Abstract
Noninvasive brain stimulation of the dorsolateral prefrontal cortex with repetitive transcranial magnetic stimulation and transcranial direct current stimulation can modify decision-making behaviors in healthy subjects. The same type of noninvasive brain stimulation can suppress drug craving in substance user patients, who often display impaired decision-making behaviors. We discuss the implications of these studies for the cognitive neurosciences and their translational applications to the treatment of addictions. We propose a neurocognitive model that can account for our findings and suggests a promising therapeutic role of brain stimulation in the treatment of substance abuse and addictive behavior disorders.
Keywords: addiction, craving, decision-making, neurocognition, dorsolateral prefrontal cortex, transcranial magnetic stimulation, transcranial direct current stimulation
Introduction
Addiction, previously considered a sin and then a crime (DSM REV, 1954), is now described as a chronic, often relapsing disease (DSM-IV, 1994) and as a complex condition that may be associated with brain damage, behavioral impairments, and/or environmental factors. Even among individuals with substance abuse who wish to quit the dependence, the percentage of successful cessation is very low (Centers for Disease Control and Prevention, 2004). For instance, consider nicotine addiction. In the United States, 41% of daily smokers report having stopped smoking for at least one day in the preceding year, but failed to sustain abstinence (Centers for Disease Control and Prevention, 2004). Despite numerous pharmacotherapies for nicotine dependence, such as nicotine substitution (e.g., gum, transdermal patch, lozenge, sublingual tablet, nasal spay, and vapor inhaler formulations) and non-nicotine medications (e.g., bupropion, notriptyline, clonidine, rimonabant, and varenicline), the rate of cessation is very small. Among nicotine-dependent smokers who wish to quit, only 30% sustain abstinence for a year following treatment and less than 5% achieve absolute abstinence (Centers for Disease Control and Prevention, 2004). The situation with drug, food, gambling, sex, and other addictions seem similar. However, why is addiction so difficult to overcome? More specifically, what are the critical conditions that are necessary for substance user behaviors and/or lifestyles to begin, to continue, to be sustained, to change, etc.?
We hypothesize that a critical, largely overlooked aspect relates to the cognitive neuroscience of the addict’s mind, characterized by dysfunctional inhibitory control and decision-making capacities. This paper reviews recent cognitive neuroscience studies describing the capacity of noninvasive brain stimulation to modulate decision-making functions when targeting dorsolateral prefrontal cortex. We will highlight the translational clinical relevance of such findings and present recent proof-of-principle evidence supporting the notion that noninvasive brain stimulation might be a valuable adjunct in the treatment of addiction. Finally, we propose a neurocognitive model to account for these findings coherently and inform future human interventions to understand underlying neurobiological mechanisms.
Decision-Making Is Linked to Prefrontal Cortex by Imaging and Can Be Modulated in the Healthy Brain
Decision-making is part of a complex and dynamic process (e.g., Koechlin and Hyafil, 2007; Rushworth and Behrens, 2008) and the prefrontal cortex, especially the orbitofrontal and dorsolateral cortex (DLPFC), has been repeatedly associated with decision-making processes (see meta-analysis from Krain, Wilson, Arbuckle, Castellanos, and Milham, 2006). Healthy subjects show for instance activations in the DLPFC, composed of the middle frontal gyrus, including Brodmann areas 9 and 9/46 (according to the anatomical criteria from Petrides and Pandya, 1999) while performing decision-making tasks such as the Risk Task (Rogers et al., 1999), a two-choice prediction task (Paulus et al., 2001), and the Ultimatum Game (Sanfey, Rilling, Aronson, Nystrom, and Cohen 2003); patients with prefrontal cortex damage often display abnormal decision-making behaviors (Bechara, Damasio, Damasio, and Anderson, 1994; Clark, Manes, Antoun, Sahakian, and Ribbins, 2003; Damasio, Damasio, and Christen, 1996; Manes et al., 2002).
More recently, studies have been conducted using noninvasive brain stimulation techniques—including repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS)—for the modulation of decision-making behaviors. Main findings are summarized in Table 1.
Table 1.
Review of findings of the effects of neuromodulation on decision-making in healthy subjects
| Stimulation paradigm | Findings | |
|---|---|---|
| 1Hz rTMS over the right DLPFC | ↓ | rejecting unfair offers >sham rTMS at the Ultimatum Game (van’t Wout et al., 2005 ; Knoch et al., 2006b). |
| ↓ | rejecting unfair offers >1Hz rTMS over the left DLPFC at the Ultimatum Game (Knoch et al., 2006b). | |
| ↑ | risk taking >sham rTMS at the Risk Task (Knoch et al., 2006a). | |
| ↑ | risk taking >1Hz rTMS over the left DLPFC at the Risk Task (Knoch et al. 2006a). | |
| 2 mA bilateral DLPFC tDCS | ↓ | risk taking >sham tDCS at the BART (Fecteau et al. 2007a). |
| ↓ | risk taking >unilateral DLPFC tDCS at the BART (Fecteau et al., 2007a). | |
| ↓ | risk taking >sham tDCS at the Risk Task (Fecteau et al., 2007b). | |
| ↓ | sensitivity to reward >sham tDCS at the Risk Task (Fecteau et al., 2007b). | |
In 2005, van’t Wout and colleagues were the first to report that applying low-frequency rTMS to transiently disrupt right DLPFC function in healthy volunteers resulted in accepting more frequently unfair offers and taking longer time to refuse unfair offers, compared with sham rTMS as measured by the Ultimatum Game (Güth, Schmittberger, and Schwarze, 1982). Then in 2006, Knoch and colleagues conducted a series of offline rTMS experiments demonstrating the impact of brain stimulation on decision-making behaviors. Knoch and colleagues (2006a) found that after applying low-frequency rTMS over the right DLPFC, compared with rTMS over the left DLPFC and sham rTMS, volunteers took more risk at the Risk Task1 (Rogers et al., 1999). In a following experiment (Knoch, Pascual-Leone, Meyer, Treyer, and Fehr, 2006b), they showed that applying low-frequency rTMS over the right DLPFC, compared with rTMS over the left DLPFC and sham rTMS, in healthy volunteers resulted in a reduction of willingness to reject their partners’ unfair offers as assessed by the Ultimatum Game,2 even though they still judged these offers as unfair.
In 2007, we reported that during bilateral stimulation over DLPFC using tDCS, healthy volunteers displayed a more conservative, risk-averse response style, compared with those with sham stimulation and those with unilateral DLPFC stimulation. Healthy volunteers receiving bilateral DLPFC stimulation showed lower risk taking at the Balloon Analog Risk Task3 (BART; Lejuez et al., 2002) than participants who received sham stimulation and unilateral stimulation (Fecteau et al., 2007b). This was the case regardless of whether anodal stimulation was applied to the right DLPFC (coupled with cathodal over the left DLPFC) or vice versa (anodal over the left DLPFC with cathodal over the right DLPFC). In addition, we found (Fecteau et al. 2007a) that healthy volunteers receiving anodal stimulation over the right DLPFC coupled with cathodal over the left DLPFC chose more often the safest prospects as measured by the Risk Task. Conversely, participants receiving anodal stimulation over the left DLPFC coupled with cathodal over the right DLPFC did not differ in their choice related to risk-taking behaviors from those receiving sham stimulation. Together, these findings demonstrate a causal link between the DLPFC and decision-making behaviors and reveal the capability of noninvasive brain stimulation techniques to influence decision-making processes.
