Abstract
Background and Purpose
Functional magnetic resonance imaging (fMRI) studies could provide crucial information on the neural mechanisms of motor recovery in stroke patients. Resting-state fMRI is applicable to stroke patients who are not capable of proper performance of the motor task. In this study, we explored neural correlates of motor recovery in stroke patients by investigating longitudinal changes in resting-state functional connectivity of the ipsilesional primary motor cortex (M1).
Methods
A longitudinal observational study using repeated fMRI experiments was conducted in 12 patients with stroke. Resting-state fMRI data were acquired four times over a period of 6 months. Patients participated in the first session of fMRI shortly after onset, and thereafter in subsequent sessions at 1, 3, and 6 months after onset. Resting-state functional connectivity of the ipsilesional M1 was assessed and compared with that of healthy subjects.
Results
Compared with healthy subjects, patients demonstrated higher functional connectivity with the ipsilesional frontal and parietal cortices, bilateral thalamus, and cerebellum. Instead, functional connectivity with the contralesional M1 and occipital cortex were decreased in stroke patients. Functional connectivity between the ipsilesional and contralesional M1 showed the most asymmetry at 1 month after onset to the ipsilesional side. Functional connectivity of the ipsilesional M1 with the contralesional thalamus, supplementary motor area, and middle frontal gyrus at onset was positively correlated with motor recovery at 6 months after stroke.
Conclusions
Resting-state fMRI elicited distinctive but comparable results with previous task-based fMRI, presenting complementary and practical values for use in the study of stroke patients.
Keywords: Resting-state fMRI, Stroke, Motor recovery, Functional connectivity
Introduction
Functional magnetic resonance imaging (fMRI) has played an integral role in defining the neural substrates and mechanisms underlying recovery after brain disease, such as stroke, at the system level of the brain. Cortical reorganization has been characterized by observation of changes in brain activation during motor recovery following stroke.1–6 fMRI studies using motor activation tasks have been conducted for investigation of the effects of specific therapeutic interventions, including constraint-induced movement therapy,7 treadmill training,8 and repetitive transcranial magnetic stimulation;9 these studies focused on recovery mechanisms associated with these interventions.
On the other hand, longitudinal studies have been conducted for assessment of changes in brain activation that are related to recovery after stroke. The initial contralesional shift of activation and evolution to later ipsilesional activation,1,2 recruitment of additional regions that are not activated in healthy subjects,10 and importance of ipsilesional preexisting regions11 during motor recovery have been demonstrated using task-based fMRI. However, these reports showed certain variability in brain activation results; one reason for this diversity originated from use of diverse activation paradigms, which prevent adequate comparison between results, although passive movement4 and motor imagery5 have been proposed as alternative methods. In addition, longitudinal studies using task-based fMRI are limited in their application for stroke patients with severe impairment, and results may be confounded by changes in performance during recovery as well.
Resting-state fMRI is a recently evolving method, from which functional connectivity between distant brain regions is extracted based on low-frequency fluctuations. Although the meaning of the resting-state fMRI signal has been debated since its initial trial,12 evidence has suggested that resting fluctuations correspond to neuronal activation during task performance.13 The methodological advantage of resting state is that it can be performed without an overt task or external input; therefore, it is applicable to unconscious patients, infants,14 and even to experimental animals.15
In healthy subjects, resting-state fMRI has shown remarkable consistency in functional connectivity;16,17 however, significant differences were observed within the aged population18 or after interventions such as acupuncture.19 Resting-state fMRI has demonstrated unique changes in patients with various neurologic disorders, including Alzheimer’s disease,20 attention deficit hyperactivity disorder (ADHD),21 depression,22 and schizophrenia.23
For stroke patients with severe motor impairment who could not perform the fMRI activation task at the early stage of onset, it is expected to be achieved through long-term follow-up by use of resting-state fMRI. Therefore, in this study, we aimed to carry out long-term follow-up of resting-state fMRI in stroke patients for delineation of the neural substrates of motor recovery after stroke. We analyzed functional connectivity of the ipsilesional primary motor cortex (M1) in stroke patients and compared it with that of healthy subjects. In order to propose a plausible underlying mechanism for successful stroke recovery, we also investigated neural correlates associated with long-term motor recovery at 6 months after stroke.
