Abstract
Peripheral blood stem cells (PBSC) have been increasingly used in the matched unrelated donor (MUD) transplant setting, but the impact of CD34+ cell dose on outcomes in this setting have not been well characterized. We analyzed 181 consecutive patients who underwent MUD-PBSC transplantation at City of Hope between August 2000 to December 2004. Patients were conditioned with either full-intensity regimen or reduced-intensity regimen. There was a significant inverse relationship between higher CD34+ cell dose and faster neutrophil engraftment (r= −0.16, p=0.035). By univariate analysis, a CD34+ cell dose ≥4.2x106/kg (above the lowest quartile) was associated with significantly lower relapse risk (HR=0.67; p=0.0126), with a trend for corresponding improvement for disease-free survival (HR=0.84; p=0.12) but not overall survival (HR=0.91; p=0.46). The impact of the CD34+ cell dose remained significant in multivariate analysis. The higher CD34+ cell dose was significantly associated with faster recovery of absolute lymphocyte counts on day +30 post-transplant. Subset analysis demonstrated that the higher CD34+ cell dose was associated with 1) greater reduction in relapse in myeloid malignancies than that in lymphoid malignancies, 2) greater reduction in reduced-intensity conditioning than in full-intensity conditioning, 3) greater reduction in relapse when there is a inhibitory killer-cell immunoglobulin-like receptor ligand (iKIRL)-mismatch in the GVH direction, and 4) greater reduction in relapse when there is a lack of iKIRL, suggesting that the protective effect of CD34+ cell dose against relapse may be immune-mediated, possibly through NK cell recovery.
Keywords: unrelated donor, peripheral blood hematopoietic cell transplantation, CD34+ cell dose
Introduction
Outcome after allogeneic hematopoietic stem cell transplants (HCT) is strongly influenced by many factors beyond the control of the treating physician, including patient age, disease status, and cytomegalovirus (CMV) serology, and donor type. Other factors which can be modified including the conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and the cellular content and source of the graft may influence both survival and quality of life. While the impact of the first two factors has been extensively studied, there are less data on the optimal doses of graft cellular contents.
Several studies have identified CD34+ cell dose as a critical factor affecting transplantation outcome. Higher CD34+ cell doses are generally associated with reduced transplant-related mortality (TRM) and relapse rates and superior survival and disease-free survival (DFS) rates1–6. Similar observations have been made in HCT using reduced-intensity conditioning7,8. However, the infusion of high doses of CD34+ cell has also been associated with an increased rate of acute and/or chronic GVHD9–11. While these studies focused on sibling donor HCT or matched unrelated donor (MUD) bone marrow transplantation, the impact of CD34+ cell dose on outcomes in MUD peripheral blood stem cell transplantation (PBSCT) has not been well characterized.
GCSF-mobilized PBSCT has been increasingly used in the MUD transplant setting despite no clear data on survival advantage or disadvantage. A previous case-control study showed no difference in GVHD, relapse and survival comparing peripheral stem cells to bone marrow using unrelated donors, except for an increased risk for extensive chronic GVHD with the use of PBSC graft12. Importantly, the impact of CD34+ cell dose in MUD-PBSC transplantations may differ from that of sibling donor PBSC transplants. In MUD-PBSC transplantations, the disease risk is generally higher and immunological disparity between the recipient and donor is greater. In addition, inhibitory killer-cell immunoglobulin-like receptor ligand (iKIRL) mismatch in this setting is another factor, which may potentially influence the risk of relapse13–16.
In this retrospective study, we sought to determine whether there was a correlation between the CD34+ cell dose of PBSC grafts and clinical outcomes including engraftment, survival, disease relapse, and risks of GVHD following MUD-PBSC transplantation. In addition, since the higher CD34+ cell dose is known to be associated with faster recovery of NK cells post-transplant17, we further investigated the effect of iKIRL matching combined with CD34+ cell dose on the transplant outcomes.
Methods
Patients
Between August 2000 to December 2004, a total of 204 patients with hematologic malignancies underwent MUD-PBSC transplantation at our institution under IRB-approved protocols. In this retrospective analysis, we excluded patients who received a truly non-myeloablative conditioning such as low-dose total body irradiation (TBI) +/− fludarabine because we expected the engraftment kinetics, overall outcome, relapse risk, would be highly distinct from the other transplant conditioning regimens. Characteristics of the remaining 181 patients are shown in Table 1. Of these 181 patients, 35 were considered to have low-risk disease (acute leukemia in first complete remission or chronic myelogenous leukemia in chronic phase).
Table 1.
