Abstract
Low-frequency repetitive transcranial magnetic stimulation (rTMS) can exert local and inter-hemispheric neuromodulatory effects on cortical excitability. These physiologic effects can translate into changes in motor behavior, and may offer valuable therapeutic interventions in recovery from stroke. Neuronavigated TMS can maximize accurate and consistent targeting of a given cortical region, but is a lot more involved that conventional TMS. We aimed to assess whether neuronavigation enhances the physiologic and behavioral effects of low-frequency rTMS. Ten healthy subjects underwent two experimental sessions during which they received 1600 pulses of either navigated or non-navigated 1 Hz rTMS at 90% of the resting motor threshold (RMT) intensity over the motor cortical representation for left first dorsal interosseous (FDI) muscle. We compared the effects of navigated and non-navigated rTMS on motor-evoked potentials (MEPs) to single-pulse TMS, intracortical inhibition (ICI) and intracortical facilitation (ICF) by paired-pulse TMS, and performance in various behavioral tasks (index finger tapping, simple reaction time and grip strength tasks). Following navigated rTMS, the amplitude of MEPs elicited from the contralateral (unstimulated) motor cortex was significantly increased, and was associated with an increase in ICF and a trend to decrease in ICI. In contrast, non-navigated rTMS elicited nonsignificant changes, most prominently ipsilateral to rTMS. Behaviorally, navigated rTMS significantly improved reaction time RT and pinch force with the hand ipsilateral to stimulation. Non-navigated rTMS lead to similar behavioral trends, although the effects did not reach significance. In summary, navigated rTMS leads to more robust modulation of the contralateral (unstimulated) hemisphere resulting in physiologic and behavioral effects. Our findings highlight the spatial specificity of inter-hemispheric TMS effects, illustrate the superiority of navigated rTMS for certain applications, and have implications for therapeutic applications of rTMS.
Keywords: Transcranial magnetic stimulation, Navigated brain stimulation, Motor cortex, Cortical excitability, Motor-evoked potentials, Silent period and paired-pulse stimulation
Introduction
Real-time visualization and feedback of coil position provided by neuronavigation systems can lead to superior targeting and stabilization of stimulus delivery relative to the traditional methods of transcranial magnetic stimulation (TMS) (Julkunen et al. 2008a, b; Säisänen et al. 2008). Yet, it is unclear whether neuronavigation translates into more effective neuromodulation of local and distant (e.g. transcallosal) cortical excitability and in greater behavioral effects.
Navigated brain stimulation (NBS) can reportedly achieve sub-millimeter precision (Julkunen et al. 2008a, b). However, when TMS is applied to the motor cortex, it is important to remember that motor evoked potentials (MEP) are inherently variable (Barker et al. 1985) and it is not clear how much of the variability can be explained by subtle variations in coil position and trajectory (Kiers et al. 1998; Devanne et al. 1997). Typically MEP onset latency is relatively stable, but amplitude can have high variability (Kiers et al. 1998) to the point of somewhat limiting their clinical utility (Pascual-Leone et al. 1998; Wassermann 2002; Kobayashi and Pascual-Leone 2003). Nonetheless, minimizing the physical variation in stimulus delivery and maximizing the repetition of optimally targeted stimulation leads to greater stability of MEP amplitude and more consistent modulation of local cortical excitability (Gugino et al. 2001; Noirhomme et al. 2004; Hannula et al. 2005; Julkunen et al. 2008a, b; Schmidt et al. 2009). In the present study we aimed to assess the utility of neuronavigation on inter-hemispheric effects of rTMS to primary motor cortex.
Application of 1-Hz rTMS to the hand area in M1 exerts inter-hemispheric effects and changes excitability and blood flow in the contralateral (unstimulated) primary motor cortex. These distant effects can modulate motor behavior ipsilateral to the site of stimulation (Paus et al. 1998; Kobayashi et al. 2003; Kathleen et al. 2009). Such inter-hemispheric effects might offer a valuable therapeutic approach for promotion of function after stroke (Mansur et al. 2005; Takeuchi et al. 2005; Hummel and Cohen 2005; Bernad and Doyon 2008). However, it is unknown whether neuronavigation can make such inter-hemispheric effects more robust.
Materials and Methods
Subjects and Study Design
Ten healthy subjects (6 males and 4 females, mean 26 years, age range 22–32 years), with no psychiatric, neurologic, or active medical history, and with no contraindications to TMS (Rossi et al. 2009) were enrolled in the study. The study was approved by the local Institutional Review Board and each subject gave written informed consent. All subjects were right handed according to the Edinburg Handedness Scale (Oldfield 1971).
Using a within-subjects, repeated measures, cross-over design, subjects completed two experimental sessions separated by one week, during which they underwent either navigated or non-navigated low-frequency rTMS to the right motor cortex. The order of these two rTMS conditions was randomized and counterbalanced across participants. Participants were assessed before and immediately after each rTMS intervention with the following battery of tests to evaluate corticomotor excitability and motor function of both hands: (1) physiologic measures including resting motor threshold, motor evoked potential (MEP) amplitude, silent period, intracortical inhibition (ICI), and intracortical facilitation (ICF), and (2) behavioral tasks, including simple reaction time, finger tapping and grip strength tasks. Prior to the TMS all subjects underwent a 3D-compatible magnetic resonance imaging (MRI) study. The individual brain MRI was then utilized in the navigation software (eXimia 3.1, Nexstim Ltd, Helsinki Finland).
Experimental Set-up
The stimulation setup consisted of the navigation system (Nexstim Ltd, Helsinki, Finland) combined with a magnetic stimulator (MagPro, MagVenture A/S, Farum, Denmark). Nexstim 59 mm mean winding diameter figure-of-eight TMS coil type (201514P) for monophasic pulses and TMS coil type (201383P) for biphasic pulses. We used focal monophasic TMS pulses for paired pulse stimulation and biphasic pulses otherwise. We used Nexstim coil for pre/post assessment, and Magpro for 1 Hz intervention. During stimulation, electromyography (EMG) was recorded and monitored continuously on-line (ME 6000, Mega Electronics Ltd, Kuopio, Finland) using pre-gelled, disposable Ag/AgCl electrodes. Active electrodes were attached to the skin overlying the first dorsal interosseus (FDI) and abductor pollicis brevis (APB) muscles. Reference electrodes were placed over the metacarpophalangeal joints. The EMG signals were filtered (8–500 Hz), amplified, displayed and stored for off-line analysis. The TMS system delivered trigger pulses that synchronized the TMS and EMG systems.
