Abstract
Background
A number of studies have applied transcranial magnetic stimulation (TMS) to physiologically characterize Alzheimer’s disease (AD) and to monitor effects of pharmacological agents, while others have begun to therapeutically use TMS and transcranial direct current stimulation (tDCS) to improve cognitive function in AD. These applications are still very early in development, but offer the opportunity of learning from them for future development.
Methods
We performed a systematic search of all studies using noninvasive stimulation in AD and reviewed all 29 identified articles. Twenty-four focused on measures of motor cortical reactivity and (local) plasticity and functional connectivity, with eight of these studies assessing also effects of pharmacological agents. Five studies focused on the enhancement of cognitive function in AD.
Results
Short-latency afferent inhibition (SAI) and resting motor threshold are significantly reduced in AD patients as compared to healthy elders. Results on other measures of cortical reactivity, e.g. intracortical inhibition (ICI), are more divergent. Acetylcholine-esterase inhibitors and dopaminergic drugs may increase SAI and ICI in AD. Motor cortical plasticity and connectivity are impaired in AD. TMS/tDCS can induce acute and short-duration beneficial effects on cognitive function, but the therapeutic clinical significance in AD is unclear. Safety of TMS/tDCS is supported by studies to date.
Conclusions
TMS/tDCS appears safe in AD, but longer-term risks have been insufficiently considered. TMS holds promise as a physiologic biomarker in AD to identify therapeutic targets and monitor pharmacologic effects. In addition, TMS/tDCS may have therapeutic utility in AD, though the evidence is still very preliminary and cautious interpretation is warranted.
Keywords: Alzheimer’s disease, transcranial magnetic stimulation, diagnostics, treatment, cortical reactivity, functional connectivity, cortical plasticity
1. Introduction
Alzheimer’s disease (AD) is the most common type of dementia worldwide, currently affecting 5.3 million people in the US alone (1). Approximately one in every eight individuals age 65 have AD and the prevalence rises steeply to 40-50% by the age of 85. Progressive episodic memory loss is a clinical hallmark of AD (2), associated with decline in other cognitive domains (e.g., word retrieval, language comprehension, calculation, visuospatial orientation, learning capacity, abstract thinking and judgment), as well as deterioration of sensory and motor functions. In addition, with disease progression, behavioral symptoms such as delusions, agitation, changes in personality, and mood disturbances may also occur (3). AD has devastating effects on patients and their caregivers, and poses a tremendous socioeconomic impact on families and the health systems around the world. Presently, there is no cure for AD and existing interventions (including medications) can at best delay progression by 6-12 months in half of the patients (1). The search for causes of AD, a detailed characterization of the progression of the disorder, and an improved understanding of the contributions of diverse genetic and environmental factors remain essential. However, given the increasing prevalence and debilitating impact of AD, successful diagnostic and therapeutic interventions are of immediate relevance.
Noninvasive brain stimulation with transcranial magnetic stimulation (TMS) or transcranial direct current stimulation (tDCS) is valuable in research and has potential therapeutic applications in cognitive neuroscience, neurophysiology, psychiatry, and neurology (4). TMS is a technique for noninvasive stimulation of the human brain and modulation of brain activity, while tDCS is a purely neuromodulatory intervention (4). TMS is based on electromagnetic induction and can be used to examine brain-behavior relations, map sensory, motor, and cognitive functions, and explore the excitability of different cortical regions (5). Repetitive TMS (rTMS) and tDCS have therapeutic potential in patients with neurologic and psychiatric disorders, as both can induce lasting modulation of brain activity in the targeted brain region and across brain networks through transcranial induction of electric currents in the brain (4). It is not, however, completely understood by which mechanisms of action TMS and tDCS induce these lasting effects on the brain. There is burgeoning evidence to suggest that the physiologic impact of both techniques involves synaptic plasticity, specifically long-term potentiation (LTP) and long-term depression (LTD). For instance, Hoogendam et al. (6) recently identified seven lines of evidence that strongly suggest a link between the after-effects induced by rTMS and the induction of synaptic plasticity. Similarly, Stagg and Nitsche (7) brought together the results from pharmacological, neurophysiological, and imaging studies to conclude that tDCS may indeed modulate synaptic strength within the cortex, with evidence pointing to the involvement of intracortical neurons. At present, nevertheless, it is impossible to demonstrate a direct link between rTMS or tDCS and synaptic plasticity. Therefore, the effects of rTMS and tDCS are often described as LTP- and LTD-like effects.
TMS may offer a reliable means to characterize important neurophysiologic and pathophysiologic aspects of AD. To date, several reports using TMS have claimed the detection of abnormalities in cortical reactivity, plasticity, and connectivity in AD patients, and revealed differences between patients with AD, those with other dementias, and healthy elder individuals. Moreover, noninvasive brain stimulation, including TMS and tDCS, might hold therapeutic promise in AD. Herein, we review all studies that have applied noninvasive brain stimulation in AD to provide a comprehensive perspective of past and current research, and to help guide future studies.
2. Methods
2.1. Selection of studies and inclusion criteria
A systematic search of the literature was conducted using Web of Science and PubMed databases (until November 30th, 2010). The identification of English language articles was based on the following keywords: ‘Alzheimer*’ and ‘magnetic stimulation’ or ‘direct current stimulation’. In addition, reference lists in retrieved reports were examined. Peer-reviewed studies were included if they met the following criteria: (a) human subjects were involved; (b) patients with AD were included in study samples; (c) studies were prospective in nature; and (d) TMS or tDCS was applied.
2.2. Collection of data
The data were collected using a semi-structured form for each study by one of the authors and checked by another. The following variables were extracted from all studies when pertinent and available: (a) Demographic and clinical characteristics: number of participants; study group; mean age; gender; educational level; mean duration of illness; neurologic, neuropsychiatric, and neuropsychological evaluation, including establishment of diagnosis and staging, neuropsychological testing, anatomical scanning, and medication; (b) Identification of all specific measures of cortical reactivity performed in each study; if TMS had been applied to the motor cortex, muscle from which neurophysiologic measures were obtained; (c) Mean and standard deviation (SD) of cortical reactivity measure(s) achieving statistical significance as compared with healthy elders; (d) Dose and duration of administration of pharmacological drugs; (e) Effects of pharmacological challenges on measures of cortical reactivity, and mean and SD of cortical reactivity measure(s) achieving statistical significance after the administration of medication, as opposed to before; (f) Any significant correlations between neurophysiologic findings and demographic or psychosocial characteristics (e.g., age, years of education), neuropathology (staging or duration of disease) or neuropsychological scorings; and (g) Adverse events/safety considerations. Note that we extracted approximate mean and SD from graphs when data were not provided otherwise.
3. Results
3.1. Selected studies
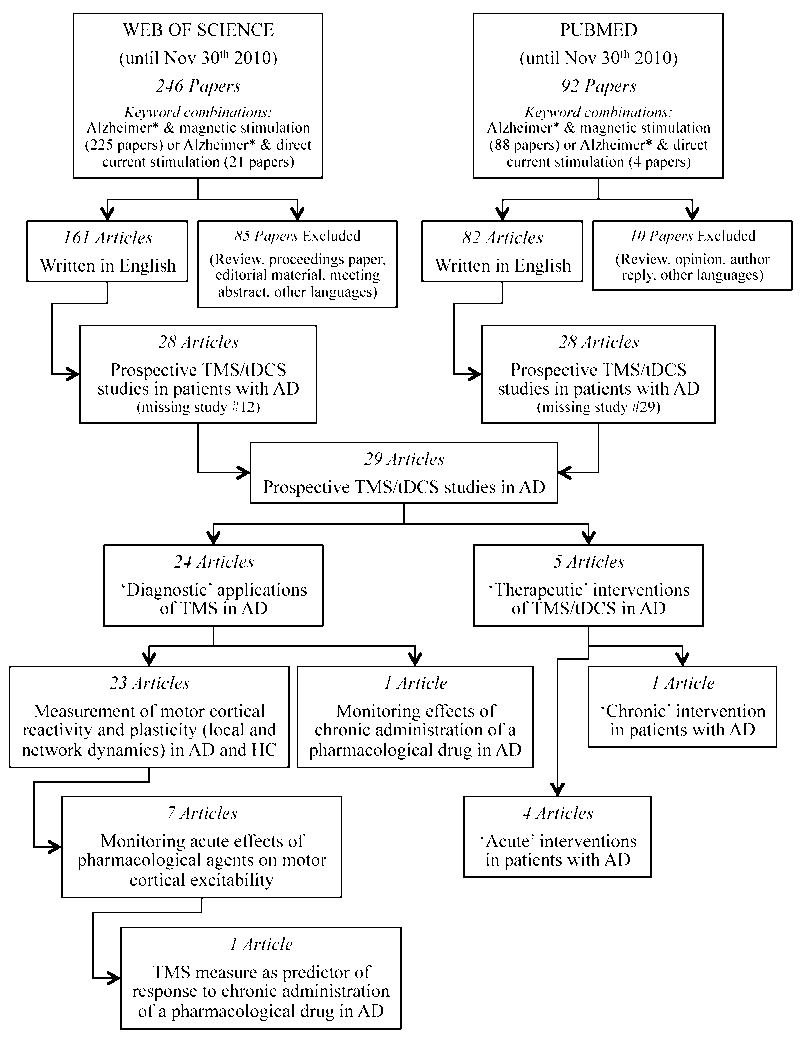
A flow diagram reflecting the process of identification and selection of studies is provided in Figure 1. The initial strategy yielded 225 and 88 peer-reviewed papers in Web of Science and PubMed, respectively, when combining ‘Alzheimer*’ and ‘magnetic stimulation’, and 21 and 4 peer-reviewed papers in Web of Science and PubMed, respectively, for the combination of ‘Alzheimer*’ and ‘direct current stimulation’. During the initial review of retrieved papers, we excluded nearly 35% from Web of Science and about 11% from PubMed as they represented review or opinion articles, or were written in languages other than English. Of these, we further excluded approximately 83% and 66% of Web of Science and PubMed retrieved articles, respectively, during a more detailed screening as TMS/tDCS were not used as a diagnostic or therapeutic tool, or patients with AD had not actually been included. Ultimately, when combining both databases, our search identified 29 articles, of which 24 were TMS studies devoted to the measurement of motor cortical reactivity, local plasticity and connectivity in patients with AD, and 5 addressed the therapeutic application of TMS or tDCS to enhance cognitive function in AD. Among the studies exploring diagnostic applications of TMS, 23 compared findings in patients with AD with those in healthy controls, while 1 study included only AD patients but presented a longitudinal, long-term follow-up (8). Overall, 7 studies examined acute effects of pharmacological agents on motor cortical excitability in AD patients. Moreover, 1 study examined the predictive value of one TMS measure for the clinical response to a long-term pharmacological treatment (9).
Figure 1.
Flow diagram of the selection process of peer-reviewed articles for systematic review.
3.2. Studies on cortical reactivity, local plasticity, and connectivity in AD
Demographic and clinical characteristics of sample populations, as well as major findings of TMS studies published to date on cortical reactivity and plasticity in AD are presented in Table 1. Overall, results on a total of 317 patients with AD were contrasted to 298 healthy older individuals. We excluded from these numbers 20 AD patients and 12 healthy controls from one study (Table 1; study #11) (9), since they appear to be the same participants included in a previous study (study #9) (10).
Table 1.
Major findings of peer-reviewed papers studying cortical reactivity and plasticity in Alzheimer’s disease (AD) using transcranial magnetic stimulation (TMS).
