Abstract
Species classified in Penicillium sect. Chrysogena are primary soil-borne and the most well-known members are P. chrysogenum and P. nalgiovense. Penicillium chrysogenum has received much attention because of its role in the production on penicillin and as a contaminant of indoor environments and various food and feedstuffs. Another biotechnologically important species is P. nalgiovense, which is used as a fungal starter culture for the production of fermented meat products. Previous taxonomic studies often had conflicting species circumscriptions. Here, we present a multigene analysis, combined with phenotypic characters and extrolite data, demonstrating that sect. Chrysogena consists of 18 species. Six of these are newly described here (P. allii-sativi, P. desertorum, P. goetzii, P. halotolerans, P. tardochrysogenum, P. vanluykii) and P. lanoscoeruleum was found to be an older name for P. aethiopicum. Each species produces a unique extrolite profile. The species share phenotypic characters, such as good growth on CYA supplemented with 5 % NaCl, ter- or quarterverticillate branched conidiophores and short, ampulliform phialides (< 9 μm). Conidial colours, production of ascomata and ascospores, shape and ornamentation of conidia and growth rates on other agar media are valuable for species identification. Eight species (P. allii-sativi, P. chrysogenum, P. dipodomyis, P. flavigenum, P. nalgiovense, P. rubens, P. tardochrysogenum and P. vanluykii) produce penicillin in culture.
Keywords: Fleming, P. chrysogenum, P. rubens, phylogeny, taxonomy
INTRODUCTION
Penicillium sect. Chrysogena was introduced by Frisvad & Samson (2004) for species having ter- or quarterverticillate branched conidiophores, relatively short phialides (< 10 μm) and smooth to finely roughened conidia. Four series and eight species (P. aethiopicum, P. chrysogenum, P. confertum, P. dipodomyis, P. flavigenum, P. mononematosum, P. nalgiovense and P. persicinum) were accepted in this section. Only species lacking a sexual state were included, but a close affinity with Eupenicillium egyptiacum was suggested. Recently, single name nomenclature was applied in Penicillium and both asexual and sexual reproducing species were included in the redefined genus (Houbraken & Samson 2011). Using a multigene approach, Penicillium was divided into 25 sections and sect. Chrysogena was expanded to include species with a sexual state (P. egyptiacum, P. kewense, P. molle and P. sinaicum), and the recently resurrected species P. rubens.
With the exception of P. chrysogenum, P. nalgiovense and P. rubens, the species of sect. Chrysogena are primary soil-borne (Frisvad & Samson 2004). Penicillium chrysogenum (and P. rubens) garner much research interest because of health ramifications that are a consequence of their occurrence in various food products (Pitt & Hocking 2009, Samson et al. 2010) and indoor environments, including damp building materials, indoor air and dust (Chang et al. 1995, Hunter & Lea 1995, Gravesen 1999, Scott et al. 2004, Bekker et al. 2012). Another biotechnologically important species of this section is P. nalgiovense, which is used as a fungal starter culture for the production of fermented meat products (Leistner 1990).
Penicillium chrysogenum is best known for the production of the antibiotic penicillin and for this reason its taxonomy has received much attention. Initially, Fleming’s penicillin producing strain was identified as P. rubrum (Fleming 1929) but because of changing taxonomic schemes, it was often called P. notatum (Thom 1945, Raper & Thom 1949), P. chrysogenum (Samson et al. 1977), P. griseoroseum (Pitt 1980) or P. rubens (Houbraken et al. 2011a). When Charles Thom was about to finish his monograph in 1930, he received the Fleming strain (CBS 205.57 = NRRL 824 = IMI 015378), then identified as P. rubrum, and re-identified it as P. notatum (Thom 1945). In the subsequent monograph of Raper & Thom (1949), series Chrysogena, based on Thom’s (1930) subsect. Radiata, was introduced and four species were accepted: P. chrysogenum, P. cyaneofulvum, P. meleagrinum and P. notatum. The name P. notatum was maintained for Fleming’s strain but the strain still used for the industrial production of penicillin (the ‘Wisconsin strain’ = NRRL 1951 = CBS 307.48) was identified as P. chrysogenum (Raper & Thom 1949). Considerable variation was observed among strains of this series, making it difficult to designate distinct phenotypic differences because of intergrading strains. Therefore, Samson et al. (1977) placed P. cyaneofulvum, P. meleagrinum, P. notatum and six additional species and varieties into synonymy with P. chrysogenum and as a result, both Fleming’s penicillin producing strains and the Wisconsin strain were classified as P. chrysogenum. Although various species similar to P. chrysogenum were examined by Samson et al. (1977), P. griseoroseum, P. brunneorubrum and P. citreoroseum were not included. These species were considered in the monograph of Pitt (1980), but the latter two species were synonymized with P. griseoroseum, Fleming’s strain was identified as P. griseoroseum and the Wisconsin strain as P. chrysogenum. Following Pitt’s monograph, various new approaches were applied to the taxonomy of P. chrysogenum. Physiological, extrolite and isozyme data suggested that P. griseoroseum and related synonyms were conspecific with P. chrysogenum (Frisvad & Filtenborg 1989, Banke et al. 1997), in which case the less commonly used name P. griseoroseum would have displaced the better known P. chrysogenum. To avoid a name change for penicillin producing strains, Kozakiewicz et al. (1992) proposed formal conservation of the name P. chrysogenum and rejection of the older P. griseoroseum, along with its synonyms P. citreoroseum and P. brunneorubrum. The proposal was accepted and the name P. chrysogenum is currently listed as a nomen conservandum (McNeill et al. 2006). More recently, the taxonomy of P. chrysogenum was subjected to multigene sequence and microsatellite analysis (Scott et al. 2004, Henk et al. 2011, Houbraken et al. 2011a). Both Scott et al. (2004) and Henk et al. (2011) show the presence of four clades within the species; however, the subdivisions are discordant. The studies agree on the existence of two main clades and based on a polyphasic approach, Houbraken et al. (2011a) named these clades P. chrysogenum and P. rubens. Interestingly, Fleming’s strain and the Wisconsin strain both reside in a clade with P. rubens (Houbraken et al. 2011a).
The first aim of the present study was to elucidate the phylogenetic relationships among species belonging to sect. Chrysogena using partial RPB1, RPB2 (RNA polymerase II genes), β-tubulin and calmodulin gene sequences. A further objective was to describe the six new species identified as belonging to this section, using a combination of sequence data, phenotypic characteristics and extrolite data, including penicillin production. In addition, an overview of species belonging to sect. Chrysogena and their synonyms is presented. The taxonomy of P. chrysogenum s.str. has often been controversial and the ultimate goal of this manuscript is to obtain a robust, reproducible and stable species concepts for this economically important species. A network analysis based on eight genes (RPB1, RPB2, calmodulin, β-tubulin, ITS, acetyl-CoA ligase (FacA), phosphoadenosine-5-phosphosulfate reductase (ParA), anthranilate synthase multifunctional protein (TrpC)) is performed in order to get insight in the haplotype diversity among P. chrysogenum, P. rubens and closely related species.
MATERIAL AND METHODS
Strains
Ex-type and representative strains were obtained from the culture collections of the CBS-KNAW Fungal Biodiversity Centre (CBS), Technical University of Denmark (IBT), USDA-ARS, National Center for Agricultural Utilization Research (NRRL) and the working collection of the department of Applied and Industrial Mycology housed at CBS (DTO). An overview of the strains is given Table 1. More information can be found in the on-line database of CBS at www.cbs.knaw.nl/databases.
Table 1.
Penicillium strains used in this study.
| Species | CBS no.1 | Other collection numbers2 | Substrate, locality and remarks | Haplotype |
|---|---|---|---|---|
| P. allii-sativi | 131541 | DTO 148-I4 = IBT 15987 | Mixed pig feed; Stora, Zagora, Bulgaria | 20 |
| 131544 | DTO 148-I8 = IBT 18101 = FRR 2818 | Sorghum malt toxic to day-old ducklings; Potchefstroom, South Africa | 21 | |
| 132071 | DTO 149-A5 = IBT 26504 = LJC 384 | Allium sativum (garlic); Anchoris, Lujan, Mendoza, Argentina | 24 | |
| 132072 | DTO 149-A6 = IBT 26505 = LJC 215 | Allium sativum (garlic); La Holanda, Lavalle, Mendoza, Argentina | 20 | |
| 132073 | DTO 149-A7 = IBT 26506 = LJC 044 | Allium sativum (garlic); Pocito, San Juan, Argentina | 25 | |
| 132074┬ | DTO 149-A8 = IBT 26507 = LJC 206 | Ex-type; Allium sativum (garlic); Lavalle, Mendoza, Argentina | 20 | |
| 132075 | DTO 149-A9 = IBT 26514 = LJC 481 | Allium sativum (garlic); La Blanca, Maipu, Mendoza, Argentina | 26 | |
| 132076 | DTO 149-B1 = IBT 26515 = LJC 394 | Allium sativum (garlic); Vistalba, Lujan, Mendoza, Argentina | 27 | |
| 132077 | DTO 149-B2 = IBT 26516 = LJC 317 | Allium sativum (garlic); Andrade, Rivadavia, Mendoza, Argentina | 25 | |
| 132198 | DTO 149-B4 = IBT 26518 = LJC 128 | Allium sativum (garlic); Lavalle, Mendoza, Argentina | 28 | |
| 132207 | DTO 149-F3 = IBT 24377 = EXF 633 | Saltern; Secovlje Saltern, Slovenia | 31 | |
| P. chrysogenum | 259.29 | DTO 071-G7 = MUCL 28649 | Representative of P. cyaneofulvum; unrecorded source | 4 |
| 282.97 | DTO 095-E6 = IBT 15162 | Barley; South Africa | 40 | |
| 289.53 | DTO 148-I9 = IBT 19373 = IMI 089373 | Gelatin, UK | 22 | |
| 302.67 | DTO 071-H6 = IBT 27042 = ATCC 18476 = IMI 129964 | Ex-type of P. aromaticum f. microsporum nom. inval.; cheese (?); Leningrad region, Russia | 12 | |
| 306.48┬ | DTO 012-I1 = IBT 5233 = NRRL 807 = IMI 24314 | Ex-lectotype; cheese, Storrs, Connecticut, USA | 6 | |
| 314.48 | DTO 071-G8 = ATCC 10431 = IMI 039764 = MUCL 28658 = MUCL 29077 = MUCL 29143 = NRRL 837 | Ex-type of P. cyaneofulvum; unrecorded source | 4 | |
| 355.48 | DTO 098-D4 = ATCC 10108 = IMI 039759 = IMI 039759ii = NRRL 821 | Ex-type of P. notatum; decaying branches of Hyssopus, Norway | 42 | |
| 412.69 | DTO 071-H9 = IBT 30174 = IBT 23022 = IMI 140340 | Ex-type of P. harmonense; soil; Syria | 37 | |
| 776.95 | DTO 095-F4 = IBT 14462 | Lechuguilla cave; Carlsbad, New Mexico; USA | ||
| 111215 | DTO 071-I8 = IBT 21928 | Mouldy leaves of Salvia officinalis (sage) plant; Farum, Denmark | 11 | |
| 116046 | DTO 001-C2 = IBT 30183 | Water used in production process of cardboard; the Netherlands | 13 | |
| 131516 | DTO 064-E8 = IBT 29739 = IBT 30133 | Air in cleanroom of vaccine production plant; the Netherlands | 33 | |
| 131517 | DTO 068-C3 = IBT 30182 | Indoor environment; Denmark | 34 | |
| 131518 | DTO 068-C4 = IBT 30176 | Indoor environment; Finland | 35 | |
| 131519 | DTO 068-C5 = IBT 30175 | Indoor environment; Finland | 36 | |
| 131520 | DTO 078-E5 = IBT 29738 | Indoor environment clean room; the Netherlands | 2 | |
| 131521 | DTO 087-I2 | Swab sample from ceiling in archive; Utrecht, the Netherlands | 11 | |
| 131522 | DTO 091-D4 | Indoor environment of pharmaceutical company; the Netherlands | 2 | |
| 131524 | DTO 098-E6 = IBT 30140 = NRRL 841 | Ex-type of P. brunneo-rubrum; unrecorded source | 12 | |
| 131525 | DTO 098-E7 = IBT 30146 = NRRL 834 | Ex-type of P. citreoroseum; unrecorded source | 4 | |
| 131526 | DTO 098-E9 = IBT 30136 = NRRL 889 | Ex-type of P. roseocitreum; unrecorded source | 4 | |
| 131527 | DTO 098-F1 = IBT 30147 = NRRL 817 | Ex-type of P. chlorophaeum; unrecorded source | 12 | |
| 131529 | DTO 100-G4 = IBT 30148 = NRRL 819 | Distributed as P. fluorescens nom. inval.; unrecorded substrate; Czech Republic | 4 | |
| 131530 | DTO 100-G6 = IBT 30150 = NRRL 822 | Sputum of a woman with a lung disease; unknown locality | 6 | |
| 131531 | DTO 100-G8 = IBT 30144 = NRRL 827 | Unrecorded source. Capable of volatilizing potassium telluride | 6 | |
| 131532 | DTO 100-H3 = IBT 30138 = NRRL 2136 | Representative of P. meleagrinum (Thom, 1930; Raper & Thom, 1949: 366); unrecorded source | 8 | |
| 131533 | DTO 102-B4 = IBT 26889 = C238 | House dust; Wallaceburg, ON, Canada. Representative of group 2 in the study of Scott et al. (2004) | 11 | |
| 131534 | DTO 102-B5 = IBT 26890 = C71.1 | House dust; Wallaceburg, ON, Canada. Representative of group 3 in the study of Scott et al. (2004) | 12 | |
| 131535 | DTO 102-B7 = IBT 26892 = C200 | House dust; Wallaceburg, ON, Canada. Representative of group 3 in the study of Scott et al. (2004) | 14 | |
| 131536 | DTO 103-E7 = IBT 30084 | Unknown substrate; Dry Valley, Antarctica | 15 | |
| 131538 | DTO 148-I1 = IBT 6041 | Dust; China | 17 | |
| 131545 | DTO 149-A1 = IBT 22435 | Bread; Italy | 4 | |
| 132068 | DTO 149-A2 = IBT 22435 | Bread; Italy | 4 | |
| 132199 | DTO 149-B5 = IBT 29402 | Damaged oil painting; Kharkov, Ukraine | 4 | |
| 132201 | DTO 149-C1 = IBT 30085 | Soil; Dry Valley, Antarctica | 15 | |