Decision-Making Is Abnormal in Addicts and Plays a Critical Role in the Chronicity of Treatment
Risky decision-making is considered to be a characteristic behavioral phenotype of addiction and plays a critical role in the maintenance and relapse of substance use and abuse (e.g., Fishbein et al., 2005; Garavan and Stout, 2005).Although addicts represent a heterogeneous population, they often display excessive risk taking (Bechara et al., 2001, 2002; Epstein, Bang, and Botvin, 2007; Grant, Contoreggi, and London, 2000; Lejuez et al., 2003). Risk taking shares similar behavioral sensations to those of substance craving (Goeders, 2002) and elicits similar neural activations. Functional neuroimaging studies have revealed fairly consistent data indicating abnormal activity in the DLPFC in addicts associated with the experience of craving (see reviews from Brody, 2006; Goldstein and Volkow, 2002;Wilson, Sayette, and Fiez, 2004), induced by substance-related cues for nicotine (Brody, Mandelkern, and London, 2002; McBride et al., 2006; Wilson, Sayette, Delgado, and Fiez, 2005), cocaine (Bonson et al., 2002; Garavan et al., 2000; Grant et al., 1996; Maas et al., 1998) and alcohol (George et al., 2001; Olbrich et al., 2006). For instance, in Brody and colleagues (2002), activations in the DLPFC were found in “heavy smokers” when exposed to cigarette-related cues compared with non-smokers. George and colleagues (2001) reported that alcoholic individuals exhibited increased activity in the left DLPFC while viewing alcohol cues, while these activations were not found when they were viewing pictures of non-alcoholic beverages. Such activations in the DLPFC associated with alcohol pictures were not observed in social drinkers. Importantly, positive correlations between the size of DLPFC activations and self-ratings of craving have been reported in several studies (Bonson et al., 2002; Brody et al., 2002; Grant et al., 1996; Maas et al., 1998; Volkow et al., 1991). Brain structural deficits have also been reported in addicts. For instance, structural deficits in smokers in prefrontal cortices were found (Domino, 2008; Gallinat et al., 2006), such as smaller grey matter volumes and lower grey matter densities (Brody et al., 2004). Moreover, the number of cigarettes smoked has been inversely related to prefrontal cortical tissue (Brody et al., 2004) and positively related to severity of regional lobar white matter signal hyperintensities (Brody et al., 2004; Fukuda and Kitani, 1996). In patients with alcoholism, an increase in prefrontal volume occurs early in the course of abstinence, such as an increase of white matter volume after 20 days of sobriety in seven patients (Agartz et al., 2003) and an increase of grey matter volume after 32 days of sobriety (Pfefferbaum et al., 1995).
The Kind of Brain Stimulation that Modifies Decision-Making in Normal Subjects Suppresses Craving in Addicts
Upregulating activity in normal participants results in more cautious, risk-averse behaviors (Fecteau et al., 2007a, 2007b). Because excessive risk taking appears linked to an increased vulnerability for addictive pathological behavior, the same neuromodulation interventions that change decision-making in normal individuals may be translated into therapeutic intervention for addictive behaviors and craving (craving is defined here as “both a stable background inclination or propensity to seek drugs and as a relatively acute and short-lived experience of an urge” as proposed by Rosenberg, 2009 and Ferguson and Shiffman, 2009). The application of brain stimulation over the DLFPC has recently been used as an investigational therapeutic tool for addicts. So far, neuromodulation findings are promising for populations who abuse nicotine, alcohol, and cocaine. Main results are presented in Table 2. These proof-of-principle findings are consistent with therapies based on molecular and cellular approaches targeting the DLPFC (Vocci, Acri, and Elkashef, 2005). Recently, in animal models, Peters, LaLumiere, and Kalivas (2008) showed that the prefrontal cortex (as well as the nucleus accumbens activity) plays a critical role in extinction training to suppress cocaine seeking, and Belin, Mar, Dalley, Robbins, and Everitt (2008) found that high impulsivity predicts compulsive cocaine taking and depends on prefrontal activity.
Table 2.
Review of findings of the effects of neuromodulation on craving and substance use in patients with addiction
| Substance Parameters Regions N of sessions |
Patients | Measurement and effects | References |
|---|---|---|---|
| Nicotine | |||
| 20 Hz rTMS | Treatment-seeking nicotine smokers | Substance intake: Decrease | Eichhammer et al. (2003) |
| L DLPFC | Craving: No change | ||
| 1 session | |||
| 2 mA tDCS | Smokers | Craving: Decrease | Fregni et al. (2008) |
| R/L DLPFC | |||
| 1 session | |||
| 2 mA tDCS | Smokers | Substance intake: Decrease | Boggio et al. (2009) |
| R/L DLPFC | Craving: Decrease | ||
| 5 sessions | |||
| 10 Hz rTMS | Smokers | Substance intake: Decrease | Amiaz et al. (2009) |
| L DLPFC | |||
| 10 sessions | Craving: Decrease | ||
| Alcohol | |||
| 2 mA tDCS | Abstinent alcoholics in out-patients detoxification clinic | Substance intake: No change | Boggio et al. (2008) |
| R/L DLPFC | Craving: Decrease | ||
| 1 session | |||
| Cocaine | |||
| 10 Hz rTMS | Abstinent cocaine abusers in in-patients detoxification clinic | Craving: Decrease | Camprodon et al. (2007) |
| R DLPFC | |||
| 1 session | |||
| 15 Hz rTMS | Abstinent cocaine abusers post-detoxification | Craving: Decrease | Politi et al. (2008) |
| L DLPFC | |||
| 10 sessions | |||
| Food | |||
| 10 Hz rTMS | Subjects reporting severe and frequent urge to eat | Caloric ingestion: No change | Uher et al. (2005) |
| L DLPFC | Craving: Decrease | ||
| 1 session | |||
| 2 mA tDCS | Subjects reporting severe and frequent urge to eat | Caloric ingestion: Decrease | Fregni et al. (2007) |
| R/L DLPFC | Craving: Decrease | ||
| 1 session |
Note: L = left; R = right.
Eichhammer and colleagues (2003) showed that after a single session of high-frequency rTMS over the left DLPFC, treatment-seeking smokers significantly decreased the number of cigarettes smoked during an ad libitum smoking period after the stimulation session compared with sham condition; however, the level of craving for nicotine remains the same after a period of acute smoking abstinence of 6 h. More recently, we conducted a placebo-controlled, randomized, double-blind, crossover tDCS study using a cue–reactivity paradigm in smokers in order to transiently reduce the level of craving for nicotine (Fregni et al., 2008). Each volunteer received three stimulation conditions in separate days (anodal stimulation over the right DLPFC coupled with cathodal stimulation over the left DLPFC (anodal right/cathodal left), anodal left/cathodal right, and sham stimulation) and were asked to rate their craving level at different time points before and after stimulation. First, we confirmed that the cue-provoked strategy was efficient, as craving was increased before and after presenting smoking cues when they receive sham stimulation. In regard to the effects of neuromodulation, there was a significant decrease in craving in both active stimulation conditions when compared with craving ratings before and after stimulation. Finally, the modulations of left and right DLPFC excitability by tDCS reduced cue-related and non-cue-related craving significantly compared with sham stimulation. Using a similar design but applying a 5-day tDCS regimen (anodal stimulation over the left DLPFC coupled with cathodal over the right DLPFC), we found a significant decrease in craving and number of cigarettes smoked as compared with sham stimulation (Boggio et al., 2009). Also, Amiaz and colleagues (2009) reported a decrease in nicotine craving, but no change in nicotine intake, in smokers after they received high-frequency rTMS over the left DLPFC.
Camprodon and colleagues (2007) conducted a randomized crossover high-frequency rTMS study over the left or the right DLPFC in order to reduce craving in cocaine addicts. Participants were willing to stop using cocaine and were hospitalized in the context of an inpatient detoxification program. They were asked to rate their craving level on visual analog scales at three time points (pre-stimulation, immediately post-stimulation, and 4 h post-stimulation). Ratings of the “desire to consume cocaine” were significantly reduced after receiving a single rTMS session over the right DLPFC as compared with pre-stimulation and the effect extinguished 4 h after stimulation as the craving level at this time point was similar to that of pre-stimulation. No significant effects were observed after stimulation over the left DLPFC. Politi and colleagues (2008) also observed a decrease in craving in addicts to cocaine after patients received high-frequency rTMS over the left DLPFC.