Methods
Subjects
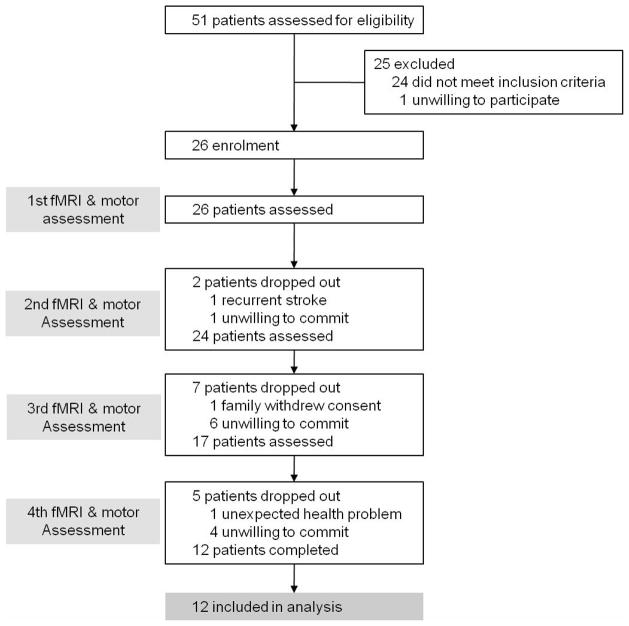
A total of 51 patients who suffered their first-ever stroke were assessed for their eligibility. Inclusion criteria were as follows: (1) less than 2 weeks from the onset of ischemic stroke, (2) unilateral supratentorial lesions, (3) moderate to severe motor deficits of the contralesional upper and lower extremities, and, (4) age older than 18 years and younger than 75 years. Exclusion criteria were as follows: (1) any clinically significant or unstable medical disorder, (2) any neuropsychiatric comorbidity other than stroke, and, (3) any contraindication to MRI. Twenty-five patients out of 51 were excluded and 26 patients were enrolled in this study. Fourteen patients dropped out during the follow-up period. Finally, 12 ischemic stroke patients (5 males and 7 females, 58.4±6.9 years) with supratentorial lesions completed longitudinal fMRI experiments, and their image data were included in the analysis (Figure 1, Table 1). Also, 11 healthy subjects (3 males and 8 females, 52.1±9.4 years) who reported no history of psychiatric or neurological problems were included as an age-matched control group. Experiments were conducted with the understanding and written consent of each participant, and ethics approval was provided by the Institutional Review Board.
Figure 1.
Patient enrollment process for a longitudinal observational study conducting repeated functional magnetic resonance imaging (fMRI) experiments. A total of 51 patients with first-ever stroke were assessed for their eligibility. Twenty-five patients were excluded and 14 patients dropped out during the follow-up fMRI experiments. Finally, 12 ischemic stroke patients completed longitudinal fMRI experiments. Acquisition of resting-state fMRI data, accompanied by behavioral assessment using Fugl-Meyer assessment, was performed within 2 weeks after onset, and then at 1, 3, and 6 months after onset.
Table 1.
Patient characteristics and motor function.
| Patients | Gender | Age (yrs.) | Lesion | FMA scores
|
FMA Change | |||
|---|---|---|---|---|---|---|---|---|
| Onset | 1 month | 3 months | 6 months | |||||
| 1 | F | 66 | Lt. MCA infarction | 8 | 8 | 19 | 27 | 19 |
| 2 | F | 61 | Lt. MCA infarction | 20 | 22 | 27 | 33 | 13 |
| 3 | F | 55 | Rt. MCA infarction | 30 | 55 | 70 | 73 | 43 |
| 4 | M | 74 | Lt. CR infarction | 16 | 22 | 17 | 21 | 5 |
| 5 | F | 58 | Lt. MCA infarction | 36 | 42 | 52 | 52 | 16 |
| 6 | F | 47 | Lt. MCA infarction | 44 | 59 | 100 | 100 | 56 |
| 7 | M | 55 | Lt. ACA infarction | 19 | 42 | 60 | 73 | 54 |
| 8 | M | 62 | Lt. MCA infarction | 19 | 22 | 52 | 57 | 38 |
| 9 | M | 59 | Rt. MCA infarction | 24 | 24 | 24 | 24 | 0 |
| 10 | F | 52 | Rt. CR infarction | 52 | 52 | 99 | 99 | 47 |
| 11 | M | 57 | Lt. MCA infarction | 13 | 13 | 52 | 52 | 39 |
| 12 | F | 55 | Rt. SC infarction | 9 | 9 | 34 | 34 | 25 |
|
| ||||||||
| Mean ± SD | M=5; F=7 | 58.4 ±6.9 | 24.2 ±13.8 | 30.8 ±18.3 | 50.5 ±28.5 | 53.8 ±27.7 | 29.6 ±19.1 | |
FMA, Fugl-Meyer assessment; F, female; M, male; Rt., right; Lt., left; MCA, middle cerebral artery; CR, corona radiata; ACA, anterior cerebral artery; SC, striatocapsular; FMA Change, FMA total scores at 6 months - FMA total scores at onset
Experimental Design
This study was designed as a longitudinal observational study for conduct of repeated fMRI experiments. A cross-sectional controlled study design was also applied for comparison of data from stroke patients to those of healthy subjects.
