Abstract
| Strategy, Management and Health Policy | ||||
|---|---|---|---|---|
| Venture Capital Enabling Technology | Preclinical Research | Preclinical Development Toxicology, Formulation Drug Delivery, Pharmacokinetics | Clinical Development Phases I-III Regulatory, Quality, Manufacturing | Postmarketing Phase IV |
Xanthine and adenosine derivatives, known to bind to recombinant rat A3 adenosine receptors stably expressed in Chinese hamster ovary cells, were characterized in a functional assay consisting of activation of A3 receptor-stimulated binding of [35S]GTPγS in rat RBL-2H3 cell membranes. 1,3-Dibutylxanthine-7-riboside-5′-N-methylcarboxamide (DBXRM, 7b), previously shown to inhibit adenylyl cyclase via rat A3 receptors with full efficacy, appeared to be a partial agonist at the rat A3 receptor of RBL-2H3 cells. Full agonists, such as Cl-IB-MECA or I-AB-MECA, were more potent and effective than the partial agonist DBXRM in causing desensitization of rat A3 receptors, as indicated by loss of [35S]GTPγS binding. At A1 receptors, antagonism of agonist-elicited inhibition of rat adipocyte adenylyl cyclase was observed for several xanthine-7-riboside derivatives that had been shown to be full agonists at rat A3 receptors. A new xanthine riboside (3′-deoxyDBXRM, 7c) was synthesized and found to be a partial agonist at rat A3 receptors and an antagonist at rat A1 receptors. Thus, it is possible for the same compound to stimulate one adenosine receptor subtype (A3) and block another subtype (A1) within the same species.
Keywords: xanthines, adenosine derivatives, nucleosides, adenylyl cyclase, guanine nucleotides
INTRODUCTION
Extracellular adenosine, acting via G protein-coupled receptors, is a ubiquitous modulator of cell function. Four subtypes of adenosine receptors (A1, A2A, A2B, and A3) have been shown to be pharmacologically and genetically distinct receptors [Jacobson et al., 1992; Olah and Stiles, 1995]. Activation of A1 receptors leads to inhibition of adenylyl cyclase and may also be coupled to other second messenger systems, including inhibition or stimulation of phosphoinositide turnover and activation of ion channels. Activation of the high-affinity A2A receptors and the low-affinity A2B receptors results in activation of adenylyl cyclase. The A3 receptor is the most recently discovered subtype [Ali et al., 1990; Meyerhof et al., 1991; Zhou et al., 1992]. It has been shown to be coupled to the inhibition of adenylyl cyclase when the receptor is expressed in Chinese hamster ovary (CHO) cells [Zhou et al., 1992] and to activation of phospholipase C (PLC) both in the rat RBL-2H3 mast cell line and in brain slices [Ali et al., 1990; Abbracchio et al., 1995; Shin et al., 1996].
In addition to possible involvement in the inflammatory response [Ramkumar et al., 1993], activation of A3 receptors is associated with cardiovascular actions. Fozard and Hannon [1994] attributed a xanthine-insensitive component of the hypotensive effects of adenosine agonists in the rat to activation of mast cell A3 receptors. Activation of A3 receptors has been shown to be involved in the cardioprotective effect of preconditioning by adenosine agonists [Liu et al., 1994; Strickler et al., 1996; Liang and Jacobson; 1998]. The occurrence of A3 receptors in the lung, brain, and testes also suggests that it may be important in the regulation of pulmonary, central nervous system (CNS), and reproductive functions [Jacobson et al., 1993; von Lubitz et al., 1994; Walker et al., 1997].
We studied in detail the structure–activity relationships for adenosine derivatives as agonists at A3 receptors [Jacobson, 1998]. An adenosine derivative substituted at N6- and 5′-positions, N6-(3-iodobenzyl)-5′-N-methylcarboxamidoadenosine (IB-MECA) [Gallo-Rodriguez et al., 1994], is 50-fold more selective for rat brain A3 receptors in binding experiments and is also selective in in vivo behavioral experiments. Even more highly selective A3 agonists, such as Cl-IB-MECA (Compound 1a), have been developed [Jacobson, 1998], but only recently were selective antagonists for A3 receptors reported [Jiang et al., 1997; Kim et al., 1996; Jacobson et al., 1997]. A selective A3 antagonist might prove to be anti-inflammatory [Ali et al., 1990; Walker et al., 1997] or cerebroprotective [von Lubitz et al., 1994], based on inference from studies with agonists. One initial approach to the design of A3 antagonists [Jacobson et al., 1995] was to truncate the ribose moiety of the structures of A3-selective agonists, such as 1a. Three such representative molecules are the 2′,3′-dideoxy adenosine derivative (2), the tetrahydrofuryl derivative (3), and the 9-methyladenine (4) (see Fig. 2 for structures). Unfortunately, such modifications resulted in the loss of potency and/or selectivity.
Fig. 2.
Adenosine, adenine, and xanthine derivatives studied as A3 receptor ligands.