| PAPER | n | Grp | Age (yrs†) |
Gender (% M) |
Education (yrs†) |
Disease duration (mo†) |
Neurologic, Neuropsychiatric, and Neuropsychological Evaluation |
Cortical Reactivity | Cortical Plasticity and Connectiv |
Assoc W/ STAGING / COGNITION |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||
| Diagnosis & Staging |
Anat scan |
NeuroΨ testing |
Meds | Muscle | MT (%) |
MEP (mV) |
CM CT |
SP (ms) |
SAI | ICI | ICF | |||||||||
| #1 Perretti et al., 1996 | 15 | AD | 67.2 ± 7.8 | 26.67 | 7.3 ± 4.5 | [Decline at least 1 yr] | DSM-III GDS1 [HIS] | MRI [atrophy in all] | MMSE: 15.7 ± 5.8 NTB TOKEN | No drugs affecting CNS | Right APB Right TA | SIGN [RMT ⇑ in AD] | N.S. [I/O curve, per M] | N.S. | SIGN [cSP ⇓ in AD] N.S. [pSP] | - | - | - | 5/6 patients w/high MT were in severe stage | |
| 10 | HC | 67.8 ± 5.0 | 30 | . | - | - | MMSE [.] | - | ||||||||||||
|
| ||||||||||||||||||||
| #2 de Carvalho et al., 1997 | 14 | AD | 67.8 ± 6.0 | 28.57 | - | - | NINCDS-ADRADA [Probable] BDS: 9.8 ± 4.2 | CT | - | No drugs affecting CNS | Right / Left ADM | SIGN [R & L RMT ⇓ in AD] | SIGN [R & L per M ⇑ in AD] | N.S. [R & L] | - | - | - | - | - | - |
| 11 | HC | 66.1 ± 7.7 | 27.27 | - | - | - | - | - | ||||||||||||
|
| ||||||||||||||||||||
| #3 Pepin et al., 1999 | 17 | AD | 70.7 ± 6.6 | 23.53 | - | - | NINCDS-ADRADA [Probable] GDS1 | - | - | - | Right ADM | SIGN [RMT & AMT ⇓ in AD] | SIGN [per M ⇑ in AD] | - | - | - | N.S. [SICI] | N.S. | - | - |
| 22 | HC | 68.5 ± 9.2 | 27.27 | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #4 Alagona et al., 2001 | 21 | AD | 70.5 ± 7.8 | 42.86 | - | - | NINCDS-ADRADA [Probable] BDS | CT or MRI [atrophy in all] | - | No drugs affecting CNS | Right / Left FDI | SIGN [RMT ⇓ in AD] | SIGN [L & R I/O & per M ⇑ in AD] | N.S. | SIGN [L & R ⇓ in AD] | - | - | - | - | MT correlated w/stage severity; SP not |
| 18 | HC | [69]’ | 72.22 | - | - | - | - | MMSE [.] | ||||||||||||
|
| ||||||||||||||||||||
| #5 Liepert et al., 2001 | 11 | AD | 74.8 ± 9.7 | 18.18 | - | - | CDR: 1.64 ± 0.64 | CT or MRI | MMSE WMT + other | No drugs affecting CNS | Right FDI | N.S. [RMT & AMT] | - | N.S. | N.S. | - | SIGN [SICI ⇓ in AD] | N.S. | - | ICI correlated w/CDR |
| [Donepezil] | 10 | HC | 70.0 ± 6.4 | 30.0 | - | - | - | - | Test battery | - | ||||||||||
|
| ||||||||||||||||||||
| #6 Di Lazzaro et al., 2002 | 15 | AD | 69.0 ± 5.3 | 60.0 | 9.3 ± 3.4 | 28.4 ± 14.6 | NINCDS-ADRADA [Probable] | - | MMSE: 18.6 ± 3.5 RAVLT + Others | Naïve to AChEIs | Left FDI | SIGN [RMT ⇓ in AD] N.S. [AMT] | N.S. [I/O curve] | N.S. | N.S. | SIGN [⇓ in AD] | N.S. | N.S. | - | No measure correlated w/disease duration |
| [Rivastigm] | 12 | HC | 73.1 ± 5.4 | . | . | - | - | - | MMSE: > 24 RAVLT + Others | - | ||||||||||
|
| ||||||||||||||||||||
| #7 Ferreri et al., 2003 | 16 | AD | 75.0 ± 6.9 | 6.25 | - | [Sympts < 4 yrs] | NINCDS-ADRADA [Possible or probable] CDR: 1-2 | Head techneti um or MRI | MMSE MDB IADLS GDS2 | No AChEIs or other | Right / Left ADM & ECD | SIGN [RMT from ECD ⇓ in AD] | - | - | - | - | - | - | - | No correlation w/MMSE, CDR, IADL |
| 13 | HC | 72.0 ± 2.0 | 38.46 | - | - | - | - | - | ||||||||||||
|
| ||||||||||||||||||||
| #8 Alagona et al., 2004 | 20 | AD | 72.2 ± 7.5 | 35.0 | - | - | NINCDS-ADRADA [Probable] | MRI | - | No AChEIs or other | Right / Left FDI | SIGN [RMT ⇓ in AD] | N.S. [per M] | - | N.S. | - | - | - | - | - |
| 20 | HC | 68.6 ± 8.0 | 40.0 | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #9 Di Lazzaro et al., 2004 | 28 | AD | 71.3 ± 6.8 | . | 8.2 ± 2.3 | 32.0 ± 16.8 | NINCDS-ADRADA [Probable] | - | MMSE: 19.35 ± 3.8 RAVLT + Others | No AChEIs/no other 30 days | Left FDI | SIGN [RMT ⇓ in AD] | - | - | - | SIGN [⇓ in AD] | N.S. [SICI] | - | - | - |
| [Rivastigm] | 12 | HC | 73.1 ± 5.4 | . | . | - | - | - | . | - | ||||||||||
|
| ||||||||||||||||||||
| #10 Pierantozzi et al., 2004 | 12 | AD | 65.2 ± 3.5 | . | - | [Sympt not > 18 mo] | NINCDS-ADRADA [Possible] CDR: ≤ 1 | MRI | MMSE: 21.8 ± 2.1 NPB BDI / NPI | No AChEIs or other | Right / Left APB | N.S. [RMT & AMT] | N.S. [I/O curve] | - | - | - | SIGN [⇓ in AD] | N.S. | - | - |
| [Galantam] | 12 | HC | 64.5 ± 3.2 | . | - | - | - | - | - | - | ||||||||||
|
| ||||||||||||||||||||
| #11 Di Lazzaro et al., 2005 | 20 | AD | 70.5 ± 6.9 | 40.0 | 7.9 ± 2.9 | 26.8 ± 16.4 | NINCDS-ADRADA [Probable] | - | MMSE: 19.1 ± 5.5 RAVLT + Others | Naïve to AChEIs/no other 2 wks | Left FDI | - | - | - | - | SIGN [⇓ in AD] | - | - | - | - |
| [Rivastigm] | 12 | HC | 73.1 ± 5.4 | . | . | - | - | - | MMSE [> 24]RAVLT + Others | - | ||||||||||
|
| ||||||||||||||||||||
| #12 Di Lazzaro et al., 2006 | 20 | AD | 69.5 ± 6.5 | 50 | 8.9 ± 4.5 | 31.9 ± 16.3 | NINCDS-ADRADA [Probable] | - | MMSE: 20.1 ± 3.5 MDB | Naïve to AChEIs/no other 2 wks | Left FDI | SIGN [RMT ⇓ in AD] | - | - | - | SIGN [⇓ in AD] | - | - | - | - |
| 20 | HC | 71.8 ± 5.7 | . | . | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #13 Inghilleri et al., 2006 | 20 | AD | 71.0 ± 2.1 | . | - | 44.0 ± 18.9 | DSM-IV | CT and/or MRI | MMSE: 16.4 ± 3.5 ADAS-Cog: 38.7 ± 10.9 + Others | No AChEIs /no other 2 wks | Right FDI | SIGN [RMT ⇓ in AD] | N.S. [I/O curve] | - | N.S. [before or after rTMS] | - | - | - | SIGN 5Hz rTMS: lack of MEP ⇑ in AD] N.S. [1 Hz] | No correlation w/clinical scores |
| 20 | HC | 69.0 ± 1.6 | . | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #14 Nardone et al., 2006 | 13 | AD | 69.6 ± 6.6 | 53.85 | 14.2 ± 2.8 | 32.2 ± 15.5 | NINCDS-ADRADA [Probable] DRS: 125.8 ± 8.0 | - | MMSE: 24.2 ± 2.8 | No AChEIs, dopamin/no other 2 wks | Domin FDI | N.S. [RMT & AMT] | - | N.S. | - | SIGN [⇓ in AD] | SIGN [SICI ⇓ in AD] | N.S. | - | SAI & SICI not correlated w/age, disease duration, MMSE, DRS |
| 15 | HC | 67.5 ± 7.2 | 53.33 | . | - | - | - | . | ||||||||||||
|
| ||||||||||||||||||||
| #15 Battaglia et al., 2007 | 10 | AD | 70.1 ± 7.4 | 60.0 | - | [1.2 ± 0.7 yrs] | DSM-IV NINCDS-ADRADA [Probable] | MRI | MMSE: 20.02 ± 3.9 ADAS-Cog: 24.4 ± 8.0 MADRS | No AChEIs/no other 2 wks | Right APB | N.S. [RMT] | N.S. [I/O curve] | - | - | - | - | - | SIGN [PAS: LTP-like plasticity ⇓ in AD] | - |
| 10 | HC | 68.4 ± 6.1 | 60.0 | - | - | - | MMSE: 27.9 ± 1.8 ADAS-Cog: 8.8 ± 3.5 MADRS | - | ||||||||||||
|
| ||||||||||||||||||||
| #16 Di Lazzaro et al., 2007 | 10 | AD | 72.1 ± 4.4 | 60.0 | 9.2 ± 4.9 | 32.0 ± 13.1 | NINCDS-ADRADA [Probable] | MRI | MMSE: 17.8 ± 1.8 RAVLT + Others | Naïve to AchEIs/no other 2 wks | Left FDI | [RMT & AMT ⇓ in AD] | N.S. [I/O curve] | - | - | SIGN [⇓ in AD] | N.S. [SICI] | - | - | SAI negatively correlated w/Raven matrices |
| 10 | HC | 72.0 ± 5.1 | . | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #17 Sakuma et al., 2007 | 12 | AD | . | . | - | - | NINCDS-ADRADA [Probable] | MRI | MMSE: 21.8 ± 2.8 | No AChEIs | Right FDI | N.S. [RMT] | - | - | SIGN [⇓ in AD] | - | - | - | - | |
| 15 | HC | . | . | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #18 Alberici et al., 2008 | 8 | AD | 74.5 ± 7.3 | 37.5 | 4.8 ± 0.4 | 30.3 ± 7.3 | NINCDS-ADRADA | MRI | MMSE: 20.2 ± 4.0 UPDRS IADL/BADL FBI DeRenzi | Chronic AChEIs | Right / Left FDI | N.S. [RMT] | - | - | - | - | N.S. [SICI] | N.S. | - | - |
| 8 | HC | 63.1 ± 7.5 | 50 | 7.1 ± 2.6 | - | - | - | MMSE: 27.9 ± 1.2 | - | |||||||||||
|
| ||||||||||||||||||||
| #19 Di Lazzaro et al., 2008 | 12 | AD | 69.3 ± 7.3 | . | 9.1 ± 4.3 | 32.0 ± 13.1 | NINCDS-ADRADA [Probable] [HIS] | MRI | MMSE 22.7 ± 2.7 MDB + other GDS | Naïve to AChEIs/no other 2 wks | Left FDI | SIGN [RMT ⇓ in AD] N.S [AMT] | - | - | - | SIGN [⇓ in AD] | N.S. [SICI] | - | - | SAI negatively correlated w/RAVLT delayed, Raven + other |
| 12 | HC | 73.1 ± 5.4 | . | . | - | - | - | MMSE: ≥ 29 | - | |||||||||||
|
| ||||||||||||||||||||
| #20 Julkunen et al., 2008 | 5 | AD | 73.2 ± 8.1 | 60.0 | 9.6 ± 2.1 | - | NINCDS-ADRADA [Probable] CDR: 0.6 ± 0.2 | MRI | MMSE: 22.0 ± 5.1 | AChEIs | Right / Left Hypo thenar | SIGN [Aver both R/L ⇓ in AD] | N.S. [I/O curve] | - | - | - | - | - | SIGN [EEG: TMS-evoked P30 ⇓ in AD; ≠ FC & TPC; less sync?] | - |
| 4 | HC | 77.8 ± 2.6 | 25.0 | 6.5 ± 1.0 | - | CDR: 0 ± 0 | - | MMSE: 27.0 ± 4.1 | - | |||||||||||
|
| ||||||||||||||||||||
| #21 Martorana et al., 2008 | 11 | AD | 73.0 ± 9.2 | . | - | - | DSM-IV NINCDS-ADRADA CDR: ≥ 1.5 | MRI | MMSE: 15.74 ± 1.6 NPB/NPI UPDRS | No AChEIs or others | Right APB | SIGN [RMT ⇓ in AD] N.S. [AMT] | - | - | - | - | SIGN [⇓ in AD] | N.S. | - | - |
| [Melevod] | 12 | HC | 68.0 ± 5.8 | . | - | - | - | - | . | - | ||||||||||
|
| ||||||||||||||||||||
| #22 Nardone et al., 2008 | 17 | AD | 68.4 ± . | 58.82 | - | [Sympt > 6 mo] | NINCDS-ADRADA [Probable] CDR: 0.5-1 | - | - | Naïve to AChEIs/no other - 2 wks | Domin FDI | N.S. [RMT& AMT] | - | N.S. | - | SIGN [⇓ in AD] | N.S. [SICI] | N.S. | - | SAI not correlated w/age, disease duration, CDR |
| 22 | HC | 70.4 ± . | 54.55 | - | - | - | - | - | - | |||||||||||
|
| ||||||||||||||||||||
| #23 Martorana et al., 2009 | 10 | AD | 72.5 ± 6.1 | . | - | - | NINCDS-ADRADA [Probable] CDR: 1.75 ± 1.6 [HIS] | MRI | MMSE: 18.23 ± 3.2 NPB | Naïve to AChEIs/no other 30 days | Right FDI | SIGN [RMT ⇓ in AD] | - | - | - | SIGN [⇓ in AD] | - | - | - | - |
| [L-dopa] | 10 | HC | 71.7 ± 4.9 | . | - | - | - | - | - | - | ||||||||||
Legend. n: Number of subjects; yrs: Years; % M: Percentage of males; mo: Months; Anat scan: Anatomical scanning; NeuroΨ: Neuropsychologic; Meds: Medications; Pharmacol: Pharmacological; MT: Motor threshold; MEP: Motor evoked potential; mV: Microvolts; CMCT: Central motor conduction time; SP: Silent period; ms: Miliseconds; SAI: Short-latency afferent inhibition; ICI: Intracortical inhibition; ICF: Intracortical facilitation; Connectiv: Connectivity; AD: Alzheimer’s disease; HC: Healthy controls; DSM-III: Diagnostic and Statistical Manual of Mental Disorders, 3rd edition; GDS1: Reisberg’s Global Deterioration Scale; HIS: Hachinski Ischemic Score; MRI: Magnetic resonance imaging; MMSE: Mini-Mental State Examination; NTB: Neuropsychological Test Battery; TOKEN: Token Test; CNS: Central Nervous System; APB: Abductor pollicis brevis; TA: Tibialis anterior; SIGN: Significant; RMT: Resting motor threshold; N.S.: Non significant; I/O curve: Input-output curve; per M: peripheral M response; cSP: Central silent period; pSP: Peripheral silent period; NINCDS-ADRADA: National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association; [Probale/Possible]: Probable/possible AD; BDS: Blessed Demential Scale; CT: Computerized tomography; ADM: Abductor digiti minimi muscle; R: Right; L: Left; AMT: Active motor threshold; SICI: Short latency intracortical inhibition; FDI: First dorsal interosseus muscle; CDR: Clinical Dementia Rating; WMT: Wechsler Memory Test; RAVLT: Rey Auditory Verbal Learning Test; AChEIs: Acetylcholinesterase Inhibitors; Sympts: Symptoms; MDB: Mental Deterioration Battery; IADLS: Instrumental Activity of Daily Living scale; GDS2: Geriatric Depression Scale; ECD: Extensor digitorum communis; mo: Months; NPB: Neuropsychological battery; BDI: Beck depression inventory; NPI: Neuropsychiatry Inventory; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; ADAS-Cog: Cognitive subscale of the Alzheimer Disease Assessment Scale; wks: Weeks; PAS: Paired associative stimulation; LTP: Long-term potentiation; plat: Plasticity; UPDRS: Unified Parkinson Disease Rating Scale; BADL: Basic Activities of Daily Living; FBI: Frontal Behavioral Inventory; FC: Frontocentral; TPC: Temporoparietal cortex; sync: Synchronization; Domin: Dominant. Symbols. †: Mean ± standard deviation; %: Percent; >: more than; ≤: equal or less than; ≥: equal or more than; . : No data; –: Not done or not applicable; ⇑: Increased; *: 14 out of the initial 20 received single-dose of rivastigmine, specifically those who had low baseline SAI; **: 16 out of the initial 20 received rivastigmine for 1 year (treatment stopped in 4); ⇓: Decreased.