| 132202 | DTO 149-C2 = IBT 30086 | Soil; Dry Valley, Antarctica | 15 | |
| 132203 | DTO 149-C3 = IBT 30087 | Soil; Dry Valley, Antarctica | 15 | |
| 132205 | DTO 149-C5 = IBT 30737 | Bee; USA | 11 | |
| 132208 | DTO 100-H2 = IBT 30139 = NRRL 842 | Representative of P. brunneorubrum; unrecorded source | 7 | |
| 132209 | DTO 100-G5 = IBT 30143 = NRRL 820 | Ex-lectotype of P. griseoroseum; unrecorded source | 5 | |
| 132211 | DTO 100-F7 = DTO 086-I4 = IBT 30177 | Surface of operating room; the Netherlands | ||
| 132212 | DTO 102-B9 = IBT 27840 | Indoor environment; Wallaceburg, Ontario, Canada | 13 | |
| 132213 | DTO 102-B2 = IBT 26887 = C317.2 | Indoor environment; Wallaceburg, Ontario, Canada; representative of group 3 in the study of Scott et al. (2004) | 9 | |
| 132214 | DTO 102-B6 = IBT 26891 = C77.2 | Indoor environment; Wallaceburg, Ontario, Canada; representative of group 3 in the study of Scott et al. (2004) | 13 | |
| 132215 | DTO 013-E6 = IBT 30181 | Flour for production of tortillas; USA | 16 | |
| 132216 | DTO 068-B8 = IBT 30179 | Industrial environment; Germany | 8 | |
| 132217 | DTO 102-B3 = IBT 26888 = C8.18 | Indoor environment; Wallaceburg, Ontario, Canada; Scott et al. (2004) | 10 | |
| DTO 100-F7 = DTO 086-I4 = IBT 30177 | Unrecorded source | 2 | ||
| DTO 100-H1 = IBT 30149 = NRRL 839 | Representative of P. cyaneofulvum (Raper & Thom 1949: 372); unrecorded source | 4 | ||
| DTO 100-G9 = IBT 30141 = NRRL 837 | Ex-type of P. cyaneofulvum; unrecorded source | 4 | ||
| Contaminant in Postia placenta MAD 698R culture. No strain available, full genome sequenced | 13 | |||
| P. confertum | 171.87┬ | DTO 072-A9 = IBT 21515 = IBT 3098 = IBT 5672 = IMI 296930 = NRRL 13488 = NRRL A-26904 | Ex-type; cheek pouch; Arizona, USA | |
| P. desertorum | 129469 | IBT 20395 | A1 horizon soil; Utah, USA | |
| 130050 | IBT 14084 = IMI 297544 | Shrub land soil; Wyoming, USA | ||
| 131229 | IBT 14452 | A1 horizon grass land soil; Wyoming, USA | ||
| 131514 | DTO 015-H9 | Soil; Chubut, Argentina | ||
| 131515 | DTO 016-B5 | Soil; Chubut, Argentina | ||
| 131542 | DTO 148-I5 = IBT 16313 | Soil under Artemisia tridentata, cool desert; 16 km north of Rawlins, Wyoming, USA | ||
| 131543┬ | DTO 148-I6 = IBT 16321 | Ex-type; soil under Oryzopsis hymenoides, cool desert; 20 km east of Little America, Wyoming, USA | ||
| P. dipodomyis | 170.87 | DTO 217-B4 = IBT 21522 | Cheek pouch; Arizona | |
| 110412┬ | DTO 072-B6 = IBT 5333 = IMI 296926 = NRRL 13485 = NRRL A-26136 | Ex-type; cheek pouch of kangaroo rat; Arizona, USA | ||
| 110413 | DTO 217-B5 = IBT 17759 | Barley; Starr Valley, Wyoming, USA | ||
| 110414 | DTO 217-B6 = IBT 12700 | Kangaroo rat; Socorro County, Seviletta Natl. Wildlife Refuge, New Mexico, USA | ||
| 110415 | DTO 217-B7 = IBT 11425 | Saddle, mouldy leather, leather probably from Saudi Arabia | ||
| 112570 | DTO 217-B8 = IBT 3353 | Soil; Walnut Crater, Arizona, USA | ||
| P. egyptiacum | 137.70 | DTO 092-B7 = IBT 14685 | Unknown source; Izmir, Bornova, Turkey | |
| 244.32NT | DTO 088-F6 = IBT 14684 = ATCC 10441 = IMI 040580 = NRRL 2090 | Neotype of P. egyptiacum; holotype of P. nilense; soil; Cairo, Egypt | ||
| 457.72 | DTO 088-G5 = NRRL 22307 = IBT 30195 | Desert soil; Egypt | ||
| 458.72 | IBT 14687 | Desert soil; Egypt | ||
| 867.70 | DTO 088-G2 = IBT 14686 | Root; Israel | ||
| 456.72 | DTO 088-G4 = ATCC 24075 = IMI 084589 = IBT 14682 | Ex-type of E. molle and P. molle; soil; Pakistan | ||
| P. flavigenum | 419.89┬ | DTO 072-B4 = IBT 21526 = IBT 3091 = IMI 293207 | Ex-type, wheat flour; Denmark | |
| 110406 | IBT 16616 | Soil under Chrysothmnus nauseosus; Table rock road/highway 80, Wyoming, USA | ||
| 110407 | DTO 217-C5 = IBT 14060 | White beans; USA | ||
| 110409 | DTO 217-C6 = IBT 3230 | Sand; Tunisia | ||
| 110411 | DTO 217-C7 = IBT 11693 | Barley; Canada | ||
| 132247 | DTO 149-C7 = IBT 30948 | Painting on canvas (lining); Provost church, Ljubljana, Slovenia | ||
| P. goetzii | 285.73┬ | DTO 088-G6 = IBT 30199 | Ex-type; soil; Calgary, Alberta | |
| 581.67 | DTO 088-F8 = NRRL 3556 = IBT 4980 = IBT 4993 | Soil; Lahore, Pakistan | ||
| 635.70 | DTO 088-F9 = IBT 30200 | Soil; USA | ||
| 812.70 | DTO 088-G1 = IBT 30196 | Culture contaminant, in Spiromastix warcupii CBS 576.63 | ||
| DTO 055-H1 = IBT 30198 | Endophyte from roots of Pinus ponderosa (ponderosa pine) and Pseudotsuga menziesii (Douglas-fir); Mission | |||
| Creek watershed, Okanogan-Wenatchee National Forest, north-central Washington state, USA | ||||
| DTO 055-H2 | Endophyte from roots of Pinus ponderosa (ponderosa pine) and Pseudotsuga menziesii (Douglas-fir); Mission | |||
| Creek watershed, Okanogan-Wenatchee National Forest, north-central Washington state, USA | ||||
| DTO 055-H3 | Endophyte from roots of Pinus ponderosa (ponderosa pine) and Pseudotsuga menziesii (Douglas-fir); Mission | |||
| Creek watershed, Okanogan-Wenatchee National Forest, north-central Washington state, USA | ||||
| P. griseofulvum | 185.27NT | DTO 072-A5 = IBT 6740 = ATCC 11885 = IMI 075832 = IMI 075832ii = | Ex-neotype; unrecorded substrate; Belgium | |
| NRRL 2152 = NRRL 2300 | ||||
| P. halotolerans | 131537┬ | DTO 148-H9 = IBT 4315 | Ex-type; salt marsh; Egypt | |
| P. kewense | 183.72 | DTO 092-B8 = IBT 14680 | Soil; the Netherlands | |
| 344.61IsoT | DTO 088-F7 = ATCC 18240 = IMI 086561 = NRRL 3332 = IBT 24547 | Isotype; culture contaminant of mineral oil CMI 1959; Surrey, Kew, England | ||
| P. lanosocoeruleum | 215.30┬ | DTO 035-H4 = IBT 3545 = ATCC 10459 = CBS 334.48 = IMI 039818 = NRRL 888 | Ex-type; culture contaminant of P. cyclopium culture; USA | |
| 484.84 | DTO 072-A8 = IBT 21501 = IBT 5903 = IMI 285524 | Ex-type of P. aethiopicum; Hordeum vulgare (barley); Addis Abeba, Ethiopia | ||
| P. mononematosum | 172.87┬ | DTO 072-B2 = IBT 21535 = IMI 296925 = NRRL 13482 | Ex-type; burrow system of Dipodomys spectabilis (banner-tailed kangaroo rat); Arizona, USA | |
| 109616 | DTO 217-B9 = IBT 4309 = IBT 4310 = IBT 5509 | Salt marsh soil; Egypt | ||
| 112104 | DTO 217-C1 = IBT 3073 = IBT 5521 = IBT 5522 = IBT 6071 = | Kangaroo rat; 8 km east of Portal, Arizona, USA | ||
| NRRL A-26910 = NRRL 13483 | ||||
| 112105 | DTO 217-C2 = IBT 11891 | Squash; France | ||
| 112106 | DTO 217-C3 = IBT 11682 | Jerusalem artichoke; Denmark | ||
| 112575 | DTO 217-C4 = IBT 4308 = IBT 4391 = IBT 5507 | Marsh soil; Egypt | ||
| P. nalgiovense | 318.92 | IBT 12383 | Sausage, imported from Italy; Denmark | |
| 352.48NT | DTO 072-A6 = IBT 21536 = ATCC 10472 = IMI 039804 = NRRL 911 | Neotype; Ellischauer cheese; Czech Republic | ||
| 109610 | DTO 217-C9 = IBT 11965 = FRR 3284 | Salami; Germany | ||
| 112438 | DTO 217-D1 = IBT 23346 | Ice; Svalbard, Norway | ||
| P. persicinum | 111235┬ | DTO 072-B8 = IBT 24565 | Ex-type; soil; Qinghai Province, China | |
| P. rubens | 197.46 | DTO 065-B3 | Must contaminant, Belgium. The strain first used for producing penicillin in submerged culture (Raper & Thom 1949: 368–370) | 1 |
| 205.57 | DTO 065-B1 = IBT 30143 = IMI 015378 | Culture contaminant in bacterial culture, UK. Fleming’s original penicillin producing strain | 1 | |
| 307.48 | DTO 065-B2 = IBT 5857 = NRRL 1951 = IMI 40233 | Mouldy cantaloupe Peoria, Illinois, USA. ‘Wisconsin strain’, parent of most high yielding penicillin producing strains; full genome sequenced | 1 | |
| 319.59 | DTO 098-D2 = ATCC 18226 = IMI 068231 | Ex-type of P. chrysogenum mut. fulvescens; soil, Japan; cinnamon-coloured conidia | 39 | |
| 339.52 | DTO 071-H2 = IBT 30130 = ATCC 22349 = IMI 041606 = IMI 041606ii | Ex-type of P. camerunense nom. inval.; root of Elaeis guineensis, together with Chalara paradoxa | 41 | |
| 349.48 | DTO 098-G1 = IBT 4350 = ATCC 10468 = IMI 039762 = NRRL 836 | Unrecorded substrate; Scotland. Representative of P. meleagrinum (Thom 1930, Raper & Thom 1949: 366) | 1 | |
| 401.92 | DTO 001-C6 | Gypsum, building materials; the Netherlands (used as model organism; e.g. Bekker et al. 2012) | 19 | |
| 478.84 | DTO 071-I2 = IBT 21511 | Air in fruit store; Denmark | 19 | |
| 111216 | DTO 071-I9 = IBT 22809 | Saltern; Slovenia | 19 | |
| 129667┬ | DTO 098-E8 = IBT 30129 = NRRL 792 = ATCC 9783 | Ex-lectotype; unrecorded source | 1 | |
| 131513 | DTO 015-F3 = IBT 30659 | Tattoo paint; the Netherlands | 32 | |
| 131523 | DTO 095-E9 = IBT 30661 | Cap of PET bear bottle; Kaulile, Belgium | 38 | |
| 131528 | DTO 100-G3 = IBT 30145 = NRRL 812 | Solution containing 4 percent iron-alum; USA | 3 | |
| 131540 | DTO 148-I3 = IBT 14508 | Lechuguilla Cave; Carlsbad, New Mexico, USA | 19 | |
| 132069 | DTO 149-A3 = IBT 22703 | Soil under Larix; 3 km west of Uthoss, Russia | 1 | |
| 132204 | DTO 149-C4 = IBT 30427 | Unrecorded substrate; Germany | 19 | |
| 132206 | DTO 149-C6 = IBT 30738 | Bee; USA | 30 | |
| 132210 | DTO 100-F6 = NRRL 843 = IBT 5303 | Unrecorded source; approximated P. baculatum (Raper & Thom 1949: 363) | 1 | |
| DTO 100-G7 = NRRL 824 = IBT 30142 | Culture contaminant in bacterial culture, UK; Fleming’s original penicillin producing strain | 1 | ||
| DAOM 234047 | Indoor air; Saskatchewan, Canada | |||
| DAOM 234052 | Pipe wrap in a house; Ontario, Canada | |||
| DAOM 234054 | House dust; Alberta, Canada | |||
| P. sinaicum | 279.82┬ | DTO 097-D3 | Ex-type; marine sludge; Suez Canal, 30 km N of Port Said, Sinai Peninsula, Egypt | |
| P. tardochrysogenum | 132200┬ | DTO 149-B9 = IBT 30075 | Ex-type; soil; McMurdo Dry Valley, Antarctica | 29 |
| P. vanluykii | 131539┬ | DTO 148-I2 = IBT 14505 | Ex-type; Lechuguilla Cave; Carlsbad, New Mexico, USA | 18 |
| 132070 | DTO 149-A4 = IBT 23469 | Soil; Bose Jubony, Isla 25 da Mugo, Shetland del Sur, Antarctica | 23 | |
| 132197 | DTO 149-B3 = IBT 26517 = LJC 005 | Garlic; Villa Aberastain, Pocito, San Juan, Argentina | 18 | |
| Penicillium sp. (near P. kewense) | 103.71 | Soil of wheat field; Kiel, Germany | ||
| 227.81 | DTO 103-D7 = CBS 653.82 = NRRL 2094 | Unknown source. Intermediate between P. brefeldianum and members of the Carpenteles series such as | ||
| P. egyptiacum (Raper & Thom 1949: 146) | ||||
| 653.82 | DTO 088-G7 = CBS 227.81 = NRRL 2094 | Unknown source. Intermediate between P. brefeldianum and members of the Carpenteles series such as P. egyptiacum (Raper & Thom 1949: 146) |
1 CBS: culture collection of the CBS-Fungal Biodiversity Centre, Utrecht, The Netherlands.
2 ATCC: American Type Culture Collection, Manassas, VA, USA; DAOM: Canadian Collection of Fungal Cultures, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada; DTO: internal culture collection of CBS-Fungal Biodiversity Centre; IBT: culture collection of Center for Microbial Biotechnology (CMB) at Department of Systems Biology, Technical University of Denmark; IHEM: culture collection of the Scientific Institute of Public Health – Mycology section, Brussels, Belgium; IMI: CABI Genetic Resources Collection, Surrey, UK; LJC: Coleccion de fitopatogenos de cultivos horticolas, Mendoza, Argentina; NRRL: Agricultural Research Service Culture Collection, National Center for Agricultural Utilization Research, Peoria, Illinois, USA.
DNA extraction, PCR amplification, sequencing and data analysis
Total genomic DNA was extracted using the Ultraclean™ Microbial DNA isolation kit (MoBio, Solana Beach, USA) according to the manufacturer’s instructions. To estimate phylogenetic relationships among species of sect. Chrysogena, parts of the RPB1 (RNA polymerase II largest subunit; regions E and F, according Matheny et al. 2002), RPB2 (polymerase II second largest subunit; regions 5–7), calmodulin (cmd) and β-tubulin genes (benA) were amplified and sequenced according the methods described previously (Houbraken & Samson 2011, Houbraken et al. 2012). To test the applicability of ITS sequencing for species identification, sequences were generated of the strains listed in Table 1 using primers V9G and LS266 (de Hoog & Gerrits van den Ende 1998).
Each individual dataset was aligned using the Muscle software as implemented in MEGA5 (Tamura et al. 2011). Prior to combining datasets, each individual dataset was analysed using Neighbour Joining (NJ) analysis in MEGA5. The number of bootstrap replicates was set to 1000 and P. griseofulvum CBS 185.27NT was used as outgroup. The combined RPB1, RPB2, benA and cmd dataset was used to study the phylogeny of sect. Chrysogena. Statistical support was measured by Bayesian tree inference (BI) analysis using MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). To identify the most suitable substitution model for the Bayesian analyses, we used MrModeltest v. 2.3 (Nylander 2004), utilizing the Akaike information criterion (AIC). The Bayesian analysis was performed with two sets of four chains (one cold and three heated) and the STOPRULE option, stopping the analyses at an average standard deviation of split frequencies of 0.01. The sample frequency was set to 100; the first 25 % of trees were removed as burnin. Statistical support was also measured by Maximum Likelihood (ML) analysis using the RAxML (randomized axelerated maximum likelihood) software (Stamatakis 2008). The phylogram obtained with RAxML was used for presenting the data.
