Abstract
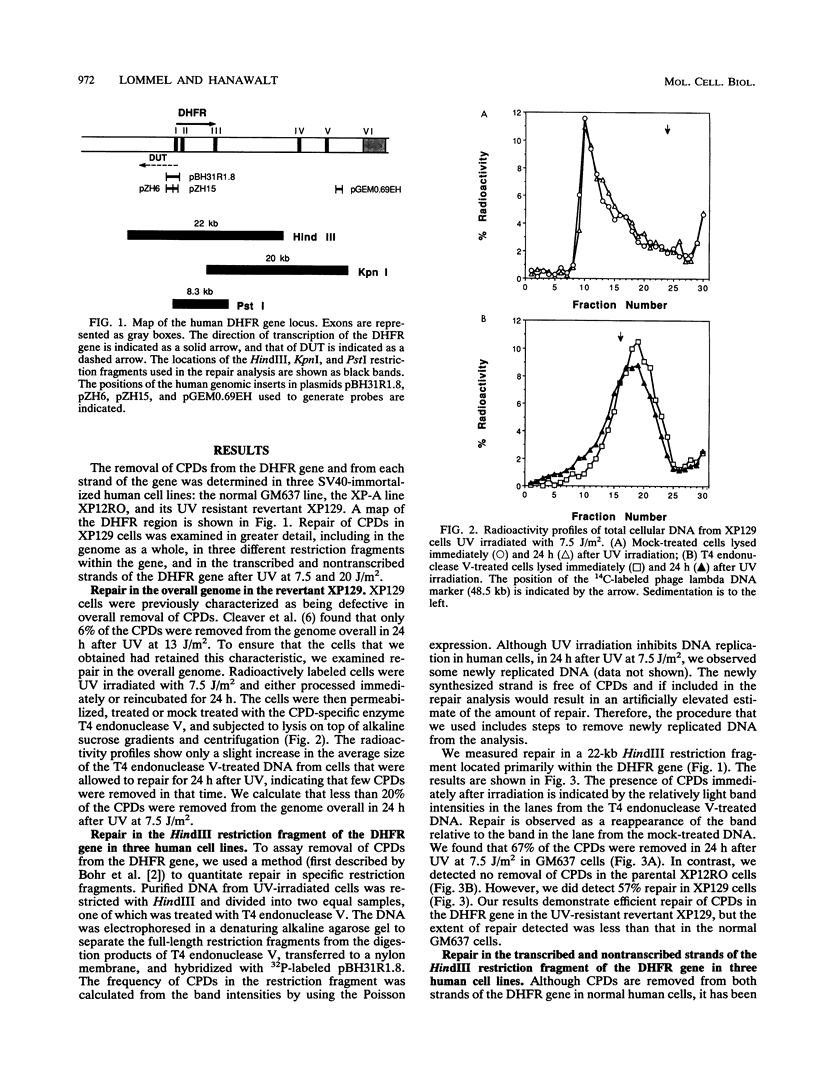
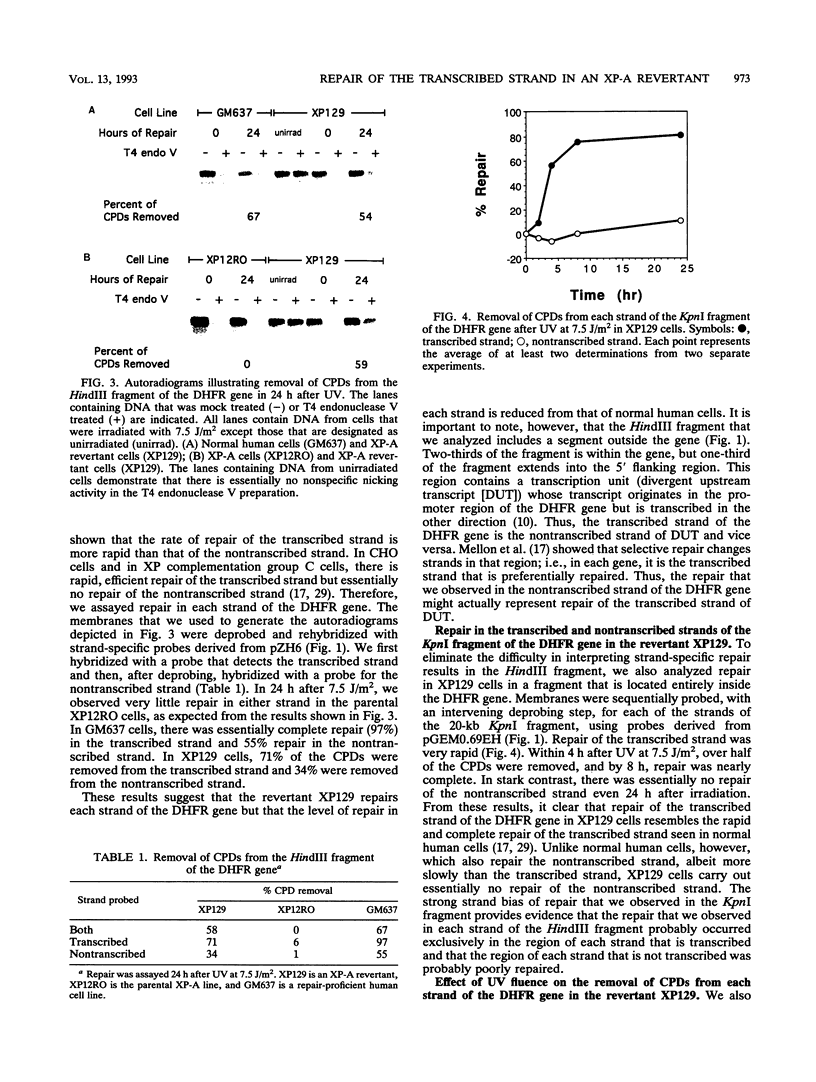
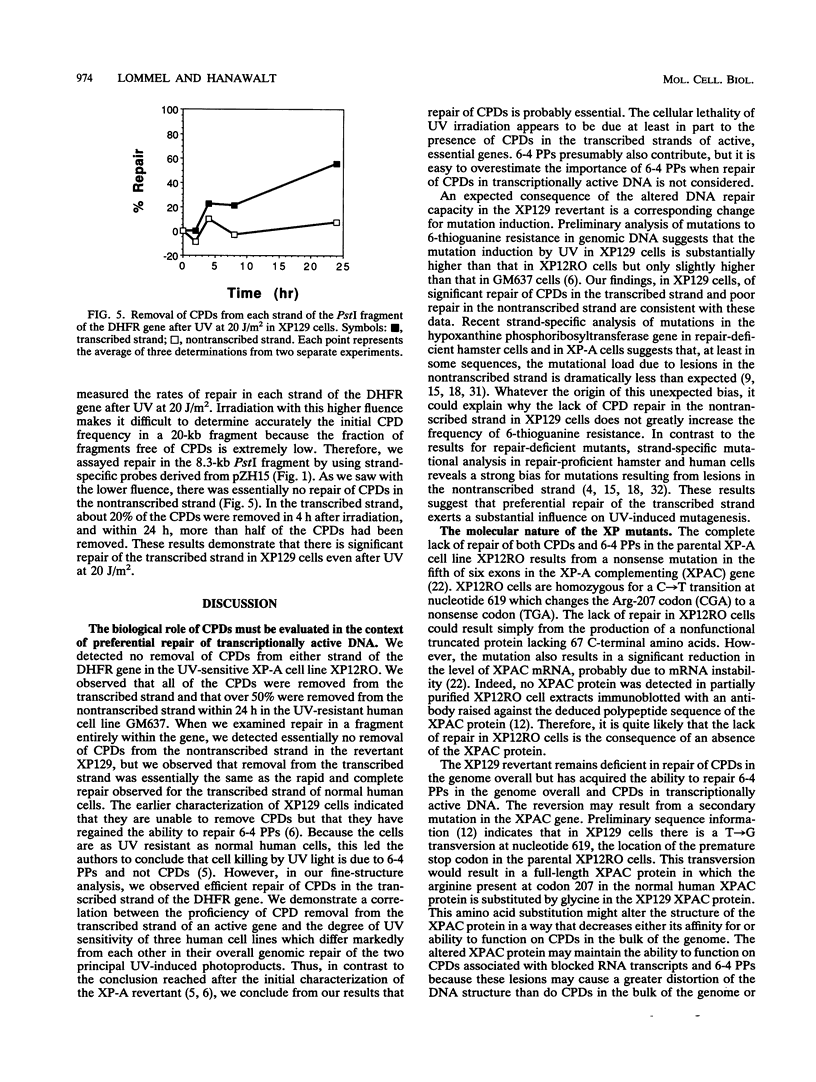
A UV-resistant revertant (XP129) of a xeroderma pigmentosum group A cell line has been reported to be totally deficient in repair of cyclobutane pyrimidine dimers (CPDs) but proficient in repair of 6-4 photoproducts. This finding has been interpreted to mean that CPDs play no role in cell killing by UV. We have analyzed the fine structure of repair of CPDs in the dihydrofolate reductase gene in the revertant. In this essential, active gene, we observe that repair of the transcribed strand is as efficient as that in normal, repair-proficient human cells, but repair of the nontranscribed strand is not. Within 4 h after UV at 7.5 J/m2, over 50% of the CPDs were removed, and by 8 h, 80% of the CPDs were removed. In contrast, there was essentially no removal from the nontranscribed strand even by 24 h. Our results demonstrate that overall repair measurements can be misleading, and they support the hypothesis that removal of CPDs from the transcribed strands of expressed genes is essential for UV resistance.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohr V. A., Smith C. A., Okumoto D. S., Hanawalt P. C. DNA repair in an active gene: removal of pyrimidine dimers from the DHFR gene of CHO cells is much more efficient than in the genome overall. Cell. 1985 Feb;40(2):359–369. doi: 10.1016/0092-8674(85)90150-3. [DOI] [PubMed] [Google Scholar]
- Bootsma D., Keijzer W., Jung E. G., Bohnert E. Xeroderma pigmentosum complementation group XP-I withdrawn. Mutat Res. 1989 Sep;218(2):149–151. doi: 10.1016/0921-8777(89)90021-9. [DOI] [PubMed] [Google Scholar]
