Abstract
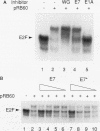
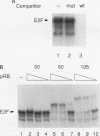
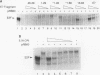
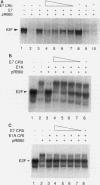
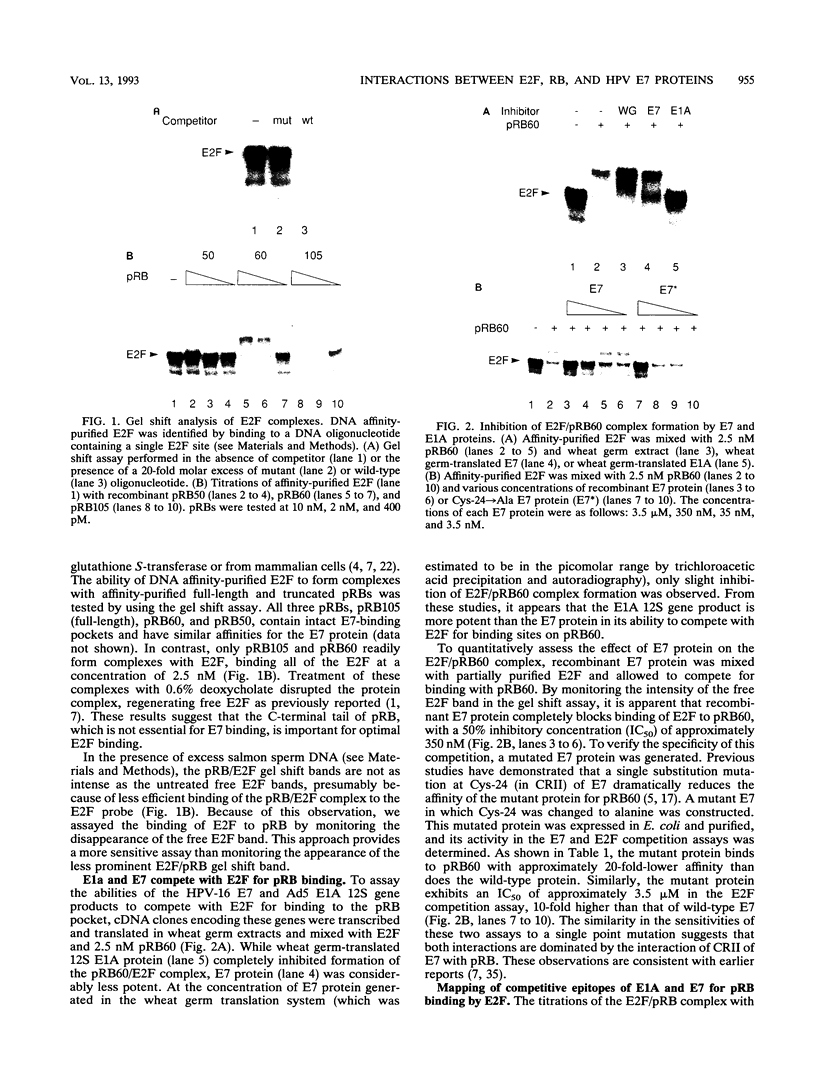
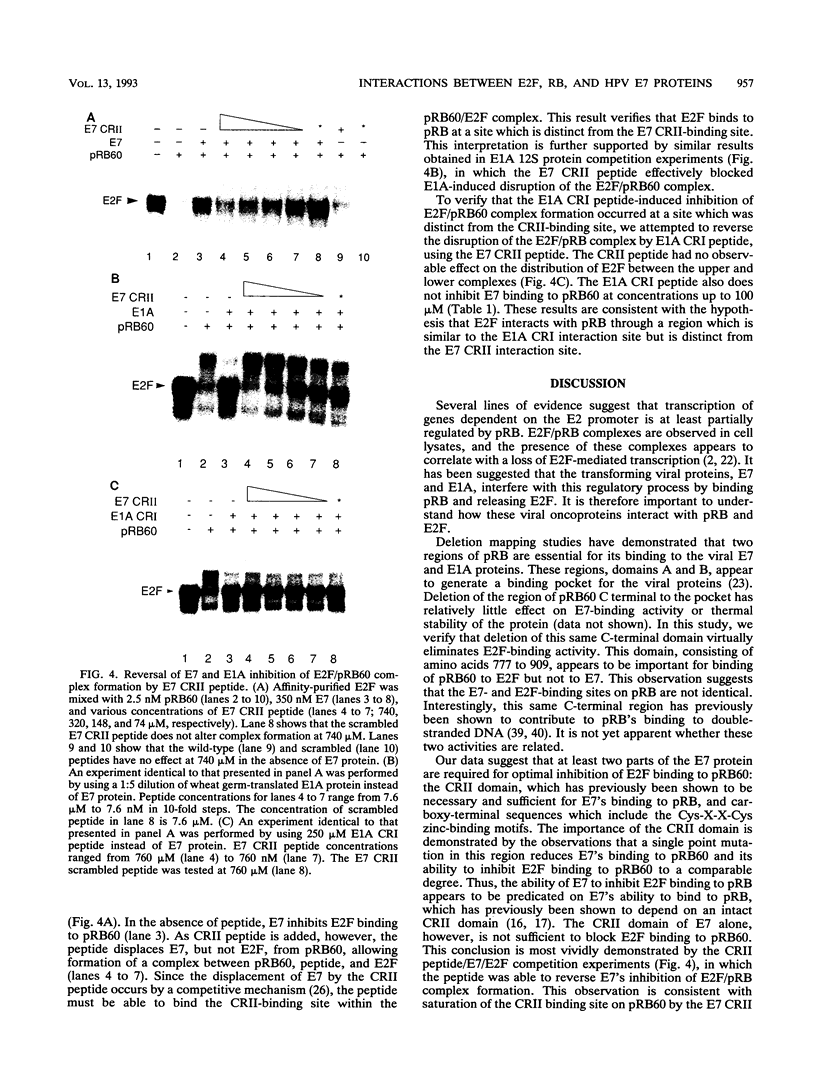
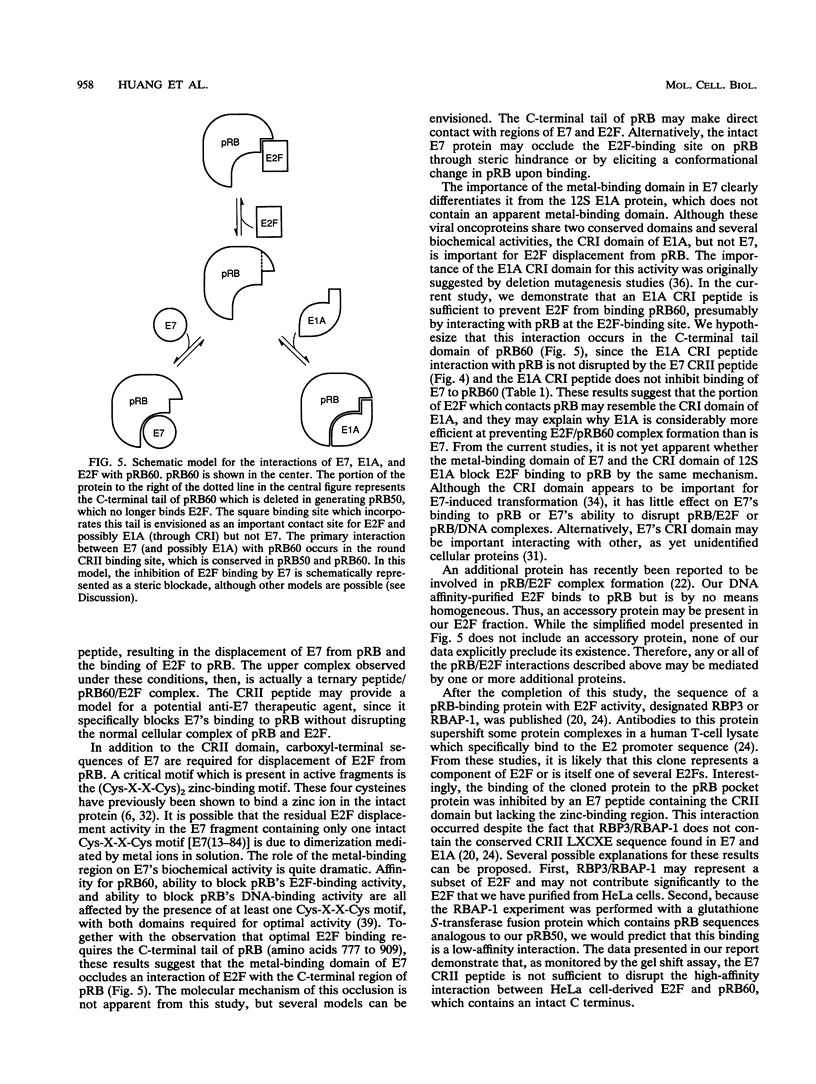
Human papillomaviruses (HPVs) are the etiological agents for genital warts and contribute to the development of cervical cancer in humans. The HPV E7 gene product is expressed in these diseases, and the E7 genes from HPV types 16 and 18 contribute to transformation in mammalian cells. Mutation and deletion analysis of this gene suggests that the transforming activity of the protein product resides in the same domain as that which is directly involved in complex formation with the retinoblastoma gene product (pRB). This domain is one of two conserved regions (designated CRI and CRII) shared by E7 and other viral oncoproteins which bind pRB, including adenovirus E1A protein. Binding of HPV type 16 E7 protein to pRB has previously been shown to affect pRB's ability to bind DNA and to form complexes with other cellular proteins. In the current study, we map the functional interaction between E7 protein and pRB by monitoring the association between a 60-kDa version of the pRB, pRB60, and the cellular transcription factor E2F. We observe that CRII of E7 (amino acids 20 to 29), which completely blocks binding of full-length E7 protein, is necessary but not sufficient to inhibit E2F/pRB60 complex formation. While CRI of E1A (amino acids 37 to 55) appears to be sufficient to compete with E2F for binding to pRB60, the equivalent region of E7 is neither necessary nor sufficient. Only E7 fragments that contained both CRII and at least a portion of the zinc-binding domain (amino acids 60 to 98) inhibited E2F/pRB60 complex formation. These results suggest that pRB60 associates with E7 and E2F through overlapping but distinct domains.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Bagchi S., Weinmann R., Raychaudhuri P. The retinoblastoma protein copurifies with E2F-I, an E1A-regulated inhibitor of the transcription factor E2F. Cell. 1991 Jun 14;65(6):1063–1072. doi: 10.1016/0092-8674(91)90558-g. [DOI] [PubMed] [Google Scholar]