Morphological analysis and extrolite analysis
For macromorphological analysis, strains were inoculated at three points onto Czapek yeast agar (CYA), CYA supplemented with 5 % NaCl (CYAS), yeast extract sucrose agar (YES), malt extract agar (MEA), creatine agar (CREA), dichloran 18 % glycerol agar (DG18) and oatmeal agar (OA). Plates were incubated in the dark for 7 d at 25 °C. In addition, CYA plates were inoculated and incubated for 7 d at 15, 30 and 37 °C in darkness. After incubation, colony diameters were measured and the degree of sporulation, obverse and reverse colony colours and the production of soluble pigments were determined. Colony photographs were taken with a Canon 400D camera under incandescent light. Furthermore, isolates were examined for the production of alkaloids reacting with Ehrlich reagent, using the filter paper method described by Lund (1995). Microscopic observations were made using Olympus BH-2 or Zeiss Axioskop 2 Plus microscopes. Mounts were made in 85 % lactic acid and excess conidia were washed away with a drop of ethanol. Manual measurements were made for at least 20 conidia, ascospores, phialides, metulae, branches and ascomata. Detailed analysis of the ornamentation of the ascospores was performed using scanning electron microscopy (SEM) using the method described by Houbraken et al. (2011b).
For extrolite analyses, cultures were grown on CYA and YES for 7 d at 25 °C. After incubation, five plugs were taken from each agar medium, pooled and extracted according the method described by Smedsgaard (1997). The extracts were subsequently analysed according the HPLC-diode array detection method (Frisvad & Thrane 1987) as modified by Houbraken et al. (2012). Penicillin production was tested according the method described by Andersen & Frisvad (1994).
ITS barcoding
To assess the sequence diversity of the ITS locus of strains belonging to sect. Chrysogena, an UPGMA (unweighted pair group method with arithmetic mean) dendrogram based on Kimura 2-parameter distances (K2P, recommended by CBOL, www.barcoding.si.edu) was constructed in MEGA5.
Haplotype diversity
In order to study the haplotype diversity among P. chrysogenum, P. rubens and closely related species, the RPB1, RPB2, calmodulin and β-tubulin sequence datasets were expanded with ITS, FacA (acetyl-CoA ligase; (facA-F_Pc (TGGAAGTGGTACTTCGAG), facA-R_Pc (ACACGACCGCGGATCCAGTA))), ParA (3-phosphoadenosine-5-phosphosulfate reductase; (parA-F_Pc (CCCGAGATTGTTTTCACCAA), parA-R_Pc (ACCTTGGCCACCCAGTCGTA))) and TrpC (anthranilate synthase multifunctional protein; (trpC-F_Pc (GCAGTGGAGGGTGTTCAGTT), trpC-R_Pc (TTAACCTCGACCAGAGGCTCCAT))) gene sequences. These datasets were supplemented with sequences obtained from the two full genome initiatives (van den Berg et al. 2008, http://genome.jgi.doe.gov/). The software programme DnaSP v. 5.10 (Librado & Rozas 2009) was used to find the different haplotypes in the alignment. Gaps and missing data were not considered during this calculation. Network v. 4.6.1.0 (www.fluxus-engineering.com) was used to generate a haplotype network using the median-joining network algorithm. Sequences were deposited in the GenBank nucleotide database under accession numbers JX996198–JX997117.
RESULTS
Phylogeny
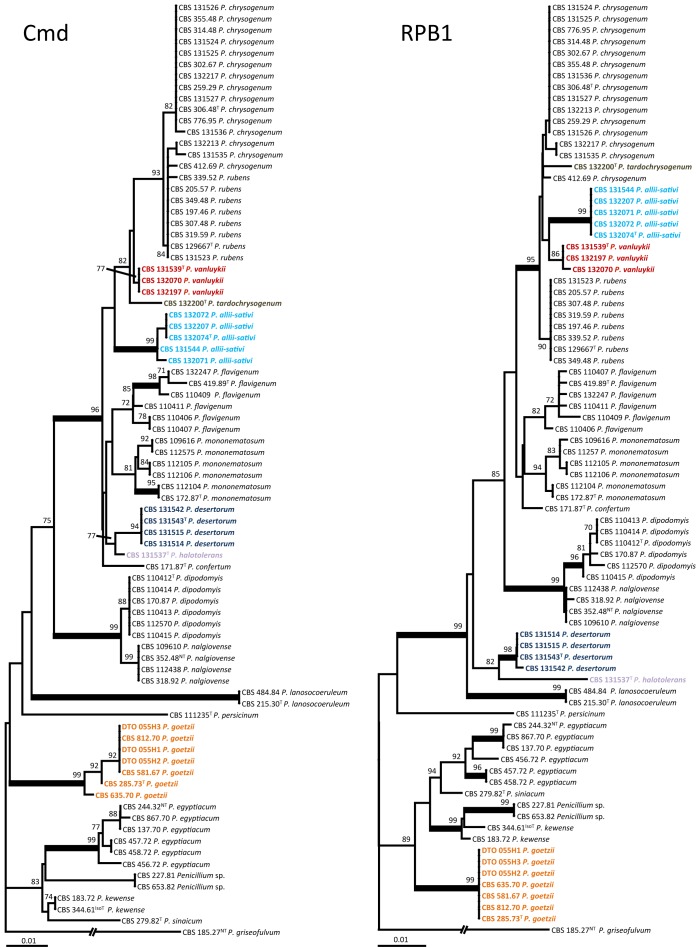
The phylogenetic relationship among members of sect. Chrysogena was studied by combining the RPB1, RPB2, cmd and benA datasets. Prior to this analysis, NJ analysis was performed on each individual dataset to determine incongruences. The individual RPB1, RPB2, cmd and benA datasets were 722, 958, 505 and 446 bp positions long, respectively. The optimal model was determined using MrModeltest and the SYM+G model was optimal for the calmodulin and RPB2 dataset, the model SYM+I+G for the RPB1 and HKY+I+G model for the BenA partition.
Eighteen lineages were observed among isolates assigned to sect. Chrysogena (Fig. 1) (Houbraken & Samson 2011) and six represent new species, named here as P. allii-sativi, P. desertorum, P. goetzii, P. halotolerans, P. tardochrysogenum and P. vanluykii. Penicillium allii-sativi, P. chrysogenum, P. rubens, P. tardochrysogenum and P. vanluykii together formed a well-supported clade in each analysis of the individual genes (> 87 % bootstrap support), except for calmodulin (Fig. 2). The clustering within this clade was generally poorly supported and varied among the examined datasets; however, analysis of the combined dataset generated highly supported clades (Fig. 1). Analysis of the combined dataset shows that P. allii-sativi is basal to the other species of this clade, and that P. chrysogenum and P. rubens are sister species with P. vanluykii basal to them. The position of isolates CBS 412.69 (ex-type of P. harmonense), DTO 102-B2 and DTO 102-B7 were in conflict between the cmd dataset and the phylogram based on combined nucleotide data. This set of isolates resides in the P. rubens clade in the cmd dataset with statistical support (bootstrap value 84 %), while they were positioned in the P. chrysogenum clade in the combined phylogram.
Fig. 1.
Best-scoring Maximum Likelihood (ML) tree using RAxML based on a combination of partial calmodulin, β-tubulin, RPB1 and RPB2 sequences, showing the relationship among members of Penicillium section Chrysogena. The bootstrap (bs) values of the ML analysis and the BI posterior probabilities (pp) values are presented at the nodes (bs/pp). Values less than 70 % supported in the ML analysis or less than 0.95 in the BI analysis are omitted, whereas asterisks indicate full support (100 % bs, 1.00 pp). The branches with more than 95 % bootstrap support and 1.00 pp values are thickened. The phylogram is rooted with Penicillium griseofulvum CBS 185.27NT.
Fig. 2.
Best-scoring Neighbour Joining (NJ) phylograms based on calmodulin, RPB1, RPB2 and β-tubulin datasets using MEGA5. Well-supported branches (> 95 % bootstrap supported) are in bold, values less than 70 % bootstrap support are not shown. Penicillium griseofulvum CBS 185.27NT was used as outgroup.
All P. dipodomyis, P. flavigenum and P. nalgiovense isolates formed distinct well-supported clades in all datasets, and all P. desertorum strains clustered in a well-supported clade except in the β-tubulin gene tree. Penicillium dipodomyis and P. nalgiovense are sister species in all individual datasets and some variation was observed within the P. dipodomyis clade; however, this pattern was incongruent among datasets. Fig. 1 (combined analysis) shows that P. confertum is basal to P. mono-nematosum, but it renders the P. mononematosum clade paraphyletic in the β-tubulin dataset. Three subclades are consistently formed in P. mononematosum phylograms. One clade includes two strains from a salt marsh in Egypt (CBS 109616, CBS 112575) while CBS 112105 and CBS 112106 also form a separate clade, as do CBS 172.87┬ and CBS 112104. The ex-type of P. lanoscoeruleum CBS 215.30┬ and P. aethiopicum CBS 484.84┬ resolve in a single clade and priority is given to the former (oldest) name.
Four species are known to form ascospores and three of these, P. egyptiacum, P. kewense and P. sinaicum, group in a clade with full support (100 % bootstrap support, 1.00 pp). Three line-ages were observed within the P. egyptiacum clade. One lineage was centred on the neotype of P. egyptiacum (CBS 244.32NT), another on CBS 457.72 and CBS 458.72 and the third consisted of CBS 456.72┬, the ex-type strain of both Penicillium molle and Eupenicillium molle. These lineages were present in all analysed individual datasets. The combined analysis placed P. goetzii basal to the asexual Penicillium species; however, this is not the case for individual datasets. In the RPB1 dataset, this species was grouped together with other ascospore producing species (89 % bootstrap support).
Morphology, physiology and extrolites
Penicillium chrysogenum, P. rubens, P. tardochrysogenum, P. vanluykii and P. allii-sativi are phenotypically similar and share characters such as a fast growth rate on YES with dense sporulation (except P. tardochrysogenum), a CYAS : CYA ratio greater than 1, ter- or quarterverticillate divergently branched conidiophores, and relatively short phialides (< 9 μm). Penicillin is produced by all species and roquefortine C, D and meleagrin by all except P. tardochrysogenum. There are also differences among the species of this section. Penicillium vanluykii produces dark green conidia on MEA and CYA, yellow soluble pigments on CYA incubated at 30 °C and a series of characteristic unidentified extrolites. In common with P. vanluykii, P. allii-sativi also produces conidia in shades of dark green on CYA; however, there is no yellow soluble pigment production on CYA incubated at 30 °C or in insignificant amounts. This species also produces a diagnostic array of extrolites (Table 2) including the potent mycotoxin verrucosidin. Penicillium tardochrysogenum is represented by one strain (CBS 132200┬). It is unique in this clade for its more restricted and floccose colonies on MEA, a lack of sporulation on YES and the production of finely roughened conidia. This species does not produce yellow soluble pigments on CYA when incubated at 30 °C and produces the asperentins, a series of compounds not produced by other members of series Chrysogena.
Table 2.
Overview of extrolites produced by species belonging to Penicillium section Chrysogena.
| Species | Extrolites |
|---|---|
| P. allii-sativi | 1) atlantinone A; 2) chrysogenamide; 3) 2-(4-hydroxyphenyl)-2-oxo acetaldehyde oxim; 4) a naptho-γ-pyrone; 5) penicillins; 6) 2-pyruvoylaminobenzamide; 7) roquefortine C, D & meleagrin; 8) verrucosidin, normethylverrucosidin, deoxyverrucosidin & verrucosidinol; 9) ‘ALKONA’; 10) ‘AURCH’; 11) ‘CRYPT’; 12) ‘DERH’, ‘GULLA’ & ‘KUTZ’ (atromentins?); 13) ‘OTOF’; 14) ‘SENGAX’; 15) ‘SNORL’; 16) ‘SPOFI’; 17) ‘VERNX’ |
| P. chrysogenum | 1) andrastrin A & B; 2) chrysogine, 2-pyruvoylaminobenzamide, 2-acetyl-quinazolin-4(3H)-one & 2-(2-hydroxypropionylamino)-benzamide; 3) citreoisocoumarin; 4) penicillins; 5) roquefortine C, D & meleagrin; 6) secalonic acid D & F; 7) sorbicillins; 8) xanthocillins; 9) ‘met Ø’; 10) ‘DOLDO’ |
| P. confertum | 1) asteltoxin; 2) roquefortine C, D & meleagrin; 3) secalonic acid D |
| P. desertorum | 1) austalides?; 2) 2-(4-hydroxyphenyl)-2-oxo acetaldehyde oxim; 3) Raistrick phenols; 4) ‘FOL’ |
| P. dipodomyis | 1) diaporthins (citreoisocoumarin, diaportinic acid, diaportinol, dichlorodiaporthin & 6-methyl-citreoisocoumarin); 2) dipodazin; 3) penicillins; 4) ‘CD’ 1-5 & ‘CRYPT’; 5) ‘CDU’; 6) ‘DI’ (an indol-alkaloid); 7) ‘DIOR’; 8) ‘DIPA’; 9) ‘FCD’; 10) ‘GNALDI’; 11) ‘met Ø’; 12) ‘TOLO’; 13) ‘VIK’ |
| P. egyptiacum (= incl. E. molle) | 1) 10,23-dihydro-24,25-dehydroaflavinine (Wang et al. 1995, also seen in this study); 2) macrophorin H (Wang et al. 1995); 3) mollenines A and B (Wang et al. 1998); 4) penicillic acid; 5) Raistrick phenols; 6) secalonic acid D & F; 7) tetronic acids; 8) xanthocillin X (Vesonder 1979, NRRL 1022, not seen in this study) |
| P. flavigenum | 1) penicillins; 2) penitrem A; 3) roquefortine C & meleagrin; 4) sorbicillins |
| P. goetzii | 1) andrastin A; 2) citreoisocoumarin; 3) fumitremorgin A, verruculogen; 4) isoepoxydon; 5) 10,23-dihydro-24,25-dehydroaflavinine & 10,23,24,25-tetrahydro-24-hydroxyaflavinine; 6) ‘GLAD’ |
| P. halotolerans | 1) andrastin A; 2) Raistrick phenols, roquefortine C, D and meleagrin; 3) ‘CUCU’ and other polar polyketides; 4) ‘PLIL’ |
| P. kewense | 1) andrastin A; 2) fumitremorgin A & verruculogen; 3) 10,23-dihydro-24,25-dehydroaflavinine & 10,23,24,25-tetrahydro-24-hydroxyaflavinine; 4) isoepoxydon; 5) 4’-oxomacrophorin A & D; 6) roquefortine C; 7) ‘KEWS’ 1-3 |
| P. cf. kewense | 1) andrastin A; 2) isoepoxydon; 3) 10,23-dihydro-24,25-dehydroaflavinine |
| P. lanosocoeruleum (=P. aethiopicum) | 1) griseofulvins (dechlorogriseofulvin, dehydrogriseofulvin griseofulvin, griseophenone C etc.), de; 2) isoepoxydon; 3) tryptoquialanins & tryptoquialanons; 4) viridicatumtoxin; 5) ‘BR’; 6) ‘met U’; 7) ‘PRU’; 8) ‘RAIS’; 9) ‘SNOK’; 10) ‘VERNX’ |
| P. mononematosum | 1) andrastin A & B; 2) citreoisocoumarin; 3) cyclopaldic acid & derived chromanols; 4) fumitremorgin A, B, C, TR-2 & verruculogen; 5) isochromantoxins; 6) viriditoxin; 7) ‘ASTYL’; 8) ‘GULLA’; 9) ‘MER’; 10) ‘MONTI’; 11) ‘PJIM’; 12) ‘PLOT’; 13) ‘OKA’ 1 & 2 (okaramins?); 14) ‘PAEL’; 15) ’PYTO’; 16) ‘SNAT’; 17) ‘TRYP’ (= dehydrocurvularin?); 18) ‘VERNX’ |
| P. nalgiovense | 1) chrysogine, 2-pyruvoylaminobenzamide, 2-acetyl-quinazolin-4(3H)-one & 2-(2-hydroxypropionylamino)-benzamide; 2) citreoisocoumarin; 3) diaporthins (citreoisocoumarin, diaportinic acid, diaportinol, dichlorodiaporthin & 6-methyl-citreoisocoumarin); 4) dipodazin; 5) nalgiovensin, nalgiolaxin and bisanthron-derivatives of those; 6) penicillins |
| P. persicinum | 1) andrastin A & B; 2) chrysogine, 2-pyrovoylaminobenzamide, 2-acetyl-quinazolin-4(3H)-one & 2-(2-hydroxypropionylamino)-benzamide; 3) griseofulvins; 4) roquefortine C & D; 5) ‘AURIN’; 6) ‘DOLDO’; 7) ‘MURA’; 8) ‘XYLA’ |
| P. rubens | 1) andrastin A & B; 2) chrysogine, 2-pyruvoylaminobenzamide, 2-acetyl-quinazolin-4(3H)-one & 2-(2-hydroxypropionylamino)-benzamide; 3) citreoisocoumarin; 4) 7-deacetoxyyanuthone; 5) penicillins; 6) roquefortine C, D & meleagrin; 7) sorbicillins (including bisorbibutenolide, bisorbicillinol, bisvertinoquinol, bisvertinolone,2’,3’-dihydrosorbicillin, oxosorbicillinol tautomer, sorhinones A, B, & C, rezishanones A, B, C & D, sorbicillin); 8) xanthocillins; 9) PR-toxin; 10) quinazolone X (based on UV spectrum, not yet structure elucidated); 11) ‘DOLDO’ |
| P. sinaicum | 1) 10,23-dihydro-24,25-dehydroaflavinine; 2) isoepoxydon or similar compound; 3) ML-236A; 4) pseurotin A; 5) indolalkaloids; 6) HO6; 7) ‘FOPT’; 8) ‘FORN’ 1, 2 & 3 |
| P. tardochrysogenum | 1) asperentins; 2) penicillins; 3) secalonic acid D & F; 4) ‘met Ø’ |
| P. vanluykii | 1) andrastin A; 2) chrysogine; 3) penicillins; 4) roquefortine C, D and meleagrin, and the uncharacterized extrolites ‘CRYPT’ (4 compounds), ‘POO’, ‘KNOLF’, ‘TBRE’, ‘FJOR’ (2 compounds). |
Phylogenetic analyses show that P. halotolerans and P. desertorum are sister species (Fig. 1) and phenotypic characters support their classification in sect. Chrysogena (CYAS : CYA ratio > 1; velvety colonies and production of short, ampulliform phialides). Penicillium halotolerans can be differentiated from P. desertorum by the production of yellow soluble pigments on CYA incubated at 30 °C. Furthermore, the conidiophores of P. desertorum have various short, divaricate branches at various levels along the stipe, while P. halotolerans has ter- or quarterverticillate branched conidiophores like other species of sect. Chrysogena. Strains of P. desertorum consistently produce species-specific profiles of extrolites (Table 2). Some of these extrolites are partially characterised and details on retention time, retention index and UV maxima (nm) are given in Table 3. Penicillium halotolerans is only known from its ex-type strain (CBS 131537┬) and this isolate produces a unique combination of extrolites, namely andrastin A, roquefortine C & D, meleagrin and Raistrick phenols.
