Abstract
Intestinal lipid transport plays a central role in fat homeostasis. Here we review the pathways regulating intestinal absorption and delivery of dietary and biliary lipid substrates, principally long-chain fatty acid, cholesterol, and other sterols. We discuss the regulation and functions of CD36 in fatty acid absorption, NPC1L1 in cholesterol absorption, as well as other lipid transporters including FATP4 and SRB1. We discuss the pathways of intestinal sterol efflux via ABCG5/G8 and ABCA1 as well as the role of the small intestine in high-density lipoprotein (HDL) biogenesis and reverse cholesterol transport. We review the pathways and genetic regulation of chylomicron assembly, the role of dominant restriction points such as microsomal triglyceride transfer protein and apolipoprotein B, and the role of CD36, L-FABP, and other proteins in formation of the prechylomicron complex. We will summarize current concepts of regulated lipoprotein secretion (including HDL and chylomicron pathways) and include lessons learned from families with genetic mutations in dominant pathways (i.e., abetalipoproteinemia, chylomicron retention disease, and familial hypobetalipoproteinemia). Finally, we will provide an integrative view of intestinal lipid homeostasis through recent findings on the role of lipid flux and fatty acid signaling via diverse receptor pathways in regulating absorption and production of satiety factors.
I. INTRODUCTION
The current pandemic of obesity, coupled to increased consumption of fat and energy-rich foods, has led to renewed interest in the role of the small intestine in the integrated regulation of lipid homeostasis. This review examines how the evolutionary adaptations that promote efficiency in coordinating dietary and biliary lipid absorption and processing in turn influence lipid homeostatic mechanisms throughout the body. We examine the specific transporters and pathways that determine substrate specificity in uptake across the brush-border membrane and review the biochemical and genetic mechanisms that modulate the net transintestinal transport of fatty acids and cholesterol, as well as other lipids. We review the regulatory mechanisms and pathways that modulate villus enterocyte cholesterol uptake and the formation of intestinal HDL as well as integrating these pathways with those involved in canalicular cholesterol secretion and cholesterol efflux. We also describe the mechanisms for complex lipid reassembly within the enterocyte and review the pathways that lead to lipid droplet formation and mobilization. We discuss the mechanisms by which lipid droplets are mobilized into the lumen of the endoplasmic reticulum and the key steps that involve microsomal triglyceride transfer protein (MTTP) and the structural protein apolipoprotein B (apoB). We review the steps in maturation of the primordial lipoprotein particle through the distal elements of the secretory pathway and the formation of chylomicron particles. In a final section, we present an overview of recent findings related to important signaling roles for intestinal lipids in the regulation of lipid absorption and satiety. These themes will frame a summary discussion that outlines future research directions in the field.
II. FATTY ACID AND STEROL TRAFFICKING
Dietary fats are important for human health, providing a densely caloric energy source in addition to essential fatty acids (FA) and fat-soluble vitamins. However, changes in the amount and composition of dietary fat brought about by the industrial revolution have contributed to the current epidemic of obesity (37). Fat content of the Western diet averages ~35% of energy intake and is mostly in the form of triglycerides (TG). Typically the fat contains equal amounts of saturated and monounsaturated FA (~14% of energy) and 6% polyunsaturated FA (PUFA). Alterations in dietary fatty acid composition, notably an increase in the ratio of omega 6 to omega 3 PUFA (reflecting increased use of vegetable oils), may also contribute to adipogenesis and obesity (3). While evolution resulted in the gut gaining highly effective mechanisms for fat absorption, there is intense focus nowadays on interventions designed to reduce fat intake by inhibiting intestinal absorption (233) or by surgically reducing the absorptive area (178). These interventions are often associated with dramatic metabolic changes but also raise some health concerns. A better understanding of the complex absorptive functions of the gut and the role they play in regulating overall energy homeostasis is more important than ever before. In addition, there have been significant advances in our understanding of the mechanisms by which long-chain FA (LCFA), cholesterol, and other sterols traverse the aqueous milieu of the mucus layer adjacent to the microvillus membrane and are then subsequently transported into the subapical domain of the villus enterocyte. Key among these has been the functional genetic and biochemical characterization of several classes of apical transporters that function in the selective uptake or export of lipid substrates.
A. Brush-Border FA Transporters
Hydrolysis of dietary TG in the intestinal lumen by pancreatic lipases generates two FA and one sn-2-monoacylglycerol (2-MG). These products are absorbed through the apical membrane of enterocytes and directed to the endoplasmic reticulum (ER) for resynthesis into TG. The newly synthesized TG are packaged for secretion into chylomicrons, the lipoproteins unique to the intestine. In the circulation, the TG of chylomicrons are hydrolyzed by lipoprotein lipase (LPL), which is located at the surface of capillaries, and the released FAs are rapidly taken up by peripheral tissues where they are used for various cellular pathways (70). In view of the importance of dietary TG as a nutrient, attention has focused on the uptake and processing of its component LCFA and also but to a lesser extent on the uptake and processing of 2-MG.
1. Uptake of LCFA by enterocytes: transporters versus diffusional mechanisms
Small intestinal cells absorb LCFA both via passive diffusion as well as protein-facilitated FA transfer. LCFA diffuse across the brush-border membrane via “flip-flop” of protonated FA down a favorable concentration gradient. Trapping of FA through binding to abundant intracellular FA binding proteins (205) and/or metabolic trapping by conversion to acyl-CoA derivatives are likely to play an important role in the initial phases of LCFA transport into small intestinal enterocytes (141). This section of the review is focused on the protein-mediated component of the membrane transfer of FA. This component may not contribute quantitatively to LCFA uptake by the intestine, but it appears to exert a regulatory role with respect to the downstream metabolic targeting of the absorbed FA.
Membrane FA permeation was documented in many cell types to exhibit features of a protein-facilitated process. This includes specificity for LCFA (with hydrocarbon chains longer than 8 carbons) and a transport Km in the low nanomolar range (207) that is well suited to the concentration of unbound FA dissociated from albumin in the circulation (175). However, enterocytes are a special case in view of the distinctive mode of presentation of FA for uptake by these cells. In the intestine, the FA released from TG digestion by lipases is incorporated into bile salt micelles. Like serum albumin, micelles solubilize millimolar concentrations of FA and are in equilibrium with FA monomers in solution. The concentration of monomeric FA is estimated in the low micromolar range (154, 225), which is orders of magnitude higher than that of unbound FA in the circulation. Early studies argued against cellular uptake of whole micelles (237), and more recent work showed that enterocyte FA uptake is a saturable function of the FA monomer (154). In addition, enterocyte processing of FA was shown to be dependent on the cellular entry site, i.e., apical (AP) versus basolateral (BL) (206). The ratio of TG to phospholipid formed was 10-fold higher for AP uptake, while a 3-fold higher level of FA oxidation was measured for BL delivery. There was also twofold greater FA incorporation into phosphatidylethanolamine (PE) with a threefold decrease in the phosphatidylcholine:PE ratio for AP compared with BL delivery (206). These observations suggested that apical membrane proteins on enterocytes may facilitate FA transfer and direct the FA to the appropriate metabolic sites. Regulatory effects of protein-mediated transport were also suggested by the adaptive changes in brush-border membrane FA uptake in response to environmental challenges (208, 221) and by the variability, possibly genetically determined, of FA uptake by intestinal segments from various mice strains (103). In the following discussion we will try to integrate the data related to intestinal FA transport into a working model that could provide a starting point for further work. Among the candidate LCFA transporters identified in the small intestine, we will discuss the role of CD36 (45-47) as well as one member of the very long chain acyl-CoA synthetases, otherwise known as fatty acid transport proteins (FATPs), namely FATP4 (198).
A) FAT/CD36: A TRANSPORTER OR A TRANSPORT REGULATOR
A role for CD36 in LCFA uptake was uncovered in 1993 (1), and in the same year it was identified as a receptor for oxidized low-density lipoproteins (52). CD36 is a 75- to 88-kDa, 472-amino acid heavily glycosylated transmembrane protein with broad ligand specificity that now includes LCFA, native or modified lipoproteins, glycated proteins, thrombospondin-1, collagen, amyloid B, and malaria-infected erythrocytes (192). CD36 is ubiquitously expressed, abundant in the heart, skeletal muscle, adipose tissue, intestine (1), and the capillary endothelium (73). There is now extensive in vivo evidence both in rodents and humans for an important role of CD36 in facilitating tissue FA uptake and for its contribution to diet-induced metabolic pathology. Studies in CD36-deficient mice (36, 78) and humans (83, 236, 244) have documented a defect in tissue FA uptake and abnormalities of FA metabolism. Polymorphisms in the CD36 gene have been linked to alterations in plasma lipid levels and susceptibility to the metabolic syndrome (133-135). For recent reviews on CD36’s role in FA transport, refer to References 184 and 207. CD36 is abundantly expressed in the small intestine where it was shown to play a number of roles related to fat absorption (discussion below) and also in fat taste perception and the regulation of food intake (see sect. IV).
I) CD36’s role in chylomicron formation
In the small intestines of mice (155) and humans (132), CD36 is most abundant in the proximal third of the intestine. In both species CD36 is detected on the brush-border membranes of duodenal and jejunal villi (132, 170). However, epithelial cell immunochemical staining of CD36 is almost absent in the ileum and colon, although CD36 is detectable in blood vessels throughout the whole intestine of humans (132) and mice (Sundaresan and Abumrad, unpublished data). This expression pattern with proximal abundance and apical localization to intestinal villus tips supports a function in lipid absorption. The nature of this function is still incompletely understood, but it is now well established that CD36 plays an important role in directing the FA absorbed proximally to chylomicron formation (46, 156, 190).
There is no evidence of lipid malabsorption in CD36 null mice based on blood appearance of intestinally derived TG (46, 71) or using the 24 h fecal lipid recovery (46, 156) except for very-long-chain FA (VLCFA) (47). However, CD36 deficiency shifts more of lipid absorption to the distal parts of the small intestine (156). In addition, a larger part of the absorbed lipid may bypass the lymphatic system. Intestinal lipid secretion into the mesenteric lymph duct is decreased by 50%, the lipoproteins secreted are 35% smaller (46, 156), and as a result their clearance from the blood is significantly delayed in CD36 null mice (46). In humans deficient in CD36, there is postprandial hypertriglyceridemia with intestinal secretion of lipoproteins smaller than chylomicrons, suggesting impaired chylomicron formation (142).
How does CD36 deficiency impair chylomicron production without affecting net FA absorption? One possibility is that the proximal intestinal defect in FA uptake is compensated for by non-CD36-mediated uptake in distal segments. Primary enterocytes isolated from the proximal but not distal intestine of CD36 null mice showed a 50% reduction in FA uptake compared with enterocytes from wild-type mice (155). Also intragastric administration of triolein to CD36 null mice was associated with reduced oleic acid enrichment of mucosal lipids in the proximal intestine (155). Similarly, CD36 deletion suppressed oleic acid uptake for oleoylethanolamide (OEA) formation by the duodenum of mice fed after a 6-h fast, which may in turn play a role in modulating the integrated satiety response following lipid ingestion (183). Thus CD36 appears to function in high-affinity uptake of FA in proximal enterocytes similar to its role in other cell types (207). However, despite evidence for its participation in enterocyte FA uptake, the contribution of CD36 to net intestinal FA absorption is likely to be small based on two considerations. 1) The Km for FA uptake by enterocyte CD36 is in the nanomolar range (155), meaning CD36 will be rapidly saturated and its function would be important only in very early stages of the digestive process. In addition, there is evidence that FAs downregulate levels of the CD36 protein by promoting its ubiquitination (196, 207). This regulation that was observed in muscle (196) and liver cells (F. Nassir and N. A. Abumrad, unpublished observations) also occurs in enterocytes (138, 223) where it would tend to further minimize CD36 contribution to net LCFA absorption. Based on all the above, the current view of CD36-mediated FA uptake in intestinal absorption would favor that of a regulatory nature designed to optimize the packaging of FA and cholesterol into chylomicrons (FIGURE 1A). Recently, CD36 was identified together with liver fatty acid binding protein (L-Fabp), a member of the cytosolic lipid binding protein family, to be required for assembly of the multiprotein complex that generates the prechylomicron transport vesicle from the intestinal ER (190). The intestinal peptide glucagon-like peptide-2 (GLP-2) was also recently reported to regulate intestinal lipid absorption and the assembly and secretion of TG-rich lipoproteins in a CD36-dependent manner (88). GLP-2 was found to promote glycosylation and membrane localization of enterocyte CD36, where it could conceivably modulate lipid trafficking (158).
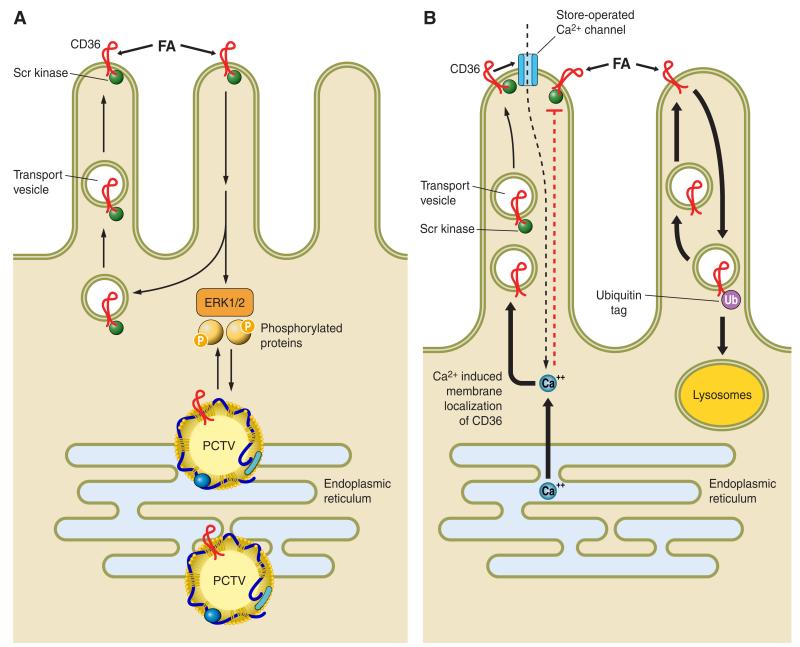
FIGURE 1.
Aspects of CD36 signaling and trafficking that may have potential relevance to the regulation of intestinal lipid metabolism. The figure proposes some hypothetical working concepts regarding steps that may be involved in the effect of intestinal CD36 to facilitate fatty acid (FA) uptake and chylomicron formation. A: CD36 is highly expressed on enterocytes of the proximal intestine and localizes to the apical side of intestinal villi (132, 155, 170) where it interacts with FA released from the digestion of dietary triglycerides. While CD36 functions in facilitating uptake of FA by proximal enterocytes (155, 183), it does not contribute quantitatively to FA absorption (47, 156), since it would be rapidly saturated at the levels of monomeric FA present in the lumen. However, CD36-mediated FA uptake and associated signaling, operating at the very early stages of absorption, may promote intracellular events that facilitate chylomicron assembly (46, 88, 156). CD36 signaling is in most cases initiated via the CD36-associated Src kinases and downstream via the extracellular regulated kinase ERK1/2 (109). This signaling pathway may be important for phosphorylating proteins required for coordinating ER processing of prechylomicron vesicles (PCTV). CD36 has also been identified in the protein complex that is required for formation of the PCTV (190). B: recent data indicate that CD36 signaling may also be mediated by a rise in intracellular calcium. Calcium release from the ER promotes membrane CD36 localization, which regulates calcium influx via the store-operated calcium channel. The sustained increase in intracellular calcium could influence multiple events related to lipid processing or secretion (16, 109). B also illustrates the concept that CD36 is downregulated by FA via ubiquitination and lysosomal degradation (196), and this feedback loop may work to reduce CD36 function in the presence of high concentrations of long-chain fatty acids (LCFA) (207). In the enterocytes, the LCFA-induced decrease in CD36 associates with reduced activation of ERK1/2 which may serve to upregulate MTTP abundance (138).
As alluded to above, the mechanisms involved in CD36 promotion of chylomicron formation remain unclear. One mechanism may involve CD36 signaling possibly to the extracellular regulated kinase 1/2 (ERK1/2) (109, 223), leading to the phosphorylation of proteins important for ER lipid processing (FIGURE 1A). Recent studies demonstrated that CD36 internalization was promoted upon binding LCFA but prevented by a rise in cellular calcium (109) (FIGURE 1B). These events may be related to intracellular lipid transport, but the details remain to be elucidated. It can be speculated that CD36’s ability to influence store-operated calcium flux (FIGURE 1B) and the secretion of bioactive compounds (50, 109) may contribute to its effects on chylomicron production by influencing vesicular transport and/or the release of intestinal regulatory peptides.
In summary, the findings to date suggest that CD36 functions at different stages of fat digestion. CD36 mediates perception of FA in lingual taste bud cells and the initiation of the cephalic phase of digestion. In the small intestine, CD36 transport provides the oleic acid needed for generation of OEA, which reduces food intake. CD36 also facilitates uptake of FA, but its contribution to net absorption is small and under excess fat supply may be manifested in a shift of fat absorption to more distal parts of the intestine.
However, CD36, most likely via its signal transduction capabilities, plays an important role in coordinating the incorporation of FA and cholesterol into TG for chylomicron production and appears required for assembly of the prechylomicron transport vesicle and its secretion from the ER. As a result, CD36 may mediate the effect of some regulatory influences on lipoprotein assembly like in the case of GLP-2.
II) CD36 in humans: deficiency and genetic polymorphisms
In humans, CD36 deficiency is relatively common (2-6%) in persons of Asian and African descent (38, 83) and is associated with abnormalities of plasma lipids (63, 148, 245). Single nucleotide polymorphisms (SNPs) in the CD36 gene influence susceptibility to the metabolic syndrome (134) and the risk of stroke (93) and are associated with alterations in the concentrations of serum FA (135) and TGs that in many cases reflect the effect of the SNP on CD36 protein levels (133).
The abnormal plasma lipids in humans with CD36 deficiency or with polymorphisms in the CD36 gene reflect at least in part abnormal peripheral clearance of plasma FA, since impaired tissue FA uptake has been documented in humans with CD36 deficiency (161, 213, 220). A contribution of defective lipid processing by the small intestine to lipid abnormalities is strongly suggested by findings of postprandial lipemia and of high levels of apoB48 in CD36-deficient subjects (111). A more recent study (142) showed CD36-deficient humans to have high levels of plasma TG, apolipoprotein B-48, free FA, and glycerol. The authors suggested that CD36 deficiency might increase atherosclerotic risk by enhancing plasma level of lipoprotein remnants due to intestinal secretion of particles that are smaller in size than chylomicrons (142).
B) IS FATP4 REQUIRED OR DISPENSABLE FOR INTESTINAL FA UPTAKE?
