D in lymphomagenesis and merits further investigation.
Despite the considerable clinical heterogeneity of the NHL subtypes [49], this and similar studies to date have been designed to evaluate the association with all NHL subtypes combined as the primary hypothesis. It is known that the NHLs are molecularly, clinically, and likely etiologically heterogeneous [49], and evaluation of distinct etiologic processes within the NHL subtypes is a major ongoing challenge in epidemiologic research. We did find some evidence of potential heterogeneity of both the sun exposure and vitamin D-related gene-variant main effects by subtype, although statistical power was limited. It is possible that the role of sun exposure and vitamin D status could be variable among the NHL subtypes, and analyses of overall NHL effects could mask associations with individual subtypes. This study was not powered to analyze the main effects by NHL subtype, so the differential subtype patterns could be due to chance alone. However, further investigation of the biological mechanism underlying the potential variability of these relationships by subtype is warranted.
Strengths of our analysis include a carefully designed case–control study, central pathology review, and high-quality genotyping. Although this study was not population-based, both case and control participation was restricted to those residing in the region surrounding Mayo Clinic (Minnesota, Iowa, and Wisconsin), thus minimizing the effects of referral and selection bias and increasing the internal validity of using frequency-matched general medicine controls from the same region [27]. However, this restriction also limited the variability of sun exposure and thus generalizability of these findings to those living in regions with higher or lower ambient sun exposure. Common HapMap SNPs were used to tag the VDR genes, and through other genotyping projects, we have ruled out the presence of significant population stratification [26]. Major limitations are the use of an exclusively Caucasian population, which limits generalizability, and the relatively small sample size, which limits our ability to estimate NHL subtype associations. Additionally, our estimates of the main effects of sun exposure rely on a self-reported average sun exposure during different age periods in the past, and it is possible that the season of recruitment along with the slight imbalance in consent season between cases and controls could bias recall of average sun exposure, although we found no evidence for any impact on our reported association when stratified on season of consent. Finally, we emphasize that these findings could be due to chance alone based on the number of hypotheses being tested. In fact, only the main effects association between the intronic VDR SNPs rs3819545 met the stated Bonferroni-adjusted significance threshold for this analysis (p < 0.001). Moreover, the gene coverage, based on the SNPs that we have genotype data on for this analysis, is variable. Therefore, an association between CYP27B1 or RXR and NHL risk cannot yet be ruled out.
In conclusion, we observed both an inverse association between the early life sun exposure and NHL risk and main effects associations of genetic variation in VDR and CYP24A1 with NHL risk. Exploratory analysis by NHL subtype indicated potential heterogeneity of these associations by NHL subtype. The observed significant interaction of effect between early life sun exposure and germline variation in VDR suggests that the vitamin D pathway may mediate an association between early life sun exposure and NHL risk.
Acknowledgments
We thank Sondra Buehler for her editorial assistance. This work was supported by awards from the National Institutes of Health, National Cancer Institute [R01 CA92153; P50 CA97274]. Dr. Kelly was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute [HL007152], National Cancer Institute [P50 CA130805] and is a Lymphoma Research Foundation Fellow.
Footnotes
Conflicts of interest No potential conflicts of interest were disclosed.
Contributor Information
Jennifer L. Kelly, School of Medicine and Dentistry, University of Rochester, 601 Elmwood Avenue, Box 704, Rochester, NY, USA
Matthew T. Drake, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Zachary S. Fredericksen, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Yan W. Asmann, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Mark Liebow, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA.