- Carothers A. M., Mucha J., Grunberger D. DNA strand-specific mutations induced by (+/-)-3 alpha,4 beta-dihydroxy- 1 alpha,2 alpha-epoxy-1,2,3,4-tetrahydrobenzo[c]phenanthrene in the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5749–5753. doi: 10.1073/pnas.88.13.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Cortés F., Lutze L. H., Morgan W. F., Player A. N., Mitchell D. L. Unique DNA repair properties of a xeroderma pigmentosum revertant. Mol Cell Biol. 1987 Sep;7(9):3353–3357. doi: 10.1128/mcb.7.9.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Jen J., Charles W. C., Mitchell D. L. Cyclobutane dimers and (6-4) photoproducts in human cells are mended with the same patch sizes. Photochem Photobiol. 1991 Sep;54(3):393–402. doi: 10.1111/j.1751-1097.1991.tb02033.x. [DOI] [PubMed] [Google Scholar]
- Dorado G., Steingrimsdottir H., Arlett C. F., Lehmann A. R. Molecular analysis of ultraviolet-induced mutations in a xeroderma pigmentosum cell line. J Mol Biol. 1991 Jan 20;217(2):217–222. doi: 10.1016/0022-2836(91)90533-c. [DOI] [PubMed] [Google Scholar]
- Fujii H., Shimada T. Isolation and characterization of cDNA clones derived from the divergently transcribed gene in the region upstream from the human dihydrofolate reductase gene. J Biol Chem. 1989 Jun 15;264(17):10057–10064. [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Comparison of the cleavage of pyrimidine dimers by the bacteriophage T4 and Micrococcus luteus UV-specific endonucleases. J Biol Chem. 1980 Dec 25;255(24):12047–12050. [PubMed] [Google Scholar]
- Jones C. J., Cleaver J. E., Wood R. D. Repair of damaged DNA by extracts from a xeroderma pigmentosum complementation group A revertant and expression of a protein absent in its parental cell line. Nucleic Acids Res. 1992 Mar 11;20(5):991–995. doi: 10.1093/nar/20.5.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann A. R., Arlett C. F., Broughton B. C., Harcourt S. A., Steingrimsdottir H., Stefanini M., Malcolm A., Taylor R., Natarajan A. T., Green S. Trichothiodystrophy, a human DNA repair disorder with heterogeneity in the cellular response to ultraviolet light. Cancer Res. 1988 Nov 1;48(21):6090–6096. [PubMed] [Google Scholar]