The classical adenosine antagonists, the xanthines, display much weaker affinity at rat A3 receptors than at other adenosine receptor subtypes [Linden et al., 1993; Salvatore et al., 1993; Ji et al., 1994]. Numerous xanthines that are potent antagonists at rat, rabbit, and human A1 and A2 receptors only weakly displaced the binding of the radioligand [125I]APNEA (N6-[2-(4-amino-3-iodophenyl)ethyl]adenosine) from cloned rat A3 receptors even at concentrations in the 10−4 M range. The human and sheep A3 receptors are similar in the respect that many xanthines bind in the micromolar range. Thus, for xanthines, an unusually large species dependence of affinity at A3 receptors is apparent, perhaps even suggesting multiple subtypes of A3 receptors. The anionic xanthine BWA522 (5) (IABOPX, Fig. 2), which contains nonequivalent substitution at the xanthine N1 and N3 positions, was reported to have Ki values of 3 and 18 nM at sheep and human A3 receptors, respectively [Linden et al., 1993; Salvatore et al., 1993], and to antagonize A3 receptor activation in the rat [Fozard and Hannon, 1994]. Ji et al. [1994] found Compound 5 to be much weaker at rat A3 receptors (Ki 1.2 μM), and thus it is not a generally useful antagonist at this subtype. At bovine brain A1 receptors this xanthine is considerably more potent, with a Ki value of 37 nM [Linden et al., 1988]. In a study of structure–activity relationships (SAR) of xanthines at rat A3 receptors, the presence of a carboxylate group did not result in a significant enhancement of affinity, although, as previously observed [Kim et al., 1994a], an anionic group tended to diminish potency at A1 and A2A receptors. Two anionic 2-thioxanthines, 6a and 6b, were among the more potent competitive inhibitors of binding at rat A3 receptors, with Ki values of 9.4 and 6.8 μM. Several xanthine derivatives which displaced radioligand at concentrations of 1–10 μM failed to functionally antagonize the inhibition of adenylyl cyclase by rat A3 receptors in CHO cells.
We demonstrated that 1,3-dialkylxanthine-7-ribosides [Kim et al., 1994b] displayed greatly enhanced affinity compared to the parent xanthines at rat A3 receptors. 1,3-Dibutylxanthine-7-riboside-5′-N-methylcarboxamide, DBXRM (7b, Fig. 1), has a Ki value of 230 nM at recombinant rat A3 receptors and is 160-fold and >400-fold selective for A3 vs. rat A1 and A2A receptors, respectively. While the affinity of xanthine-7-ribosides increases compared to the parent xanthine, so does the efficacy as agonists at rat A3 receptors. Thus, 1,3-dibutylxanthine-7-riboside (DBXR, 7a) proved to be a partial agonist at this subtype, while DBXRM proved to be an agonist of full efficacy. For the present study, a new xanthine riboside (3′-deoxyDBXRM, 7c) was synthesized and found to be an antagonist at A1 receptors, while being a partial agonist at A3 receptors.
Fig. 1.

Compound 7c, synthesized by standard methods.
The present study explores the properties of adenine derivatives, xanthines, and xanthine-7-ribosides at adenosine receptors in both in binding and in functional assays. In the present study, agonist/antagonist activity was assessed in functional assays measuring stimulation of binding of [35S]GTPγS to A3 receptor coupled G-proteins. For A1 receptors, agonist/antagonist activity was assessed in a functional assay measuring inhibition of adenylyl cyclase in rat adipocyte membranes. In general, the xanthine-7-riboside ligands were partial or full agonists at A3 receptors, while they were partial agonists [IJzerman et al., 1994] or antagonists at A1 receptors.
MATERIALS AND METHODS
Chemistry
New compounds were characterized (and resonances assigned) by 300 MHz proton nuclear magnetic resonance spectroscopy using a Varian GEMINI-300 FT-NMR spectrometer. Unless noted, chemical shifts are expressed as ppm downfield from tetramethylsilane. Synthetic intermediates were characterized by chemical ionization mass spectrometry (NH3) on a JEOL SX102 mass spectrometer. High-resolution mass was determined using a VG7070F mass spectrometer in the electron impact mode. C, H, and N analyses were carried out by Atlantic Microlabs (Norcross, GA), and ±0.4% was acceptable. 1,3-Dibutylxanthine was prepared as described [Kim et al., 1994a].