Among the various candidate intestinal FA transporters, FATP4 attracted considerable attention by virtue of its high expression in mammalian villus enterocytes and, by virtue of its endogenous acyl CoA synthetase (ACS) activity, the capacity to function not only in FA transport but also in the process of metabolic trapping of LCFA through conversion to the CoA derivative (198). It is worth noting that FATP4 is not strictly a brush-border transporter, since its location is confined to the ER and subapical membranes where it participates in FA uptake that is mediated through metabolic trapping of the acyl-CoA product mediated by its ACS activity (147). Unlike the native LCFA substrate, which is freely diffusible, fatty acyl CoA products are hydrophilic and undergo rapid metabolic conversion into complex lipid species (di- and triglycerides, phospholipids). Germline deletion of FATP4 led variably to embryonic lethality or perinatal death that was associated with thickened, abnormal skin that led to restricted breathing and fatal dehydration (152) and which complicated understanding of the role of intestinal FATP4. Rescue of the perinatal lethality was accomplished by keratinocyte-specific transgenic expression of FATP4 (Fatp4−/−; Ivl-Fatp4tg/+), which permitted an evaluation of the role of intestinal FATP4 since these “rescued” mice only expressed FATP4 in the skin, with no expression in the small intestine (151). However, there was no detectable effect of intestinal FATP4 deletion on any aspect of intestinal fat (cholesterol or triglyceride) absorption in the Fatp4−/−; Ivl-Fatp4tg/+ mice, no protection from high-fat diet-induced weight gain, or other evidence for a physiological role of intestinal FATP4 in dietary lipid transport (188). The findings do not imply that FATP4 is without a role in intestinal lipid transport, but strongly suggest that FATP4 appears dispensable for the process.
2. Monoglyceride transport into enterocytes
Digestion of dietary TG within the intestinal lumen yields 2-MG together with unesterified FA. Studies that examined 2-MG transport into Caco-2 cells as a model of enterocytes showed that uptake was a saturable function of the MG monomer concentration and exhibited sensitivity to trypsin digestion, implying that a membrane protein might be involved in mediating 2-MG uptake (153, 154). An interesting observation was that 2-MG inhibited cellular FA uptake while triolein (glyceryltrioleate), glycerol, diacylglycerol, and monooctanoate had no effect. This suggested that the transport of FA and 2-MG may be coordinated to optimize TG resynthesis inside the cell. Further support for this interpretation is provided by data showing that the metabolic fate of 2-MG in enterocytes, like that of FA, is dependent on the site of cellular entry (206). Esterification of 2-MG delivered in vivo either to the AP or the BL surface of rat and mouse intestine demonstrated differences that reflect the entry site. The ratio of TG to phospholipid (TG:PL) formed from 2-MG was ~10-fold higher for AP compared with BL delivery, findings that were qualitatively similar to those with FA (206). More studies are needed to understand what membrane proteins may facilitate uptake of 2-MG and how this may be linked to its metabolic processing.
3. Intracellular FA transport by cytosolic FA binding proteins and FA esterification
Once inside the small intestinal enterocyte, fatty acids are targeted and delivered to specific metabolic sites, a process which may be mediated by FA binding to small (~14 kDa) cytosolic fatty acid binding proteins (FABP). Two proteins of the FABP mutigene family are expressed in the intestine where they comprise 4-6% of the cytosolic proteins: liver-FABP (LFABP or FABP1) and intestinal-FABP (IFABP or FABP2) (12). The two FABPs were shown to deliver FA to membranes by different mechanisms, namely, diffusion versus collision for LFABP and IFABP, respectively (222). A collisional mechanism has been conclusively demonstrated for IFABP (204, 205). However, LFABP may also interact with membranes and in particular with ER membranes, where it helps initiate budding of lipid transport vesicles (157). The specific roles of LFABP versus IFABP in lipid absorption remain poorly defined, but are unlikely to be redundant based on the distinctive properties of the two proteins. While IFABP binds one FA molecule, LFABP binds two FA molecules in addition to its capacity to bind a variety of other lipids, including bile salts, lysophospholipids, cholesterol, acyl-CoA, and monoacylglycerol (MG) (79, 205).
Recent work using murine models with genetic deletion of LFABP or IFABP suggests a minor role of the two proteins in intestinal fat absorption but supports their involvement in the differential metabolic targeting of FA (114, 140, 159). Mice null for either protein have normal intestinal fat absorption, but LFABP null mice exhibit a reduction of chylomicron output (159), probably reflecting LFABP contribution to enterocyte chylomicron trafficking (157). There is evidence that LFABP may target FA toward oxidation while IFABP would favor FA incorporation into triacylglycerols. Fasted LFABP-null mice lose less lean mass while fasted IFABP-null mice lose more fat mass compared with wild-type mice (114).
Processing of FA by enterocytes is initiated by activation of the FA to its CoA derivative by the acyl-CoA synthetase enzymes specific for long chain (ACSL) or very-long-chain (ACSVL) FA (51). In the intestine, ACLS3 and ACLS5 are the major synthetases present, with lower expression levels found for ACSL1 (61, 163, 209). These ACSLs may contribute to the metabolic channeling of FA as there is evidence from studies in the liver or with hepatocyte cell models that ACLS3 delivers FA-CoA for phospholipid synthesis while ACLS5 delivers acyl FA for TGs, as reviewed in Reference 51.
The FA-acyl-CoA is transferred to MG to generate diacylgycerol (DG) by the MG acyltransferase (MGAT) enzymes. MGAT activity is important for TG absorption, since ~75% of the secreted intestinal TG may be synthesized from absorbed MG (4). Of the 3 MGAT isoenzymes, MGAT2 is expressed in both mouse and human intestine (249), while MGAT3 is also present in the human ileum (33). MGAT2 null mice absorb dietary fat, but the secretion of dietary TG into the circulation occurs at a reduced rate. This appears to favor more partitioning of dietary fat toward energy dissipation, which protects the mice from high fat induced obesity, glucose intolerance, and fatty liver (248).
The FA-acyl-CoA is transferred to DG to form TG by diacylglycerol acyltransferases (DGAT), the enzymes that catalyze the terminal step in TG synthesis (250). The intestine expresses two DGATs (DGAT1 and DGAT2). DGAT1 is a member of the same family as acyl-CoA cholesterol acyltransferase (ACAT) while DGAT2 is considered part of the same family as MGAT2 (250).
DGAT1 knockout mice are resistant to high fat induced obesity (197) and exhibit reduced chylomicron output (25), which is reversed by rescue of intestinal DGAT1 expression (120). DGAT1 contributes to the formation of a major fraction of the postprandial TG secreted by the intestine (32). Assigning a specific role for DGAT2 in intestinal fat absorption is challenging since the DGAT2 null mice die shortly after birth due to loss of skin barrier function and to markedly low nutrient levels (203). This phenotype reflects the importance of DGAT2 for TG synthesis in most tissues other than the intestine (203). DGAT2 localizes to a subdomain of the ER, and its active site is presumed to face the cytosol, in contrast to DGAT1 where the active site faces the ER lumen. DGAT2 is also present in mitochondria-associated ER membranes, subdomains that are highly enriched in lipid synthetic enzymes. The NH2 terminus of DGAT2 contains domains that promote its association with mitochondria and thus favor its subcellular localization (202).
B. Cholesterol and Sterol Transporters
Intestinal cholesterol homeostasis is regulated through an intricate balance of sterol uptake, absorption, and de novo synthesis, daily losses through shedding of enterocytes and through the regulated export of intestinal lipoproteins, principally chylomicrons. Evidence from in vitro studies, however, suggests that intestinal cholesterol transport may occur through an alternative pathway involving HDL formation, mediated through the efflux pump ABCA1 (FIGURE 2) (95). The functional importance of ABCA1 is illustrated through the loss-of-function phenotype in patients with Tangier disease, an autosomal recessive allele associated with mutations in ABCA1 that cause cholesterol accumulation in the intestine, spleen, and tonsils with virtually no detectable plasma HDL, because of defective cholesterol transfer to an extracellular apolipoprotein A-1 acceptor particle (86, 100). Advances in the understanding of cholesterol transporters and their role in the integrated regulation of intestinal and hepatobiliary cholesterol metabolism are discussed below.
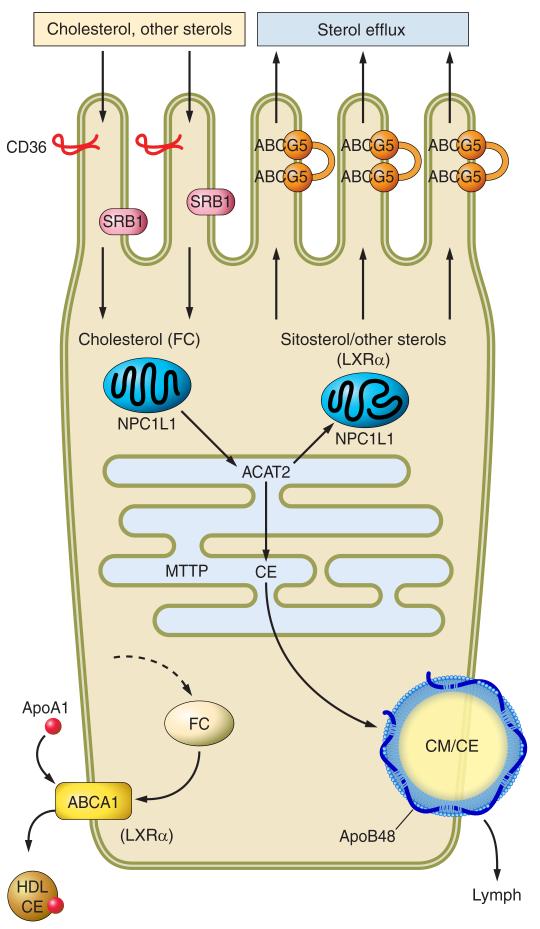
FIGURE 2.
Pathways regulating intestinal sterol absorption and homeostasis. Dietary/biliary cholesterol and other sterols are transported across the brush border principally via NPC1L1, a 13-transmembrane domain protein (shown only schematically here) with a sterol-sensing domain, that functions possibly in association with CD36. Note that the figure illustrates a conformational change in NPC1L1 upon sterol binding. NPC1L1 is expressed in subapical domains but not within the brush-border membrane and undergoes clathrin-mediated internalization following sterol binding, after which the sterol cargo is vectorially delivered to the endoplasmic reticulum for esterification via acyl cholesterol acyltransferase-2 (ACAT2). NPC1L1 then recycles back in proximity to the brush border. Sitosterol and other sterols are less effective substrates for ACAT2 and are preferentially secreted back into the intestinal lumen through the paired half-transporters ABCG5/G8. A portion of cytoplasmic intestinal free cholesterol is also selectively secreted back into the lumen through ABCG5/G8. An alternative pathway has been proposed to account for the portion of intestinal cholesterol excretion that operates independently of ABCG5/G8, referred to as transintestinal cholesterol efflux (TICE). The transporters involved and the metabolic source of cholesterol are unknown. Other apical transporters have been described, including SR-B1 and CD-36, but the significance of these two transporters in absorption of cholesterol and other sterols is yet to be established. Intestinal cholesterol ester (CE) is partitioned into distinct metabolic pools, including a dominant apoB48-dependent chylomicron pathway (CM) that requires microsomal triglyceride transfer protein (MTTP). Cholesterol, in the form of cholesterol ester (CE), can also be secreted via apoA1-dependent pathways, although the quantitative importance of this pathway is unknown. The metabolic pool of free cholesterol (FC) arises through de novo synthesis, from receptor-dependent lipoprotein uptake and also through mobilization of membrane free cholesterol. These pathways are summarized schematically by the dashed arrow. Intracellular free cholesterol from any of these sources may also enter the metabolic pool from which ACAT2 derives its substrate. In addition, membrane free cholesterol may be mobilized, along with phospholipid, through the export pump ABCA1, resulting in transfer to an extracellular apoA-I acceptor with the formation of discoidal HDL particles that enter the lymphatic circulation.
1. NPC1L1
The mechanisms by which sterols interact with brush-border membrane transporters are still debated, but evidence suggests that interaction with the NPC1L1 transporter is a key step (8). NPC1L1 was identified as a molecular target for ezetimibe, a pharmacological agent that inhibits intestinal cholesterol absorption by binding to the NPC1L1 transporter and blocking its sterol-dependent internalization via clathrin-coated vesicles (41, 66). NPC1L1 has 13 predicted transmembrane domains (reviewed in Ref. 15; FIGURE 2) that include a sterol-sensing domain in the NH2-terminal half of the protein, whose structure and function is proposed to be similar to that demonstrated in a homolgous sterol transporter, NPC1 (112). It is likely that conformational specificity for sterol binding (i.e., cholesterol versus plant and other sterols) resides within the sterol-sensing domain and that, following binding of cholesterol, there is a conformational change in the protein that in turn functions to direct its subcellular localization and intracellular transport (reviewed in Ref. 15). Recent information on human variants of NPC1L1 associated with low cholesterol absorption indeed suggests that a range of posttranslational modifications including alterations in glycosylation and recycling appear to influence the function of the protein (234). Other studies have demonstrated that the function of NPC1L1 is independent of caveolin-1, as evidenced by the finding that cholesterol absorption and NPC1L1 expression are unaltered in Caveolin1 (Cav1) null mice (229). More recent studies have provided insight into the mechanisms and pathways that regulate NPC1L1 trafficking in response to cholesterol binding, specifically the demonstration that the small Rho GTPase Cdc42 plays a key role in recycling activity in response to cholesterol binding (240). Other studies have demonstrated an important role for Flottilins, essential lipid raft components, in forming cholesterol-enriched domains that function with NPC1L1 in cholesterol delivery (65).
Endogenous NPC1L1 mRNA is expressed in murine proximal small intestine villi and at much lower levels in the liver (8). In contrast, human NPC1L1 mRNA is expressed at equivalent levels in the small intestine and liver as well as tissues not involved in cholesterol absorption such as ovary, lung, and muscle (8). Intestinal NPC1L1 expression is modulated in response to cholesterol feeding in mice (41), but details of its physiological regulation in humans are still to be defined. In contrast, emerging information suggests that NPC1L1 is expressed on the canalicular membrane in human liver and may play a role in the fine regulation of biliary cholesterol secretion (218). Intestinal NPC1L1 expression is regulated at least in part through peroxisome proliferator-activated receptor α (PPARα)-dependent pathways, as evidenced by the decreased expression of NPC1L1 and decreased cholesterol absorption in mice treated over several days with fenofibrate (228). The mechanisms that account for the reduced expression of NPC1L1 following fenofibrate treatment remain to be elucidated, particularly whether intestinal PPARα activation leads to modulation of other factors that in turn downregulate NPC1L1 expression.
Insight into the physiological importance of NPC1L1 in intestinal cholesterol homeostasis emerged from studies showing greatly reduced cholesterol absorption in NPC1L1−/− mice (40), a phenotype similar to wild-type mice treated with ezetimibe. In regard to sterol substrate selectivity, both sitosterol and cholesterol absorption were similarly impaired in NPC1L1−/− mice (40), although structural selectivity for NPC1L1 in phytosterol transport was inferred using a gain-of-function approach in hepatoma cells (21). While there was no evidence of overt triglyceride malabsorption in NPC1L1−/− mice (40), other studies have suggested a defect in saturated FA absorption in NPC1L1−/− mice (113). More recent work has demonstrated attenuated postprandial hypertriglyceridemia in wild-type and CD36−/− mice treated with ezetimibe, again suggesting that intracellular trafficking of LCFA and chylomicron secretion may be impaired (180). The implications for patients treated with ezetimibe, in whom there has been no evidence of intestinal TG malabsorption, remain to be determined.
As alluded to above, NPC1L1 undergoes a highly regulated intracellular itinerary (FIGURE 2), which is initiated following sterol uptake across the microvillus membrane, where-upon the liganded NPC1L1 is directed through a recycling endocytic compartment that eventually allows presentation of cholesterol as well as other sterols from the NPC1L1-sterol complex to the ER membrane (15, 66). Studies using intestinal organ explant cultures have demonstrated that cholesterol binding may induce the trafficking of NPC1L1 from domains close to the apical brush border towards endosomal compartments in a process that appears distinct from lipid rafts (195). Once delivered to the ER, sterols bound to NPC1L1, including cholesterol and other plant (sitosterol, campesterol, sitostanol) and shellfish sterols (brassicasterol), are then selectively esterified by acyl-CoA cholesterol acyl transferase 2 (ACAT2) (179). There are remaining questions regarding the role of NPC1L1 in intracellular cholesterol trafficking, however, as highlighted by recent studies demonstrating that the accumulation and transport of a fluorescent cholesterol analog (NBD-cholesterol) was similar in wild-type and NPC1L1−/− mice, suggesting that there may be an NPC1L1-independent pathway for intestinal trafficking of some sterol substrates (2). Sitosterol and other noncholesterol sterols are less effective substrates for ACAT2 (FIGURE 2) and are directed to the efflux pump ABCG5/G8 for resecretion back into the lumen. This efflux mechanism also accounts for excretion of at least some excess intracellular free cholesterol (FIGURE 2).
2. ABCG5/G8
The sterol export pump ABCG5/G8 is composed of a functional heterodimer (FIGURE 2), whose genes are organized in a head to head (i.e., 3′ to 5′ and 5′ to 3′) configuration and coordinately regulated by a shared promoter promoter (13, 122). Ho-modimeric complexes have not been demonstrated, and the functional transporter is normally composed of an obligate 1:1 heteromeric complex of ABCG5 and ABCG8, encoding a 12 membrane-spanning protein found in the brush-border membrane of villus enterocytes (72), the canalicular membrane of hepatocytes, and also the apical membrane of gallbladder epithelial cells (107). Most ABCG5/ABCG8 mutant proteins are retained in the ER rather than inserting into the apical membrane of the enterocyte (72). Cholesterol transporter gene expression is upregulated by dietary sterol intake, through production of oxysterol derivatives that signal through the orphan nuclear receptors LXRα and LRH-1 (60, 174). In addition, adaptive changes in cholesterol transporters were found in mice fed cholesterol-free high-fat diets and through pathways that are LXR independent (42). Plant sterols and stanol esters reduce cholesterol absorption in mice at least in part through upregulation of ABCG5/8 (174). However, species-specific differences emerged in other studies where ABCG5/G8 transporter mRNA was found to decrease following stanol ester supplementation in hamsters, and the decreased cholesterol absorption noted in these stanol ester supplemented animals occurred independent of changes in ABC transporter or NPC1L1 expression (56). Which of these two possible responses occurs in human intestine is unknown, but becomes relevant because of widespread use of plant stanol supplementation in commercial oils and fats. These findings suggest that intestinal cholesterol transport is regulated by multiple pathways and highlight the potential for therapeutic interventions. This suggestion is particularly relevant in terms of strategies that target LXRα in an intestine-restricted manner, since the gain-of-function phenotype in mice expressing the activated form of LXRα in the small intestine demonstrate reduced cholesterol absorption (through upregulation of ABCG5/G8), enhanced cholesterol efflux (through upregulation of ABCA1), and protection from atherosclerosis (FIGURE 2) (131). Those findings are consistent with earlier findings that LXRα/β double knockout mice have reduced cholesterol absorption, observations consistent with a role for LXR expression in the regulation of cholesterol absorption via an effect on ABCG5/G8 expression (228). It is worth noting that ABCG5/G8 knockout mice demonstrated a 3-fold increase in plant sterol absorption and a 30-fold increase in plasma sitosterol levels (251), suggesting that ABCG5/G8 functions in the regulated efflux of a range of sterols from enterocytes back into the intestinal lumen. These findings were confirmed in transgenic mice overexpressing ABCG5/G8, which demonstrated reduced cholesterol absorption, consistent with the predicted role of the apical heterodimer in promoting cholesterol efflux back into the lumen (252). Despite the array of evidence that modulation of ABCG5/G8 expression in murine intestine is associated with changes in cholesterol absorption and sterol efflux, the findings from human studies imply only a modest role for genetic variations at the ABCG5/8 locus as a contributor to population-wide variations in cholesterol homeostasis (97).