We also conducted a randomized, sham-controlled, double-blind, crossover tDCS study in patients with alcohol dependence to reduce alcohol craving (Boggio et al., 2008). We used an urge-elicitation strategy, which was successful as there was an increase in craving after presenting alcohol cues for sham condition. After receiving active stimulation over bilateral DLPFC (anodal left/cathodal right or anodal left/cathodal right), patients rated levels of craving lower, compared with when they received sham stimulation. Specifically, the mean craving change was −2%, +20%, and +27% for sham, anodal left/cathodal right, and anodal right/cathodal left stimulation. There was no difference between the two active stimulation conditions.
Although overeating is not considered as an addiction by the DSM-IV (APA, 1994), overeating in obese individuals shares similarities with the loss of control and compulsive drug-taking behaviors observed in drug-addicted individuals. Increasing evidence suggests that the decision control of eating origins in neural networks associated with decision-making (Alonso-Alonso and Pascual-Leone, 2006; Pignatti et al., 2006). Not only have impaired decision-making behaviors been reported in obese patients as assessed by the Gambling Task (Davis, Levitan, Muglia, Bewell, and Kennedy, 2004; Pignatti et al., 2006), but they have also been correlated to the body mass index (Davis et al., 2004). Moreover, substance taking in drug-addict individuals and overeating in individuals with obesity share neural similarities, such as reduced striatal dopamine (DA) D2 receptors (Wang, Volkow, Thanos, and Fowler, 2004). In 2005, Uher and colleagues (2005) conducted a high-frequency rTMS study in women with frequent food craving. They observed a significant diminished food craving after receiving a single session of 10 Hz rTMS over the left DLPFC. However, in the ad libitum eating period, the amount of energy content consumed did not differ between active and sham stimulation. In 2007, we applied bilateral stimulation of DLPFC using tDCS to reduce food craving (Fregni et al., 2007).We performed a placebo-controlled, randomized, double-blind, crossover study with individuals who had frequent and strong urges to eat but without clinical eating disorders. They received three types of stimulation: anodal right/cathodal left, anodal left/cathodal right, and sham stimulation. They rated their craving level on visual analog scales. We also used an eye-tracking system to measure the fixation time on food items while participants were viewing pictures of food and non-food items. After the sham stimulation, craving was significantly increased by presentation of food-related cues. After receiving anodal right/cathodal left stimulation, participants showed a significant reduced craving as compared to before stimulation. However, the craving level remained the same before and after anodal left/cathodal right stimulation. Importantly, after both active stimulations, the fixation time for food items significantly decreased as compared with an increase after sham stimulation. Finally, caloric ingestion after active stimulation was significantly lower than that after sham stimulation.
A decrease in craving symptoms using neuromodulation may carry potential therapeutic benefits for population with addiction. Importantly, both rTMS and tDCS techniques appear to be safe in humans as shown by neuropsychological testing (Iyer et al., 2005), EEG assessment (Iyer et al., 2005), neuroimaging study (Nitsche et al., 2004), and brain metabolites evaluation (Nitsche and Paulus, 2001). For rTMS, the most severe adverse effect is a seizure. However, the overall risk of this complication is very low, and recommended guidelines largely prevent it (Rossini and Rossi, 2007; Wassermann, 1998). For tDCS, because this technique only induces a small electric current, chances of inducing seizures are not likely (Poreisz, Boros, Antal, and Paulus, 2007). Other mild adverse effects such as headache and neck pain are common to both techniques (Machii, Cohen, Ramos-Estebanez, and Pascual-Leone, 2006; Rossi, Hallett, Rossini, Pascual-Leone, Safety of TMS Consensus Group, 2009), but can be readily treated with analgesics and their incidence minimized following proper precautions (Wagner et al., 2007b). Neuromodulation studies in addicts have reported only few mild adverse effects and were similar in groups receiving active or placebo tDCS stimulation (Fregni et al., 2008). The most frequent adverse effects were headache and local itching.
Conceptual Models of the Role of DLPFC in Decision-Making and Addiction
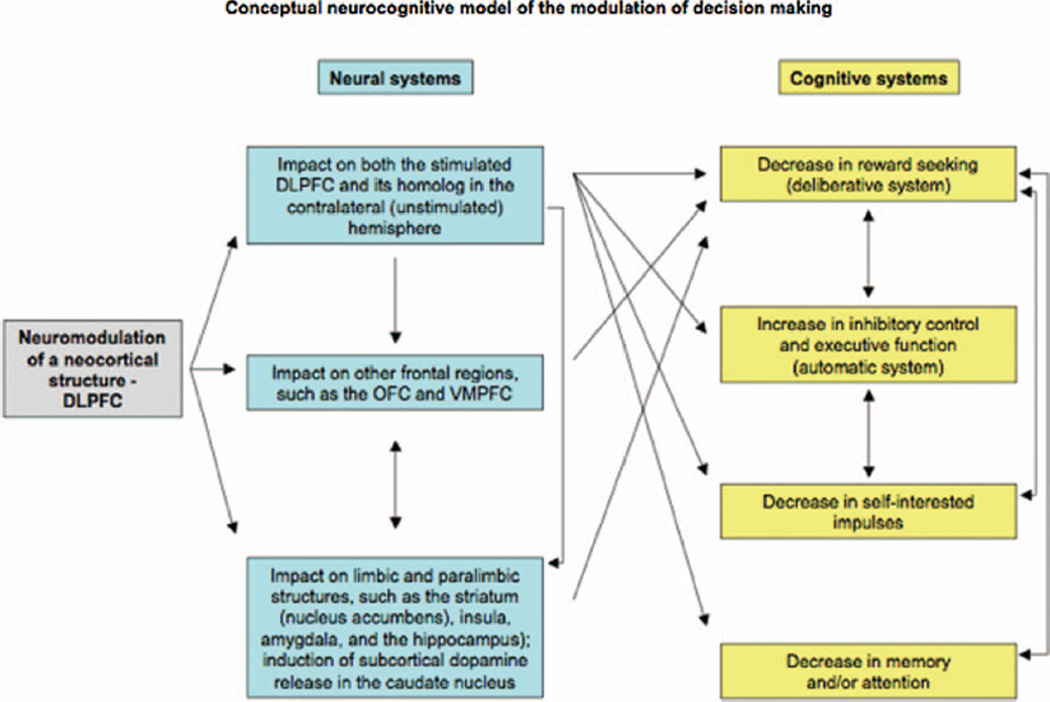
Most work has been focusing on the neuroplasticity of limbic and striatal/subcortical structures to account for addiction. This is certainly a very important concept; however, we believe that there is a neocortical control mechanism that is cognitive and also fails in the addictive brain. Modulation of DLPFC activity can lead to changes in decision-making behaviors and craving reduction. These results are in line with studies suggesting that risk taking and craving induce similar behavioral, physiological, and neural responses. For example, in a rat model of cocaine addiction, Belin and colleagues (2008) found that “whereas high reactivity to novelty predicts the propensity to initiate cocaine self-administration, high impulsivity predicts development of addiction-like behavior (…), including persistent or compulsive drug-taking in the face of aversive outcomes.” Here we propose a neurocognitive model of the modulation of decision-making which suggests translational approaches to addictive behavior in humans (Figure 1).
Figure 1.
Basic neural and cognitive cascade of events impacted by modulation of DLPFC on decision-making. Importantly, in our conceptual model, hypothetical systems involved are not mutually exclusive; they share commonalities and overlap in several aspects. OFC, orbitofrontal cortex; VMPFC, ventromedial prefrontal cortex.