- Lommel L., Hanawalt P. C. The genetic defect in the Chinese hamster ovary cell mutant UV61 permits moderate selective repair of cyclobutane pyrimidine dimers in an expressed gene. Mutat Res. 1991 Sep;255(2):183–191. doi: 10.1016/0921-8777(91)90052-q. [DOI] [PubMed] [Google Scholar]
- McGregor W. G., Chen R. H., Lukash L., Maher V. M., McCormick J. J. Cell cycle-dependent strand bias for UV-induced mutations in the transcribed strand of excision repair-proficient human fibroblasts but not in repair-deficient cells. Mol Cell Biol. 1991 Apr;11(4):1927–1934. doi: 10.1128/mcb.11.4.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon I., Hanawalt P. C. Induction of the Escherichia coli lactose operon selectively increases repair of its transcribed DNA strand. Nature. 1989 Nov 2;342(6245):95–98. doi: 10.1038/342095a0. [DOI] [PubMed] [Google Scholar]
- Mellon I., Spivak G., Hanawalt P. C. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987 Oct 23;51(2):241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- Menichini P., Vrieling H., van Zeeland A. A. Strand-specific mutation spectra in repair-proficient and repair-deficient hamster cells. Mutat Res. 1991 Nov;251(1):143–155. doi: 10.1016/0027-5107(91)90224-c. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Nairn R. S. The biology of the (6-4) photoproduct. Photochem Photobiol. 1989 Jun;49(6):805–819. doi: 10.1111/j.1751-1097.1989.tb05578.x. [DOI] [PubMed] [Google Scholar]
- Mitchell D. L., Zdzienicka M. Z., van Zeeland A. A., Nairn R. S. Intermediate (6-4) photoproduct repair in Chinese hamster V79 mutant V-H1 correlates with intermediate levels of DNA incision and repair replication. Mutat Res. 1989 May;226(1):43–47. doi: 10.1016/0165-7992(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Thompson L. H., Carrier W. L., Weber C. A., Francis A. A., Zdzienicka M. Z. Cyclobutane-pyrimidine dimer excision in UV-sensitive CHO mutants and the effect of the human ERCC2 repair gene. Mutat Res. 1990 May;235(3):157–163. doi: 10.1016/0921-8777(90)90069-h. [DOI] [PubMed] [Google Scholar]
