Abstract
The core species of the family Planistromellaceae are included in the teleomorphic genera Planistroma and Planistromella and the connected anamorphic, coelomycetous genera Alpakesa, Kellermania, and Piptarthron. These genera have been defined primarily on the basis of ascospore septation or number of conidial appendages. Due to a lack of DNA sequence data, phylogenetic placement of these genera within the Dothideomycetes, evaluation of monophyly, and questions about generic boundaries could not be adequately addressed in the past. Isolates of nearly all of the known species in these genera were studied genetically and morphologically. DNA sequence data were generated for the nSSU, ITS, nLSU, and RPB1 markers and analysed phylogenetically. These results placed the Planistromellaceae, herein recognised as a distinct family, in an unresolved position relative to other genera within the order Botryosphaeriales. Species representing the core genera of the Planistromellaceae formed a clade and evaluation of its topology revealed that previous morphology-based definitions of genera resulted in an artificial classification system. Alpakesa, Kellermania, Piptarthron, Planistroma, and Planistromella are herein recognised as belonging to the single genus Kellermania. The following new combinations are proposed: Kellermania crassispora, K. dasylirionis, K. macrospora, K. plurilocularis, and K. unilocularis. Five new species are described, namely K. con- fusa, K. dasylirionicola, K. micranthae, K. ramaleyae, and K. rostratae. Descriptions of species in vitro and a key to species known from culture are provided.
Keywords: Agavaceae, Ascomycota, Asparagaceae, Botryosphaeriaceae, Botryosphaeriales, coelomycetes, Dothideomycetes, molecular phylogeny, Planistromellaceae, Septoplaca, taxonomy
INTRODUCTION
Kellermania was established by Ellis & Everhart (1885) to accommodate an unusual coelomycete, K. yuccigena, with large, cylindrical, and septate conidia that were considered stipitate, and it occurred on dead leaves of Yucca (Asparagaceae, subfamily Agavoideae sensu APG III (2009) = Agavaceae in earlier classifications). The stipes of the conidia described by Ellis & Everhart (1885) are interpreted as apical appendages (Sutton 1968). Höhnel (1918a) adopted the generic name Piptarthron that was provisionally suggested by Montagne for Septoria macrospora, a coelomycetous species without appendages that occurred on senescing leaves of Agave (Asparagaceae, subfamily Agavoideae = Agavaceae in earlier classifications). He provided a generic diagnosis and comparison to Kellermania. Subramanian & Ramakrishnan (1954) established the coelomycetous genus Alpakesa, which was characterised in part by conidia with multiple apical appendages, when their study revealed that Neottiospora yuccifolia, a species found on dead leaves of Yucca, was not congeneric with the type of Neottiospora.
Several other studies have added new species to Alpakesa, Kellermania, and Piptarthron and/or provided revised generic circumscriptions. Sutton (1968) restricted Kellermania to species with simple, blastic conidiophores; septate, hyaline, and appendage-bearing conidia; and sclerotioid, pycnidial conidiomata, while discussing segregate genera and excluding many names classified in Kellermania. Morgan-Jones et al. (1972a) re-examined the type species of Alpakesa, modified the generic circumscription, and added two species. Morgan-Jones et al. (1972b) revised the concepts of the two Kellermania species that were accepted by Sutton (1968), added two new species, and included taxa with or without a single apical conidial appendage. Sutton (1977) synonymised the Yucca-inhabiting genus Septoplaca with Piptarthron, but he was unable to determine which species was represented by the type, S. limbata. Sutton (1980) later treated S. limbata as Piptarthron limbatum and subsequently as P. yuccae (Sutton 1983). Sutton (1980) maintained Alpakesa, Kellermania, and Piptarthron for genera having multiple, single, or no conidial appendages, respectively. Sutton (1980) also noted the possibility of a broadly expanded Kellermania for all species in the complex. Nag Raj (1993) treated Alpakesa as a later synonym of Kellermania and listed the unexamined or excluded taxa.
Ramaley (1991, 1992, 1993, 1995, 1998) provided the next major advances in the study of this group of fungi by discovering and describing the sexual states of Kellermania and Piptarthron, adding new species, and reviewing several coelomycetous species. The genus Planistroma, the sexual state of Piptarthron, was characterised by subepidermal, ostiolate ascomata in multilocular stromata with bitunicate asci and lackling paraphyses (Ramaley 1991). Ramaley (1992) added another species of Planistroma, P. obtusilunatum, with unilocular conidiomata more typical of Kellermania. She noted the intermediate nature of the asexual state in that the lack of apical appendages suggested placement in Piptarthron while the presence of unilocular conidiomata supported placement in Kellermania. Ramaley (1993) circumscribed Planistromella, the sexual state of Kellermania, and noted that it was similar to Planistroma but distinct in having septate ascospores. The genus originally included two new species that were correlated with one new and one existing species of Kellermania (Ramaley 1993). She did not embrace synonymising the asexual and sexual states into a broadly defined Planistroma with Kellermania anamorphs. Ramaley (1995) added five new species based on both asexual and sexual states and provided a key to the species of Kellermania and Piptarthron. Most notably among the new species was Planistromella torsifolium, the first sexual state having Alpakesa-type conidia, which provided further support for the synonymy of Alpakesa and Kellermania (Ramaley 1995). Lastly, Ramaley (1998) named two more teleomorphic species connected with known species of Kellermania and illustrated another undescribed species with both Piptarthron and Planistroma states. Planistroma kellermaniae, which has aseptate ascospores, was connected to the anamorphic Kellermania nolinae, a species with Alpakesa-type conidia, providing further confusion in regard to generic boundaries and Ramaley (1998) suggested that a re-evaluation was necessary.
Barr (1996) observed that several genera in the Dothideales were not classified easily into any family, and she established the family Planistromellaceae to accommodate “taxa having ascostromata, interthecial tissues, and schizogenously formed, periphysate ostioles”. Planistromella and Planistroma were included and three species were transferred to the former genus along with circumstantial links to anamorphs for two species (Barr 1996). The genera Loratospora, Eruptio, Microcyclus, and Mycosphaerellopsis were also classified in Planistromellaceae.
Due to the availability of a number of Ramaley’s cultures that represent nearly all of the known species of Alpakesa, Kellermania, and Piptarthron and additional cultures obtained from plant disease interceptions at U.S. ports of entry, a systematic study was made to address the following questions: 1) What are the phylogenetic relationships between members of the Planistromellaceae and the Dothideomycetes?; 2) are the Planistromellaceae and its genera monophyletic?; 3) does morphology of conidial appendages or ascospore septation correlate with phylogeny?; and 4) are slight morphological differences among otherwise similar isolates, which are often obtained from different hosts, indications of distinct phylogenetic species? To answer these questions, nuclear protein-coding DNA and nuclear ribosomal DNA sequence data were generated for several loci and analysed phylogenetically. Additionally, detailed studies were made of these species in culture, including micromorphological characters. Herbarium specimens were examined whenever possible and/or necessary.
MATERIALS AND METHODS
Morphology and herbarium material
Dried herbarium material was rehydrated and viewed in 3 % KOH (Largent et al. 1977), and microscopic observations of cultures were made of material mounted in 3 % KOH or buffered Shear’s mounting fluid (Graham 1959). Length to width ratios are given as Q. Mean values for length, width and Q are indicated by Lm, Wm, and Qm, respectively, based on n = 30. Herbarium acronyms follow Thiers (2012). See Farr & Rossman (2012) for additional information about collections housed at the U.S. National Fungus Collections (BPI). New specimens were deposited at BPI. The Ramaley collections from which many of the cultures utilized in this study were obtained, if extant, are missing.
Cultures
Isolates were grown in plastic Petri plates on Difco potato-dextrose agar (PDA), which was prepared according to the manufacturers’ instructions. Growth conditions were 24 °C with a 12 h light/dark regimen. Cultures were measured and photographed after 2 wk. Notes on colour and general appearance were made after 2–3 wk. Terminology for colour includes general terms as well as standard terminology with the sample reference code in parentheses from Kornerup & Wanscher (1967). Cultures that had not sporulated after 3 wk were continuously incubated under the same conditions for up to several months and periodically re-examined. Reference cultures were deposited at the Centraalbureau voor Schimmelcultures (CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; Table 1).
Table 1.
Isolates and sequences employed in this study. Taxon names in bold indicate newly generated strains and data.
| GenBank accesion no.2 |
|||||||
|---|---|---|---|---|---|---|---|
| Species | Isolate culture no.1 | Host | Locality | SSU | LSU | ITS | RPB1 |
| Bagnisiella examinans | CBS 551.66 | Lantana camara | India | GU296139 | EU167562 | GU301803 | n/a |
| Barriopsis fusca | CBS 174.26 | Citrus sp. | Cuba | EU673182 | EU673330 | DQ377857 | n/a |
| Botryosphaeria corticis | ATCC 22927 | Vaccinium sp. | USA | EU673176 | DQ299247 | EU673245 | n/a |
| Botryosphaeria dothidea | CBS 110302 | Vitis vinifera | Portugal | EU673174 | AY259092 | EU673243 | n/a |
| ‘Botryosphaeria’ tsugae | CBS 418.64 | Tsuga heterophylla | Canada | EU673208 | DQ458888 | DQ377867 | n/a |
| Diplodia corticola | CBS 112546 | Quercus ilex | Spain | EU673207 | AY259090 | EU673262 | n/a |
| Diplodia cupressi | CBS 168.87 | Cupressus sempervirens | Israel | EU673209 | DQ458893 | EU673263 | n/a |
| Diplodia mutila | CBS 112553 | Vitis vinifera | Portugal | EU673213 | AY259093 | AY928049 | n/a |
| Diplodia pinea A | CBS 109727 | Pinus radiata | South Africa | EU673220 | DQ458897 | EU673269 | n/a |
| Diplodia rosulata | CBS 116470 | Prunus africana | Ethiopia | EU673211 | EU430265 | DQ377896 | n/a |
| Diplodia scrobiculata | CBS 109944 | Pinus greggii | Mexico | EU673218 | DQ458899 | EU673268 | n/a |
| Diplodia seriata | CBS 119049 | Vitis sp. | Italy | EU673216 | DQ458889 | EU673266 | n/a |
| Dothiorella iberica | CBS 113188 | Quercus suber | Spain | EU673156 | AY573198 | EU673230 | n/a |
| Dothiorella sarmentorum | CBS 115038 | Malus pumila | Netherlands | EU673159 | AY573206 | DQ377860 | n/a |
| Guignardia bidwellii | CBS 111645 | Parthenocissus quinquefolia | USA | EU673223 | FJ824766 | DQ377876 | n/a |
| Guignardia citricarpa | CBS 102374 | Citrus aurantium | Brasil | FJ824759 | FJ824767 | DQ377877 | n/a |
| Guignardia philoprina | CBS 447.68 | Taxus baccata | Netherlands | FJ824760 | FJ824768 | DQ377878 | n/a |
| Helicomyces roseus | CBS 283.51 | submerged bark, in brook | Switzerland | AY856928 | AY916464 | AY856881 | n/a |
| Kellermania anomala | AR 3471, CBS 132218 | Yucca brevifolia | USA | JX444899 | JX444853 | JX444869 | JX444884 |
| Kellermania confusa | AR 3469, CBS 131723 | Yucca thornberi | USA | n/a | JX444854 | JX444870 | JX444885 |
| Kellermania crassispora | AR 3463, CBS 131714 | Nolina micrantha | USA | JX444900 | JX444855 | JX444871 | JX444886 |
| Kellermania dasylirionicola | AR 3465, CBS 131720 | Dasylirion leiophyllum | USA | JX444901 | JX444856 | JX444872 | JX444887 |
| Kellermania dasylirionis | AR 3464, CBS 131715 | Dasylirion leiophyllum | USA | n/a | JX444857 | JX444873 | JX444888 |
| Kellermania macrospora | AR 3468, CBS 131716 | Agave sp. | USA | JX444902 | JX444858 | JX444874 | JX444889 |
| Kellermania micranthae | AR 3474, CBS 131724 | Nolina micrantha | USA | JX444903 | JX444859 | JX444875 | JX444890 |