7-(3-Deoxy-5-methylcarboxamido-β-D-ribofuranosyl)-1,3-dibutylxanthine (7c)
A mixture of 1,3-dibutylxanthine (8, Fig. 1, 0.11 g, 0.42 mmol), ammonium sulfate (catalytic amount), and HMDS (10 ml) was refluxed for 1 h under nitrogen. After the reaction mixture was concentrated to dryness, the residue (7-silylated dibutylxanthine, 9) was dissolved in dry acetonitrile (3 ml) and a solution of 1,2-di-O-acetyl-3-deoxy-5-methylcarbamoyl-D-ribofuranoside (10 [Jacobson et al., 1995], 0.1 g, 0.41 mmol) in dry acetonitrile (5 ml), potassium nonaflate (0.55 g, 1.63 mmol), and trichlorosilane (0.12 ml, 1.2 mmol) were added. The reaction mixture was refluxed for 24 h under nitrogen. After the reaction mixture was cooled, saturated NaHCO3 (50 ml) and chloroform (50 ml) were added and it was stirred for 10 min. Two layers separated and the aqueous layer was extracted with chloroform (3 × 30 ml). Organic layer and extracts were combined, washed with saturated NaHCO3, brine, dried over anhydrous MgSO4, filtered, and concentrated to dryness. The residue was purified on a preparative TLC (chloroform-methanol, 20:1 → hexanes-ethyl acetate, 1:1) to give 7-(2-O-acetyl-3-deoxy-5-methylcarbamoyl-β-D-ribofuranosyl)-1,3-dibutylxanthine (11, 69 mg, 37%) as a semisolid, mass (EI) m/z 449 (M+). 1H NMR (DMSO-d6) 0.89 (m, 6 H, CH3), 1.30 (m, 4 H, CH2), 1.50 and 1.62 (each: m, 2 H, CH2), 2.10 (s, 3 H, OAc), 2.30 and 2.45 (each: m, 1 H), 2.63 (d, J -= 4.4 Hz, 3 H, NHCH3), 3.80 and 3.98 (each: m, 2 H, N-CH2Pr), 4.68 (m, 1 H), 5.50 (m, 1 H), 6.38 (s, 1 H), 8.15 (br s, 1 H), 8.61 (s, 1 H, H-8).
A mixture of 7-(2-O-acetyl-3-deoxy-5-methylcarbamoyl-β-D-ribofuranosyl)-1,3-dibutylxanthine (54 mg, 0.12 mmol) and methanolic ammonia (saturated at 0°C, 30 ml) was stirred at room temperature overnight. After the reaction mixture was concentrated to dryness, the residue was purified by preparative TLC (chloroform-methanol, 10:1) to give Compound 7c (40.2 mg, 84%) as a foam. Mass (CI NH3) m/z 408 (M+ + 1). 1H NMR (DMSO-d6) 0.89 (m, 6 H, CH3), 1.30 (m, 4 H, CH2), 1.50 and 1.62 (each: m, 2 H, CH2), 2.10 (m, 2 H), 2.63 (d, J = 4.6 Hz, 3 H, NHCH3), 3.87 and 3.99 (each: t, J = 7.4 Hz, 2 H, N-CH2Pr), 4.47 (s, 1 H), 4.70 (pseudo t, J = 9 and 6.9 Hz, 1 H), 5.82 (d, J = 3.9 Hz, 1 H, OH), 6.17 (s, 1 H), 8.22 (br s, 1 H, NH), 8.71 (s, 1 H, H-8). CHN analysis.
Biological Methods
Materials
F-12 (Ham’s) medium, fetal bovine serum (FBS) and penicillin/streptomycin were from Gibco BRL (Gaithersburg, MD). [125I]AB-MECA (1000 Ci/mmol) and [35S]guanosine 5′-(γ-thio)triphosphate (1,000–1,500 Ci/mmol) were from DuPont NEN (Boston, MA). Adenosine deaminase (ADA) was from Boehringer Mannheim (In-dianapolis, IN). BSA was from Sigma Chemical Co. (St. Louis, MO). All other materials were from standard local sources and of the highest grade commercially available.
Cell culture and membrane preparation
Preparation of membranes
RBL-2H3 cells (gift of Dr. Michael Beaven, NIH) were cultured in Earl’s modified Eagles medium supplemented with 10% fetal bovine serum. Cells were maintained as monolayer cultures. Cells were treated with various chemicals in the presence of 0.2 U/ml adenosine deaminase for 24 h. Cells were washed twice with 10 ml ice-cold phosphate buffered saline, lysed in lysis buffer (10 mM Tris.HCl buffer, pH 7.4, containing 2 mM MgCl2 and 0.5 mM EDTA), and homogenized in a Polytron homogenizer. The crude membranes were prepared by centrifuging the homogenate at 1,000g for 10 min followed by centrifugation of the supernatant at 40,000g for 15 min. The pellet was washed once with the lysis buffer and recentrifuged at 40,000g for 15 min. The final pellets were resuspended in 50 mM Tris.HCl buffer, pH 7.4, containing 10 mM MgCl2 and 0.1 mM EDTA and stored at −70°C.
Receptor binding
Determination of [125I]AB-MECA binding
Determination of A3 adenosine receptor binding to RBL-2H3 cell membranes was carried out using [125I]AB-MECA by the method of Olah et al. [1994]. Briefly, aliquots of crude RBL-2H3 membranes (approximately 40 μg protein/tube) were incubated with 0.3 to 10 nM (0.5 nM in the competition binding assay) [125I]AB-MECA, 10 mM MgCl2, 2 units/ml adenosine deaminase, 50 mM Tris.HCl (pH 7.4) at 37°C for 60 min. The total volume of the reaction mixture was 125 μl. Bound and free ligands were separated by rapid filtration of the reaction mixture through Whatman GF/B glass filters. The filters were immediately washed with two 5 ml-portions of ice-cold 50 mM Tris.HCl buffer (pH 7.4). The radioactivity bound to the filters was determined in a Beckman γ counter. Specific binding was defined as the amount of the radioligand bound in the absence of competing ligand minus the amount of that bound in the presence of 100 μM NECA. Ki-values were calculated according to Cheng and Prusoff [1973], using the Kd for [125I]AB-MECA binding of 2.60 nM as determined in RBL-2H3 cell membranes.