Tait D. Shanafelt, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Andrew L. Feldman, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Stephen M. Ansell, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
William R. Macon, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Megan M. Herr, School of Medicine and Dentistry, University of Rochester, 601 Elmwood Avenue, Box 704, Rochester, NY, USA
Alice H. Wang, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Grzegorz S. Nowakowski, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Timothy G. Call, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Thomas M. Habermann, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Susan L. Slager, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
Thomas E. Witzig, Department of Health Sciences Research, College of Medicine, Mayo Clinic, 200 First St SW, Rochester, MN 55905, USA
James R. Cerhan, Email: cerhan.james@mayo.edu.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Alexander DD, Mink PJ, Adami HO, Chang ET, Cole P, Mandel JS, Trichopoulos D. The non-Hodgkin lymphomas: a review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 3.Hartge P, Lim U, Freedman DM, Colt JS, Cerhan JR, Cozen W, Severson RK, Davis S. Ultraviolet radiation, dietary vitamin D, and risk of non-Hodgkin lymphoma (United States) Cancer Causes Control. 2006;17(8):1045–1052. doi: 10.1007/s10552-006-0040-8. [DOI] [PubMed] [Google Scholar]
- 4.Soni LK, Hou L, Gapstur SM, Evens AM, Weisenburger DD, Chiu BC. Sun exposure and non-Hodgkin lymphoma: a population-based, case-control study. Eur J Cancer. 2007;43(16):2388–2395. doi: 10.1016/j.ejca.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 5.Weihkopf T, Becker N, Nieters A, Mester B, Deeg E, Elsner G, Blettner M, Seidler A. Sun exposure and malignant lymphoma: a population-based case-control study in Germany. Int J Cancer. 2007;120(11):2445–2451. doi: 10.1002/ijc.22492. [DOI] [PubMed] [Google Scholar]
- 6.Hughes AM, Armstrong BK, Vajdic CM, Turner J, Grulich AE, Fritschi L, Milliken S, Kaldor J, Benke G, Kricker A. Sun exposure may protect against non-Hodgkin lymphoma: a case-control study. Int J Cancer. 2004;112(5):865–871. doi: 10.1002/ijc.20470. [DOI] [PubMed] [Google Scholar]
- 7.Smedby KE, Hjalgrim H, Melbye M, Torrang A, Rostgaard K, Munksgaard L, Adami J, Hansen M, Porwit-MacDonald A, Jensen BA, Roos G, Pedersen BB, Sundstrom C, Glimelius B, Adami HO. Ultraviolet radiation exposure and risk of malignant lymphomas. J Natl Cancer Inst. 2005;97(3):199–209. doi: 10.1093/jnci/dji022. [DOI] [PubMed] [Google Scholar]
- 8.Chang ET, Canchola AJ, Cockburn M, Lu Y, Wang SS, Bernstein L, Clarke CA, Horn-Ross PL. Adulthood residential ultraviolet radiation, sun sensitivity, dietary vitamin D, and risk of lymphoid malignancies in the California Teachers Study. Blood. 2011;118(6):1591–1599. doi: 10.1182/blood-2011-02-336065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Holford TR, Leaderer B, Boyle P, Zhu Y, Wang R, Zou K, Zhang B, Wise JP, Sr, Qin Q, Kilfoy B, Han J, Zheng T. Ultraviolet radiation exposure and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2007;165(11):1255–1264. doi: 10.1093/aje/kwm020. [DOI] [PubMed] [Google Scholar]
- 10.Veierod MB, Smedby KE, Lund E, Adami HO, Weiderpass E. Pigmentary characteristics, UV radiation exposure, and risk of non-Hodgkin lymphoma: a prospective study among Scandinavian women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1569–1576. doi: 10.1158/1055-9965.EPI-10-0115. [DOI] [PubMed] [Google Scholar]
- 11.Freedman DM, Kimlin MG, Hoffbeck RW, Alexander BH, Linet MS. Multiple indicators of ambient and personal ultraviolet radiation exposure and risk of non-Hodgkin lymphoma (United States) J Photochem Photobiol, B. 2010;101(3):321–325. doi: 10.1016/j.jphotobiol.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertrand KA, Chang ET, Abel GA, Zhang SM, Spiegelman D, Qureshi AA, Laden F. Sunlight exposure, vitamin D, and risk of non-Hodgkin lymphoma in the Nurses’ Health Study. Cancer Causes Control. 2011;22(12):1731–1741. doi: 10.1007/s10552-011-9849-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kricker A, Armstrong BK, Hughes AM, Goumas C, Smedby KE, Zheng T, Spinelli JJ, De Sanjose S, Hartge P, Melbye M, Willett EV, Becker N, Chiu BC, Cerhan JR, Maynadie M, Staines A, Cocco P, Boffeta P. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 14.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 15.Kelly JL, Friedberg JW, Calvi LM, van Wijngaarden E, Fisher S. Vitamin D and non-Hodgkin lymphoma risk in adults: a review. Cancer Invest. 2009;27(9):942–951. doi: 10.3109/07357900902849632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee HV, Coebergh JW, Vries ED. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur J Cancer Prev. 2009;18:458–475. doi: 10.1097/CEJ.0b013e32832f9bb1. [DOI] [PubMed] [Google Scholar]