| Kellermania nolinae | AR 3475, CBS 131717 | Nolina erumpens | USA | JX444904 | JX444860 | JX444876 | JX444891 |
| Kellermania nolinifoliorum | AR 3473, CBS 31718 | Nolina microcarpa | USA | JX444905 | JX444861 | JX444877 | JX444892 |
| Kellermania plurilocularis | AR 3467, CBS 131719 | Yucca baccata | USA | n/a | JX444862 | JX444878 | JX444893 |
| Kellermania ramaleyae | MEP 1260, CBS 131722 | Yucca sp. | Mexico | n/a | JX444863 | JX444879 | JX444894 |
| Kellermania rostratae | JB 5.16.11-01, CBS 131721 | Yucca rostrata | Mexico | n/a | JX444864 | JX444880 | JX444895 |
| Kellermania unilocularis | AR 3466 (dead) | Yucca baccata | USA | n/a | JX444865 | n/a | n/a |
| Kellermania uniseptata | AR 3476, CBS 131725 | Yucca rupicola | USA | JX444906 | JX444866 | JX444881 | JX444896 |
| Kellermania yuccifoliorum | AR 3472, CBS 131726 | Yucca brevifolia | USA | JX444907 | JX444867 | JX444882 | JX444897 |
| Kellermania yuccigena | AR 3470, CBS 131727 | Yucca filamentosa | USA | JX444908 | JX444868 | JX444883 | JX444898 |
| Lasiodiplodia crassispora | CBS 110492 | unknown | unknown | EU673189 | EF622086 | EU673251 | n/a |
| Lasiodiplodia gonubiensis | CBS 115812 | Syzygium cordatum | South Africa | EU673193 | DQ458892 | DQ377902 | n/a |
| Lasiodiplodia parva | CBS 356.59 | Theobroma cacao | Sri Lanka | EU673200 | EF622082 | EU673257 | n/a |
| Lasiodiplodia pseudotheobromae | CBS 116459 | Gmelina arborea | Costa Rica | EU673199 | EF622077 | EU673256 | n/a |
| Lasiodiplodia rubropurpurea | CBS 118740 | Eucalyptus grandis | Australia | EU673191 | DQ103553 | DQ377903 | n/a |
| Lasiodiplodia theobromae | CAA 006 | Vitis vinifera | USA | EU673197 | DQ458891 | EU673254 | n/a |
| Lasiodiplodia venezuelensis | CBS 118739 | Acacia mangium | Venezuela | EU673192 | DQ103547 | DQ377904 | n/a |
| Macrophomina phaseolina | AFTOL 1783, CBS 227.33 | Zea mays | unknown | DQ678037 | n/a | DQ377906 | n/a |
| Melanops sp. | CBS 118.39 | Quercus borealis | USA | FJ824763 | FJ824771 | DQ377856 | n/a |
| Melanops tulasnei | CBS 116805 | Quercus robur | Germany | FJ824761 | FJ824769 | FJ824764 | n/a |
| Neodeightonia phoenicum | CBS 122528 | Phoenix dactylifera | Spain | EU673205 | EU673340 | EU673261 | n/a |
| Neodeightonia subglobosa | CBS 448.91 | keratomycosis in human eye | United Kingdom | EU673202 | EU673337 | DQ377866 | n/a |
| Neofusicoccum luteum | CBS 110299 | Vitis vinifera | Portugal | EU673148 | AY259091 | AY928043 | n/a |
| Neofusicoccum mangiferae | CBS 118531 | Mangifera indica | Australia | EU673153 | AY615185 | DQ377920 | n/a |
| Neofusicoccum parvum | CBS 110301 | Vitis vinifera | Portugal | EU673150 | AY259098 | AY928046 | n/a |
| Phaeobotryon mamane | CPC 12264 | Sophora chrysophylla | USA (Hawaii) | EU673183 | EU673331 | DQ377898 | n/a |
| Phaeobotryosphaeria citrigena | ICMP 16812 | Citrus sinensis | New Zealand | EU673180 | EU673328 | EU673246 | n/a |
| Phaeobotryosphaeria porosa | CBS 110496 | Vitis vinifera | South Africa | EU673179 | AY343379 | DQ377894 | n/a |
| Phaeobotryosphaeria visci | CBS 100163 | Viscum album | Luxembourg | EU673177 | EU673324 | DQ377870 | n/a |
| Pseudofusicoccum stromaticum | CBS 117448 | Eucalyptus sp. | Venezuela | EU673146 | AY693974 | DQ377931 | n/a |
| Saccharata proteae | CBS 115206 | Protea sp. | Australia | GU296194 | n/a | GU301869 | n/a |
| Spencermartinsia viticola | CBS 117006 | Vitis vinifera | Spain | EU673166 | AY905555 | EU673236 | n/a |
1 AFTOL: Assembling the Fungal Tree of Life; AR: Culture collection of Amy Rossman, housed at U.S. National Fungus Collections (BPI), Beltsville, MD, USA; ATCC: American Type Culture Collection, Manassas, VA, USA; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CAA: Artur Alves, Universidade de Aveiro, Aveiro, Portugal; ICMP: International Collection of Microorganisms from Plants, Auckland, New Zealand; JB: Joseph Bischoff, cultures housed at BPI; MEP: Mary E. Palm, cultures housed at BPI.
2 SSU: partial 18S rRNA gene; LSU: partial 28S rRNA gene; ITS: internal transcribed spacer regions 1 & 2 including 5.8S rRNA gene; RPB1: partial RNA Polymerase II subunit 1 gene.
DNA extraction, PCR amplification, and sequencing
DNA was extracted from fresh mycelium using Qiagen’s DNeasy Plant Mini Kit (Germantown, MD). Ribosomal DNA from the nuclear small subunit (SSU), the internal transcribed spacer region (ITS; ITS1, 5.8S, ITS2), and the nuclear large subunit (LSU) were PCR amplified using the primer pairs NS1 and NS4 (White et al. 1990), ITS5 and ITS4 (White et al. 1990), LROR (Moncalvo et al. 2000) and LR7 or LR5 (Vilgalys & Hester 1990), respectively. Additionally, a portion of the largest subunit of the RNA polymerase II (RPB1) was amplified using the primer pair RPB1-Ac and RPB1-Cr (Matheny et al. 2002). Each region was amplified using GoTaq (Promega, Madison, WI) and associated standard reagents following the manufacturer’s recommendations including 2.0 mM MgCl2 and 1.5 μM of each primer. Thermal cycling conditions for RPB1 and LSU were according to Malkus et al. (2006) and Reeb et al. (2004), respectively. Thermal cycling conditions for the SSU and ITS were: 95 °C for 60 s; 35 cycles at 95 °C for 15 s, 50 °C (55 °C for ITS) for 20 s, and 72 °C for 60 s; and 72 °C for 180 s. Cycle sequencing and fluorescent labelling was conducted with the same corresponding PCR primers that were used for each locus and the BigDye Terminator v. 3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA). The purified products were then sequenced on an ABI 3730 automated DNA sequencer. Geneious Pro v. 5 (Drummond et al. 2010) was used to edit electropherograms and to build consensus sequences that were submitted to GenBank (http://www.ncbi.nlm.nih.gov; Table 1).
Data matrix and phylogenetic analysis
For the purpose of determining the phylogenetic position of Planistromellaceae among the Dothideomycetes, sequences of the SSU and LSU from the type species of Kellermania, Piptarthron, Planistroma, and Planistromella were manually incorporated into the alignment of Schoch et al. (2009) using the program Geneious Pro v. 5 (Drummond et al. 2010; i.e., Dothideomycetes alignment). Identical sequences were removed from this alignment. A Maximum Likelihood (ML) analysis was conducted in RAxML v. 7.3.0 (Stamatakis 2006) using the ‘RAxML-HPC2 on XSEDE’ tool via the CIPRES Science Gateway (Miller et al. 2010), selecting the GTRGAMMA nucleotide substitution model and Opegrapha dolomitica and Schismatomma decolorans as outgroups (Schoch et al. 2009). A separate rapid bootstrap (bs) analysis of 1 000 iterations was conducted with identical settings.
Multiple-sequence alignments were conducted for each of the datasets from the Planistromellaceae in Geneious Pro v. 5 (Drummond et al. 2010) using MUSCLE v. 3.6 (Edgar 2004), adjusted manually, and then concatenated (i.e., Kellermania alignment). Congruence among these four data partitions (SSU, ITS, LSU, RPB1) was evaluated by comparing their topologies in search of well-supported clades (posterior probability > 0.85) with conflicting compositions, and separately with the Incongruence Length Difference test (ILD; Farris et al. 1994). The individual topologies were constructed with Bayesian inference (BI) in MrBayes v. 3.1.2 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003) where best-fitting models for each dataset were determined in MrModeltest v. 2.2 (Nylander 2004) by the Akaike Information Criterion (AIC; Posada & Buckley 2004). All other parameters were left as default. The posterior probability (pp) distribution of trees was estimated from those collected until the standard deviation of split frequencies reached less than 0.01 (1 million generations) minus the burn-in (10 %), which was determined in the program Tracer v. 1.5 (Rambaut & Drummond 2007). The ILD test was implemented in PAUP v. 4.0 b10 (Swofford 2003) as the Partition Homogeneity Test with 1 000 homogeneity replicates. Each replicate searched tree space with 100 random addition sequences (RAS) saving 10 trees per RAS while ignoring uninformative characters.
The monophyly of core Planistromellaceae was tested by conducting a phylogenetic analysis of a matrix comprised of SSU, ITS, and LSU from a wide range of Botryosphaeriaceae selected from the analyses of Crous et al. (2006), Phillips & Alves (2009), and Schoch et al. (2009); plus type species of genera of Kellermania, Piptarthron, Planistroma, and Planistromella as well as at least one representative of each major clade of core Planistromellaceae; and Helicomyces roseus (CBS 283.51) as outgroup (i.e., Botryosphaeriaceae alignment). This matrix was aligned with MUSCLE v. 3.6 (Edgar 2004) and analysed with BI, as described above.
The major clades of the core Planistromellaceae were identified from the results of a mixed-model BI analysis of the Kellermania alignment plus Helicomyces roseus (CBS 283.51) in MrBayes v. 3.1.2 using the methods outlined above. This phylogeny was also used to i) test the monophyly of generic concepts in Planistromellaceae; ii) determine relationships among its species including novel taxa; and iii) evaluate the evolution of key morphological characters and host relationships. All alignments and resulting trees were deposited into TreeBASE (S13234), and nomenclatural novelties in MycoBank (Crous et al. 2004).
RESULTS
Data matrix and phylogenetic analyses
Sequencing was successful for all core Planistromellaceae taxa with living cultures except for the SSU of Kellermania confusa AR 3469, K. dasylirionis AR 3464, K. plurilocularis AR 3467, K. ramaleyae MEP 1260, and K. rostratae JB 5.16.11-01, which were treated as missing data in all alignments. The Dothideomycetes alignment contained 342 ingroup taxa with a total length of 2 880 characters, 837 (29 %) of which were phylogenetically informative (Table 2). The Botryosphaeriaceae alignment included 45 ingroup taxa (Table 1) with a total length of 2 091 characters, 208 (10 %) of which were phylogenetically informative (Table 2). The Kellermania alignment contained 15 ingroup taxa with a total length of 3 054 characters, 210 (7 %) of which were phylogenetically informative (Table 2).
Table 2.
A summary of matrix partition statistics (ingroup only) for each alignment which was analyzed phylogenetically in the present study. N = the number of taxonomic units in the alignment, Length = the number of nucleotide characters in the alignment, No. V.C. = the number of variable characters, No. I.C. = the number of phylogenetically informative characters, DNA model = the model of nucleotide substition used in an analysis for the corresponding partition.
| Dothideomycetes alignment |
Botryosphaeriaceae alignment |
Kellermania alignment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Length | No. V.C. | No. I.C. | DNA Model | N | Length | No. V.C. | No. I.C. | DNA Model | N | Length | No. V.C. | No. I.C. | DNA Model | |
| SSU | 283 | 1027 | 473 (46%) | 295 (29%) | N.A. | 44 | 1133 | 94 (8%) | 42 (4%) | GTR+I+G | 10 | 1025 | 9 (1%) | 5 (0.5%) | HKY+I |
| ITS | N.A. | N.A. | N.A. | N.A. | N.A. | 42 | 377 | 130 (34%) | 92 (24%) | SYM+I+G | 15 | 351 | 45 (13%) | 27 (8%) | K80+I+G |
| LSU | 327 | 1853 | 689 (37%) | 542 (29%) | N.A. | 45 | 584 | 134 (23%) | 82 (14%) | GTR+I+G | 15 | 883 | 29 (3%) | 18 (2%) | GTR+I+G |
| RPB1 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. | 15 | 795 | 221 (28%) | 160 (20%) | GTR+G |
| Combined | 342 | 2880 | 1162 (40%) | 837 (29%) | GTRGAMMA | 45 | 2091 | 302 (14%) | 208 (10%) | Mixed | 15 | 3054 | 304 (10%) | 210 (7%) | Mixed |
Phylogenetic relationships of the Planistromellaceae
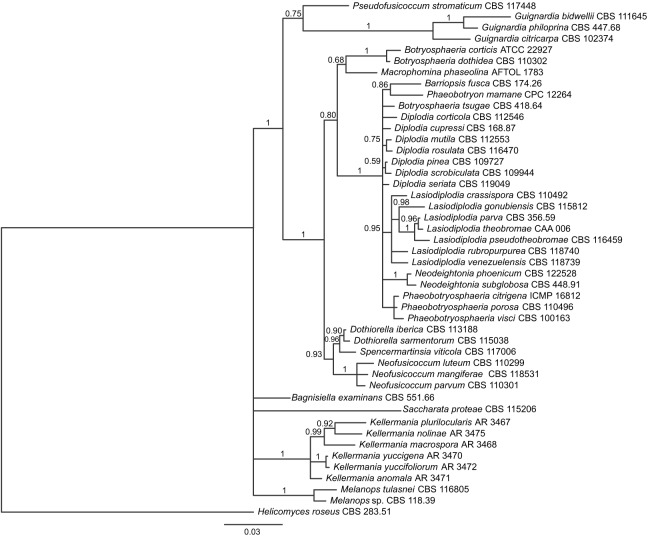
Maximum Likelihood analysis of Dothideomycetes revealed high support (bs = 91 %) for a clade comprised strictly of Botryosphaeriaceae that was congruent with Schoch et al. (2009) with the exception of a well-supported clade (bs = 99 %) containing the type species of the genera Kellermania, Piptarthron, Planistroma, and Planistromella of Planistromellaceae (phylogram not shown). Bayesian inference of the Botryosphaeriaceae alignment suggested that Planistromellaceae is one of five monophyletic members of Botryosphaeriaceae s.l. (see Discussion): i) Bagnisella examinans; ii) Saccharata proteae; iii) Melanops (pp = 1.0); iv) Planistromellaceae (pp = 1.0); and v) the remaining members of the family including the core members of Botryosphaeriaceae (pp = 1.0; Fig. 1).