Functional assays
Effects on adenylyl cyclase
Adenylyl cyclase was measured as previously described in A1 receptor-containing membranes of rat adipocytes [Ukena et al., 1986].
Determination of [35S]GTPγS binding
[35S]GTPγS binding was determined by the method of Lorenzen et al. [1996]. The incubation mixture contained in a total volume of 125 μl, 50 mM Tris.HCl (pH 7.4), 1 mM EDTA, 10 mM MgCl2, 10 μM guanosine 5′-diphosphate, 1 mM dithiothreitol, 100 mM NaCl, 0.2 units/ml adenosine deaminase, 0.16 nM [35S]GTPγS (about 50,000 cpm), and 0.5% BSA. The incubation was carried out at 37°C. The membranes were preincubated with the above-mentioned assay mixture for 1 h and further incubated for 1 h after the addition of [35S]GTPγS. Incubations were terminated by rapid filtration of the samples through glass fiber filters (Whatman GF/B), followed by two 5-ml washes of the same buffer. After transferring the filters into a vial containing 3 ml of scintillation cocktail, the radioactivity was determined in a scintillation counter.
Determination of protein concentrations
Protein concentrations were determined by the method of Bradford [1976] using BSA as standard.
Data analysis
Analyses of saturation binding assays and concentration–response curves were carried out using the GraphPad Prism (GraphPad Software, San Diego, CA). Comparisons between groups were carried out using the unpaired Student’s t-test.
RESULTS
Chemical Synthesis
Compound 7c was synthesized by standard methods (Fig. 1) used previously to synthesize 7a and 7b [Jacobson et al., 1995; Kim et al., 1994b].
Binding Affinity for Rat A3 Receptors
The affinity of four nucleosides, two adenine (Compounds 1a and 1b, Fig. 2) and two xanthine riboside derivatives (Compounds 7a and 7b), was measured in radioligand binding experiments at rat A3 receptors in RBL-2H3 cell membranes (Fig. 3). Displacement of binding of the radioligand [125I]AB-MECA was monophasic, as indicated by Hill coefficients of approximately 1. As anticipated from previous binding experiments utilizing recombinant rat A3 receptors [Gallo-Rodriguez et al., 1994; Kim et al., 1994b], Cl-IB-MECA (Ki = 4.9 nM) and I-AB-MECA (Ki = 5.8 nM) were considerably more potent than DBXRM (Ki = 0.60 μM) and DBXR (Ki = 6.6 μM). Ki values for 3′-deoxyDBXRM, 7c, were determined to be: 3.57 ± 0.99 at rat cortical A, receptors (vs. [3H]R-PIA), 30.8 ± 4.3 at rat striatal A2A receptors (vs. [3H]CGS21680), and 37.6 ± 6.2 at recombinant rat A3 receptors as described [Olah et al., 1994].
Fig. 3.
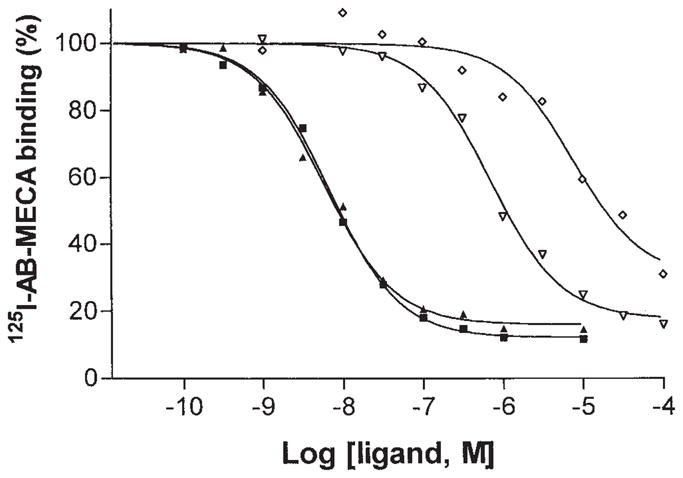
Agonist competition for [125I]AB-MECA binding in RBL-2H3 cell membranes. Ki values for I-AB-MECA (■), Cl-IB-MECA (▲), DBXRM (▽), and DBXR (◇) were 5.8 nM, 4.9 nM, 0.6 μM, and 6.6 ± 0.6 μM, respectively.
Stimulation of [35S]GTPγS Binding via A3 Receptors
A functional assay based on stimulation of binding of [35S]GTPγS to G-proteins [Lorenzen et al., 1996] in RBL-2H3 cell membranes was used. Stimulation of [35S]GTPγS binding by Cl-IB-MECA and other A3 agonists at human A3 receptors expressed in HEK-293 cell membranes has been shown to be dependent on activation of an A3 receptor [Jacobson et al., 1997]. In the present study, binding of [35S]GTPγS was measured in response to five nucleosides, including two adenosine derivatives, known to be A3 agonists, Cl-IB-MECA and I-AB-MECA (1a and 1b), and three xanthine riboside derivatives, DBXR, DBXRM, and 3′-deoxy-DBXRM (7a–c) (Fig. 4). Cl-IB-MECA and I-AB-MECA elicited maximal agonist effects at concentrations ≥10−5 M. The response to DBXRM at >50 μM exceeded 50% of the maximal stimulation of binding, but full agonist stimulation was not reached at the highest concentration examined (100 μM). DBXR and 3′-dDBXRM (Fig. 4) elicited only very small stimulation of binding of [35S]GTPγS, corresponding to ~5% and 10% of maximal, respectively. Thus, the latter two xanthine ribosides appear to be partial agonists of low efficacy. For DBXR, this is consistent with results obtained on stimulation of PLC in striatal slices [data not shown, Abbracchio et al., 1995].