fMRI Data Acquisition
Resting-state fMRI data were longitudinally acquired four times over a period of 6 months in patients with stroke. Patients participated in the first session of fMRI shortly after onset (10.5±4.3 days), and thereafter in subsequent sessions at 1, 3, and 6 months after onset. In healthy subjects, we obtained one time resting-state fMRI data.
During the resting-state, subjects were instructed to keep their eyes closed and to remain motionless. fMRI data were acquired using a Philips ACHIEVA MR scanner (Philips Medical Systems, Best, The Netherlands) operating at 3 Tesla. At each session, a total of 100 whole-brain images were collected using a T2*-weighted gradient echo echo-planar imaging sequence (repetition time=3,000 msec, echo time=35 msec, number of slices=35, slice thickness=4 mm, matrix size=128×128, field of view=220 mm×220 mm).
Behavioral Assessment
Degree of motor impairment was scored using the Fugl-Meyer assessment (FMA) for upper and lower extremities24 on the same day as fMRI data acquisition.
fMRI DataAnalysis
fMRI data were preprocessed using SPM8 (Wellcome Trust Centre for Neuroimaging, University College London, London, UK) and AFNI (Scientific and Statistical Computing Core, National Institute of Mental Health, Bethesda, MD, US) software. Preprocessing steps included spatial realignment to the mean volume of a series of images, normalization into the same coordinate frame as the MNI-template brain, band-pass filtering between 0.01–0.08 Hz, and smoothing using a Gaussian filter of 8 mm FWHM.
Correlation analysis between the reference time course of the M1 and the time course of every voxel in the brain was performed for acquisition of a map of correlation coefficients that revealed functional connectivity of the M1. The reference time course was extracted from the ipsilesional M1 in stroke patients and the left M1 in healthy subjects. M1 was defined to include voxels covering approximately the caudal half of the precentral gyrus along the anterior wall of the central sulcus. Correction of time courses was made by regressing out the time courses that corresponded to head motions and global fluctuations.
A map of correlation coefficients was converted to a map of Gaussian distributed values through Fisher’s z-transformation defined by z = tanh−1 r, or z = (1/2)(ln((1+r)/(1-r))), where r is a correlation coefficient, z is an approximately Gaussian distributed value, tanh−1 is the inverse hyperbolic tangent function, and ln is the natural logarithm function.25 The lesion-side of the correlation map was set to the left-side by flipping the map from right to left about the mid-sagittal line for patients with lesions on the right side.
Fisher’s z-transformed and flipped correlation maps were used for random-effects analysis. Two-sample t-tests were performed in order to find areas that showed significant differences in functional connectivity between patients and healthy subjects. Also, to search for brain regions correlated with motor improvement, correlation maps of patients at onset were regressed with increases in the FMA score at 6 months after stroke. We determined the significance using height (uncorrected P < 0.001 at the voxel-level) and extent (uncorrected P < 0.05 at the cluster-level) thresholds.
Lateralization Index
As a quantitative measure of functional connectivity, the lateralization index (LI) was calculated for each correlation map. The LI was introduced for the purpose of providing a specific description of the asymmetry of functional connectivity between the ipsilesional and contralesional M1 according to the following definition: (number of connected voxels in the ipsilesional M1/total number of voxels in the ipsilesional M1) - (number of connected voxels in the contralesional M1/total number of voxels in the contralesional M1). If functional connectivity of the ipsilesional M1 with any voxel had a value above the 95th percentile of the Gaussian distribution when considering all Gaussian distributed values in a map, the voxel was determined to be connected. This approach yielded LIs that ranged between −1 and 1, where −1 referred to contralesional connectivity only, 1 ipsilesional connectivity only, and values close to 0 referred to symmetric connectivity. The LI of patients was assessed at each time point and compared with that of healthy subjects.