Fig. 1.
Bayesian inference 50 % majority-rule phylogram of the Botryosphaeriaceae alignment based on analysis of the combined SSU, ITS, and LSU data. This tree reveals a monophyletic Kellermania (core Planistromellaceae) in an unresolved position among four other major ingroup lineages of Botryosphaeriales.
Phylogenetic diversity and relationships within Kellermania
Inspection of the individual phylogenies resulting from BI analyses of SSU, ITS, LSU, and RPB1 revealed a single well-supported incongruence. This incongruence was between the SSU and RPB1 trees where Kellermania macrospora AR 3468 was sister with a clade containing K. crassispora AR 3463, K. dasylirionicola AR 3465, K. micranthae AR 3474, and K. nolinae AR 3475 in the SSU analysis (pp = 0.94); whereas K. macrospora was part of a polytomy (pp = 1.0) in the RPB1 analysis containing two other clades, one comprised of K. uniseptata AR 3476, K. yuccifoliorum AR 3472, and K. yuccigena AR 3470, and another containing K. anomala AR 3471 and K. nolinifoliorum AR 3473. Results of the ILD test (Farris et al. 1994) suggested that the SSU, ITS, LSU, and RPB1 data from Planistromellaceae were congruent (P = 0.230) and thus suitable for combined analysis.
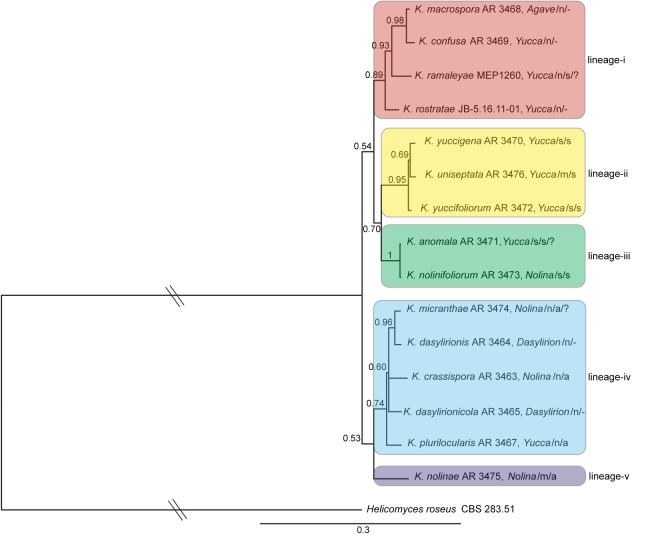
Our BI analysis of the Kellermania alignment resulted in a consensus phylogram (Fig. 2) comprised of five major lineages: i) Kellermania macrospora AR 3468, K. confusa AR 3469, K. ramaleyae MEP 1260, and K. rostratae JB5.16.11-01 (pp = 0.89); ii) K. yuccigena AR 3470, K. uniseptata AR 3476, and K. yuccifoliorum AR 3472 (pp = 0.95); iii) K. anomala AR 3471, K. nolinifoliorum AR 3473 (pp = 1.0); iv) K. micranthae AR 3474, K. dasylirionis AR 3464, K. crassispora AR 3463, K. dasylirionicola AR 3465, and K. pluilocularis AR 3467 (pp = 0.74); and v) K. nolinae AR 3475 (Fig. 2). Lineage v, which is based upon a single isolate, is herein labelled as distinct because its position among the other lineages is unresolved and varied in different analyses. The relationships among these five lineages are not well resolved though some major groupings are weakly supported and a sister relationship of lineages ii–iii receives some support (pp = 0.70). Members of lineages i–iii were all isolated from either Agave or Yucca (Asparagaceae, subfamily Agavoideae), with the exception of K. nolinifoliorum AR 3473 on Nolina (Asparagaceae, subfamily Nolinoideae). Members of lineages iv–v were isolated from members of Nolina or Dasylirionis (subfamily Nolinoideae) with the exception of K. pluri- locularis AR 3467 on Yucca (Fig. 2). Lineages i–iii possess septate ascospores and lineages iv–v possess aseptate ascospores, but see K. unilocularis below and in Fig. 3. Lineage i possesses conidia without conidial appendages, lineage ii possesses conidia with single or multiple appendages, lineage iii possesses conidia with single appendages, lineage iv possesses conidia without appendages, and lineage v possesses conidia with multiple appendages.
Fig. 2.
Bayesian inference 50 % majority-rule phylogram of Kellermania (core Planistromellaceae) based on analysis of the combined SSU, ITS, LSU, and RPB1 alignment. Hatch marks indicate that the branch length was shortened by 50 % for presentation purposes. This tree reveals five lineages, i–v. Host genus, numbers of conidial appendages, and ascospore septation are given next to taxon names in that order. For conidial appendages, n = none, s = single, m = multiple. For ascospore septation, a = aseptate, s = septate, - = unknown. A question mark indicates uncertainty in the link of asexual and sexual states.
Fig. 3.
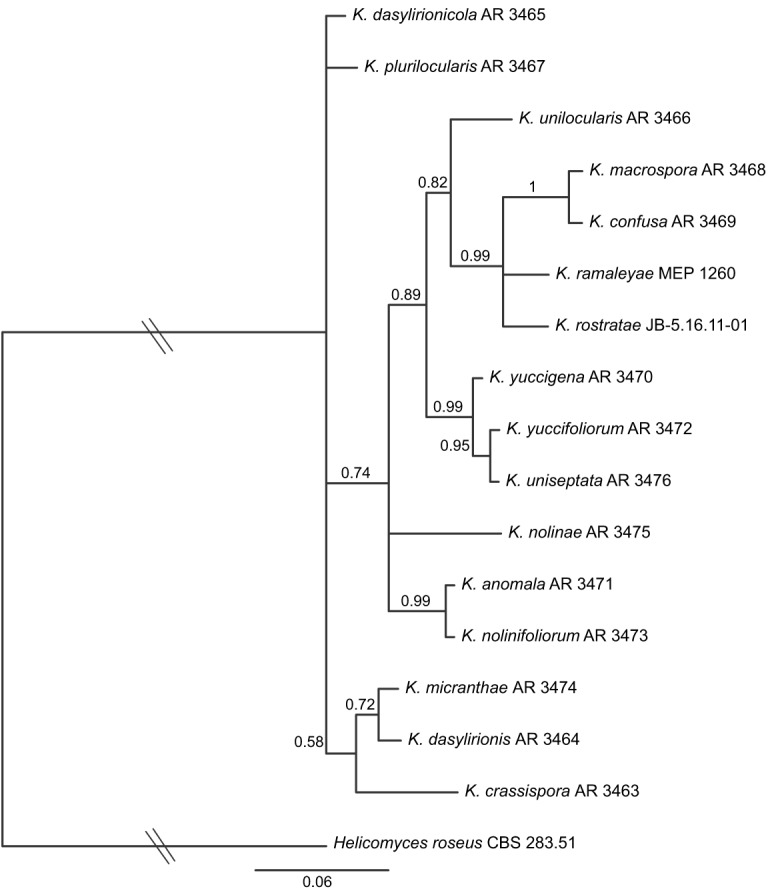
Bayesian inference 50 % majority-rule phylogram of Kellermania based on analysis of the ITS, including K. unilocularis. Hatch marks indicate that the branch was shortened by 50 % for presentation purposes.
Colony characteristics and micromorphology of Kellermania
Colony colour typically ranged from greenish to greyish tones, but K. crassispora uniquely remained pink. Differences were observed in growth rates, but in general slight differences in growth rates and overall colony appearances were observed from subculture to subculture. Thus, tendencies toward slower vs faster growth rates are more useful than exact measurements. Conidiomata were produced in culture by all species except K. crassispora. They are characterised as: superficial or immersed, scattered to densely gregarious, at times confluent becoming fused, discrete or associated with stromatal growths including irregularly column-like and rarely mat-like structures, frequently more or less round in shape, with or without necks, black, at times covered with whitish, greenish, or green grey hyphae. Conidiogenous cells lining the inner conidiomatal walls were holoblastic, determinate, discrete or integrated on short single-celled conidiophores, doliiform to cylindrical, hyaline, and smooth. Conidia were variable in size and shape, aseptate, single to multiseptate, and bear 0–multiple apical appendages. A less useful character included the presence of a frill at the base of conidia. Conidia, which developed within weeks or sometimes only after months, were exuded from conidiomata in whitish, mucilaginous masses. Conidia often germinate quickly and, in some several month old cultures, nearly all conidia in the mucilaginous masses had either germinated or desiccated. In the descriptions of cultures, colony appearance, growth rate, and conidia are the most valuable characters for distinguishing species.
Taxonomy
Planistromellaceae M.E. Barr, Mycotaxon 60: 433. 1996.
Type genus. Planistromella A.W. Ramaley.
Kellermania Ellis & Everh., J. Mycol. 1: 153. 1885.
Type species. Kellermania yuccigena Ellis & Everh.
= Piptarthron Mont. ex Höhn., Hedwigia: 60: 203. 1918.
Type species. Piptarthron macrosporum (Durieu & Mont.) Höhn.
= Alpakesa Subram. & K. Ramakr., J. Indian Bot. Soc. 33: 204. 1954.
Type species. Alpakesa yuccifolia (J.G. Hall) Subram. & K. Ramakr. as ‘yuccaefolia’.
?= Septoplaca Petr., Sydowia 17: 271. 1964 ‘1963’.
Type species. Septoplaca limbata Petr.
= Planistroma A.W. Ramaley, Mycotaxon 42: 69. 1991.
Type species. Planistroma yuccigenum A.W. Ramaley as ‘yuccigena’.
= Planistromella A.W. Ramaley, Mycotaxon 47: 260. 1993.
Type species. Planistromella yuccifoliorum A.W. Ramaley.
Kellermania anomala (Cooke) Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 124: 84. 1915. — Fig. 4, 5
Fig. 4.
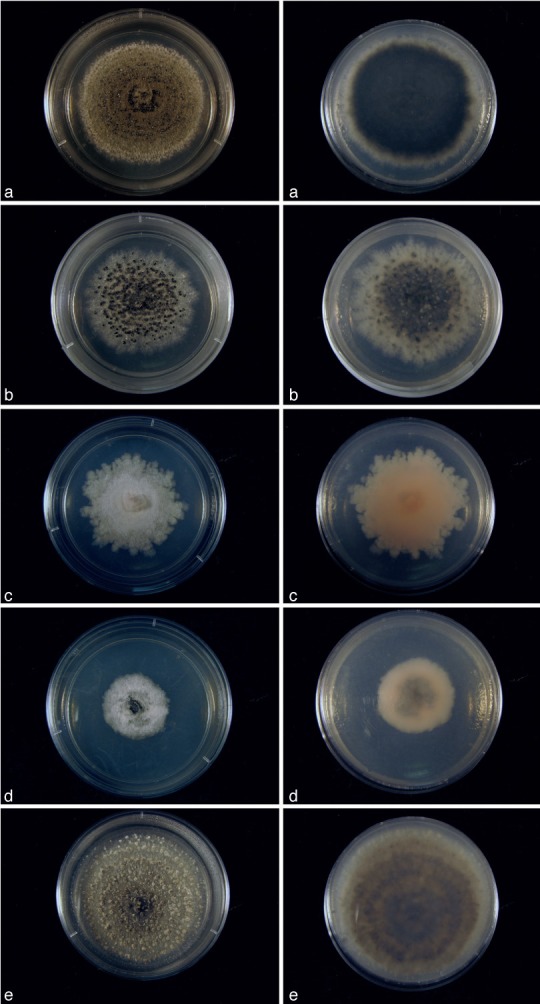
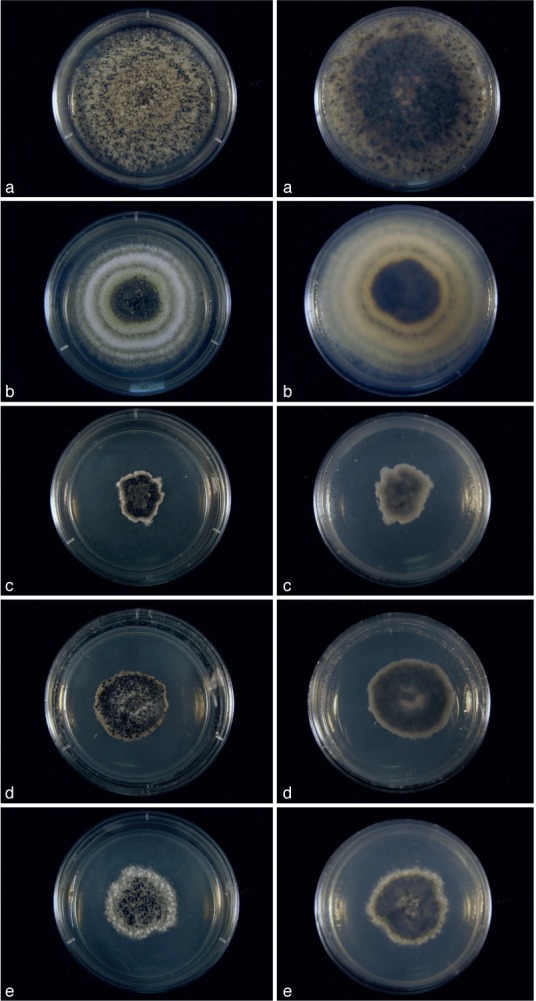
Cultures of Cultures of Kellermania species. a–e. Cultures (surface and reverse) on PDA at 2 wk after incubation at 24 °C with a 12 h light/dark regime; a. K. ano- mala (AR 3471); b. K. confusa (AR 3469); c. K. crassispora (AR 3463); d. K. dasylirionicola (AR 3465); e. K. dasylirionis (AR 3464).
