Abstract
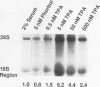
The synthesis of ribosomes is an essential cellular process which requires the transcription of the rRNA genes by RNA polymerase I (Pol I). The regulation of rRNA synthesis is known to be coupled to growth regulation. In nongrowing, slowly growing, and rapidly growing Drosophila cells, exposure to the tumor-promoting phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA) increases the synthesis of precursor and mature rRNAs. Using nuclear run-on assays, we show that TPA enhances transcription of the rRNA genes. These results suggest that TPA regulates expression of RNA genes transcribed by Pol I, irrespective of the growth state of the cells. In slowly dividing Drosophila cells, increasing the serum concentration rapidly alters the accumulation of rRNA by enhancing rDNA transcription within 1 h. Thus, TPA and serum are each able to rapidly regulate rRNA gene expression in Drosophila cells. These results indicate that the RNA Pol I transcription system can be regulated by agents which have previously been shown to effect specific genes transcribed by the RNA Pol II system.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allo S. N., McDermott P. J., Carl L. L., Morgan H. E. Phorbol ester stimulation of protein kinase C activity and ribosomal DNA transcription. Role in hypertrophic growth of cultured cardiomyocytes. J Biol Chem. 1991 Nov 15;266(32):22003–22009. [PubMed] [Google Scholar]
- Bell J. R., Bogardus A. M., Schmidt T., Pellegrini M. A new copia-like transposable element found in a Drosophila rDNA gene unit. Nucleic Acids Res. 1985 Jun 11;13(11):3861–3871. doi: 10.1093/nar/13.11.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J., Neilson L., Pellegrini M. Effect of heat shock on ribosome synthesis in Drosophila melanogaster. Mol Cell Biol. 1988 Jan;8(1):91–95. doi: 10.1128/mcb.8.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bowman L. H. rDNA transcription and pre-rRNA processing during the differentiation of a mouse myoblast cell line. Dev Biol. 1987 Jan;119(1):152–163. doi: 10.1016/0012-1606(87)90217-x. [DOI] [PubMed] [Google Scholar]
- Buttgereit D., Pflugfelder G., Grummt I. Growth-dependent regulation of rRNA synthesis is mediated by a transcription initiation factor (TIF-IA). Nucleic Acids Res. 1985 Nov 25;13(22):8165–8180. doi: 10.1093/nar/13.22.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chao Y., Pellegrini M. In vitro transcription of Drosophila rRNA genes shows stimulation by a phorbol ester and serum. Mol Cell Biol. 1993 Feb;13(2):934–941. doi: 10.1128/mcb.13.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Garber M., Panchanathan S., Fan R. S., Johnson D. L. The phorbol ester, 12-O-tetradecanoylphorbol-13-acetate, induces specific transcription by RNA polymerase III in Drosophila Schneider cells. J Biol Chem. 1991 Nov 5;266(31):20598–20601. [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Liebhaber S. A., Wolf S., Schlessinger D. Differences in rRNA metabolism of primary and SV40-transformed human fibroblasts. Cell. 1978 Jan;13(1):121–127. doi: 10.1016/0092-8674(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Long E. O., Dawid I. B. Alternative pathways in the processing of ribosomal RNA precursor in Drosophila melanogaster. J Mol Biol. 1980 Apr 25;138(4):873–878. doi: 10.1016/0022-2836(80)90070-4. [DOI] [PubMed] [Google Scholar]
- Martelly I., Castagna M. Protein kinase C and tumor promoters. Curr Opin Cell Biol. 1989 Apr;1(2):206–210. doi: 10.1016/0955-0674(89)90088-4. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Gourse R., Baughman G. Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem. 1984;53:75–117. doi: 10.1146/annurev.bi.53.070184.000451. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schmidt T., Chen P. S., Pellegrini M. The induction of ribosome biosynthesis in a nonmitotic secretory tissue. J Biol Chem. 1985 Jun 25;260(12):7645–7650. [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Soprano K. J., Baserga R. Reactivation of ribosomal RNA genes in human-mouse hybrid cells by 12-O-tetradecanoylphorbol 13-acetate. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1566–1569. doi: 10.1073/pnas.77.3.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Polymerase III transcription factor B activity is reduced in extracts of growth-restricted cells. Mol Cell Biol. 1988 Feb;8(2):1001–1005. doi: 10.1128/mcb.8.2.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Warner J. R. Ribosomal proteins are synthesized preferentially in cells commencing growth. J Cell Physiol. 1982 Jul;112(1):128–135. doi: 10.1002/jcp.1041120119. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Phillips W. H., Grainger R. M. Drosophila ribosomal RNA stability increases during slow growth conditions. J Biol Chem. 1985 Jun 25;260(12):7716–7720. [PubMed] [Google Scholar]
- Yamamoto K., Chadarevian A., Pellegrini M. Juvenile hormone action mediated in male accessory glands of Drosophila by calcium and kinase C. Science. 1988 Feb 19;239(4842):916–919. doi: 10.1126/science.3124270. [DOI] [PubMed] [Google Scholar]