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandara L. R., Adamczewski J. P., Hunt T., La Thangue N. B. Cyclin A and the retinoblastoma gene product complex with a common transcription factor. Nature. 1991 Jul 18;352(6332):249–251. doi: 10.1038/352249a0. [DOI] [PubMed] [Google Scholar]
- Barbosa M. S., Edmonds C., Fisher C., Schiller J. T., Lowy D. R., Vousden K. H. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and Sv40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 1990 Jan;9(1):153–160. doi: 10.1002/j.1460-2075.1990.tb08091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Lowy D. R., Schiller J. T. Papillomavirus polypeptides E6 and E7 are zinc-binding proteins. J Virol. 1989 Mar;63(3):1404–1407. doi: 10.1128/jvi.63.3.1404-1407.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellappan S. P., Hiebert S., Mudryj M., Horowitz J. M., Nevins J. R. The E2F transcription factor is a cellular target for the RB protein. Cell. 1991 Jun 14;65(6):1053–1061. doi: 10.1016/0092-8674(91)90557-f. [DOI] [PubMed] [Google Scholar]
- Chellappan S., Kraus V. B., Kroger B., Munger K., Howley P. M., Phelps W. C., Nevins J. R. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4549–4553. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T., Livingston D. M., Kaelin W. G., Jr The T/E1A-binding domain of the retinoblastoma product can interact selectively with a sequence-specific DNA-binding protein. Cell. 1991 Jun 14;65(6):1073–1082. doi: 10.1016/0092-8674(91)90559-h. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Whyte P. RB and the cell cycle: entrance or exit? Cell. 1989 Sep 22;58(6):1009–1011. doi: 10.1016/0092-8674(89)90495-9. [DOI] [PubMed] [Google Scholar]
- Coutlée F., Shah K. V., Rader J. S., Currie J. L., Viscidi R. P. Detection of transcripts of human papillomaviruses 16 and 18 in cancer-derived cell lines and cervical biopsies by enzyme immunoassay for DNA-RNA hybrids following solution hybridization. J Clin Microbiol. 1991 May;29(5):968–974. doi: 10.1128/jcm.29.5.968-974.