During the measurements, the subjects sat in a comfortable recliner and held their hands in a supine position on their laps. Earplugs were used to protect the subjects from the loud click associated with TMS (Rossi et al. 2009). The subjects remained silent during the study to avoid speech-induced modulation of cortical excitability. The subjects were also monitored for drowsiness and asked to keep their eyes open throughout the experiment. Relaxation of the measured muscle was controlled by continuous visual EMG monitoring.
Mapping Protocol
In each session, the motor cortical output (presumed to represent primary motor cortex representation and referred to as primary motor cortex was mapped carefully for the optimal representation of the FDI muscle on both hemispheres (Fig. 1). Each individual’s resting motor threshold intensity was determined as the minimum TMS intensity that produced at least five MEPs of ≥50 µV peak-to-peak amplitude out of 10 consecutive stimuli delivered at a rate of 4–6 s interstimulus interval (Chen et al. 2008; Rossini et al. 1994). Following determination of RMT, 10 single stimuli 4–6 s apart were delivered to the optimal scalp location at an intensity of 120% of resting motor threshold to determine baseline MEP amplitude and latency.
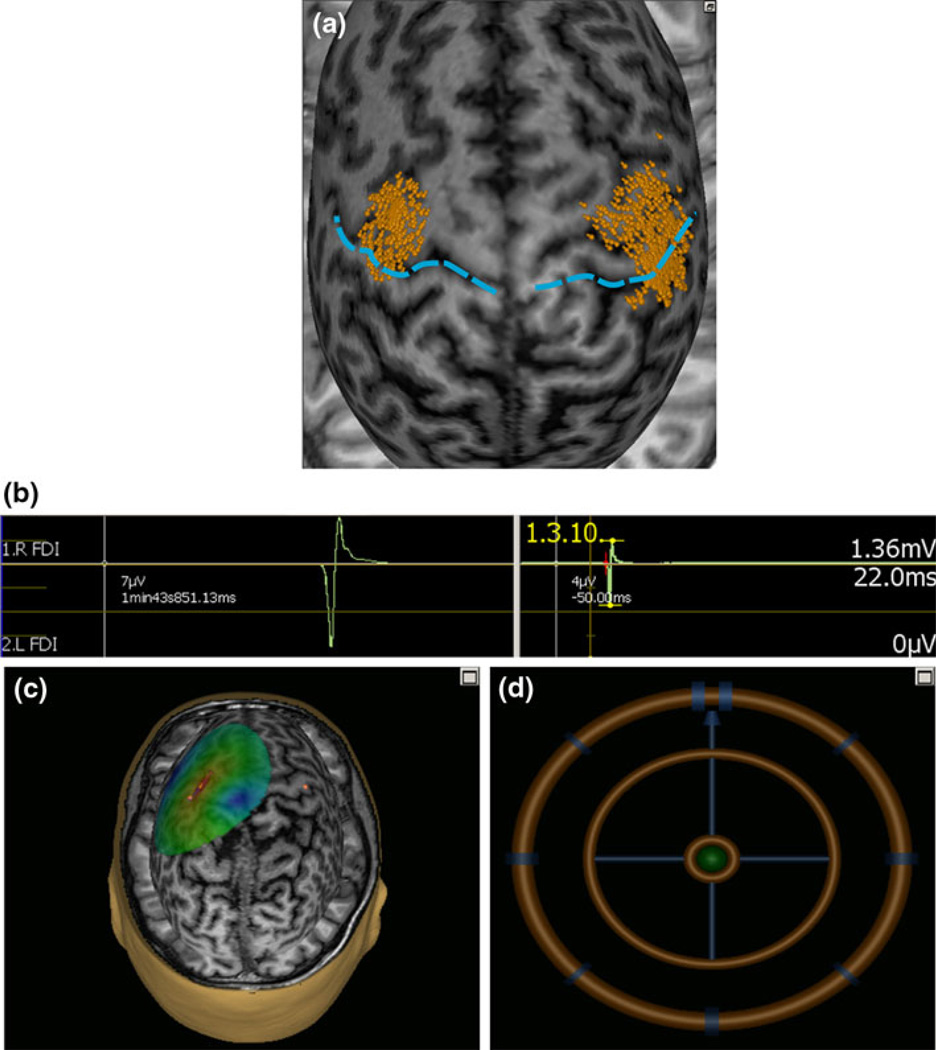
Fig. 1.
An sample screenshot in one subject illustrating (a) the area mapped to identify the motor optimal location (hot-spot) for first dorsal interosseus (FDI) muscle relative to the central sulcus (blue dashed line). The each dot on the scalp are visualized as small ball and the head of ball showed the orientation of a single pulse. b A single evoked response in FDI measured as peak to peak amplitude and onset latency. c The colors show the relative strength of the E-field (red high and blue low E-field strength). The color-code intensity map representing interpolated motor evoked potential amplitude. d The position feedback indicator, providing real time feedback surface location, roll, pitch and yaw for consistent and reliable targeting. The brain is “peeled” into 25 mm depth, i.e., the visualized stimulation surface resides at this depth from the scalp
Active motor threshold was determined as the minimum single pulse intensity required to produce an MEP of greater than 200 µV on more than five out of ten consecutive trials from the contralateral FDI muscle while the subject contracts the target muscle to 10 of maximal voluntary contraction (as assessed by an electronic force transducer).
Paired-Pulse Paradigm
Paired pulse paradigms following the design by Kujirai et al. (1993) were recorded at two interstimulus intervals (ISI): 3 ms to assess ICI and 12 ms to assess ICF. The conditioning stimulus was applied at an intensity of 90% of resting motor threshold, the intensity of the test stimulus was set at the intensity of 120% of resting motor threshold, and these intensities were kept the same in all paired-pulse TMS trials. The interval between pairs of stimuli was at least 6 s to avoid carry-over effects.
Silent Period
The contraction force was measured using a pinch grip simultaneously with both hands in a series of brief (4–5 s) maximal isometric efforts (maximum voluntary contraction, MVC). To assess the ipsilateral and the contralateral silent period (SP), force level was set to 20% of MVC. The muscle contraction was controlled by the subject with online force monitoring. TMS-pulses were delivered during active tonic muscle contraction of approximately 5 s with the instruction to maintain contraction at least 2 s after the stimulus. TMS intensity was set at 120% of resting motor threshold.
