Figure 3. Active Site Architecture of Ath α-DOX.
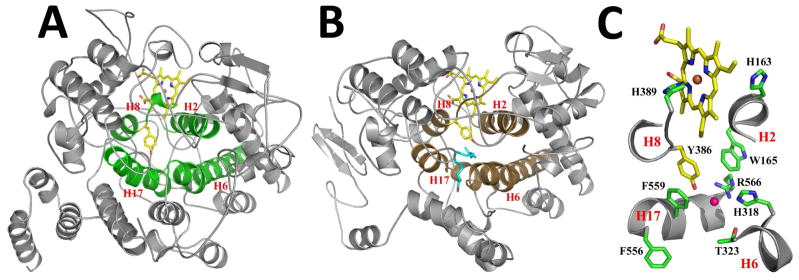
Cartoon representation of the crystal structures of (A) Ath α-DOX and (B) murine COX-2 (PDB id 3HS5), highlighting the four conserved helices (H2, H6, H8, and H17) that make up the fatty acid binding channel (green) and the cyclooxygenase channel (brown) in each structure. The side chain of the catalytic tyrosine and the heme moiety within each active site are depicted in stick form, with carbon, oxygen, nitrogen, and iron atoms colored yellow, red, blue, and orange, respectively. Arachidonic acid is shown in stick form (blue carbon atoms and red oxygen atoms) bound within the cyclooxygenase channel of COX-2 in B. (C) Close up of the active site residues in Ath α-DOX. The view is zoomed and rotated ~90° from the orientation depicted in A. Portions of helix H2, H6, H8, and H17 have been removed for clarity. Residues His-163, Trp-165, His-318, Thr-323, Tyr-386, His-389, Phe-556, Phe-559, and Arg-566 are labeled accordingly. The chloride ion (pink) is shown bound in the channel of Ath α-DOX.