3. Regulation of intestinal sterol transporters and the role of transintestinal cholesterol export
Emerging evidence suggests that the small intestine plays an important role in the overall homeostatic regulation of cholesterol dynamics by regulating both cholesterol uptake and delivery to the systemic circulation as well as its regulated efflux back into the intestinal lumen. Elements of intestinal cholesterol uptake and efflux are therefore likely to represent key components of a homeostatic mechanism previously designated as reverse cholesterol transport (RCT). While the concepts of RCT date back several decades (232), there is great interest in the role of the intestine in facilitating cholesterol efflux from the body (186) and also in its role in the biogenesis of HDL as a carrier of cholesterol destined for selective excretion (105). Cellular free cholesterol (including intestinal cholesterol) may be mobilized for secretion through the actions of the basolateral efflux pump ABC1A1 (FIGURE 2), whose actions facilitate the transfer of membrane cholesterol to an apolipoprotein A-1 acceptor molecule with the net formation of discoidal HDL particles (100). A crucial role of the ABCA1 transporter in HDL formation was established by findings (alluded to above) in patients with Tangier Disease in whom mutations in the ABCA1 gene were associated with virtual absence of plasma HDL (100). As noted above, ABCA1 is a transcriptional target of intestinal LXRα, and it is possible that under certain circumstances (such as LXR agonist treatment), intestinal ABCA1 may play a role in intestinal cholesterol mobilization and HDL formation. However, there is conflicting data on the role of ABCA1 in cholesterol absorption from germline Abca1 knockout mice (144, 216), and the findings in intestine-specific Abca1 knockout mice showed no change in cholesterol absorption (22). Taken together, the findings suggest a minor role, if any, for ABCA1 in the physiological regulation of cholesterol absorption, although the expression of ABCA1 in the intestine plays a key role in HDL biogenesis (23). As alluded to above, HDL biogenesis is regulated in mice by LXR agonist administration, and work has demonstrated that this may further represent a viable strategy to promote cholesterol efflux by attenuating cholesterol absorption (131, 247). In this regard, recent studies have suggested that cholesterol absorption per se is required for the LXR agonist-mediated increase in intestinal HDL biogenesis, because Npc1l1−/− mice (where cholesterol absorption was greatly decreased) were found to demonstrate no increased HDL biogenesis upon administration of an LXR agonist (214). Those findings imply a role for luminal cholesterol delivery, likely via NPC1L1-mediated pathways, in providing the substrate for ABCA1-mediated HDL biogenesis. Further study will be required to resolve the metabolic compartmentalization of intestinal cholesterol in relation to HDL biogenesis and reverse cholesterol transport.
In regard to other potential transport pathways for cholesterol absorption, the HDL receptor SR-B1 was also considered a candidate gene in regulating intestinal cholesterol uptake, but Srb1−/− mice demonstrated no change in intestinal cholesterol absorption from their wild-type controls (7). The multifunctional ligand receptor CD36 is expressed on the microvillus membrane of mammalian enterocytes and has been implicated in intestinal lipid transport and in the regulation of NPC1L1 expression (155). Cd36−/− mice, however, exhibited no alteration in intestinal cholesterol absorption compared with wild-type controls (despite upregulation of NPC1L1 expression), suggesting a complex role in regulating FA versus cholesterol transport, which remains to be fully understood (156, 158). Furthermore, mice with combined deletion of both Srb1 and Cd36 (Srb1−/− Cd36−/−) also exhibited no decrease in cholesterol absorption (160), suggesting that neither transporter is rate limiting for intestinal cholesterol uptake under physiological conditions. Interestingly, findings in humans support a role of CD36 in cholesterol homeostasis. Multiple polymorphisms in the CD36 gene exhibited strong association with plasma HDL which were inversely related to CD36 protein levels determined on monocytes (133, 134). CD36 may influence cholesterol flux by an effect on translocation of membrane phospholipid or by altering localization/function of ABCA1 (253). A role for ACAT2 in intestinal cholesterol transport emerged from studies in ACAT2 knockout mice where mice were protected from diet-induced hypercholesterolemia and gallstones, in association with reduced cholesterol absorption (24). Other studies have demonstrated that a range of adaptive mechanisms function to limit cholesterol absorption and intestinal cholesterol accumulation in ACAT2 knockout mice (including decreased NPC1L1 expression and upregulation of ABCG5/G8), which together promote cholesterol efflux (227). Finally, in regard to the dominant pathways that regulate intestinal cholesterol transport, it is worth noting that most dietary cholesterol is transported in the form of chylomicrons and, as will be discussed in a later section, blocking chylomicron assembly results in the virtual elimination of cholesterol absorption (243).
There is yet another possible pathway for cholesterol efflux through the intestine, whose molecular and biochemical characterization is still very much in progress. This pathway, designated “transintestinal cholesterol export” (TICE), has been implicated in reverse cholesterol transport, through studies in mice that have utilized intestinal perfusion to examine sterol efflux across the intestine (217, 231). Those studies suggest that a luminal acceptor particle (bile salt plus phospholipid micelles) facilitates net efflux of cholesterol from the blood to the intestinal lumen (230). Studies have demonstrated that TICE is independent of ABCG5/G8, suggesting that this particular apical cholesterol efflux pathway is not required, while other studies demonstrated that TICE was increased in Srb1−/− mice (230, 231). It is important to emphasize that details of the transporter(s) involved, the lipoprotein carriers implicated, and the physiological regulation (if any) of this plasma-to-enterocyte cholesterol efflux pathway are yet to be determined. The observation that biliary cholesterol secretion is not required for the augmented efflux of cholesterol (217) challenges the concept that biliary cholesterol secretion is required for reverse cholesterol transport (at least in mice), but the implications remain to be tested in humans. Other recent studies have demonstrated a key role for intestinal LXR activation in reverse cholesterol transport (131). These studies demonstrated augmented cholesterol secretion of macrophage cholesterol into both bile and feces (reflecting upregulation of ABCG5/G8) along with decreased cholesterol absorption in mice expressing a constitutively active LXR in small intestinal (but not liver) epithelium (131).
III. LIPOPROTEIN ASSEMBLY, VECTORIAL TRANSPORT, AND SECRETION
Following lipid uptake (FA, cholesterol) across the brush-border membrane, there exist dominant genetic restriction points that regulate intestinal lipoprotein assembly. Fatty acids, their CoA derivatives, monoglyceride, and sterols are transported by a network of transporters and metabolically regulated pathways (outlined above) that ultimately provide the substrate for complex lipid biosynthesis and the assembly of lipoproteins within the enterocyte. These metabolic pathways intersect at the apical domains of the ER and involve crucial interactions between apoB and MTTP that facilitate complex lipid delivery into the secretory pathway (FIGURE 3). ApoB is the requisite surface structural protein that facilitates the formation of lipoproteins within the ER. MTTP promotes lipoprotein biogenesis by shuttling neutral lipid from within the bilayer of the ER to an acceptor apoB molecule. The current concept is that both apoB and MTTP are obligate requirements for intestinal lipoprotein biogenesis. Genetic mutations or pharmacological inhibition of either protein greatly attenuates the formation of lipoproteins. Key to the mechanisms regulating intestinal lipoprotein biogenesis is a detailed understanding of the tissue-specific production of distinctive isoforms of apoB, the result of posttranscriptional RNA editing. There also exists an important metabolic partitioning of complex lipid between their sites of formation within the bilayer of the ER membrane and lipid droplets within the cytosol and the ER lumen. Finally, lipoprotein biogenesis involves maturation and transport of the prechylomicron transport vesicle from the ER to Golgi compartments and eventually vectorial delivery of a mature chylomicron particle for secretion into the lymphatic circulation.
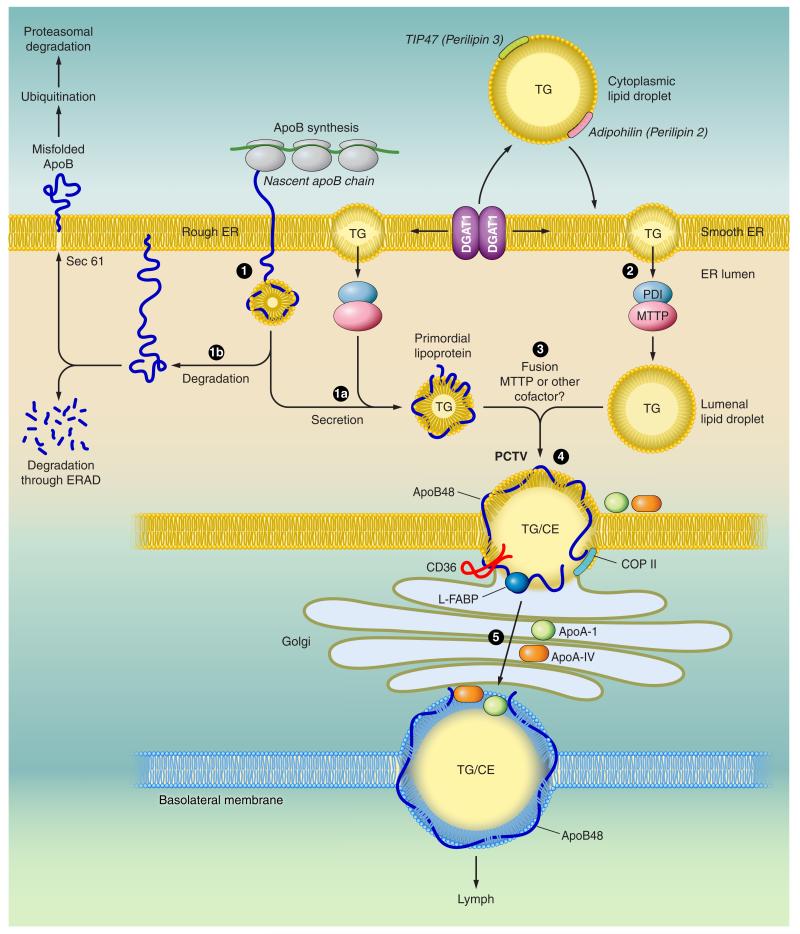
FIGURE 3.
Model of intestinal triglyceride-rich lipoprotein assembly. The nascent apoB polypeptide is cotranslationally translocated across the rough endoplasmic reticulum (ER) membrane (1). The membranes of the ER are shown in yellow. When lipid (triglyceride, TG) is available, physical interactions between the NH2-terminal domain of apoB and the microsomal triglyceride transfer protein (MTTP) promote optimal folding and direct biogenesis of a primordial lipoprotein particle (1a). MTTP exists as a heterodimeric complex with the chaperone protein protein disulfide isomerase (PDI). MTTP also promotes mobilization of triglyceride-rich lipid droplets from the bilayer of the adjacent smooth ER into the lumen of the ER to become luminal lipid droplets (2). When lipid availability is limited or MTTP function is impaired, nascent apoB becomes misfolded (1b) and is degraded, either within the ER lumen via ER-associated degradation pathways (ERAD), or the misfolded protein undergoes ubiquitination and is degraded by the proteasomal pathway. During the second step of lipoprotein assembly, the primordial lipoprotein particle fuses with endoluminal lipid droplets, resulting in prechylomicron transport vesicle (PCTV) formation (3). Other proteins, including CD36 and L-Fabp, also participate in PCTV formation. After fusion with key vesicular transport proteins, including COP II proteins, the prechylomicron particles are incorporated into a vesicular complex that buds from the ER and fuses with Golgi membranes. Vectorial transport of these prechylomicron particles (4) results in their continued maturation and eventually secretion (5) of the mature, nascent chylomicron particles into the pericellular spaces adjacent to lymphatic fenestrae. The basolateral membrane (shown schematically with a mature chylomicron traversing into the lymphatic fenestrae) is illustrated in blue.
A. ApoB and MTTP: Functional Domains Involved in Lipoprotein Biogenesis
The regulated assembly of chylomicrons brings together apoB and complex lipids in a fixed temporal sequence, followed by progressive maturation in the ER with vesicle formation and fusion steps involving Golgi membranes (FIGURE 3). The assembly of apoB-containing lipoprotein particles begins with translocation of nascent apoB into the ER lumen while still attached to the polysome and before translation of apoB48 is complete (57, 168). The NH2 terminus of apoB contains almost all the N-glycosylation sites as well as a region for physical interaction with MTTP (89, 98). In addition, the α1 domain contained within apoB48 contains seven of the eight paired disulfide bonds and plays a critical role in the optimal folding of hydrophobic domains of apoB mediated by physical interactions with MTTP (91). The first step in lipoprotein biogenesis thus occurs with transmembrane forms of the nascent apoB peptide undergoing partial lipidation and organization into a primordial particle within the rough ER. This primordial particle is further modified through a second-step “fusion” process that requires MTTP, resulting in lipid enrichment and expansion of the lipoprotein particle. Accordingly, the size range of lipoprotein particles varies depending on lipid availability and the size of lipid droplets within the smooth ER (27). These initial steps in apoB-containing lipoprotein biogenesis likely also govern the number of lipoprotein particles produced and may be influenced by physiological variations in apoB gene expression, the rates of apoB synthesis, and the rates and efficiency of secretion of the newly synthesized apoB protein. Genetic defects associated with abnormal apoB are clinically heterogeneous, but most cases are caused by nonsense and frameshift mutations in the APOB gene that lead to formation of COOH-terminal-truncated apoB (181). The population frequency of APOB gene mutations causing truncated apoB proteins is ~1:3,000, but by clinical criteria heterozygous FHBL may occur in 1:500-1: 1,000, although homozygous FHBL is very rare (215).
MTTP is a 97-kDa heterodimeric protein found in complex with the resident ER chaperone protein disulfide isomerase (90) (FIGURE 3). MTTP is most abundantly expressed in small intestinal enterocytes and hepatocytes (90); in cardiomyocytes (11), placenta (136), retinal (124), and proximal renal tubular epithelial cells (108); and also in CD1 restricted T cells, where there is no apoB and presumably no lipid export (44). Mammalian MTTP principally transfers triglycerides to facilitate optimal folding of nascent apoB, but also shuttles other lipid classes such as cholesteryl esters, free cholesterol, and phospholipids to further promote lipoprotein formation (173). In the case of CD1 restricted T cells, MTTP transfers lipid antigens to a variety of CD1 molecules and may play an important evolutionary role in antigen presentation, since human subjects with genetic deficiencies in MTTP exhibit impaired activation of CD1 restricted T cells and invariant natural killer T cells (44, 254). MTTP is necessary and (with apoB) sufficient for lipoprotein formation and secretion and confers lipid secretion capability to non-lipoprotein-secreting cell lines when coexpressed with lipid-binding competent forms of apoB (98). In contrast, pharmacological MTTP inhibition (110), or conditional genetic deletion, blocks both apoB and lipoprotein secretion from hepatocytes (171, 172) and enterocytes (243). In humans, homozygous or compound heterozygous truncating or missense mutations in the MTTP gene result in abetalipoproteinemia (14), a rare disorder characterized by extremely low TG and cholesterol levels in serum and lipid droplet accumulation in the liver and small intestine. Intestinal MTTP exhibits diurnal regulation in concert with the diurnal changes in plasma TG levels (166), which involve CLOCK genes and the transcriptional repressor SHP (short heterodimeric partner) (167). These findings suggest that both nutritional and visual cues work in concert to accommodate rapid fluxes in dietary lipid availability. Other findings demonstrated a role for leptin signaling in the regulation of intestinal MTTP, involving leptin receptor and melanocortin 4 receptor pathways (96). A theme of emerging importance in lipid homeostasis concerns the regulation of ER stress (176). Recent studies demonstrated that interruption with one of the effector limbs of the unfolded protein response (176), specifically using inositol requiring enzyme 1β knockout mice, revealed increased intestinal MTTP expression and augmented lipid mobilization, along with increased chylomicron secretion (94).
B. Requisite Structural Components for Intestinal Lipoprotein Biogenesis: Molecular Isoforms of ApoB
ApoB is the requisite scaffold for intestinal TG-rich lipoprotein formation, whose regulation is coordinated in a developmental and tissue-specific manner. The dominant form of apoB expressed in the small intestine is referred to as apoB48. Mammalian APOB gene transcription yields a ~14 kb mRNA, in which C to U RNA editing, mediated by apobec-1, introduces a translational stop codon into the nuclear transcript and directs production of apoB48 in the small intestine (17). The unedited apoB mRNA (found in human liver, which does not express apobec-1) encodes apoB100, while mouse and rat liver (as well as other species that express apobec-1 more widely) synthesize both apoB100 and B48 (17, 74). Intestinal apoB mRNA editing is mediated by a heterodimeric complex containing apo-bec-1, the catalytic deaminase and an obligate RNA binding subunit, apobec-1 complementation factor (ACF) (123, 145, 219). The tissue-specific distribution of apoB mRNA editing reflects the tissue- and cell-specific distribution of apobec-1, which is expressed in enterocytes and subepithelial cells throughout the luminal gastrointestinal tract (68, 76). ACF is predominantly expressed in the liver, kidney, and intestine, but low levels are found in almost all tissues in humans, rats, and mice (39, 48, 82). Apobec-1 and ACF shuttle between nuclear and cytoplasmic compartments (19, 34), but whether ACF and apobec-1 shuttle independently or together has yet to be resolved. Recombinant apo-bec-1 and ACF can each be isolated as homodimers (64, 117), and in addition, ACF forms multimers in complex with apoB RNA (64).
Apobec-1 mediates C to U deamination of a cytidine base in apoB RNA, which is embedded in an A+U rich context, raising the question of whether alternate targets (beyond apoB RNA) exist. Earlier work identified a canonical apo-bec-1 binding site containing an UUUN[A/U]U motif (9), which has been confirmed in transcriptome-wide RNA sequencing of intestinal RNA from the Apobec-1−/− mice, which revealed other RNA targets for C to U editing, all within 3′ untranslated regions (UTRs) (177). The physiological relevance of apobec-1-mediated RNA editing in these 3′ UTRs remains to be defined. Other work has examined the parallel possibility that alternate (non-apoB RNA) targets exist for ACF, particularly in view of the observation that germline deletion of ACF is early embryonic lethal (18). These findings were extended recently with the finding that ACF modulates cytokine (specifically interleukin 6) mRNA stability (20). Taken together, the findings suggest that apobec-1 and ACF each have a range of targets distinct from apoB mRNA, whose biological implications have yet to be formally defined.
There are functional consequences for intestinal lipid absorption of the different apoB isoforms associated with alterations in apoB RNA editing. Apobec-1−/− mice (which express only apoB100) absorb TG over a longer time interval than wild-type (apoB48) controls, and apoB100-containing chylomicron are paradoxically larger than those from wild-type (i.e., apoB48-expressing) controls (130, 242). These findings suggest that intestinal chylomicron production in an apoB100 background results in fewer, larger particles and less efficient intestinal TG delivery than wild-type (apoB48) controls (130). A major consequence of eliminating intestinal apoB RNA editing, however, emerged from the striking intestinal lipotoxicity following compound genetic deletion of intestinal Mttp in the Apobec-1−/− background with an exaggerated unfolded protein response and death (241). Considered together, the findings suggest that intestinal apoB mRNA editing, and the production of apoB48, is a critical adaptation for accommodating high fat intake or under conditions where intestinal MTTP abundance may be limiting (210).