Low-Frequency rTMS Intervention
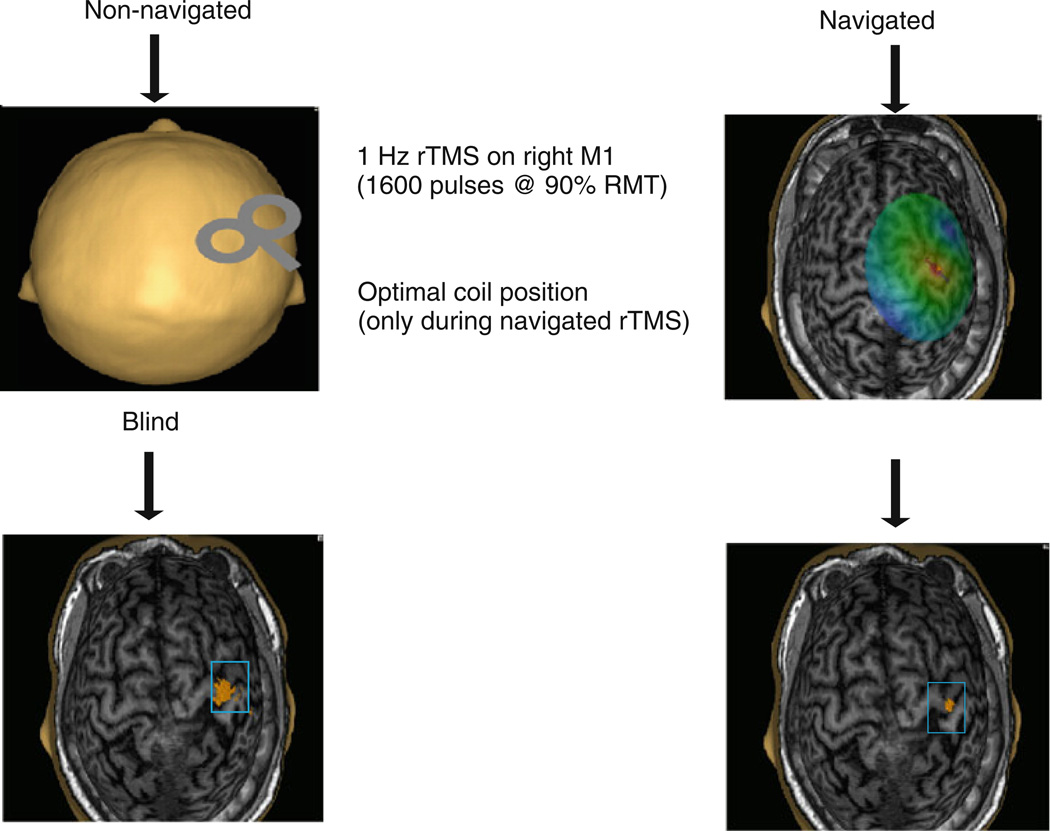
Repetitive TMS was delivered to the right primary motor cortex over the optimal site for induction of MEPs in the left FDI muscle as defined by the mapping procedure described above. A single train of 1600 stimuli of 1 Hz rTMS at 90% resting motor threshold was delivered (Fig. 2). During the navigated and non-navigated rTMS, the location of each stimulus was recorded using the navigation software. However, in the non-navigated rTMS condition, the display of the navigation was switched off and thus not accessible to the experimenter during the stimulation session. In the navigated rTMS intervention procedure, the cortex and hot spot were visualized by navigated brain stimulation software to aid the delivery of rTMS (Fig. 2).
Fig. 2.
The study protocol, which was followed in both navigated and non-navigated rTMS intervention. Ten healthy volunteers received 1-Hz rTMS (1600 pulses) to the right M1 cortex. Stimulus intensity was set at 90% of resting motor threshold (RMT). In the non-navigated session the study protocol was similar like navigated session. In the non-navigated rTMS intervention (left), visualization was neglected. The visualization used for navigated intervention is presented on the right. The each dot on the scalp are visualized as small ball and the head of ball showed the orientation of a single pulse. In navigated intervention the dispersion of the stimulus was less and more focal compare to the non-navigated intervention
In both instances, non-navigated and navigated rTMS, the coil was hand-held, and neither a mechanical coil support nor a head-rest were employed during the stimulation procedures.
Reaction Time Task
The subjects were trained to perform index finger flexion movements in response to a ‘GO’ signal: when the blue circle appeared on the screen, subjects pressed the space bar with their right or left index finger as fast as possible. After training (2 blocks of 40 trials each), subjects had to press a response key as quickly as possible in response to a randomly displayed GO signal preceded by a warning signal. A total of 80 trials were recorded before and after rTMS. Performance with both, left and right hands was assessed.
Grip Strength
Pinch force was measured according to a previously described protocol that exhibits good validity and test–retest reliability (Mathiowetz et al. 1984). Subjects performed 6 pinch trials lasting 6 s each with 1 min of rest between. Subjects grasped a pinch key with the pad of the thumb opposed against the lateral aspect of the middle phalanx of the index finger. The peak force was recorded for both, right and left hands, before and following rTMS.
Finger Tapping Task (FTT)
The FTT required subjects to tap a button with their index finger of each hand as quickly as possible. The tapping assessment comprised 5 × 10 s trials, with 15 s rest between each trial.
Data Analyses
For MEP determination in response to TMS, continuous EMG was sampled in 350 ms epochs, 50 ms before and 300 ms after each TMS pulse. MEP latencies were marked manually by visual inspection and determination of the onset of the MEP. Muscle responses were analyzed by using MegaWin software (Mega Electronics Ltd, Kuopio, Finland). Corticospinal excitability for right (stimulated) and left (unstimulated) primary motor cortex was assessed by measuring peak-to-peak amplitude of MEPs in the contralateral FDI muscle in response to navigated, single-pulse TMS pulses. To minimize the variability of single responses, the largest and smallest amplitude responses were excluded from analysis. Baseline MEP amplitude and latency were defined from the mean response of ten trials in each subject.
For the determination of the SP we defined the beginning of the EMG silence (i.e., the offset of MEP) and the return of any level of EMG activity following the post-MEP silence. This way, both the absolute SP (excluding MEP) and the relative SP (including MEP) could be defined.
For each subject an average ICI and ICF response was determined by calculating the mean of 10 trials of paired-pulse TMS for each. These paired stimulations were then compared to the mean of 10 MEPs in response to single pulses at 120% resting motor threshold, to create a ratio indicating the degree of suppression in the MEP during ICI and ICF as compared to single (test) pulses (Kujirai et al. 1993).