Results
Differences in Connectivity between Patients and Healthy Subjects
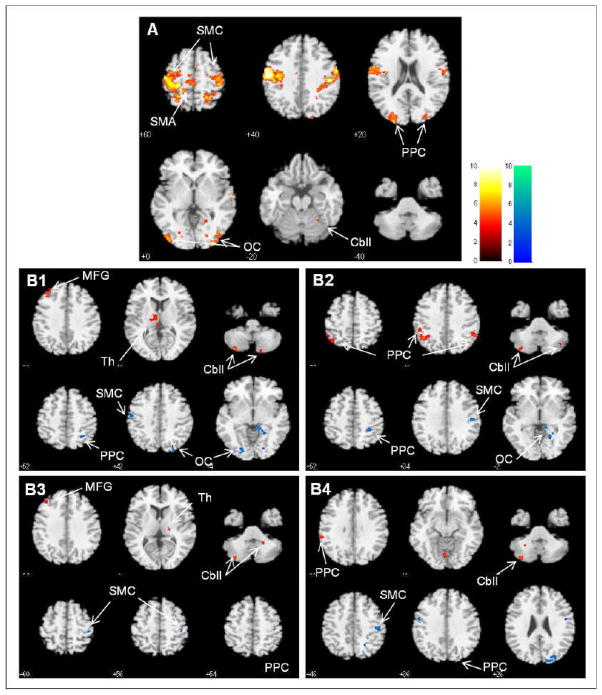
Correlation analysis data acquired from 11 healthy subjects demonstrated the discrete network, namely sensorimotor network (SMN), which is displayed in Figure 2A. SMN of healthy subjects included motor-sensory related regions, such as the primary sensorimotor cortex (SMC), premotor cortex, supplementary motor area (SMA), cingulate motor area, secondary somatosensory cortex, cerebellum, basal ganglia, thalamus, frontal and parietal cortices, and striate and extrastriate cortices. SMN in patients with stroke showed asymmetrical involvement, and other regions were additionally included throughout a period of 6 months. Figure 2B shows comparisons of connectivity between stroke patients and healthy subjects at four time points. Significant differences of connectivity in the SMN are summarized (Supplemental Table, please see http://stroke.ahajournals.org). Stroke patients displayed decreased connectivity of the ipsilesional M1 with the SMC, occipital cortex, Middle frontal gyrus (MFG), and posterior parietal cortex (PPC) since onset. On the other hand, stroke patients showed increased connectivity of the ipsilesional M1 with the cerebellum, thalamus, MFG, and PPC since onset. In particular, decreased connectivity with the ipsilesional SMC and increased connectivity with the cerebellum persisted throughout a period of 6 months after onset. In general, it is conceivable that connectivity of the ipsilesional M1 increased within ipsilesional brain regions, whereas it decreased within contralesional brain regions.
Figure 2.
A. Sensorimotor networks acquired by resting-state functional connectivity with the ipsilesional primary motor cortex in healthy subjects. B. Significant differences in resting-state functional connectivity between patients and healthy subjects over four time points of onset (B1), 1 month (B2), 3 months (B3), and 6 months (B4) after onset. Red-yellow blobs and blue-green blobs indicate increased and decreased functional connectivity in patients, compared to healthy subjects, respectively. The left-side of the brain is the ipsilesional hemisphere. SMC, Sensorimotor cortex; SMA, Supplementary motor area; PPC, Posterior parietal cortex; OC, Occipital cortex; Cbll, Cerebellum; MFG, Middle frontal gyrus; Th, Thalamus.
