Abstract
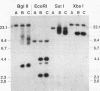
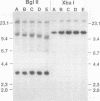
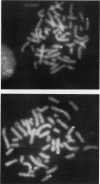
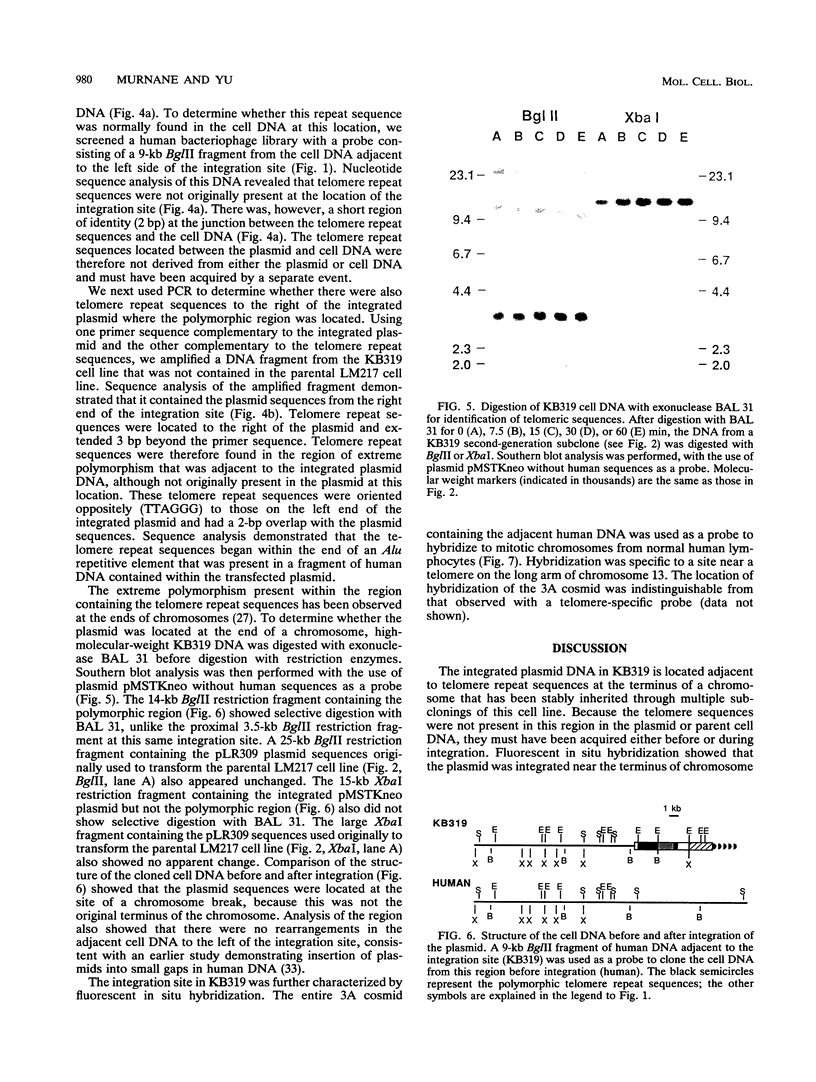
Previous analysis of plasmid DNA transfected into 108 cell clones demonstrated extensive polymorphism near the integration site in one clone. This polymorphism was apparent by Southern blot analysis as diffuse bands that extended over 30 kb. In the present study, nucleotide sequence analysis of cloned DNA from the integration site revealed telomere repeat sequences at the ends of the integrated plasmid DNA. The telomere repeat sequences at one end were located at the junction between the plasmid and cell DNA. The telomere repeat sequences at the other end were located in the opposite orientation in the polymorphic region and were shown by digestion with BAL 31 to be at the end of the chromosome. Telomere repeat sequences were not found at this location in the plasmid or parent cell DNA. Although the repeat sequences may have been acquired by recombination, a more likely explanation is that they were added to the ends of the plasmid by telomerase before integration. Comparison of the cell DNA before and after integration revealed that a chromosome break had occurred at the integration site, which was shown by fluorescent in situ hybridization to be located near the telomere of chromosome 13. These results demonstrate that chromosome breakage and rearrangement can result in interstitial telomere repeat sequences within the human genome. These sequences could promote genomic instability, because short repeat sequences can be recombinational hotspots. The results also show that DNA rearrangements involving telomere repeat sequences can be associated with chromosome breaks. The introduction of telomere repeat sequences at spontaneous or ionizing radiation-induced DNA strand breaks may therefore also be a mechanism of chromosome fragmentation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell L. The mutagenic and carcinogenic effects of gene transfer. Mutagenesis. 1989 Jul;4(4):245–253. doi: 10.1093/mutage/4.4.245. [DOI] [PubMed] [Google Scholar]
- Bedford J. S. Sublethal damage, potentially lethal damage, and chromosomal aberrations in mammalian cells exposed to ionizing radiations. Int J Radiat Oncol Biol Phys. 1991 Nov;21(6):1457–1469. doi: 10.1016/0360-3016(91)90320-4. [DOI] [PubMed] [Google Scholar]
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Brown W. R., MacKinnon P. J., Villasanté A., Spurr N., Buckle V. J., Dobson M. J. Structure and polymorphism of human telomere-associated DNA. Cell. 1990 Oct 5;63(1):119–132. doi: 10.1016/0092-8674(90)90293-n. [DOI] [PubMed] [Google Scholar]
- Cao L., Alani E., Kleckner N. A pathway for generation and processing of double-strand breaks during meiotic recombination in S. cerevisiae. Cell. 1990 Jun 15;61(6):1089–1101. doi: 10.1016/0092-8674(90)90072-m. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991 Sep;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Evidence that DNA double-strand breaks initiate the phenotype of delayed reproductive death in Chinese hamster ovary cells. Radiat Res. 1992 Jul;131(1):53–59. [PubMed] [Google Scholar]
- Chang W. P., Little J. B. Persistently elevated frequency of spontaneous mutations in progeny of CHO clones surviving X-irradiation: association with delayed reproductive death phenotype. Mutat Res. 1992 Nov 16;270(2):191–199. doi: 10.1016/0027-5107(92)90130-t. [DOI] [PubMed] [Google Scholar]
- Colbère-Garapin F., Ryhiner M. L., Stephany I., Kourilsky P., Garapin A. C. Patterns of integration of exogenous DNA sequences transfected into mammalian cells of primate and rodent origin. Gene. 1986;50(1-3):279–288. doi: 10.1016/0378-1119(86)90332-x. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drets M. E., Therman E. Human telomeric 6; 19 translocation chromosome with a tendency to break at the fusion point. Chromosoma. 1983;88(2):139–144. doi: 10.1007/BF00327334. [DOI] [PubMed] [Google Scholar]