Index finger tapping was calculated by summing the total number of taps in a 10 s period. Reaction time was calculated as the time (in ms) between visual GO-stimulus onset and response. All measures were averaged across all trials performed by each participant.
Data are presented as mean ± SD before (pre), and immediately after (post) rTMS intervention. For each parameter, data were normalized as post-intervention relative to pre-intervention for each subject and are expressed as a percentage.
Statistical Analyses
Statistical analyses were done with SPSS (version 16.0, Chicago, IL, USA). Data were analyzed separately for stimulated and non-stimulated M1. Multiple factor, repeated measures analysis of variance was calculated for each parameter with the factors ‘stimulation type’ [levels: (1) navigated-rTMS (2) non-navigated rTMS], ‘hand’ [levels: (1) right and (2) left hand], ‘intervention’ [levels: (1) pre rTMS, (2) post rTMS], and ‘motor cortex’ [levels: (1) rTMS stimulated and (2) non-stimulated motor cortex]. Post hoc pair-wise comparisons between conditions were performed using t-tests. A P value of ≤0.05 was considered significant.
Results
All participants tolerated the TMS and rTMS without any side-effects or complications. All neurophysiologic results are summarized in Table 1, and the behavioral results are summarized in Table 2.
Table 1.
Effects of 1 Hz navigated and non-navigated rTMS on RMT resting motor threshold, MEP motor evoked potential, SP silent period, ICI intracortical inhabitation and ICF intracortical facilitation in the stimulated and non-stimulated M1
| Navigated | Non-navigated | |||||||
|---|---|---|---|---|---|---|---|---|
| Hemisphere | Non-stimulated | Stimulated | Non-stimulated | Stimulated | ||||
| Condition | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| RMT (% maximal) | 40 ± 6 | 41 ± 7 | 41 ± 6 | 40 ± 6 | 41 ± 7 | 40 ± 5 | 40 ± 9 | 41 ± 8 |
| MEP amplitude at 120% RMT | 1226 ± 690 | 1620 ± 899** | 1293 ± 818 | 1224 ± 1353 | 1336 ± 784 | 1522 ± 953 | 1424 ± 850 | 1394 ± 620 |
| MEP latency at 120% RMT | 22.02 ± 1.86 | 21.8 ± 2.48 | 21.7 ± 1.7 | 22.5 ± 1.27 | 21.9 ± 1.2 | 22.5 ± 1.27 | 22.07 ± 1.38 | 22.3 ± 1.29 |
| MEP for silent period | 2084 ± 540 | 2285 ± 611 | 2254 ± 603 | 2043 ± 675 | 1922 ± 744 | 2156 ± 1154 | 2372 ± 996 | 2167 ± 502 |
| Latency at SP | 21.35 ± 0.70 | 21.58 ± 0.91 | 21.27 ± 0.88 | 21.17 ± 0.46 | 21.19 ± 1.05 | 21.79 ± 0.96 | 21.9 ± 0.67 | 21.23 ± 0.59 |
| Absolute SP | 157 ± 41 | 160 ± 46 | 161 ± 54 | 163 ± 59 | 155 ± 53 | 166 ± 54 | 161 ± 54 | 159 ± 55 |
| ICI | 340 ± 137 | 418 ± 123 | 375 ± 116 | 351 ± 93 | 364 ± 127 | 374 ± 141 | 383 ± 132 | 422 ± 133 |
| ICF | 1016 ± 206 | 1116 ± 244 | 1225 ± 591 | 1087 ± 580 | 975 ± 236 | 1013 ± 247 | 1208 ± 341 | 1187 ± 498 |
The significance levels are *** P < 0.001, ** P < 0.01 and * P < 0.05
Table 2.
Effects of 1 Hz navigated and non-navigated rTMS on hand performing tasks (finger tapping, grip strength, pinch force and RT) in the stimulated and non-stimulated M1
| Hemisphere | Non-stimulated | Stimulated | Non-stimulated | Stimulated | ||||
|---|---|---|---|---|---|---|---|---|
| Condition | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Finger tapping | 47.6 ± 9 | 52 ± 7* | 38 ± 6.2 | 36 ± 5 | 46 ± 8 | 49 ± 7 | 37 ± 8 | 36 ± 6 |
| Grip strength | 27 ± 10.56 | 31.5 ± 9.61* | 28.3 ± 10.8 | 29.3 ± 11.5 | 27.6 ± 8.4 | 32.8 ± 7.7* | 27.1 ± 9.14 | 27 ± 6.81 |
| Pinch force | 23 ± 14.5 | 28 ± 7.2* | 21 ± 8.7 | 20 ± 8.2 | 22 ± 8.2 | 23.1 ± 7.8 | 20 ± 6.4 | 21 ± 7.1 |
| Reaction time | 384 ± 104 | 343 ± 84* | 420 ± 88 | 456 ± 76 | 378 ± 82 | 352 ± 58 | 444 ± 76 | 458 ± 63 |
The significance levels are *** P < 0.001, ** P < 0.01 and * P < 0.05
Corticospinal Excitability Measures
No inter-hemispheric differences were observed within subjects for RMTs (Table 1). RMTs were comparable after navigated and non-navigated rTMS for both the stimulated and the non-stimulated primary motor cortex (M1) (for experimental values see Table 1.