3.2.1. Cortical reactivity measures
3.2.1.1. Short-latency afferent inhibition
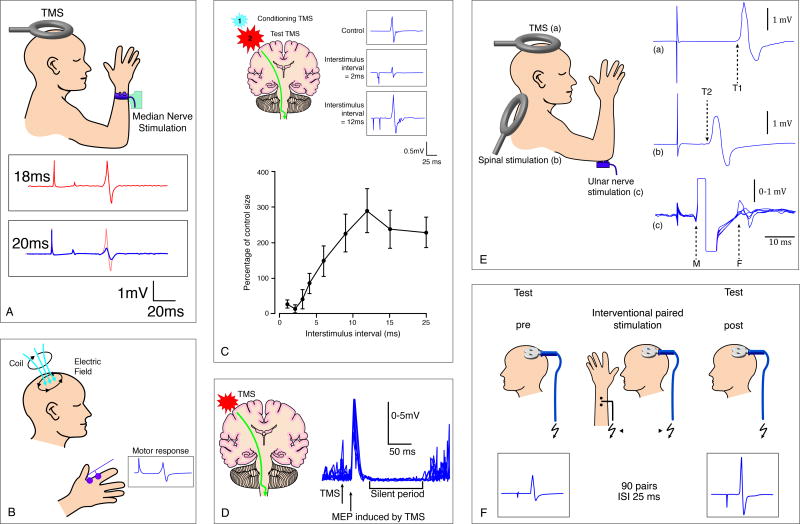
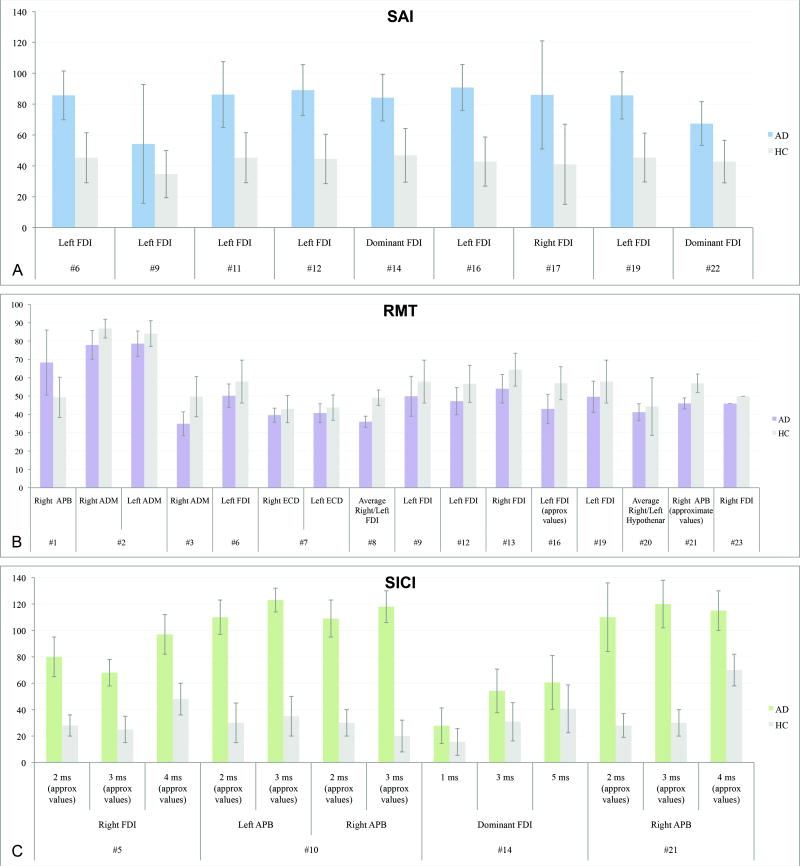
The most consistent finding of altered motor cortical reactivity in AD regarded short-latency afferent inhibition (SAI). SAI refers to the suppression of the amplitude of a motor evoked potential (MEP) produced by a conditioning afferent electrical stimulus applied to the median nerve at the wrist approximately 20ms prior to TMS of the hand area of the contralateral motor cortex (11). Figure 2A provides a schematic illustration of the experimental set-up. SAI is thought to reflect the integrity of central cholinergic neural circuits, as it has been shown to be reduced or abolished by the muscarinic antagonist scopolamine in healthy subjects (12). It has long been suggested that the pathogenesis of AD may involve deficits in the cholinergic circuits (13, 14), and agents that enhance cholinergic neurotransmission – e.g., acetylcholine-esterase inhibitors (AChEIs) – are associated with improvements in cognitive function and activities of daily living (15). All 10 identified studies assessing SAI (Figure 3A) found significant reductions in AD as compared to healthy individuals (studies #6,9,11,12,14,16,17,19,22,23) (9, 10, 16-23). Results from TMS studies measuring SAI thus suggest that afferent inhibition may be a useful marker of central cholinergic dysfunction even in early stages of AD (21).
Figure 2.
Schematics of TMS measures of cortical reactivity (A-E) and plasticity (F). A: Short-latency afferent inhibition (SAI); B: Motor evoked potential (MEP); C: Intracortical inhibition (ICI) and intracortical facilitation (ICF), represented at inter-stimulus intervals of 2 ms and 12 ms, respectively; D: Cortical silent period (cSP); E: Central motor conduction time (CMCT); F: Paired associative stimulation (PAS). Schematic A partially composed from Tokimura et al. (11); schematics B-E adapted from Kobayashi and Pascual-Leone (25); schematic F adapted from Stefan et al. (45).
Figure 3.
Measures of cortical reactivity found in all (SAI) or most (RMT) studies to be significantly different in patients with AD as compared to healthy elders. As for ICI, although fewer studies found significant differences than those that did not, significant results are also presented. A: Neurophysiologic findings for SAI in 9 out of the 10 studies measuring it, all showing significantly reduced SAI in AD as opposed to healthy elderly; study #23 (23) was not included for lack of data conform with all others. B: Neurophysiologic findings (means and SDs) for RMT of all 15 studies of 22 finding significant differences between AD versus healthy elder individuals. C: Neurophysiologic findings for ICI of 4 studies, from a total of 11, in which significant differences between AD versus healthy elder individuals were obtained. Study numbers correspond to studies presented in Table 1. Results are presented in means and SDs. Note: A few studies did not provide exact means and SDs; we thus extracted approximate values from graphs whenever necessary.
Abnormal SAI may differentiate AD from frontotemporal dementia (study #12) (17) or vascular dementia (study #19) (19). However, in neurodegenerative dementias in which cholinergic deficits have been demonstrated, such as dementia with Lewy bodies (DLB), results to date are conflicting. Nardone et al. (study #14) (20) found that SAI could differentiate AD from DLB, the latter presenting with normal SAI, although a clear tendency toward a reduced SAI was present in DLB patients as compared to controls. Di Lazzaro et al. (study #16) (18) showed a significant reduction in SAI in both AD and DLB patients, thus suggesting that SAI testing may be useful in differentiating cholinergic versus non-cholinergic forms of dementia. The discrepancy between studies might be due to major limitations in Nardone et al.’s study that make their results less conclusive: (a) SAI testing was performed without randomization of different conditions, which might have resulted in less accurate SAI evaluation; (b) patient selection seems to have been less strict than in the study by Di Lazzaro and co-workers because the diagnosis of DLB was based on criteria proposed in 1996 instead of the revised ones (24); (c) neuroradiological studies, which are extremely helpful to support the diagnosis, appear not to have been conducted; (d) given the less strict patient selection, it is possible that some patients with Parkinson’s disease (PD) associated with dementia might have been included in the DLB group, and since SAI appears to be normal or even enhanced in PD, this may have resulted in an overall normal mean; (e) it should also be noted that the mean values of SAI reported are different (for each ISI) from those reported by Di Lazzaro and colleagues (grand mean of all ISIs), making it difficult to evaluate them.
On the other hand, SAI appears to be normal in patients with mild cognitive impairment (MCI) (study #17) (22) and may thus not be useful to anticipate risk for development of AD. Similarly, no significant correlations were shown between SAI and age or duration and staging of AD (study #22) (21). Nevertheless, SAI was found to be negatively correlated with performance in abstract thinking (studies #16,19) (18, 19) and long-term memory (study #19) (19) in AD patients.
3.2.1.2. Motor threshold
Another particularly consistent result across studies of TMS in AD regards resting motor threshold (RMT). When TMS is applied to the motor cortex at appropriate stimulation intensity, MEPs can be recorded from contralateral extremity muscles (Figure 2B). RMT refers to the lowest TMS intensity necessary to evoke MEPs in the target muscle when single-pulse stimuli are applied to the motor cortex, and it is commonly defined as the minimum stimulus intensity required to elicit MEPs of more than 50 μV peak-to-peak amplitude in at least 50% of successive trials, in resting target muscles (25).
One study (study #11) (9) did not report data on this measure, but of the others, most (14 of 22) found significantly reduced RMT in AD as compared with healthy controls (studies #2,3,4,6,7,8,9,12,13,16,19,20,21,23) (10, 16-19, 23, 26-33) (Figure 3B). However, in one study (study #1) (34), RMT was found to be higher in AD patients than controls (Figure 3B). RMT is believed to reflect membrane excitability of corticospinal neurons and interneurons projecting onto these neurons in the motor cortex (25). Overall, findings to date on this TMS measure suggest an increased excitability of the corticospinal projections or motor cortical outputs in AD.
Active motor threshold (AMT) differs from RMT in that excitability of motoneurons in the spinal cord is enhanced by voluntary contraction of the target muscle, thus providing a measure of corticospinal excitability with greater dependence on the spinal segmental level excitability (25). AMT is usually defined as the minimum stimulus intensity to produce MEPs of approximately 200 μV in 50% of consecutive trials during isometric contraction of the tested muscle, at about 20% of maximum voluntary contraction. AMT was assessed in 9 studies, with results being somewhat divergent from those for RMT, even within the same study. Two studies found significant decreases in AMT, along with RMT, in AD patients as opposed to healthy controls (studies #3,16) (18, 27). The remaining studies found no significant differences in AMT in AD versus controls. However, while in 4 studies that absence of differences was paralleled by a lack of difference in RMT (studies #5,10,14,22) (20, 21, 35, 36), 3 other studies reported significant decreases in RMT but no differences in AMT findings (studies #6,19,21) (16, 19, 33). Thus, it seems that the excitability of spinal projections is relatively preserved during early-course AD.
3.2.1.3. Motor evoked potential amplitude
The amplitude of the MEP reflects not only the integrity of the corticospinal tract and the excitability of motor cortex and spinal level, but also the conduction along the peripheral motor pathway to the muscles. That is, dysfunction at any level along the corticospinal pathway may reveal abnormal MEPs, while the presence of intact MEPs suggests integrity of the pyramidal tract (25). The amplitude of MEPs can be assessed in relation to the maximal MEP amplitude to progressively higher TMS intensities (input-output curve; Figure 2B) or to the maximum peripheral M response. Either way, 11 studies measuring MEP amplitude in AD were identified. When using the former approach, most of the studies (7 of 8) found no significant differences between AD patients and healthy individuals in MEP amplitude (studies #1,6,10,13,15,16,20) (16, 18, 31, 32, 34, 36, 37), whereas one study found MEP amplitude to be significantly higher among patients with AD than healthy controls (study #4) (28). When using the latter approach, significant increases in MEP amplitude in AD patients were found in 3 studies (studies #2,3,4) (26-28), whilst in 2 other studies (studies #1,8) (30, 34) no significant differences were detected. Overall, however, these results suggest that the integrity of the corticospinal tract is not compromised in earlier stages of AD.
3.2.1.4. Central motor conduction time
Central motor conduction time (CMCT) is defined as the latency difference between the MEPs induced by stimulation of the motor cortex and those evoked by spinal (motor root) stimulation, and is calculated by subtracting the latency of the motor potential induced by stimulation of the spinal motor root from that of the response to motor cortex stimulation (Figure 2E). Lengthening of CMCT suggests demyelination of pathways, while low amplitude responses with little delay or absence of responses are more suggestive of loss of neurons or axons (25). None of the 7 studies that examined CMCT in AD (studies #1,2,4,5,6,14,22) (16, 20, 21, 26, 28, 34, 35) found statistically significant differences between patients and healthy elders. Once again, these results support that the integrity of the corticospinal tract is unaffected in mild-to-moderate stages of AD.