Fig. 4.
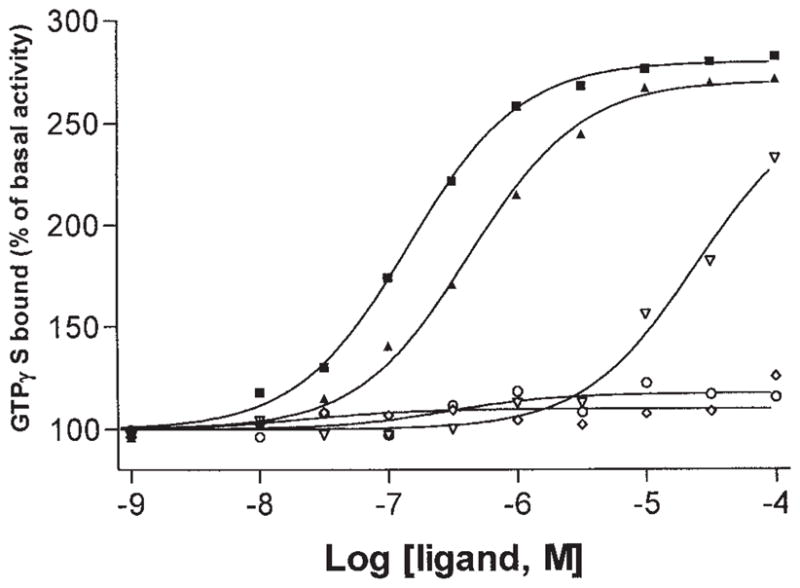
Stimulation of [35S]GTPγS binding in RBL-2H3 cell membranes by various A3 adenosine agonists: I-AB-MECA (■), Cl-IB-MECA (▲), DBXRM (▽), DBXR (◇), and 3′-deoxy DBXRM (○). Data are means from two experiments performed in duplicate. Basal activity was 32.4 ± 1.1 pmol/mg protein.
The effect of DBXR on Cl-IB-MECA-stimulated [35S]GTPγS-binding in RBL-2H3 cell membranes was examined (Fig. 5). Membranes were coincubated with DBXR, and then the ability of the full agonist Cl-IB-MECA to stimulate binding of [35S]GTPγS was measured. At relatively low concentrations of Cl-IB-MECA (<10−7 M), the functional activity was slightly augmented by the presence of 100 μM DBXR. At high concentrations of Cl-IB-MECA (≥10−6 M) the maximal effect of Cl-IB-MECA appeared slightly diminished, but this difference was not statistically significant.
Fig. 5.

Inhibition of Cl-IB-MECA-stimulated [35S]GTPγS binding by DBXR in RBL-2H3 cell membranes. Stimulation of [35S]GTPγS binding by Cl-IB-MECA was determined in the absence (■) and presence (▲) of 100 μM DBXR. Data are means from two experiments performed in triplicate. Basal activity was 32.7 ± 1.4 pmol/mg protein.
The [35S]GTPγS binding assay was also used to assess the effects of A3 agonists on receptor desensitization in RBL-2H3 cells. Preincubation with the full agonist Cl-IB-MECA caused a substantial desensitization of the functional response to I-AB-MECA (Fig. 6A). Preincubation with 10 nM and 100 nM Cl-IB-MECA desensitized the maximal response by approximately 60% and 90%, respectively. DBXRM caused a similar desensitization (Fig. 6B), but higher concentrations were required. Preincubation with 0.1 μM and 1.0 μM DBXRM desensitized the maximal response by approximately 20% and 40%, respectively.
Fig. 6.

(A) Desensitization of A3 adenosine receptor by Cl-IB-MECA in RBL-2H3 cell membranes. RBL-2H3 cells were incubated with 0 (squares), 10 (▲) or 100 (▼) nM of Cl-IB-MECA for 24 h, and stimulation of [35S]GTPγS binding by Cl-IB-MECA was determined in crude membranes prepared as described in Materials and Methods. (B) Desensitization of A3 adenosine receptor by DBXRM in RBL-2H3 membranes. RBL-2H3 cells were incubated with 0 (squares), 0.1 (▼) or 1.0 (▲) μM of DBXRM for 24 h, and stimulation of [35S]GTPγS binding by Cl-IB-MECA was determined in crude membranes prepared as described in Materials and Methods.
Curiously, although this xanthine riboside has only low agonist efficacy in stimulating the binding of [35S]GTPγS in RBL-2H3 cell membranes, DBXR at high micromolar concentrations elicited a substantial desensitization of the response to Cl-IB-MECA. Thus, preincubation with 10 μM and 100 μM DBXR depressed the maximal response to Cl-IB-MECA by approximately 30% and 70%, respectively (Fig. 7A). Saturation of binding of [125I]AB-MECA was determined following desensitization (Table I). This desensitization altered the density of binding (Bmax), but not the affinity of the radioligand for the remaining sites (Kd).