Time-Dependent Changes in Connectivity
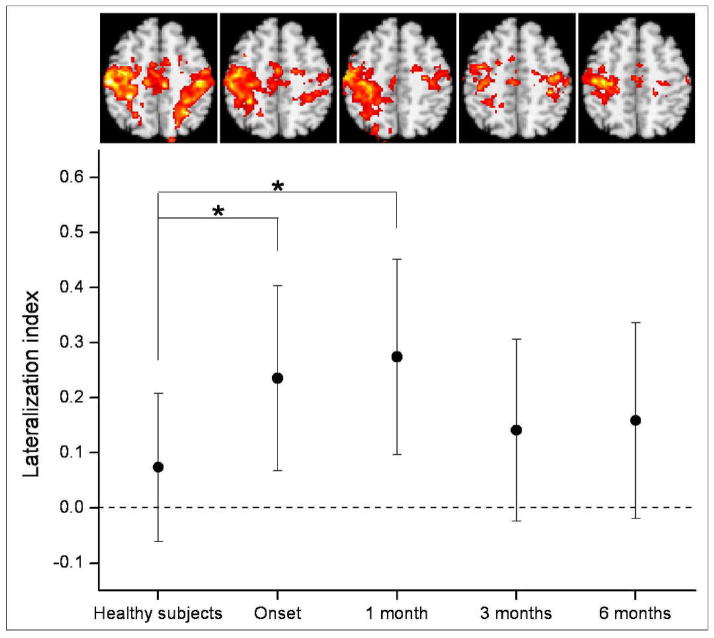
Figure 3 shows time-dependent changes in the LI, together with corresponding maps of functional connectivity. The LI of patients was larger at onset and even larger at 1 month after onset, compared with that of healthy subjects. At 3 months and 6 months after onset, the LI of patients had decreased, so that it did not differ significantly from that of healthy subjects. Corresponding maps of functional connectivity also showed that asymmetry of functional connectivity between ipsilesional and contralesional M1 increased until 1 month after onset and then decreased.
Figure 3.
Time-dependent changes in resting-state functional connectivity. Quantitative changes were exhibited by the lateralization index (LI) and corresponding maps of functional connectivity were also displayed. The LI was compared between patients and healthy subjects over four time points, including onset, 1 month, 3 months, and 6 months after onset. In the graph of the LI, points represent means, error bars represent standard deviations, and stars represent significant differences between patients and healthy subjects at a threshold of P < 0.05.
Correlation of Connectivity at Onset with Later Motor Improvement
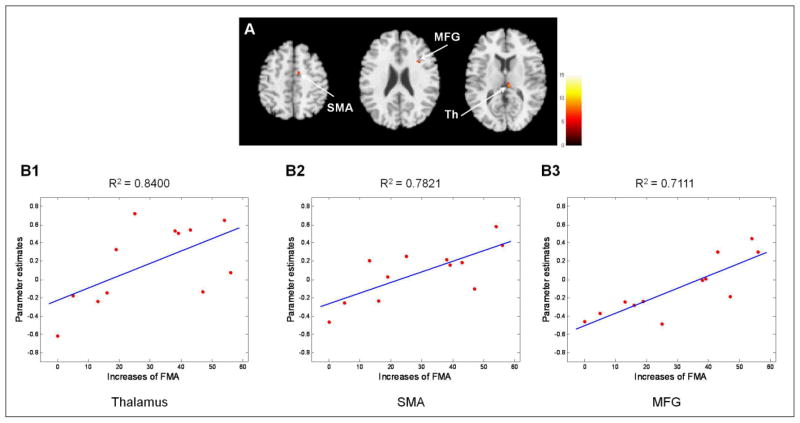
Figure 4 shows brain regions in which functional connectivity at onset was positively correlated with later motor improvement, as measured by increases in the FMA score at 6 months after onset. Brain areas demonstrating significant correlation with FMA changes are summarized in Table 2. Connectivity of the ipsilesional M1 with the contralesional thalamus, SMA, and MFG showed positive correlation with later motor improvement. R2 statistics were 0.8400, 0.7821, and 0.7111 for the thalamus, SMA, and MFG, respectively, in linear regression analysis, or partial correlation coefficients were 0.8998, 0.8822, and 0.8311 for the thalamus, SMA, and MFG, respectively, in partial correlation analysis for control of FMA scores at onset.
Figure 4.
A. Significant positive correlations of patients’ resting-state functional connectivity at onset with later motor improvement, as indexed by changes in the Fugl-Meyer assessment score for 6 months after onset. B. Linear regression of functional connectivity in the thalamus (B1), SMA (B2), and MFG (B3) on increases in the Fugl-Meyer Assessment score. The goodness of fit for each linear regression was given by the R2 statistic. Th, Thalamus; SMA, Supplementary motor area; MFG, Middle frontal gyrus.