3.2.1.5. Intracortical inhibition and silent period
More divergent results between studies have been obtained for intracortical inhibition (ICI; Figure 2C) and silent period (SP; Figure 2D) measures. Both short-latency ICI (SICI) and cortical SP (cSP) are thought to reflect the excitability of inhibitory GABAergic cortical circuits (38). In SICI, inhibitory interactions in the cortex can be studied by combining a subthreshold (60–80% of RMT) conditioning stimulus with a suprathreshold test stimulus, a technique known as paired-pulse TMS, at different short inter-stimulus intervals. Maximum inhibitory effects are generally found with interstimuli intervals between 1 and 4ms, and the maximum amount of this inhibition is commonly 20–40% of the test MEP. SICI is thought to reflect mostly GABAA-mediated intracortical inhibitory interactions (39). In SP, when an individual is instructed to maintain muscle contraction and a single suprathreshold TMS pulse is applied to the motor cortex contralateral to the target muscle, the electromyographic (EMG) activity is arrested for a few hundred milliseconds after the MEP. This period of EMG suppression is referred to as a silent period, normally defined as the time from the end of the MEP to the return of voluntary EMG activity (25). Whereas spinal inhibition contributes to the early part of the SP (its first 50-75ms), the late part originates most likely in the motor cortex (40).
Significant reductions of SICI (Figure 3C) to paired-pulse TMS were found by some investigators (studies #5,10,14,21) (20, 33, 35, 36). However, most (7 of 11) studies did not find differences in SICI between AD patients and controls (studies #3,6,9,16,18,19,22) (10, 16, 18, 19, 21, 27, 41). Nonetheless, when present, the amount of disinhibition can correlate with the severity of AD (study #5) (35). On the other hand, of the 6 studies assessing cSP, most (studies #5,6,8,13) (16, 30, 31, 35), but not all (studies #1,4) (28, 34), failed to find a significant deficit in this measure in AD versus healthy participants. Peripheral SP, assessed in a single study, was not altered in AD patients (study #1) (34). Taken together, these findings do not strongly support impairments in GABAergic inhibition in AD.
3.2.1.6. Intracortical facilitation
Intracortical facilitation (ICF) is studied with paired-pulse TMS (Figure 2C), but the inter-stimulus intervals at which facilitatory effects of the conditioning subthreshold TMS pulse on the suprathreshold test MEP can be observed are longer, usually between 7-20ms. The magnitude of this facilitation can be quite variable among individuals, from 120% to 300% of the test MEPs (25). ICF is thought to reflect excitatory neurotransmission in human motor cortex largely mediated by N-methyl-D-aspartate receptors (NMDARs) (40). No study has found significant changes in ICF in AD patients as compared to healthy controls volunteers (studies #3,5,6,10,14,18,21,22) (16, 20, 21, 27, 33, 35, 36, 41). Findings of all 8 studies point, therefore, to a normal NMDAR-dependent glutamate excitatory activity in AD, as tested by this cortical reactivity measure.
3.2.2. Cortical plasticity and functional connectivity
Three studies have examined cortical plasticity (studies #13,15) (31, 37) or functional connectivity (study #20) (32) in AD, involving a total of 35 patients with AD and 34 healthy elders. Several TMS techniques can be used to measure brain plasticity noninvasively in humans. These include cortical responses to repetitive TMS (rTMS) and paired-associative stimulation (PAS), and each provides information about different aspects of cortical plasticity (38, 42, 43).
Repetitive TMS consists of the application of a train of TMS pulses of the same intensity to a single brain area at a given frequency that can range from 1 to 20 or more stimuli per second. Such a train of rTMS can induce a modulation of cortical excitability beyond the duration of the train itself. Depending on the stimulation parameters, particularly frequency of stimulation, cortical excitability is rendered facilitated or suppressed. In general, a continuous train of lower frequencies of rTMS, in the 1 Hz range, leads to a transient suppression of excitability in the targeted cortical area, while bursts of high-frequency stimulation (≥ 5 Hz) lead to a temporary increase in cortical excitability (25). This modulation can last for several minutes (depending on the duration of the train itself) and provides an index of plasticity.
PAS (Figure 2F) refers to a paradigm consisting of pairing low-frequency repetitive median nerve electric stimulation with timed TMS over the contralateral the motor cortex, resembling models of associative LTP as developed in animal studies (44). PAS was shown to be able to modulate the excitability of the motor system, leading to a lasting (≥ 60min) increase in MEP amplitude, when the interval between peripheral nerve stimulation and TMS was set at 25ms (PAS25), an inter-stimulus interval slightly longer than the time needed for the afferent inputs to reach the cerebral cortex (45). However, changing the interval between the two associative stimuli to 10ms (PAS10), i.e., an inter-stimulus interval shorter than the time needed for the afferent inputs to reach the cerebral cortex, leads to a depression of TMS-evoked MEPs (46).
3.2.2.1. Studies of plasticity in motor cortical areas
Inghilleri and colleagues (31) tested the effects of modulation of cortical motor areas induced by suprathreshold high-frequency (5 Hz) rTMS. During rTMS, the amplitude of MEPs progressively decreased in patients while increasing in controls. Interestingly, 5 Hz rTMS induced an increase in cSP in both groups, thus pointing to normal plasticity in cortical inhibitory circuits in the patient group. These findings suggest that the mechanisms supporting facilitatory cortical plasticity might be abnormal in AD. It is noteworthy, though, that such modulatory effects of rTMS show great inter-individual variability (47).
Other findings (37) seem to support impaired glutamatergic neurotransmission in AD, likely through NMDAR dysfunction. Employing PAS25, Battaglia and colleagues (37) studied corticomotor LTP-like plasticity in AD and healthy individuals, and also performed biochemical analyses in brain slices of a symptomatic APP/PS1 model. PAS-induced plasticity was found to be significantly reduced in AD patients. Moreover, the authors also showed that 4-4.5 month-old APP/PS1 mice exhibited deficits of NMDAR-dependent neocortical (motor and medial prefrontal) and hippocampal LTP, and a marked alteration of NMDAR activity. Therefore, it was suggested that decreased plasticity might underlie motor symptoms in AD and result from a deficit of NMDAR-dependent neurotransmission.
3.2.2.2. Studies of connectivity between motor and non-motor cortical areas
To date, no study has measured cortical reactivity or plasticity outside the motor cortex. However, Julkunen and colleagues (32) have studied functional connectivity between the motor cortex and other cortical regions. They delivered 50 single TMS pulses 3s apart to the motor cortex to assess spreading of navigated TMS-evoked electroencephalography (EEG) responses throughout the brain. They found significant differences in motor cortical reactivity (decreased MT from averaged left and right hemispheres) in AD subjects as compared with healthy controls. In addition, they obtained proof-of-concept findings of prominent changes in cortical connectivity in patients with AD using real-time integration of TMS and EEG. In particular, the TMS-evoked response at 30-50ms decreased significantly in AD patients compared to both healthy elders and subjects with MCI over widespread brain regions, with significant differences detected in the ipsilateral parietal cortex and contralateral frontocentral areas (Figure 4A). These findings of diminished reactivity and connectivity between regions suggest a dysfunction of large-scale sensorimotor networks perhaps with reduced synchronization of EEG activity in patients with AD. Moreover, amplitudes of P30 and P200 in MCI patients were halfway between the values of AD and control groups, thus suggesting that the combination of EEG with rTMS might detect abnormalities during prodromal stages of AD.
Figure 4.
Measuring cortical reactivity and plasticity, and functional connectivity and synchrony with TMS-EEG. A: Results from study #20 (32) to illustrate the feasibility of combining TMS with EEG; B: Schematic representation of experimental paradigm to measure local and network plasticity in motor (TMS-EMG and TMS-EEG) and non-motor (TMS-EEG) cortical regions.
3.3. Studies on the monitoring of effects of pharmacological agents on motor cortical excitability
Table 2 presents major findings of TMS studies published to date assessing the acute and long-term effects of pharmacological agents, along with demographic and clinical characteristics of study samples. Overall, 77 patients with AD and 25 healthy, age-matched subjects have been tested before and after the administration of a single oral dose of medication, while 33 AD patients were tested prior to and following prolonged treatment.
Table 2.
Major findings of peer-reviewed papers assessing the effects of acute and chronic administration of pharmacological drugs on cortical reactivity in Alzheimer’s disease (AD), and assessing the predictive value of TMS measures for response to chronic administration of medication.
| PAPER | n | Age (yrs †) |
Gender (% M) |
Education (yrs†) |
Disease duration (mo †) |
Neurologic, Neuropsychiatric, and Neuropsychological Evaluation |
Pharmacol Challenge |
Effects on Cortical Reactivity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis & Staging |
Anat scan |
NeuroΨ testing |
Meds | Muscle | MT (%) |
MEP (mV) |
CM CT |
SP (ms) |
SAI | ICI | ICF | |||||||
|
Acute administration of pharmacological drugs
| ||||||||||||||||||
| #5 Liepert et al., 2001 | 10 | [n = 11: 74.8 ± 9.7] | [n = 11: 18.18] | - | - | [n = 11: CDR: 1.64 ± 0.64] | CT or MRI | MMSE WMT + other | No drugs affecting CNS | Donepezil 1 wk [5 mg/d] + 1 wk [10 mg/d] in 5 patients | Right FDI | - | - | - | N.S. | - | SIGN [SICI ⇑ after 10 mg] | N.S. |
|
| ||||||||||||||||||
| #6 Di Lazzaro et al., 2002 | 6 | [n = 15: 69.0 ± 5.3] | [n = 15: 60.0] | [n = 15: 9.3 ± 3.4] | [n = 15: 28.4 ± 14.6] | NINCDS-ADRADA [Probable] | - | MMSE: 18.6 ± 3.5 RAVLT + Others | Naïve to AChEIs | Rivastigmine Single-dose [3 mg] | Left FDI | N.S. [RMT, AMT] | - | - | - | SIGN [⇑ in AD] N.S. [HC] | - | - |
|
| ||||||||||||||||||
| 3 [HC] | 30.6 ± 2.3 | - | - | - | - | - | ||||||||||||
|
| ||||||||||||||||||
| #9 Di Lazzaro et al., 2004 | 14 | [n = 28: 71.3 ± 6.8] | . | [n = 28: 8.2 ± 2.3] | [n = 28: 32.0 ± 16.8] | NINCDS-ADRADA [Probable] | - | MMSE: [n = 28: 19.35 ± 3.8] RAVLT + Others | Naïve to AchEIs/no other 30 days | Rivastigmine Single-dose [3 mg] | Left FDI | N.S. [RMT] | - | - | - | SIGN [⇑] | N.S. [SICI] | - |
|
| ||||||||||||||||||
| #10 Pierantozzi et al., 2004 | 12 | 65.2 ± 3.5 | . | - | [Sympt not > 18 mo] | NINCDS-ADRADA [Possible] CDR: ≤ 1 | MRI | MMSE: 21.8 ± 2.1 NPB BDI / NPI | No AchEIs or other | Galantamine Single-dose [4 mg] | Right / Left APB | N.S. [RMT & AMT] | N.S. [MEP amp] | - | - | - | SIGN [⇑ SICI] | - |
|
| ||||||||||||||||||
| #11 Di Lazzaro et al., 2005 | 14* [⇓ bas SAI] | [n = 20: 70.5 ± 6.9] | [n = 20: 40.0] | [n = 20: 7.9 ± 2.9] | [n = 20: 26.8 ± 16.4] | NINCDS-ADRADA [Possible] | - | MMSE: [n = 20: 19.1 ± 5.5] RAVLT + Others | Naïve to AchEIs/no other 30 days | Rivastigmine Single-dose [3 mg] | Left FDI | - | - | - | - | SIGN [⇑] | - | - |
|
| ||||||||||||||||||
| #21 Martorana. et al., 2008 | 11 | 73.0 ± 9.2 | . | - | - | DSM-IV NINCDS-ADRADA CDR: ≥ 1.5 | MRI | MMSE: 15.74 ± 1.6 NPB/NPI UPDRS | No AChEIs or others | Melevodopa Single-dose [250 mg] + [Placebo in AD] | Right APB | N.S. [RMT & AMT in AD or HC] | - | - | - | - | SIGN [⇑SICI in AD] N.S. [HC] | N.S. [AD & HC] |
|
| ||||||||||||||||||
| 12 [HC] | 68.0 ± 5.8 | . | - | - | - | - | . | - | ||||||||||
|
| ||||||||||||||||||
| #23 Martorana et al., 2009 | 10 | 72.5 ± 6.1 | . | - | - | NINCDS-ADRADA [Probable] CDR: 1.75 ± 1.6 | MRI | MMSE: 18.23 ± 3.2 NPB | Naïve to AchEIs/no other 30 days | L-dopa Single-dose [125 mg] | Right FDI | N.S. [RMT in AD or HC] | N.S. [MEP amp in AD or HC] | - | - | SIGN [⇑ in AD] N.S. [HC] | - | - |
|
| ||||||||||||||||||
| 10 [HC] | 71.7 ± 4.9 | . | - | - | - | - | - | - | ||||||||||
|
| ||||||||||||||||||
|
Chronic administration of pharmacological drugs
| ||||||||||||||||||
| #24 Pennisi et al., 2002 | 17 | [Range: 55-82] | 41.2 | - | - | NINCDS-ADRADA [Probable] | CT/MRI [diffuse atrophy in all; repeated at 1 yr] | MMSE: 11.76 ± 5.68 MMSE (1yr): 8.53 ± 5.14 | AChEIs 1 yr | Right / Left FDI | SIGN [⇓ R/L RMT] | N.S. | N.S. | - | - | - | - | |
|
| ||||||||||||||||||
|
Predictors of response to chronic administration of pharmacological drugs
| ||||||||||||||||||
| #11 Di Lazzaro et al., 2005 | 16** | [n = 20: 70.5 ± 6.9] | [n = 20: 40.0] | [n = 20: 7.9 ± 2.9] | [n = 20: 26.8 ± 16.4] | NINCDS-ADRADA [Possible] | - | MMSE [n = 20: 19.1 ± 5.5] RAVLT + Others | Rivastigmine 1 yr chronic tt | Left FDI | - | - | - | - | SIGN [⇑ in those w/⇓ bas SAI or ⇑ after s-d] | - | - | |
Legend. n: Number of subjects; yrs: Years; % M: Percentage of males; mo: Months; Anat scan: Anatomical scanning; NeuroΨ: Neuropsychologic; Meds: Medications; Pharmacol: Pharmacological; MT: Motor threshold; MEP: Motor evoked potential; mV: Microvolts; CMCT: Central motor conduction time; SP: Silent period; ms: Miliseconds; SAI: Short-latency afferent inhibition; ICI: Intracortical inhibition; ICF: Intracortical facilitation; CDR: Clinical Dementia Rating; CT: Computerized tomography; MRI: Magnetic resonance imaging; MMSE: Mini-Mental State Examination; WMT: Wechsler Memory Test; CNS: Central nervous system; wk: Week; mg/d: Miligram/day; FDI: First dorsal interosseus muscle; N.S.: Non-significant; SIGN: Significant; HC: Healthy control; NINCDS-ADRADA: National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association; [Probable]: Probable AD; RAVLT: Rey Auditory Verbal Learning Test; AChEIs: Acetylcholinesterase Inhibitors; RMT: Resting motor threshold; AMT: Active motor threshold; SICI: Short-latency intracortical inhibition; Sympt: Symptoms; [Possible]: Possible AD; NPB: Neuropsychological battery; BDI: Beck Depression Inventory; NPI: Neuropsychiatry Inventory; APB: Abductor pollicis brevis muscle; amp: Amplitude; bas: baseline; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; UPDRS: Unified Parkinson Disease Rating Scale; R/L: Right/left; tt: Treatment; w/: With; s-d: Single-dose. Symbols. †: Mean ± standard deviation; %: Percent; >: more than; ≤: equal or less than; ≥: equal or more than; . : No data; –: Not done or not applicable; ⇑: Increased; *: 14 out of the initial 20 received single-dose of rivastigmine, specifically those who had low baseline SAI; ** 16 out of the initial 20 received rivastigmine for 1 year (treatment stopped in 4); ⇓: Decreased.