Fig. 7.

(A) Desensitization of A3 adenosine receptor by DBXR in RBL-2H3 cell membranes. RBL-2H3 cells were incubated with 0 (■), 10 (▲) or 100 (▼) μM of DBXR for 24 h, and stimulation of [35S]GTPγS binding by Cl-IB-MECA were determined in crude membranes prepared as described in Materials and Methods. Basal activities for control, 10, and 100 mM DBXR-treated groups were 33.0 ± 1.0, 32.9 ± 1.9, and 34.6 ± 1.1 pmol/mg protein, respectively. (B) Inhibition of Cl-IB-MECA-induced desensitization of A3 adenosine receptor in RBL-2H3 cells. RBL-2H3 cells were incubated alone (■) or in the presence of: 1 μM Cl-IB-MECA (▼), 100 μM DBXR (▲), or 1 μM Cl-IB-MECA plus 100 μM DBXR (◆) for 24 h. Stimulation of [35S]GTPγS binding by Cl-IB-MECA was then determined in crude membranes made from each treated cell preparation as described in Materials and Methods. Basal activities were (all pmol/mg protein): For the control group (30.7 ± 3.3), and for groups treated with Cl-IB-MECA (34.8 ± 1.2), DBXR (41.7 ± 1.2), and both Cl-IB-MECA and DBXR (40.1 ± 2.0).
TABLE 1.
Change in Radioligand Saturation Binding Parameters at A3 Adenosine Receptors in RBL-2H3 Cell Membranes Following Prolonged Exposure to DBXR, 7aa
| Pre-treatment with DBXR (μM) | Kd (nM) | Bmax (fmol/mg protein) |
|---|---|---|
| 0 | 2.60 ± 0.31 | 358 ± 16 |
| 10 | 1.71 ± 0.72 | 197 ± 28* |
| 100 | 2.67 ± 0.26 | 96 ± 4* |
RBL-2H3 cell membranes were incubated with DBXR for 24 hrs, and saturation binding of [125I]AB-MECA were determined (n = 3). Crude membranes prepared as described in the Materials and Methods.
p < 0.05.
The ability of DBXR, as a partial agonist, to blunt the desensitization elicited by a full agonist, Cl-IB-MECA, was examined (Fig. 7B). Preincubation with a concentration of 1 μM of Cl-IB-MECA caused a nearly complete desensitization of the binding of [35S]GTPγS in RBL-2H3 cell membranes elicited later by Cl-IB-MECA. However, preincubation with the same concentration of Cl-IB-MECA in the presence of 100 μM DBXR resulted in only 80% desensitization of the maximal response.
Inhibition of Adenylyl Cyclase via A1 Receptors
At rat A1 receptors, antagonism of R-PIA-elicited inhibition of adipocyte adenylyl cyclase was observed for several xanthine-7-riboside derivatives that had been shown to be full agonists at rat A3 receptors. The KB values of DBXRM (7b) and its 3′-deoxy analog, 7c (Fig. 8), vs. the A1-agonist elicited inhibition of adenylyl cyclase in rat adipocytes were 12.5 ± 0.6 and 3.12 ± 1.18 μM, respectively (n = 3).
Fig. 8.

Antagonism by the xanthine-7-riboside derivative 3′-deoxy-DBXRM (7c) of the inhibition by R-PIA of isoproterenol-stimulated adenylyl cyclase of rat adipocyte membranes. Basal enzyme activity was 120 pmol cyclic AMP per min per mg protein. A representative determination is shown, in which the IC50 values for R-PIA for inibition of isoproterenol (10 μM)-stimulated adenylyl cyclase activity were 22 nM in the absence (○) and 68 nM in the presence of 10 μM 7c (■).
DISCUSSION
A3 receptors are involved in a variety of physiological functions and agonists/antagonists could have therapeutic applications [Jacobson, 1998]. Potent agonists have been developed.
In light of the tremendous species differences for antagonists at A3 receptors, the development of antagonists of general applicability across species remains a challenge. At the human A3 receptor, effective and selective antagonists have been reported [Jiang et al., 1997; Jacobson et al., 1997]. Such antagonists might be suitable for therapeutic needs; however, antagonists selective in the rat are still needed as research tools. Ideally, selectivity across multiple species would permit the required preclinical research to be carried out in laboratory animals prior to human trials with the same compound.
The present study principally utilized stimulation of binding of [35S]GTPγS as a functional activity at A3 receptors. This response was also used to study desensitization. We verified that Compounds 1a and 1b act as full agonists.