- Satokata I., Tanaka K., Miura N., Narita M., Mimaki T., Satoh Y., Kondo S., Okada Y. Three nonsense mutations responsible for group A xeroderma pigmentosum. Mutat Res. 1992 Mar;273(2):193–202. doi: 10.1016/0921-8777(92)90080-m. [DOI] [PubMed] [Google Scholar]
- Smerdon M. J., Thoma F. Site-specific DNA repair at the nucleosome level in a yeast minichromosome. Cell. 1990 May 18;61(4):675–684. doi: 10.1016/0092-8674(90)90479-x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Miura N., Satokata I., Miyamoto I., Yoshida M. C., Satoh Y., Kondo S., Yasui A., Okayama H., Okada Y. Analysis of a human DNA excision repair gene involved in group A xeroderma pigmentosum and containing a zinc-finger domain. Nature. 1990 Nov 1;348(6296):73–76. doi: 10.1038/348073a0. [DOI] [PubMed] [Google Scholar]
- Terleth C., van Sluis C. A., van de Putte P. Differential repair of UV damage in Saccharomyces cerevisiae. Nucleic Acids Res. 1989 Jun 26;17(12):4433–4439. doi: 10.1093/nar/17.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Mitchell D. L., Regan J. D., Bouffler S. D., Stewart S. A., Carrier W. L., Nairn R. S., Johnson R. T. CHO mutant UV61 removes (6-4) photoproducts but not cyclobutane dimers. Mutagenesis. 1989 Mar;4(2):140–146. doi: 10.1093/mutage/4.2.140. [DOI] [PubMed] [Google Scholar]
- Venema J., van Hoffen A., Karcagi V., Natarajan A. T., van Zeeland A. A., Mullenders L. H. Xeroderma pigmentosum complementation group C cells remove pyrimidine dimers selectively from the transcribed strand of active genes. Mol Cell Biol. 1991 Aug;11(8):4128–4134. doi: 10.1128/mcb.11.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen W., Stefanini M., Giliani S., Hoeijmakers J. H., Bootsma D. Xeroderma pigmentosum complementation group H falls into complementation group D. Mutat Res. 1991 Sep;255(2):201–208. doi: 10.1016/0921-8777(91)90054-s. [DOI] [PubMed] [Google Scholar]