C. ApoB Degradation Pathways and the Regulation of Lipoprotein Biogenesis
As modeled in hepatoma cell lines, primary murine hepatocytes, and enterocyte cell culture experiments, several distinctive pathways have emerged to account for apoB degradation, including ER-associated degradation (ERAD), autophagy (59), and the proteasomal pathway (57, 58) (FIGURE 3). The proteasomal degradation pathway involves ubiquitination of the nascent apoB polypeptide, typically due to misfolding as a consequence of insufficient lipidation or defective MTTP function (125). The misfolded apoB100 undergoes retrograde transport via a Sec61-mediated mechanism across the ER membrane (dislocation) and is ubiquinylated and targeted for degradation (57, 58). Studies in Caco-2 cells also demonstrate that intracellular apoB degradation occurs when MTTP activity is blocked using pharmacological inhibitors (126). Other studies revealed extensive apoB100 degradation (range 70-90%) in enterocytes from Apobec-1−/− mice, particularly with conditional intestinal Mttp deletion (241-243). A small fraction of apoB48 in the enterocytes of wild-type mice is degraded, but to a lesser extent (~30%) than noted for apoB100 (242, 243). Similarly, apoB48 is more efficiently secreted than apoB100 and appears less susceptible to presecretory degradation in murine hepatocytes (110). Thus, despite the shared NH2 terminus between apoB48 and apoB100 and the requirement for physical interaction with MTTP to initiate lipoprotein biogenesis, the findings suggest that apoB48 undergoes translocation more efficiently than apoB100 and requires less lipidation (via MTTP) to become secretion-competent (187).
D. Lipid Droplet Formation and Metabolism
One of the cardinal features of intestinal lipid homeostatic function is the presence of cytoplasmic lipid droplets, whose emergence as metabolically active structures in other tissues has stimulated interest in their metabolic origin and fate within enterocytes (54). TG-rich lipid droplets are formed within the ER bilayer, synthesized by DGAT, especially DGAT-1, which is located within the ER membrane (250) (FIGURE 3). Mice that lack DGAT1 (Dgat1−/− mice) have no obvious defect in dietary fat absorption yet are protected against high-fat diet-induced obesity, at least in part due to increased energy expenditure (25). However, Dgat1−/− mice also exhibit a reduced surge in postprandial plasma TG levels and accumulate numerous large cytoplasmic lipid droplets (25). This phenotype was rescued in transgenic mice that express Dgat-1 only in enterocytes (Dgat-1IntONLY), where over-expression of DGAT1 resulted in normalization of postprandial TG accumulation in plasma and reversal of the protection against high-fat diet-induced obesity along with a decrease in the accumulation of cytosolic lipid droplets (120). The results of these studies suggest that DGAT1 stimulates and promotes TG mobilization from enterocytes and in turn plays a key role in whole body energy homeostasis. Detailed understanding of the mechanisms for intestinal cytoplasmic lipid droplet formation and mobilization awaits further study, but recent findings have emphasized the dynamic formation of lipid droplets in vivo following lipid administration along with demonstrating the presence of perilipin proteins in association with these droplets (FIGURE 3) (121, 256). Studies suggest that perilipin 2 (Adipophilin) and perilipin 3 (TIP47) play distinctive roles in lipid droplet formation and metabolic compartmentalization, specifically in regard to storage versus rapid mobilization with perilipin 2 coating lipid droplets in response to sustained high-fat intake while perilipin 3 but not perilipin 2 coats lipid droplets following an acute lipid challenge (121).
Findings suggest that MTTP plays a critical role in extracting TGs from the ER bilayer membrane (110, 172) into lipid droplets within the ER lumen and ultimately promotes fusion with primordial lipoprotein particles to allow their progressive lipid engorgement (FIGURE 3). These conclusions are in accord with earlier findings from electron microscopic observations showing tubules and vesicles of a typical hepatocyte smooth ER did not contain apoB but contained lipid droplets, suggesting that the TG-rich lipid droplets originate from the smooth ER and fuse with apoB synthesized within the rough ER (6). These findings were extended in electron microscopic studies of intestinal apoB-deficient mice, which showed that even without apoB synthesis there were large lipid particles present in the ER lumen (80). These lipid droplets were on occasion even larger than chylomicron-sized particles, from enterocytes of wild-type mice. In other words, the ability of the enterocyte to synthesize apoB does not affect the formation of large lipid droplets per se in the ER lumen, but rather plays a critical role in their mobilization and secretion.
The role of MTTP in the formation of intraluminal ER-associated lipid droplets was further established in hepatocytes from liver-specific MTTP-deficient (Mttp-LKO) mice (172). Mttp-LKO mice accumulated large cytosolic TG droplets, reflecting the block in VLDL assembly. Similarly, intraluminal lipid droplets were also absent from the rough and smooth ER in Mttp-LKO hepatocytes compared with wild-type controls (172). Indistinguishable findings were elicited in enterocytes from intestine-specific MTTP-deficient mice (Mttp-IKO), where enormous cytosolic lipid droplets were present in the apical cytoplasmic compartment but no lipid droplets were found within profiles of the ER or Golgi, suggesting a crucial role for MTTP in lipid droplet formation and partitioning between cytosol and ER (243). In summary, intestinal lipoprotein biogenesis requires two simultaneous events (both considered part of the so-called “first step” of lipoprotein biogenesis), namely, cotranslational lipidation of the nascent apoB polypeptide and MTTP-mediated accumulation of luminal lipid droplets. The first of these events requires both apoB and MTTP, while the second event requires only MTTP.
E. Distal Elements of Chylomicron Formation
Once the initial steps of chylomicron formation are completed, the nascent intracellular prechylomicron particles leave the ER and are transported through the secretory pathway and further modified. The COPII machinery plays a role in vesicular budding and transport of ER cargo destined for secretion through the Golgi apparatus (137). Studies using rat enterocytes demonstrated the sequential interaction of heterodimeric COPII proteins (Sec 23/24 and Sec 13/31) to be required for budding of prechylomicrons from ER to the Golgi apparatus (190, 191). Interestingly, liver fatty acid-binding protein (l-FABP) promotes budding of prechylomicron transport vesicles (PCTV) from the ER, with decreased budding and fusion in enterocyte extracts from l-Fabp−/− mice (157). PCTV budding was also impaired in intestinal extracts prepared from CD36−/− mice, suggesting a role for this multifunctional receptor (190). These findings together are of interest since the kinetics of intestinal TG secretion were significantly slower in l-Fabp−/− mice and in CD36−/− mice, suggesting an important functional counterpart to the kinetic delays in PCTV formation (46, 47, 159). Studies in intestinal extracts suggest that COPII proteins are required for the step involving Golgi fusion but not at the step of ER budding and formation of the prechylomicron transport vesicle (190). On the other hand, studies in a rat hepatoma cell model demonstrated that a functional COPII complex was required for ER budding of the nascent apoB100-VLDL particle (75). The reasons for this apparent discrepancy in the role of COPII proteins in ER export of TG-rich lipoproteins is not known but may reflect the different models examined. From the perspective of human disease, an integral component of the COPII machinery, SAR1B, a member of the Sar1/Arf family of small GTPases, has been implicated in defective chylomicron formation (189). Mutations in the SARA2 gene, encoding SAR1B, are associated with Anderson/chylomicron-retention disease (CRD) (99), in which enterocytes assemble prechylomicrons in the ER but fail to transport them through the secretory pathway, causing intestinal lipid droplet accumulation (28, 30, 99, 189). In addition, compensatory increases in SAR1A that have been shown to accompany defective SAR1B expression in some CRD subjects fail to complement the functional block in intestinal lipid mobilization despite 90% sequence identity (67). CRD subjects have no defect in hepatic VLDL secretion, suggesting that the mechanisms of lipid droplet formation and mobilization in enterocytes are distinct from that in hepatocytes. Whether this observation reflects a tissue-specific defect or an effect mediated by the intrinsic difference between apoB48 versus apoB100 is currently unknown.
IV. LCFA SENSING BY THE GUT AND ITS SIGNALING FUNCTIONS
The homeostatic mechanisms that maintain body weight in the face of fluctuating energy rely on a complex network of afferent and efferent signals that connect various metabolic organs to the hypothalamus and brain stem. Afferent signals from the gastrointestinal tract are transmitted to the brain via the blood or vagal afferent fibers. Dietary fat in the small intestine is associated with suppression of the secretion of ghrelin (55), a hormone with positive effects on appetite and food intake at the same time it stimulates secretion of a number of intestinal peptides that promote satiety and reduce intake (5, 106; FIGURE 4) as discussed in following sections. Fat-induced suppression of ghrelin requires digestion, but it is unclear whether it is mediated by LCFA (55).
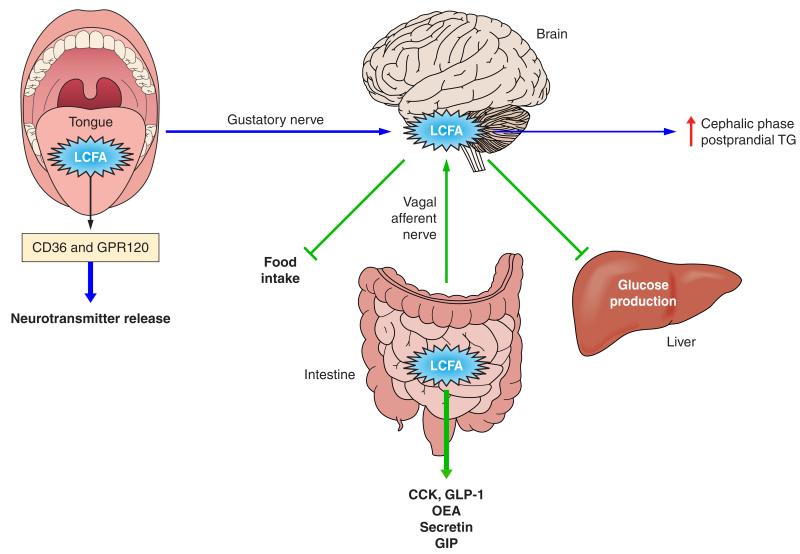
FIGURE 4.
Signaling functions of LCFA. Dietary triglyceride hydrolysis during digestion releases LCFA that have important signaling functions at various levels of the digestive tract. In the oral cavity, LCFA receptors (CD36, GPR120) present on the apical surface of taste bud cells in the tongue (49, 102) contribute to fat taste perception which associates with secretion of neurotransmitters, signal transmission to brain centers, and induction of the early cephalic phase of digestion (102) (blue arrows). In the intestinal lumen, dietary lipids have satiety effects (green arrows) mediated by LCFA signaling to release peptides with inhibitory effects on food intake (GLP-1, CCK, OEA). GLP-1 and CCK also delay gastric emptying and lipid absorption (23). GLP-1 and GIP enhance insulin release from the pancreas and glucose metabolism (10). Lipid sensing in the proximal gut mediated by accumulation of long-chain fatty acyl-coenzyme A also activates an intestine-brain-liver axis to inhibit glucose production by the liver (194).
Dietary fat intake has been shown to contribute to obesity in a manner related to both its quantity and composition, with the gut playing an important role in this contribution. The gut links fat sensing to the regulation of food intake and energy utilization. This section will be restricted to high-lights of the major concepts involved, and the reader is referred to a number of excellent reviews that delve at length into this topic (115, 143, 199, 201).
In the case of dietary fat, the nutrient that is sensed by the digestive system is LCFA, which have the capacity for cellular signal transduction and are generated from intraluminal digestion of TGs. In regard to FA sensing, several studies have demonstrated the presence of membrane receptors (149, 207). Studies in rats have demonstrated that it is oral sensing of free fatty acids rather than TG that may be involved (101). Those findings were confirmed more recently in humans (169), with the data showing that inhibition of lingual lipase, which releases FA from TG in the oral cavity, reduces preference for TG but not for FA. LCFA are recognized on the cell surface by membrane receptors that include CD36 (discussed earlier) and a number of G protein-coupled receptors (GPRs) (162, 182, 211).
A. GPRs for FA, CD36, and Fat Perception
The GPRs are members of a large family of receptors possessing several transmembrane segments that can activate heterotrimeric G proteins to stimulate activation of signal transduction pathways (84). As shown in TABLE 1, LCFAs can activate GPR40 and GPR120, while GPR84 responds to medium-chain FAs and GPR41 and GPR43 to short-chain FA (84).
Table 1.
Specificity of various fatty acid receptors expressed in the gut
| Receptor | Fatty Acid Specificity | Distribution |
|---|---|---|
| GPR40 | Medium and LCFA | Taste bud cells; K, L, I, and S cells |
| GPR120 | Medium and LCFA | Taste bud cells; I and L cells |
| GPR119 | OEA | L and K cells |
| GPR41 | Short-chain fatty acid | L cells |
| CD36 | LCFA | ? |
LCFA, long-chain fatty acid; OEA, oleoyl ethanolamide.
CD36 was identified on the apical surface of taste bud cells in the tongue (62, 119) where it was shown to contribute to fat taste perception/preference and to the early cephalic phase of digestion (119, 138). These events are mediated by a signaling cascade triggered by LCFA binding to CD36, which involves the CD36-associated src kinase fyn, resulting in neurotransmitter release and signal relay to the central nervous system (50). Mice taste buds express both CD36 and GPR120, but only CD36 is responsive to regulation by food intake, which produces parallel reductions in CD36 level and in preference for FA (139). Consistent with this, the 50% reduction of CD36 expression in mice heterozygous for CD36 deficiency was associated with a significant blunting of fat preference (139). The possibility that these findings may apply to humans has been recently suggested by the observations that human taste buds express CD36 (193) and humans carrying a genetic polymorphism which associates with reduced CD36 levels have a severalfold lower sensitivity for perceiving the taste of oleic acid (169).
High fat feeding downregulates CD36 expression on taste buds, and it has been proposed that this would increase fat consumption as more fat is needed for a satisfactory fat taste (255). In the small intestine, CD36 contributes to fat-induced satiety by providing oleic acid for production of the messenger OEA, which prolongs the intermeal interval (183). OEA, in turn, increases intestinal CD36 expression and FA uptake (246). Although this might suggest that CD36 deficiency would enhance food intake, studies demonstrate that food intake was decreased in CD36-deficient mice (77), possibly a postingestive effect (185) of delayed fat absorption as discussed in earlier sections.
GPR40 and GPR120, which bind LCFA (TABLE 1), have been implicated in fat taste perception, and mice null for these receptors exhibit reduced sensitivity to FA (26). Whether these receptors also play a role in fat taste perception in humans remains unexplored. There is a wealth of studies to document that humans can detect various FA in the oral cavity and that this triggers the cephalic digestive response possibly influencing subsequent absorption (143). There is also large interindividual variability in FA perception, which may play a potentially important role in food intake and obesity (200). The identification of taste receptors for FA should allow a mechanistic examination of the steps involved in fat perception and how they influence fat preference and digestion in humans.
In addition to influencing perception and possibly preference for fat, as already alluded to, an important physiological function of taste bud LCFA receptors is to prepare the digestive system for fat intake to optimize absorption. Taste perception triggers the first phase of the digestion (i.e., cephalic phase, FIGURE 4), which is characterized by an increase of pancreatic secretion and a rise in serum TG (138, 143). The role of neural input from taste receptors to facilitate absorption was demonstrated in early studies (146) and later firmly linked to higher levels of postprandial TGs in the blood (143). The increase in serum TG in response to oral fat detection appears to reflect stimulation of intestinal chylomicron secretion and may be influenced by enterocyte lipid stores (31).
Fat-specific cephalic phase responses also include secretion of lipases; transient increases of intestinal cholecystokinin (CCK), pancreatic polypeptide (PP), and PYY; and the release of insulin (143, 201). As demonstrated for CD36 (104), gustatory nerves generate central signals to initiate secretions that prepare the organism for absorption of the fat and its peripheral utilization.
In humans and rodents, there is an association between taste sensitivity to FA, fat intake, and susceptibility to obesity (143, 201). It is suggested that excess fat intake that is prevalent among obese subjects could result in attenuated sensitivity to LCFA, which would encourage even more fat consumption to reach satisfactory taste perception.
B. Intestinal FA Sensing and Lipid Absorption
In addition to the oral cavity, LCFA receptors are distributed throughout the gastrointestinal tract (162) where they may act to coordinate absorption with nutrient processing and to trigger the release of gut peptides (116) (TABLE 2). As discussed in a previous section, CD36 is abundantly expressed in the proximal intestine and contributes to processes that optimize chylomicron formation, probably operating at the very beginning of digestion when luminal FA is still low (223). The small fraction of FA uptake that occurs early and the signal transduction triggered by FA interaction with CD36 appear important to initiate TG packaging into lipoproteins (223). Interestingly, both CD36 (109) and GPR120 signal to ERK1/2 (85), which might be a convergence point for receptor-mediated FA signaling. However, the distribution patterns of CD36 and GPR120 in the intestine do not overlap. GPR120 is not detectable in the proximal intestine, where CD36 is prominently expressed, while it is abundant in the colon and to a lesser extent the ileum (85), locations where epithelial CD36 expression is relatively low (132). This would suggest that these two receptors might act in parallel rather than cooperatively in FA-mediated signaling in the intestine.
Table 2.
Enteroendocrine cell peptides that are induced by fatty acids
| Cell | Peptide | Most Abundant Site | Fat Type | Fatty Acid Receptor |
|---|---|---|---|---|
| K | GIP | Duodenum/jejunum | LCFA (10) | GPR40, 119, 120 |
| S | Secretin | Duodenum/jejunum | LCFA (35) | |
| I | CCK | Duodenum/jejunum | LCFA (127) | GPR120 |
| L | PYY | Ileum/colon | ShFA (129) | GPR41, 43 |
| GLP-1 | LCFA, OEA (10) | GPR40, 119, 120 |
Reference numbers are given in parentheses.
LCFA released from fat digestion in the intestinal lumen influences secretion of a number of intestinal peptides that modulate the digestive process (49, 149, 201) (TABLE 2). Among the most studied in the context of responsiveness to LCFA are CCK and GLP-1. Enteroendocrine cells in the intestine are the primary chemosensors and constitute <1% of the epithelial cell population (TABLE 2). These cells express a number of FA receptors including the LCFA receptor GPR40 and GPR120, and upon stimulation secrete GLP-1 and CCK (49, 149, 201). CCK is secreted by enteroendocrine cells (I cells), which are found in the mucosa of the duodenum, jejunum, and proximal ileum. CCK is important for optimizing fat digestion as it plays a key role in regulating gastric emptying, gallbladder contraction, pancreatic secretion, and intestinal motility (29). Secretion of CCK is stimulated by LCFA (129), although the mechanisms underlying this effect are only partially understood. Studies in the enteroendocrine cells STC-1 showed that fatty acids of at least 12-carbon length stimulate CCK secretion by these cells via two separate pathways, one involving FA monomers in solution and one involving FA aggregates (102). The two LCFA receptors GPR120 (212) and GPR40 (127) have been implicated in mediating the effect of FA on CCK. In the case of GPR40, using fluorescence-activated cell sorting to isolate I cells from the duodenal mucosa of mice, it could be shown that I cells that express GPR40 respond to linoleic acid addition with increases in intracellular calcium and CCK release, and these are reduced in I cells from GPR40 null mice. In vivo, secretion of CCK in response to oleic acid is also reduced in these mice (127). In addition to CCK, LCFA stimulate S and K cells present in the duodenum and jejunum to release secretin and glucose insulinotropic peptide (GIP), respectively. Secretin inhibits gastric emptying and synergizes with CCK to regulate pancreatic secretions (35), while GIP together with GLP-1 has insulinotropic effects (10).