- Farr C., Fantes J., Goodfellow P., Cooke H. Functional reintroduction of human telomeres into mammalian cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7006–7010. doi: 10.1073/pnas.88.16.7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Hahn P., Morgan W. F., Painter R. B. The role of acentric chromosome fragments in gene amplification. Somat Cell Mol Genet. 1987 Nov;13(6):597–608. doi: 10.1007/BF01534480. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Allshire R. C. Human telomeres: fusion and interstitial sites. Trends Genet. 1989 Oct;5(10):326–331. doi: 10.1016/0168-9525(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Chang C., Bradley A. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol Cell Biol. 1991 Sep;11(9):4509–4517. doi: 10.1128/mcb.11.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Odijk H., Westerveld A. Differences between rodent and human cell lines in the amount of integrated DNA after transfection. Exp Cell Res. 1987 Mar;169(1):111–119. doi: 10.1016/0014-4827(87)90230-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Neumann R., Wilson V. Repeat unit sequence variation in minisatellites: a novel source of DNA polymorphism for studying variation and mutation by single molecule analysis. Cell. 1990 Feb 9;60(3):473–485. doi: 10.1016/0092-8674(90)90598-9. [DOI] [PubMed] [Google Scholar]
- Kato S., Anderson R. A., Camerini-Otero R. D. Foreign DNA introduced by calcium phosphate is integrated into repetitive DNA elements of the mouse L cell genome. Mol Cell Biol. 1986 May;6(5):1787–1795. doi: 10.1128/mcb.6.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., McCormick F., Denny C. Molecular mechanisms of cancer. West J Med. 1991 Nov;155(5):505–514. [PMC free article] [PubMed] [Google Scholar]
- Little J. B., Gorgojo L., Vetrovs H. Delayed appearance of lethal and specific gene mutations in irradiated mammalian cells. Int J Radiat Oncol Biol Phys. 1990 Dec;19(6):1425–1429. doi: 10.1016/0360-3016(90)90354-m. [DOI] [PubMed] [Google Scholar]
- Lohrer H., Blum M., Herrlich P. Ataxia telangiectasia resists gene cloning: an account of parameters determining gene transfer into human recipient cells. Mol Gen Genet. 1988 Jun;212(3):474–480. doi: 10.1007/BF00330852. [DOI] [PubMed] [Google Scholar]
- Mayne L. V., Jones T., Dean S. W., Harcourt S. A., Lowe J. E., Priestley A., Steingrimsdottir H., Sykes H., Green M. H., Lehmann A. R. SV 40-transformed normal and DNA-repair-deficient human fibroblasts can be transfected with high frequency but retain only limited amounts of integrated DNA. Gene. 1988 Jun 15;66(1):65–76. doi: 10.1016/0378-1119(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Morin G. B. Recognition of a chromosome truncation site associated with alpha-thalassaemia by human telomerase. Nature. 1991 Oct 3;353(6343):454–456. doi: 10.1038/353454a0. [DOI] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Moyzis R. K., Buckingham J. M., Cram L. S., Dani M., Deaven L. L., Jones M. D., Meyne J., Ratliff R. L., Wu J. R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P., Fuller L. F., Painter R. B. Establishment and characterization of a permanent pSV ori--transformed ataxia-telangiectasia cell line. Exp Cell Res. 1985 May;158(1):119–126. doi: 10.1016/0014-4827(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Murnane J. P. Inducible gene expression by DNA rearrangements in human cells. Mol Cell Biol. 1986 Feb;6(2):549–558. doi: 10.1128/mcb.6.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P. Influence of cellular sequences on instability of plasmid integration sites in human cells. Somat Cell Mol Genet. 1990 May;16(3):195–209. doi: 10.1007/BF01233356. [DOI] [PubMed] [Google Scholar]
- Murnane J. P. The role of recombinational hotspots in genome instability in mammalian cells. Bioessays. 1990 Dec;12(12):577–581. doi: 10.1002/bies.950121204. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Yezzi M. J. Association of high rate of recombination with amplification of dominant selectable gene in human cells. Somat Cell Mol Genet. 1988 May;14(3):273–286. doi: 10.1007/BF01534588. [DOI] [PubMed] [Google Scholar]