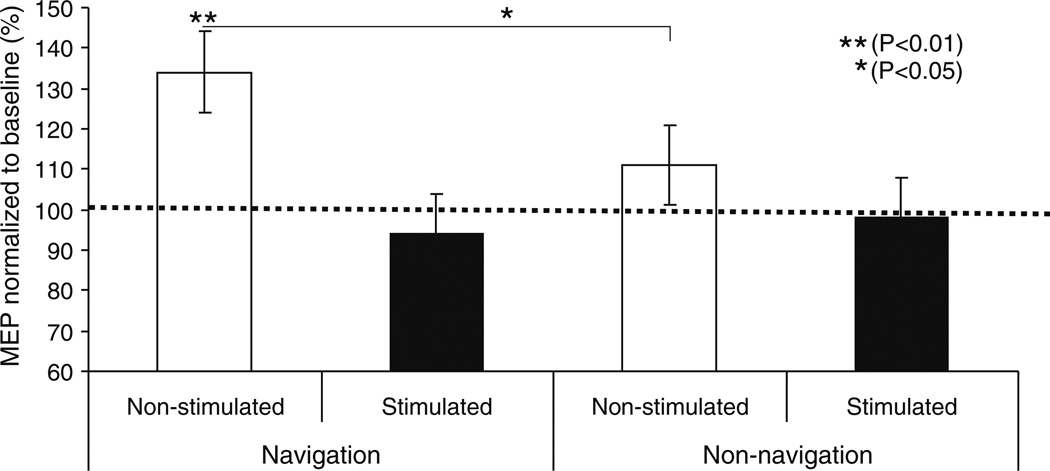
The amplitude of MEPs evoked in the right FDI by single pulse TMS to the left (non-stimulated) M1 increased significantly (F = 10.74, P = 0.004) (~34%) following navigated 1 Hz rTMS to the right M1. On the other hand, the amplitude of MEPs evoked in the right FDI by single pulse TMS to the left (non-stimulated) M1 following non-navigated 1 Hz rTMS to the right M1 also increased, but not significantly (F = 0.982, P = 0.335)(for experimental values see Fig. 3, Table 1). It should be noted, that on average the baseline (pre) MEP amplitude was slightly larger in the non-navigated than in the navigated rTMS experiment. This difference however was not significant.
Fig. 3.
Mean Motor evoked potential (MEP) amplitude at 120% of resting motor threshold (RMT) measure in (mV), before (solid horizontal line at 100 of Y-value normalized as pre rTMS value and expressed as 100% level), immediately after navigated rTMS (left two columns) and non-navigated rTMS (right two columns) stimulated and non-stimulated motor cortex. The significant change represent by (* P<0.05 and ** P<0.01). Data are means from ten subjects. Bars show standard deviations
For both navigated and non-navigated rTMS, the amplitude of MEPs evoked in the left FDI by single pulse TMS to the right (stimulated) M1 decreased slightly, though non-significantly.
The ANOVA showed a significant interactions between stimulation type (navigated versus non-navigated rTMS) and intervention (pre- versus post-rTMS): F = 4.102, P = 0.050. Mean MEP amplitude increased significantly more after navigated than non-navigated rTMS. This effect was dependent on the motor cortex tested. The effect of navigated rTMS was greater than that of non-navigated rTMS for the unstimulated (left) M1: motor cortex * intervention: F = 7.25, P = 0.009 and motor cortex * intervention type: F = 4.755, P = 0.032 (Fig. 3).
The latency of MEPs elicited by TMS at 120% RMT in the relaxed muscle were comparable after navigated and non-navigated rTMS from both the stimulated and the non-stimulated M1 (for experimental values see Table 1). There was a trend for MEP latencies to be shorter after navigated rTMS, but this did not reach significance (F = 3.747, P = 0.069).
When TMS was applied during sustained 20% of maximum voluntary contraction, amplitude and latency of the MEPs induced from the stimulated and the non-stimulated M1 were also not different after navigated or non-navigated rTMS (for experimental values see Table 1).
Silent Period
The range in the absolute duration of silent period (SP) was between 98 and 192 ms across all subjects. Absolute duration of the SP induced from the stimulated M1 was prolonged by navigated rTMS but not significantly. The mean relative SP duration showed the same non-significant effects (for experimental values see Table 1). No significant differences in SP were found after navigated or non-navigated rTMS.
Intracortical Inhibition (ICI)
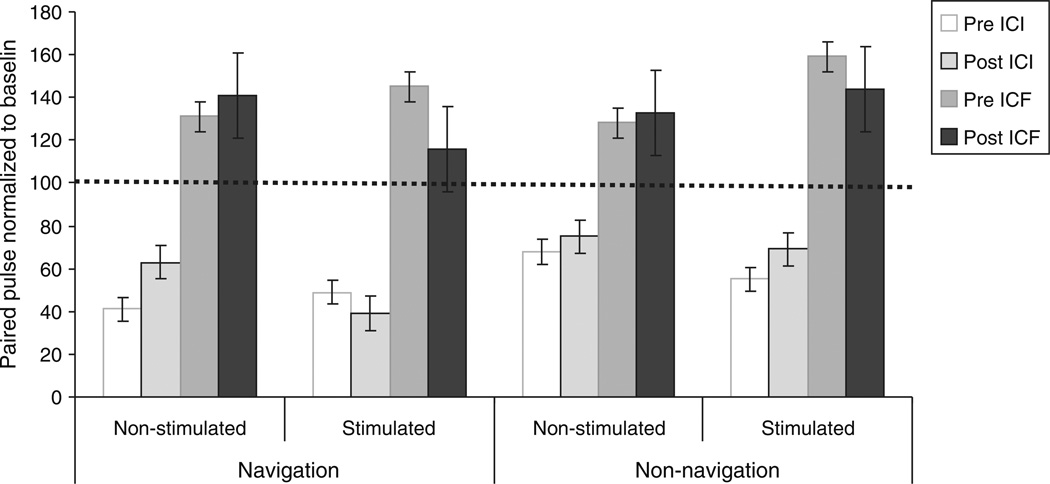
ICI decreased by an average of 23% in the non-stimulated hemisphere following navigated rTMS (F = 3.416, P = 0.056). This trend was absent following non-navigated rTMS, where the ICI decrease in the non-stimulated hemisphere was only 4% (F = 0.582, P = 0.455). Changes in ICI in the stimulated hemisphere were not significant regardless of rTMS intervention (Table 1, Fig. 4).
Fig. 4.
ICI at interstimulus intervals of 3 ms and ICF at 12 ms before, immediately after navigated and non-navigated repetitive transcranial magnetic stimulation (rTMS) to stimulated and non-stimulated motor cortex. The size of the conditioned MEPs is expressed as percentage of the unconditioned MEP (express as 100% level on y-axis). Data are means from ten subjects. Bars show standard deviations
Intracortical Facilitation (ICF)
ICF in the stimulated M1 was decreased, though non-significantly, after navigated (18%), and after non-navigated rTMS (4%, Table 1). ICF increased in the non-stimulated M1. This effect was also greater after navigated than non-navigated rTMS, but the difference was not significant either (Fig. 4).