GLP-1 is formed in intestinal L-cells in the jejunum, distal ileum, and colon from the posttranslational processing of a proglucagon precursor (87). GLP-1 influences digestion by delaying gastric emptying and inhibiting gastrointestinal secretion and motility (81, 87). Release of GLP-1 is stimulated in vivo by FA reaching the distal intestine (or administered directly into it) and was shown to require protein kinase C-ζ (92). GLP-1 release from the distal intestine, in response to predominantly PUFA, involves the FA receptor GPR120 (85). GPR40 has been colocalized with enteroendocrine cells that express GLP-1, GIP, and CCK-expressing cells, and its disruption reduces secretion of these hormones (49).
C. Satiety Effect of Intestinal Dietary Lipid
Dietary lipid while present in the gut inhibits food intake in both rats and humans, and this is mediated by LCFA signaling to release peptides that have regulatory effects on food intake or energy utilization (116, 128, 182) (FIGURE 4). As already mentioned, the release of CCK is sensitive to nutrients including LCFA, and CCK has satiety-inducing actions in addition to its effects in the gut to influence the digestive process. CCK binds to its receptors on vagal afferents in the duodenum, and a central signal is transmitted to the nucleus of tractus solitarius in the brain to induce satiety and reduce the feeling of hunger (29). Similarly GLP-1 induces satiety and reduces food intake in addition to its gastrointestinal effects (87). This section is focused on the satiety-inducing role of LCFA, but it is worth mentioning that short-chain FA were shown to induce PYY release from intestinal L cells of (129) which has central prosatiety effects (194) (TABLE 2). PYY release in vivo may in addition be responsive to LCFA (F. Nassir and N. A. Abumrad, unpublished observations).
As noted above, LCFA also induce satiety via the production of OEA, a bioactive lipid that is generated by the small intestine from absorbed oleic acid and acts centrally to reduce food intake and body weight gain in rodents (165, 182). Generation of OEA from dietary oleic acid is CD36 dependent, and disruption of CD36 or PPARα blunts fat-induced satiety (182). Some of the effects of OEA may be mediated via inducing release of GLP-1. This effect is mediated by GPR119, which binds OEA (118). In addition to OEA, fat ingestion promotes secretion by the intestine of N-acylphosphatidylethanolamine (NAPE) (69) and of apoA-IV (226), which have centrally mediated satiety effects. It is not clear whether LCFA sensing plays a role in these events.
In summary, dietary LCFA in the gut signal the release of various intestinal peptides that influence gut function and also act at the level of the brain to promote satiety. This would seem paradoxical considering the established link between fat intake and obesity. However, the satiety signaling property of fat can be blunted or overridden by other factors (194, 238). Consumption of fat together with other foods such as carbohydrates reduces the response of satiety peptides. In addition, the release of endocannabinoids by the palatable fat-sugar mix in food will promote hunger and blunt the fat-induced satiety response. Furthermore, the satiety-inducing property of fat requires complete digestion to release the LCFA, which then induce secretion of the satiety peptides. These peptide effectors delay gastric emptying and decrease the rate of fat absorption. Furthermore, there is good evidence to suggest that FA oxidation (FAO) may suppress eating, an effect based on the use of the FAO inhibitor mercaptoacetate (MA). The effect of MA was initially ascribed to inhibition of liver FAO acting via a vagal afferent signal. However, evidence against an effect of hepatic FAO led to the proposal that mercaptoacetate may be acting by inhibiting FAO in enterocytes and that changes in intestinal FAO can affect eating. Indeed, the effect of MA to increase eating was blocked by surgical elimination of vagal afferents from the upper gut (116). This adds further complexity to fat regulation of food intake, which involves a number of factors with positive (e.g., ghrelin, galanin) versus negative (CCK, PYY, OEA, FAO) effects (53).
D. LCFA Sensing and Glucose Metabolism
Sensing of LCFA by the gut-brain axis contributes to the regulation of glucose homeostasis (115) (FIGURE 4). GIP and GLP-1 are secreted by enteroendocrine cells early during the digestive process in response to LCFA released from digestion of dietary fat and have important effects on glucose homeostasis. Both act on pancreatic β-cells to enhance glucose-dependent insulin secretion, dubbed the incretin effect. In addition, GLP-1 has antihyperglycemic action via inhibition of liver glucagon secretion after a meal and by slowing gastric emptying (43). GIP has direct effects on adipose tissue to promote glucose uptake and energy storage. GLP-1 receptor agonists that are resistant to hydrolysis by the enzyme dipeptidyl peptidase-4 are current effective therapies for the treatment of type 2 diabetes (10). As discussed earlier, several of the intestinal hormones released by LCFA inhibit gastric emptying, which influences levels of blood glucose (239), and contribute to the glucoregulatory actions of gut FA sensing. Lipid sensing in the upper gut also activates an intestine-brain-liver axis to inhibit hepatic glucose production (FIGURE 4). This particular axis was shown to be dependent on generation and accumulation of LCFA-CoA and becomes defective in fat-fed rodents (235). In the brain, LCFA accumulation as LCFA-CoA and the subsequent activation of PKC directly regulate the centers responsible for modulating glucose production and energy homeostasis (115) (FIGURE 4).
V. SUMMARY, CONCLUSIONS, AND FUTURE QUESTIONS
As highlighted in this review, emerging evidence strongly suggests that the gut plays an important role in the regulation of fat homeostasis and of energy balance in general, and there is evidence that this regulation is impaired in obesity and diabetes. A complete understanding of the steps involved in intestinal fat absorption as related to the function of various membrane and intracellular proteins is still lacking. The events involved in chylomicron formation also remain to be fully elucidated. How, for example, are cytosolic lipid droplets formed and what if any relationship do they have on the formation of luminal lipid droplets within the ER? Do intestinal lipid droplet contents undergo a similar cycle of hydrolysis and metabolic partitioning to that described for hepatocytes? What are the pathways involved in partitioning intracellular cholesterol mobilization for chylomicron versus HDL biogenesis? How is intestinal cholesterol efflux promoted and what are the intracellular carriers and pathways involved? To what extent is HDL formation by the intestine a regulated event? How is the overall homeostatic regulation of canalicular cholesterol output integrated with intestinal pathways for cholesterol efflux? How does apoB48 promote chylomicron formation and result in the production of particles that are so much larger than those formed with apoB100? What proteins are involved in the distal elements of the secretory pathway and how are they regulated? Recent evidence suggests a role for LCFA sensing in chylomicron formation, but the molecular details and even the precise site(s) of action are unknown. The identification and cloning of several LCFA membrane receptors have stimulated studies into the mechanisms of lipid-induced secretion of intestinal hormones, but much more remains to be learned about the role of these receptors in mediating physiological and pathophysiological effects of various FAs. There is little information on whether these receptors are regulated and if their function is impacted by fat intake, over-feeding, diabetes, or a combination of all the above. For example, does the activity or regulation of intestinal MTTP play a permissive or pathogenic role in type 2 diabetes? Information on the potential role of the LCFA receptor CD36 in intestinal nutrient sensing and how this influences chylomicron production and possibly intestinal peptide secretion also remain to be elucidated. Why does fat intake promote obesity despite the well-established ability of lipid in the intestine to signal for satiety? Is the food-reducing property of dietary fat overridden by fat perception and the brain reward system, and what are the molecular steps involved in this regulation? Much remains to be learned in this area, and the findings are likely to have major implications with respect to the etiology of obesity.
ACKNOWLEDGMENTS
We apologize to those colleagues whose work we were unable to cite due to space limitations.
GRANTS
Work cited in this review from the authors’ laboratories was supported by National Institutes of Health Grants DK-56260, DK-52574, and HL-38180 (to N. O. Davidson) and DK-033301, DK-60022, and HL-057278C (to N. A. Abumrad).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268:17665–17668. [PubMed] [Google Scholar]
- 2.Adams MR, Konaniah E, Cash JG, Hui DY. Use of NBD-cholesterol to identify a minor but NPC1L1-independent cholesterol absorption pathway in mouse intestine. Am J Physiol Gastrointest Liver Physiol. 2011;300:G164–G169. doi: 10.1152/ajpgi.00392.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ailhaud G, Guesnet P, Cunnane SC. An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr. 2008;100:461–470. doi: 10.1017/S0007114508911569. [DOI] [PubMed] [Google Scholar]
- 4.Akesson B, Gronowitz S, Herslof B, Ohlson R. Absorption of synthetic, stereochemically defined acylglycerols in the rat. Lipids. 1978;13:338–343. doi: 10.1007/BF02533725. [DOI] [PubMed] [Google Scholar]
- 5.Al Massadi O, Tschop MH, Tong J. Ghrelin acylation and metabolic control. Peptides. 2011;32:2301–2308. doi: 10.1016/j.peptides.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Alexander CA, Hamilton RL, Havel RJ. Subcellular localization of B apoprotein of plasma lipoproteins in rat liver. J Cell Biol. 1976;69:241–263. doi: 10.1083/jcb.69.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altmann SW, Davis HR, Jr, Yao X, Laverty M, Compton DS, Zhu LJ, Crona JH, Caplen MA, Hoos LM, Tetzloff G, Priestley T, Burnett DA, Strader CD, Graziano MP. The identification of intestinal scavenger receptor class B, type I (SR-BI) by expression cloning and its role in cholesterol absorption. Biochim Biophys Acta. 2002;1580:77–93. doi: 10.1016/s1388-1981(01)00190-1. [DOI] [PubMed] [Google Scholar]
- 8.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-Pick C1 Like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 9.Anant S, Davidson NO. An AU-rich sequence element (UUUN[A/U]U) downstream of the edited C in apolipoprotein B mRNA is a high-affinity binding site for Apobec-1: binding of Apobec-1 to this motif in the 3 untranslated region of c-myc increases mRNA stability. Mol Cell Biol. 2000;20:1982–1992. doi: 10.1128/mcb.20.6.1982-1992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 11.Bartels ED, Nielsen JM, Hellgren LI, Ploug T, Nielsen LB. Cardiac expression of microsomal triglyceride transfer protein is increased in obesity and serves to attenuate cardiac triglyceride accumulation. PLoS One. 2009;4:e5300. doi: 10.1371/journal.pone.0005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bass NM. Function and regulation of hepatic and intestinal fatty acid binding proteins. Chem Phys Lipids. 1985;38:95–114. doi: 10.1016/0009-3084(85)90060-x. [DOI] [PubMed] [Google Scholar]
- 13.Berge KE, Tian H, Graf GA, Yu L, Grishin NV, Schultz J, Kwiterovich P, Shan B, Barnes R, Hobbs HH. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 14.Berriot-Varoqueaux N, Aggerbeck LP, Samson-Bouma M, Wetterau JR. The role of the microsomal triglygeride transfer protein in abetalipoproteinemia. Annu Rev Nutr. 2000;20:663–697. doi: 10.1146/annurev.nutr.20.1.663. [DOI] [PubMed] [Google Scholar]
- 15.Betters JL, Yu L. NPC1L1 and cholesterol transport. FEBS Lett. 2010;584:2740–2747. doi: 10.1016/j.febslet.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornsson OG, Bourgeois CS, Gibbons GF. Varying very low-density lipoprotein secretion of rat hepatocytes by altering cellular levels of calcium and the activity of protein kinase C. Eur J Clin Invest. 1998;28:720–729. doi: 10.1046/j.1365-2362.1998.00354.x. [DOI] [PubMed] [Google Scholar]
- 17.Blanc V, Davidson NO. C-to-U RNA editing: mechanisms leading to genetic diversity. J Biol Chem. 2003;278:1395–1398. doi: 10.1074/jbc.R200024200. [DOI] [PubMed] [Google Scholar]
- 18.Blanc V, Henderson JO, Newberry EP, Kennedy S, Luo J, Davidson NO. Targeted deletion of the murine apobec-1 complementation factor (acf) gene results in embryonic lethality. Mol Cell Biol. 2005;25:7260–7269. doi: 10.1128/MCB.25.16.7260-7269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanc V, Kennedy S, Davidson NO. A novel nuclear localization signal in the auxiliary domain of apobec-1 complementation factor regulates nucleocytoplasmic import and shuttling. J Biol Chem. 2003;278:41198–41204. doi: 10.1074/jbc.M302951200. [DOI] [PubMed] [Google Scholar]
- 20.Blanc V, Sessa KJ, Kennedy S, Luo J, Davidson NO. Apobec-1 complementation factor modulates liver regeneration by post-transcriptional regulation of interleukin-6 mRNA stability. J Biol Chem. 2010;285:19184–19192. doi: 10.1074/jbc.M110.115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JM, Rudel LL, Yu L. NPC1L1 (Niemann-Pick C1-like 1) mediates sterol-specific unidirectional transport of non-esterified cholesterol in McArdle-RH7777 hepatoma cells. Biochem J. 2007;406:273–283. doi: 10.1042/BJ20070168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunham LR, Kruit JK, Iqbal J, Fievet C, Timmins JM, Pape TD, Coburn BA, Bissada N, Staels B, Groen AK, Hussain MM, Parks JS, Kuipers F, Hayden MR. Intestinal ABCA1 directly contributes to HDL biogenesis in vivo. J Clin Invest. 2006;116:1052–1062. doi: 10.1172/JCI27352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunham LR, Kruit JK, Pape TD, Parks JS, Kuipers F, Hayden MR. Tissue-specific induction of intestinal ABCA1 expression with a liver X receptor agonist raises plasma HDL cholesterol levels. Circ Res. 2006;99:672–674. doi: 10.1161/01.RES.0000244014.19589.8e. [DOI] [PubMed] [Google Scholar]
- 24.Buhman KK, Accad M, Novak S, Choi RS, Wong JS, Hamilton RL, Turley S, Farese RV., Jr. Resistance to diet-induced hypercholesterolemia and gallstone formation in ACAT2-deficient mice. Nat Med. 2000;6:1341–1347. doi: 10.1038/82153. [DOI] [PubMed] [Google Scholar]
- 25.Buhman KK, Smith SJ, Stone SJ, Repa JJ, Wong JS, Knapp FF, Jr, Burri BJ, Hamilton RL, Abumrad NA, Farese RV., Jr. DGAT1 is not essential for intestinal triacylglycerol absorption or chylomicron synthesis. J Biol Chem. 2002;277:25474–25479. doi: 10.1074/jbc.M202013200. [DOI] [PubMed] [Google Scholar]
- 26.Cartoni C, Yasumatsu K, Ohkuri T, Shigemura N, Yoshida R, Godinot N, le Coutre J, Ninomiya Y, Damak S. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartwright IJ, Higgins JA. Direct evidence for a two-step assembly of ApoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J Biol Chem. 2001;276:48048–48057. doi: 10.1074/jbc.M104229200. [DOI] [PubMed] [Google Scholar]
- 28.Cefalu AB, Calvo PL, Noto D, Baldi M, Valenti V, Lerro P, Tramuto F, Lezo A, Morra I, Cenacchi G, Barbera C, Averna MR. Variable phenotypic expression of chylomicron retention disease in a kindred carrying a mutation of the Sara2 gene. Metabolism. 2010;59:463–467. doi: 10.1016/j.metabol.2009.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes. 2007;14:63–67. doi: 10.1097/MED.0b013e3280122850. [DOI] [PubMed] [Google Scholar]
- 30.Charcosset M, Sassolas A, Peretti N, Roy CC, Deslandres C, Sinnett D, Levy E, Lachaux A. Anderson or chylomicron retention disease: molecular impact of five mutations in the SAR1B gene on the structure and the functionality of Sar1b protein. Mol Genet Metab. 2008;93:74–84. doi: 10.1016/j.ymgme.2007.08.120. [DOI] [PubMed] [Google Scholar]
- 31.Chavez-Jauregui RN, Mattes RD, Parks EJ. Dynamics of fat absorption and effect of sham feeding on postprandial lipema. Gastroenterology. 2010;139:1538–1548. doi: 10.1053/j.gastro.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng D, Iqbal J, Devenny J, Chu CH, Chen L, Dong J, Seethala R, Keim WJ, Azzara AV, Lawrence RM, Pelleymounter MA, Hussain MM. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J Biol Chem. 2008;283:29802–29811. doi: 10.1074/jbc.M800494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng D, Nelson TC, Chen J, Walker SG, Wardwell-Swanson J, Meegalla R, Taub R, Billheimer JT, Ramaker M, Feder JN. Identification of acyl coenzyme A:monoacylglycerol acyltransferase 3, an intestinal specific enzyme implicated in dietary fat absorption. J Biol Chem. 2003;278:13611–13614. doi: 10.1074/jbc.C300042200. [DOI] [PubMed] [Google Scholar]
- 34.Chester A, Somasekaram A, Tzimina M, Jarmuz A, Gisbourne J, O’Keefe R, Scott J, Navaratnam N. The apolipoprotein B mRNA editing complex performs a multifunctional cycle and suppresses nonsense-mediated decay. EMBO J. 2003;22:3971–3982. doi: 10.1093/emboj/cdg369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chey WY, Chang TM. Secretin, 100 years later. J Gastroenterol. 2003;38:1025–1035. doi: 10.1007/s00535-003-1235-3. [DOI] [PubMed] [Google Scholar]
- 36.Coburn CT, Knapp FF, Jr, Febbraio M, Beets AL, Silverstein RL, Abumrad NA. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J Biol Chem. 2000;275:32523–32529. doi: 10.1074/jbc.M003826200. [DOI] [PubMed] [Google Scholar]