Table 3.
A partial characterisation of extrolites from Penicillium section Chrysogena which have not yet been fully structure elucidated based on HPLC-DAD.
| Extrolite | Retention time | Retention index | UV maxima (nm) (sh: shoulder) | Ref. |
|---|---|---|---|---|
| ‘CRYPT1’ | 10.62 | 769 | 200, 271 | A1 |
| ‘CRYPT2’ | 10.87 | 774 | 200, 271 | A |
| ‘CRYPT3’ | 12.66 | 812 | 200, 271 | A |
| ‘CRYPT4’ | 14.66 | 855 | 200, 271 | A |
| ‘FJOR1’ | 6.43 | 799 | 200, 335 | A |
| ‘FJOR2’ | 6.67 | 810 | 200, 228, 270, 330 | A |
| ‘KNOLF’ | 16.76 | 900 | 202, 235sh, 270, 337 | A |
| ‘POO’ | 15.07 | 864 | 202, 266, 319 | A |
| ‘TBRE’ | 7.60 | 705 | 221, 267, 331 | A |
| ‘KEWS1’ | 2.67 | 692 | 220, 275, 297, 380, 400sh | A |
| ‘KEWS2’ | 6.96 | 802 | 200, 225, 250, 275, 311sh, 378, 400sh | A |
| ‘KEWS3’ | 13.11 | 967 | 226, 250sh, 260, 276sh, 355sh, 376, 385 | A |
| ‘AURIN’ | 6.48 | 814 | 200, 235, 311 | A |
| ‘DOLDOX’ | 2.86 | 710 | 265 | A |
| ‘MURA’ | 25.39 | 1479 | 201, 212sh, 265, 310 | A |
| ‘XYLA’ | 5.03 | 773 | 201, 231, 281, 320 | A |
| ‘FOPT’ | 12.54 | 976 | 200, 240sh, 319 | A |
| ‘FORN1’ | 20.84 | 1280 | 204, 239, 292 | A |
| ‘FORN2’ | 25.57 | 1494 | 204, 239, 292 | A |
| ‘FORN3’ | 16.58 | 1111 | 204, 239, 292 | A |
| ‘HO6’ | 8.22 | 847 | 200, 225, 242, 274sh, 323 | A |
| ‘DOLDO’ | 4.63 | 710 | 280 | B2 |
| ‘met Ø’ | 7.95 | 852 | 210, 255, 275sh | B |
| ‘ALKONA’ | 11.60 | 1974 | 200, 215sh, 265, 287sh | B |
| ‘AURCH’ | 6.89 | 796 | 200, 228, 310 | B |
| a naphtho-γ-pyrone | 6.77 | 814 | 202, 232, 280, 328, 338, 405 | B |
| chrysogenamide | 9.87 | 963 | 221, 273, 280sh | B |
| ‘DERH’ | 8.02 | 869 | 223, 280, 359, 440sh | B |
| ‘GULLA’ | 8.15 | 875 | 220, 272, 359, 481sh | B |
| ‘KUTZ’ | 12.70 | 1057 | 220, 269, 320, 412 | B |
| ‘OTOF’ | 12.48 | 1038 | 217, 271, 315 | B |
| ‘SENGAX’ | 15.48 | 1360 | 220, 277, 330 | B |
| ‘SNORL’ | 15.84 | 1380 | 210, 225, 264, 323 | B |
| ‘SPOFI’ | 12.21 | 1106 | 200, 227sh | B |
| ‘CD1’ | 11.460 | 892 | 200, 273 | C3 |
| ‘CD2’ | 12.782 | 919 | 200, 273 | C |
| ‘CD3’ | 13.612 | 935 | 200, 273 | C |
| ‘CD4’ | 13.972 | 942 | 200, 273 | C |
| ‘CD5’ | 15.960 | 981 | 200, 273 | C |
| ‘CDU’ | 9.854 | 858 | 200, 220, 275 | C |
| ‘CRYPT’ | 10.769 | 877 | 200, 269 | C |
| ‘DI’ | 11.891 | 886 | 200, 240, 270, 325 | C |
| ‘DIOR’ | 14.143 | 947 | 200, 261, 425 | C |
| ‘DIPA’ | 17.554 | 995 | 200, 213, 236, 259, 295, 331 | C |
| ‘FCD’ | 7.359 | 808 | 200, 215, 280, 341 | C |
| ‘GNALDI’ | 10.174 | 865 | 200, 224, 335 | C |
| ‘TOLO’ | 16.828 | 990 | 207, 250, 281, 376 | C |
| ‘VIK’ | 25.092 | 1163 | 200, 210sh, 280sh, 330-375 | C |
| Tetronic acid, P. egyptiacum 1 | 1.390 | 6974 | 227, 261, 322sh | C |
| Tetronic acid, P. egyptiacum 1 | 1.623 | 7014 | 200, 223, 270sh, 303 | C |
| Tetronic acid, P. egyptiacum 1 | 1.918 | 7084 | 200, 225sh, 275 | C |
| ‘BR’ | 3.846 | 733 | 200, 225, 271, 320sh, 421 | C |
| ‘met U’ | 2.679 | 711 | 200, 230+, 263, 364 | C |
| ‘PRU’ | 1.892 | 697 | 200, 235, 280 | C |
| ‘RAIS’ | 3.51 | 716 | 214, 222sh, 270, 310 | C |
| ‘SNOK’ | 14.15 | 911 | (200), 275 | C |
| ‘VERNX’ | 2.285 | 704 | 202, 285 | C |
| ‘ASTYL’ | 16.819 | 994 | 263, 359 | C |
| ‘GULLA’ | 15.283 | 964 | 220, 272, 359, 431sh | C |
| ‘MER’ | 7.351 | 798 | 222, 225sh, 263, 318 | C |
| ‘MONTI’ | 17.416 | 992 | 200, 210sh, 266, 280sh, 372, 440sh | C |
| ‘PJIM’ | 13.656 | 993 | 200, 218, 270 | C |
| ‘PLOT’ | 17.571 | 987 | 202, 265, 281. 360 | C |
| ‘PAEL’ | 29.533 | 1291 | 230 | C |
| ‘PYTO’ | 4.384 | 741 | 200, 276, 370 | C |
| ‘SNAT’ | 19.394 | 1043 | 200, 224sh, 275 | C |
| ‘TRYP’ | 12.304 | 893 | 202, 225, 279, 300sh | C |
| ‘VERNX2’ | 1.877 | 694 | 202, 285 | C |
| ‘CUCU’ | 1.519 | 683 | 202, 222sh, 277, 300sh | C |
| ‘PLIL’ | 23.405 | 1218 | 200, 223sh, 299 | C |
1 A: Nielsen & Smedsgaard 2003; 2 B: Nielsen et al. 2011; 3 C: Frisvad & Thrane 1987; 4 Inaccurate RI values, as chromatographic peaks were broad.
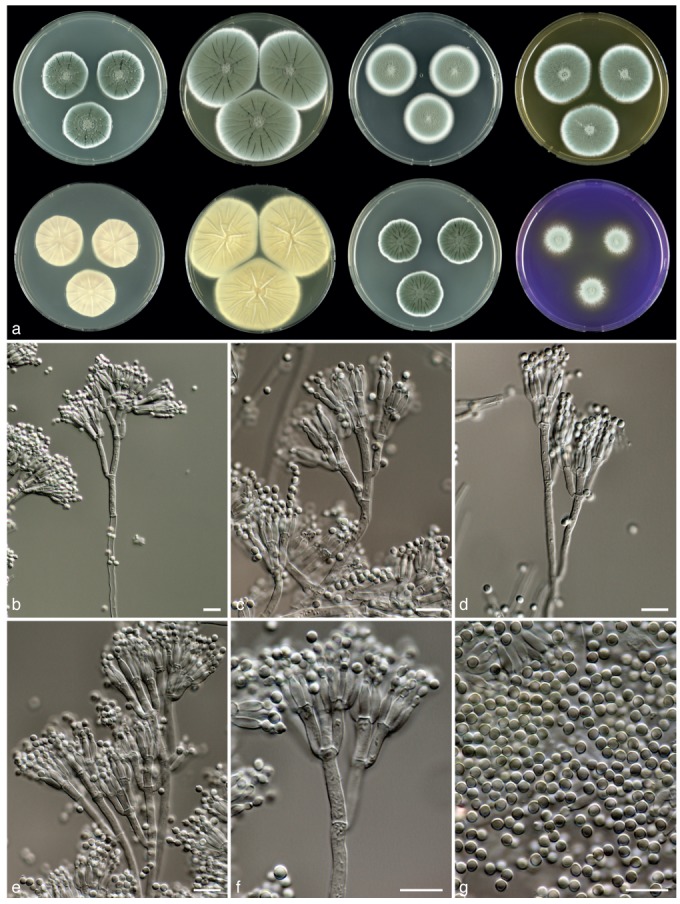
Four of the 18 species (P. egyptiacum, P. goetzii, P. kewense and P. sinaicum) are capable of forming a sexual state. These species are characterised by the production of creamish, avellaneous or ochraceous ascomata, ter- to quarterverticillate branched conidiophores and globose to subglobose conidia. Isolates grown on CYA for 7 d at 25 °C typically produce brown or red-brown soluble pigments. However, they differ from each other by various characters. Penicillium egyptiacum is a good acid producer on CREA, while the other species do not or produce limited amounts of acidic compounds. These species also differ in ascospore size and ornamentation (Fig. 3). The ascospores of P. egyptiacum measure 2–3×2.5–3.5 μm, but vary in their ornamentation. CBS 244.32NT and CBS 137.70 have inconspicuous ridges and smooth-walled valves, while ascospores of CBS 457.72 have closely separated equatorial ridges, with prominent secondary ridges and roughened valves. In contrast, the ascospores of P. goetzii are larger, 3–4.5×2.5–4 μm, with two distinct equatorial ridges and often two secondary ridges that are connected by transverse ribs and valves ornamented with a reticulate pattern. The ascospores of P. kewense take an intermediate position between those of P. egyptiacum and P. goetzii, and P. sinaicum is unique in having ascospores without a distinct equatorial ridge and reticulate valves (Fig. 3). Penicillium egyptiacum, P. goetzii, P. kewense and P. sinaicum also produce species-specific patterns of extrolites. Penicillic acid, Raistrick phenols and secalonic acids D & F are produced by P. egyptiacum but not by the other ascospore producing species. On the other hand, andrastin A, fumitremorgin A and verruculogen are produced by P. goetzii and P. kewense, and the uncharacterised compound ‘GLAD’ is only produced by P. goetzii.
Fig. 3.

Scanning Electron Micrographs of ascospores of species belonging to Penicillium section Chrysogena. a. P. egyptiacum CBS 244.32; b. P. egyptiacum CBS 457.72; c. P. sinaicum CBS 279.82; d. P. goetzii CBS 285.73; e. P. kewense CBS 344.61; f. Penicillium sp. CBS 103.71 — Scale bars = 2 μm.
ITS barcoding
ITS sequences were generated to assess the suitability of this locus for species identification in sect. Chrysogena and 44 % of the species can unequivocally be identified with this locus. Penicillium confertum, P. goetzii, P. halotolerans, P. lanoscoeruleum, P. mononematosum, P. nalgiovense and P. percisinum can be reliably identified by ITS sequencing. Five ITS sequence variants are present in our revised concept of P. chrysogenum. A total of 61 % of the P. chrysogenum strains have identical ITS sequences and this sequence is also present in P. tardochrysogenum (100 %) and P. allii-sativi (100 %). A different P. chrysogenum sequence was observed in 15 % of the examined strains (e.g. CBS 776.95, CBS 131522, CBS 132211). This sequence is shared with P. rubens, all strains of which have identical ITS sequences. The three other unique P. chrysogenum sequences were represented by CBS 131538 (2 %), CBS 131516 (10 %) and CBS 111215 (12 %). The three investigated P. vanluykii isolates have a ITS sequence that is shared with NRRL 3710, a strain identified as P. chrysogenum by Henk et al. (2011). ITS sequences could not distinguish P. dipodomyis and P. flavigenum, while P. kewense and P. sinaicum share sequences with P. egyptiacum CBS 456.72, CBS 457.72 and CBS 458.72.