Muscle Strength
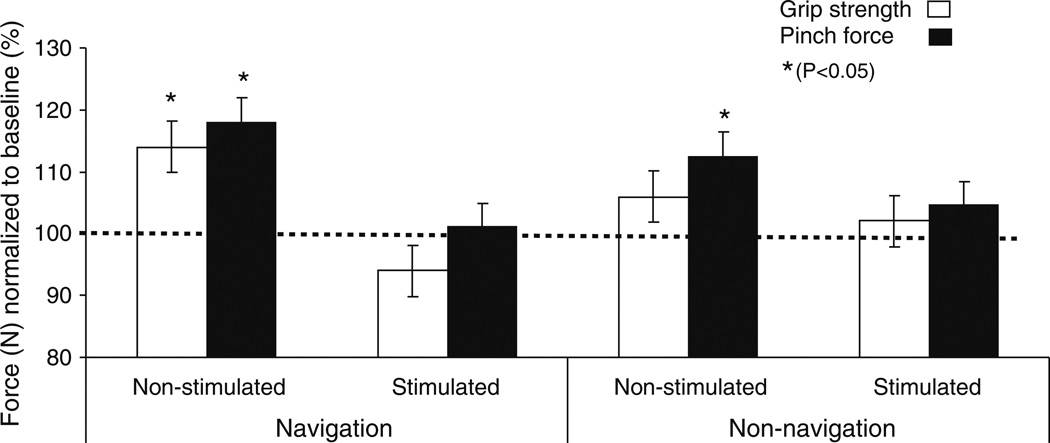
Strength in the right hand (ipsilateral to the rTMS) improved following both, navigated and non-navigated rTMS. However, the effect was stronger following navigated rTMS. Specifically, pinch force increased significantly more following navigated than non-navigated rTMS (P = 0.044, Table 2 and Fig. 5), and whole-hand grip strength improved only after navigated but not after non-navigated stimulation (Table 2, Fig. 5). Strength measures for the left hand (contralateral to the rTMS) were not significantly different following either navigated or non-navigated stimulation (Table 2). The interaction between stimulation TYPE and hand showed a trend towards significance in the ANOVA (F = 2.640, P = 0.058) with the effects being greater for the navigated rTMS.
Fig. 5.
Mean of grip strength and pinch force (thumb and index finger) in (N) task illustrated for each hand and intervention. Significant differences in performance after repetitive transcranial magnetic stimulation (rTMS) over stimulated and non-stimulated M1 compared to baseline (solid horizontal line at 100 of Y-value normalized as baseline value and express as 100% level) values. The significant change represent by (* P<0.05 and ** P<0.01). Data are means from ten subjects. Bars show standard deviations
Reaction Time Task
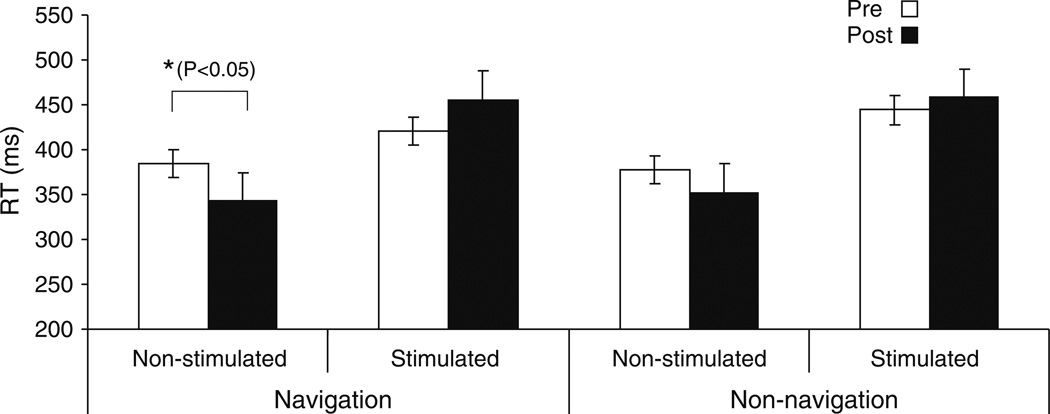
There was a significant (F = 3.51, P = 0.047) decrease in reaction time with the hand ipsilateral to the stimulated hemisphere after navigated rTMS (Table 2, Fig. 6), but only a non-significant (F = 2.09, P = 0.062) trend following the non-navigated intervention. No significant changes in reaction times were evident with the hand contralateral to the stimulated hemisphere following either navigated or non-navigated rTMS.
Fig. 6.
Summarizes mean of the reaction time (RT) in (ms) before and after repetitive transcranial magnetic stimulation (rTMS) illustrated for each hand and intervention over stimulated and non-stimulated M1. Data are means from ten subjects. The significant change represent by (* P<0.05 and ** P<0.01). Bars show standard deviations
Finger Tapping
Following rTMS, the finger tapping rate with the right hand (ipsilateral to the stimulated hemisphere), increased after the non-navigation condition (# taps pre = 47 ± 9 SD, post = 52 ± 7 SD, F = 1.921, P = 0.052). A similar, though much weaker trend occurred in the same direction following navigated rTMS (pre = 46 ± 8, post = 49 ± 7, F = 0.877, P = 0.152, Table 2). For the left hand (contralateral to the stimulated hemisphere), there was no significant change or trend in finger tapping rate regardless of rTMS condition (Table 2).
Discussion
In the present study we show that the use of neuronavigation during the application of low-frequency rTMS in healthy subjects can lead to stronger neurophysiologic impact onto the unstimulated, homologous contralateral cortex, and greater functional impact in motor behavior compared to the same intervention without neuronavigation. In contrast, the inhibitory cortical effects ipsilateral to rTMS seem stronger after non-navigated rTMS. The inevitable scattering of the stimulus locations, even with experienced TMS operators, during non-navigated rTMS may explain this disparity. Greater precision in the targeting of a given cortical location appears to increase the transynaptic, inter-hemispheric impact. On the other hand, greater scatter may result larger modulation of intracortical interneuronal populations leading to greater local physiologic effects.
We found that in the non-stimulated M1, MEP amplitude increased by 34% after rTMS. This finding is in accordance with previous studies (Gilio et al. 2003; Schambra et al. 2003), although others found no change (Plewnia et al. 2003), or even a decrease of MEP amplitude (Wassermann 2002), probably due to the use of lower rTMS intensity or lower number of stimuli in the rTMS train. None of these previous studies utilized individual MRI-based navigation of TMS delivery. However, we show that neuronavigated rTMS led to greater inter-hemispheric modulator effects. The finding of MEPs of greater amplitude induced by single-pulse TMS to the contralateral (unstimulated) hemisphere after navigated than non-navigated rTMS provides direct evidence that inter-hemispheric modulatory effects are increased by precise and consistent stimulation. The consistent targeting of a given brain area, which can be best achieved during navigated rTMS, appears thus important to maximize distant, inter-hemispheric impact.