- 37.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 38.Curtis BR, Ali S, Glazier AM, Ebert DD, Aitman TJ, Aster RH. Isoimmunization against CD36 (glycoprotein IV): description of four cases of neonatal isoimmune thrombocytopenia and brief review of the literature. Transfusion. 2002;42:1173–1179. doi: 10.1046/j.1537-2995.2002.00176.x. [DOI] [PubMed] [Google Scholar]
- 39.Dance GS, Sowden MP, Cartegni L, Cooper E, Krainer AR, Smith HC. Two proteins essential for apolipoprotein B mRNA editing are expressed from a single gene through alternative splicing. J Biol Chem. 2002;277:12703–12709. doi: 10.1074/jbc.M111337200. [DOI] [PubMed] [Google Scholar]
- 40.Davies JP, Scott C, Oishi K, Liapis A, Ioannou YA. Inactivation of NPC1L1 causes multiple lipid transport defects and protects against diet-induced hypercholesterolemia. J Biol Chem. 2005;280:12710–12720. doi: 10.1074/jbc.M409110200. [DOI] [PubMed] [Google Scholar]
- 41.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-Pick C1 Like 1 (NPC1L1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 42.De Vogel-van den Bosch HM, de Wit NJ, Hooiveld GJ, Vermeulen H, van der Veen JN, Houten SM, Kuipers F, Muller M, van der Meer R. A cholesterol-free, high-fat diet suppresses gene expression of cholesterol transporters in murine small intestine. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1171–G1180. doi: 10.1152/ajpgi.00360.2007. [DOI] [PubMed] [Google Scholar]
- 43.Donnelly D. The structure and function of the glucagon-like peptide-1 receptor and its ligands. Br J Pharmacol. 2011;2011 doi: 10.1111/j.1476-5381.2011.01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dougan SK, Salas A, Rava P, Agyemang A, Kaser A, Morrison J, Khurana A, Kronenberg M, Johnson C, Exley M, Hussain MM, Blumberg RS. Microsomal triglyceride transfer protein lipidation and control of CD1d on antigen-presenting cells. J Exp Med. 2005;202:529–539. doi: 10.1084/jem.20050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drover VA, Abumrad NA. CD36-dependent fatty acid uptake regulates expression of peroxisome proliferator activated receptors. Biochem Soc Trans. 2005;33:311–315. doi: 10.1042/BST0330311. [DOI] [PubMed] [Google Scholar]
- 46.Drover VA, Ajmal M, Nassir F, Davidson NO, Nauli AM, Sahoo D, Tso P, Abumrad NA. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drover VA, Nguyen DV, Bastie CC, Darlington YF, Abumrad NA, Pessin JE, London E, Sahoo D, Phillips MC. CD36 mediates both cellular uptake of very long chain fatty acids and their intestinal absorption in mice. J Biol Chem. 2008;283:13108–13115. doi: 10.1074/jbc.M708086200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dur S, Krause K, Pluntke N, Greeve J. Gene structure and expression of the mouse APOBEC-1 complementation factor: multiple transcriptional initiation sites and a spliced variant with a premature stop translation codon. Biochim Biophys Acta. 2004;1680:11–23. doi: 10.1016/j.bbaexp.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 49.Edfalk S, Steneberg P, Edlund H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes. 2008;57:2280–2287. doi: 10.2337/db08-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El-Yassimi A, Hichami A, Besnard P, Khan NA. Linoleic acid induces calcium signaling, Src kinase phosphorylation, and neurotransmitter release in mouse CD36-positive gustatory cells. J Biol Chem. 2008;283:12949–12959. doi: 10.1074/jbc.M707478200. [DOI] [PubMed] [Google Scholar]
- 51.Ellis JM, Frahm JL, Li LO, Coleman RA. Acyl-coenzyme A synthetases in metabolic control. Curr Opin Lipidol. 2010;21:212–217. doi: 10.1097/mol.0b013e32833884bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Endemann G, Stanton LW, Madden KS, Bryant CM, White RT, Protter AA. CD36 is a receptor for oxidized low density lipoprotein. J Biol Chem. 1993;268:11811–11816. [PubMed] [Google Scholar]
- 53.Erlanson-Albertsson C. Fat-rich food palatability and appetite regulation. In: Montmayeru J, le Coutre J, editors. Fat Detection: Taste, Texture, and Post Ingestive Effects. CRC; Boca Raton, FL: 2010. [PubMed] [Google Scholar]
- 54.Farese RV, Jr, Walther TC. Lipid droplets finally get a little R-E-S-P-E-C-T. Cell. 2009;139:855–860. doi: 10.1016/j.cell.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Feinle-Bisset C, Patterson M, Ghatei MA, Bloom SR, Horowitz M. Fat digestion is required for suppression of ghrelin and stimulation of peptide YY and pancreatic polypeptide secretion by intraduodenal lipid. Am J Physiol Endocrinol Metab. 2005;289:E948–E953. doi: 10.1152/ajpendo.00220.2005. [DOI] [PubMed] [Google Scholar]
- 56.Field FJ, Born E, Mathur SN. Stanol esters decrease plasma cholesterol independently of intestinal ABC sterol transporters and Niemann-Pick C1-like 1 protein gene expression. J Lipid Res. 2004;45:2252–2259. doi: 10.1194/jlr.M400208-JLR200. [DOI] [PubMed] [Google Scholar]
- 57.Fisher EA, Ginsberg HN. Complexity in the secretory pathway: the assembly and secretion of apolipoprotein B-containing lipoproteins. J Biol Chem. 2002;277:17377–17380. doi: 10.1074/jbc.R100068200. [DOI] [PubMed] [Google Scholar]
- 58.Fisher EA, Pan M, Chen X, Wu X, Wang H, Jamil H, Sparks JD, Williams KJ. The triple threat to nascent apolipoprotein B. Evidence for multiple, distinct degradative pathways. J Biol Chem. 2001;276:27855–27863. doi: 10.1074/jbc.M008885200. [DOI] [PubMed] [Google Scholar]
- 59.Fisher EA, Williams KJ. Autophagy of an oxidized, aggregated protein beyond the ER: a pathway for remarkably late-stage quality control. Autophagy. 2008;4:721–723. doi: 10.4161/auto.6346. [DOI] [PubMed] [Google Scholar]
- 60.Freeman LA, Kennedy A, Wu J, Bark S, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr. The orphan nuclear receptor LRH-1 activates the ABCG5/ABCG8 intergenic promoter. J Lipid Res. 2004;45:1197–1206. doi: 10.1194/jlr.C400002-JLR200. [DOI] [PubMed] [Google Scholar]
- 61.Fujino T, Kang MJ, Suzuki H, Iijima H, Yamamoto T. Molecular characterization and expression of rat acyl-CoA synthetase 3. J Biol Chem. 1996;271:16748–16752. doi: 10.1074/jbc.271.28.16748. [DOI] [PubMed] [Google Scholar]
- 62.Fukuwatari T, Kawada T, Tsuruta M, Hiraoka T, Iwanaga T, Sugimoto E, Fushiki T. Expression of the putative membrane fatty acid transporter (FAT) in taste buds of the circumvallate papillae in rats. FEBS Lett. 1997;414:461–464. doi: 10.1016/s0014-5793(97)01055-7. [DOI] [PubMed] [Google Scholar]
- 63.Furuhashi M, Ura N, Nakata T, Shimamoto K. Insulin sensitivity and lipid metabolism in human CD36 deficiency. Diabetes Care. 2003;26:471–474. doi: 10.2337/diacare.26.2.471. [DOI] [PubMed] [Google Scholar]
- 64.Galloway CA, Kumar A, Krucinska J, Smith HC. APOBEC-1 complementation factor (ACF) forms RNA-dependent multimers. Biochem Biophys Res Commun. 2010;398:38–43. doi: 10.1016/j.bbrc.2010.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ge L, Qi W, Wang LJ, Miao HH, Qu YX, Li BL, Song BL. Flotillins play an essential role in Niemann-Pick C1-like 1-mediated cholesterol uptake. Proc Natl Acad Sci USA. 2011;108:551–556. doi: 10.1073/pnas.1014434108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ge L, Wang J, Qi W, Miao HH, Cao J, Qu YX, Li BL, Song BL. The cholesterol absorption inhibitor ezetimibe acts by blocking the sterol-induced internalization of NPC1L1. Cell Metab. 2008;7:508–519. doi: 10.1016/j.cmet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Georges A, Bonneau J, Bonnefont-Rousselot D, Champigneulle J, Rabes JP, Abifadel M, Aparicio T, Guenedet JC, Bruckert E, Boileau C, Morali A, Varret M, Aggerbeck LP, Samson-Bouma ME. Molecular analysis and intestinal expression of SAR1 genes and proteins in Anderson’s disease (Chylomicron retention disease) Orphanet J Rare Dis. 2011;6:1. doi: 10.1186/1750-1172-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giannoni F, Chou SC, Skarosi SF, Verp MS, Field FJ, Coleman RA, Davidson NO. Developmental regulation of the catalytic subunit of the apolipoprotein B mRNA editing enzyme (APOBEC-1) in human small intestine. J Lipid Res. 1995;36:1664–1675. [PubMed] [Google Scholar]
- 69.Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI. N-acylphosphatidylethanolamine, a gut-derived circulating factor induced by fat ingestion, inhibits food intake. Cell. 2008;135:813–824. doi: 10.1016/j.cell.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldberg IJ, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2009;50(Suppl):S86–90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goudriaan JR, Dahlmans VE, Febbraio M, Teusink B, Romijn JA, Havekes LM, Voshol PJ. Intestinal lipid absorption is not affected in CD36 deficient mice. Mol Cell Biochem. 2002;239:199–202. [PubMed] [Google Scholar]
- 72.Graf GA, Yu L, Li WP, Gerard R, Tuma PL, Cohen JC, Hobbs HH. ABCG5 and ABCG8 are obligate heterodimers for protein trafficking and biliary cholesterol excretion. J Biol Chem. 2003;278:48275–48282. doi: 10.1074/jbc.M310223200. [DOI] [PubMed] [Google Scholar]
- 73.Greenwalt DE, Scheck SH, Rhinehart-Jones T. Heart CD36 expression is increased in murine models of diabetes and in mice fed a high fat diet. J Clin Invest. 1995;96:1382–1388. doi: 10.1172/JCI118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greeve J, Altkemper I, Dieterich JH, Greten H, Windler E. Apolipoprotein B mRNA editing in 12 different mammalian species: hepatic expression is reflected in low concentrations of apoB-containing plasma lipoproteins. J Lipid Res. 1993;34:1367–1383. [PubMed] [Google Scholar]
- 75.Gusarova V, Brodsky JL, Fisher EA. Apolipoprotein B100 exit from the endoplasmic reticulum (ER) is COPII-dependent, its lipidation to very low density lipoprotein occurs post-ER. J Biol Chem. 2003;278:48051–48058. doi: 10.1074/jbc.M306898200. [DOI] [PubMed] [Google Scholar]
- 76.Hadjiagapiou C, Giannoni F, Funahashi T, Skarosi SF, Davidson NO. Molecular cloning of a human small intestinal apolipoprotein B mRNA editing protein. Nucleic Acids Res. 1994;22:1874–1879. doi: 10.1093/nar/22.10.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hajri T, Hall AM, Jensen DR, Pietka TA, Drover VA, Tao H, Eckel R, Abumrad NA. CD36-facilitated fatty acid uptake inhibits leptin production and signaling in adipose tissue. Diabetes. 2007;56:1872–1880. doi: 10.2337/db06-1699. [DOI] [PubMed] [Google Scholar]
- 78.Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002;109:1381–1389. doi: 10.1172/JCI14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamilton JA. Fatty acid interactions with proteins: what X-ray crystal and NMR solution structures tell us. Prog Lipid Res. 2004;43:177–199. doi: 10.1016/j.plipres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Hamilton RL, Wong JS, Cham CM, Nielsen LB, Young SG. Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J Lipid Res. 1998;39:1543–1557. [PubMed] [Google Scholar]
- 81.Hellstrom PM. GLP-1 playing the role of a gut regulatory compound. Acta Physiol. 2011;201:151–156. doi: 10.1111/j.1748-1716.2010.02150.x. [DOI] [PubMed] [Google Scholar]
- 82.Henderson JO, Blanc V, Davidson NO. Isolation, characterization and developmental regulation of the human apobec-1 complementation factor (ACF) gene. Biochim Biophys Acta. 2001;1522:22–30. doi: 10.1016/s0167-4781(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 83.Hirano K, Kuwasako T, Nakagawa-Toyama Y, Janabi M, Yamashita S, Matsuzawa Y. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 84.Hirasawa A, Hara T, Katsuma S, Adachi T, Tsujimoto G. Free fatty acid receptors and drug discovery. Biol Pharm Bull. 2008;31:1847–1851. doi: 10.1248/bpb.31.1847. [DOI] [PubMed] [Google Scholar]
- 85.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med. 2005;11:90–94. doi: 10.1038/nm1168. [DOI] [PubMed] [Google Scholar]
- 86.Hoffman HN, Fredrickson DS. Tangier disease (familial high density lipoprotein deficiency). Clinical and genetic features in two adults. Am J Med. 1965;39:582–593. doi: 10.1016/0002-9343(65)90081-1. [DOI] [PubMed] [Google Scholar]
- 87.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 88.Hsieh J, Longuet C, Maida A, Bahrami J, Xu E, Baker CL, Brubaker PL, Drucker DJ, Adeli K. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 2009;137:997–1005. doi: 10.1053/j.gastro.2009.05.051. [DOI] [PubMed] [Google Scholar]
- 89.Huang XF, Shelness GS. Efficient glycosylation site utilization by intracellular apolipoprotein B. Implications for proteasomal degradation. J Lipid Res. 1999;40:2212–2222. [PubMed] [Google Scholar]
- 90.Hussain MM, Rava P, Pan X, Dai K, Dougan SK, Iqbal J, Lazare F, Khatun I. Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr Opin Lipidol. 2008;19:277–284. doi: 10.1097/MOL.0b013e3282feea85. [DOI] [PubMed] [Google Scholar]
- 91.Hussain MM, Shi J, Dreizen P. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J Lipid Res. 2003;44:22–32. doi: 10.1194/jlr.r200014-jlr200. [DOI] [PubMed] [Google Scholar]
- 92.Iakoubov R, Ahmed A, Lauffer LM, Bazinet RP, Brubaker PL. Essential role for protein kinase Cζ in oleic acid-induced glucagon-like peptide-1 secretion in vivo in the rat. Endocrinology. 2011;152:1244–1252. doi: 10.1210/en.2010-1352. [DOI] [PubMed] [Google Scholar]
- 93.Ikram MA, Seshadri S, Bis JC, Fornage M, DeStefano AL, Aulchenko YS, Debette S, Lumley T, Folsom AR, van den Herik EG, Bos MJ, Beiser A, Cushman M, Launer LJ, Shahar E, Struchalin M, Du Y, Glazer NL, Rosamond WD, Rivadeneira F, Kelly-Hayes M, Lopez OL, Coresh J, Hofman A, DeCarli C, Heckbert SR, Koudstaal PJ, Yang Q, Smith NL, Kase CS, Rice K, Haritunians T, Roks G, de Kort PL, Taylor KD, de Lau LM, Oostra BA, Uitterlinden AG, Rotter JI, Boerwinkle E, Psaty BM, Mosley TH, van Duijn CM, Breteler MM, Longstreth WT, Jr, Wolf PA. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iqbal J, Dai K, Seimon T, Jungreis R, Oyadomari M, Kuriakose G, Ron D, Tabas I, Hussain MM. IRE1beta inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 2008;7:445–455. doi: 10.1016/j.cmet.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iqbal J, Hussain MM. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J Lipid Res. 2005;46:1491–1501. doi: 10.1194/jlr.M500023-JLR200. [DOI] [PubMed] [Google Scholar]
- 96.Iqbal J, Li X, Chang BH, Chan L, Schwartz GJ, Chua SC, Jr, Hussain MM. An intrinsic gut leptin-melanocortin pathway modulates intestinal microsomal triglyceride transfer protein and lipid absorption. J Lipid Res. 2010;51:1929–1942. doi: 10.1194/jlr.M005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jakulj L, Vissers MN, Tanck MW, Hutten BA, Stellaard F, Kastelein JJ, Dallinga-Thie GM. ABCG5/G8 polymorphisms and markers of cholesterol metabolism: systematic review and meta-analysis. J Lipid Res. 2010;51:3016–3023. doi: 10.1194/jlr.M008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jiang ZG, Liu Y, Hussain MM, Atkinson D, McKnight CJ. Reconstituting initial events during the assembly of apolipoprotein B-containing lipoproteins in a cell-free system. J Mol Biol. 2008;383:1181–1194. doi: 10.1016/j.jmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jones B, Jones EL, Bonney SA, Patel HN, Mensenkamp AR, Eichenbaum-Voline S, Rudling M, Myrdal U, Annesi G, Naik S, Meadows N, Quattrone A, Islam SA, Naoumova RP, Angelin B, Infante R, Levy E, Roy CC, Freemont PS, Scott J, Shoulders CC. Mutations in a Sar1 GTPase of COPII vesicles are associated with lipid absorption disorders. Nat Genet. 2003;34:29–31. doi: 10.1038/ng1145. [DOI] [PubMed] [Google Scholar]
- 100.Kang MH, Singaraja R, Hayden MR. Adenosine-triphosphate-binding cassette transporter-1 trafficking and function. Trends Cardiovasc Med. 2010;20:41–49. doi: 10.1016/j.tcm.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 101.Kawai T, Fushiki T. Importance of lipolysis in oral cavity for orosensory detection of fat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R447–R454. doi: 10.1152/ajpregu.00729.2002. [DOI] [PubMed] [Google Scholar]
- 102.Kazmi S, Sidhu SS, Donohoe TJ, Wickham M, Jones MN, Thompson DG, Case RM, Benson RS. Calcium mobilisation and CCK secretion induced by modified fatty acids and latex microspheres reveal dual receptor mechanisms for lipid stimulation of STC-1 cells. J Physiol. 2003;553:759–773. doi: 10.1113/jphysiol.2003.051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Keelan M, Hui DY, Wild G, Clandinin MT, Thomson AB. Variability of the intestinal uptake of lipids is genetically determined in mice. Lipids. 2000;35:833–837. doi: 10.1007/s11745-000-0592-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Khan NA, Besnard P. Oro-sensory perception of dietary lipids: new insights into the fat taste transduction. Biochim Biophys Acta. 2009;1791:149–155. doi: 10.1016/j.bbalip.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 105.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kirchner H, Gutierrez JA, Solenberg PJ, Pfluger PT, Czyzyk TA, Willency JA, Schurmann A, Joost HG, Jandacek RJ, Hale JE, Heiman ML, Tschop MH. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med. 2009;15:741–745. doi: 10.1038/nm.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klett EL, Lee MH, Adams DB, Chavin KD, Patel SB. Localization of ABCG5 and ABCG8 proteins in human liver, gall bladder and intestine. BMC Gastroenterol. 2004;4:21. doi: 10.1186/1471-230X-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krzystanek M, Pedersen TX, Bartels ED, Kjaehr J, Straarup EM, Nielsen LB. Expression of apolipoprotein B in the kidney attenuates renal lipid accumulation. J Biol Chem. 2010;285:10583–10590. doi: 10.1074/jbc.M109.078006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuda O, Jenkins CM, Skinner JR, Moon SH, Su X, Gross RW, Abumrad NA. CD36 is involved in store operated calcium flux, phospholipase A2 activation and production of prostaglandin E2. J Biol Chem. 2011;286:17785–17795. doi: 10.1074/jbc.M111.232975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 2002;277:31516–31525. doi: 10.1074/jbc.M202015200. [DOI] [PubMed] [Google Scholar]