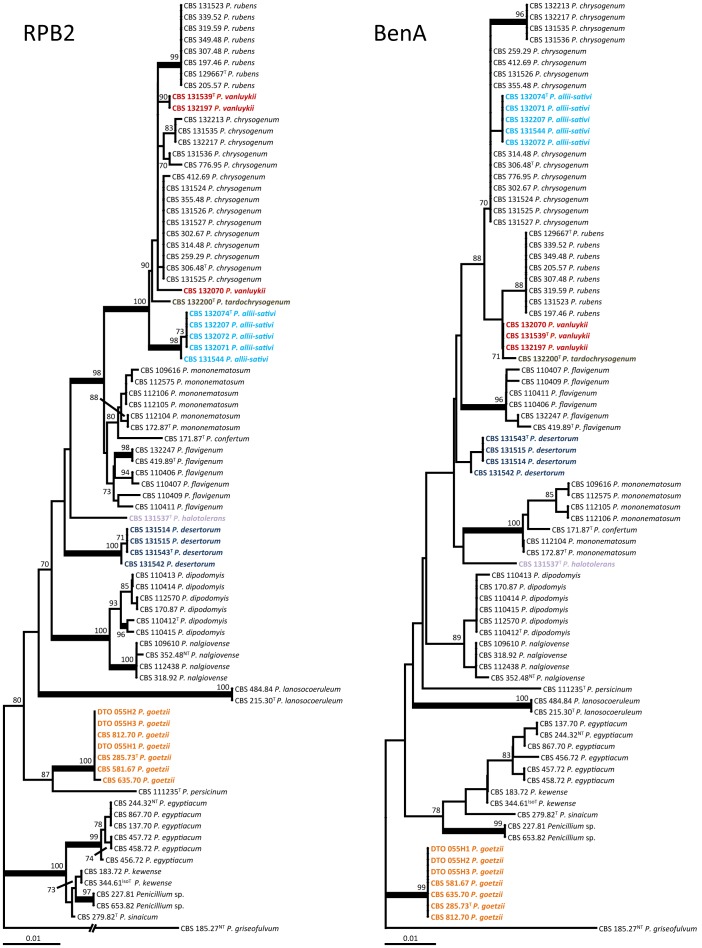
Haplotype diversity
A detailed analysis was performed on 88 P. allii-sativi, P. chrysogenum, P. rubens, P. tardochrysogenum and P. vanluykii isolates, including ex-type and authentic strains, supplemented with isolates used in other taxonomic studies (Samson et al. 1977, Scott et al. 2004, Houbraken et al. 2011a) and other representative strains from culture collections. Haplotypic groups were defined based on the combined sequence alignment of eight loci (cmd, RPB1, RPB2, benA, TrpC, parA, FacA, ITS). Forty-three haplotype groups were detected, most containing only one strain. The haplotype network is shown in Fig. 4 and the haplotype assignment of each strain is included in Table 1. This data demonstrates that haplotype diversity among P. chrysogenum strains is higher than among P. rubens strains. The full genome sequenced P. rubens strain Wisconsin 54-1255 belongs to haplotype 1. This haplotype includes most of the other P. rubens strains, including the ex-type strain CBS 129667┬ (9/20 P. rubens isolates). Serendipitously, a strain of P. chrysogenum for which no culture is available, had its full genome sequenced unexpectedly as a contaminant of a Postia placenta MAD 698R culture (http://genome.jgi.doe.gov/Pench1/Pench1.info.html). Our haplotype analysis shows that this strain belongs to haplotype group 13, together with strains CBS 132214, CBS 132212 and CBS 116046; perhaps one of these strains could be selected as ‘epitype’ kind of voucher to represent this genome strain. However, CBS 116046 is a good penicillin producer, but no penicillin production was observed in CBS 132214 and CBS 132212. In contrast, both CBS 132214 and CBS 132212 produce roquefortine C, but CBS 116046 does not. CBS 132214 was the only strain producing the uncharacterised compound ‘met Ø’. These results suggest that even with 8 loci, the resulting haplotype assignments may not be precise enough to correlate with a precise genome.
Fig. 4.
Haplotype network of P. chrysogenum (yellow), P. rubens (blue), P. vanluykii (purple), P. allii-sativi (green) and P. tardochrysogenum (black) strains based on cmd, benA, RPB1, RPB2, TrpC, ParA, FacA and ITS sequences. In total, 43 different haplotypes were detected and a detailed list is given in Table 1. Red coloured circles represent median vectors. The lines between the groups connecting the haplotypes show the number of nucleotides differing.
TAXONOMY
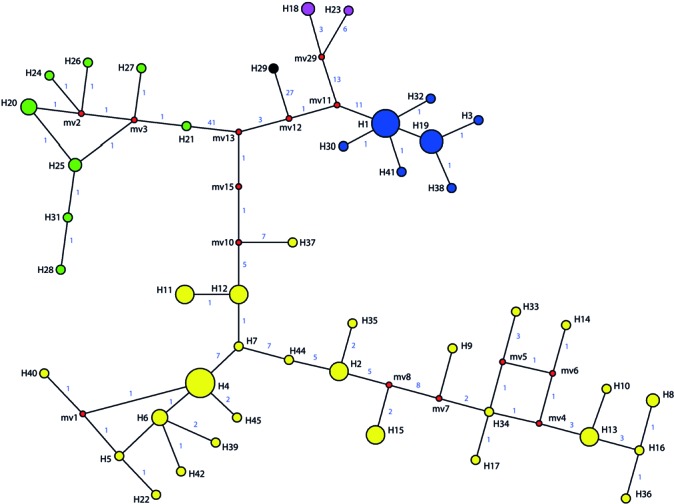
Penicillium allii-sativi Frisvad, Houbraken & Samson, sp. nov. —MycoBank MB801873; Fig. 5
Fig. 5.
Penicillium allii-sativi, CBS 132198. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b–f. conidiophores; g. conidia. — Scale bars = 10 μm.
Typus. ARGENTINA, Mendoza, Lavalle, Col 3 de Mayo, on bulbs of Allium sativum (garlic), M. Makuch & J. Valdez (CBS H-21058 holotype, cultures ex-type CBS 132074 = IBT 26507 = DTO 149-A8 = LJC 206).
Etymology. Referring to Allium sativum (garlic), the substrate where the type strain was isolated from.
Sporulation on CYA dense; colonies slightly polygonal in outline, velvety; mycelium white, sporulation in shades of dark green, exudate droplets large, clear, pale yellow or light brown; soluble pigments absent or occasionally present, light brown; colony reverse pale brown. Soluble pigments on YES absent; mycelium white; sporulation dense; sporulation dark green; exudate absent, reverse beige. Sporulation on DG18 dense; sporulation grey-green or dull green; reverse pale. Colonies on MEA velvety or slightly floccose; sporulation variable, grey-green, dark green or dull green; exudate droplets large, clear, pale yellow or light brown, reverse yellow-brown. No violet reaction with Ehrlich reagents. Sclerotia absent. Conidiophores borne from the agar surface, ter- or quarterverticillate, divaricate. Stipes 200–400×3–4 μm, smooth walled. Branches 15–25(−35) ×3–4 μm. Metulae unequal in length, in verticils of 3–8, 10–12(−16) ×2.5–3.5 μm. Phialides ampulliform, in verticils of 4–10, closely packed, 7.5–8.5×2–5.5 μm. Conidia globose to subglobose, smooth, 2.5–3.5 μm.
Diagnosis — Penicillium allii-sativi is phenotypically similar to P. chrysogenum and P. vanluykii. Isolates of this species produce conidia in shades of dark green on CYA and yellow soluble pigment usually absent on CYA incubated at 30 °C.
Colony morphology — Colony diam, 7 d, in mm: CYA 26–38; CYA 15 °C 18–25; CYA 30 °C 22–32; CYA 37 °C: no growth–4; MEA 31–42; YES 45–58; DG18 26–40; CYAS 37–45(−60); creatine agar 18–30, weak or moderate growth, weak acid production.
Extrolites — Penicillins, Atlantinone A, chrysogenamide, 2-(4-hydroxyphenyl)-2-oxo acetaldehydeoxim, a naptho-γ-pyrone, 2-pyruvoylaminobenzamide, roquefortine C, D, meleagrin, verrucosidin, normethylverrucosidin, deoxyverrucosidin, verrucosidinol and the uncharacterised compounds ‘ALKONA’, ‘AURCH’, ‘CRYPT’, ‘DERH’, ‘GULLA’, ‘KUTZ’ (atromentins?), ‘OTOF’, ‘SENGAX’, ‘SNORL’, ‘SPOFI’, ‘VERNX’.
Distribution & Ecology — This species has a broad distribution (Argentina, Bulgaria, France, Portugal, South Africa, UK) and has been isolated from garlic, soil, salterns, sorghum malt and mixed pig feed (Henk et al. 2011, this study). This species is not a pathogen on garlic like P. allii (Valdez et al. 2009).
Barcode & Molecular based ID — ITS sequencing is imprecise for species identification because all investigated strains of P. allii-sativi and P. chrysogenum CBS 306.48┬ share the same ITS sequence (GenBank JX997021).
Penicillium desertorum Frisvad, Houbraken & Samson, sp. nov. — MycoBank MB801874; Fig. 6
Fig. 6.
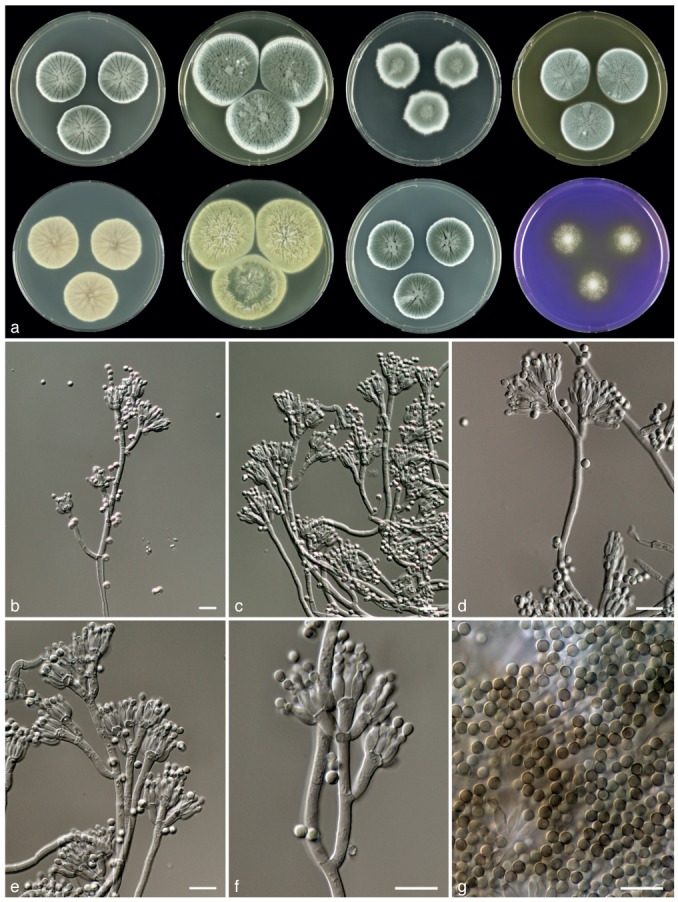
Penicillium desertorum, CBS 131543┬. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b–f. conidiophores; g. conidia. — Scale bars = 10 μm.
Typus. USA, Wyoming, 20 km east of Little America, ex cool desert soil under Oryzopsis hymenoides, J.C. Frisvad (CBS H-21056 holotype, cultures ex-type CBS 131543 = IBT 16321 = DTO 148-I6).
Etymology. Referring to desert; because this species is common in desert soil.
Sporulation on CYA dense; colonies entire or slightly polygonal in outline, velvety, radially sulcate; mycelium white, conidia dull green or greyish dull green, exudate absent or sparsely produced; soluble pigments absent; colony reverse brown. Soluble pigments on YES absent; mycelium white; sporulation dense; sporulation dark green; exudate absent, reverse beige with a brown centre or brown, with cerebriform sulcations. Sporulation on DG18 dense; sporulation grey en masse; reverse pale, transparent. Colonies on MEA velvety or slightly floccose; sporulation dense, conidia grey-green with a blue shade; exudate droplets absent, reverse unaffected or becoming brown. No violet reaction with Ehrlich reagent. Sclerotia absent. Conidiophores borne from surface, with (short) divaricate branches at various levels along the stipe. Stipes long, 200–400×2.5–3.5 μm, smooth walled and occasionally very finely roughened. Branches 8–15 (−25) ×2.5–3.5 μm. Metulae equal in length, occasionally inflated, densely packed, 3–8, 8–10(−15) ×2.5–3.5 μm. Phialides ampulliform, in verticils of 4–10, closely packed, 6–7.5×2–3 μm. Conidia globose, smooth, 2.5–3(−3.5) μm.
Diagnosis — Isolates of P. desertorum do not produce yellow pigments on CYA incubated at 25 °C and 30 °C and colonies on YES have a beige-brown or brown, cerebriform, sulcate reverse. This species is unique in sect. Chrysogena by the production of conidiophores that have several short, divaricate branches at various levels along the stipe.
Colony morphology — Colony diam, 7 d, in mm: CYA (20−) 24–37; CYA 15 °C 17–25; CYA 30 °C (15−)20–32; CYA 37 °C: no growth–4; MEA 20–37; YES 37–55; DG18 20–30; CYAS 24–38; creatine agar 10–23, weak growth, weak to moderate acid production.
Extrolites — 2-(4-hydroxyphenyl)-2-oxo acetaldehyde oxim, Raistrick phenols, austalides?, ‘FOL’.
Distribution & Ecology — This species has a world-wide distribution and has been found in Argentina, Iran, USA (Wyoming, New Mexico), Canada (British Columbia), Puerto Rica and Costa Rica. Arid or desert soil seems to be the primary substrate of this species. Only a selected number of strains are included in Table 1.
Barcode & Molecular based ID — Two ITS sequence types are detected in P. desertorum. DTO 016-B5, DTO 148-I5 and DTO 148-I6 share the same ITS sequence and this type is species specific (GenBank JX997010). DTO 015-H9 shares its ITS sequence with the type of P. chrysogenum CBS 306.48┬ (GenBank JX997038) and therefore ITS sequencing is imprecise for identification of P. desertorum. Partial β-tubulin, calmodulin, RPB1 or RPB2 sequences are recommended for species identification.
Penicillium goetzii J. Rogers, Frisvad, Houbraken & Samson, sp. nov. — MycoBank MB801876; Fig. 7
Fig. 7.