MEP amplitudes show high intra- and inter-individual variation (Rösler et al. 2008; Weber and Eisen 2002). Although there are many physiological factors that influence such variation (Rösler et al. 2008), we have shown that the intraindividual variation can be significantly decreased by using navigated TMS.
The observed MEP changes were not paralleled by changes in resting motor threshold (RMT). RMT is considered as a measure of membrane excitability (Ziemann et al. 1996). However, ketamine, which indirectly enhances neurotransmission through ionotropic non-NMDA glutamatergic receptors, results in a RMT decrease (Di Lazzaro et al. 2003), indicating that excitatory synaptic projections onto corticospinal neurons can affect motor threshold. We did not find rTMS-induced changes in RMT in the unstimulated M1, which is consistent with some prior 1 Hz rTMS studies (Siebner et al. 1998; Bagnato et al. 2005). Other studies have found changes in RMT in the unstimulated M1 following 1 Hz rTMS, but the duration of the RMT increase was shorter than the change in MEP amplitude (Muellbacher et al. 2000), or the RMT increase occurred without a change in MEPs (Fitzgerald et al. 2002). Thus, consistent with current views on the underlying neurophysiologic substrates, RMT changes, if they occur, are dissociable from changes in MEP amplitude.
We found that navigated rTMS had a greater impact than non-navigated rTMS on ICI and ICF in the unstimulated hemisphere. This finding also supports the notion that the inter-hemispheric effects of rTMS benefit form a consistent modulation of a given brain region and are thus driven by highly spatially specific connections. Our results on the effects of 1 Hz rTMS onto ICI and ICF in the stimulated and unstimulated motor cortex are overall consistent with prior studies (e.g. Boroojerdi et al. 2000; Hammond et al. 2004; Ziemann 2004; Vucic et al. 2009). ICI and ICF are complex phenomena with different contributing neurobiologic mechanisms. At an ISI of 3–5 ms, the paired-pulse TMS effects appear to reflect primarily the GAB Aergic inhibitory system, and the resulting measure of short-latency ICI is not be contaminated by ICF (Vucic et al. 2009). On the other hand, at an ISI of 12 ms, the paired-pulse TMS effects are thought to be driven primarily by Glutamatergic, NMDA-receptor dependant, facilitatory infuences (Heide et al. 2006), and the resulting ICF measure is independent from ICI.
We found no effect of rTMS onto the SP. However, our assessment of the SP could be improved by more quantitative approaches, and in any case, the dissociation in the neurobiologic substrates of the SP and other measures of corticospinal excitability has been repeatedly supported.
Behaviorally, we found that navigated rTMS applied over the right improved index finger tapping as well as the grip strength and reaction time (RT) tasks performed with the right (ipsilateral) hand. These effects were greater than following non-navigated rTMS and are consistent with the neurophysiologic findings of a greater inter-hemispheric impact of navigated than non-navigated rTMS. Our results expand prior findings that show that suppression of M1 cortical excitability by 1 Hz rTMS can result in faster execution of a serial button pressing task with the ipsilateral hand in healthy humans (Kobayashi et al. 2003). 1 Hz rTMS applied over right M1 was also found to modify several kinematic parameters of a sequential finger opposition task performed with the ipsilateral hand (Avanzino et al. 2008).
Conclusion
Our study is in line with the dominating view of the local and inter-hemispheric effects of 1 Hz rTMS, yet extends prior findings by providing a comparison of the physiologic and behavioral effects of MRI-guided, neuronavigated versus non-navigated delivery of this rTMS intervention. We found that navigated rTMS led, for the most part, to stronger physiologic and behavioral effects. Our findings provide support for the use of neuronavigation in therapeutic trials of rTMS aimed at achieving a more robust and stable impact, and highlight that non-navigation may not only lead to more variable results, but also promote certain effects which may be undesired.
Acknowledgements
Work on this study was supported by grants from the National Center for Research Resources: Harvard-Thorndike General Clinical Research Center at BIDMC (NCRR MO1 RR01032) and Harvard Clinical and Translational Science Center (UL1 RR025758); NIH grant K24 RR018875 and a grant from the Nancy Lurie Marks Family Foundation to A.P.-L. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Nancy Lurie Marks Family Foundation, National Center for Research Resources or the National Institutes of Health. The authors would like to thank Jari Karhu* valuable comments on a preliminary draft of the present manuscript, Jarmo Laine** and Tuomas Neuvonen** for technical support and Andrea Vatulas and Mark Thivierge for their administrative help. * Jari Karhu is employed part-time as professor in the Faculty of Medicine at the University of Kuopio and part-time as Chief Medical Officer of Nexstim Ltd. ** Jarmo Laine and Tuomas Neuvonen are employees for Nexstim Ltd.
Contributor Information
S. Bashir, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA
D. Edwards, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA Burke-Cornell Medical Research Institute, White Plains, NY, USA; Centre for Neuromuscular and Neurological Disorders, University of Western Australia, Crawley, WA, Australia.
A. Pascual-Leone, Email: apleone@bidmc.harvard.edu, Berenson-Allen Center for Noninvasive Brain Stimulation, Division of Cognitive Neurology, Department of Neurology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA, USA; Institute Guttmann de Neurorrehabilitación, Universidad Autónoma de Barcelona, Barcelona, Spain.