- Murnane J. P., Yezzi M. J., Young B. R. Recombination events during integration of transfected DNA into normal human cells. Nucleic Acids Res. 1990 May 11;18(9):2733–2738. doi: 10.1093/nar/18.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P., Young B. R. Nucleotide sequence analysis of novel junctions near an unstable integrated plasmid in human cells. Gene. 1989 Dec 7;84(1):201–205. doi: 10.1016/0378-1119(89)90157-1. [DOI] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Straume T., Gray J. W. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci U S A. 1986 May;83(9):2934–2938. doi: 10.1073/pnas.83.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid L. H., Shesely E. G., Kim H. S., Smithies O. Cotransformation and gene targeting in mouse embryonic stem cells. Mol Cell Biol. 1991 May;11(5):2769–2777. doi: 10.1128/mcb.11.5.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Roth D. B., Chang X. B., Wilson J. H. Comparison of filler DNA at immune, nonimmune, and oncogenic rearrangements suggests multiple mechanisms of formation. Mol Cell Biol. 1989 Jul;9(7):3049–3057. doi: 10.1128/mcb.9.7.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle N. J., Clarkson R. E., Wong Z., Jeffreys A. J. Clustering of hypervariable minisatellites in the proterminal regions of human autosomes. Genomics. 1988 Nov;3(4):352–360. doi: 10.1016/0888-7543(88)90127-9. [DOI] [PubMed] [Google Scholar]
- Sager R., Gadi I. K., Stephens L., Grabowy C. T. Gene amplification: an example of accelerated evolution in tumorigenic cells. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7015–7019. doi: 10.1073/pnas.82.20.7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A., Gorman P. A., Stark M. B., Groves R. P., Stark G. R. Distinctive chromosomal structures are formed very early in the amplification of CAD genes in Syrian hamster cells. Cell. 1990 Dec 21;63(6):1219–1227. doi: 10.1016/0092-8674(90)90417-d. [DOI] [PubMed] [Google Scholar]
- Starling J. A., Maule J., Hastie N. D., Allshire R. C. Extensive telomere repeat arrays in mouse are hypervariable. Nucleic Acids Res. 1990 Dec 11;18(23):6881–6888. doi: 10.1093/nar/18.23.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D. Normal diploid human and rodent cells lack a detectable frequency of gene amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trask B. J., Hamlin J. L. Early dihydrofolate reductase gene amplification events in CHO cells usually occur on the same chromosome arm as the original locus. Genes Dev. 1989 Dec;3(12A):1913–1925. doi: 10.1101/gad.3.12a.1913. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Massa H., Kenwrick S., Gitschier J. Mapping of human chromosome Xq28 by two-color fluorescence in situ hybridization of DNA sequences to interphase cell nuclei. Am J Hum Genet. 1991 Jan;48(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Wang S. S., Zakian V. A. Telomere-telomere recombination provides an express pathway for telomere acquisition. Nature. 1990 May 31;345(6274):456–458. doi: 10.1038/345456a0. [DOI] [PubMed] [Google Scholar]
- Weber B., Collins C., Robbins C., Magenis R. E., Delaney A. D., Gray J. W., Hayden M. R. Characterization and organization of DNA sequences adjacent to the human telomere associated repeat (TTAGGG)n. Nucleic Acids Res. 1990 Jun 11;18(11):3353–3361. doi: 10.1093/nar/18.11.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie A. O., Lamb J., Harris P. C., Finney R. D., Higgs D. R. A truncated human chromosome 16 associated with alpha thalassaemia is stabilized by addition of telomeric repeat (TTAGGG)n. Nature. 1990 Aug 30;346(6287):868–871. doi: 10.1038/346868a0. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Smith H. S., Watt F. M., Hancock M. C., Hudson D. L., Stark G. R. DNA amplification is rare in normal human cells. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1791–1795. doi: 10.1073/pnas.87.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis J. J. High resolution of human chromosomes. Science. 1976 Mar 26;191(4233):1268–1270. doi: 10.1126/science.1257746. [DOI] [PubMed] [Google Scholar]