Fig. 5.
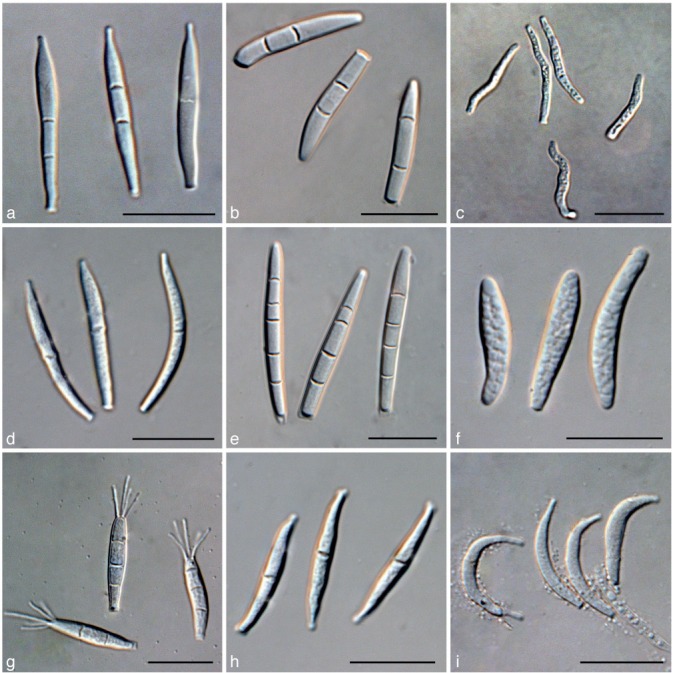
a. Conidia of Kellermania anomala from culture (AR 3471); b. conidia of K. confusa from culture (AR 3469); c. conidia of K. dasylirionicola from culture (AR 3465); d. conidia of K. dasylirionis from culture (AR 3464); e. conidia of K. macrospora from culture (AR 3468); f. conidia of K. micranthae from culture (AR 3474); g. conidia of K. nolinae from culture (AR 3475); h. conidia of K. nolinifoliorum from culture (AR 3473); i. conidia of K. plurilocularis from culture (AR 3467). — Scale bars = 30 μm for all.
Basionym. Discella anomala Cooke, Grevillea 7: 11. 1878.
≡Discula anomala (Cooke) Sacc., Syll. Fung. 3: 677. 1884.
?= Wettsteinina yuccigena M.E. Barr, Contr. Univ. Michigan Herb. 9: 547. 1972 as ‘yuccaegena’.
≡Planistromella yuccigena (M.E. Barr) M.E. Barr, Mycotaxon 60: 434. 1996.
Culture characteristics — Colonies on PDA 43–44 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near dull green (30E4), covered with low, cobwebby to velutinous, greenish hyphae; margin uneven, whitish; reverse black with margin uncoloured. Conidia 40–69 × 5–8 μm, Q = 5–14.3 (Lm = 52.3 m, W = 52.3 μm, Wm = 7.3 m, Q = 7.3 μm, Qm = 7.4), cylindrical to fusiform, apices tapering to a relatively acute point, tapering towards and typically truncate at bases that may bear an indistinct frill, 1–2(–3)-septate; walls smooth, thin and hyaline, and not significantly constricted at septa; contents hyaline; appendages absent or present, at times scarcely visible, up to 11 μm long, apical, single, appearing as a short, filiform, hyaline mucro.
Habitat & Distribution — Dead leaves of Yucca spp. (Morgan-Jones et al. 1972b, Nag Raj 1993, Farr & Rossman 2012). The type was found on Y. draconis (Cooke 1878), probably correctly named Y. brevifolia. This fungal species is widely distributed in the western half of the USA (Morgan-Jones et al. 1972b, Farr & Rossman 2012).
Specimen examined. USA, Arizona, Mohave Co., 0.3 miles from Gem Acres Rd., exit mile 20 from U.S. Hwy. 40, on dead leaves of Yucca brevifolia, 3 June 1992, coll. A.W. Ramaley, AR 3471 (CBS 132218) isolated by A.W. Ramaley from AWR 9228 (9229 on tube), dried culture on PDA (BPI 882814).
Notes — Ramaley (1993) noted that the appendage may be hard to observe or absent and that the middle cell in conidia with two septa is regularly shorter. We observed that the appendage (when present in culture) was shorter than in previous reports from material in nature (Morgan-Jones et al. 1972b, Nag Raj 1993). Shoemaker & Babcock (1987) redescribed P. yuccigena on Yucca glauca. Barr (1996) provisionally linked Planistromella yuccigena to K. anomala based on the circumstantial occurrence of both on the same leaves. This link is indirectly supported by the morphological similarity of the sexual state to that of K. nolinifoliorum, a sister species.
Kellermania confusa Minnis & A.H. Kenn., sp. nov. — MycoBank MB801095; Fig. 4, 5
Etymology. The name refers to confusion in regard to the identity of this fungus.
Culture characteristics — Colonies on PDA 35–40 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near pale grey (30B1), covered with low, white cobwebby to velutinous hyphae; margin uneven, whitish; reverse grey with black conidiomata visible. Conidia 38.5–64 × 6.5–9.5 μm, Q = 4.8–8 (Lm = 49.9 m, W = 49.9 μm, Wm = 8.3 m, Q = 8.3 μm, Qm = 6.0), obclavate, at times curved, apices tapering towards and slightly acute, typically truncate at bases, (1–)2(–3)-septate; walls smooth, thin, hyaline, and not constricted at septa; contents hyaline; appendages absent.
Habitat & Distribution — Dead leaves of Yucca thornberi. It is known only from the type locality in the USA: AZ.
Specimen examined. USA, Arizona, Santa Cruz Co., Interstate 10, mile 283.6, north side of road, on dead leaves of Yucca thornberi, 13 Apr. 1992, coll. A.W. Ramaley, AR 3469 (CBS 131723) isolated by A.W. Ramaley from AWR 9212, dried culture on PDA (holotype, BPI 882824).
Notes — This species is distinguished from the others that occur on Yucca by its (1–)2(–3)-septate conidia that lack appendages. Ramaley (in litt.) noted that two isolates identified as Piptarthron macrosporum, this one from Yucca and another from Agave, had consistent differences in conidial morphology. Morphological and DNA sequence data support the separation of this species from Kellermania macrospora on Agave.
Kellermania crassispora (A.W. Ramaley) Minnis & A.H. Kenn., comb. nov. — MycoBank MB801096; Fig. 4
Basionym. Piptarthron crassisporum A.W. Ramaley, Mycotaxon 55: 261. 1995.
= Planistroma nolinae A.W. Ramaley, Mycotaxon 55: 258. 1995.
Culture characteristics — Colonies on PDA 30–36 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near pinkish white (10A2) to cotton candy-pink, covered with low, velutinous hyphae; margin uneven, whitish to pale pink; reverse near pale red (7A3). No conidiomata observed. No conidia observed.
Habitat & Distribution — Dead leaves of Nolina micrantha, Nolina sp. (Ramaley 1995, this study). It is known from the USA: NM, TX (Ramaley 1995, this study).
Specimen examined. USA, Texas, Culberson Co., Guadalupe Mtns. National Park, 5.6 miles from highway along Williams Ranch Rd., on dead leaves of Nolina micrantha, 23 Oct. 1995, coll. A.W. Ramaley, AR 3463 (CBS 131714) isolated by A.W. Ramaley from AWR 9536, dried culture on PDA (BPI 882815).
Notes — The culture that we studied did not sporulate though Ramaley (1995) observed conidia in culture. Ramaley (1995) stated that conidia on the host were 56.8–78.4 × 12.8–14.4 μm, cylindrical with rounded apices, aseptate, and without appendages. The link between the asexual and sexual states is based on cultural similarities that were noted previously (Ramaley 1995).
Kellermania dasylirionicola Minnis & A.H. Kenn., sp. nov. — MycoBank MB801097; Fig. 4, 5
Etymology. This species is named for its occurrence on Dasylirion leiophyllum.
Culture characteristics — Colonies on PDA 22–25 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface whitish to near pinkish white (10A2), covered with low, tomentose to velutinous hyphae; margin uneven, whitish; reverse near orange white (5A2). Conidia 32–45 × 3–5 μm, Q = 6.7–12.5 (Lm = 39.4 m, W = 39.4 μm, Wm = 4.5 μm, Qm = 9.0), more or less cylindrical with irregularly curved shape, flexuous, apices tapering to a somewhat acute point, typically truncate at bases, aseptate; walls smooth, thin, and hyaline; contents hyaline; appendages absent.
Habitat & Distribution — Dead leaves of Dasylirion leiophyllum. It is known only from the type locality in the USA: TX.
Specimen examined. USA, Texas, Pecos Co., 1.2 miles north of T 2400 on U.S. Hwy. 285, roadside plant, on dead leaves of Dasylirion leiophyllum, 7 May 1994, coll. A.W. Ramaley, AR 3465 (CBS 131720) isolated by A.W. Ramaley from AWR 9413, dried culture on PDA (holotype, BPI 882821).
Notes — Although similar to Kellermania dasylirionis, this second species on Dasylirion is distinguished by its aseptate conidia with irregular, curved shapes and slower growth on PDA. Ramaley (in litt.) noted aseptate, curly conidia in material from the host in nature and speculated that the collection may represent an undescribed species. DNA sequence data and associated analyses confirm this as a new species.
Kellermania dasylirionis (A.W. Ramaley) Minnis & A.H. Kenn., comb. nov. — MycoBank MB801098; Fig. 4, 5
Basionym. Piptarthron dasylirionis A.W. Ramaley, Mycotaxon 55: 263. 1995.
Culture characteristics — Colonies on PDA 45–52 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near greenish grey (30C2), covered with low, velutinous hyphae that often covers conidiomata; margin uneven, whitish; reverse near brownish grey (7E2) to greyish orange (5B3). Conidia 45–64 × 5–9.5 μm, Q = 6.3–12.3 (Lm = 57.1 m, W = 57.1 μm, Wm = 7.2 m, Q = 7.2 μm, Qm = 8.1), cylindrical to narrowly fusiform with some degree of curvature, apices tapering to a relatively acute point, tapering towards and typically truncate at bases, 0–1-septate, septa approx. median when present; walls smooth, thin, hyaline, and not constricted at septa; contents hyaline, at times granular; appendages absent.
Habitat & Distribution — Dead leaves of Dasylirion leiophyllum, D. wheeleri, Dasylirion sp. (Ramaley 1995, this study). This species is known from Mexico and the USA: AZ, TX (Ramaley 1995, this study).
Specimen examined. USA, Texas, Brewster Co., Big Bend National Park, Sotol Vista, on dead leaves of Dasylirion leiophyllum, 25 Oct. 1994, coll. A.W. Ramaley, AR 3464 (CBS 131715) isolated by A.W. Ramaley from AWR 9441, dried culture on PDA (BPI 882816).
Notes — Ramaley (1995) noted that conidia in culture were shorter and narrower than those on the host in nature.
Kellermania macrospora (Durieu & Mont.) Minnis & A.H. Kenn., comb. nov. — MycoBank MB801099; Fig. 5, 6
Fig. 6.
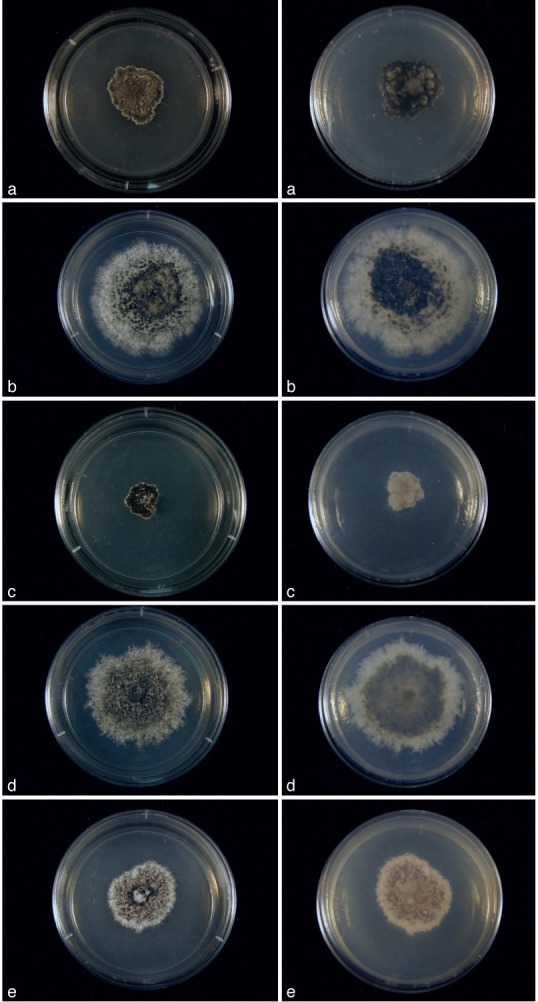
Cultures of Cultures of Kellermania species. a–e. Cultures (surface and reverse) on PDA at 2 wk after incubation at 24 °C with a 12 h light/dark regime; a. K. macro- spora (AR 3468); b. K. micranthae (AR 3474); c. K. nolinae (AR 3475); d. K. nolinifoliroum (AR 3473); e. K. plurilocularis (AR 3467).