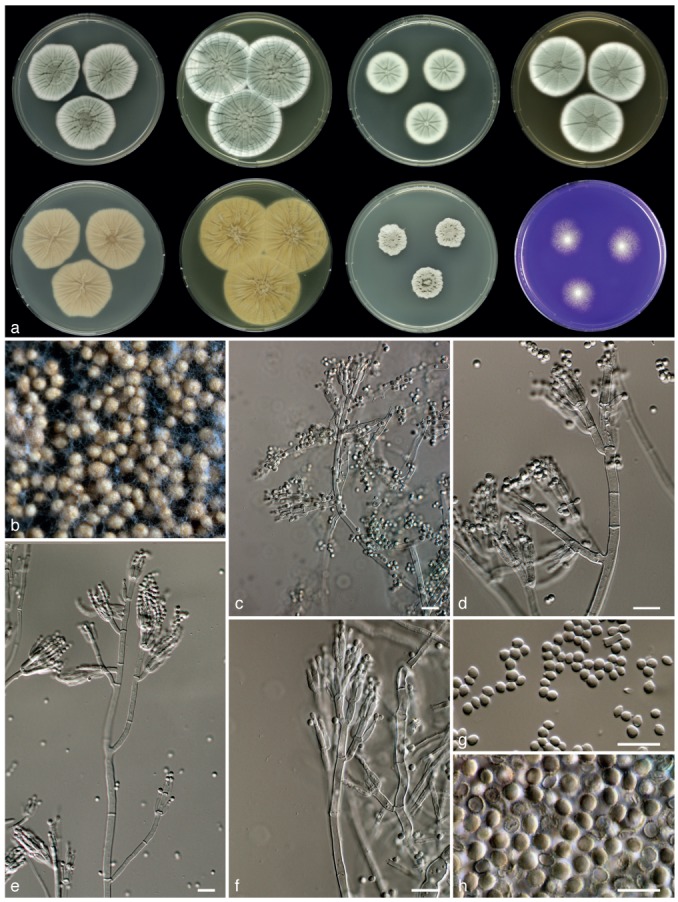
Penicillium goetzii, CBS 581.67. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b. ascomata; c–f. conidiophores; g. conidia; h. ascospores. — Scale bars = 10 μm.
Typus. CANADA, Calgary, ex soil, J. Bissett (CBS H-21061 holotype, cultures ex-type CBS 285.73 = DTO 088-G6).
Etymology. Named after John Richard Goetz III, a student of Jack Rogers who isolated this species (isolates DTO 055-H1, DTO 055-H2 and DTO 055-H3) and performed experiments with it.
Sporulation on CYA variable, absent to dense; velvety or slightly floccose, colonies with a feathery edge, radially sulcate; mycelium white and occasionally pinkish, conidia grey-green, exudate sparsely produced, clear, light brown or reddish brown; soluble pigments brown or reddish brown; colony reverse beige, sometimes with a reddish brown centre. Soluble pigments not produced on YES; mycelium white; sporulation often absent, occasionally present and dense, grey green en masse; exudate absent, reverse yellow, sometimes with a yellow-orange centre. Sporulation on DG18 absent or poor; sporulation grey en masse; mycelium white, reverse pale or bright yellow. Colonies on MEA floccose; sporulation variable, absent to dense, conidia grey-green en masse; mycelium white, exudate droplets absent or produced as clear or light brown droplets, reverse unaffected or becoming yellow. No violet reaction with Ehrlich reagent. Ascomata white when young, becoming creamish brown in time, maturing within 3–6 wk, 150–350 μm. Asci 6.5–11×5.5–8 μm. Ascospores ellipsoidal, with two distinct equatorial ridges and often two secondary ridges which are connected by transverse ribs, valves ornamented with a reticulate pattern, 3–5×3–4.5 μm. Conidiophores borne from surface and aerial mycelium, ter- to quarterverticillate, 200–400×2.5–3.5 μm, smooth walled. Branches 12–20×2.5–3.5 μm. Metulae equal in length, slightly inflated, 2–6, 8–12(−15) ×2.5–3.5 μm. Phialides ampulliform, in verticils of 4–10, closely packed, 7–9(−10) × 2–3 μm. Conidia broadly ellipsoidal, smooth, 2–2.5×2–3 μm.
Diagnosis — Penicillium goetzii is characterised by fast growth rate on CYA, production of brown soluble pigments on CYA and ascospores measuring 3–4.5×2.5–4 μm. It forms larger colonies on DG18 after 7 d of incubation at 25 °C (22–30 mm) than P. kewense (12–19 mm) and differs from P. egyptiacum by ascospore size and ornamentation.
Colony morphology — Colony diam, 7 d, in mm: CYA (30−) 33–42; CYA 15 °C 18–28; CYA 30 °C (10−)15–27; CYA 37 °C: no growth; MEA 33–42; YES 40–55; DG18 22–30; CYAS 30–40; creatine agar 15–30, weak growth, acid production absent or weak.
Extrolites — Andrastin A, citreoisocoumarin, fumitremorgin A, verruculogen, isoepoxydon, 10,23-dihydro-24,25-dehydroaflavinine & 10,23,24,25-tetrahydro-24-hydroxyaflavinine and the uncharacterised compound ‘GLAD’.
Distribution & Ecology — The primary substrate seems to be soil, but this species was also isolated as an endophyte in coniferous roots (Goetz 2006) and culture contaminant of a Spiromastix warcupii culture. The species has a broad distribution and has been isolated from Canada (Alberta, British Colombia), Pakistan and the USA.
Barcode & Molecular based ID — This species can be identified reliably by ITS sequencing. Two ITS sequence types were detected. CBS 581.67, 812.70, 285.73┬ and DTO 055-H1, DTO 055-H2 and DTO 055-H3 share the same ITS sequence (e.g. GenBank JX997042) and CBS 635.70 has a unique ITS sequence type (GenBank JX997112).
Penicillium halotolerans Frisvad, Houbraken & Samson, sp. nov. — MycoBank MB801875; Fig. 8
Fig. 8.
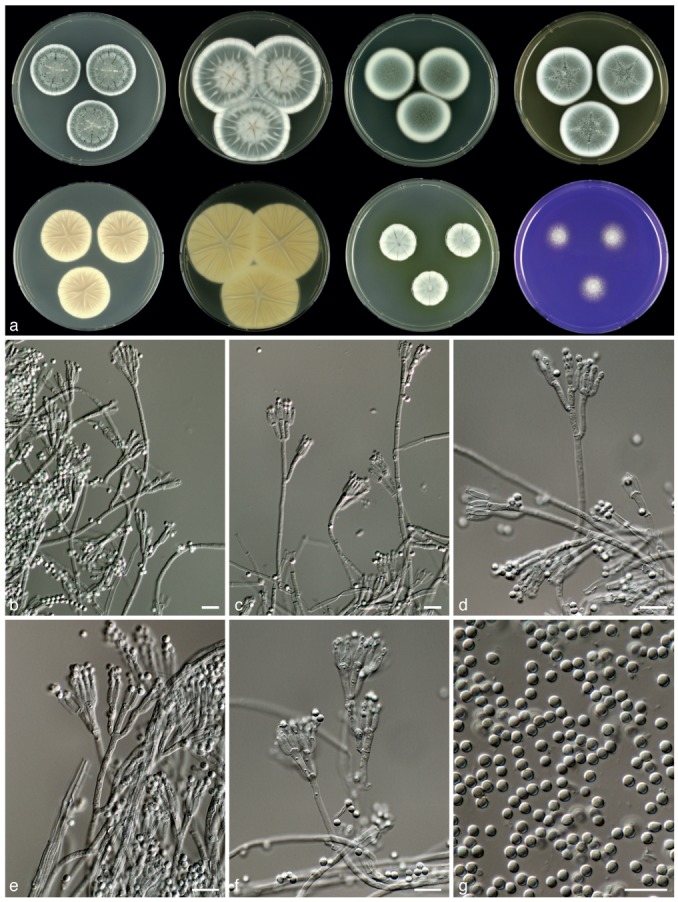
Penicillium halotolerans, CBS 131537┬. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b–f. conidiophores; g. conidia. — Scale bars = 10 μm.
Typus. EGYPT, ex salt marsh, A.H. Moubasher (CBS H-21060 holotype, cultures ex-type CBS 131537 = IBT 4315 = DTO 148-H9 = MOUS S42).
Etymology. Named after its ability to grow well in the presence of 5 % NaCl.
Sporulation on CYA dense; colonies entire, velvety; mycelium white, sporulation dull green with a blue tinge; exudate droplets clear, small; soluble pigments absent; colony reverse light brown. Soluble pigments on YES absent; mycelium white; sporulation moderate to dense; sporulation dull green to blue-green; exudate absent, reverse cream. Sporulation on DG18 moderate dense; sporulation blue-green; reverse pale. Colonies on MEA velvety, slightly floccose in centre; sporulation green to grey-green, reverse yellow-brown. No violet reaction with Ehrlich reagent. Sclerotia absent. Conidiophores borne from surface; stipes (100−)200–300(−500) ×2–3.5 μm, smooth walled, ter- to quarterverticillate, bearing terminal verticils of 2–4 metulae. Branches divaricate, 10–20(−40) ×2–3.5 μm. Metulae unequal in length, (8−)10–15×2–3 μm. Phialides ampulliform to cylindrical, in verticils of 2–6, 7.5–9×2–2.5 μm. Conidia globose, smooth, 2–3 μm.
Diagnosis — Penicillium halotolerans can be distinguished from P. desertorum by the production of yellow soluble pigments on CYA when incubated at 30 °C, slightly smaller conidia and the production of the extrolites andrastin A, roquefortine C & D, meleagrin and Raistrick phenols.
Colony morphology — Colony diam, 7 d, in mm: CYA 27–35; CYA 15 °C 19–23; CYA 30 °C 20–25; CYA 37 °C: germination (0–2); MEA 31–39; YES 41–51; DG18 26–32; CYAS 32–38; creatine agar 16–22, weak growth, no acid production.
Extrolites — Andrastin A, roquefortine C & D, meleagrin, Raistrick phenols and the uncharacterised compounds such as ‘CUCU’ and ‘PLIL’.
Distribution & Ecology — This species is known only from its type, isolated from a salt march in Egypt. An ITS sequence deposited in GenBank (HQ607840) and obtained from a strain (ATT111) isolated from a nest of the ant Atta texana in Texas, USA, was identical to that generated from CBS 131537┬.
Barcode & Molecular based ID — This species can be reliably identified using ITS barcoding (GenBank JX997005).
Penicillium tardochrysogenum Frisvad, Houbraken & Samson, sp. nov. — MycoBank MB801877; Fig. 9
Fig. 9.

Penicillium tardochrysogenum, CBS 132200┬. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b–f. conidiophores; g. conidia. — Scale bars = 10 μm.
Typus. ANTARCTICA, McMurdo Dry Valley, S. Onofri (CBS H-21057 holotype, cultures ex-type CBS 132200 = IBT 30075 = DTO 149-B9.
Etymology. Named after its resemblance to P. chrysogenum and its relative slow growth rate.
Sporulation on CYA dense; colonies entire, velvety to slightly floccose, distinctly radially sulcate; mycelium white, sporulation grey green; exudate droplets clear or pale brown, large; soluble pigments absent; colony reverse brown. Soluble pigments on YES absent; mycelium white; sporulation absent; exudate absent, reverse yellow-brown. Sporulation on DG18 dense; conidia grey green en masse; reverse pale. Colonies on MEA floccose with a wide, non-sporulating edge (4–8 mm); exudate droplets large in centre, smaller towards the rim of colony, hyaline; sporulation bluish grey green, reverse brown and in yellow-brown in valves of sulcations. No violet reaction with Ehrlich reagent. Sclerotia absent. Conidiophores mainly borne from aerial mycelium, sometimes direct from agar surface, ter- to quarterverticillate; stipes 150–400×2–3 μm, smooth walled. Branches divaricate, 10–20(−25) ×2–3 μm. Metulae equal in length, occasionally unequal, in verticils of 2–4, 10–13(−18) ×2.5–3.5 μm. Phialides ampulliform, in verticils of 3–8, closely packed, short, 7–9×2–3 μm. Conidia globose, finely roughened, 2.7–3.5 μm.
Diagnosis — Penicillium tardochrysogenum differs from other members of series Chrysogena by more restricted and floccose colonies on MEA, lack of sporulation on YES and finely roughened conidia. It does not produce yellow soluble pigments on CYA incubated at 30 °C. The species produces the asperentins, a series of compounds not produced by other members of series Chrysogena.
Colony morphology — Colony diam, 7 d, in mm: CYA 29–37; CYA 15 °C 16–20; CYA 30 °C 20–25; CYA 37 °C: germination (0–2); MEA 18–24; YES 35–45; DG18 34–40; CYAS 36–44; creatine agar 8–12, weak growth, no or poor acid production.
Extrolites — Penicillins, secalonic acids D & F, asperentins and the uncharacterised extrolite met Ø.
Distribution & Ecology — This species is only known from its type, which was isolated from the McMurdo Dry Valley, Antarctica.
Barcode & Molecular based ID — This species shares ITS sequences with the type of P. chrysogenum CBS 306.48┬ (GenBank JX997093). Partial β-tubulin, calmodulin, RPB1 or RPB2 can be used for species identification.
Penicillium vanluykii Frisvad, Houbraken & Samson, sp. nov. — MycoBank MB801878; Fig. 10
Fig. 10.
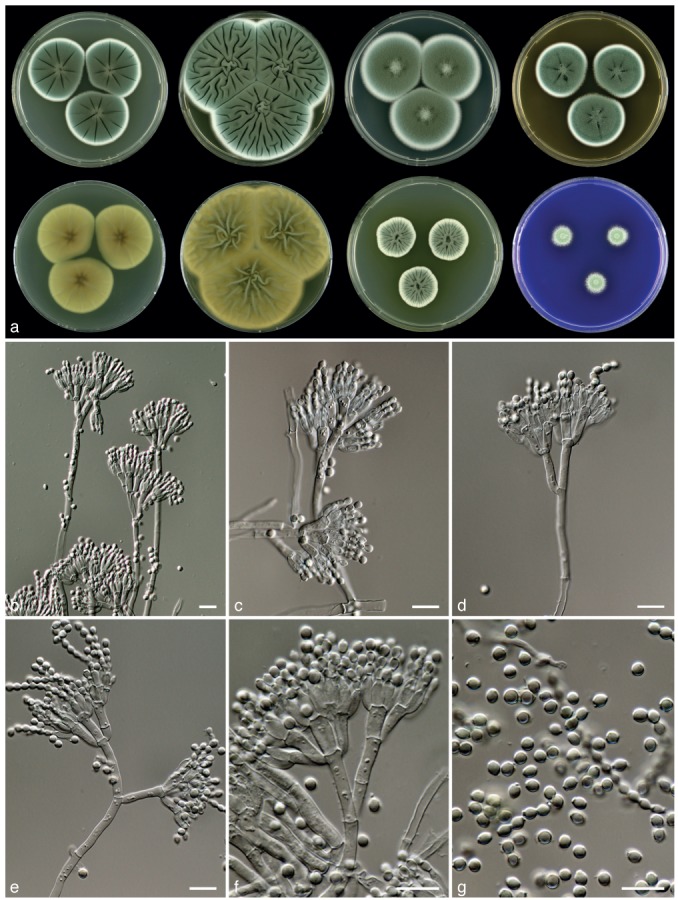
Penicillium vanluykii, CBS 132070. a. 7 d old cultures at 25 °C unless stated otherwise, left to right, first row, all obverse, CYA, YES, DG18, MEA; second row, CYA reverse, YES reverse, CYA incubated at 30 °C obverse, CREA obverse; b–f. conidiophores; g. conidia. — Scale bars = 10 μm.
Typus. USA, New Mexico, Carlsbad, ex Lechuguilla Cave, D. Northup (CBS H-21059 holotype, cultures ex-type CBS 131539 = IBT 14505 = DTO 148-I2).
Etymology. Named after Abraham van Luyk, a CBS mycologist who worked on the antibiotic activity of Penicillium in the 1940s.