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Morgenstern J. P., Crawford L., Banks L. Continued expression of HPV-16 E7 protein is required for maintenance of the transformed phenotype of cells co-transformed by HPV-16 plus EJ-ras. EMBO J. 1989 Feb;8(2):513–519. doi: 10.1002/j.1460-2075.1989.tb03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo-Jones D., Huang P. S., Jones R. E., Haskell K. M., Vuocolo G. A., Hanobik M. G., Huber H. E., Oliff A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991 Jul 18;352(6332):251–254. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Bernards R., Friend S. H., Gooding L. R., Hassell J. A., Major E. O., Pipas J. M., Vandyke T., Harlow E. Large T antigens of many polyomaviruses are able to form complexes with the retinoblastoma protein. J Virol. 1990 Mar;64(3):1353–1356. doi: 10.1128/jvi.64.3.1353-1356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Edmonds C., Vousden K. H. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol. 1989 Jun;63(6):2650–2656. doi: 10.1128/jvi.63.6.2650-2656.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. M., Huber H. E., DeFeo-Jones D., Vuocolo G., Goodhart P. J., Maigetter R. Z., Sanyal G., Oliff A., Heimbrook D. C. Purification and characterization of a functionally homogeneous 60-kDa species of the retinoblastoma gene product. J Biol Chem. 1992 Apr 25;267(12):7971–7974. [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Helin K., Lees J. A., Vidal M., Dyson N., Harlow E., Fattaey A. A cDNA encoding a pRB-binding protein with properties of the transcription factor E2F. Cell. 1992 Jul 24;70(2):337–350. doi: 10.1016/0092-8674(92)90107-n. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W., Blake M., Azizkhan J., Nevins J. R. Role of E2F transcription factor in E1A-mediated trans activation of cellular genes. J Virol. 1991 Jul;65(7):3547–3552. doi: 10.1128/jvi.65.7.3547-3552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. E., Heimbrook D. C., Huber H. E., Wegrzyn R. J., Rotberg N. S., Stauffer K. J., Lumma P. K., Garsky V. M., Oliff A. Specific N-methylations of HPV-16 E7 peptides alter binding to the retinoblastoma suppressor protein. J Biol Chem. 1992 Jan 15;267(2):908–912. [PubMed] [Google Scholar]
- Jones R. E., Wegrzyn R. J., Patrick D. R., Balishin N. L., Vuocolo G. A., Riemen M. W., Defeo-Jones D., Garsky V. M., Heimbrook D. C., Oliff A. Identification of HPV-16 E7 peptides that are potent antagonists of E7 binding to the retinoblastoma suppressor protein. J Biol Chem. 1990 Aug 5;265(22):12782–12785. [PubMed] [Google Scholar]