Table 2.
Cluster maxima showing a significant positive correlation between patients’ resting-state functional connectivity at onset and later motor improvement as indexed by changes in the FMA score for 6 months after onset.
| Brain region | BA | Side | Peak MNI coordinates (mm)
|
Voxel count | Z-score | p-value | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Thalamus | C | 8 | −26 | 12 | 18 | 3.7726 | 0.0001 | |
| SMA | 6 | C | 10 | −6 | 54 | 15 | 3.5941 | 0.0002 |
| MFG | 48 | C | 34 | 16 | 26 | 16 | 3.1698 | 0.0008 |
BA, Brodmann’s area; MNI, Montreal Neurological Institute; SMA, Supplementary motor area; MFG, Middle frontal gyrus; I, Ipsilesional; C, Contralesional
Discussion
In the current study, we investigated 1) differences in resting-state functional connectivity between patients and healthy subjects during the period after stroke, and 2) a prognostic value of initial resting-state functional connectivity for assessment of later motor improvement. Our results demonstrated characteristic asymmetry of resting-state functional connectivity of the ipsilesional M1 in stroke patients, which lasted until 6 months after onset. Connectivity with subcortical SMN areas, such as the cerebellum and thalamus, increased at the early stage of stroke. On the other hand, connectivity with ipsilesional cortical areas increased and connectivity with contralesional cortical areas decreased. Preservation of connectivity with contralesional thalamus, SMA, and MFG and the thalamus at an early stage of stroke was meaningful for later motor recovery in these patients.
If resting-state fMRI activity reflects neuronal baseline activation, changes in resting-state connectivity may be related to functional changes in the brain. Previous studies using resting-state fMRI have demonstrated differences in the default-mode network in Alzheimer’s disease,26 and connectivity of the dorsal anterior cingulate cortex in ADHD,27 implying pathophysiology of disease. Correspondence of the regions involved in the current resting-state connectivity study with previous motor task activation studies implies that stroke also influences resting-state connectivity in reference to functional impairment. In previous task-based fMRI studies, activation of the contralesional SMC showed an initial increase and then decreased or vanished in correspondence with functional restoration of the perilesional cortex and the ipsilesional M1.2 In the current study, decreased connectivity between the ipsilesional M1 and contralesional hemispheric cortex was demonstrated after unilateral ischemic injury of the motor network. This finding implies that breakdown of harmonious interaction between two hemispheres at resting state may lead to alteration of the activity of the contralesional hemisphere in response to ipsilesional M1 activity.
Specifically, breakdown of harmonious interaction between both M1 could be quantitatively characterized in terms of the LI. Patients’ functional connectivity between the ipsilesional and contralesional M1 was more highly lateralized to the ipsilesional M1 at onset, compared with healthy subjects, and showed the greatest asymmetry at 1 month after onset. Restoration of relatively symmetric connectivity since 3 months after onset may be achieved after widespread reorganization in the sensorimotor system. That is, in the process of recovery following stroke, increased asymmetry in functional connectivity between both hemispheres in resting-state fMRI is considered to correspond to rearrangements of activation over the bihemispheric sensorimotor system in task-based fMRI.
Changes in connectivity of the ipsilesional M1 with the non-primary SMN regions, such as the frontal and parietal cortices and occipital cortex, were observed; these may reflect plastic changes to compensate for impaired connectivity with the contralesional hemisphere, or response to disconnection of transcallosal inhibition. These findings coincide with previous task-based fMRI studies that reported increased activation of the fronto-parietal cortex10 and other non-motor brain areas, such as the occipital cortex,6 in association with motor tasks in stroke patients. Changes in involvement of the cerebellum and thalamus after stroke have also been demonstrated in previous task-based fMRI studies of motor recovery.2,6,10 In particular, activation of the cerebellum was correlated with later motor recovery.28 Taken together, resting-state SMN connectivity appears to reflect abnormalities of motor network interaction after stroke, as well as plastic changes in response to motor network impairment. In addition, these changes appear to have an association with changes in brain activation provoked by performance of overt motor tasks.