3.3.1. Acethylcoline-esterase inhibitors (AChEIs)
Five studies examined the acute effects of AchEIs on motor cortical excitability in patients with AD (Table 2; studies #5,6,9,10,11) (9, 10, 16, 35, 36). Specifically, single doses of rivastigmine were administered in 3 studies (9, 10, 16), galantamine in another (36), and in one study a smaller dose of donezepil was given to 10 patients and a higher dosage to five (35). In this latter study, SICI was increased in patients who were given the higher dose of donezepil (10 mg). A reversal of SICI was also detected following administration of galantamine (36), but not after rivastigmine (10). In contrast, rivastigmine appears to augment SAI (9, 10, 16), while having no effects on healthy controls (10). Neither rivastigmine nor galantamine changed MT (studies #6,9,10) (10, 16, 36).
3.3.2. Dopaminergic agents
Two studies assessed the acute effects of dopaminergic challenges on motor cortical excitability (studies #21,23) (23, 33) in AD patients. The studies were motivated by the hypothesis that the dopaminergic system, involved in learning and memory processes, might be dysfunctional in patients with AD, eventually disrupting the cholinergic system. In the first study, Martorana and colleagues (33) examined the effects of a single dose of melevodopa on RMT, AMT, ICI, and ICF in patients with AD and healthy elders. While melevodopa had no significant effect on RMT, AMT, and ICF in either group, it significantly changed SICI in AD patients, whereas no changes were observed among controls. Therefore, dopamine may modulate cortical excitability in AD via intracortical inhibitory circuits. In a second intervention, Martorana et al. (23) measured SAI after administration of a single dose of L-dopa in both AD and healthy individuals. Normalization of SAI was observed in AD, while no effect was noted in controls. This suggests that the relationship between acetylcholine and dopamine systems may be abnormal in AD. Interestingly, the amount of increase of SAI by L-dopa correlated with cognitive impairment, as assessed by the Mini-mental State Examination (MMSE).
3.3.3. Long-term effects of medications on motor cortical excitability in AD
One study has investigated the effects of long-term administration of AChEIs on motor cortical excitability. Indeed, Pennisi and colleagues (study #24) (8) showed that mean RMT, which was positively correlated with disease severity at baseline, significantly decreased over both hemispheres after 1 year of treatment with AChEIs at various dosages, while no significant changes were observed on MEP amplitude or CMCT.
3.3.4. Predictors of response to chronic administration of medications
Di Lazzaro et al. (study #11) (9) assessed the predictive value of SAI for positive clinical response to 1 year of treatment with rivastigmine in patients with AD. They found that most patients with abnormal SAI at baseline and who had had an acute increase in SAI after a single oral dose of rivastigmine benefited from prolonged administration of rivastigmine. In contrast, patients with normal SAI at baseline or abnormal SAI at baseline but presenting a small change in SAI after a single dose of rivastigmine showed progression of disease. Moreover, patients with abnormal SAI at baseline improved or remained stable on average in almost three-fold more neuropsychological tests than patients with normal baseline SAI. The acute change in SAI after single-dose rivastigmine was positively correlated with an improvement in a number of neuropsychological tests after 1 year of treatment. It was, therefore, suggested that abnormal baseline SAI—conceivably reflecting dysfunction in central cholinergic circuits—and a positive response to the acute effects of a single dose of rivastigmine may be predictive of long-term response to rivastigmine, so that the evaluation of SAI and the effects of a single dose of rivastigmine on SAI could be useful to identify responders. Eventually, similar strategies may prove to be similarly useful with other pharmacological agents for AD.
3.4. Studies on the enhancement of cognitive function in AD
Table 3 compiles major findings of TMS and tDCS studies published to date to enhance cognitive function in AD, as well as demographic and clinical characteristics of study samples. Overall, 54 patients with AD have been studied (this includes 9 new patients in study #26, as the remaining were the same 15 patients of study #25).
Table 3.
Major findings of peer-reviewed papers aiming at enhancing cognitive function in patients with Alzheimer’s disease (AD) using noninvasive brain stimulation.
| PAPER |
Study design |
n | Age (yrs †) |
Gender (% M) |
Education (yrs †) |
Disease duration (yrs †) |
Neurologic, Neuropsychiatric, and Neuropsychological Evaluation |
Study Design | Cognitive Function Enhanced |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis & Staging |
Anat scan |
NeuroΨ testing |
Meds | Noninvasive modality |
Parameters | Brain target |
No. of sessions |
||||||||
| #25 Cotelli et al., 2006 | Crossover Sham-controlled | 15 | 76.6 ± 6.0 | . | 6.0 ± 2.0 | - | NINCDS-ADRADA [Probable] | MRI [all atrophy MTL & TPC] | MMSE: 17.8 ± 3.7 | AChEIs [don / riv; no memant] | rTMS [online] | 20 Hz 90% MT 600 ms [+ sham] | Left / Right DLPFC | 1 | ⇑ action naming; no effects on object naming |
|
| |||||||||||||||
| #26 Cotelli et al., 2008 | Crossover Sham-controlled | 12 [Mi] | 75.0 ± 6.2 | . | 6.8 ± 3.1 | - | NINCDS-ADRADA [Probable] | MRI | MMSE: 19.7 ± 1.6 | AChEIs [don / riv; no memant] | rTMS [online] | 20 Hz 90% MT 500 ms [+ Sham] | Left / Right DLPFC | 1 | ⇑action naming in Mi AD; ⇑action & object naming in M-S AD |
|
|
|
||||||||||||||
| 12 [M-S] | 77.6 ± 5.8 | . | 5.7 ± 2.6 | - | MMSE: 14.3 ± 2.6 | ||||||||||
|
| |||||||||||||||
| #27 Ferrucci et al., 2008 | Crossover Sham-controlled | 10 | 75.2 ± 7.3 | 30 | 10.9 ± 4.8 | - | DSM-IV NINCDS-ADRADA [Probable] | - | MMSE: 22.7 ± 1.8 | Stable on AchEIs for 3 mo. | tDCS | Anodal / cathodal / sham 1.5 mA, 15m | Left / Right TPC | 3 | ⇑word recognit memory [after anodal tDCS] |
|
| |||||||||||||||
| #28 Boggio et al., 2009 | Crossover Sham-controlled | 10 | 79.1 ± 8.8 | 40 | 8.7 ± 4.9 | 4.5 ± 2.2 | CDR: [1-3] 1.7 ± 0.9 | - | MMSE: 17.0 ± 4.9 | AChEIs + others | tDCS | Anodal / sham 2 mA, 30m | Left DLPFC Left TC | 3 | ⇑ visual recognit memory; no effect on working memory |
|
| |||||||||||||||
| #29 Cotelli et al., 2010 | Parallel Sham-controlled | 5 [Real] | 71.2 ± 6.1 | . | 6.4 ± 1.3 | - | NINCDS-ADRADA [Probable] | - | MMSE: 16.2 ± 2.7 + Others | Stable on AchEIs [don / riv] for 6 mo. | rTMS [off-line] | 20 Hz 100% MT 2000 stim/s [+ sham] | Left DLPFC | 20 [Follow-up 8 wks after] | ⇑ auditory sentence comprehension; no effect on naming perform |
|
|
|
||||||||||||||
| 5 [Sham] | 74.4 ± 3.8 | . | 4.8 ± 0.4 | - | MMSE: 16.0 ± 2.0 + Others | ||||||||||
Legend. n: Number of subjects; yrs: Years; % M: Percentage of males; Anat scan: Anatomical scanning; NeuroΨ: Neuropsychological; Meds: medications; No.: Number; NINCDS-ADRADA: National Institute of Neurological and Communicative Disorders and Stroke – Alzheimer’s Disease and Related Disorders Association; Probable: Probable AD; MRI: Magnetic resonance imaging; MTL: Medial temporal lobe; TPC: Temporoparietal cortex; MMSE: Mini-Mental State Examination; AChEIs: Acetylcholinesterase inhibitors; don: Donezepil; riv: Rivastigmine; memant: Memantine; rTMS: Repetitive transcranial magnetic stimulation; Hz: Hertz; MT: Motor threshold; ms: Miliseconds; DLPFC: Dorsolateral prefrontal cortex; CDR: Clinical Dementia Rating; tDCS: Transcranial direct current stimulation; mA: Miliamperes; m: Minutes; TC: Temporal cortex; recognit: Recognition; Mi: Mild; M-S: Moderate-to-severe; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders, 4th edition; mo.: Months; stim/s: Stimuli per second; wks: Weeks. Symbols. †: Mean ± standard deviation; .: No data; –: Not done or not applicable; %: Percent; ⇑: Increase/improvement.
Both, rTMS and tDCS are noninvasive brain stimulation techniques capable of modulating cortical excitability and inducing lasting effects (48, 49). The mechanisms of action of rTMS and tDCS remain unclear, but both show promise as therapeutic applications in neuropsychiatric disorders (50-53), including the approval by the US Food and Drug Administration of the Neuronetics’ Neurostar TMS intervention for the treatment of medication-resistant depression (54). Repetitive TMS can be applied as continuous trains of low-frequency (1 Hz) or bursts of higher frequency (≥ 5Hz) rTMS, while tDCS can be applied as anodal or cathodal stimulation (4). In general, low frequency rTMS and cathodal tDCS are thought to reduce, and high-frequency rTMS and anodal tDCS to enhance excitability in the targeted cortical region.
Three studies, all conducted by the same group (Table 3; studies #25,26,29) (55-57), have been carried out to assess the effects of rTMS over the dorsolateral prefrontal cortex (DLPFC) on naming and language performance in patients with AD. Two crossover, sham-controlled, single-session studies (55, 56) used online rTMS, i.e., rTMS was applied during the execution of naming tasks. Significant improvements in action naming, but not in object naming, were found following high-frequency stimulation of either left or right DLPFC in each of the 15 AD patients in the earlier study (55). In their second study, when dividing AD patients according to disease staging based on MMSE scoring, Cotelli and colleagues (56) found that previous results were replicated only in mild AD patients (MMSE ≥ 17/30).
In contrast, in patients with moderate-to-severe AD (MMSE < 17/30), both action and object naming were improved after both left and right DLPFC rTMS. This led the investigators to suggest that the lack of benefit of rTMS on object naming in early-stage AD might be related to a ‘ceiling’ effect. In a later, parallel study (57), no significant effects of off-line rTMS were observed on naming performance (rTMS intervention consisted of a total of 4 weeks of daily stimulation) in moderate AD. A significant effect was nonetheless observed on auditory sentence comprehension after 2 weeks of real rTMS sessions, as compared to sham. Two additional weeks of daily rTMS sessions resulted in no additional improvements, although a significant benefit on auditory sentence comprehension was still detected 8 weeks after the end of the rTMS intervention as compared to baseline. These results were thought to be specific to the language network, therefore not due to a non-specific effect on cognitive processing in general, as no effects on memory and executive functions were found. None of these three studies report any side effects of the intervention, but it is not clear what safety evaluations (if any) were completed.
Two other, crossover-designed studies used tDCS to enhance recognition memory in patients with AD (studies #27,28) (58, 59). Ferrucci et al. (58) also studied patients with mild AD before and after 3 sessions of tDCS targeting bilateral temporoparietal areas with anodal, cathodal, or sham tDCS. In either case, a third electrode (second cathodal) was placed over the right deltoid muscle (extracephalic reference). Separate sessions (15 min at 1.5 mA) were at least one week apart. Anodal tDCS to the temporoparietal region significantly increased accuracy in a word recognition task; cathodal tDCS significantly decreased accuracy, while sham tDCS did not change it. Moreover, no effects were observed in a visual attention task, suggesting that the effects of tDCS were likely specific for recognition memory. No safety considerations were reported, and no modeling of the actual current distributions was presented. Boggio et al. (59) exposed patients with mild to moderately severe AD to a session of anodal tDCS to the left DLPFC, anodal tDCS to the left temporal cortex (cathode electrode was placed over the right supraorbital area for these 2 sessions), or a session of sham stimulation. Sessions were 48 hours apart, and patients were tested during each of the stimulation sessions, starting 10 min after stimulation onset and lasting until the end (30 min at 2 mA). No adverse effects occurred. Stimulation over both prefrontal and temporal areas resulted in a significant improvement of visual recognition memory, which was not attributable to attentional, non-specific processes, as assessed by the Stroop task. Nevertheless, contrary to the investigators’ expectations, no effects were obtained on working memory, which was however measured by a digit span task, more indicative of attentional than working memory functions.