- Vrieling H., Van Rooijen M. L., Groen N. A., Zdzienicka M. Z., Simons J. W., Lohman P. H., van Zeeland A. A. DNA strand specificity for UV-induced mutations in mammalian cells. Mol Cell Biol. 1989 Mar;9(3):1277–1283. doi: 10.1128/mcb.9.3.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieling H., Venema J., van Rooyen M. L., van Hoffen A., Menichini P., Zdzienicka M. Z., Simons J. W., Mullenders L. H., van Zeeland A. A. Strand specificity for UV-induced DNA repair and mutations in the Chinese hamster HPRT gene. Nucleic Acids Res. 1991 May 11;19(9):2411–2415. doi: 10.1093/nar/19.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will C. L., Dolnick B. J. 5-Fluorouracil augmentation of dihydrofolate reductase gene transcripts containing intervening sequences in methotrexate-resistant KB cells. Mol Pharmacol. 1986 Jun;29(6):643–648. [PubMed] [Google Scholar]
- Yang J. K., Masters J. N., Attardi G. Human dihydrofolate reductase gene organization. Extensive conservation of the G + C-rich 5' non-coding sequence and strong intron size divergence from homologous mammalian genes. J Mol Biol. 1984 Jun 25;176(2):169–187. doi: 10.1016/0022-2836(84)90419-4. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., Venema J., Mitchell D. L., van Hoffen A., van Zeeland A. A., Vrieling H., Mullenders L. H., Lohman P. H., Simons J. W. (6-4) photoproducts and not cyclobutane pyrimidine dimers are the main UV-induced mutagenic lesions in Chinese hamster cells. Mutat Res. 1992 Jan;273(1):73–83. doi: 10.1016/0921-8777(92)90051-4. [DOI] [PubMed] [Google Scholar]
- van Zeeland A. A., Smith C. A., Hanawalt P. C. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat Res. 1981 Jun;82(1):173–189. doi: 10.1016/0027-5107(81)90148-2. [DOI] [PubMed] [Google Scholar]