- 17.Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis. 2009;30(7):1170–1180. doi: 10.1093/carcin/bgp103. [DOI] [PubMed] [Google Scholar]
- 18.Purdue MP, Lan Q, Kricker A, Vajdic CM, Rothman N, Armstrong BK. Vitamin D receptor gene polymorphisms and risk of non-Hodgkin’s lymphoma. Haematologica. 2007;92(8):1145–1146. doi: 10.3324/haematol.11053. [DOI] [PubMed] [Google Scholar]
- 19.Purdue MP, Hartge P, Davis S, Cerhan JR, Colt JS, Cozen W, Severson RK, Li Y, Chanock SJ, Rothman N, Wang SS. Sun exposure, vitamin D receptor gene polymorphisms and risk of non-Hodgkin lymphoma. Cancer Causes Control. 2007;18(9):989–999. doi: 10.1007/s10552-007-9039-z. [DOI] [PubMed] [Google Scholar]
- 20.Smedby KE, Eloranta S, Duvefelt K, Melbye M, Humphreys K, Hjalgrim H, Chang ET. Vitamin D receptor genotypes, ultraviolet radiation exposure, and risk of non-Hodgkin lymphoma. Am J Epidemiol. 2011;173(1):48–54. doi: 10.1093/aje/kwq340. [DOI] [PubMed] [Google Scholar]
- 21.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66(10 Suppl 2):S116–S124. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 22.Bikle DD. Vitamin D: newly discovered actions require reconsideration of physiologic requirements. Trends Endocrinol Metab. 2010;21(6):375–384. doi: 10.1016/j.tem.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88(2):296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- 24.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M. Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab. 2001;86(2):888–894. doi: 10.1210/jcem.86.2.7220. [DOI] [PubMed] [Google Scholar]
- 25.Hickish T, Cunningham D, Colston K, Millar BC, Sandle J, Mackay AG, Soukop M, Sloane J. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–672. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerhan JR, Ansell SM, Fredericksen ZS, Kay NE, Liebow M, Call TG, Dogan A, Cunningham JM, Wang AH, Liu-Mares W, Macon WR, Jelinek D, Witzig TE, Habermann TM, Slager SL. Genetic variation in 1253 immune and inflammation genes and risk of non-Hodgkin lymphoma. Blood. 2007;110(13):4455–4463. doi: 10.1182/blood-2007-05-088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerhan JR, Fredericksen ZS, Wang AH, Habermann TM, Kay NE, Macon WR, Cunningham JM, Shanafelt TD, Ansell SM, Call TG, Witzig TE, Slager SL, Liebow M. Design and validity of a clinic-based case-control study on the molecular epidemiology of lymphoma. Int J Mol Epidemiol Genet. 2011;2(2):95–113. [PMC free article] [PubMed] [Google Scholar]
- 28.Jaffe ES, Harris N, Stein H, Vardiman J. World Health Organization classification of tumours pathology and genetics, tumors of hematopoietic and lymphoid tissues. IARC Press; Lyon: 2001. [Google Scholar]
- 29.Holick CN, Stanford JL, Kwon EM, Ostrander EA, Nejentsev S, Peters U. Comprehensive association analysis of the vitamin D pathway genes, VDR, CYP27B1, and CYP24A1, in prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):1990–1999. doi: 10.1158/1055-9965.EPI-07-0487. [DOI] [PubMed] [Google Scholar]
- 30.McCullough ML, Bostick RM, Mayo TL. Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr. 2009;29:111–132. doi: 10.1146/annurev-nutr-080508-141248. [DOI] [PubMed] [Google Scholar]
- 31.Oliphant A, Barker DL, Stuelpnagel JR, Chee MS. BeadArray technology: enabling an accurate, cost-effective approach to high-throughput genotyping. Biotechniques Suppl. 2002:56–58. 60–51. [PubMed] [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Applied logistic regression. 2. Wiley; New York: 2000. [Google Scholar]
- 33.Pan SY, Morrison H. Physical activity and hematologic cancer prevention. Recent Results Cancer Res. 2011;186:135–158. doi: 10.1007/978-3-642-04231-7_6. [DOI] [PubMed] [Google Scholar]