Sporulation on CYA dense; colonies entire, velvety, sulcate radially; mycelium white, sporulation dark green to green; exudate droplets hyaline, light brown or absent, small; soluble pigments absent, in 149A4 yellow soluble pigments produced; colony reverse pale brown or yellow-brown. Soluble pigments on YES absent; mycelium white; sporulation dense; conidia dark green or green en masse; exudate absent, reverse greenish brown in centre with pale brown edge. Sporulation on DG18 moderate dense; conidia green or dull green en masse; reverse unaffected or pale brown. Colonies on MEA velvety; exudate droplets absent; sporulation green to dark green, reverse unaffected, sometimes with dark brown centre. No violet reaction with Ehrlich reagent. Sclerotia absent. Conidiophores borne from surface; quarterverticillate. Stipes 100–300 × 2.5–3.5 μm smooth walled. Branches divaricate, 15–25 × 2.5–3.5 μm. Metulae equal in length, 3–8, 8–12×3–3.5(−4) μm. Phialides ampulliform, in verticils of 4–10, closely packed, short, 6.5–8.5× 2–3 μm. Conidia globose to subglobose, smooth, with distinct connectives, 2.5–3.5 μm.
Diagnosis — Penicillium vanluykii is phenotypically similar to P. allii-sativi and P. chrysogenum. This species is characterised by the production of dark green conidia on MEA and CYA, yellow soluble pigment production on CYA incubated at 30 °C and a series of incompletely characterised extrolites.
Colony morphology — Colony diam, 7 d, in mm: CYA 30–45; CYA 15 °C 18–25; CYA 30 °C 18–27; CYA 37 °C: germination–4; MEA 30–40; YES 50–65; DG18 35–47; CYAS 40–55; creatine agar 15–20, weak to moderate growth, weak acid production.
Extrolites — Penicillins, chrysogine, roquefortine C and meleagrin, and andrastin A and the uncharacterised extrolites ‘CRYPT’ (4 compounds), ‘POO’, ‘KNOLF’, ‘TBRE’, ‘FJOR’ (2 compounds).
Distribution & Ecology — This species has a world-wide distribution and is found in the USA (Florida, New Mexico, Ohio), South Shetland Islands, Antarctica, Argentina (San Juan), the UK (Henk et al. 2011, this study).
Barcode & Molecular based ID — DTO 148-I2, DTO 149-A4 and DTO 149-B3 share the same ITS sequence, which can be used for precise species identification (GenBank JX997025).
LIST OF SPECIES CURRENTLY ACCEPTED IN PENICILLIUM SECTION CHRYSOGENA
The following list includes accepted species in sect. Chrysogena and their presently accepted synonyms. Our data indicate that more species might exist in this section. For example, three phylogenetic species are present in P. mononematosum (according the PSC) and also P. egyptiacum might represent three taxa.
Penicillium allii-sativi Frisvad, Houbraken & Samson, this study.
Typus. ARGENTINA, Mendoza, Lavalle, Col 3 de Mayo, garlic, M. Makuch & J. Valdez (CBS H-21058).
Penicillium chrysogenum Thom, Bull. Bur. Anim. Ind. USDA 118: 58, 1910; nom. cons.
Typus. USA, Connecticut, Storrs, ex cheese, 1904, C. Thom (IMI 24314 typ. cons.).
= Penicillium citreoroseum Dierckx, Ann. Soc. Sci. Bruxelles 25: 86. 1901; nom. rej.
= Penicillium griseoroseum Dierckx, Ann. Soc. Sci. Bruxelles 25: 86. 1901; nom. rej.
= Penicillium brunneorubrum Dierckx, Ann. Soc. Sci. Bruxelles 25: 88. 1901; nom. rej.
= Penicillium notatum Westling, Ark. Bot. 11, 1: 95. 1911.
= Penicillium cyaneofulvum Biourge, Cellule 33: 171. 1923.
= Penicillium roseocitreum Biourge, Cellule 33: 184. 1923.
= Penicillium chlorophaeum Biourge, Cellule 33: 249. 1923.
? = Penicillium chrysogenum var. brevisterigma Forster, Brit. Pat. 691: 242. 1953; (nom. inval. Art. 36.1; without Latin diagnosis).
= Penicillium aromaticum f. microsporum Romankova, Uchen. Zap. Lenin. Univ. Zhdanov 191: 102. 1955; (nom. inval. Art. 36.1; without Latin diagnosis).
= Penicillium harmonense Baghdadi, Novosti Sist. Nizsh. Rast. 5: 102. 1968.
Notes — Penicillium brunneorubrum, P. citreoroseum and P. griseoroseum predate P. chrysogenum but these names are formally nomina rejicienda (McNeill et al. 2006). No (ex-type) material was available of P. chrysogenum var. brevisterigma and this invalidly described species is tentatively placed in synonymy with P. chrysogenum.
Penicillium confertum (Frisvad et al.) Frisvad, Mycologia 81: 852. 1989.
Typus: USA, Arizona, 6 km east of Portal, cheek pouch of Dipodomys spectabilis (IMI 296930).
= Penicillium glandicola var. confertum Frisvad, Filt. & Wicklow, Canad. J. Bot. 65: 769. 1987.
Penicillium desertorum Frisvad, Houbraken & Samson, this study.
Typus: USA, Wyoming, 20 km east of Little America, cool desert soil under Oryzopsis hymenoides, J.C. Frisvad (CBS H-21056).
Penicillium dipodomyis (Frisvad, Filt. & Wicklow) Banke, Frisvad & S. Rosend., Int. Mod. Meth. Pen. Asp. Clas.: 270. 2000.
Typus. USA, Arizona, 6 km east of Portal, cheek pouch of Dipodomys spectabilis (IMI 296926).
= Penicillium chrysogenum var. dipodomyis Frisvad, Filt. & Wicklow, Canad. J. Bot. 65: 766. 1987.
= Penicillium dipodomyis (Frisvad, Filt. & Wicklow) Banke, Frisvad & S. Rosend., Mycol. Res. 101: 622. 1997 (nom. inval. Art. 33.3, basionym not cited).
Penicillium egyptiacum J.F.H. Beyma, Zentralbl. Bakteriol., 2. Abt., 88: 137. 1933.
Typus. EGYPT, Cairo, soil, Y.S. Sabet (CBS 344.32).
= Eupenicillium egyptiacum (J.F.H. Beyma) Stolk & D.B. Scott, Persoonia 4: 401. 1967.
= Eupenicillium molle Malloch & Cain, Canad. J. Bot. 50: 62. 1972.
= Penicillium nilense Pitt, The genus Penicillium: 145. 1980, ‘1979’.
= Penicillium molle Pitt, The genus Penicillium: 148. 1980, ‘1979’.
Penicillium flavigenum Frisvad & Samson, Mycol. Res. 101: 620. 1997.
Typus. DENMARK, wheat flour, J.C. Frisvad, 1985 (CBS 419.89).
Penicillium goetzii J. Rogers, Frisvad, Houbraken & Samson, this study.
Typus. CANADA, Calgary, soil, J. Bissett (CBS H-21061).
Penicillium halotolerans Frisvad, Houbraken & Samson, this study.
Typus. EGYPT, salt marsh, A.H. Moubasher (CBS H-21060).
Penicillium kewense G. Sm., Trans. Brit. Mycol. Soc. 44: 42. 1961.
Typus. Contaminant of a culture stored under mineral oil, G. Smith (LSHTM BB400).
= Eupenicillium crustaceum F. Ludw., Lehrb. Nied. Krypt.: 263. 1892.
Notes — Penicillium crustaceum was described by Fries (1829: 407). Crusts of conidia are formed by several species in Penicillium and Fries’ description of this species is not informative enough for to characterise it in modern terms. Although its exact identity cannot be established, Raper & Thom (1949: 515) indicated that this species could be the same as P. expansum. Brefeld (1874) described the formation of sclerotioid cleistothecia in detail, in a species he identified as “Penicillium crustaceum Fries, Penicillum glaucum Link”. It is unlikely that Brefeld’s fungus represented the species described by Link and Fries. The illustrations of the conidial state strongly suggest that Brefeld dealt with mixed cultures (Stolk & Scott 1967). Winter (1887) included Brefeld’s fungus in his work on ascomycetes (as P. crustaceum) and later, Ludwig (1892) introduced the generic name Eupenicillium based on the name used by Winter and named this species Eupenicillium crustaceum. Penicillium kewense most closely resembles the species described by Brefeld (Scott & Stolk 1967) and therefore, applying single name nomenclature, we use this epithet for strains formerly identified as E. crustaceum.
Penicillium lanosocoeruleum Thom, the Penicillia: 322. 1930.
Typus. USA, culture contaminant of P. cyclopium culture, C. Thom (NRRL 888).
= Penicillium aethiopicum Frisvad, Mycologia 81: 848. 1990.
Notes — Penicillium aethiopicum CBS 484.84┬ and P. lanosocoeruleum CBS 215.30┬ are conspecific. This is supported by molecular data, phenotypic characteristics and extrolite data. Both species form ellipsoidal conidia (Raper & Thom 1949: 436) and produce the extrolites griseofulvin, tryptoquialanins and viridicatumtoxin (Frisvad et al. 2004, Chooi et al. 2010, Gao et al. 2011). Strain IBT 5753 is fully genome sequenced (Chooi et al. 2010).
Penicillium mononematosum (Frisvad et al.) Frisvad, Mycologia 81: 857. 1990.
Typus. USA, Arizona, 6 km east of Portal, burrow system of Dipodomys spectabilis (IMI 296925).
= Penicillium glandicola var. mononematosa Frisvad, Filt. & Wicklow, Canad. J. Bot. 65: 767. 1987.
= Penicillium granulatum var. mononematosa (Frisvad, Filt. & Wicklow) Bridge, Kozak. & R.R.M. Paterson, Mycol. Pap. 165: 38. 1992.
Notes — Our phylogenetic analyses (Fig. 1, 2) reveal three distinct clades within P. mononematosum. The occurrence of two types (I and II) was described by Frisvad & Samson (2004: 126). Both ‘type II’ isolates (CBS 112575, CBS 10916) were isolated from salt marsh soil in Egypt and cluster together in our phylogenetic analysis.
Penicillium nalgiovense Laxa, Zentralbl. Bakteriol., 2. Abt., 86: 160. 1932.
Typus. CZECH REPUBLIC, Ellischauer cheese (CBS 352.48 neotype).
Penicillium persicinum L. Wang, H.B. Zhou, Frisvad & Samson, Antonie van Leeuwenhoek 86: 177. 2004.
Typus. CHINA, Qinghai: soil (HMAS 80638-1-4).
Penicillium rubens Biourge, Cellule 33: 265. 1923.
Typus. Unrecorded source, P. Biourge, CBS H-20595 (NRRL 792 = IBT 30129 = ATCC 9783 = CBS 129667).
? = Penicillium baculatum Westling, Svensk Bot. Tidskr. 14: 139. 1910.
? = Penicillium meleagrinum Biourge, Cellule 33: 184. 1923.
= Penicillium camerunense R. Heim, Nouvel & Saccas, Bull. Acad. Belg. C1. Sci., Ser. 5, 35: 52. 1949 (nom. inval. Art. 36, without Latin diagnosis).
= Penicillium chrysogenum mut. fulvescens Takash., Arima & S. Abe, J. Gen. Appl. Microbiol. 2, 1-2: 92. 1956 (nom. inval. Art. 36, without Latin diagnosis).
= Penicillium chrysogenum mut. fulvescens Takash., Arima & S. Abe ex C. Ramírez , Man. Atlas Penicil.: 364. 1982.
Notes — Raper & Thom (1949: 363) stated that NRRL 843 (= CBS 132210 = DTO 100-F6 = IBT 5303) was similar to P. baculatum, but no ex-type of P. baculatum has been saved in culture collections. Therefore, we decided to place this species in synonymy with P. rubens. Similarly, no type material of P. meleagrinum is available. Raper & Thom (1949) based their description of P. meleagrinum on NRRL 836 (= CBS 349.48 = DTO 098-G1 = IBT 4350) and NRRL 2136 (= CBS 131532 = DTO 100-H3 = IBT 30138 = NRRL 2136). The former strain is re-identified here as P. rubens and the latter as P. chrysogenum. The exact position of P. meleagrinum is uncertain.
Penicillium sinaicum Udagawa & S. Ueda, Mycotaxon 14: 266. 1982.
Typus. EGYPT, Sinai Peninsula, Suez Canal, 30 km north from Port Said, marine sludge, H. Komatsu (NHL 2894).
= Eupenicillium sinaicum Udagawa & S. Ueda, Mycotaxon 14: 266. 1982.
Penicillium tardochrysogenum Frisvad, Houbraken & Samson, this study.
Typus. ANTARCTICA, Dry Valley, S. Onofri (CBS H-21057).
Penicillium vanluykii Frisvad, Houbraken & Samson, this study.
Typus. USA, New Mexico, Carlsbad, Lechuguilla Cave, D. Northup (CBS H-21059).
DISCUSSION
With this revision, Penicillium sect. Chrysogena now consists of 18 phylogenetic and phenotypic species, most of which are also diagnosable morphologically. Compared with the classification of Houbraken & Samson (2011), six new species are added to this section and P. molle is synonymised with P. egyptiacum and P. aethiopicum with P. lanosocoeruleum. Recent taxonomic studies on P. chrysogenum determined the presence of four lineages within this species (Scott et al. 2004, Henk et al. 2011, Houbraken et al. 2011a). Our results confirm those of Houbraken et al. (2011a), demonstrating that one lineage is centred on the ex-type strain of P. chrysogenum CBS 306.48 (= ‘clade 1’ in Scott et al. (2004)) and another on P. rubens CBS 129667 (= ‘Fleming species’ fide Henk et al. (2011); ‘clade 4’ in Scott et al. (2004)). The other two lineages found by Scott et al. (2004) and Henk et al. (2011) do not correspond with each other. Our data support those of Henk et al. (2011) and show that the two other lineages recognised by Scott et al. (2004; ‘clade 2’ and ‘clade 3’) still represent P. chrysogenum. A comparison of sequences deposited in GenBank show that the two groups of isolates listed as ‘species A’ and ‘species B’ by Henk et al. (2011) correspond with the newly described species P. vanluykii and P. allii-sativi. A large number of species resembling P. chrysogenum have been described historically (Samson et al. 1977, Pitt 1980) and all are placed here in synonymy with P. chrysogenum or P. rubens. Houbraken et al. (2011a) focused on penicillin producing strains and also included the ex-type strains of P. griseoroseum, P. notatum and P. rubens. Various synonyms of P. chrysogenum were included in the study of Henk et al. (2011) and their species designations largely correspond with the current study. The only exception is the placement of P. camerunense CBS 339.52┬ in synonymy with P. rubens, whereas Henk et al. (2011) treated this species as P. chrysogenum. Our multigene phylogeny (Fig. 1) and the haplotype network analysis (Fig. 4) demonstrate that P. aromaticum f. microsporum (nom. inval.), P. brunneorubrum, P. chlorophaeum, P. citreorosum, P. cyaneofulvum, P. griseoroseum, P. harmonense, P. notatum and P. roseocitreum, are all synonyms of P. chrysogenum. Additionally, P. camerunense (nom. inval.) and P. chrysogenum mut. fulvescens should be placed in synonymy with P. rubens. An overview of accepted species and their synonyms is given in the Taxonomy part of this paper.
Pitt (1974, 1980) treated E. egyptiacum, P. kewense (as E. crustaceum) and E. molle as distinct species based on the ornamentation and size of the ascospores. In contrast, Stolk & Samson (1983) defined P. kewense (as E. crustaceum) as one variable species. Although Stolk & Samson (1983) included five ascospore patterns in their circumscription of P. kewense, they treated E. molle and E. egyptiacum as small-spored strains of P. kewense. They also observed the same ornamentation, but the ribs and ridges on ascospores of E. egyptiacum were less pronounced. Our results show that P. kewense sensu Stolk & Samson (1983) can be divided into at least three species: P. egyptiacum, P. goetzii and P. kewense, while P. molle is placed in synonymy with P. egyptiacum. Our study also shows that the isolates with large ascospores represent a separate species, here named P. goetzii. Phylogenetic analyses indicate that this group of related species probably contains additional new species. For example, three lineages occur in P. egyptiacum, which might represent distinct species. Also, CBS 653.82 (= CBS 227.81 = NRRL 2094) forms a single strain lineage and Raper & Thom (1949: 146) noted that this strain is intermediate between P. brefeldianum and P. egyptiacum. The description of this species is deferred until more strains of this tentative new species are collected.