In addition, regression analysis showed that preservation of connectivity of the ipsilesional M1 with the contralesional thalamus, SMA, and MFG at an early stage of stroke was positively correlated with later motor improvement at 6 months after stroke. The crucial role of the SMA in motor recovery has been demonstrated in previous task-based fMRI studies of stroke patients, in which early involvement of the SMA in the process of stroke recovery2 and correlation of initial activation of the SMA with motor recovery29 were described. The MFG is not regarded as a primary SMN region; however, recruitment of the MFG may be helpful in reinforcement of the management of cognitive load required for motor performance.10 In the case of the thalamus, despite its important contribution to processing and relay of sensorimotor information, the role of the thalamus in recovery of motor function has not yet been established. Strong recruitment of regions related to sensory integration, such as the thalamus, at an early stage of stroke, as shown in the current study, may suggest a beneficial effect of sensory related areas upon later motor restoration in stroke patients. For detailed clarification of the role of those regions, further investigation should be invited.
With a view that motor recovery corresponds to reorganization of surviving neuronal networks over the bihemispheric sensorimotor system, overall patterns of utilization of neuronal resources should be examined with respect to functional specialization and integration. Results of the current study are distinctive; however, they are comparable with those of previous task-based fMRI studies by a plausible association between resting-state connectivity and motor task activation.
Despite its novel results, the current study has some limitations in presenting results that cover various patterns of stroke recovery. Due to a high drop-out rate in long-term follow-up over a period of 6 months, we only had final resting-state fMRI data for 12 patients. Most drop-outs were due to patients’ circumstances. Still, with resting-state fMRI, recruitment of different subgroups of patients with uniform characteristics and careful control during follow-up appear to be requirements for successful explanation of different stroke recovery patterns.
Another limitation is that, in the current study, we did not specifically measure physiologic noise, such as cardiac and respiratory cycles. It has previously been proclaimed that cardiac30 and respiratory31 cycles can obscure detection of low-frequency fluctuations in resting-state fMRI, and, thus, induce changes in resting-state connectivity, although resting-state connectivity cannot be explained by cardiorespiratory effects alone.32 Therefore, investigation of resting-state connectivity corrected for cardiorespiratory effects would provide us with better information and is recommended for future study.
Conclusions
Stroke recovery might be time-dependent and affected according to task parameters. In this study, we attempted to overcome these critical issues through longitudinal resting-state fMRI. Although the implications of resting-state fMRI are still under dispute, systematic assessment of initial resting-state functional connectivity may provide prognostic insight for later motor recovery. In addition, practical values of the resting-state fMRI study, free from a number of confounds that are associated with task performances, may enable thorough long-term follow-up in patients with severe motor impairment at onset of stroke.
Supplementary Material
Acknowledgments
Sources of Funding
This study was supported by a KOSEF grant funded by the Korean government (MOST) (No. M10644000022-06N4400-02210) and by a grant from the Samsung Biomedical Research Institute (#SBRI C-A7-407-1). APL was supported in part by Grant UL1 RR025758 -Harvard Clinical and Translational Science Center, from the National Center for Research Resources and National Institutes of Health grant K 24 RR018875. The funders had no role in the design and conduct of the study; in collection, management, analysis, and interpretation of the data; or in preparation, review, or approval of the manuscript.
Footnotes
Disclosures
None.