Cognitive Systems
The DLPFC appears to be centrally involved in decision-making by integrating cognitive (inhibitory control/executive function) and emotionally relevant information (reward processing/motivation, deliberative systems). Its activity is involved in the cognitive control of the emotional impulse by providing adequate inhibition in the context of seductive options (Bechara, 2005; Ernst and Paulus, 2005; Evans, 2008; Groenewegen and Uylings, 2000; Krawczyk, 2002). One possible mechanism underlying changes in risk taking and craving is that modulation of DLPFC activity increases the level of inhibitory control of prepotent, impulsive responses required for appropriate decision-making behaviors.
Another possibility is that modulation of DLPFC activity might alter the reward-seeking behavior and mimic craving-related processes as suggested by Eichhammer and colleagues (2003). In support of this hypothesis, we found (Fecteau et al., 2007a) that healthy volunteers receiving bilateral DLPFC modulation were significantly less influenced by the reward options at the Risk Task (described earlier) than those with sham stimulation. In this task, response times are usually related to the level of risk and the size of reward, and subjects take more deliberation time for choosing the most likely outcome when its associated reward is decreased in comparison with that associated with the least likely outcome (Rogers et al., 1999). However, participants receiving bilateral DLPFC modulation were significantly faster to make their choice than all other participants, suggesting that they were so reward insensitive that they did not even seriously consider the choice of greater reward even in the less risky context (reward of 10:90 points in the context of risk of 4:2). Modulated DLPFC activity may thus simply abolish sensitivity to reward.
In line with this, modulation of the DLPFC activity seems to impact self-interested impulses, which could lead to reduced craving. Transient suppression of the DLPFC activity appears to override self-interested impulses. Knoch et al. (2006b) found that healthy volunteers behaved more selfishly at the Ultimatum Game (described earlier) after receiving a single session of low-frequency rTMS over the right DLPFC. Importantly, volunteers were accepting unfair offers from their partners although they still judged the offers as unfair. Some addicted user patients manifested similar patterns: although they are aware that their addiction has significant negative consequences for themselves and their peers, they keep abusing the substance. The relative loss of such impulses might be one of the reasons that patients have difficulties to quit their substance addiction. Therefore, the final outcome of neuromodulation may be a decrease in the impulsive self-interest motives, which leads to decreased craving. In support with this, Fregni et al. (2007) found that prefrontal stimulation did not change how the volunteers rated the smell and appearance of food, however craving and caloric ingestion were significantly decreased.
Finally, modulation of DLPFC might also impact addiction-relevant memory. DLPFC is a crucial area for memory, planning, and sustained attention (Cabeza and Nyberg, 2000; Passingham, 1993; Petrides, 1994), and cue-associated anticipation and planning of imminent substance use elicit DLPFC activations (Grant et al., 1996). In addicts, abnormal overactivations in the prefrontal cortex were found during a simple visual attentional task (Tomasi et al., 2007; see also review from Hyman, Malenka, and Nestler, 2006). Behaviorally, stronger memories and a greater sustained attention during substance-related cues are associated with levels of craving (Brody et al., 2002). Neuromodulation may suppress these memories or attentional biases resulting in decreased craving. For instance, Fregni and colleagues (2007) found that DLPFC modulation, which decreased food craving, also resulted in an attentional shift to non-food stimuli even in the presence of food stimuli that would otherwise preferentially capture attention as assessed by eye-tracking data.
Neural Systems
Future studies must measure the neural changes associated with beneficial effects of prefrontal neuromodulation for addiction. The identification of the specific neural networks externally activated and/or deactivated by brain stimulation will certainly contribute to deciphering the specific mechanisms responsible for diminishing risky behaviors and craving. Certainly, not only are the effects of neuromodulation local but they also spread trans-synaptically across bi-hemispheric cortico-subcortical networks connected with the targeted area (Nahas et al., 2001; Valero-Cabré, Payne, and Pascual-Leone, 2007; Wagner et al., 2007).
The Role of Dopaminergic Pathways
Neuromodulation might suppress the reward-seeking process via modulation of the dopaminergic systems or other neurotransmitters associated with the mesolimbic dopaminergic reward system. Most drugs of abuse target the neural reward system by rapidly flooding the circuit with dopamine (DA). Because of the overstimulation of the system by the drug, the addicted state is marked by a weak dopamine function by reductions in striatal D2 DA receptors. Therefore, the brain produces less dopamine or reduces the number of dopamine receptors in the reward circuit (see review from Volkow, Fowler, Wang, Swanson, and Telang, 2007). For instance, smoking withdrawal is associated with a hypo-dopaminergic state in humans (Smolka, Budde, Karow, and Schmidt, 2004) and animals (Epping-Jordan, Watkins, Koob, and Markou, 1998; Fung, Schmid, Anderson, and Lau, 1996). Pathologically obese subjects also display a reduced level of striatal D2 DA receptors, which is inversely related to the body mass index (Wang et al., 2004). Thus, addicts seek substances that will compensate for this reduced sensitivity of neurotransmitters of reward circuits. Dopaminergic antagonists such as clozapine and olanzapine can decrease substance craving (Green, Zimmet, Strous, and Schildkraut, 1999; Hutchison et al., 2006). The dopaminergic mesocorticolimbic pathway arises in the ventral tegmental area and connects brain structures including the DLPFC and nucleus accumbens (Berridge and Robinson, 1998); thus, a reduction of the sensitivity or number of dopamine receptors is likely to impact DLPFC activity. On the other hand, modulation of the DLPFC activity via brain stimulation techniques may modify this cascade of events. High-frequency rTMS over the DLPFC can induce dopamine release in the caudate nucleus (Strafella, Paus, Barrett, and Dagher, 2001) as well as in the ipsilateral anterior cingulate and orbitofrontal cortex (Cho and Strafella, 2009), which may provide a possible mechanism of action. More recently, Nitsche and colleagues (2006) showed that sulpiride, a D2 receptor blocker, abolished the induction of tDCS effects nearly completely after stimulation.
The Role of the Orbitofrontal Cortex
Modulation of the DLPFC may also coactivate other frontal regions such as the orbitofrontal/ventromedial cortex because they are densely interconnected (Ghashghaei and Barbas, 2002) and spatially close. The orbitofrontal region appears to be especially involved in inhibitory control functions (Elliot and Deakin, 2005) and craving (Fowler and Volkow, 1998; London, Bonson, Ernst, and Grant, 2000), and is known to have extensive connections to other brain areas such as the striatum and amygdala. Thus, the orbitofrontal cortex may serve to integrate cortical and subcortical processing of motivational behavior and reward. According to the model of Ernst and Paulus (2005), the DLPFC is involved in the cognitive process of decision-making, whereas the ventrolateral and ventromedial prefrontal cortex, the striatum, and the amygdala are involved in the affective process of decision-making. Given the tight and reciprocal interaction between DLPFC and orbitofrontal cortex, modulation of the DLPFC might simultaneously modulate both cognitive and affective neural-related processes of decision-making.