Polyphasic characterisation of Penicillium species allows identification using several different types of data, including colony characters and micromorphology (morphological species concept), extrolite profiles (phenotypic species concept) and correlations among multigene phylogenies (phylogenetic species concept). The new species described here meet all of these criteria as distinct species, although their morphological characters are similar to other species of Penicillium sect. Chrysogena, which are notoriously difficult to identify using classical taxonomic techniques. In common with other species level studies of Penicillium subgenus Penicillium, sequences of the ITS region have minimal resolution for distinguishing closely related species in sect. Chrysogena (Skouboe et al. 1999, Houbraken et al. 2011b). The individual gene trees based on RPB1 and β-tubulin sequence data generated the best clustering of species, and these genes are therefore promising loci for barcoding within this genus. Neither gene tree correlates well with the series proposed within sect. Chrysogena by Frisvad & Samson (2004), as already noted by Samson et al. (2004).
Both sexual and asexual species are accommodated in the currently defined sect. Chrysogena. The sexually competent members (P. kewense, P. goetzii, P. egyptiacum, P. sinaicum) are all homothallic and there are indications that P. chrysogenum, P. dipodomyis and P. rubens may reproduce in a heterothallic manner (Hoff et al. 2008, Henk et al. 2011, Henk & Fischer 2011). Repeated attempts to induce a sexual state in P. chrysogenum and related species were unsuccessful (Hoff et al. 2008, Eagle 2009, Henk & Fischer 2011, Henk et al. 2011, J. Houbraken unpubl. res., K.A. Seifert unpubl. res.). However, some unpublished crossing experiments with P. chrysogenum isolates have apparently resulted in the production of cleistothecia and ascospores, similar to those described recently for P. kewense (Böhm et al. 2012). The limited number of successful mating experiments in P. chrysogenum might be explained by the strains used in these experiments. Perhaps strains maintained for long periods in culture collections lose their fertility. For example, the heterothallic Histoplasma capsulatum lost fertility rapidly during laboratory passage, leading to speculation that selective pressures might serve to maintain fertility in the environment (Kwon-Chung et al. 1974, Fraser et al. 2007). For the heterothallic and heat resistant Byssochlamys spectabilis (syn. Paecilomyces variotii), it was shown that only strains derived from pasteurised products were fertile (Houbraken et al. 2008). It will therefore be promising to repeat the mating experiments with Penicillium strains freshly isolated from nature. Another possible reason for unsuccessful crossing experiments may be stringent conditions required for successful mating. Various growth factors induce the formation of cleistothecia, such as temperature, light, nutrient and oxygen levels (Han et al. 2003). Recently, Houbraken et al. (2010) showed that P. psychrosexualis, a species related to P. roqueforti, produces abundant cleistothecia at low temperatures (9–15 °C). The production of a sexual stage at low temperatures might be more widespread in Penicillium, and mating experiments at this temperature might result in the discovery of a sexual stage in other species.
Acknowledgments
We are grateful to Uwe Braun for his suggestions on the species names. Jelle Bos is thanked for generating sequences of various P. chrysogenum isolates and Sharon Groenen is acknowledged for editing the haplotype network figure.
REFERENCES
- Andersen SJ, Frisvad JC. 1994. Penicillin production by Penicillium nalgiovense. Letters in Applied Microbiology 19: 486–488 [DOI] [PubMed] [Google Scholar]
- Banke S, Rosendahl S, Frisvad JC. 1997. Taxonomy of Penicillium chrysogenum and related xerophilic species, based on isozyme analysis. Mycological Research 101: 617–624 [Google Scholar]
- Bekker M, Huinink HP, Adan OC, Samson RA, Wyatt T, Dijksterhuis J. 2012. Production of an extracellular matrix as an isotropic growth phase of Penicillium rubens on gypsum. Applied and Environmental Microbiology 78: 6930–6937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg MA van den, Albang R, Albermann K, Badger JH, Daran JM, et al. 2008. Genome sequencing and analysis of the filamentous fungus Penicillium chrysogenum. Nature Biotechnology 26: 1161–1168 [DOI] [PubMed] [Google Scholar]
- Böhm J, Hoff B, O’Gorman C, Wolfers S, Pöggeler S, Kück U. 2012. Evidence for sexual recombination in Penicillium chrysogenum. 11th European Conference on Fungal Genetics, Programme & Abstract book. [Google Scholar]
- Brefeld O. Botanische Untersuchungen uber Schimmelpilze. Heft 2. ‘Die Entwicklungsgeschichte von Penicillium’; Felix, Leipzig: 1874. [Google Scholar]
- Chang JCS, Foarde KK, Vanosdell DW. 1995. Growth evaluation of fungi on ceiling tiles. Atmospheric Environment 29: 2331–2337 [Google Scholar]
- Chooi YH, Cacho R, Tang T. 2010. Identification of the viridicatumtoxin and griseofulvin gene clusters from Penicillium aethiopicum. Chemistry & Biology 17: 483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle CE. 2009. Mating-type genes and sexual potential in the Ascomycete genera Aspergillus and Penicillium. PhD thesis, University of Nottingham [Google Scholar]
- Fleming A. 1929. On the antibacterial action of cultures of a Penicillium, with special reference to their use in the isolation of B. influenzae. British Journal of Experimental Pathology 10: 226–236 [PubMed] [Google Scholar]
- Fraser JA, Stajich JE, Tarcha EJ, Cole GT, Inglis DO, et al. 2007. Evolution of the mating type locus: insights gained from the dimorphic primary fungal pathogens Histoplasma capsulatum, Coccidioides immitis, and Coccidioides posadasii. Eukaryotic Cell 6: 622–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries EM. 1821–1832. ‘Systema mycologicum’ 3 vols. Lund & Griefswald [Google Scholar]
- Frisvad JC, Filtenborg O. 1989. Terverticillate penicillia: chemotaxonomy and mycotoxin production. Mycologia 81: 836–861 [Google Scholar]
- Frisvad JC, Samson RA. 2004. Polyphasic taxonomy of Penicillium subgenus Penicillium: a guide to identification of food and airborne terverticillate penicillia and their mycotoxins. Studies in Mycology 49: 1–173 [Google Scholar]
- Frisvad JC, Smedsgaard J, Larsen TO, Samson RA. 2004. Mycotoxins, drugs and other extrolites produced by species in Penicillium subgenus Penicillium. Studies in Mycology 49: 201–241 [Google Scholar]
- Frisvad JC, Thrane U. 1987. Standardized highperformance liquid chromatography of 182 mycotoxins and other fungal metabolites based on alkylphenone indices and UVVIS spectra (diodearray detection). Journal of Chromatography 404: 195–214 [DOI] [PubMed] [Google Scholar]
- Gao X, Chooi YH, Amnes BD, Wang P, Walsh CT, Tang Y. 2011. Fungal indole alkaloid biosynthesis: genetic and biochemical investigation of the tryptoquialanine pathway in Penicillium aethiopicum. Journal of the American Chemical Society 133: 2729–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz JR. 2006. Fungal endophytes isolated from large roots of Douglas-fir (Pseudotsuga menziesii) and ponderosa pine (Pinus ponderosa). MSc Plant Pathology. [Google Scholar]
- Gravesen S. 1999. Microfungal contamination of damp buildings. In: Johanning E. (ed), Bioaerosols, Fungi and Mycotoxins: 505–515. (Proceedings of the Third International Conference on Fungi, Mycotoxins and Bioaerosols. Saratoga Springs, New York. September 23–25, 1998.) Albany, NY, Eastern New York Occupational and Environmental Health Center [Google Scholar]
- Han K-H, Lee D-B, Kim J-H, Kim M-S, Han K-Y, et al. 2003. Environmental factors affecting development of Aspergillus nidulans. Journal of Microbiology 41: 34–40 [Google Scholar]
- Henk DA, Eagle CE, Brown K, Berg MA van den, Dyer PS, et al. 2011. Speciation despite globally overlapping distributions in Penicillium chrysogenum: the population genetics of Alexander Fleming’s lucky fungus. Molecular Ecology 20: 4288–4301 [DOI] [PubMed] [Google Scholar]
- Henk DA, Fisher MC. 2011. Genetic diversity, recombination, and divergence in animal associated Penicillium dipodomyis. PLoS ONE 6: e22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoff B, Pöggeler S, Kück U. 2008. Eighty years after its discovery, Fleming’s Penicillium strain discloses the secret of its sex. Eukaryotic Cell 7: 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoog GS de, Gerrits van den Ende AHG. 1998. Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189 [DOI] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. 2010. Sex in Penicillium series Roqueforti. IMA Fungus 2: 171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. 2011a. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2: 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Frisvad JC, Samson RA. 2011b. Taxonomy of Penicillium section Citrina. Studies in Mycology 70: 53–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Samson R. 2011. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Studies in Mycology 70: 1–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Spierenburg H, Frisvad JC. 2012. Rasamsonia, a new genus comprising thermotolerant and thermophilic Talaromyces and Geosmithia species. Antonie van Leeuwenhoek 101: 403–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houbraken J, Varga J, Rico-Munoz E, Johnson S, Samson RA. 2008. Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph Paecilomyces variotii). Applied and Environmental Microbiology 74: 1613–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CA, Lea RG. 1995. The airborne fungal population of representative British homes. Air Quality Monographs 2: 141–153 [Google Scholar]
- Kozakiewicz Z, Frisvad JC, Hawksworth DL, Pitt JI, Samson RA, Stolk AC. 1992. Proposals for nomina specifica conservanda and rejicienda in Aspergillus and Penicillium (Fungi). Taxon 41: 109–113 [Google Scholar]
- Kwon-Chung KJ, Weeks RJ, Larsh HW. 1974. Studies on Emmonsiella capsulata (Histoplasma capsulatum). II. Distribution of the two mating types in 13 endemic states of the United States. American Journal of Epidemiology 99: 44–49 [DOI] [PubMed] [Google Scholar]
- Leistner L. 1990. Mould-fermented foods: recent developments. Food Biotechnology 4: 433–441 [Google Scholar]
- Librado P, Rozas J. 2009. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25: 1451–1452 [DOI] [PubMed] [Google Scholar]
- Ludwig F. Eupenicillium. Lehrbuch der niederen Krypogamen; Stuttgart: 1892. [Google Scholar]
- Lund F. 1995. Differentiating Penicillium species by detection of indole metabolites using a filter paper method. Letters in Applied Microbiology 20: 228–231 [Google Scholar]
- Matheny BP, Liu YJ, Ammirati JF, Hall BD. 2002. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). American Journal of Botany 89: 688–698 [DOI] [PubMed] [Google Scholar]
- McNeill J, Barrie FR, Burdet HM, Demoulin V, Hawksworth DL, et al. 2006. International Code of Botanical Nomenclature (Vienna Code): adopted by the Seventeenth International Botanical Congress Vienna, Austria, July 2005. (Regnum Vegetabile vol. 146.) ARG Gantner Verlag KG, Ruggell [Google Scholar]
- Nielsen KF, Månsson M, Rank C, Frisvad JC, Larsen TO. 2011. Dereplication of microbial natural products by LC-DAD-TOFMS. Journal of Natural Products 74: 2338–2348 [DOI] [PubMed] [Google Scholar]
- Nielsen KF, Smedsgaard J. 2003. Fungal metabolite screening: database of 474 mycotoxins and fungal metabolites for dereplication by standardized liquid chromatography-UV-mass spectrometry methodology. Journal of Chromatography A 1002: 111–136 [DOI] [PubMed] [Google Scholar]
- Nylander JAA. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University; 2004. [Google Scholar]
- Pitt JI. 1974. A synoptic key to the genus Eupenicillium and to sclerotigenic Penicillium species. Canadian Journal of Botany 52: 2231–2236 [Google Scholar]
- Pitt JI. 1980, ‘1979’. The genus Penicillium and its teleomorphic states Eupenicillium and Talaromyces. London, Academic Press [Google Scholar]
- Pitt JI, Hocking AD. 2009. Fungi and food spoilage. Berlin, Springer Science – Business Media [Google Scholar]
- Raper KB, Thom C. 1949. Manual of the Penicillia. Williams & Wilkins, Baltimore, USA [Google Scholar]
- Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19: 1572e–1574. [DOI] [PubMed] [Google Scholar]
- Samson RA, Hadlok R, Stolk AC. 1977. A taxonomic study of the Penicillium chrysogenum series. Antonie van Leeuwenhoek 43: 169–175 [DOI] [PubMed] [Google Scholar]
- Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. 2010. Food and indoor fungi. CBS Laboratory Manual series no. 2. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands [Google Scholar]
- Samson RA, Seifert K, Kuijpers A, Houbraken J, Frisvad JC. 2004. Phylogenetic analyses of Penicillium subgenus Penicillium using partial β-tubulin sequences. Studies in Mycology 49: 175–200 [Google Scholar]
- Scott J, Untereiner WA, Wong B, Strauss NA, Malloch D. 2004. Genotypic variation in Penicillium chrysogenum from indoor environments. Mycologia 96: 1095–1105 [PubMed] [Google Scholar]
- Skouboe P, Frisvad JC, Lauritsen D, Boysen M, Taylor JW, Rossen L. 1999. Nucleotide sequences from the ITS region of Penicillium species. Mycological Research 103: 873–881 [Google Scholar]
- Smedsgaard J. 1997. Micro-scale extraction procedure for standardized screening of fungal metabolite production in cultures. Journal of Chromatography A 760: 264–270 [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web-servers. Systematic Biology 75: 758–771 [DOI] [PubMed] [Google Scholar]
- Stolk AC, Samson RA. 1983. The ascomycete genus Eupenicillium and related Penicillium anamorphs. Studies in Mycology 23: 1–149 [Google Scholar]
- Stolk AC, Scott B. 1967. Studies on the genus Eupenicillium Ludwig. I. Taxonomy and nomenclature of Penicillia in relation to their sclerotioid ascocarpic states. Persoonia 4: 391–405 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom C. 1930. The Penicillia. Williams & Wilkins, Baltimore, USA [Google Scholar]
- Thom C. 1945. Mycology presents penicillin. Mycologia 37: 460–475 [Google Scholar]
- Valdez JG, Makuch MA, Ordovini AF, Masuelli RW, Frisvad JC, et al. 2009. Identification, pathogenicity and distribution of Penicillia isolated from garlic in Mendoza and San Juan provinces, Argentine. Plant Pathology 58: 352–361 [Google Scholar]
- Vesonder RF.1979. Xanthocillin, a metabolite of Eupenicillium egyptiacum NRRL 1022. Journal of Natural Products 42: 232–233 [DOI] [PubMed] [Google Scholar]
- Wang HJ, Gloer JB, Wicklow DT, Dowd PF. 1995. Aflavinines and other anti-insectan metabolites from the ascostromata of Eupenicillium crustaceum and related species. Applied and Environmental Microbiology 61: 4429–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HJ, Gloer JB, Wicklow DT, Dowd PF. 1998. Mollenines A and B: new dioxomorpholines from the ascostromata of Eupenicillium molle. Journal of Natural Products 61: 804–807 [DOI] [PubMed] [Google Scholar]
- Winter HG. 1887. Ascomyceten: Gymnoascaceen und Pyrenomyceten. Dr. L. Rabenhorst’s Kryptogamen-Flora von Deutschland, Österreich und der Schweiz 2, 1: 918–925 [Google Scholar]