Basionym. Septoria macrospora Durieu & Mont., Expl. Sci. Algerie 1: 589. 1849.
≡Hendersonia montagnei Cooke, Nuovo Giorn. Bot. Ital. 10: 19. 1878. Note: This nom. nov. was established since the epithet ‘macrospora’ was occupied by H. macrospora Berk. & Broome 1850.
≡Hendersonia piptarthra Sacc., Michelia 2: 111. 1880. Note: This nom. nov. was established since the epithet ‘macrospora’ is occupied by H. macrospora Berk. & Broome 1850. It is a nom. illeg. via superfluous, ICBN Art. 52 (McNeill et al. 2006), since H. montagnei was already published as a replacement name.
≡Stagonospora macrospora (Durieu & Mont.) Sacc., Syll. Fung. 3: 450. 1883.
≡Piptarthron macrosporum (Durieu & Mont.) Höhn., Hedwigia 60: 203. 1918.
Culture characteristics — Colonies on PDA 52 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near light grey (30C1), with scattered low, hyphae between conidiomata; margin uneven, whitish; reverse uncoloured, black, to near greyish brown (6D3). Conidia 54.5–93 × 6.5–11 μm, Q = 4.9–13 (Lm = 72.2 m, W = 72.2 μm, Wm = 9.0 m, Q = 9.0 μm, Qm = 8.3), cylindrical to obclavate, at times slightly curved, apices tapering towards and obtuse to slightly acute, typically truncate at bases that frequently bear a marginal frill, 3–5(–7)-septate; walls smooth, thin, hyaline, and not constricted at septa; contents hyaline; appendages absent.
Habitat & Distribution — Dead leaves of Agave americana, Agave sp. (Sutton 1980, Farr & Rossman 2012). Based on scattered reports, this species is known from Africa (Algeria), Europe, and North America (USA) (Höhnel 1918a, Sutton 1980, Farr & Rossman 2012). This species has also been reported from species of Yucca from various locations (Sutton 1980, Farr & Rossman 2012), but we have found no specimens on Yucca.
Specimen examined. USA, Arizona, Cochise Co., north side of road, I 10 mile 322.5, on dead leaves of Agave sp., 13 Apr. 1992, coll. A.W. Ramaley, AR 3468 (CBS 131716) isolated by A.W. Ramaley from AWR 9205, dried culture on PDA (BPI 882817).
Notes — This species was described originally from Agave. Collections on Yucca, including the type of Kellermania multiseptata, have been treated as conspecific (e.g. Sutton 1980), but it appears that K. macrospora is limited to Agave. Planistromella parryi, described originally from Agave shawii (Cooke 1885), Plowrightia agaves, described from Agave sp. (Maublanc 1903), and Plowrightia williamsoniana, described from Agave americana (Kellerman 1906), are potential synonyms (Barr 1996) as well as possibly the sexual state of K. macrospora, but these teleomorphs are poorly known.
Kellermania micranthae Minnis & A.H. Kenn., sp. nov. — MycoBank MB801100; Fig. 5, 6
Etymology. The name is derived from Nolina micrantha, the host.
Culture characteristics — Colonies on PDA 38–48 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near greyish green to greenish grey (30B6–30B2) to greyish green to dull green (30E5–30E4), at times portions covered with low, white, cottony hyphae; margin more or less even, whitish; reverse near greyish green to greenish grey (30C3–30C2). Conidia 43–61 × 6.5–9.5 μm, Q = 4.5–7.8 (Lm = 49.2 m, W = 49.2 μm, Wm = 8.6 m, Q = 8.6 μm, Qm = 5.8), cylindrical to obclavate, at times curved, apices tapering towards and obtuse, rounded or truncate at bases, aseptate; walls smooth, thin, hyaline; contents hyaline, at times granular; appendages absent.
Habitat & Distribution — Dead leaves of Nolina micrantha. This species is known only from the type locality in the USA: TX.
Specimen examined. USA, Texas, Culberson Co., Guadalupe Mtns. National Park, 5.6 miles from highway along Williams Ranch Rd., on dead leaves of Nolina micrantha, 23 Oct. 1995, coll. A.W. Ramaley, AR 3474 (CBS 131724) isolated by A.W. Ramaley from AWR 9536, dried culture on PDA (holotype, BPI 882825).
Notes — This species is distinguished from the others known from Nolina by the combination of its aseptate conidia that lack appendages and its greenish colouration in culture. Ramaley (in litt.) stated that this was an undescribed species and gave it the provisional name, Piptarthron sotoli. An undescribed species (Ramaley 1998) with aseptate, Planistroma-type ascospores, which was found on the type specimen of P. kellermaniae on Nolina erumpens in Texas, has an anamorph that is similar to K. micranthae and the two species may be conspecific.
Kellermania nolinae (Pollack) Nag Raj, in Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 442. 1993. — Fig. 5, 6
Basionym. Bartalinia nolinae Pollack, Mycologia 39: 620. 1947.
≡Alpakesa nolinae (Pollack) Morgan-Jones, Nag Raj & W.B. Kendr., Canad. J. Bot. 50: 879. 1972.
= Planistroma kellermaniae A.W. Ramaley, Mycotaxon 66: 510. 1998.
Culture characteristics — Colonies on PDA 9–20 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near greenish grey (25C2), forming tiers of raised mounds, smooth to grainy, at times portions covered with scattered white, velutinous, aerial hyphae; margin uneven, whitish; reverse near greyish white (25B1). Conidia 35–48 × 8–11 μm, Q = 3.7–6 (Lm = 40.5 m, W = 40.5 μm, Wm = 8.8 m, Q = 8.8 μm, Qm = 4.7), fusiform to obclavate, at times slightly curved, tapering or not towards generally obtuse apices, tapering towards and typically truncate at bases that frequently bear a frill, 2–3-septate; walls smooth, thin and hyaline, and not constricted at septa; contents hyaline; appendages present, 8–24 μm long, apical, 3–5, filiform, unbranched, hyaline.
Habitat & Distribution — Dead leaves of Nolina erumpens, N. microcarpa (Pollack 1947, Ramaley 1998, this study). It is known from the USA: AZ, TX (Pollack 1947, Ramaley 1998, this study).
Specimen examined. USA, Texas, Brewster Co., Big Bend National Park, 3.75 miles from U.S. Hwy. 385 on road to The Basin, on dead leaves of Nolina erumpens, 9 May 1994, coll. A.W. Ramaley, AR 3475 (CBS 131717) isolated by A.W. Ramaley from AWR 9408, dried culture on PDA (BPI 882818).
Notes — The link between the asexual and sexual states is based on the production of characteristic conidia in cultures derived from asci (Ramaley 1998).
Kellermania nolinifoliorum A.W. Ramaley, Mycotaxon 55: 255. 1995. — Fig. 5, 6
= Planistromella nolinifoliorum A.W. Ramaley, Mycotaxon 66: 509. 1998.
Culture characteristics — Colonies on PDA 23–26 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near dark grey (28F1), at times portions covered with scattered white, cobwebby, aerial hyphae; margin uneven, whitish, somewhat slimy in appearance; reverse near light grey to dark grey (28D1–28E1) with off white margin. Conidia 37–57.5 × 5–9.5 μm, Q = 5.6–9.3 (Lm = 49.1 m, W = 49.1 μm, Wm = 7.1 m, Q = 7.1 μm, Qm = 7.0), fusiform, straight, slightly curved to somewhat sigmoid, apices tapering to a relatively acute point, tapering towards and typically truncate at bases that may bear mucilaginous material, approx. medianly 1-septate; walls smooth, thin and hyaline, and not significantly constricted at septa; contents hyaline; appendages present or scarcely visible, perhaps absent, up to 5 μm long, apical, single, appearing as a blunt, mucilaginous mucro.
Habitat & Distribution — Dead leaves of Nolina microcarpa, N. micrantha, Nolina sp. (Ramaley 1995, 1998, this study). This fungus is known from the USA: AZ, NM, TX (Ramaley 1995, 1998, this study).
Specimen examined. USA, Arizona, Yarapai Co., 0.1 mile north of Big Creek, west side of Hwy. 17 at mile 262.1, on dead leaves of Nolina microcarpa, 19 July 1996, coll. A.W. Ramaley, AR 3473 (CBS 131718) isolated by A.W. Ramaley from AWR 9610, dried culture on PDA (BPI 882819).
Notes — The connection between the asexual and sexual state is based on the production of characteristic conidia in cultures obtained from asci (Ramaley 1998).
Kellermania plurilocularis (A.W. Ramaley) Minnis & A.H. Kenn., comb. nov. — MycoBank MB801101; Fig. 5, 6
Basionym. Piptarthron pluriloculare A.W. Ramaley, Mycotaxon 42: 63. 1991.
= Planistroma yuccigenum A.W. Ramaley, Mycotaxon 42: 69. 1991 as ‘yuccigena’.
Culture characteristics — Colonies on PDA 20–29 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near greenish grey (30D2), at times portions covered with white, cottony hyphae; margin uneven, whitish; reverse near light grey (18D1). Conidia 30.5–56 × 5–8 μm, Q = 4.2–11.7 (Lm = 41.5 m, W = 41.5 μm, Wm = 6.0 m, Q = 6.0 μm, Qm = 7.2), slightly curved, falcate, to strongly curved nearing U-shapes, apices tapering to a relatively acute point, typically truncate at bases, aseptate; walls smooth, thin and hyaline; contents hyaline; appendages absent.
Habitat & Distribution — Dead leaves of Yucca baccata (Ramaley 1991, this study). This fungus is known only from the USA: CO (Ramaley 1991, this study).
Specimen examined. USA, Colorado, La Plata Co., Durango, along bike trail between Chapman Hill ski slope and Lion’s Den, on dead leaves of Yucca baccata, 8 June 2000, coll. A.W. Ramaley, AR 3467 (CBS 131719) isolated by A.W. Ramaley from AWR 2001b, dried culture on PDA (BPI 882820).
Notes — The size range of the conidia of the holotype of Piptarthron pluriloculare was reported as (48–)59–76(–98) × (4–)5.5–7(–8) μm (Ramaley 1991). The conidial length of the present isolate (AR 3467) in culture was much shorter, but the overall appearance of the conidia was basically the same as those of the holotype. The link between the asexual and sexual states is based on cultural similarities (Ramaley 1991).
Kellermania ramaleyae Minnis, M.E. Palm & Rossman, sp. nov. — MycoBank MB801102; Fig. 7, 8
Fig. 7.
Cultures of Cultures of Kellermania species. a–e. Cultures (surface and reverse) on PDA at 2 wk after incubation at 24 °C with a 12 h light/dark regime; a. K. rama- leyae (MEP 1260); b. K. rostratae (JB 5.16.11-01); c. K. uniseptata (AR 3476); d. K. yuccifoliorum (AR 3472); e. K. yuccigena (AR 3470).
Fig. 8.
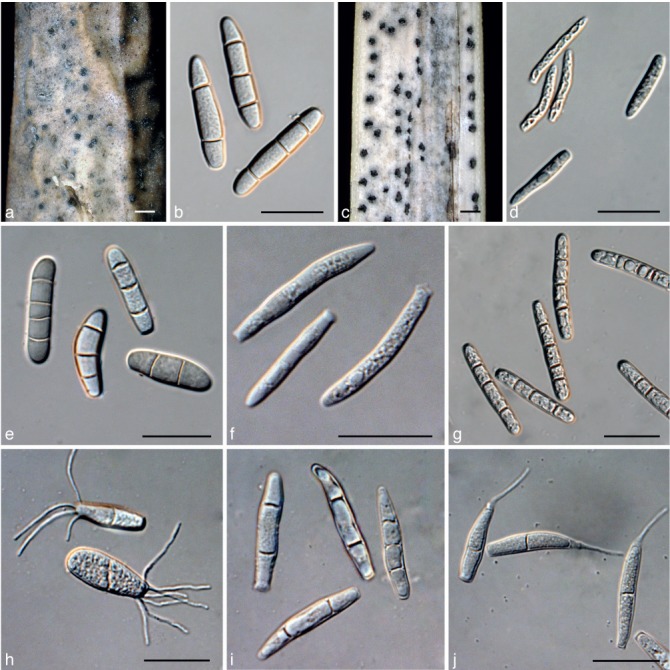
a. Stromata of Kellermania ramaleyae (holotype, BPI 525045) on leaf of Yucca sp.; b. conidia of K. ramaleyae (holotype, BPI 525045) on leaf of Yucca sp.; c. stromata of K. rostratae (holotype, BPI 884092) on leaf of Y. rostrata; d. conidia of K. rostratae (holotype, BPI 884092) on leaf of Y. rostrata; e. conidia of K. ramaleyae from culture (MEP 1260); f. conidia of K. rostratae from culture (JB 5.16.11-01); g. conidia of K. unilocularis (isotype of Piptarthron uniloculare, BPI 1110167) on leaf of Y. baccata; h. conidia of K. uniseptata from culture (AR 3476); i. conidia of K. yuccifoliorum from culture (AR 3472); j. conidia of K. yucci- gena from culture (AR 3470). — Scale bars = 1 mm for stromata, = 30 μm for conidia.