References
- Avanzino L, Bove M, Trompetto C, Tacchino A, Ogliastro C, Abbruzzese G. 1-Hz repetitive TMS over ipsilateral motor cortex influences the performance of sequential finger movements of different complexity. Eur J Neurosci. 2008;27:1285–1291. doi: 10.1111/j.1460-9568.2008.06086.x. [DOI] [PubMed] [Google Scholar]
- Bagnato S, Curraà A, Modugno N, Gilio F, Quartarone A, Rizzo V, Girlanda P, Inghillieri M, Berardelli A. One-hertz subthreshold TMS increases the threshold for evoking inhibition in the human motor cortex. Exp Brain Res. 2005;160:368–374. doi: 10.1007/s00221-004-2020-0. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–1107. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Bernad DM, Doyon J. The role of noninvasive techniques in stroke therapy. Int J Biomed Imaging. 2008;2008 doi: 10.1155/2008/672582. 672582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boroojerdi B, Kopylev L, Battaglia F, Facchini S, Ziemann U, Muellbacher W, Cohen LG. Reproducibility of intracortical inhibition and facilitation using the paired-pulse paradigm. Muscle Nerve. 2000;23:1594–1597. doi: 10.1002/1097-4598(200010)23:10<1594::aid-mus19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Chen R, Cros D, Curra A, Di Lazzaro V, Lefaucheur JP, Magistris MR, Mills K, Rosler KM, Triggs WJ, Ugawa Y, Ziemann U. The clinical diagnostic utility of transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2008;119:504–532. doi: 10.1016/j.clinph.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input-output properties and gain changes in the human corticospinal pathway. Exp Brain Res. 1997;114:329–338. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J. Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol. 2002;113:1136–1141. doi: 10.1016/s1388-2457(02)00145-1. [DOI] [PubMed] [Google Scholar]
- Gilio F, Rizzo V, Siebner HR, Rothwell JC. Effects on the right motor hand-area excitability produced by low-frequency rTMS over human contralateral homologous cortex. J Physiol. 2003;551:563–573. doi: 10.1113/jphysiol.2003.044313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugino LD, Romero JR, Aglio L, Titone D, Ramirez M, Pascual-Leone A, Grimson E, Weisenfeld N, Kikinis R, Shenton ME. Transcranial magnetic stimulation coregistered with MRI: a comparison of a guided versus blind stimulation technique and its effect on evoked compound muscle action potentials. Clin Neurophysiol. 2001;112:1781–1792. doi: 10.1016/s1388-2457(01)00633-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G, Faulkner D, Byrnes M, Mastaglia F, Thickbroom G. Transcranial magnetic stimulation reveals asymmetrical efficacy of intracortical circuits in primary motor cortex. Exp Brain Res. 2004;155:19–23. doi: 10.1007/s00221-003-1696-x. [DOI] [PubMed] [Google Scholar]
- Hannula H, Ylioja S, Pertovaara A, Korvenoja A, Ruohonen J, Ilmoniemi RJ, Carlson S. Somatotopic blocking of sensation with navigated transcranial magnetic stimulation of the primary somatosensory cortex. Hum Brain Mapp. 2005;26:100–109. doi: 10.1002/hbm.20142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heide G, Witte OW, Ziemann U. Physiology of modulation of motor cortex excitability by low-frequency suprathreshold repetitive transcranial magnetic stimulation. Exp Brain Res. 2006;171:26–34. doi: 10.1007/s00221-005-0262-0. [DOI] [PubMed] [Google Scholar]
- Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehab Neural Re. 2005;19(1):14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Jauhiainen AM, Westereén-Punnonen S, Pirinen E, Soininen H, Könönen M, Pääkkönen A, Määttä S, Karhu J. Navigated TMS combined with EEG in mild cognitive impairment and Alzheimer’s disease: a pilot study. J Neurosci Methods. 2008a;172:270–276. doi: 10.1016/j.jneumeth.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Paakkonen A, Hukkanen T, Kononen M, Tiihonen P, Vanhatalo S, Karhu J. Efficient reduction of stimulus artefact in TMS-EEG by epithelial short-circuiting by mini-punctures. Clin Neurophysiol. 2008b;119:475–481. doi: 10.1016/j.clinph.2007.09.139. [DOI] [PubMed] [Google Scholar]
- Kathleen YH, Sydney Y, Schaefer RT, Knight JA, Alvaro M, Joseph S, Robert LS. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp Brain Res. 2009;196(2):195–204. doi: 10.1007/s00221-009-1836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers L, Cros D, Chiappa KH, Fang J. Variability of motor potentials evoked by transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;89:415–423. doi: 10.1016/0168-5597(93)90115-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage. 2003;20:2259–2270. doi: 10.1016/s1053-8119(03)00220-9. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS. A sham stimulation controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64:1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9(2):222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol. 2000;111:1002–1007. doi: 10.1016/s1388-2457(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Noirhomme Q, Ferrant M, Vandermeeren Y, Olivier E, Macq B, Cuisenaire O. Registration and real-time visualization of transcranial magnetic stimulation with 3-D MR images. IEEE Trans Biomed Eng. 2004;51:1994–2005. doi: 10.1109/TBME.2004.834266. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial agnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. (Review) [DOI] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeaur R, Peters T, Evans AC. Dose-dependent reduction of cerebral blood flow during rapid-rate transcranial magnetic stimulation of the human sensorimotor cortex. J Neurophysiol. 1998;79:1102–1107. doi: 10.1152/jn.1998.79.2.1102. [DOI] [PubMed] [Google Scholar]
- Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport. 2003;14:609–612. doi: 10.1097/00001756-200303240-00017. [DOI] [PubMed] [Google Scholar]
- Rösler KM, Roth DM, Magistris MR. Trial-to-trial size variability of motor-evoked potentials. A study using the triple stimulation technique. Exp Brain Res. 2008;187:51–59. doi: 10.1007/s00221-008-1278-z. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Säisänen L, Pirinen E, Teitti S, Könönen M, Julkunen P, Määttä S, Karhu J. Factors influencing cortical silent period: optimized stimulus location, intensity and muscle contraction. J Neurosci Methods. 2008;169:231–238. doi: 10.1016/j.jneumeth.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol. 2003;114:130–133. doi: 10.1016/s1388-2457(02)00342-5. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Cichy RM, Kraft A, Brocke J, Irlbacher K, Brandt SA. An initial transient state and reliable measures of corticospinal excitability in TMS studies. Clin Neurophysiol. 2009;120(5):987–993. doi: 10.1016/j.clinph.2009.02.164. Epub 2009 Apr 8. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36:2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Vucic S, Cheah BC, Krishnan AV, Burke D, Kiernan MC. The effects of alterations in conditioning stimulus intensity on short interval intracortical inhibition. Brain Res. 2009;1273:39–47. doi: 10.1016/j.brainres.2009.03.043. [DOI] [PubMed] [Google Scholar]
- Wassermann EA. Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol. 2002;113(2002):1165–1171. doi: 10.1016/s1388-2457(02)00144-x. [DOI] [PubMed] [Google Scholar]
- Weber M, Eisen AA. Magnetic stimulation of the central and peripheral nervous systems. Muscle Nerve. 2002;25:160–175. doi: 10.1002/mus.10038. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC, Ridding MC. Interaction between intracortical inhibition and facilitation in human motor cortex. J Physiol. 1996;496(Pt 3):873–881. doi: 10.1113/jphysiol.1996.sp021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]