Bihemispheric Contributions and Issues of Lateralization
Finally, modulating activity in one DLPFC is presumed to have transcallosal (and opposite) effects on the activity of its homolog in the contralateral (unstimulated) hemisphere. For instance, George and colleagues (1999) demonstrated that high-frequency rTMS over the left DLPFC reduced activity in the right DLPFC as measured by PET. Therefore, even when stimulation is applied unilaterally, there is likely modulation of activity in the contralateral hemisphere that may, in fact, contribute to the observed behavioral effects. In Fecteau and colleagues (2007b), changes in risk-taking behaviors were observed only when applying bilateral modulation of the DLPFC (anodal tDCS coupled with cathodal tDCS), whereas no significant change was found when applying unilateral stimulation (anodal over one DLPFC coupled with cathodal tDCS over the contralateral supraorbital area). One possible explanation is that the effects are mediated by the balance of activity across the hemispheres and that brain stimulation exerts its effects by altering the relative balance of the two DLPFCs: relative hyperactivation of one DLPFC and suppression of cortical excitability of the contralateral DLPFC. There may be a critical cross-hemisphere interplay between the right and left DLPFC during decision-making that is altered by the bilateral electrode placement. In regard to the addictive brain, beneficial effects on craving were observed with bilateral tDCS stimulation (Boggio et al., 2008; Fregni et al., 2008), high-frequency rTMS over the left DLPFC (Eichhammer et al., 2003) and over the right DLPFC (Camprodon et al., 2007). Interestingly, after receiving left anodal/right cathodal stimulation, subjects displayed no change in food craving ratings; however, the amount of caloric ingestion was diminished after active stimulation regardless of whether the anodal electrode was placed over the right or left DLPFC coupled with the cathodal electrode over the contralateral DLPFC (Fregni et al., 2007). Modulation of either right or left DLPFC might disrupt the cross-hemispheric interplay during decision-making that might be necessary for craving states and substance use. Support for this notion of balanced bilateral activity of DLPFC during craving states has been shown in neuroimaging studies (Wilson et al., 2004).
Inter-individual Differences and the Role of Expectation
Certainly, neuromodulation might be more effective for some patients than others, as addicts, a heterogeneous population, display different neural patterns related to their substance use and misuse. For instance, treatment-seeking status and state of expectancy to use the substance in a near future appear to induce differential patterns of DLPFC activity elicited by drug cues (Wilson et al., 2004). In a meta-analysis including 19 cue-induced studies of drug craving, Wilson et al. (2004) noted that activations in the DLPFC associated with drug cues were mainly observed in addicts not seeking treatment, who likely expect to use the drug after the experiment. Conversely, studies with addicts undergoing treatment fail to show significant DLPFC activation by drug cues. In McBride and colleagues (2006), smoking cue-induced DLPFC response in actively using addicts was modulated by both expectancy to smoke in a near future and craving levels. Only addicts with expectancy of smoking in the near future showed significant bilateral DLPFC responses, compared with those with no expectancy. Moreover, greater activity in the left DLPFC was correlated with smoking expectancy and a higher level of craving. Interestingly, craving is reported by addicts in the presence and absence of DLPFC activation (Wilson et al., 2004). Wilson and colleagues (2004) concluded that the DLPFC is a region most influenced by perceived drug use opportunity. It is also worth mentioning that behavioral and physiological responses are also different in regard to the state of expectancy to use the substance. When instructed that drugs were available for consumption during the experiment, addicts produced distinct affective (Carter and Tiffany, 2001; Sayette, Martin, Hull, Wertz, and Perrott, 2003) and physiological (Carter and Tiffany, 2001; Lazev, Herzog, and Brandon, 1999; Zinser, Fiore, Davidson, and Baker, 1999) responses, and report a higher level of craving (Carter and Tiffany, 2001; Droungas, Ehrman, Childress, and O’Brien, 1995; Juliano and Brandon, 1998; Sayette et al., 2003) when instructed that drugs were not available for an extended period of time (Wertz and Sayette, 2001). Future work that correlates the change on decision-making with the clinical impact on craving and on substance use and abuse is warranted to support the conceptual framework we are offering in this paper.
Summary
In sum, experimental proof-of-principle findings reveal that modulation of the DLPFC might be a valuable adjunct in the treatment of addiction. We hypothesize that such effects are linked to shifts in decision-making related to the role of the DLPFC in impulsivity control, reward, motivation, and expectation.
In the last two decades, the use of brain stimulation has generated a groundbreaking research field for the treatment of the neuropsychiatric disorders (Daskalakis, Christensen, Fitzgerald, and Chen, 2002; Lisanby, Kinnunen, and Crupain, 2002). Because risky decision-making seems to be, in part, responsible for the maintenance and relapse of addiction, a neuromodulation-based approach to modulate decision-making is particularly interesting. Moreover, the neuromodulation-based approach has the advantage that the effects are immediate. Craving and substance use and abuse are highly variable and dependent on environmental influences and a single treatment of brain stimulation can transiently and immediately block or reduce this drug-seeking process.
It is also worth mentioning that modulation of DLPFC activity appears to have inhibitory effects on craving regardless of the substance, consistent with the concept that drug and food craving share a common neurobiological substrate (Wang et al., 2004). This suggests that other populations displaying detrimental decision-making behaviors or abnormal responses to reward, such as pathological gamblers (Brand, Franke-Sievert, Jacoby, Markowitsch, and Tuschen-Caffier, 2005; Cavedini, Riboldi, Keller, D’Annucci, and Bellodi, 2002; Goudriaan, Oosterlaan, de Beurs, and Van Den Brink, 2006), patients with bulimia, or those with anorexia nervosa (Davis and Woodside, 2002), might similarly benefit from neuromodulation-based approaches.
Acknowledgments
SF was supported by a fellowship from the Canadian Institutes of Health Research. JAC was supported by grants from the Fundación Rafael del Pino and the Harvard NeuroDiscovery Center (AY05). APL was supported by grants from the National Institutes of Health (K24 RR018875, RO1-NS 20068, R01-EB 005047), the National Alliance for Autism Research, the Nancy Lurie Marks Family Foundation, and the Klarman Foundation.
Glossary
- Repetitive transcranial magnetic stimulation
rTMS is a neurotechnique that enables modulation of cortical activity through the application of focal, repeated magnetic fields. Opposite modulatory effects on cortical activity can be induced depending on the frequency of stimulation; low-frequency rTMS has an inhibitory effect, whereas high-frequency rTMS facilitates activity (Chen, 1997; Gangitano, 2002; Romero, Anschel, Sparing, Gangitano, and Pascual-Leone, 2002). Finally, its effect on physiologic, neural, and behavioral responses outlasts the period of stimulation (Pascual-Leone, Rubio, Pallardo, and Catala, 1996; Walsh and Pascual-Leone, 2003).
- Transcranial direct current stimulation
tDCS is a neurotechnique that uses a weak electric current using large electrodes to induce neural changes (Gandiga, Hummel, and Cohen, 2006). Its effects depend on the polarity of stimulation. Anodal stimulation increases cortical excitability and cathodal stimulation decreases it (Antal, Nitsche, and Paulus, 2001; Ardolino, Bossi, Barbieri, and Priori, 2005; Nitsche and Paulus, 2000, 2001). The after-effects of a single session can last for more than an hour (Nitsche and Paulus, 2000, 2001; Nitsche et al., 2003).
Biographies

Shirley Fecteau, PhD, is an Instructor of Neurology at Harvard Medical School and a member of the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center. She was awarded a CIHR fellowship (first percentile). Her research aims at promoting cognitive functions and behaviors related to the prefrontal cortex in neurological and psychiatric populations. In order to achieve this, she has been characterizing and modulating brain plasticity related to prefrontal functions in healthy individuals and patients using neuromodulation and neuroimaging techniques and real-time combination of these techniques. The motivation behind her work is to provide proof-of-concept studies suggesting potential improvement of cognitive functions and behaviors that may ultimately lead to beneficial interventions for clinical populations.

Felipe Fregni, MD, PhD, MPH, is an Assistant Professor of Neurology at Harvard Medical School, the Director of Clinical Trials Network at the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center, and the Director of the Continuing Education for Scholars in Clinical Science Program at Harvard Medical School.

Paulo S. Boggio, PhD, is an Adjunct Professor at Mackenzie Presbyterian University, Sao Paulo, Brazil. He is the Director of Research at the Center for Health and Biological Sciences at Mackenzie Presbyterian University and Director of the Cognitive Neuroscience Laboratory at the same institution.