- 34.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. The National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 35.Polesel J, Talamini R, Montella M, Parpinel M, Dal Maso L, Crispo A, Crovatto M, Spina M, La Vecchia C, Franceschi S. Linoleic acid, vitamin D and other nutrient intakes in the risk of non-Hodgkin lymphoma: an Italian case-control study. Ann Oncol. 2006;17(4):713–718. doi: 10.1093/annonc/mdl054. [DOI] [PubMed] [Google Scholar]
- 36.Lim U, Freedman DM, Hollis BW, Horst RL, Purdue MP, Chatterjee N, Weinstein SJ, Morton LM, Schatzkin A, Virtamo J, Linet MS, Hartge P, Albanes D. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–986. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chang ET, Balter KM, Torrang A, Smedby KE, Melbye M, Sundstrom C, Glimelius B, Adami HO. Nutrient intake and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2006;164(12):1222–1232. doi: 10.1093/aje/kwj330. [DOI] [PubMed] [Google Scholar]
- 38.Freedman DM, Looker AC, Chang SC, Graubard BI. Prospective study of serum vitamin D and cancer mortality in the United States. J Natl Cancer Inst. 2007;99(21):1594–1602. doi: 10.1093/jnci/djm204. [DOI] [PubMed] [Google Scholar]
- 39.Giovannucci E, Liu Y, Willett WC. Cancer incidence and mortality and vitamin d in black and white male health professionals. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2467–2472. doi: 10.1158/1055-9965.EPI-06-0357. [DOI] [PubMed] [Google Scholar]
- 40.Purdue MP, Freedman DM, Gapstur SM, Helzlsouer KJ, Laden F, Lim U, Maskarinec G, Rothman N, Shu XO, Stevens VL, Zeleniuch-Jacquotte A, Albanes D, Bertrand K, Weinstein SJ, Yu K, Irish L, Horst RL, Hoffman-Bolton J, Giovannucci EL, Kolonel LN, Snyder K, Willett W, Arslan AA, Hayes RB, Zheng W, Xiang YB, Hartge P. Circulating 25-hydroxyvitamin D and risk of non-Hodgkin lymphoma: cohort consortium Vitamin D pooling project of rarer cancers. Am J Epidemiol. 2010;172(1):58–69. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Millen AE, Bodnar LM. Vitamin D assessment in population-based studies: a review of the issues. Am J Clin Nutr. 2008;87(4):1102S–1105S. doi: 10.1093/ajcn/87.4.1102S. [DOI] [PubMed] [Google Scholar]
- 42.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2008;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halsall JA, Osborne JE, Potter L, Pringle JH, Hutchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibility and prognosis in malignant melanoma. Br J Cancer. 2004;91(4):765–770. doi: 10.1038/sj.bjc.6602006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barroso E, Fernandez LP, Milne RL, Pita G, Sendagorta E, Floristan U, Feito M, Aviles JA, Martin-Gonzalez M, Arias JI, Zamora P, Blanco M, Lazaro P, Benitez J, Ribas G. Genetic analysis of the vitamin D receptor gene in two epithelial cancers: melanoma and breast cancer case-control studies. BMC Cancer. 2008;8:385. doi: 10.1186/1471-2407-8-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kizildag S, Ates H. Treatment of K562 cells with 1,25-dihydroxyvitamin D(3) induces distinct alterations in the expression of apoptosis-related genes BCL2, BAX, BCL(XL), and p21. Ann Hematol. 2009 doi: 10.1007/s00277-009-0766-y. [DOI] [PubMed] [Google Scholar]
- 46.Bai M, Vlachonikolis J, Agnantis NJ, Tsanou E, Dimou S, Nicolaides C, Stefanaki S, Pavlidis N, Kanavaros P. Low expression of p27 protein combined with altered p53 and Rb/p16 expression status is associated with increased expression of cyclin A and cyclin B1 in diffuse large B-cell lymphomas. Mod Pathol. 2001;14(11):1105–1113. doi: 10.1038/modpathol.3880444. [DOI] [PubMed] [Google Scholar]
- 47.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci USA. 2007;104(22):9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, Misselwitz J, Klaus G, Kuwertz-Broking E, Fehrenbach H, Wingen AM, Guran T, Hoenderop JG, Bindels RJ, Prosser DE, Jones G, Konrad M. Mutations in CYP24A1 and idiopathic infantile hypercalcemia. N Engl J Med. 2011;365(5):410–421. doi: 10.1056/NEJMoa1103864. [DOI] [PubMed] [Google Scholar]
- 49.Morton LM, Wang SS, Cozen W, Linet MS, Chatterjee N, Davis S, Severson RK, Colt JS, Vasef MA, Rothman N, Blair A, Bernstein L, Cross AJ, De Roos AJ, Engels EA, Hein DW, Hill DA, Kelemen LE, Lim U, Lynch CF, Schenk M, Wacholder S, Ward MH, Zahm SH, Chanock SJ, Cerhan JR, Hartge P. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes. Blood. 2008;112(13):5150–5160. doi: 10.1182/blood-2008-01-133587. [DOI] [PMC free article] [PubMed] [Google Scholar]