4. Discussion
We performed a systematic review of prospective studies using noninvasive brain stimulation in AD. Overall, 388 patients with varying stages of severity of AD were involved in 29 studies, and 298 healthy elders served as controls. A number of studies have applied TMS to physiologically characterize AD and to monitor effects of pharmacological agents, while others have begun to therapeutically use TMS or tDCS to improve cognitive function in AD. We thus report on two main types of studies: neurophysiologic measures and therapeutic applications. The former comprise either diagnostic studies, in which comparisons were made between patients with AD and age-matched healthy controls (though generally not against patients with other forms of dementia or neurodegenerative illnesses), or studies monitoring the effects of certain drugs, in which within subject comparisons were made between pre- and post-drug intake. The therapeutic application studies were sham-controlled, but they were small proof-of-principle trials, not adequately powered to establish evidence for therapeutic efficacy. Furthermore, given the difficulties of a reliable sham TMS intervention, some of these studies lacked reliable blinding of subjects and none seem to have blinded the technicians or experimenters applying the TMS (though the clinical raters were blinded). Overall, applications of TMS to characterize motor system pathophysiology in AD appear to be safe and may be developed in valuable biomarkers. Among the studies focusing on motor cortical reactivity measures, the most consistent finding is a significant reduction of SAI in AD patients in comparison to healthy elders. The reduction of SAI in AD was found in all studies that assessed it, and thus appears to provide a reliable marker of cortical cholinergic dysfunction in AD, eventually capable of monitoring the effect of AChEIs and dopaminergic drugs (9, 10, 16, 23). Significantly lower RMT in AD patients as compared to age-matched controls was detected in about 2/3 of the studies. This seems to support the hypothesis of cortical hyperexcitability in AD and may inversely relate to the stage of severity of cognitive involvement (8, 28). However, the reason for somewhat variable results is worth considering in relation to this and other neurophysiologic measures. Encouraging findings, showing impaired functional connectivity between motor and non-motor brain regions in AD, have also been obtained (32). Finally, TMS and tDCS therapeutic approaches seeking to enhance cognitive function in AD appear safe and promising, but have to be considered very preliminary.
In the following subchapters, we shall discuss several different topics which we think are scarcely, if at all, addressed in studies conducted to date, while some others may provide lines in which future research can be of interest.
4.1. The role of atrophy
Although some studies have conducted magnetic resonance imaging (MRI) scanning to rule out other potential causes of dementia (e.g., vascular lesions) or to assess anatomical brain atrophy in AD patients, no study has performed more detailed analyses of the potential influence of brain atrophy or distance-to-coil on their results. It has been shown that the impact of TMS depends on the distance between cortex and scalp, as the magnetic field (i.e., the induced current in the brain) decreases with distance (60, 61). Furthermore, modeling work suggests that current density distribution is critically influenced by brain morphology and tissue characteristics (4, 61). Brain atrophy can thus substantially alter the effect of TMS (4), not only because of greater scalp-to-brain distance but also due to increased current shunting in the CSF compartment. On the other hand, regional cortical thinning has been demonstrated in AD (62) and even during prodromal stages (63). Therefore, it is worth considering the possibility that the differences of TMS effects in patients with AD versus healthy elders might simply be confounded by and possible be simply the consequence of brain atrophy and tissue shrinkage. Volumetric studies of cerebrospinal fluid (CSF) layer, white matter volume, and cortical thinning should be included in future studies and will assist in the interpretation of TMS results involving subjects with cerebral atrophy.
4.2. Role of cortical target
Neuropathologic and neuroimaging studies in AD suggest that distinct cortical areas are differently affected. For instance, positron emission tomography (PET) studies with the amyloid-ß binding 11C-labelled Pittsburgh Compound B (PiB) show that patients with AD have higher 11C-PiB binding in prefrontal, precuneus and cingulate, and lateral temporal and parietal cortices, with minimal binding differences in medial temporal lobe and visual, sensory, and motor cortices (64-66). PET studies with the glucose ligand 18F-labelled fluorodeoxyglucose (FDG) show characteristics patterns of cerebral glucose hypometabolism (67), with primary cortices being relatively spared. In addition, metabolic deficits appear to occur in a progressive manner, from the hippocampus to temporoparietal and posterior cingulate cortices (42), to frontal lobes (68), and eventually extending to the occipital lobes (67). Furthermore, even during transitional stages between normal aging and AD, including patients with MCI converting to AD, an ‘AD-like’ pattern of FDG hypometabolism—in hippocampus, posterior cingulate and temporoparietal cortex, with milder magnitude of reduced metabolism than in clinical AD patients—is frequent [for review, (69)]. Similarly, PiB retention follows an intermediate pattern between healthy controls and AD (70), being significantly increased in frontal, parietal, and lateral temporal association cortices, and precuneus in amnestic MCI patients converting to AD as compared to healthy control individuals (71, 72). A similar distribution is also observed in PiB-positive non-amnestic MCI patients (72). Based on such neuroimaging findings, the motor cortex does not seem to be the best cortical area to assess in prodromal and early stages of AD. Rather, non-motor cortical regions where abnormalities may be particularly profound or early in the course of disease, e.g. lateral temporoparietal and frontal association cortices, may provide earlier physiologic biomarkers.
Cortical reactivity and plasticity measures outside the motor cortex are possible when combining TMS with EEG (73-75). EEG provides exquisite temporal resolution, a direct measure of neuronal activity, and is capable of differentiating between inhibitory and facilitatory effects. The TMS-EEG integration provides real-time information on cortical reactivity and connectivity. A noninvasive input (TMS) of known spatial and temporal characteristics can be applied to study local reactivity of the brain and interactions between different brain regions with directional and precise chronometric information. Thus, measures of cortical reactivity, including ICI or ICF, can be obtained in non-motor cortical areas with TMS-EEG looking at TMS-evoked potentials (EPs). Analysis of time-domain phase synchrony and coherence between different cortical regions can be directly estimated from high-density EEG recording (76-79). Further, a variety of sophisticated techniques is available to identify and characterize connectivity networks using EEG data (80-82). Additionally, the study of cortical plasticity mechanisms outside the motor cortex is also possible. In particular, the application of theta burst stimulation (TBS), a unique rTMS protocol capable of more robustly inducing LTP- and LTD-like plasticity than traditional rTMS protocols (83), followed by TMS-EPs over time can provide an index of synaptic and neuronal plasticity, analogously to the approach in animal models and brain slice preparations. Thus, contrasting the results of TMS on EEG before and after TMS can provide information about cortical reactivity and plasticity (depending on the TMS protocols applied) at the site of stimulation and resulting network dynamic adaptation (75, 84). In addition to enabling the systematic exploration of local cortical reactivity and plasticity across different brain regions, the combination of TMS with EEG is promising as it allows the characterization of functional connectivity and synchrony across different neural networks. Such findings may promote the understanding of the neurobiological underpinnings of AD, while eventually constituting valuable biological markers of AD risk and progression.
4.3. Population variability
The studies reviewed reveal that most of the TMS measures show considerable variability between studies. The cause for such variability remains unclear, but some factors, in addition to TMS methodological issues, are worth discussing. Baseline assessment of patients using standardized evaluations, as suggested by The Alzheimer’s Disease Centers’ Uniform Data Set [UDS; Initial Visit Packet and Neuropsychological Test Battery; www.alz.washington.edu; see also (85)] might be worth implementing to enable comparison between studies.
Genetic factors may contribute significantly to variability in TMS findings. For instance, the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene has been shown (86) to differentially modulate human cortical plasticity and the response to noninvasive brain stimulation. Furthermore, the impact of the BDNF Val66Met polymorphism might differ according to the noninvasive brain stimulation technique utilized (87). In the context of AD research, the low-activity Met66 allele was shown to be an additional risk factor for rapid disease progression during the preclinical period of AD (88), and may also constitute a risk factor for the development of psychotic symptoms in AD (89). Conceivably, the presence of BDNF Val66Met polymorphism may have distinct repercussions in the studied measures of reactivity and plasticity in normal and pathologic aging, thus deserving particular attention in future studies.
Apolipoprotein E (APOE) and its ε4 allele is thought to raise the risk of AD (90) in a dose-dependent manner (91), and may also influence brain responses to TMS measures. APOE-ε4 allele is associated with an earlier age of AD onset (92, 93), an accelerated onset of amyloid-ß deposition into amyloid plaques (94), higher amyloid load (95), and lower glucose metabolism (96, 97). Moreover, while some studies have found that the APOE gene does not influence the rates of disease progression in cognitive and functional domains (98-100), others have associated an accelerated cognitive (101-103) and motor (104) decline to the APOE-ε4 allele. APOE status, and the resulting differences in cognitive and motor decline, may be associated with differences in brain activity. Indeed, Wolk and colleagues (105) recently showed that the presence of APOE-ε4 distinctively modulates the clinical phenotype of AD by differentially influencing large-scale brain networks as assessed by functional neuroimaging. It is, therefore, also plausible that the presence of APOE-ε4 may influence cortical reactivity and local and network plasticity as measured by TMS. Research on the impact of APOE-ε4 in TMS measures in AD seems, thus, to be of great importance.
Age at disease onset may also contribute to the prominent between-study variability. Mean patient age in some studies was in the mid-sixties, in others in the mid-seventies. Moreover, duration of illness was not always reported and varied considerably across studies. Age-at-onset for patients in some studies was in the 50’s, while in others it was past 70 years of age. Rabinovici et al. (97) showed that sporadic AD patients with early-onset of disease (< 65 years) have decreased glucose metabolism in posterior regions as compared to those with late-onset of disease (> 65 years), whilst there is no difference in burden and distribution of amyloid-ß as measured by PiB. Age-of-onset may also relate to the concept of cognitive reserve, the brain’s capacity to minimize the clinical manifestations of cerebral damage. For instance, high-educated mild AD patients have more advanced pathological (increased PiB uptake in the lateral frontal cortex) and functional (lower glucose metabolic rate in the temporoparietal cortex) brain changes than less educated patients with the same degree of cognitive deterioration (71). Therefore, age at disease onset and the associated differences in brain network dynamics seem important to control for in future studies.
Assessing cognitive performance, particularly for domains associated to the function of the TMS targeted brain region, may also help explain some of the between-study discrepancies. Some studies have investigated the relationship between TMS findings and staging of disease (8, 20, 21, 28, 29, 31, 35) or performance in neuropsychological tests (9, 18, 19). However, the results have been conflicting, perhaps because of lack of specificity of the tasks used. Since motor cortex was targeted in all TMS measures reviewed, quantitative evaluation of motor function, either with specific motor skill tasks (106), measures of performance on daily activities (107), or motor learning tasks (108), particularly relevant in plasticity studies, seem important to include in future studies. Such measures will address the functional significance of the neurophysiologic findings and potentially disentangle some of the discrepancies observed among study populations.
4.4. Finding the earliest biomarkers of AD pathology
SAI shows very consistent results among patients with AD, and can be used to predict medication effects, but appears to be normal in MCI (22). Thus, it would be desirable to consider noninvasive neurophysiologic measures that could show earlier abnormalities. Animal studies reveal that the neurobiological substrate of age-associated cognitive decline likely involves an alteration of synaptic plasticity. For instance, deficits in late maintenance of hippocampal LTP in older rodents are characterized by a more rapid decay of LTP (109-111), and fastest decay rates of LTP are associated with greater degree of forgetfulness (112, 113). Additionally, deficits in the balance between LTP and LTD may lead to impaired learning and memory (114-116), therefore potentially underlying deterioration of cognitive functions.
Aberrantly reduced local and network plasticity, promoted by a plethora of factors (genetic influences such as APOE-ε4, and biological and environmental factors such as age, poor cognitive reserve, traumatic brain injury, diabetes mellitus, chronic adverse stress, poor diet, etc.), may also be related to age-related cognitive decline in humans. In addition, recent evidence demonstrates that oligomers of soluble amyloid-ß disrupt LTP and LTD (117, 118), and thus altered plasticity may critically contribute to the development of AD. TMS paradigms to study brain plasticity, particularly outside the motor cortex, might therefore be particularly worth investigating.
TBS is thought to mimic paradigms used to induce LTP and LTD in slice preparations and animal models (119, 120). When applied to the motor cortex, this TMS protocol has been shown to induce robust responses across subjects and to lead to enhancement or depression of MEP amplitudes to about half, depending on stimulation parameters. Physiologic and pharmacologic studies of TBS in humans show involvement of glutamatergic and GABAergic mediators, and the effects and their time-course are consistent with the notion that TBS does indeed engage mechanisms of cortical synaptic plasticity (121). Thus, post-TBS enhancement (following intermittent TBS) or suppression (after continuous TBS) of the cortical activity is considered an index of LTP or LTD-like induction of synaptic plasticity, respectively, in the targeted brain area. On the other hand, as noted above, real-time integration of image-guided TMS with EEG can provide information about the integrity of human brain plasticity (75), and is well suited for parallel studies in animals. Such an experimental approach, combining TMS protocols capable of inducing plasticity with other methods able to register changes throughout the brain, likely offers a potential metric of local and network plasticity. Figure 4B displays a schematic representation of this experimental paradigm.
4.5. Designing robust therapeutic interventions
Although promising, results of noninvasive stimulation to enhance cognitive function in AD to date have to be considered extremely preliminary. Most interventions have been acute or of short-duration, the effects seem short-lived, and were not replicated after longer-duration interventions. On the other hand, some of the effects obtained after longer-lasting interventions were not detected after single stimulation session (55-57). Others failed to show predicted improvements (59), stressing the importance of careful choice of outcome measures. Overall, short studies are essential (for safety, for proof-of-principle, for cost efficacy). However, the translation to long-term studies has to be done carefully. For example, the specific intervention that helps in short-term studies may not help in long-term ones (though this should be experimentally tested). In long-term studies, multiple TMS or tDCS sessions may interact, and metaplasticity effects may affect outcome.