Etymology. This species is named in honour of Annette W. Ramaley for her outstanding contributions towards the study of Planistromellaceae and coelomycetous anamorphs.
On host (holotype): Foliicolous, stromata approx. 0.5–0.75 mm diam, scattered to gregarious, round in top view, subepidermal, immersed becoming erumpent after peeling back of disc-shaped epidermal tissue, black, multilocular. Conidiophores absent. Conidiogenous cells lining the basal and lateral, locular walls, more or less doliiform to slightly cylindrical. Conidia 48–70.5 × 11–16 μm, Q = 4.3–5.3 (Lm = 58.8 m, W = 58.8 μm, Wm = 12.6 m, Q = 12.6 μm, Qm = 4.7), cylindrical to slightly clavate or rarely curved, apices obtuse, typically truncate at bases, 2–4(–5)-septate; walls smooth, thin, hyaline, and not significantly constricted at septa; contents hyaline, at times granular; appendages absent.
Culture characteristics — Colonies on PDA 7–21 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near dull green (26D3, 29E4, 30E4,) to dark green (29F4), covered with low, cottony to velutinous hyphae; margin uneven, whitish; reverse multi-coloured: uncoloured, black, near greyish turquoise (24D5), and dull green (27E3). Conidia 35–51 × 9.5–16 μm, Q = 2.6–4.6 (Lm = 42.7 m, W = 42.7 μm, Wm = 12.3 m, Q = 12.3 μm, Qm = 3.5), cylindrical to slightly clavate or rarely curved, apices obtuse, typically truncate at bases, 2–4-septate; walls smooth, thin, hyaline, and not constricted at septa; contents hyaline, at times granular; appendages absent.
Habitat & Distribution — Dead leaves of Yucca sp. It is known only from the material from Mexico intercepted at the Laredo Plant Inspection Station.
Specimen examined. MEXICO, Intercepted at Laredo, Texas, USA on dead leaves of Yucca sp., 2 Dec. 1985, coll. S. Vesper, MEP 1260 (CBS 131722) isolated by M.E. Palm from BPI 525045 (holotype, as ‘P. macrosporum’), dried culture on PDA (BPI 882823).
Notes — This species is distinguished from the others that occur on Yucca by its conidia in culture with 2–4 septa, Q = 2.6–4.6, and appendages lacking. Some differences were observed in dimensions of conidia produced on the host vs in culture. A loculoascomycete producing tardily septate ascospores that was found on the host may be the sexual state of this species.
Kellermania rostratae Minnis, A.H. Kenn. & J.F. Bisch., sp. nov. — MycoBank MB801103; Fig. 7, 8
Etymology. The name is derived from Yucca rostrata, the host.
On host (holotype): Foliicolous, stromata approx. 0.5–1 mm diam, scattered to gregarious, round to ellipsoid in top view, subepidermal, immersed becoming erumpent after peeling back of disc-shaped epidermal tissue, black, multilocular. Conidiophores absent. Conidiogenous cells lining the basal and lateral, locular walls, more or less doliiform to slightly cylindrical. Conidia 30.5–48 × 5–6.5 μm, Q = 6.3–9.3 (Lm = 41.3 μm, Wm = 5.3 m, Q = 5.3 μm, Qm = 7.8), cylindrical to obclavate, at times curved, apices tapering towards and obtuse to slightly acute, typically truncate at bases, aseptate; walls smooth, thin, and hyaline; contents hyaline, at times granular; appendages absent.
Culture characteristics — Colonies on PDA 52 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface whitish to near greenish grey (30C2), at times portions covered with scattered low, wispy, white hyphae; margin uneven, whitish; reverse uncoloured with conidiomata visible. Conidia 33.5–62.5 × 5–9.5 μm, Q = 4.2–9 (Lm = 44.1 m, W = 44.1 μm, Wm = 7.1 m, Q = 7.1 μm, Qm = 6.3), cylindrical to obclavate, at times curved, apices tapering towards and obtuse to slightly acute, typically truncate at bases, 0–1(–3)-septate; walls smooth, thin, hyaline, and not constricted at septa; contents hyaline, at times granular; appendages absent.
Habitat & Distribution — Dead leaves of Yucca rostrata. This species is known only from an interception of Mexican material.
Specimen examined. MEXICO, Intercepted at Los Indios, Texas, USA on dead leaves of Yucca rostrata, 12 May 2011, coll. A. Garza, JB 5.16.11-01 (CBS 131721) isolated by J.F. Bischoff from BPI 884092 (holotype), dried culture on PDA (BPI 882822).
Notes — This species is distinguished from the others that occur on Yucca by its typically cylindrical to obclavate, 0–1(–3)-septate conidia in culture that lack appendages. Conidia produced on the host were aseptate. In culture, septa were observed on a small percentage of conidia. Some differences in size of conidia were observed between material from host and in culture.
Kellermania unilocularis (A.W. Ramaley) Minnis & A.H. Kenn., comb. nov. — MycoBank MB801104; Fig. 8
Basionym. Piptarthron uniloculare A.W. Ramaley, Mycotaxon 45: 451. 1992.
= Planistroma obtusilunatum A.W. Ramaley, Mycotaxon 45: 450. 1992.
On host (isotype of Piptarthron uniloculare): Conidia 48–72 × 8–9.5 μm, Q = 5–7.6 (Lm = 56.4 m, W = 56.4 μm, Wm = 9.0 m, Q = 9.0 μm, Qm = 6.3), cylindrical, at times curved, apices straight, obtuse, less commonly tapering towards, typically rounded to inconspicuously truncate at bases, 2–4(mostly 3)-septate; walls smooth, thin, hyaline, and not significantly constricted at septa; contents hyaline; appendages absent.
Culture characteristics — Only sterile, irregular stromatal growths observed in the dried culture.
Habitat & Distribution — Dead leaves of several Yucca spp. (Ramaley 1992). The types of both the asexual and sexual states were found on Yucca baccata (Ramaley 1992). This species has been reported from the USA: CO, NV (Ramaley 1992, this study).
Specimens examined. USA, Colorado, La Plata Co., Durango, along bike trail between Chapman Hill ski slope and Lion’s Den, on dead leaves of Yucca baccata, 8 June 2000, coll. A.W. Ramaley, AR 3466 isolated by A.W. Ramaley from AWR 2001a, dried culture (BPI 883224); Ridge S. of Smelter Mountain, Cactus ridge, on dead leaves of Yucca baccata, 10 Feb. 1992, coll. A.W. Ramaley, AWR 9018 (isotype of Piptarthron uniloculare, BPI 1110167).
Notes — The conidia of the isotype were observed to be slightly shorter than reported by Ramaley (1992), and no conidia were observed with 5 septa although Ramaley (1992) reported that some had 5 septa. The culture of this species is dead and only a dried culture of this isolate is extant, but Ramaley (1992) reported that typical conidia were formed in culture. The link between the asexual and sexual states is based on the production of typical conidia in cultures obtained from asci (Ramaley 1992).
An additional analysis was performed as the Kellermania analysis above but with the addition of an existing ITS sequence from K. unilocularis AR 3466. The results suggested a sister relationship between K. unilocularis and lineage i (pp = 0.82). This expanded clade with lineage i was member of a larger clade (pp = 0.89) also containing lineage ii (Fig. 3). The unilocular stromata of K. unilocularis is shared by members of lineage ii having conidia with appendages. Its lack of conidial appendages is shared by members of lineage i having plurilocular stromata. Its aseptate ascospores distinguish it from both lineages. This is the only species in or closely related to lineages i–iii (Fig. 2) with aseptate ascospores. It was excluded from other phylogenetic analyses due to missing data.
Kellermania uniseptata (Morgan-Jones, Nag Raj & W.B. Kendr.) Nag Raj, in Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 443. 1993. — Fig. 7, 8
Basionym. Alpakesa uniseptata Morgan-Jones, Nag Raj & W.B. Kendr., Canad. J. Bot. 50: 879. 1972.
= Planistromella torsifoliorum A.W. Ramaley, Mycotaxon 55: 265. 1995.
Culture characteristics — Colonies on PDA 10–13 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near orange white (6A2), slightly slimy in appearance, at times portions covered with low, cottony hyphae; margin uneven, whitish; reverse off white to nearly concolourous with upper surface. Conidia 27–45 × 8–17.5 μm, Q = 2–4.2 (Lm = 35.8 m, W = 35.8 μm, Wm = 12.4 m, Q = 12.4 μm, Qm = 3.0), cylindrical, fusiform, obclavate, to ellipsoid, apices tapering or not, obtuse, tapering or not towards and typically truncate at bases bearing indistinct frills, approx. medianly 1-septate; walls smooth, thin and hyaline, and not significantly constricted at septa; contents hyaline, at times granular; appendages present, 13–41.5 μm long, apical, 3–5(–8), filiform, unbranched, flexuous, hyaline.
Habitat & Distribution — Dead leaves of Yucca rupicola (Morgan-Jones et al. 1972a, Ramaley 1995, this study). This species is known only from the USA: TX (Morgan-Jones et al. 1972a, Ramaley 1995, this study).
Specimen examined. USA, Texas, Kimble Co., junction of I 10 and U.S. Hwy. 290, on dead leaves of Yucca rupicola, 24 Oct. 1994, coll. A.W. Ramaley, AR 3476 (CBS 131725) isolated by A.W. Ramaley from AWR 9432, dried culture on PDA (BPI 882826).
Notes — The connection of the asexual and sexual states is circumstantial (Ramaley 1995).
Kellermania yuccifolia (J.G. Hall) Nag Raj, in Nag Raj, Coelomycetous anamorphs with appendage-bearing conidia: 443. 1993.
Basionym. Neottiospora yuccifolia J.G. Hall, Phytopathology 5: 57. 1915 as ‘yuccaeafolia’.
≡Alpakesa yuccifolia (J.G. Hall) Subram. & K. Ramakr., J. Indian Bot. Soc. 33: 205. 1954 as ‘yuccaefolia’.
Culture characteristics — This species is not known from culture. It is characterised by its aseptate conidia with multiple appendages (Hall 1915, Morgan-Jones et al. 1972a, Nag Raj 1993).
Habitat & Distribution — Dead leaves of Yucca filamentosa, Y. gloriosa, and Yucca sp. (Morgan-Jones et al. 1972a, Sutton 1980). This species is widespread in the USA based on scattered reports (Morgan-Jones et al. 1972a, Sutton 1980, Farr & Rossman 2012). The type was collected on Yucca sp. in the USA: WA (Hall 1915).
Specimen examined — None.
Notes — No DNA sequence data exist for this species, which is the type of the genus Alpakesa (Subramanian & Ramakrishnan 1954). Considering that the data presented here for other species of Kellermania indicate that conidial septation and appendage number are not important for distinguishing genera, this species belongs in the genus Kellermania. The occurrence of this species on Yucca and its multiple conidial appendages suggest a probable phylogenetic placement in lineage ii.
Kellermania yuccifoliorum A.W. Ramaley, Mycotaxon 47: 262. 1993. — Fig. 7, 8
= Planistromella yuccifoliorum A.W. Ramaley, Mycotaxon 47: 261. 1993.
Culture characteristics — Colonies on PDA 27–40 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface near dark green (30F5–30F3), covered with low, cottony to velutinous hyphae; margin uneven, whitish; reverse grey to black. Conidia 40–65.5 × 8–11 μm, Q = 3.9–7.4 (Lm = 52.6 m, W = 52.6 μm, Wm = 9.8 m, Q = 9.8 μm, Qm = 5.4), cylindrical to obclavate, apices tapering towards and obtuse, typically truncate to less commonly somewhat rounded at bases, 2–3-septate; walls smooth, thin and hyaline, and not significantly constricted at septa; contents hyaline; appendages present, at times scarcely visible, possibly absent, up to 1.5 μm long, apical, single, appearing as a short, dome-like mound.
Habitat & Distribution — Dead leaves of Yucca baccata, Y. brevifolia (holotypes of both names), Y. schidigera, Y. thornberi (Ramaley 1993, this study). This species has been reported from the USA: AZ, CA, UT (Ramaley 1993, this study).
Specimen examined. USA, Arizona, Mohave Co., 0.3 miles from Gem Acres Rd., exit mile 20 from U.S. Hwy. 40, on dead leaves of Yucca brevifolia, 3 June 1992, coll. A.W. Ramaley, AR 3472 (CBS 131726) isolated by A.W. Ramaley from AWR 9229, dried culture on PDA (BPI 882827).