At sheep A3 receptors, the xanthine BWA 1433 was found to antagonize the effects of an adenosine agonist [Linden et al., 1993]. However, xanthine-7-ribosides have been explored as partial agonists at rat A1 receptors [IJzerman et al., 1994] and in preliminary pharmacological characterization appeared to be either partial or full agonists at rat A3 receptors [Kim et al., 1994b]. We synthesized a novel xanthine-7-riboside (7c) in an effort to reduce the efficacy of 7b, the removal of the 3′-hydroxyl group potentially leading to an A3 receptor-selective antagonist. Evidently the efficacy at rat A3 receptors was diminished by this structural change (Fig. 4); however, it is nevertheless not a pure antagonist. Among the three xanthine-7-ribosides examined in the [35S]GTP-γ-S binding assay, either robust (in the A3 receptor-selective 7b) or weak partial agonism (7a and 7c) was observed. Compound 7b appeared to be a full agonist in inhibiting adenylyl cyclase via the rat A3 receptor [Kim et al., 1994b]. As agonists, both 7a and 7b caused desensitization of the rat A3 receptor. In contrast, the xanthine ribosides appear to be effective antagonists at the rat A1 receptor (Fig. 8).
The observation that the rat A3 receptor is easily activated by a wide range of substances, including xanthine-7-riboside derivatives that are adenosine antagonists at the human A3 or the rat A1 receptor, may reflect a fundamental structural characteristic of the receptor. The energy required to activate the rat A3 receptor upon ligand binding may be considerably less than required for activation of the other adenosine receptors, i.e., the native state of the rat A3 receptor may be closer in conformation to the activated state. Alternatively, it has been speculated that the rat and human A3 receptors may be distinct subtypes, rather than species homologs of the same receptor [Jacobson, 1998].
The present study has led to the identification of several A3 receptor partial agonists. Partial agonists at A3 receptors may be useful as pharmacological probes, and potentially as therapeutic agents. In theory, a partial agonist may combine certain pharmacological properties of agonists and antagonists; for example, they might modestly stimulate the receptor, without producing desensitization. Conversely, while producing a sub-threshold biological response, they might downregulate a receptor. This study has identified several xanthine-riboside derivatives 7a [Kim et al., 1994b] and 7c as partial A3 receptor agonists. These compounds were not able to fully activate the receptor to couple to the G-protein, as indicated in GTPγS binding in RBL-2H3 cell membranes. However, 7a caused a partial receptor desensitization and blocked the ability of full agonists to desensitize the receptor. In contrast to the partial effects of preincubation with 7a, preincubation with the full agonist Cl-IB-MECA caused a substantial desensitization of the functional response to I-AB-MECA.
The present study has highlighted the contrast in functional structure activity requirements between A3 and other adenosine receptor subtypes. Thus, it is possible for the same compound, e.g., 7c, to stimulate one adenosine receptor subtype (A3) and block another subtype (A1) within the same species.
Abbreviations
- [125I]AB-MECA
N6-(4-amino-3-iodobenzyl)-adenosine-5′-N-methyluronamide
- [125I]APNEA
N6-[2-(4-amino-3-iodophenyl)ethyl]adenosine
- BSA
bovine serum albumin
- BWA522
3-(4-aminobenzyl)-8-[4-[[[carboxy]methyl]oxy]phenyl]-1-propylxanthine
- DBXR
1,3-dibutylxanthine-7-riboside
- DBXRM
1,3-dibutylxanthine-7-riboside-5′-N-methylcarboxamide
- DMF
N,N-dimethylformamide
- DMSO
dimethylsulfoxide
- EDAC
1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride
- FBS
fetal bovine serum
- HMDS
hexamethyldisilazane
- IABOPX
see BWA522
- NECA
5′-N-ethylcarboxamidoadenosine
- R-PIA
R-N6-phenylisopropyladenosine
- PLC
phospholipase C
- Tris
tris(hydroxymethyl)aminomethane
References
- Abbracchio MP, Brambilla R, Ceruti S, Kim HO, von Lubitz DKJE, Jacobson KA, Cattabeni F. G-protein-dependent activation of phospholipase C by adenosine A3 receptors in rat brain. Mol Pharmacol. 1995;48:1038–1045. [PubMed] [Google Scholar]
- Ali H, Cunha-Melo JR, Saul WF, Beavan MF. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen stimulated RBL-2H3 cells. J Biol Chem. 1990;265:745–753. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng YC, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzyme reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Fozard JR, Hannon JP. BW-A522 blocks adenosine A3 receptor-mediated hypotensive responses in the rat. Eur J Pharmacol. 1994;252:R5–R6. doi: 10.1016/0014-2999(94)90604-1. [DOI] [PubMed] [Google Scholar]
- Gallo-Rodriguez C, Ji X-D, Melman N, Siegman BD, Sanders LH, Orlina J, Pu Q-L, Olah ME, van Galen PJM, Stiles GL, Jacobson KA. Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J Med Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IJzerman AP, van der Wenden EM, von Frijtag Drabbe Kunzel JK, Mathot RA, Danhof M, Borea PA, Varani K. Partial agonism of theophylline-7-riboside on adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:638–645. doi: 10.1007/BF00169369. [DOI] [PubMed] [Google Scholar]