Thus, it seems that careful experimental design is needed and it should consider patient selection aspects, stimulation parameters, and clinical, cognitive and behavioral assessment tools. For example, it would be valuable for all future studies examining therapeutic efficacy of TMS or tDCS in AD to employ (in addition to any other desired outcome measures) the neuropsychological battery of the UDS or outcome scales commonly used in trials of pharmacological agents for AD, such as the Cognitive subscale of the Alzheimer’s Disease Assessment Scale (ADAS-Cog) (122), despite its several limitations (123). This will enable comparison across studies.
Furthermore, the rationale for treating AD with neuromodulatory techniques remains debatable, and it might be a paradox that, while TMS studies show cortical hyperexcitability, the therapeutic attempts are based on techniques aimed at enhancing cortical excitability (anodal tDCS, high-frequency rTMS). It is plausible that hyperexcitability may be the consequence of other underlying pathophysiologic mechanisms, such as decreased synaptic efficiency or hypoplasticity. If so, appropriately testing cortical physiology before and after therapeutic interventions would make sense. In addition, the assumption that high-frequency rTMS, for instance, will enhance cortical excitability in AD patients may be wrong. Indeed, rTMS effects are thought to be state-dependent, dependent on the state of activity of the brain at the time of stimulation (124). High-frequency rTMS has also been shown to have desirable symptomatic effects on other conditions characterized by cortical hyperexcitability, such as auditory verbal hallucinations (125, 126). It is, therefore, possible that the effects of neuromodulatory techniques in the brain of AD patients may differ to those in normal subjects, and studies to physiologically characterize this would be important to guide future therapeutic trials.
Importantly, studies to date have failed to be evidence-based. For example, the assumption that the mechanisms of plasticity might be aberrantly diminished in the DLPFC or temporoparietal regions (brain areas targeted in the published studies) in AD is yet to be demonstrated and, thus, the concomitant assumption that plasticity enhancement is needed for amelioration of the AD condition remains conjectural. Consequently, whether plasticity-based interventions with high-frequency rTMS or anodal tDCS, or other protocols, would result in benefit to the patients seems to require more solid investigation before embarking upon long-standing treatments. Furthermore, it seems questionable whether improving performance in one task actually represents cognitive enhancement. More comprehensive outcome measures are needed to assess the clinical significance of TMS or tDCS in AD and appropriately powered studies with sound blinding procedures are necessary. Moreover, although merely speculative, it is unlikely that stimulations over a single brain area will heavily contribute to betterment of the cognitive status of AD patients, particularly those with more advanced disease. Eventually, well-planned, multiple-target stimulation protocols may be necessary in order to overcome the multitude of cognitive deficits characterizing moderate or severe AD.
5. Conclusions and perspectives for the future
Noninvasive stimulation has been steadily increasing its potential utility for the diagnosis, monitoring, and enhancement of cognitive function in AD. TMS may contribute to the understanding of the neurobiological substrates underlying cognitive decline and AD pathology. However, establishment of noninvasive brain stimulation techniques as diagnostic and therapeutic tools in AD requires systematic and thorough investigation of a variety of factors (e.g., volumetric studies of cortical thinning, white matter volume and integrity, genetic variants, learning capacity, age at disease onset, cognitive reserve) and novel TMS protocols (e.g., TBS, assessment of non-motor cortical regions, TMS combined with EEG or other neuroimaging modalities). We thus envision future novel diagnostic and therapeutic interventions for not only clinical AD but also its prodromal stages, including MCI, based on the overarching hypothesis that aberrant mechanisms of cortical plasticity and altered brain networks are the proximal causes of cognitive decline and critical contributors to the development of AD. If so, assessment of cortical plasticity mechanisms and network dynamics might ultimately provide an earliest predictive biomarker of cognitive decline and AD, and lead to novel interventions that might forestall neurocognitive decline during aging and prevent, or at least drastically delay, development of symptomatic AD.
Research highlights.
TMS holds promise as a physiologic biomarker in AD to identify therapeutic targets and monitor pharmacologic effects.
Short-latency afferent inhibition and resting motor threshold are reduced in AD.
Motor cortical plasticity and connectivity are impaired in AD.
TMS/tDCS may have therapeutic utility in AD, but evidence is still very preliminary.
TMS/tDCS appear safe in AD, but longer-term risks have been insufficiently considered.
Acknowledgments
Funding Work on this study was supported in part by the Berenson-Allen Foundation, the Harvard Clinical and Translational Science Center (Harvard Catalyst; NCRR-NIH UL1 RR025758), and a K24-RR018875 award from the National Institutes of Health to APL. CF was supported by a post-doctoral grant (SFRH/BPD/66846/2009) from the Foundation for Science and Technology, Portugal, co-financed by the European Social Fund. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Footnotes
Conflict of interest APL serves on the scientific advisory board for Nexstim, Neuronix, Starlab, and Neosync, and holds IP for the combination of TMS with EEG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alzheimer’s Association’s Facts and Figures’ Report 2010. ( www.alz.org/documents_custom/reportalzfactsfigures2010.pdf)
- 2.Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings JL, Vinters HV, Cole GM, Khachaturian ZS. Alzheimer’s disease: etiologies, pathophysiology, cognitive reserve, and treatment opportunities. Neurology. 1998;51:S2–17. doi: 10.1212/wnl.51.1_suppl_1.s2. discussion S65-17. [DOI] [PubMed] [Google Scholar]
- 4.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 5.Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–199. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 6.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010;3:95–118. doi: 10.1016/j.brs.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- 8.Pennisi G, Alagona G, Ferri R, Greco S, Santonocito D, Pappalardo A, et al. Motor cortex excitability in Alzheimer disease: one year follow-up study. Neurosci Lett. 2002;329:293–296. doi: 10.1016/s0304-3940(02)00701-2. [DOI] [PubMed] [Google Scholar]
- 9.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Neurophysiological predictors of long term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiatry. 2005;76:1064–1069. doi: 10.1136/jnnp.2004.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, et al. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:555–559. doi: 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, et al. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523(Pt 2):503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, et al. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- 13.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;2:1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 14.Coyle JT, Price DL, DeLong MR. Alzheimer’s disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 15.Birks J, Grimley Evans J, Iakovidou V, Tsolaki M, Holt FE. Rivastigmine for Alzheimer’s disease. Cochrane Database Syst Rev. 2009:CD001191. doi: 10.1002/14651858.CD001191. [DOI] [PubMed] [Google Scholar]
- 16.Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, et al. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- 17.Di Lazzaro V, Pilato F, Dileone M, Saturno E, Oliviero A, Marra C, et al. In vivo cholinergic circuit evaluation in frontotemporal and Alzheimer dementias. Neurology. 2006;66:1111–1113. doi: 10.1212/01.wnl.0000204183.26231.23. [DOI] [PubMed] [Google Scholar]
- 18.Di Lazzaro V, Pilato F, Dileone M, Saturno E, Profice P, Marra C, et al. Functional evaluation of cerebral cortex in dementia with Lewy bodies. Neuroimage. 2007;37:422–429. doi: 10.1016/j.neuroimage.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Di Lazzaro V, Pilato F, Dileone M, Profice P, Marra C, Ranieri F, et al. In vivo functional evaluation of central cholinergic circuits in vascular dementia. Clin Neurophysiol. 2008;119:2494–2500. doi: 10.1016/j.clinph.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 20.Nardone R, Bratti A, Tezzon F. Motor cortex inhibitory circuits in dementia with Lewy bodies and in Alzheimer’s disease. J Neural Transm. 2006;113:1679–1684. doi: 10.1007/s00702-006-0551-1. [DOI] [PubMed] [Google Scholar]
- 21.Nardone R, Bergmann J, Kronbichler M, Kunz A, Klein S, Caleri F, et al. Abnormal short latency afferent inhibition in early Alzheimer’s disease: a transcranial magnetic demonstration. J Neural Transm. 2008;115:1557–1562. doi: 10.1007/s00702-008-0129-1. [DOI] [PubMed] [Google Scholar]
- 22.Sakuma K, Murakami T, Nakashima K. Short latency afferent inhibition is not impaired in mild cognitive impairment. Clin Neurophysiol. 2007;118:1460–1463. doi: 10.1016/j.clinph.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 23.Martorana A, Mori F, Esposito Z, Kusayanagi H, Monteleone F, Codeca C, et al. Dopamine modulates cholinergic cortical excitability in Alzheimer’s disease patients. Neuropsychopharmacology. 2009;34:2323–2328. doi: 10.1038/npp.2009.60. [DOI] [PubMed] [Google Scholar]
- 24.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 26.de Carvalho M, de Mendonca A, Miranda PC, Garcia C, Luis ML. Magnetic stimulation in Alzheimer’s disease. J Neurol. 1997;244:304–307. doi: 10.1007/s004150050091. [DOI] [PubMed] [Google Scholar]
- 27.Pepin JL, Bogacz D, de Pasqua V, Delwaide PJ. Motor cortex inhibition is not impaired in patients with Alzheimer’s disease: evidence from paired transcranial magnetic stimulation. J Neurol Sci. 1999;170:119–123. doi: 10.1016/s0022-510x(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 28.Alagona G, Bella R, Ferri R, Carnemolla A, Pappalardo A, Costanzo E, et al. Transcranial magnetic stimulation in Alzheimer disease: motor cortex excitability and cognitive severity. Neurosci Lett. 2001;314:57–60. doi: 10.1016/s0304-3940(01)02288-1. [DOI] [PubMed] [Google Scholar]
- 29.Ferreri F, Pauri F, Pasqualetti P, Fini R, Dal Forno G, Rossini PM. Motor cortex excitability in Alzheimer’s disease: a transcranial magnetic stimulation study. Ann Neurol. 2003;53:102–108. doi: 10.1002/ana.10416. [DOI] [PubMed] [Google Scholar]
- 30.Alagona G, Ferri R, Pennisi G, Carnemolla A, Maci T, Domina E, et al. Motor cortex excitability in Alzheimer’s disease and in subcortical ischemic vascular dementia. Neurosci Lett. 2004;362:95–98. doi: 10.1016/j.neulet.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Inghilleri M, Conte A, Frasca V, Scaldaferri N, Gilio F, Santini M, et al. Altered response to rTMS in patients with Alzheimer’s disease. Clin Neurophysiol. 2006;117:103–109. doi: 10.1016/j.clinph.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Julkunen P, Jauhiainen AM, Westeren-Punnonen S, Pirinen E, Soininen H, Kononen M, et al. Navigated TMS combined with EEG in mild cognitive impairment and Alzheimer’s disease: a pilot study. J Neurosci Methods. 2008;172:270–276. doi: 10.1016/j.jneumeth.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Martorana A, Stefani A, Palmieri MG, Esposito Z, Bernardi G, Sancesario G, et al. L-dopa modulates motor cortex excitability in Alzheimer’s disease patients. J Neural Transm. 2008;115:1313–1319. doi: 10.1007/s00702-008-0082-z. [DOI] [PubMed] [Google Scholar]
- 34.Perretti A, Grossi D, Fragassi N, Lanzillo B, Nolano M, Pisacreta AI, et al. Evaluation of the motor cortex by magnetic stimulation in patients with Alzheimer disease. J Neurol Sci. 1996;135:31–37. doi: 10.1016/0022-510x(95)00244-v. [DOI] [PubMed] [Google Scholar]
- 35.Liepert J, Bar KJ, Meske U, Weiller C. Motor cortex disinhibition in Alzheimer’s disease. Clin Neurophysiol. 2001;112:1436–1441. doi: 10.1016/s1388-2457(01)00554-5. [DOI] [PubMed] [Google Scholar]
- 36.Pierantozzi M, Panella M, Palmieri MG, Koch G, Giordano A, Marciani MG, et al. Different TMS patterns of intracortical inhibition in early onset Alzheimer dementia and frontotemporal dementia. Clin Neurophysiol. 2004;115:2410–2418. doi: 10.1016/j.clinph.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 37.Battaglia F, Wang HY, Ghilardi MF, Gashi E, Quartarone A, Friedman E, et al. Cortical plasticity in Alzheimer’s disease in humans and rodents. Biol Psychiatry. 2007;62:1405–1412. doi: 10.1016/j.biopsych.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 38.Hallett M. Transcranial magnetic stimulation and the human brain. Nature. 2000;406:147–150. doi: 10.1038/35018000. [DOI] [PubMed] [Google Scholar]
- 39.Paulus W, Classen J, Cohen LG, Large CH, Di Lazzaro V, Nitsche M, et al. State of the art: Pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 2008;1:151–163. doi: 10.1016/j.brs.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Ziemann U. Pharmacology of TMS measures. In: Wassermann EM, Z U, Walsh V, Paus T, Lisanby S, editors. The Oxford Handbook of Transcranial Stimulation. Vol. 2008. New York: Oxford University Press Inc.; pp. 135–151. [Google Scholar]
- 41.Alberici A, Bonato C, Calabria M, Agosti C, Zanetti O, Miniussi C, et al. The contribution of TMS to frontotemporal dementia variants. Acta Neurol Scand. 2008;118:275–280. doi: 10.1111/j.1600-0404.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 42.Ziemann U, Paulus W, Nitsche MA, Pascual-Leone A, Byblow WD, Berardelli A, et al. Consensus: Motor cortex plasticity protocols. Brain Stimul. 2008;1:164–182. doi: 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Udupa K. Measurement and modulation of plasticity of the motor system in humans using transcranial magnetic stimulation. Motor Control. 2009;13:442–453. doi: 10.1123/mcj.13.4.442. [DOI] [PubMed] [Google Scholar]
- 44.Classen J, S S. Changes in TMS measures induced by repetitive TMS. In: Wassermann EM, Z U, Walsh V, Paus T, Lisanby S, editors. The Oxford Handbook of Transcranial Stimulation. Vol. 2008. New York: Oxford University Press Inc.; pp. 185–200. [Google Scholar]