References
- 1.Kim YH, You SH, Kwon YH, Hallett M, Kim JH, Jang SH. Longitudinal fmri study for locomotor recovery in patients with stroke. Neurology. 2006;67:330–333. doi: 10.1212/01.wnl.0000225178.85833.0d. [DOI] [PubMed] [Google Scholar]
- 2.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F. A longitudinal fmri study: In recovering and then in clinically stable subcortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 3.Loubinoux I, Dechaumont-Palacin S, Castel-Lacanal E, De Boissezon X, Marque P, Pariente J, Albucher JF, Berry I, Chollet F. Prognostic value of fmri in recovery of hand function in subcortical stroke patients. Cereb Cortex. 2007;17:2980–2987. doi: 10.1093/cercor/bhm023. [DOI] [PubMed] [Google Scholar]
- 4.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Longitudinal changes in cerebral response to proprioceptive input in individual patients after stroke: An fmri study. Neurorehabil Neural Repair. 2006;20:398–405. doi: 10.1177/1545968306286322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nair DG, Fuchs A, Burkart S, Steinberg FL, Kelso JA. Assessing recovery in middle cerebral artery stroke using functional mri. Brain Inj. 2005;19:1165–1176. doi: 10.1080/02699050500149858. [DOI] [PubMed] [Google Scholar]
- 6.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: A longitudinal fmri study. Brain. 2003;126:2476–2496. doi: 10.1093/brain/awg245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng B, Lin M. A longitudinal study of functional magnetic resonance imaging in upper-limb hemiplegia after stroke treated with constraint-induced movement therapy. Brain Inj. 2009;23:65–70. doi: 10.1080/02699050802635299. [DOI] [PubMed] [Google Scholar]
- 8.Enzinger C, Dawes H, Johansen-Berg H, Wade D, Bogdanovic M, Collett J, Guy C, Kischka U, Ropele S, Fazekas F, Matthews PM. Brain activity changes associated with treadmill training after stroke. Stroke. 2009;40:2460–2467. doi: 10.1161/STROKEAHA.109.550053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nowak DA, Grefkes C, Dafotakis M, Eickhoff S, Kust J, Karbe H, Fink GR. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65:741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- 10.Puh U, Vovk A, Sevsek F, Suput D. Increased cognitive load during simple and complex motor tasks in acute stage after stroke. Int J Psychophysiol. 2007;63:173–180. doi: 10.1016/j.ijpsycho.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Ward NS. Functional reorganization of the cerebral motor system after stroke. Curr Opin Neurol. 2004;17:725–730. doi: 10.1097/00019052-200412000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 13.Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH. Modulation of spontaneous fmri activity in human visual cortex by behavioral state. Neuroimage. 2009;45:160–168. doi: 10.1016/j.neuroimage.2008.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fransson P, Skiold B, Horsch S, Nordell A, Blennow M, Lagercrantz H, Aden U. Resting-state networks in the infant brain. Proc Natl Acad Sci U S A. 2007;104:15531–15536. doi: 10.1073/pnas.0704380104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pawela CP, Biswal BB, Cho YR, Kao DS, Li R, Jones SR, Schulte ML, Matloub HS, Hudetz AG, Hyde JS. Resting-state functional connectivity of the rat brain. Magn Reson Med. 2008;59:1021–1029. doi: 10.1002/mrm.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Ross TJ, Zhan W, Myers CS, Chuang KS, Heishman SJ, Stein EA, Yang Y. Group independent component analysis reveals consistent resting-state networks across multiple sessions. Brain Res. 2008;1239:141–151. doi: 10.1016/j.brainres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex. 2008;18:1856–1864. doi: 10.1093/cercor/bhm207. [DOI] [PubMed] [Google Scholar]
- 19.Dhond RP, Yeh C, Park K, Kettner N, Napadow V. Acupuncture modulates resting state connectivity in default and sensorimotor brain networks. Pain. 2008;136:407–418. doi: 10.1016/j.pain.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with adhd revealed by resting-state functional mri. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Anand A, Li Y, Wang Y, Gardner K, Lowe MJ. Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: An fmri study. J Neuropsychiatry Clin Neurosci. 2007;19:274–282. doi: 10.1176/appi.neuropsych.19.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- 24.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 25.Fisher RA. Frequency distribution of the values of the correlation coefficient in samples from an indefinitely large population. Biometrika. 1915;10:507–521. [Google Scholar]
- 26.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Drzezga A, Forstl H, Kurz A, Zimmer C, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tian LX, Jiang TZ, Wang YF, Zang YF, He Y, Liang M, Sui MQ, Cao QJ, Hu SY, Peng M, Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience Letters. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fmri activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- 29.Loubinoux I, Carel C, Pariente J, Dechaumont S, Albucher JF, Marque P, Manelfe C, Chollet F. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20:2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Bhattacharyya PK, Lowe MJ. Cardiac-induced physiologic noise in tissue is a direct observation of cardiac-induced fluctuations. Magnetic Resonance Imaging. 2004;22:9–13. doi: 10.1016/j.mri.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Birn RM, Diamond JB, Smith MA, Bandettini PA. Separating respiratory-variation-related fluctuations from neuronal-activity-related fluctuations in fmri. Neuroimage. 2006;31:1536–1548. doi: 10.1016/j.neuroimage.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 32.Van Buuren M, Gladwin TE, Zandbelt BB, Van Den Heuvel M, Ramsey NF, Kahn RS, Vink M. Cardiorespiratory effects on default-mode network activity as measured with fmri. Human Brain Mapping. 2009;30:3031–3042. doi: 10.1002/hbm.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.