Joan A. Camprodon, PhD, was born in Barcelona (Spain) and studied Medicine at the University of Barcelona and the Humboldt University in Berlin. He did a PhD in Neuroscience with Alvaro Pascual-Leone and a Master in Public Health at the Harvard School of Public Health. He obtained his first faculty appointment as Instructor of Neurology at Harvard Medical School and continued working at the Berenson-Allen Center for Noninvasive Brain Stimulation. He is currently a resident physician and researcher at the Department of Psychiatry of the Massachusetts General Hospital. His research focuses on the study of neural connectivity and plasticity in health and disease. He uses TMS, fMRI, and the simultaneous combination of both to study dynamic neural networks and their properties in the context of systems and cognitive neuroscience. He also works on the application of these paradigms and methods to patient-oriented questions in Translational Neuropsychiatry. This latter research particularly focuses on combining neuroimaging with neurostimulation to study the role of plasticity in the pathophysiology of neuropsychiatric disorders such as addictions, depression or neglect, and explore the therapeutic potential of neuromodulation. His clinical interests are in neuropsychiatry and behavioral neurology, at the interface between psychiatry, neurology, and medicine.

Alvaro Pascual-Leone is a Professor of Neurology and Director of the Berenson-Allen Center for Noninvasive Brain Stimulation at Beth Israel Deaconess Medical Center and Harvard Medical School, where he also serves as Program Director of the Harvard-Thorndike Clinical Research Center. He has authored over 350 scientific papers and is the recipient of several international honors and awards, including the Ramon y Cajal Award in Neuroscience (Spain), the Norman Geschwind Prize in Behavioral Neurology from the American Academy of Neurology, the Friedrich Wilhelm Bessel Research Award from The Alexander von Humboldt Foundation (Germany), and the Jean Signoret Prize from the Ipsen Foundation (France). His research aims at understanding the mechanisms that control brain plasticity across the lifespan to be able to modify them for the subjects’ optimal behavioral outcome. Pascual-Leone combines various brain imaging and brain stimulation methodologies to establish a causal relationship and a precise chronometry between regional brain activation and behavior, and uses noninvasive brain stimulation techniques to modulate brain plasticity, suppressing some changes and enhancing others, to gain a clinical benefit and behavioral advantage for a given individual. Such noninvasive approaches can lead to clinically relevant therapeutic effects in neuropsychiatry and neurorehabilitation, and serve as proof-of-principle prior to more invasive neuromodulatory interventions.
Footnotes
In the Risk Task, subjects are presented with six horizontally arranged boxes that could be pink or blue. The ration of pink and blue boxes varies from trial to trial and can be 5:1, 4:2, or 3:3. Participants have to pick the color of the box that hides the winning token. They are told that the token is equally likely to be hidden in any of the boxes. Therefore, for each trial, the ratio of pink to blue boxes (referred to as level of risk) effectively determines the probability of finding the winning token and thus the level of risk of the choice. Participants are rewarded with points for correctly guessing the color of the box hiding the winning token and punished by losing points for picking the incorrect color. The amount of reward (or penalty) points associated with any scenario varies and is clearly indicated on the computer screen. Here, the conflict inherent in risk taking is reflected by the fact that the largest reward is always associated with the least likely of the two outcomes (i.e., the most risky option).
In the Ultimatum Game, a proposer (here the investigator) offers the subject to split a certain amount of money. The subject can either accept or reject the offer. If he or she accepts the offer, the money is split as proposed, but if he or she rejects, none of them receive the money.
In the BART, subjects have to make a choice in a context of increasing risk. They are invited to inflate a computerized balloon by pushing a “pump.” The balloon can explode at any moment. Participants have to decide after each pump whether to keep pumping and risk explosion of the balloon, or to stop. Subjects accumulate money in a temporary bank with each pump (e.g., 5 cents for each pump). When the subject decides to stop pumping, the accumulated money is transferred to a permanent bank. However, if the balloon explodes, all of the money accumulated in the temporary bank is lost. Therefore, the probability of losing the money, as well as the potential loss (i.e., the amount of money), increases with each pump.
Declaration of Interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- Agartz I, Brag S, Franck J, Hammarberg A, Okugawa G, Svinhufvud K, et al. MR volumetry during acute alcohol withdrawal and abstinence: a descriptive study. Alcohol and Alcoholism. 2003;38:71–78. doi: 10.1093/alcalc/agg020. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. Journal of American Medical Association. 2006;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV) 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Amiaz R, Levy D, Vainiger D, Grunhaus L, Zangen A. Repeated high-frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction. 2009;104(4):653–660. doi: 10.1111/j.1360-0443.2008.02448.x. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Paulus W. External modulation of visual perception in humans. Neuroreport. 2001;12:3553–3555. doi: 10.1097/00001756-200111160-00036. [DOI] [PubMed] [Google Scholar]
- Ardolino G, Bossi B, Barbieri S, Priori A. Non-synaptic mechanisms underlie the after-effects of cathodal transcutaneous direct current stimulation of the human brain. Journal of Physiology. 2005;568:653–663. doi: 10.1113/jphysiol.2005.088310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Review. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing current on the cerebral cortex of the rat during current flow and in the production of long-lasting after-effects. Journal of Physiology. 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Liguori P, Sultani N, Rezende L, Fecteau S, Fegni F. Cumulative priming effects of cortical stimulation on smoking cue-induced craving. Neuroscience Letters. 2009;463:82–86. doi: 10.1016/j.neulet.2009.07.041. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual-Leone A, et al. Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drugs and Alcohol Dependence. 2008;92:55–60. doi: 10.1016/j.drugalcdep.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Brand M, Franke-Sievert C, Jacoby GE, Markowitsch HJ, Tuschen-Caffier B. Neuropsychological correlates of decision making in patients with bulimia nervosa. Neuropsychology. 2005;1:742–750. doi: 10.1037/0894-4105.21.6.742. [DOI] [PubMed] [Google Scholar]
- Brody AL. Functional brain imaging of tobacco use and dependence. Journal of Psychiatric Research. 2006;40:404–418. doi: 10.1016/j.jpsychires.2005.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Jarvik ME, Lee GS, Smith EC, Huang JC, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biological Psychiatry. 2004;55(1):77–84. doi: 10.1016/s0006-3223(03)00610-3. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED. Brain metabolic changes during cigarette craving. Archives of General Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug and Alcohol Dependence. 2007;86:91–94. doi: 10.1016/j.drugalcdep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. The cue-availability paradigm: the effects of cigarette availability on cue reactivity in smokers. Experimental and Clinical Psychopharmacology. 2001;9:183–190. doi: 10.1037//1064-1297.9.2.183. [DOI] [PubMed] [Google Scholar]
- Cavedini P, Riboldi G, Keller R, D’Annucci A, Bellodi L. Frontal lobe dysfunction in pathological gambling patients. Biological Psychiatry. 2002;51:334–341. doi: 10.1016/s0006-3223(01)01227-6. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual smoking-attributable mortality, years of potential life lost, and economic costs – United States 1995–1999. Morbidity and Mortality Weekly Report. 2002;51:300–303. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults – United States. Morbidity and Mortality Weekly Report. 2004;53:427–431. [PubMed] [Google Scholar]
- Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE. 2009;4(8):e6725. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L, Manes F, Antoun N, Sahakian BJ, Ribbins TW. The contributions of lesion laterality and lesion volume to decision-making impairment following frontal lobe damage. Neuropsychologia. 2003;41:1474–1483. doi: 10.1016/s0028-3932(03)00081-2. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Damasio H, Christen Y. Neurobiology of decision-making. Berlin and New York: Springer; 1996. [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Transcranial magnetic stimulation: a new investigational and treatment tool in psychiatry. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:406–415. doi: 10.1176/jnp.14.4.406. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Muglia P, Bewell C, Kennedy JL. Decision-making deficits and overeating: a risk model for obesity. Obesity Research. 2004;12:929–935. doi: 10.1038/oby.2004.113. [DOI] [PubMed] [Google Scholar]
- Davis C, Woodside DB. Sensitivity to the rewarding effects of food and exercise in the eating disorders. Comprehensive Psychiatry. 2002;43:189–194. doi: 10.1053/comp.2002.32356. [DOI] [PubMed] [Google Scholar]
- Domino EF. Tobacco smoking and MRI/MRS brain abnormalities compared to nonsmokers. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32(8):1778–1781. doi: 10.1016/j.pnpbp.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addictive Behaviors. 1995;20:657–673. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Eichhammer P, Johann M, Kharraz A, Binder H, Pittrow D, Wodarz N, et al. High-frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. Journal of Clinical Psychiatry. 2003;64:951–953. doi: 10.4088/jcp.v64n0815. [DOI] [PubMed] [Google Scholar]
- Elliot R, Deakin B. Role of the orbitofrontal cortex in reinforcement processing and inhibitory control: evidence from functional magnetic resonance imaging studies in healthy human subjects. International Review of Neurobiology. 2005;65:89–116. doi: 10.1016/S0074-7742(04)65004-5. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- Epstein JA, Bang H, Botvin GJ. Which psychosocial factors moderate or directly affect substance use among inner-city adolescents? Addictive Behaviors. 2007;32:700–713. doi: 10.1016/j.addbeh.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Ernest M, Bolla K, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, et al. Decision-making in a risk-taking task: a PET study. Neuropsychopharmacology. 2002;26:682–691. doi: 10.1016/S0893-133X(01)00414-6. [DOI] [PubMed] [Google Scholar]
- Ernst M, Heischman SJ, Spurrgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Evans JS. Dual-processing accounts of reasoning, judgment, and social cognition. Annual Review of Psychology. 2008;59:255–278. doi: 10.1146/annurev.psych.59.103006.093629. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Knoch D, Fregni F, Sultani N, Boggio PS, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex. A direct current stimulation study. Journal of Neuroscience. 2007a;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Zald D, Liguori P, Théoret H, Boggio PS, et al. Activation of prefrontal cortex by transcranial direct current stimulation reduces appetite for risk during ambiguous decision making. Journal of Neuroscience. 2007b;27:6212–6218. doi: 10.1523/JNEUROSCI.0314-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Shiffman S. The relevance and treatment of cue-induced cravings in tobacco dependence. Journal of Substance Abuse Treatment. 2009;36:235–243. doi: 10.1016/j.jsat.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Fishbein DH, Eldreth DL, Hyde C, Matochik JA, London ED, Contoreggi C, et al. Risky decision making and the anterior cingulate cortex in abstinent drug abusers and nonusers. Cognitive Brain Research. 2005;23(1):119–136. doi: 10.1016/j.cogbrainres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND. PET imaging studies in drug abuse. Journal of Toxicology. Clinical Toxicology. 1998;36:163–174. doi: 10.3109/15563659809028936. [DOI] [PubMed] [Google Scholar]
- Fregni F, Liguori P, Fecteau S, Nitsche MA, Pascual-Leone A, Boggio PS. Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. Journal of Clinical Psychiatry. 2008;69:32–40. doi: 10.4088/jcp.v69n0105. [DOI] [PubMed] [Google Scholar]
- Fregni F, Orsati F, Pedrosa W, Fecteau S, Tome FA, Nitsche MA, et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2007;51(1):34–41. doi: 10.1016/j.appet.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Repetitive transcranial magnetic stimulation for the treatment of depression. Journal of Psychiatry and Neuroscience. 2005;30:434. [PMC free article] [PubMed] [Google Scholar]
- Fregni F, Pascual-Leone A. Hand motor recovery alter stroke: tuning the orchestra to improve hand motor function. Cognitive and Behavioral Neurology. 2006;19:21–33. doi: 10.1097/00146965-200603000-00003. [DOI] [PubMed] [Google Scholar]
- Fregni F, Simon D, Wu A, Pascual-Leone A. Noninvasive brain stimulation for Parkinson’s disease: a systematic review and meta-analysis of the literature. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:1614–1623. doi: 10.1136/jnnp.2005.069849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke. 1996;27:645–649. doi: 10.1161/01.str.27.4.645. [DOI] [PubMed] [Google Scholar]
- Fung YK, Schmid MJ, Anderson TM, Lau YS. Effects of nicotine withdrawal on central dopaminergic systems. Pharmacology, Biochemistry, and Behavior. 1996;53:635–640. doi: 10.1016/0091-3057(95)02063-2. [DOI] [PubMed] [Google Scholar]
- Gallinat J, Meisenzahl E, Jacobsen LK, Kalus P, Kienast T, Witthaus H, et al. Smoking and structural brain deficits: a volumetric MR investigation. European Journal of Neuroscience. 2006;24:1744–1750. doi: 10.1111/j.1460-9568.2006.05050.x. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS) A tool for double-blind sham-controlled clinical studies in brain stimulation. Clinical Neurophysiology. 2006;117:845–850. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends in Cognitive Sciences. 2005;9(4):195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, et al. Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry. 2001;58(4):345–352. doi: 10.1001/archpsyc.58.4.345. [DOI] [PubMed] [Google Scholar]
- George MS, Stallings LE, Speer AM, Nahas Z, Spicer KM, Vincent DJ, et al. Prefrontal repetitive transcranial magnetic stimulation (rTMS) changes relative perfusion locally and remotely. Human Psychopharmacology: Clinical and Experimental. 1999;14:161–170. [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. Psychoneuroendocrinology. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. American Journal of Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, Van Den Brink W. Psychophysiological determinants and concomitants of deficient decision making in pathological gamblers. Drug and Alcohol Dependence. 2006;84:231–239. doi: 10.1016/j.drugalcdep.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, et al. Activation of memory circuits during cue-elicited cocaine craving. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AL, Zimmet SV, Strous RD, Schildkraut JJ. Clozapine for comorbid substance use disorder and schizophrenia: do patients with schizophrenia have a reward-deficiency syndrome that can be ameliorated by clozapine? Harvard Review of Psychiatry. 1999;6:287–296. doi: 10.3109/10673229909017206. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Uylings HB. The prefrontal cortex and the integration of sensory, limbic and autonomic information. Progress in Brain Research. 2000;126:3–28. doi: 10.1016/S0079-6123(00)26003-2. [DOI] [PubMed] [Google Scholar]
- Güth W, Schmittberger R, Schwarze B. An experimental analysis of ultimatum bargaining. Journal of Economic Behavior and Organization. 1982;3:367–388. [Google Scholar]
- Hutchison KE, Ray L, Sandman E, Rutter MC, Peters A, Davidson D, et al. The effect of olanzapine on craving and alcohol consumption. Neuropsychopharmacology. 2006;31:1310–1317. doi: 10.1038/sj.npp.1300917. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Iyer MB, Mattu U, Grafman J, Lomarev M, Sato S, Wassermann EM. Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology. 2005;64:872–875. doi: 10.1212/01.WNL.0000152986.07469.E9. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. Reactivity to instructed smoking availability and environmental cues: evidence with urge and reaction time. Experimental and Clinical Psychopharmacology. 1998;6:45–53. doi: 10.1037//1064-1297.6.1.45. [DOI] [PubMed] [Google Scholar]
- Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. Journal of Neuroscience. 2006a;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006b;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32:477–484. doi: 10.1016/j.neuroimage.2006.02.047. [DOI] [PubMed] [Google Scholar]
- Krawczyk DC. Contributions of the prefrontal cortex to the neural basis of human decision making. Neuroscience and Biobehavioral Reviews. 2002;26:631–664. doi: 10.1016/s0149-7634(02)00021-0. [DOI] [PubMed] [Google Scholar]