- 45.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 46.Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, et al. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- 47.Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- 48.Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008;1:326–336. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 51.Sparing R, Mottaghy FM. Noninvasive brain stimulation with transcranial magnetic or direct current stimulation (TMS/tDCS)-From insights into human memory to therapy of its dysfunction. Methods. 2008;44:329–337. doi: 10.1016/j.ymeth.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Nitsche MA, Boggio PS, Fregni F, Pascual-Leone A. Treatment of depression with transcranial direct current stimulation (tDCS): a review. Exp Neurol. 2009;219:14–19. doi: 10.1016/j.expneurol.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 53.Slotema CW, Blom JD, Hoek HW, Sommer IE. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J Clin Psychiatry. 2010;71:873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- 54.O’Reardon JP, Solvason HB, Janicak PG, Sampson S, Isenberg KE, Nahas Z, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62:1208–1216. doi: 10.1016/j.biopsych.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 55.Cotelli M, Manenti R, Cappa SF, Geroldi C, Zanetti O, Rossini PM, et al. Effect of transcranial magnetic stimulation on action naming in patients with Alzheimer disease. Arch Neurol. 2006;63:1602–1604. doi: 10.1001/archneur.63.11.1602. [DOI] [PubMed] [Google Scholar]
- 56.Cotelli M, Manenti R, Cappa SF, Zanetti O, Miniussi C. Transcranial magnetic stimulation improves naming in Alzheimer disease patients at different stages of cognitive decline. Eur J Neurol. 2008;15:1286–1292. doi: 10.1111/j.1468-1331.2008.02202.x. [DOI] [PubMed] [Google Scholar]
- 57.Cotelli M, Calabria M, Manenti R, Rosini S, Zanetti O, Cappa SF, et al. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2010 doi: 10.1136/jnnp.2009.197848. [DOI] [PubMed] [Google Scholar]
- 58.Ferrucci R, Mameli F, Guidi I, Mrakic-Sposta S, Vergari M, Marceglia S, et al. Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology. 2008;71:493–498. doi: 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- 59.Boggio PS, Khoury LP, Martins DC, Martins OE, de Macedo EC, Fregni F. Temporal cortex direct current stimulation enhances performance on a visual recognition memory task in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80:444–447. doi: 10.1136/jnnp.2007.141853. [DOI] [PubMed] [Google Scholar]
- 60.Wagner T, Gangitano M, Romero R, Theoret H, Kobayashi M, Anschel D, et al. Intracranial measurement of current densities induced by transcranial magnetic stimulation in the human brain. Neurosci Lett. 2004;354:91–94. doi: 10.1016/s0304-3940(03)00861-9. [DOI] [PubMed] [Google Scholar]
- 61.Wagner T, Eden U, Fregni F, Valero-Cabre A, Ramos-Estebanez C, Pronio-Stelluto V, et al. Transcranial magnetic stimulation and brain atrophy: a computer-based human brain model study. Exp Brain Res. 2008;186:539–550. doi: 10.1007/s00221-007-1258-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72:1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 65.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 66.Devanand DP, Mikhno A, Pelton GH, Cuasay K, Pradhaban G, Dileep Kumar JS, et al. Pittsburgh compound B (11C-PIB) and fluorodeoxyglucose (18 F-FDG) PET in patients with Alzheimer disease, mild cognitive impairment, and healthy controls. J Geriatr Psychiatry Neurol. 2010;23:185–198. doi: 10.1177/0891988710363715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. FDG-PET studies in MCI and AD. Eur J Nucl Med Mol Imaging. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 69.Herholz K. Cerebral glucose metabolism in preclinical and prodromal Alzheimer’s disease. Expert Rev Neurother. 2010;10:1667–1673. doi: 10.1586/ern.10.136. [DOI] [PubMed] [Google Scholar]
- 70.Forsberg A, Engler H, Almkvist O, Blomquist G, Hagman G, Wall A, et al. PET imaging of amyloid deposition in patients with mild cognitive impairment. Neurobiol Aging. 2008;29:1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 71.Kemppainen NM, Aalto S, Karrasch M, Nagren K, Savisto N, Oikonen V, et al. Cognitive reserve hypothesis: Pittsburgh Compound B and fluorodeoxyglucose positron emission tomography in relation to education in mild Alzheimer’s disease. Ann Neurol. 2008;63:112–118. doi: 10.1002/ana.21212. [DOI] [PubMed] [Google Scholar]
- 72.Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, et al. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thut G, Ives JR, Kampmann F, Pastor MA, Pascual-Leone A. A new device and protocol for combining TMS and online recordings of EEG and evoked potentials. J Neurosci Methods. 2005;141:207–217. doi: 10.1016/j.jneumeth.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 74.Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clin Neurophysiol. 2006;117:1870–1875. doi: 10.1016/j.clinph.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 75.Thut G, Pascual-Leone A. Integrating TMS with EEG: How and what for? Brain Topogr. 2010;22:215–218. doi: 10.1007/s10548-009-0128-z. [DOI] [PubMed] [Google Scholar]
- 76.Jing H, Takigawa M. Observation of EEG coherence after repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:1620–1631. doi: 10.1016/s1388-2457(00)00357-6. [DOI] [PubMed] [Google Scholar]
- 77.Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–1285. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]
- 78.Oliviero A, Strens LH, Di Lazzaro V, Tonali PA, Brown P. Persistent effects of high frequency repetitive TMS on the coupling between motor areas in the human. Exp Brain Res. 2003;149:107–113. doi: 10.1007/s00221-002-1344-x. [DOI] [PubMed] [Google Scholar]
- 79.Schindler K, Nyffeler T, Wiest R, Hauf M, Mathis J, Hess Ch W, et al. Theta burst transcranial magnetic stimulation is associated with increased EEG synchronization in the stimulated relative to unstimulated cerebral hemisphere. Neurosci Lett. 2008;436:31–34. doi: 10.1016/j.neulet.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 80.Sameshima K, Baccala LA. Using partial directed coherence to describe neuronal ensemble interactions. J Neurosci Methods. 1999;94:93–103. doi: 10.1016/s0165-0270(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 81.Kaminski M, Ding M, Truccolo WA, Bressler SL. Evaluating causal relations in neural systems: granger causality, directed transfer function and statistical assessment of significance. Biol Cybern. 2001;85:145–157. doi: 10.1007/s004220000235. [DOI] [PubMed] [Google Scholar]
- 82.Kramer MA, Eden UT, Cash SS, Kolaczyk ED. Network inference with confidence from multivariate time series. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:061916. doi: 10.1103/PhysRevE.79.061916. [DOI] [PubMed] [Google Scholar]
- 83.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thut G, Pascual-Leone A. A review of combined TMS-EEG studies to characterize lasting effects of repetitive TMS and assess their usefulness in cognitive and clinical neuroscience. Brain Topogr. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586:5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Antal A, Chaieb L, Moliadze V, Monte-Silva K, Poreisz C, Thirugnanasambandam N, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain Stimul. 2010;3:230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 88.Hashimoto R, Hirata Y, Asada T, Yamashita F, Nemoto K, Mori T, et al. Effect of the brain-derived neurotrophic factor and the apolipoprotein E polymorphisms on disease progression in preclinical Alzheimer’s disease. Genes Brain Behav. 2009;8:43–52. doi: 10.1111/j.1601-183X.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- 89.Pivac N, Nikolac M, Nedic G, Mustapic M, Borovecki F, Hajnsek S, et al. Brain derived neurotrophic factor Val66Met polymorphism and psychotic symptoms in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2010 doi: 10.1016/j.pnpbp.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 90.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 91.Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- 92.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 93.Sando SB, Melquist S, Cannon A, Hutton ML, Sletvold O, Saltvedt I, et al. APOE epsilon 4 lowers age at onset and is a high risk factor for Alzheimer’s disease; a case control study from central Norway. BMC Neurol. 2008;8:9. doi: 10.1186/1471-2377-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62:1977–1983. doi: 10.1212/01.wnl.0000128091.92139.0f. [DOI] [PubMed] [Google Scholar]
- 95.Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, et al. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 96.Drzezga A, Riemenschneider M, Strassner B, Grimmer T, Peller M, Knoll A, et al. Cerebral glucose metabolism in patients with AD and different APOE genotypes. Neurology. 2005;64:102–107. doi: 10.1212/01.WNL.0000148478.39691.D3. [DOI] [PubMed] [Google Scholar]
- 97.Rabinovici GD, Roberson ED. Beyond diagnosis: what biomarkers are teaching us about the “bio”logy of Alzheimer disease. Ann Neurol. 2010;67:283–285. doi: 10.1002/ana.22020. [DOI] [PubMed] [Google Scholar]
- 98.Growdon JH, Locascio JJ, Corkin S, Gomez-Isla T, Hyman BT. Apolipoprotein E genotype does not influence rates of cognitive decline in Alzheimer’s disease. Neurology. 1996;47:444–448. doi: 10.1212/wnl.47.2.444. [DOI] [PubMed] [Google Scholar]
- 99.Murphy GM, Jr, Taylor J, Kraemer HC, Yesavage J, Tinklenberg JR. No association between apolipoprotein E epsilon 4 allele and rate of decline in Alzheimer’s disease. Am J Psychiatry. 1997;154:603–608. doi: 10.1176/ajp.154.5.603. [DOI] [PubMed] [Google Scholar]
- 100.Kleiman T, Zdanys K, Black B, Rightmer T, Grey M, Garman K, et al. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer’s disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006;22:73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]
- 101.Dal Forno G, Rasmusson DX, Brandt J, Carson KA, Brookmeyer R, Troncoso J, et al. Apolipoprotein E genotype and rate of decline in probable Alzheimer’s disease. Arch Neurol. 1996;53:345–350. doi: 10.1001/archneur.1996.00550040085017. [DOI] [PubMed] [Google Scholar]
- 102.Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, et al. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology. 1998;51:149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- 103.Martins CA, Oulhaj A, de Jager CA, Williams JH. APOE alleles predict the rate of cognitive decline in Alzheimer disease: a nonlinear model. Neurology. 2005;65:1888–1893. doi: 10.1212/01.wnl.0000188871.74093.12. [DOI] [PubMed] [Google Scholar]
- 104.Buchman AS, Boyle PA, Wilson RS, Beck TL, Kelly JF, Bennett DA. Apolipoprotein E e4 allele is associated with more rapid motor decline in older persons. Alzheimer Dis Assoc Disord. 2009;23:63–69. doi: 10.1097/wad.0b013e31818877b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wolk DA, Dickerson BC. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2010;107:10256–10261. doi: 10.1073/pnas.1001412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Crutch SJ, Rossor MN, Warrington EK. A novel technique for the quantitative assessment of apraxic deficits: application to individuals with mild cognitive impairment. J Neuropsychol. 2007;1:237–257. doi: 10.1348/174866407x209943. [DOI] [PubMed] [Google Scholar]
- 107.Bouwens SF, van Heugten CM, Aalten P, Wolfs CA, Baarends EM, van Menxel DA, et al. Relationship between measures of dementia severity and observation of daily life functioning as measured with the Assessment of Motor and Process Skills (AMPS) Dement Geriatr Cogn Disord. 2008;25:81–87. doi: 10.1159/000111694. [DOI] [PubMed] [Google Scholar]
- 108.Brown RM, Robertson EM, Press DZ. Sequence skill acquisition and off-line learning in normal aging. PLoS One. 2009;4:e6683. doi: 10.1371/journal.pone.0006683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 110.Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- 111.Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelly KM, Nadon NL, Morrison JH, Thibault O, Barnes CA, Blalock EM. The neurobiology of aging. Epilepsy Res. 2006;68(Suppl 1):S5–20. doi: 10.1016/j.eplepsyres.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 114.Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- 115.Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418:221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- 116.Bliss TV, Collingridge GL, Morris RG. Introduction. Long-term potentiation and structure of the issue. Philos Trans R Soc Lond B Biol Sci. 2003;358:607–611. doi: 10.1098/rstb.2003.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li S, Hong S, Shepardson NE, Walsh DM, Shankar GM, Selkoe D. Soluble oligomers of amyloid Beta protein facilitate hippocampal long-term depression by disrupting neuronal glutamate uptake. Neuron. 2009;62:788–801. doi: 10.1016/j.neuron.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 120.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex. 2008;18:563–570. doi: 10.1093/cercor/bhm087. [DOI] [PubMed] [Google Scholar]
- 121.Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C. Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr. 2010;22:294–306. doi: 10.1007/s10548-009-0084-7. [DOI] [PubMed] [Google Scholar]
- 122.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. Am J Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 123.Cano SJ, Posner HB, Moline ML, Hurt SW, Swartz J, Hsu T, et al. The ADAS-cog in Alzheimer’s disease clinical trials: psychometric evaluation of the sum and its parts. J Neurol Neurosurg Psychiatry. 2010;81:1363–1368. doi: 10.1136/jnnp.2009.204008. [DOI] [PubMed] [Google Scholar]
- 124.Silvanto J, Pascual-Leone A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 2008;21:1–10. doi: 10.1007/s10548-008-0067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Montagne-Larmurier A, Etard O, Razafimandimby A, Morello R, Dollfus S. Two-day treatment of auditory hallucinations by high frequency rTMS guided by cerebral imaging: a 6 month follow-up pilot study. Schizophr Res. 2009;113:77–83. doi: 10.1016/j.schres.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 126.Dollfus S, Larmurier-Montagne A, Razafimandimby A, Allio G, Membrey JM, Delcroix N, et al. Treatment of auditory hallucinations by combining high-frequency repetitive transcranial magnetic stimulation and functional magnetic resonance imaging. Schizophr Res. 2008;102:348–351. doi: 10.1016/j.schres.2008.04.012. [DOI] [PubMed] [Google Scholar]