- Jacobson KA. A3 adenosine receptors: Novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998;19:184–191. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, van Galen PJM, Williams M. Perspective. Adenosine receptors: Pharmacology, structure-activity relationships and therapeutic potential. J Med Chem. 1992;35:407–422. doi: 10.1021/jm00081a001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Nikodijevic O, Shi D, Gallo-Rodriguez C, Olah ME, Stiles GL, Daly JW. A role for central A3-adenosine receptors: Mediation of behavioral depressant effects. FEBS Lett. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Siddiqi SM, Olah ME, Ji Xd, Melman N, Bellamkonda K, Meshulam Y, Stiles GL, Kim HO. Structure-activity relationships of 9-alkyladenine and ribose-modified adenosine derivatives at rat A3 adenosine receptors. J Med Chem. 1995;38:1720–1735. doi: 10.1021/jm00010a017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Park KS, Jiang J-l, Kim Y-C, Olah ME, Stiles GL, Ji Xd. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997;36:1157–1165. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X-d, von Lubitz D, Olah ME, Stiles GL, Jacobson KA. Species differences in ligand affinity at central A3-adenosine receptors. Drug Dev Res. 1994;33:51–59. doi: 10.1002/ddr.430330109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J-l, van Rhee AM, Chang L, Patchornik A, Evans P, Melman N, Jacobson KA. Structure activity relationships of 4-phenylethynyl-6-phenyl-1,4-dihydropyridines as highly selective A3 adenosine receptor antagonists. J Med Chem. 1997;40:2596–2608. doi: 10.1021/jm970091j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji X-d, Melman N, Olah ME, Stiles GL, Jacobson KA. Structure activity relationships of 1,3-dialkylxanthine derivatives at rat A3-adenosine receptors. J Med Chem. 1994a;37:3373–3382. doi: 10.1021/jm00046a022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HO, Ji X-d, Melman N, Olah ME, Stiles GL, Jacobson KA. Selective ligands for rat A3-adenosine receptors: Structure-activity relationships of 1,3-dialkylxanthine-7-riboside derivatives. J Med Chem. 1994b;37:4020–4030. doi: 10.1021/jm00049a021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-C, Ji Xd, Jacobson KA. Derivatives of the triazoloquinazoline adenosine antagonist (CGS15943) are selective for the human A3 receptor subtype. J Med Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang BT, Jacobson KA. A physiological role of the adenosine A3 receptor: Sustained cardioprotection. Proc Natl Acad Sci USA. 1998;95:6995–6999. doi: 10.1073/pnas.95.12.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden J, Patel A, Earl CQ, Craig RH, Daluge SM. 125I-labeled 8-phenylxanthine derivatives: Antagonist radioligands for adenosine A1 receptors. J Med Chem. 1988;31:745–751. doi: 10.1021/jm00399a010. [DOI] [PubMed] [Google Scholar]
- Linden J, Taylor HE, Robeva AS, Tucker AL, Stehle JH, Rivkees SA, Fink JS, Reppert SM. Molecular cloning and functional expression of a sheep A3 adenosine receptor with widespread tissue distribution. Mol Pharmacol. 1993;44:524–532. [PubMed] [Google Scholar]
- Liu GS, Richards RA, Olsson RA, Mullane K, Walsh RS, Downey JM. Evidence that the adenosine A3 receptor can mediate preconditioning’s protection in isolated rabbit hearts. Cardiovasc Res. 1994;28:1057–1061. doi: 10.1093/cvr/28.7.1057. [DOI] [PubMed] [Google Scholar]
- Lorenzen A, Guerra L, Vogt H, Schwabe U. Interaction of full and partial agonists of the A1 adenosine receptor with receptor/G protein complexes in rat-brain membranes. Mol Pharmacol. 1996;49:915–926. [PubMed] [Google Scholar]
- Meyerhof W, Müller-Brechlin R, Richter D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991;284:155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- Olah ME, Stiles GL. Adenosine receptor subtypes—Characterization and therapeutic regulation. Annu Rev Pharmacol Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- Olah ME, Gallo-Rodriguez C, Jacobson KA, Stiles GL. [125I]AB-MECA, a high affinity radioligand for the rat A3 adenosine receptor. Mol Pharmacol. 1994;45:978–982. [PMC free article] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3AR is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–16890. [PubMed] [Google Scholar]
- Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci. 1993;90:10365–10369. doi: 10.1073/pnas.90.21.10365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y, Daly JW, Jacobson KA. Activation of phosphoinositide breakdown in a rat RBL-2H3 mast cell line by adenosine analogues: Lack of correlation with affinity for A3-adenosine receptor. Drug Dev Res. 1996;39:36–46. doi: 10.1002/(sici)1098-2299(19960901)39:1<36::aid-ddr5>3.0.co;2-l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickler J, Jacobson KA, Liang BT. Direct preconditioning of cultured chick ventricular myocytes: Novel functions of cardiac adenosine A2A and A3 receptors. J Clin Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukena D, Daly JW, Kirk KL, Jacobson KA. Functionalized congeners of 1,3-dipropyl-8-phenylxanthine: Potent antagonists for adenosine receptors that modulate membrane adenylate cyclase in pheochromocytoma cells, platelets and fat cells. Life Sci. 1986;38:797–807. doi: 10.1016/0024-3205(86)90596-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Lubitz DKJE, Lin RC-S, Popik P, Carter MF, Jacobson KA. Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BA, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, Bai TR. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol. 1997;16:531–737. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- Zhou QY, Li CY, Olah ME, Johnson RA, Stiles GL, Civelli O. Molecular cloning and characterization of an adenosine receptor—The A3 adenosine receptor. Proc Natl Acad Sci USA. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]