Notes — Ramaley (1993) reported variation in conidial sizes between specimens found on different hosts and between fresh and dried conidia. Given the diversity found in this study, it is difficult to state with certainty that all specimens from all hosts are conspecific. The conidia from the culture examined here were smaller than those reported from the host in nature (Ramaley 1993). The connection of the asexual and sexual states is circumstantial (Ramaley 1993).
Kellermania yuccigena Ellis & Everh., J. Mycol. 1: 154. 1885 as ‘yuccaegena’. — Fig. 7, 8
= Planistromella uniseptata A.W. Ramaley, Mycotaxon 47: 267. 1993
Culture characteristics — Colonies on PDA 19–25 mm after 2 wk at 24 °C with a 12 h light/dark regimen; surface whitish to near pale grey (30B1), covered with cottony hyphae; margin uneven, whitish; reverse greyish with black conidiomata visible. Conidia 37–56 × 8–13 μm, Q = 3.3–6.2 (Lm = 48.2 m, W = 48.2 μm, Wm = 10.1 m, Q = 10.1 μm, Qm = 4.8), cylindrical to somewhat obclavate, apices tapering towards or not and typically obtuse but sometimes slightly acute, tapering or not, typically truncate at bases, approx. medianly 1(–2)-septate; walls smooth, thin and hyaline, and not or slightly constricted at septa; contents hyaline; appendages present, 5–35 μm (Lm = 19.6 μm) long, apical, single, filiform, unbranched to rarely branched with a single bifurcation, flexuous, hyaline.
Habitat & Distribution — Dead leaves of several species of Yucca (Morgan-Jones et al. 1972b, Nag Raj 1993, Ramaley 1993, Farr & Rossman 2012). The type of the asexual state was found on Y. glauca (as Y. angustifolia) (Ellis & Everhart 1885) and the type of the sexual state was found on Y. elata (Ramaley 1993). This species is widely distributed in the western half of the USA (Morgan-Jones et al. 1972b, Ramaley 1993, Farr & Rossman 2012).
Specimen examined. USA, New Mexico, Chaves Co., mile 302.05 on U.S. Hwy. 380, on dead leaves of Yucca filamentosa? , 24 Oct. 1993, coll. A.W. Ramaley, AR 3470 (CBS 131727) isolated by A.W. Ramaley from AWR 9325, dried culture on PDA (BPI 882828).
Notes — The asexual and sexual states were linked through the observation of typical conidia in cultures derived from asci (Ramaley 1993).
Excluded, poorly known and uncertain taxa
Diatrype acervata Ellis & Everh., J. Mycol. 4: 75. 1888.
≡Planistromella acervata (Ellis & Everh.) M.E. Barr, Mycotaxon 60: 434. 1996.
Notes — This species described from Yucca filamentosa in New Jersey (Ellis & Everhart 1888) could be the sexual state of a Kellermania, but confusion around a species complex as well as a Stigmina anamorph have hindered the development of a proper species concept (Barr 1996). Other homotypic synonyms listed in MycoBank are not listed here.
Endothia parryi Farl. ex Cooke, Grevillea 13: 102. 1885.
≡Planistromella parryi (Farl. ex Cooke) M.E. Barr, Mycotaxon 60: 435. 1996.
Notes — This species found on Agave shawii (Cooke 1885) has plurilocular stromata with septate ascospores (Barr 1996). It may represent a distinct species of Kellermania or the sexual state of K. macrospora. Other homotypic synonyms listed in MycoBank are not listed here.
Hypocrea agaves Maubl., Bull. Soc. Mycol. France 19: 292. 1903.
≡Plowrightia agaves (Maubl.) Maubl., Bull. Soc. Mycol. France 23: 143. 1907.
Notes — This species based on material from Agave sp. in Mexico (Maublanc 1903) may be the sexual state of a Kellermania, possibly K. macrospora. It has been treated as a synonym of Planistromella parryi (Barr 1996).
Kellermania attenuata Morgan-Jones, Nag Raj & W.B. Kendr., Canad. J. Bot. 50: 1643. 1972.
Notes — In the protologue, this species was reported to have conidia 70–85 × 3–5 μm, cylindrical, aseptate, appendages lacking (Morgan-Jones et al. 1972b). The type from Mexico occurs on Yucca filifera, but other collections from widespread North American localities occur on other Yucca spp. (Morgan-Jones et al. 1972b). Based on the figure of the conidia in the protologue (Morgan-Jones et al. 1972b), the length to width ratio is 12–15. Though treated as a synonym of Piptarthron limbatum by Sutton (1980), who later treated both of these as synonyms of Piptarthron yuccae (Sutton 1983), this seems to be a distinct species on Yucca.
Kellermania major Dearn. & Barthol., Mycologia 16: 163. 1924.
Notes — In the protologue, this species was reported to have conidia 55–75 × 11–14 μm, 2-septate with the middle cell half the size of the end cells with 1–2 appendages, 15–18 × 3–4 μm (Dearness 1924). According to Dearness (1924), the type from the USA: CA occurs on Hesperoyucca whipplei (as Yucca whipplei; subfam. Agavoideae). There are a few other reports of this species on Yucca spp. from the western USA (Farr & Rossman 2012). Though treated as a synonym of Kellermania anomala by Morgan-Jones et al. (1972b), Sutton (1980), and Nag Raj (1993), this is almost certainly a distinct species given the importance of host associations since it is the only species described originally from the host genus Hesperoyucca.
Kellermania multiseptata Morgan-Jones, Nag Raj & W.B. Kendr., Canad. J. Bot. 50: 1644. 1972.
Notes — In the protologue, this plurilocular species was reported to have conidia 50–68 × 6–7.5 μm, cylindrical to obclavate, aseptate, 3–4-septate, appendages lacking (Morgan-Jones et al. 1972b). The type from the USA: TX occurs on Yucca macrocarpa and an additional collection was reported from the USA: AZ on Yucca brevifolia (Morgan-Jones et al. 1972b). Based on the figure of the conidia in the protologue (Morgan-Jones et al. 1972b), the length to width ratio is 8.2–12. Though treated as a synonym of Piptarthron macrosporum by Sutton (1980), this seems to be a distinct species on Yucca.
Phyllachora yuccae Ellis & Everh., Bull. Torrey Bot. Club 22: 440. 1895.
≡ Piptarthron yuccae (Ellis & Everh.) B. Sutton, Trans. Brit. Mycol. Soc. 81: 407. 1983.
Notes — In the protologue, this species was reported to be an immature ascomycete and a question mark was placed next to the genus to indicate uncertainty about its classification (Ellis & Everhart 1895). According to their observations, the immature asci, if interpreted as conidia, are given as 50–60 × 7–8 μm, cylindrical, tapered towards apices, aseptate. The type from Mexico occurs on Yucca glauca (as Y. angustifolia) (Ellis & Everhart 1895). Based on correspondence with M.E. Barr, Sutton (1983) determined that this species represented a Piptarthron and that P. limbatum and K. attenuata were later synonyms. If the type material is a coelomycete bearing aseptate conidia that lack appendages, this would likely be a distinct species correctly classified in Kellermania. However, M.E. Barr’s annotation of the type specimen from 1970 (NY) states that the specimen is an immature K. anomala.
Plowrightia williamsoniana Kellerm., J. Mycol. 12: 186. 1906.
Notes — Kellerman (1906) described this species from Guatemalan material found on Agave americana. The combination of host, plurilocular stromata, and septate ascospores suggests a possible link with Kellermania macrospora. It has been treated as a synonym of Planistromella parryi and Plowrightia agaves (Barr 1996).
Septoplaca limbata Petr., Sydowia 17: 272. 1964 ‘1963’.
≡ Piptarthron limbatum (Petr.) B. Sutton, in Sutton, The coelomycetes: 54. 1980.
Notes — In the protologue, this species was designated as the type of the genus Septoplaca, and it was reported to have indistinctly pseudoseptate conidia measuring 35–60(–78) × 3–3.5 (Petrak 1964). The type from the USA: AZ occurs on Yucca macrocarpa (Petrak 1964). Sutton (1977) treated Septoplaca as a synonym of Piptarthron, but he was unable to place the type species. Assuming the conidia may actually be aseptate and after considering conidial measurements, Sutton (1977) suggested that S. limbata was an earlier synonym for Kellermania attenuata. Later, Sutton (1980) formally transferred S. limbata to Piptarthron, treated K. attenuata as a synonym, and considered the species to be aseptate. Sutton (1983) treated these names as later synonyms of P. yuccae. Although Petrak (1964) may have confused pseudoseptate conidia with the euseptate conidia in Piptarthron, it seems unlikely that he would confuse aseptate with septate conidia.
KEY TO KELLERMANIA SPECIES IN CULTURE
1. Colony distinctly pink................ K. crassispora
1. Colony not pink................ 2
2. Conidia with more than one apical appendage................ 3
2. Conidia with 0–1 apical appendages................ 5
3. Conidia aseptate................ K. yuccifolia*
3. Conidia septate................ 4
4. Conidia 1-septate................ K. uniseptata
4. Conidia 2–3-septate................ K. nolinae
5. Some conidia with 1 apical appendage................ 6
5. All conidia with no apical appendages................ 9
6. Many apical appendages greater than 12 μm long................ K. yuccigena
6. Apical appendages not or rarely greater than 12 μm long 7
7. All conidia 1-septate................ K. nolinifoliorum
7. Some conidia more than 1-septate................ 8
8. Conidia 1–2(–3)-septate, Q = 5–14.3, middle cell shorter than end cells................ K. anomala
8. Conidia 2–3-septate, Q = 3.9–7.4, middle cell longer than end cells................ K. yuccifoliorum
9. Conidia aseptate................ 10
9. Some conidia septate................ 12
10. Conidia typically cylindrical to obclavate................ K. micranthae
10. Conidia typically curved................ 11
11. Conidia slightly curved, falcate, to strongly curved, nearly U-shaped................ K. plurilocularis
11. Conidia more or less cylindrical with irregularly curved shapes, flexuous................ K. dasylirionicola
12. Conidia mostly 0–1-septate................ 13
12. Conidia mostly with 2 or more septa................ 14
13. Conidia typically cylindrical to obclavate................ K. rostratae
13. Conidia typically with some degree of curvature................ K. dasylirionis
14. Conidia 1–3 (mostly 2)-septate................ K. confusa
14. Some conidia with 4 or more septa................ 15
15. Some conidia with more than 5 septa, Q = 4.9–13................ K. macrospora
15. Conidia with 5 or less septa, maximum Q = ± 7.6................ 16
16. Conidia 2–4-septate, Q = 2.6–4.6................ K. ramaleyae
16. Conidia 2–4-septate, Q = 5–7.6................ K. unilocularis**
DISCUSSION
Phylogenetic analyses support the placement and monophyly of the core Planistromellaceae in the order Botryosphaeriales of class Dothideomycetes where the Planistromellaceae represent a well-supported clade comprised of the coelomycetous genera Alpakesa, Kellermania, and Piptarthron with associated teleomorphic genera Planistroma and Planistromella. Within Botryosphaeriales, it is positioned in a polytomy with four other lineages of Botryosphaeriaceae s.l., three of which occupy basal positions in previous studies (Philips & Alves 2009, Schoch et al. 2009) relative to the fourth that includes the core diversity of Botryosphaeriaceae (Fig. 1). This core Botryosphaeriaceae lineage (pp = 1.0) is comprised of two main clades, one with Botryosphaeria (pp = 1.0) and another (pp = 0.75) with Pseudofusicoccum stromaticum and Guignardia (pp = 1.0). The position of Guignardia is significant due to its unclear position based on previous studies (Crous et al. 2006, Philips & Alves 2009). Though the family Botryosphaeriaceae has been recognised with a broad circumscription, i.e. sensu lato (Philips & Alves 2009), and Planistromellaceae could well be treated as a later synonym, we prefer to maintain the family Planistromellaceae as distinct from Botryosphaeriaceae s.str. and suggest that further sampling is needed to clarify familial classification within the Botryosphaeriales. The Planistromellaceae is significantly and phylogenetically distinct from the Botryosphaeriaceae.
All genera of core Planistromellaceae are here considered to constitute one genus, namely Kellermania. Four other genera classified in Planistromellaceae (Barr 1996) are redisposed as follows: Eruptio has been shown to be a member of Mycosphaerellaceae (Verkley et al. 2004); Loratospora has been shown to be a member of the Phaeosphaeriaceae (Schoch et al. 2009); and none of the species of Mycosphaerellopsis including the type species, M. myricariae (Höhnel 1918b), have been placed phylogenetically; Microcyclus, typified by Microcyclus angolensis (Sydow & Sydow 1904), is only represented by DNA sequence data of Microcyclus ulei, and based on BLAST searches of GenBank using available ITS sequences, it does not belong in the order Botryosphaeriales. The closest hits resulting from the BLAST searches are members of the Mycosphaerellaceae, but its familial classification is uncertain.
Among the Planistromellaceae (Kellermania) are five lineages that, with the exception of lineage iv, are each well supported. Lineage v is treated separately as its position is unresolved. These lineages were recovered in all analyses of individual genes with the exception of the SSU. The lack of resolution in the SSU tree was due to a low level of sequence divergence, and thus a lack of informative characters (Table 2). Overall, the relationships among the five lineages were not strongly supported.