- Lazev AB, Herzog TA, Brandon TH. Classical conditions of environmental cues to cigarette smoking. Experimental and Clinical Psychopharmacology. 1999;7:56–63. doi: 10.1037//1064-1297.7.1.56. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Aklin WM, Jones HA, Strong DR, Kahler CW, Read JP. The balloon analogue risk task (BART) differentiates smokers and nonsmokers. Experimental and Clinical Psychopharmacology. 2003;11:26–33. doi: 10.1037//1064-1297.11.1.26. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JR, Ramsey SE, Stuart GL, et al. Evaluation of a behavioral measure of risk-taking: the balloon analogue risk task (BART) Journal of Experimental Psychology. Applied. 2002;6:75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after effects of human motor cortex excitability. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Kinnunen LH, Crupain MJ. Applications of TMS to therapy in psychiatry. Journal of Clinical Neurophysiology. 2002;19:344–360. doi: 10.1097/00004691-200208000-00007. [DOI] [PubMed] [Google Scholar]
- London ED, Bonson KR, Ernst M, Grant S. Orbitofrontal cortex and human drug abuse: functional imaging. Cerebral Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, et al. Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. American Journal of Psychiatry. 1998;155:124–126. doi: 10.1176/ajp.155.1.124. [DOI] [PubMed] [Google Scholar]
- Machii K, Cohen R, Ramos-Estebanez C, Pascual-Leone A. Safety of rTMS to non-motor cortical areas in healthy participants and patients. Clinical Neurophysiology. 2006;117:455–471. doi: 10.1016/j.clinph.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clinical Neurophysiology. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Nahas Z, Teneback CC, Kozel A, Speer AM, DeBrux C, Molloy M, et al. Brain effects of TMS delivered over prefrontal cortex in depressed adults: role of stimulation frequency and coil-cortex distance. Journal of Neuropsychiatry and Clinical Neuroscience. 2001;13:459–470. doi: 10.1176/jnp.13.4.459. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortical neuroplasticity in humans. Cerebral Cortex. 2005;14:1240–1245. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Lampe C, Antal A, Liebetanz D, Lang N, Tergau F, et al. Dopaminergic modulation of long-lasting direct current-induced cortical excitability changes in the human motor cortex. European Journal of Neuroscience. 2006;23:1651–1617. doi: 10.1111/j.1460-9568.2006.04676.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Modulation of cortical excitability by weak direct current stimulation: technical, safety and functional aspects. Supplements to Clinical Neurophysiology. 2003;56:255–276. doi: 10.1016/s1567-424x(09)70230-2. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Niehaus L, Hoffmann KT, Hengst S, Liebetanz D, Paulus W, et al. MRI study of human brain exposed to weak direct current stimulation of the frontal cortex. Clinical Neurophysiology. 2004;115:2419–2423. doi: 10.1016/j.clinph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. Journal of Physiology. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. The Australian and New Zealand Journal of Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Rubio B, Pallardo F, Catala MD. Rapid-rate transcranial magnetic stimulation of left dorsolateral prefrontal cortex in drug-resistant depression. Lancet. 1996;348:233–237. doi: 10.1016/s0140-6736(96)01219-6. [DOI] [PubMed] [Google Scholar]
- Passingham RE. The frontal lobes and voluntary action. Oxford Psychology Series 21. Oxford: Oxford University Press; 1993. [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, et al. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. Journal of Neuroscience. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. European Journal of Neuroscience. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and behaviour. Current Opinion of Neurobiology. 1994;4:207–211. doi: 10.1016/0959-4388(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO. Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcoholism, Clinical and Experimental Research. 1995;19(5):1177–1191. doi: 10.1111/j.1530-0277.1995.tb01598.x. [DOI] [PubMed] [Google Scholar]
- Pignatti R, Bertella L, Albani G, Mauro A, Molinari E, Semenza C. Decision-making in obesity: a study using the Gambling Task. Eating and Weight Disorders. 2006;11:126–132. doi: 10.1007/BF03327557. [DOI] [PubMed] [Google Scholar]
- Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. The American Journal on Addictions. 2008;17(4):345–346. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Research Bulletin. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Purpura D, McMurtry J. Intracellular activities and evoked potential changes during polarization of motor cortex. Journal of Neurophysiology. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Ramos-Estebanez C, Machii K, Pascual-Leone A. [Retrieved 8 June, 2005];TMS in Neuropsychiatry. 2005 from http://www.cnsforum.com/magazine/nonpharmacological_treatment/tms/.
- Rogers RD, Owen AM, Middleton HC, Williams EJ, Pickard JD, Sahakian BJ, et al. Choosing between small, likely rewards and large, unlikely rewards activates inferior and orbital prefrontal cortex. Journal of Neuroscience. 1999;20:9029–9038. doi: 10.1523/JNEUROSCI.19-20-09029.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clinical Neurophysiology. 2002;113:101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg H. Clinical and laboratory assessment of the subjective experience of drug craving. Clinical Psychology Review. 2009;29(6):519–534. doi: 10.1016/j.cpr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120(12):2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:253–264. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11(4):389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling KJ, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimate Game. Science. 2003;300:1673–1675. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Martin CS, Hull JG, Wertz JM, Perrott MA. Effects of smoking opportunity on cue-elicited urge: a facial coding analysis. Journal of Abnormal Psychology. 2003;112:110–118. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Budde H, Karow AC, Schmidt LG. Neuroendocrinological and neuropsychological correlates of dopaminergic function in nicotine dependence. Psychopharmacology (Berlin) 2004;175:374–381. doi: 10.1007/s00213-004-1824-8. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. Journal of Neuroscience. 2001;21:RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. Repetitive transcranial magnetic stimulation. In: Lueders H, editor. Deep brain stimulation and epilepsy. London: Martin Dunitz; 2004. [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, et al. Thalamo-cortical dysfunction in cocaine abusers: implications in attention and perception. Psychiatry Research. 2007;155:189–201. doi: 10.1016/j.pscychresns.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, Eranti SV, Treasure J, Campbell IC, et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biological Psychiatry. 2005;58:840–842. doi: 10.1016/j.biopsych.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Payne BR, Pascual-Leone A. Opposite impact on 14C-2-deoxyglucose brain metabolism following patterns of high and low frequency repetitive transcranial magnetic stimulation in the posterior parietal cortex. Experimental Brain Research. 2007;176:603–615. doi: 10.1007/s00221-006-0639-8. [DOI] [PubMed] [Google Scholar]
- van’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16:1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. Medication development for addictive disorders: the state of the science. American Journal of Psychiatry. 2005;162:1432–1440. doi: 10.1176/appi.ajp.162.8.1432. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Archives of Neurology. 2007;64:1575–1579. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Bendriem B, Alpert R, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. American Journal of Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation. Neuroimage. 2007a;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annual Review of Biomedical Engineering. 2007b;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Neurochronometrics of mind: transcranial magnetic stimulation in cognitive science. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Wang GJ, Volkow ND, Thanos PK, Fowler JS. Similarity between obesity and drug addiction as assessed by neurofunctional imaging: a concept review. Journal of Addictive Diseases. 2004;23:39–53. doi: 10.1300/J069v23n03_04. [DOI] [PubMed] [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Delgado MR, Fiez JA. Instructed smoking expectancy modulates cue-elicited neural activity: a preliminary study. Nicotine and Tobacco Research. 2005;7:637–645. doi: 10.1080/14622200500185520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nature Neuroscience. 2004;7:211–214. doi: 10.1038/nn1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinser MC, Fiore MC, Davidson RJ, Baker TB. Manipulating smoking motivation: impact on an electrophysiological index of approach motivation. Journal of Abnormal Psychology. 1999;108:240–254. doi: 10.1037//0021-843x.108.2.240. [DOI] [PubMed] [Google Scholar]