- Kaelin W. G., Jr, Krek W., Sellers W. R., DeCaprio J. A., Ajchenbaum F., Fuchs C. S., Chittenden T., Li Y., Farnham P. J., Blanar M. A. Expression cloning of a cDNA encoding a retinoblastoma-binding protein with E2F-like properties. Cell. 1992 Jul 24;70(2):351–364. doi: 10.1016/0092-8674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Kanda T., Furuno A., Yoshiike K. Human papillomavirus type 16 open reading frame E7 encodes a transforming gene for rat 3Y1 cells. J Virol. 1988 Feb;62(2):610–613. doi: 10.1128/jvi.62.2.610-613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudryj M., Devoto S. H., Hiebert S. W., Hunter T., Pines J., Nevins J. R. Cell cycle regulation of the E2F transcription factor involves an interaction with cyclin A. Cell. 1991 Jun 28;65(7):1243–1253. doi: 10.1016/0092-8674(91)90019-u. [DOI] [PubMed] [Google Scholar]
- Münger K., Phelps W. C., Bubb V., Howley P. M., Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989 Oct;63(10):4417–4421. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger K., Yee C. L., Phelps W. C., Pietenpol J. A., Moses H. L., Howley P. M. Biochemical and biological differences between E7 oncoproteins of the high- and low-risk human papillomavirus types are determined by amino-terminal sequences. J Virol. 1991 Jul;65(7):3943–3948. doi: 10.1128/jvi.65.7.3943-3948.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Bagchi S., Barnes J. A., Raychaudhuri P., Kraus V., Münger K., Howley P. M., Nevins J. R. Analysis of trans activation by human papillomavirus type 16 E7 and adenovirus 12S E1A suggests a common mechanism. J Virol. 1991 Dec;65(12):6922–6930. doi: 10.1128/jvi.65.12.6922-6930.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Münger K., Yee C. L., Barnes J. A., Howley P. M. Structure-function analysis of the human papillomavirus type 16 E7 oncoprotein. J Virol. 1992 Apr;66(4):2418–2427. doi: 10.1128/jvi.66.4.2418-2427.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Raychaudhuri P., Bagchi S., Devoto S. H., Kraus V. B., Moran E., Nevins J. R. Domains of the adenovirus E1A protein required for oncogenic activity are also required for dissociation of E2F transcription factor complexes. Genes Dev. 1991 Jul;5(7):1200–1211. doi: 10.1101/gad.5.7.1200. [DOI] [PubMed] [Google Scholar]