- 111.Kuwasako T, Hirano K, Sakai N, Ishigami M, Hiraoka H, Yakub MJ, Yamauchi-Takihara K, Yamashita S, Matsuzawa Y. Lipoprotein abnormalities in human genetic CD36 deficiency associated with insulin resistance and abnormal fatty acid metabolism. Diabetes Care. 2003;26:1647–1648. doi: 10.2337/diacare.26.5.1647-a. [DOI] [PubMed] [Google Scholar]
- 112.Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Labonte ED, Camarota LM, Rojas JC, Jandacek RJ, Gilham DE, Davies JP, Ioannou YA, Tso P, Hui DY, Howles PN. Reduced absorption of saturated fatty acids and resistance to diet-induced obesity and diabetes by ezetimibe-treated and Npc1l1−/− mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G776–G783. doi: 10.1152/ajpgi.90275.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lagakos WS, Gajda A, Agellon L, Binas B, Choi V, Mandap B, Russnak T, Zhou YX, Storch J. Different functions of the intestinal- and liver-type fatty acid-binding proteins in the intestine and in whole body energy homeostasis. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00229.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lam CK, Chari M, Rutter GA, Lam TK. Hypothalamic nutrient sensing activates a forebrain-hindbrain neuronal circuit to regulate glucose production in vivo. Diabetes. 2011;60:107–113. doi: 10.2337/db10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Langhans W, Leitner C, Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol. 2011;300:R554–R565. doi: 10.1152/ajpregu.00610.2010. [DOI] [PubMed] [Google Scholar]
- 117.Lau PP, Zhu HJ, Baldini A, Charnsangavej C, Chan L. Dimeric structure of a human apolipoprotein B mRNA editing protein and cloning and chromosomal localization of its gene. Proc Natl Acad Sci USA. 1994;91:8522–8526. doi: 10.1073/pnas.91.18.8522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lauffer LM, Iakoubov R, Brubaker PL. GPR119 is essential for oleoylethanolamide-induced glucagon-like peptide-1 secretion from the intestinal enteroendocrine L-cell. Diabetes. 2009;58:1058–1066. doi: 10.2337/db08-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee B, Fast AM, Zhu J, Cheng JX, Buhman KK. Intestine-specific expression of acyl CoA:diacylglycerol acyltransferase 1 reverses resistance to diet-induced hepatic steatosis and obesity in Dgat1−/− mice. J Lipid Res. 2010;51:1770–1780. doi: 10.1194/jlr.M002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee B, Zhu J, Wolins NE, Cheng JX, Buhman KK. Differential association of adipophilin and TIP47 proteins with cytoplasmic lipid droplets in mouse enterocytes during dietary fat absorption. Biochim Biophys Acta. 2009;1791:1173–1180. doi: 10.1016/j.bbalip.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 122.Lee MH, Lu K, Hazard S, Yu H, Shulenin S, Hidaka H, Kojima H, Allikmets R, Sakuma N, Pegoraro R, Srivastava AK, Salen G, Dean M, Patel SB. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lellek H, Kirsten R, Diehl I, Apostel F, Buck F, Greeve J. Purification and molecular cloning of a novel essential component of the apolipoprotein B mRNA editing enzyme-complex. J Biol Chem. 2000;275:19848–19856. doi: 10.1074/jbc.M001786200. [DOI] [PubMed] [Google Scholar]
- 124.Li CM, Presley JB, Zhang X, Dashti N, Chung BH, Medeiros NE, Guidry C, Curcio CA. Retina expresses microsomal triglyceride transfer protein: implications for age-related maculopathy. J Lipid Res. 2005;46:628–640. doi: 10.1194/jlr.M400428-JLR200. [DOI] [PubMed] [Google Scholar]
- 125.Liang S, Wu X, Fisher EA, Ginsberg HN. The amino-terminal domain of apolipoprotein B does not undergo retrograde translocation from the endoplasmic reticulum to the cytosol. Proteasomal degradation of nascent apolipoprotein B begins at the carboxyl terminus of the protein, while apolipoprotein B is still in its original translocon. J Biol Chem. 2000;275:32003–32010. doi: 10.1074/jbc.M004646200. [DOI] [PubMed] [Google Scholar]
- 126.Liao W, Chan L. Apolipoprotein B, a paradigm for proteins regulated by intracellular degradation, does not undergo intracellular degradation in CaCo2 cells. J Biol Chem. 2000;275:3950–3956. doi: 10.1074/jbc.275.6.3950. [DOI] [PubMed] [Google Scholar]
- 127.Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE, Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Little TJ, Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity-oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 129.Little TJ, Russo A, Meyer JH, Horowitz M, Smyth DR, Bellon M, Wishart JM, Jones KL, Feinle-Bisset C. Free fatty acids have more potent effects on gastric emptying, gut hormones, and appetite than triacylglycerides. Gastroenterology. 2007;133:1124–1131. doi: 10.1053/j.gastro.2007.06.060. [DOI] [PubMed] [Google Scholar]
- 130.Lo CM, Nordskog BK, Nauli AM, Zheng S, Vonlehmden SB, Yang Q, Lee D, Swift LL, Davidson NO, Tso P. Why does the gut choose apolipoprotein B48 but not B100 for chylomicron formation? Am J Physiol Gastrointest Liver Physiol. 2008;294:G344–G352. doi: 10.1152/ajpgi.00123.2007. [DOI] [PubMed] [Google Scholar]
- 131.Lo Sasso G, Murzilli S, Salvatore L, D’Errico I, Petruzzelli M, Conca P, Jiang ZY, Calabresi L, Parini P, Moschetta A. Intestinal specific LXR activation stimulates reverse cholesterol transport and protects from atherosclerosis. Cell Metab. 2010;12:187–193. doi: 10.1016/j.cmet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 132.Lobo MV, Huerta L, Ruiz-Velasco N, Teixeiro E, de la Cueva P, Celdran A, Martin-Hidalgo A, Vega MA, Bragado R. Localization of the lipid receptors CD36 and CLA-1/SR-BI in the human gastrointestinal tract: towards the identification of receptors mediating the intestinal absorption of dietary lipids. J Histochem Cytochem. 2001;49:1253–1260. doi: 10.1177/002215540104901007. [DOI] [PubMed] [Google Scholar]
- 133.Love-Gregory L, Sherva R, Schappe T, Qi JS, McCrea J, Klein S, Connelly MA, Abumrad NA. Common CD36 SNPs reduce protein expression and may contribute to a protective atherogenic profile. Hum Mol Genet. 2011;20:193–201. doi: 10.1093/hmg/ddq449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Love-Gregory L, Sherva R, Sun L, Wasson J, Schappe T, Doria A, Rao DC, Hunt SC, Klein S, Neuman RJ, Permutt MA, Abumrad NA. Variants in the CD36 gene associate with the metabolic syndrome and high-density lipoprotein cholesterol. Hum Mol Genet. 2008;17:1695–1704. doi: 10.1093/hmg/ddn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ma X, Bacci S, Mlynarski W, Gottardo L, Soccio T, Menzaghi C, Iori E, Lager RA, Shroff AR, Gervino EV, Nesto RW, Johnstone MT, Abumrad NA, Avogaro A, Trischitta V, Doria A. A common haplotype at the CD36 locus is associated with high free fatty acid levels and increased cardiovascular risk in Caucasians. Hum Mol Genet. 2004;13:2197–2205. doi: 10.1093/hmg/ddh233. [DOI] [PubMed] [Google Scholar]
- 136.Madsen EM, Lindegaard ML, Andersen CB, Damm P, Nielsen LB. Human placenta secretes apolipoprotein B-100-containing lipoproteins. J Biol Chem. 2004;279:55271–55276. doi: 10.1074/jbc.M411404200. [DOI] [PubMed] [Google Scholar]
- 137.Mansbach CM, Siddiqi SA. The biogenesis of chylomicrons. Annu Rev Physiol. 2010;72:315–333. doi: 10.1146/annurev-physiol-021909-135801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Martin C, Chevrot M, Poirier H, Passilly-Degrace P, Niot I, Besnard P. CD36 as a lipid sensor. Physiol Behav. 2011;105:36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 139.Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PLoS One. 2011;6:e24014. doi: 10.1371/journal.pone.0024014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Martin GG, Atshaves BP, Huang H, McIntosh AL, Williams BJ, Pai PJ, Russell DH, Kier AB, Schroeder F. Hepatic phenotype of liver fatty acid binding protein gene-ablated mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1053–G1065. doi: 10.1152/ajpgi.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mashek DG, Coleman RA. Cellular fatty acid uptake: the contribution of metabolism. Curr Opin Lipidol. 2006;17:274–278. doi: 10.1097/01.mol.0000226119.20307.2b. [DOI] [PubMed] [Google Scholar]
- 142.Masuda D, Hirano K, Oku H, Sandoval JC, Kawase R, Yuasa-Kawase M, Yamashita Y, Takada M, Tsubakio-Yamamoto K, Tochino Y, Koseki M, Matsuura F, Nishida M, Kawamoto T, Ishigami M, Hori M, Shimomura I, Yamashita S. Chylomicron remnants are increased in the postprandial state in CD36 deficiency. J Lipid Res. 2009;50:999–1011. doi: 10.1194/jlr.P700032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mattes RD. Oral fatty acid signaling and intestinal lipid processing: support and supposition. Physiol Behav. 2011;105:27–35. doi: 10.1016/j.physbeh.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 144.McNeish J, Aiello RJ, Guyot D, Turi T, Gabel C, Aldinger C, Hoppe KL, Roach ML, Royer LJ, de Wet J, Broccardo C, Chimini G, Francone OL. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc Natl Acad Sci USA. 2000;97:4245–4250. doi: 10.1073/pnas.97.8.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mehta A, Kinter MT, Sherman NE, Driscoll DM. Molecular cloning of apobec-1 complementation factor, a novel RNA-binding protein involved in the editing of apolipoprotein B mRNA. Mol Cell Biol. 2000;20:1846–1854. doi: 10.1128/mcb.20.5.1846-1854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mendeloff AI. The effects of eating and of sham feeding upon the absorption of vitamin A palmitate in man. J Clin Invest. 1954;33:1015–1021. doi: 10.1172/JCI102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Milger K, Herrmann T, Becker C, Gotthardt D, Zickwolf J, Ehehalt R, Watkins PA, Stremmel W, Fullekrug J. Cellular uptake of fatty acids driven by the ER-localized acyl-CoA synthetase FATP4. J Cell Sci. 2006;119:4678–4688. doi: 10.1242/jcs.03280. [DOI] [PubMed] [Google Scholar]
- 148.Miyaoka K, Kuwasako T, Hirano K, Nozaki S, Yamashita S, Matsuzawa Y. CD36 deficiency associated with insulin resistance. Lancet. 2001;357:686–687. doi: 10.1016/s0140-6736(00)04138-6. [DOI] [PubMed] [Google Scholar]
- 149.Miyauchi S, Hirasawa A, Ichimura A, Hara T, Tsujimoto G. New frontiers in gut nutrient sensor research: free fatty acid sensing in the gastrointestinal tract. J Pharmacol Sci. 2010;112:19–24. doi: 10.1254/jphs.09r09fm. [DOI] [PubMed] [Google Scholar]
- 150.Mosher DF. Physiology of thrombospondin. Annu Rev Med. 1990;41:85–97. doi: 10.1146/annurev.me.41.020190.000505. [DOI] [PubMed] [Google Scholar]
- 151.Moulson CL, Lin MH, White JM, Newberry EP, Davidson NO, Miner JH. Keratinocyte-specific expression of fatty acid transport protein 4 rescues the wrinkle-free phenotype in Slc27a4/Fatp4 mutant mice. J Biol Chem. 2007;282:15912–15920. doi: 10.1074/jbc.M701779200. [DOI] [PubMed] [Google Scholar]
- 152.Moulson CL, Martin DR, Lugus JJ, Schaffer JE, Lind AC, Miner JH. Cloning of wrinkle-free, a previously uncharacterized mouse mutation, reveals crucial roles for fatty acid transport protein 4 in skin and hair development. Proc Natl Acad Sci USA. 2003;100:5274–5279. doi: 10.1073/pnas.0431186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Murota K, Matsui N, Kawada T, Takahashi N, Fushuki T. Inhibitory effect of monoacylglycerol on fatty acid uptake into rat intestinal epithelial cells. Biosci Biotechnol Biochem. 2001;65:1441–1443. doi: 10.1271/bbb.65.1441. [DOI] [PubMed] [Google Scholar]
- 154.Murota K, Storch J. Uptake of micellar long-chain fatty acid and sn-2-monoacylglycerol into human intestinal Caco-2 cells exhibits characteristics of protein-mediated transport. J Nutr. 2005;135:1626–1630. doi: 10.1093/jn/135.7.1626. [DOI] [PubMed] [Google Scholar]
- 155.Nassir F, Wilson B, Han X, Gross RW, Abumrad NA. CD36 is important for fatty acid and cholesterol uptake by the proximal but not distal intestine. J Biol Chem. 2007;282:19493–19501. doi: 10.1074/jbc.M703330200. [DOI] [PubMed] [Google Scholar]
- 156.Nauli AM, Nassir F, Zheng S, Yang Q, Lo CM, Vonlehmden SB, Lee D, Jandacek RJ, Abumrad NA, Tso P. CD36 is important for chylomicron formation and secretion and may mediate cholesterol uptake in the proximal intestine. Gastroenterology. 2006;131:1197–1207. doi: 10.1053/j.gastro.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Neeli I, Siddiqi SA, Siddiqi S, Mahan J, Lagakos WS, Binas B, Gheyi T, Storch J, Mansbach CM., 2nd Liver fatty acid-binding protein initiates budding of pre-chylomicron transport vesicles from intestinal endoplasmic reticulum. J Biol Chem. 2007;282:17974–17984. doi: 10.1074/jbc.M610765200. [DOI] [PubMed] [Google Scholar]
- 158.Newberry EP, Davidson NO. Intestinal lipid absorption, GLP-2, and CD36: still more mysteries to moving fat. Gastroenterology. 2009;137:775–778. doi: 10.1053/j.gastro.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Newberry EP, Xie Y, Kennedy SM, Luo J, Davidson NO. Protection against Western diet-induced obesity and hepatic steatosis in liver fatty acid-binding protein knockout mice. Hepatology. 2006;44:1191–1205. doi: 10.1002/hep.21369. [DOI] [PubMed] [Google Scholar]
- 160.Nguyen DV, Drover VA, Knopfel M, Dhanasekaran P, Hauser H, Phillips MC. Influence of class B scavenger receptors on cholesterol flux across the brush border membrane and intestinal absorption. J Lipid Res. 2009;50:2235–2244. doi: 10.1194/jlr.M900036-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nozaki S, Tanaka T, Yamashita S, Sohmiya K, Yoshizumi T, Okamoto F, Kitaura Y, Kotake C, Nishida H, Nakata A, Nakagawa T, Matsumoto K, Kameda-Takemura K, Tadokoro S, Kurata Y, Tomiyama Y, Kawamura K, Matsuzawa Y. CD36 mediates long-chain fatty acid transport in human myocardium: complete myocardial accumulation defect of radiolabeled long-chain fatty acid analog in subjects with CD36 deficiency. Mol Cell Biochem. 1999;192:129–135. [PubMed] [Google Scholar]
- 162.Oh da Y, Lagakos WS. The role of G-protein-coupled receptors in mediating the effect of fatty acids on inflammation and insulin sensitivity. Curr Opin Clin Nutr Metab Care. 2011;14:322–327. doi: 10.1097/MCO.0b013e3283479230. [DOI] [PubMed] [Google Scholar]
- 163.Oikawa E, Iijima H, Suzuki T, Sasano H, Sato H, Kamataki A, Nagura H, Kang MJ, Fujino T, Suzuki H, Yamamoto TT. A novel acyl-CoA synthetase, ACS5, expressed in intestinal epithelial cells and proliferating preadipocytes. J Biochem. 1998;124:679–685. doi: 10.1093/oxfordjournals.jbchem.a022165. [DOI] [PubMed] [Google Scholar]
- 164.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 165.Overton HA, Babbs AJ, Doel SM, Fyfe MC, Gardner LS, Griffin G, Jackson HC, Procter MJ, Rasamison CM, Tang-Christensen M, Widdowson PS, Williams GM, Reynet C. Deorphanization of a G protein-coupled receptor for oleoylethanolamide and its use in the discovery of small-molecule hypophagic agents. Cell Metab. 2006;3:167–175. doi: 10.1016/j.cmet.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 166.Pan X, Hussain MM. Diurnal regulation of microsomal triglyceride transfer protein and plasma lipid levels. J Biol Chem. 2007;282:24707–24719. doi: 10.1074/jbc.M701305200. [DOI] [PubMed] [Google Scholar]
- 167.Pan X, Zhang Y, Wang L, Hussain MM. Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 2010;12:174–186. doi: 10.1016/j.cmet.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pariyarath R, Wang H, Aitchison JD, Ginsberg HN, Welch WJ, Johnson AE, Fisher EA. Co-translational interactions of apoprotein B with the ribosome and translocon during lipoprotein assembly or targeting to the proteasome. J Biol Chem. 2001;276:541–550. doi: 10.1074/jbc.M007944200. [DOI] [PubMed] [Google Scholar]
- 169.Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene, CD36, and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2011 doi: 10.1194/jlr.M021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Poirier H, Degrace P, Niot I, Bernard A, Besnard P. Localization and regulation of the putative membrane fatty-acid transporter (FAT) in the small intestine. Comparison with fatty acid-binding proteins (FABP) Eur J Biochem. 1996;238:368–373. doi: 10.1111/j.1432-1033.1996.0368z.x. [DOI] [PubMed] [Google Scholar]
- 171.Raabe M, Flynn LM, Zlot CH, Wong JS, Veniant MM, Hamilton RL, Young SG. Knockout of the abetalipoproteinemia gene in mice: reduced lipoprotein secretion in heterozygotes and embryonic lethality in homozygotes. Proc Natl Acad Sci USA. 1998;95:8686–8691. doi: 10.1073/pnas.95.15.8686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Raabe M, Veniant MM, Sullivan MA, Zlot CH, Bjorkegren J, Nielsen LB, Wong JS, Hamilton RL, Young SG. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J Clin Invest. 1999;103:1287–1298. doi: 10.1172/JCI6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rava P, Hussain MM. Acquisition of triacylglycerol transfer activity by microsomal triglyceride transfer protein during evolution. Biochemistry. 2007;46:12263–12274. doi: 10.1021/bi700762z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 175.Richieri GV, Ogata RT, Kleinfeld AM. The measurement of free fatty acid concentration with the fluorescent probe ADIFAB: a practical guide for the use of the ADIFAB probe. Mol Cell Biochem. 1999;192:87–94. [PubMed] [Google Scholar]
- 176.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 177.Rosenberg BR, Hamilton CE, Mwangi MM, Dewell S, Papavasiliou FN. Transcriptome-wide sequencing reveals numerous APOBEC1 mRNA-editing targets in transcript 3′ UTRs. Nat Struct Mol Biol. 2011;18:230–236. doi: 10.1038/nsmb.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rubino F, R’Bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rudel LL, Lee RG, Cockman TL. Acyl coenzyme A: cholesterol acyltransferase types 1 and 2: structure and function in atherosclerosis. Curr Opin Lipidol. 2001;12:121–127. doi: 10.1097/00041433-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 180.Sandoval JC, Nakagawa-Toyama Y, Masuda D, Tochino Y, Nakaoka H, Kawase R, Yuasa-Kawase M, Nakatani K, Inagaki M, Tsubakio-Yamamoto K, Ohama T, Matsuyama A, Nishida M, Ishigami M, Komuro I, Yamashita S. Molecular mechanisms of ezetimibe-induced attenuation of postprandial hypertriglyceridemia. J Atheroscler Thromb. 2010;17:914–924. doi: 10.5551/jat.4929. [DOI] [PubMed] [Google Scholar]