In general, the phylogenetic groupings within the Planistromellaceae (Kellermania) do not correspond with the presence or absence and numbers of conidial appendages nor with conidial septation (e.g. multiple appendages in lineages ii and v; appendages absent in lineages i and iv). Previous generic circumscriptions of these coelomycetes based on conidial appendages alone are not well supported, a finding which was also recently observed among other genera of appendaged coelomycetes (Barber et al. 2011, Crous et al. 2012). The type species of Kellermania, K. yuccigena, and Piptarthron, P. macrosporum ( ≡K. macrospora), belong to separate lineages ii and i, respectively, that notably form a clade (pp = 0.89) with K. unilocularis in the ITS analysis (Fig. 3). Lineage ii, which includes K. yuccigena, also includes three well-known species of Planistromella for which DNA sequence data exist including the type species, Planistromella yuccifoliorum (= K. yuccifoliorum). Though lineages i–iii in Fig. 2 contain only species with septate ascospores, Planistroma obtusilunatum, linked to P. uniloculare (≡ K. unilocularis), as mentioned above appears to belong among these lineages (Fig. 3). It has aseptate ascospores (Ramaley 1992). Other species lacking ascospore septation, namely Planistroma yuccigenum, the type of the genus and linked to P. pluriloculare (≡ K. plurilocularis) and Planistroma nolinae, linked to P. crassisporum (≡ K. crassispora), belong to lineage iv and Planistroma kellermaniae, linked to Kellermania nolinae, represents lineage v. Thus, Planistroma and Planistromella do not form well-supported clades if circumscribed by ascospore septation alone. Considering the biological and phylogenetic similarities of the species in the five major lineages and the lack of resolution among the lineages, there is little pragmatic value in recognising any subclades or lineages as distinct genera. Thus, the entire clade is herein recognised as a broadly defined genus Kellermania, the generic name having priority. In accordance with the changes enacted in the Melbourne Code in regard to Art. 59 (Norvell 2011), we do not recognise separate anamorphic and teleomorphic names.
Morphological variation of conidia among isolates from different hosts or even the same host, especially in regard to appendages, septation, and shape of conidia, is frequently an indicator of the presence of distinct taxa. However, slight differences in size, septation, and appendages of conidia among material from fresh collections, herbarium collections, and culture were frequently observed for a given species (Ramaley 1993, 1995, this study). In spite of these differences, the overall appearance of conidia on the host and in culture is similar for a given species and species differences tend to be obvious when considering within species and among species morphological variation. DNA sequence data are required to distinguish species and to confirm species recognition in ambiguous cases.
From an ecological and evolutionary point of view, it is interesting to note that several lineages including lineages i, sisters ii–iii, and iv (Fig. 2) are largely correlated with plant hosts from different host clades in the Asparagaceae, subfamilies Agavoideae and Nolinoideae sensu APG III (2009). This suggests that host phylogeny is correlated with the phylogeny of these fungi to some degree. However, sampling within these fungi remains limited and it is too early to formulate final conclusions given the lack of resolution among the lineages. Although a number of Kellermania species have been recognised historically as occurring on numerous hosts, we expect that there are few, if any, plurivorous species given the evidence of host specificity and numbers of cryptic taxa found in this study. However, multiple isolates were not available for species of Kellermania reported from numerous hosts, thus, this hypothesis needs further study as many members of the Botryosphaeriales are known to inhabit multiple hosts (van Niekerk et al. 2004, Glienke et al. 2011).
Acknowledgments
The authors extend heartfelt gratitude to Annette W. Ramaley for detailed observations of these unusual fungi as well as her contribution of the cultures used in this study and her notes without which this study would not have been possible. The authors thank John H. Wiersema for providing the nomenclaturally correct names for some species of Yucca that were identified by others. Joseph F. Bischoff kindly provided fungal materials and other support from the initiation of this study to its later stages. Lisa A. Castlebury is warmly acknowledged for providing an ITS sequence of Kellermania unilocularis. Pedro W. Crous generously assisted with the publication of this manuscript and advised on matters of familial taxonomy.
Footnotes
*Not known from culture **Based on study of isotype on host (BPI 1110167)
REFERENCES
- APG III 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121 [Google Scholar]
- Barber PA, Crous PW, Groenewald JZ, Pascoe IG, Keane P. 2011. Reassessing Vermisporium (Amphisphaeriaceae), a genus of foliar pathogens of eucalypts. Persoonia 27: 90–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr ME. 1996. Planistromellaceae, a new family in the Dothideales. Mycotaxon 60: 433–442 [Google Scholar]
- Cooke MC. 1885. Synopsis pyrenomycetorum. Grevillea 13: 100–109 [Google Scholar]
- Crous PW, Gams W, Stalpers JA, Robert V, Stegehuis G. 2004. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology 50: 19–22 [Google Scholar]
- Crous PW, Slippers B, Wingfield WJ, Rheeder J, Marasas WFO, et al. 2006. Phylogenetic lineages in Botryosphaeriaceae. Studies in Mycology 55: 235–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Christensen M, Castañeda-Ruiz RF, Groenewald JZ. 2012. How important are conidial appendages? Persoonia 28: 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearness J. 1924. New and noteworthy fungi. III. Mycologia 16: 143–176 [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, Cheung M, Cooper A, et al. 2010. Geneious v5.1. Available from http://www.geneious.com [Google Scholar]
- Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JB, Everhart BM. 1885. New fungi. Journal of Mycology 1: 148–154 [Google Scholar]
- Ellis JB, Everhart BM. 1888. New species of fungi from various localities. Journal of Mycology 4: 73–82 [Google Scholar]
- Ellis JB, Everhart BM. 1895. New species of fungi. Bulletin of the Torrey Botanical Club 22: 434–440 [Google Scholar]
- Farr DF, Rossman AY. 2012. Fungal databases, systematic mycology and microbiology laboratory, ARS, USDA. Retrieved March 30, 2012 from http://nt.ars-grin.gov/fungaldatabases/ [Google Scholar]
- Farris JS, Källersjö M, Kluge AG, Bult C. 1994. Testing significance of incongruence. Cladistics 10: 315–319 [Google Scholar]
- Glienke C, Pereira OL, Stringari D, Fabris J, Kava-Cordeiro V, et al. 2011. Endophytic and pathogenic Phyllosticta species, with reference to those associated with Citrus Black Spot. Persoonia: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SO. 1959. The effects of various reagents, mounting media, and dyes on the teliospore walls of Tilletia controversa Kühn. Mycologia 51: 477–491 [Google Scholar]
- Hall JG. 1915. Notes upon Washington fungi. Phytopathology 5: 55–58 [Google Scholar]
- Höhnel F von. 1918a. Fungi imperfecti. Beiträge zur Kenntnis derselben. Hedwigia 60: 129–209 [Google Scholar]
- Höhnel F von. 1918b. Mycologische Fragmente. Annales Mycologici 16: 35–174 [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MrBayes: Bayesian inference of phylogeny. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Kellerman WA. 1906. A new Plowrightia from Guatemala. Journal of Mycology 12: 185–187 [Google Scholar]
- Kornerup A, Wanscher JH. 1967. Methuen handbook of colour, 2nd ed Methuen & Co Ltd, London, UK [Google Scholar]
- Largent D, Johnson D, Watling R. 1977. How to identify mushrooms to genus III. Microscopic features. Mad River Press, Eureka, CA [Google Scholar]
- Malkus A, Chang P-FL, Sabina MZ, Chung K, Shao J, et al. 2006. RNA polymerase II gene (RPB2) encoding the second largest protein subunit in Phaeosphaeria nodorum and P. avenaria. Mycological Research 110: 1152–1164 [DOI] [PubMed] [Google Scholar]
- Matheny PB, Liu YJ, Ammirati JF, Hall BD. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698 [DOI] [PubMed] [Google Scholar]
- Maublanc A. 1903. Sur quelques espéces nouvelles de champignons inférieurs. Bulletin de la Société Mycologique de France 19: 291–296 [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, et al. (eds). 2006. International Code of Botanical Nomenclature (Vienna Code): Adopted by the Seventeenth International Botanical Congress, Vienna, Austria, July 2005. ARG Gantner, Ruggell, Liechtenstein [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov.: 1–8 New Orleans, LA [Google Scholar]
- Moncalvo JM, Lutzoni FM, Rehner SA, Johnson J, Vilgalys R. 2000. Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. Systematic Biology 49: 278–305 [DOI] [PubMed] [Google Scholar]
- Morgan-Jones G, Nag Raj TR, Kendrick B. 1972a. Genera coelomycetarum. V. Alpakesa and Bartalinia. Canadian Journal of Botany 50: 877–882 [Google Scholar]
- Morgan-Jones G, Nag Raj TR, Kendrick B. 1972b. Genera coelomycetarum. VI. Kellermania. Canadian Journal of Botany 50: 1641–1648 [Google Scholar]
- Nag Raj TR. 1993. Coelomycetous anamorphs with appendage-bearing conidia. Mycologue Publications, Waterloo, Ontario, Canada [Google Scholar]
- Niekerk JM van, Crous PW, Groenewald JZ, Fourie PH, Halleen F. 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96: 781–798 [PubMed] [Google Scholar]
- Norvell L. 2011. Fungal nomenclature. Melbourne approves a new Code. Mycotaxon 116: 481–490 [Google Scholar]
- Nylander JAA. 2004. MrModeltest, v. 2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University [Google Scholar]
- Petrak F. 1964, ‘1963’. Septoplaca nov. gen., eine neue gattung der scolecosporen Sphaeropsideen. Sydowia 17: 271–274 [Google Scholar]
- Phillips AJL, Alves A. 2009. Taxonomy, phylogeny, and epitypification of Melanops tulasnei, the type species of Melanops. Fungal Diversity 38: 155–166 [Google Scholar]
- Pollack FG. 1947. Two additions to the fungi imperfecti. Mycologia 39: 617–621 [PubMed] [Google Scholar]
- Posada D, Buckley TR. 2004. Model selection and model averaging in phylogenetics: advantages of akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808 [DOI] [PubMed] [Google Scholar]
- Ramaley A. 1991. Fungi of Yucca baccata 1. Piptarthron pluriloculare and its teleomorph, Planistroma yuccigena. Mycotaxon 42: 63–75 [Google Scholar]
- Ramaley AW. 1992. Fungi from Yucca baccata. 2. Planistroma obtusilunatum sp. nov., and its anamorph, Piptarthron uniloculare sp. nov. Mycotaxon 45: 449–460 [Google Scholar]
- Ramaley AW. 1993. New fungi from Yucca: Planistromella yuccifoliorum, gen. et sp. nov., its anamorph, Kellermania yuccifoliorum, sp. nov., and Planistromella uniseptata, sp. nov., the teleomorph of Kellermania yuccigena. Mycotaxon 47: 259–274 [Google Scholar]
- Ramaley AW. 1995. New species of Kellermania, Piptarthron, Planistroma, and Planistromella from members of the Agavaceae. Mycotaxon 55: 255–268 [Google Scholar]
- Ramaley AW. 1998. New teleomorphs of the anamorphic genus Kellermania. Mycotaxon 66: 509–514 [Google Scholar]
- Rambaut A, Drummond AJ. 2007. Tracer v1.5. Computer program and documentation distributed by the authors at http://beast.bio.ed.ac.uk/Tracer [Google Scholar]
- Reeb V, Lutzoni F, Roux C. 2004. Multilocus phylogenetic circumscription of the lichen-forming fungi family Acarosporaceae and its position within the Ascomycota. Molecular Phylogeny and Evolution 32: 1036–1060 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572–1574 [DOI] [PubMed] [Google Scholar]
- Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, et al. 2009. A class-wide phylogenetic assessment of Dothideomycetes. Studies in Mycology 64: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RA, Babcock CE. 1987. Wettsteinina. Canadian Journal of Botany 65: 373–405 [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690 [DOI] [PubMed] [Google Scholar]
- Subramanian CV, Ramakrishnan K. 1954. Alpakesa, a new genus of the Sphaeropsidales. Journal of the Indian Botanical Society 33: 203–205 [Google Scholar]
- Sutton BC. 1968. Kellermania and its segregates. Canadian Journal of Botany 46: 181–196 [Google Scholar]
- Sutton BC. 1977. Coelomycetes VI. Nomenclature for generic names proposed for Coelomycetes. Mycological Papers 141: 1–253 [Google Scholar]
- Sutton BC. 1980. The coelomycetes. Fungi imperfecti with pycnidia, acervuli, and stromata. Commonwealth Mycological Institute, Kew, Surrey, England [Google Scholar]
- Sutton BC. 1983. Nomenclatural notes on Deuteromycetes. Transactions of the British Mycological Society 81: 407 [Google Scholar]
- Swofford DL. 2003. PAUP*. Phylogenetic Analysis Using Parsimony (* and other methods), Version 4. Sinauer Associates, Sunderland, Massachusetts [Google Scholar]
- Sydow H, Sydow P. 1904. Novae fungorum species. Annales Mycologici 2: 162–174 [Google Scholar]
- Thiers B. 2012[continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ [Google Scholar]
- Verkley GJM, Starink-Willemse M, Iperen A van, Abeln ECA. 2004. Phylogenetic analyses of Septoria species based on the ITS and LSU-D2 regions of nuclear ribosomal DNA. Mycologia 96: 558–571 [PubMed] [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor JW. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ. (eds), PCR Protocols: A guide to methods and applications. Academic Press, Inc, New York: 315–322 [Google Scholar]