- Shirodkar S., Ewen M., DeCaprio J. A., Morgan J., Livingston D. M., Chittenden T. The transcription factor E2F interacts with the retinoblastoma product and a p107-cyclin A complex in a cell cycle-regulated manner. Cell. 1992 Jan 10;68(1):157–166. doi: 10.1016/0092-8674(92)90214-w. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Ahern J. D., Oliff A., Heimbrook D. C. Retinoblastoma protein binding properties are dependent on 4 cysteine residues in the protein binding pocket. J Biol Chem. 1992 Jul 25;267(21):14846–14851. [PubMed] [Google Scholar]
- Stirdivant S. M., Huber H. E., Patrick D. R., Defeo-Jones D., McAvoy E. M., Garsky V. M., Oliff A., Heimbrook D. C. Human papillomavirus type 16 E7 protein inhibits DNA binding by the retinoblastoma gene product. Mol Cell Biol. 1992 May;12(5):1905–1914. doi: 10.1128/mcb.12.5.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N. P., Chen P. L., Huang S., Donoso L. A., Lee W. H., Lee E. Y. DNA-binding activity of retinoblastoma protein is intrinsic to its carboxyl-terminal region. Cell Growth Differ. 1990 May;1(5):233–239. [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Sato H., Furuno A., Yoshiike K. Mutational analysis of human papillomavirus type 16 E7 functions. J Virol. 1990 Jan;64(1):207–214. doi: 10.1128/jvi.64.1.207-214.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Kanda T., Yoshiike K. Human papillomavirus type 16 transformation of primary human embryonic fibroblasts requires expression of open reading frames E6 and E7. J Virol. 1989 Feb;63(2):965–969. doi: 10.1128/jvi.63.2.965-969.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Yee A. S., Raychaudhuri P., Jakoi L., Nevins J. R. The adenovirus-inducible factor E2F stimulates transcription after specific DNA binding. Mol Cell Biol. 1989 Feb;9(2):578–585. doi: 10.1128/mcb.9.2.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C., Krishnan-Hewlett I., Baker C. C., Schlegel R., Howley P. M. Presence and expression of human papillomavirus sequences in human cervical carcinoma cell lines. Am J Pathol. 1985 Jun;119(3):361–366. [PMC free article] [PubMed] [Google Scholar]