- 181.Schonfeld G. Familial hypobetalipoproteinemia: a review. J Lipid Res. 2003;44:878–883. doi: 10.1194/jlr.R300002-JLR200. [DOI] [PubMed] [Google Scholar]
- 182.Schwartz GJ. Gut fat sensing in the negative feedback control of energy balance-recent advances. Physiol Behav. 2011;104:621–623. doi: 10.1016/j.physbeh.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V, Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79:249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 185.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 186.Shah PK. Atherosclerosis: targeting endogenous apo A-I-a new approach for raising HDL. Nat Rev Cardiol. 2011;8:187–188. doi: 10.1038/nrcardio.2011.37. [DOI] [PubMed] [Google Scholar]
- 187.Shelness GS, Morris-Rogers KC, Ingram MF. Apolipoprotein B48-membrane interactions. Absence of transmembrane localization in nonhepatic cells. J Biol Chem. 1994;269:9310–9318. [PubMed] [Google Scholar]
- 188.Shim J, Moulson CL, Newberry EP, Lin MH, Xie Y, Kennedy SM, Miner JH, Davidson NO. Fatty acid transport protein 4 is dispensable for intestinal lipid absorption in mice. J Lipid Res. 2009;50:491–500. doi: 10.1194/jlr.M800400-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shoulders CC, Stephens DJ, Jones B. The intracellular transport of chylomicrons requires the small GTPase, Sar1b. Curr Opin Lipidol. 2004;15:191–197. doi: 10.1097/00041433-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 190.Siddiqi S, Saleem U, Abumrad NA, Davidson NO, Storch J, Siddiqi SA, Mansbach CM., 2nd A novel multiprotein complex is required to generate the prechylomicron transport vesicle from intestinal ER. J Lipid Res. 2010;51:1918–1928. doi: 10.1194/jlr.M005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Siddiqi S, Siddiqi SA, Mansbach CM., 2nd Sec24C is required for docking the prechylomicron transport vesicle with the Golgi. J Lipid Res. 2010;51:1093–1100. doi: 10.1194/jlr.M002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Simons PJ, Kummer JA, Luiken JJ, Boon L. Apical CD36 immunolocalization in human and porcine taste buds from circumvallate and foliate papillae. Acta Histochem. 2011;113:839–843. doi: 10.1016/j.acthis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 194.Simpson KA, Bloom SR. Appetite and hedonism: gut hormones and the brain. Endocrinol Metab Clin North Am. 2010;39:729–743. doi: 10.1016/j.ecl.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 195.Skov M, Tonnesen CK, Hansen GH, Danielsen EM. Dietary cholesterol induces trafficking of intestinal Niemann-Pick Type C1 Like 1 from the brush border to endosomes. Am J Physiol Gastrointest Liver Physiol. 2011;300:G33–G40. doi: 10.1152/ajpgi.00344.2010. [DOI] [PubMed] [Google Scholar]
- 196.Smith J, Su X, El-Maghrabi R, Stahl PD, Abumrad NA. Opposite regulation of CD36 ubiquitination by fatty acids and insulin: effects on fatty acid uptake. J Biol Chem. 2008;283:13578–13585. doi: 10.1074/jbc.M800008200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Smith SJ, Cases S, Jensen DR, Chen HC, Sande E, Tow B, Sanan DA, Raber J, Eckel RH, Farese RV., Jr Obesity resistance and multiple mechanisms of triglyceride synthesis in mice lacking Dgat. Nat Genet. 2000;25:87–90. doi: 10.1038/75651. [DOI] [PubMed] [Google Scholar]
- 198.Stahl A, Hirsch DJ, Gimeno RE, Punreddy S, Ge P, Watson N, Patel S, Kotler M, Raimondi A, Tartaglia LA, Lodish HF. Identification of the major intestinal fatty acid transport protein. Mol Cell. 1999;4:299–308. doi: 10.1016/s1097-2765(00)80332-9. [DOI] [PubMed] [Google Scholar]
- 199.Steinert RE, Beglinger C. Nutrient sensing in the gut: interactions between chemosensory cells, visceral afferents and the secretion of satiation peptides. Physiol Behav. 2011;105:62–70. doi: 10.1016/j.physbeh.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 200.Stewart JE, Feinle-Bisset C, Golding M, Delahunty C, Clifton PM, Keast RS. Oral sensitivity to fatty acids, food consumption and BMI in human subjects. Br J Nutr. 2010;104:145–152. doi: 10.1017/S0007114510000267. [DOI] [PubMed] [Google Scholar]
- 201.Stewart JE, Feinle-Bisset C, Keast RS. Fatty acid detection during food consumption and digestion: associations with ingestive behavior and obesity. Prog Lipid Res. 2011;50:225–233. doi: 10.1016/j.plipres.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 202.Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–5361. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, Farese RV., Jr Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 204.Storch J, McDermott L. Structural and functional analysis of fatty acid-binding proteins. J Lipid Res. 2009;50(Suppl):S126–131. doi: 10.1194/jlr.R800084-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285:32679–32683. doi: 10.1074/jbc.R110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Storch J, Zhou YX, Lagakos WS. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J Lipid Res. 2008;49:1762–1769. doi: 10.1194/jlr.M800116-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20:72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sukhotnik I, Gork AS, Chen M, Drongowski RA, Coran AG, Harmon CM. Effect of a high fat diet on lipid absorption and fatty acid transport in a rat model of short bowel syndrome. Pediatr Surg Int. 2003;19:385–390. doi: 10.1007/s00383-003-1016-3. [DOI] [PubMed] [Google Scholar]
- 209.Suzuki H, Kawarabayasi Y, Kondo J, Abe T, Nishikawa K, Kimura S, Hashimoto T, Yamamoto T. Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem. 1990;265:8681–8685. [PubMed] [Google Scholar]
- 210.Swift LL, Jovanovska A, Kakkad B, Ong DE. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem Cell Biol. 2005;123:475–482. doi: 10.1007/s00418-005-0772-7. [DOI] [PubMed] [Google Scholar]
- 211.Talukdar S, Olefsky JM, Osborn O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol Sci. 2011;32:543–550. doi: 10.1016/j.tips.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tanaka T, Katsuma S, Adachi T, Koshimizu TA, Hirasawa A, Tsujimoto G. Free fatty acids induce cholecystokinin secretion through GPR120. Naunyn-Schmiedebergs Arch Pharmacol. 2008;377:523–527. doi: 10.1007/s00210-007-0200-8. [DOI] [PubMed] [Google Scholar]
- 213.Tanaka T, Okamoto F, Sohmiya K, Kawamura K. Lack of myocardial iodine-123 15-(p-iodiphenyl)-3-R,S-methylpentadecanoic acid (BMIPP) uptake and CD36 abnormality-CD36 deficiency and hypertrophic cardiomyopathy. Jpn Circ J. 1997;61:724–725. doi: 10.1253/jcj.61.724. [DOI] [PubMed] [Google Scholar]
- 214.Tang W, Ma Y, Jia L, Ioannou YA, Davies JP, Yu L. Niemann-Pick C1-like 1 is required for an LXR agonist to raise plasma HDL cholesterol in mice. Arterioscler Thromb Vasc Biol. 2008;28:448–454. doi: 10.1161/ATVBAHA.107.160465. [DOI] [PubMed] [Google Scholar]
- 215.Tarugi P, Averna M, Di Leo E, Cefalu AB, Noto D, Magnolo L, Cattin L, Bertolini S, Calandra S. Molecular diagnosis of hypobetalipoproteinemia: an ENID review. Atherosclerosis. 2007;195:e19–e27. doi: 10.1016/j.atherosclerosis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 216.Temel RE, Lee RG, Kelley KL, Davis MA, Shah R, Sawyer JK, Wilson MD, Rudel LL. Intestinal cholesterol absorption is substantially reduced in mice deficient in both ABCA1 and ACAT2. J Lipid Res. 2005;46:2423–2431. doi: 10.1194/jlr.M500232-JLR200. [DOI] [PubMed] [Google Scholar]
- 217.Temel RE, Sawyer JK, Yu L, Lord C, Degirolamo C, McDaniel A, Marshall S, Wang N, Shah R, Rudel LL, Brown JM. Biliary sterol secretion is not required for macrophage reverse cholesterol transport. Cell Metab. 2010;12:96–102. doi: 10.1016/j.cmet.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic Niemann-Pick C1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Teng B, Burant CF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;260:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- 220.Teraguchi M, Ohkohchi H, Ikemoto Y, Higashino H, Kobayashi Y. CD36 deficiency and absent myocardial iodine-123-(R,S)-15-(p-iodophenyl)-3-methylpentadecanoic acid uptake in a girl with cardiomyopathy. Eur J Pediatr. 2003;162:264–266. doi: 10.1007/s00431-002-1118-2. [DOI] [PubMed] [Google Scholar]
- 221.Thomson AB, Keelan M, Clandinin MT, Walker K. Dietary fat selectively alters transport properties of rat jejunum. J Clin Invest. 1986;77:279–288. doi: 10.1172/JCI112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Thumser AE, Storch J. Liver and intestinal fatty acid-binding proteins obtain fatty acids from phospholipid membranes by different mechanisms. J Lipid Res. 2000;41:647–656. [PubMed] [Google Scholar]
- 223.Tran TT, Poirier H, Clement L, Nassir F, Pelsers MM, Petit V, Degrace P, Monnot MC, Glatz JF, Abumrad NA, Besnard P, Niot I. Luminal lipid regulates CD36 levels and downstream signaling to stimulate chylomicron synthesis. J Biol Chem. 2011;286:25201–25210. doi: 10.1074/jbc.M111.233551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tranchant T, Besson P, Hoinard C, Delarue J, Antoine JM, Couet C, Gore J. Mechanisms and kinetics of alpha-linolenic acid uptake in Caco-2 clone TC7. Biochim Biophys Acta. 1997;1345:151–161. doi: 10.1016/s0005-2760(96)00171-3. [DOI] [PubMed] [Google Scholar]
- 226.Tso P, Sun W, Liu M. Gastrointestinal satiety signals IV. Apolipoprotein A-IV. Am J Physiol Gastrointest Liver Physiol. 2004;286:G885–G890. doi: 10.1152/ajpgi.00511.2003. [DOI] [PubMed] [Google Scholar]
- 227.Turley SD, Valasek MA, Repa JJ, Dietschy JM. Multiple mechanisms limit the accumulation of unesterified cholesterol in the small intestine of mice deficient in both ACAT2 and ABCA1. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1012–G1022. doi: 10.1152/ajpgi.00190.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Valasek MA, Clarke SL, Repa JJ. Fenofibrate reduces intestinal cholesterol absorption via PPARalpha-dependent modulation of NPC1L1 expression in mouse. J Lipid Res. 2007;48:2725–2735. doi: 10.1194/jlr.M700345-JLR200. [DOI] [PubMed] [Google Scholar]
- 229.Valasek MA, Weng J, Shaul PW, Anderson RG, Repa JJ. Caveolin-1 is not required for murine intestinal cholesterol transport. J Biol Chem. 2005;280:28103–28109. doi: 10.1074/jbc.M504609200. [DOI] [PubMed] [Google Scholar]
- 230.Van der Velde AE, Vrins CL, van den Oever K, Kunne C, Oude Elferink RP, Kuipers F, Groen AK. Direct intestinal cholesterol secretion contributes significantly to total fecal neutral sterol excretion in mice. Gastroenterology. 2007;133:967–975. doi: 10.1053/j.gastro.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 231.Van der Velde AE, Vrins CL, van den Oever K, Seemann I, Oude Elferink RP, van Eck M, Kuipers F, Groen AK. Regulation of direct transintestinal cholesterol excretion in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G203–G208. doi: 10.1152/ajpgi.90231.2008. [DOI] [PubMed] [Google Scholar]
- 232.Verdery RB. Reverse cholesterol transport from fibroblasts to high density lipoproteins: computer solutions of a kinetic model. Can J Biochem. 1981;59:586–592. doi: 10.1139/o81-081. [DOI] [PubMed] [Google Scholar]
- 233.Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010;6:578–588. doi: 10.1038/nrendo.2010.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang LJ, Wang J, Li N, Ge L, Li BL, Song BL. Molecular characterization of the NPC1L1 variants identified from cholesterol low absorbers. J Biol Chem. 2011;286:7397–7408. doi: 10.1074/jbc.M110.178368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wang PY, Caspi L, Lam CK, Chari M, Li X, Light PE, Gutierrez-Juarez R, Ang M, Schwartz GJ, Lam TK. Upper intestinal lipids trigger a gut-brain-liver axis to regulate glucose production. Nature. 2008;452:1012–1016. doi: 10.1038/nature06852. [DOI] [PubMed] [Google Scholar]
- 236.Watanabe K, Ohta Y, Toba K, Ogawa Y, Hanawa H, Hirokawa Y, Kodama M, Tanabe N, Hirono S, Ohkura Y, Nakamura Y, Kato K, Aizawa Y, Fuse I, Miyajima S, Kusano Y, Nagamoto T, Hasegawa G, Naito M. Myocardial CD36 expression and fatty acid accumulation in patients with type I and II CD36 deficiency. Ann Nucl Med. 1998;12:261–266. doi: 10.1007/BF03164911. [DOI] [PubMed] [Google Scholar]
- 237.Westergaard H, Dietschy JM. The mechanism whereby bile acid micelles increase the rate of fatty acid and cholesterol uptake into the intestinal mucosal cell. J Clin Invest. 1976;58:97–108. doi: 10.1172/JCI108465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Westerterp KR. Perception, passive overfeeding and energy metabolism. Physiol Behav. 2006;89:62–65. doi: 10.1016/j.physbeh.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 239.Woerle HJ, Albrecht M, Linke R, Zschau S, Neumann C, Nicolaus M, Gerich J, Goke B, Schirra J. Importance of changes in gastric emptying for postprandial plasma glucose fluxes in healthy humans. Am J Physiol Endocrinol Metab. 2008;294:E103–E109. doi: 10.1152/ajpendo.00514.2007. [DOI] [PubMed] [Google Scholar]
- 240.Xie C, Li N, Chen ZJ, Li BL, Song BL. The small GTPase Cdc42 interacts with Niemann-Pick C1-like 1 (NPC1L1) and controls its movement from endocytic recycling compartment to plasma membrane in a cholesterol-dependent manner. J Biol Chem. 2011;286:35933–35942. doi: 10.1074/jbc.M111.270199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Xie Y, Luo J, Kennedy S, Davidson NO. Conditional intestinal lipotoxicity in Apobec-1−/− Mttp-IKO mice: a survival advantage for mammalian intestinal apolipoprotein B mRNA editing. J Biol Chem. 2007;282:33043–33051. doi: 10.1074/jbc.M705386200. [DOI] [PubMed] [Google Scholar]
- 242.Xie Y, Nassir F, Luo J, Buhman K, Davidson NO. Intestinal lipoprotein assembly in apobec-1−/− mice reveals subtle alterations in triglyceride secretion coupled with a shift to larger lipoproteins. Am J Physiol Gastrointest Liver Physiol. 2003;285:G735–G746. doi: 10.1152/ajpgi.00202.2003. [DOI] [PubMed] [Google Scholar]
- 243.Xie Y, Newberry EP, Young SG, Robine S, Hamilton RL, Wong JS, Luo J, Kennedy S, Davidson NO. Compensatory increase in hepatic lipogenesis in mice with conditional intestine-specific Mttp deficiency. J Biol Chem. 2006;281:4075–4086. doi: 10.1074/jbc.M510622200. [DOI] [PubMed] [Google Scholar]
- 244.Yamashita S, Hirano K, Kuwasako T, Janabi M, Toyama Y, Ishigami M, Sakai N. Physiological and pathological roles of a multi-ligand receptor CD36 in atherogenesis; insights from CD36-deficient patients. Mol Cell Biochem. 2007;299:19–22. doi: 10.1007/s11010-005-9031-4. [DOI] [PubMed] [Google Scholar]
- 245.Yanai H, Chiba H, Fujiwara H, Morimoto M, Takahashi Y, Hui SP, Fuda H, Akita H, Kurosawa T, Kobayashi K, Matsuno K. Metabolic changes in human CD36 deficiency displayed by glucose loading. Thromb Haemost. 2001;86:995–999. [PubMed] [Google Scholar]
- 246.Yang Y, Chen M, Georgeson KE, Harmon CM. Mechanism of oleoylethanolamide on fatty acid uptake in small intestine after food intake and body weight reduction. Am J Physiol Regul Integr Comp Physiol. 2007;292:R235–R241. doi: 10.1152/ajpregu.00270.2006. [DOI] [PubMed] [Google Scholar]
- 247.Yasuda T, Grillot D, Billheimer JT, Briand F, Delerive P, Huet S, Rader DJ. Tissue-specific liver X receptor activation promotes macrophage reverse cholesterol transport in vivo. Arterioscler Thromb Vasc Biol. 2010;30:781–786. doi: 10.1161/ATVBAHA.109.195693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Yen CL, Cheong ML, Grueter C, Zhou P, Moriwaki J, Wong JS, Hubbard B, Marmor S, Farese RV., Jr Deficiency of the intestinal enzyme acyl CoA:monoacylglycerol acyltransferase-2 protects mice from metabolic disorders induced by high-fat feeding. Nat Med. 2009;15:442–446. doi: 10.1038/nm.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Yen CL, Farese RV., Jr MGAT2, a monoacylglycerol acyltransferase expressed in the small intestine. J Biol Chem. 2003;278:18532–18537. doi: 10.1074/jbc.M301633200. [DOI] [PubMed] [Google Scholar]
- 250.Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic review series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Yu L, Hammer RE, Li-Hawkins J, Von Bergmann K, Lutjohann D, Cohen JC, Hobbs HH. Disruption of Abcg5 and Abcg8 in mice reveals their crucial role in biliary cholesterol secretion. Proc Natl Acad Sci USA. 2002;99:16237–16242. doi: 10.1073/pnas.252582399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Yu L, Li-Hawkins J, Hammer RE, Berge KE, Horton JD, Cohen JC, Hobbs HH. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yue P, Chen Z, Nassir F, Bernal-Mizrachi C, Finck B, Azhar S, Abumrad NA. Enhanced hepatic apoA-I secretion and peripheral efflux of cholesterol and phospholipid in CD36 null mice. PLoS One. 2010;5:e9906. doi: 10.1371/journal.pone.0009906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, Ho M, Mandel H, McIntyre A, Kennedy SM, Painter GF, Veerapen N, Besra GS, Cerundolo V, Yue S, Beladi S, Behar SM, Chen X, Gumperz JE, Breckpot K, Raper A, Baer A, Exley MA, Hegele RA, Cuchel M, Rader DJ, Davidson NO, Blumberg RS. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest. 2010;120:2889–2899. doi: 10.1172/JCI42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang XJ, Zhou LH, Ban X, Liu DX, Jiang W, Liu XM. Decreased expression of CD36 in circumvallate taste buds of high-fat diet induced obese rats. Acta Histochem. 2011;113:663–667. doi: 10.1016/j.acthis.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 256.Zhu J, Lee B, Buhman KK, Cheng JX. A dynamic, cytoplasmic triacylglycerol pool in enterocytes revealed by ex vivo and in vivo coherent anti-Stokes Raman scattering imaging. J Lipid Res. 2009;50:1080–1089. doi: 10.1194/jlr.M800555-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]