Patient and transplant characteristics
| Number of patients | 181 |
| Dates of transplantation | August 2000–December 2004 |
| Median follow up (range) | 18 months (range: 3–49) |
| Median age (range) | 44 (1–67) |
| Sex (F/M) | 82/99 |
| Diagnosis | |
| AML | 68 |
| ALL | 38 |
| NHL/HD | 19/4 |
| CML | 18 |
| MDS | 18 |
| CLL | 3 |
| MM | 2 |
| Myelofibrosis/MPD | 8 |
| Other acute leukemias (biphenotypic, undifferentiated) | 3 |
| Disease risk | |
| Low | 35 |
| High | 146 |
| Stem cell source | |
| PBSC | 181 (100%) |
| Conditioning regimen | |
| Full intensity (TBI-Cy: 46, TBI-VP16: 28, Bu-Cy: 9) | 83 |
| Reduced intensity (Flu-Mel: 97, Flu-Bu: 1) | 98 |
| GVHD prophylaxis | |
| FK506/MTX | 79 |
| CSA (or FK506)/MMF | 51 |
| CSA (or FK506)/MMF/MTX | 50 |
| FK506/sirolimus | 1 |
| Donor/recipient sex combination | |
| female to male | 34 |
| Other combinations | 147 |
| Donor/recipient CMV serostatus | |
| donor−/recipient+ | 64 |
| donor+/recipient+ | 53 |
| donorminus;/recipientminus; | 38 |
| donor+/recipientminus; | 26 |
| HLA matching | |
| 10 of 10 allele match | 93 |
| Others (total) | (88) |
| antigen mismatch in A | 3 |
| antigen mismatch in C+/minus;DQ | 47 |
| antigen mismatch in DQ alone | 6 |
| allele mismatch(es) without Ag mismatch | 32 |
| iKIRL matching | |
| iKIRL mismatch | |
| GVH direction | 18 |
| HVG direction | 11 |
| iKIRL match (or unknown) | 149 (3) |
| Transplant cell doses | |
| median CD34+ cells (range) | 6.7 (0.6–28) x106/kg |
| median mononuclear cells (range) | 7.1 (5.0–5.06) x1088/kg |
| median lymphocytes (range) | 4.4 (4.0–2.64)x108/kg |
AML: acute myelogenous leukemia, ALL: acute lymphoblastic leukemia, NHL/HD: non-Hodgkin’s lymphoma, HD: Hodgkin’s disease, CML: chronic myelogenous leukemia, MDS: myelodysplastic syndrome, CLL: chronic lymphocytic leukemia, MM: multiple myeloma, MPD: myloproliferative disorders, PBSC: peripheral blood stem cell, TBI: total body irradiation, Cy: cyclophosphamide, VP-16: etoposide, Bu: Busulphan, Flu: fludarabine, Mel: melphalan, iKIRL: inhibitory killer-cell immunoglobulin-like receptor ligand, GVH: graft-versus-host, HVG: host-versus-graft
Donors
Unrelated donors were consented and procured through the National Marrow Donor Program (NMDP) according to their standards and procedures. High-resolution polymerase chain reaction-sequence-specific-primers (PCR-SSP) for HLA class I and II was performed using PCR-based DNA sequencing reactions18. PBSC donors received a G-CSF at a dose ranging from 9 to 12.5 μg/kg/d, administered subcutaneously once daily for five consecutive days. All PBSC products were unmanipulated.
Criteria of HLA and iKIRL incompatibility, and patients lacking iKIRLs
HLA compatibility was classified into two different mismatch levels, the antigen level (including split antigens) and the allele level. Ninety-three pairs were 10 of 10 allele level match. In the remaining 88 pairs, antigen mismatch was seen in A (n=3), C+/-DQ (n=47), or DQ (n=6), and allele mismatch(s) without antigen mismatch was observed in 32 pairs (Table 1).
Killer-cell immunoglobulin-like receptor (KIR) genotyping was performed with a multiplex polymerase chain reaction sequence specific primer method19. Assignment of iKIRL incompatibility in either GVH or host versus graft direction basically followed the previously proposed algorithm13,20,21(Table 1). Transplants were also analyzed employing the model of the “lacking iKIRLs”29 (Table 1). We also considered the KIR genotyping results of patients and donors in both models. For example, in a homozygous HLA-Bw6 patient transplanted with a heterozygous HLA-B donor, the iKIRL mismatch was counted only if the donor genotype had KIR3DL1.
Conditioning
As preparative treatment before transplantation, 83 patients received one of the full-intensity regimens; cyclophosphamide (Cy) 60 mg/kg for two consecutive days (n=46) or etoposide 60mg/kg for one day (n=28), combined with total body irradiation (TBI) in a dose of 1320 cGy over 11 fractions, or busulfan (Bu) 16 mg/kg followed by Cy 60 mg/kg for two consecutive days (n=9). Ninety-eight patients received reduced-intensity regimens using fludarabine (Flu) 25 mg/m2 for five days and Melphalan (Mel) 140 mg/m2 (n=97) or Bu (n=1). There was no use of Campath in our regimen, but ATG was used in three patients when the risk of graft rejection or GVHD was considered to be high.
GVHD prophylaxis
For patients undergoing full-intensity transplant, GVHD prophylaxis consisted of tacrolimus (FK506) combined with a short course (4 doses) of methotrexate (MTX) at 15mg/m2 on day 1 and 10mg/m2 on days +3, +6, and +11. For reduced intensity transplants, cyclosporine (CSA) 1.5mg/kg IV twice a day and mycophenolate mofetil (MMF) at 15mg/kg IV three times a day with or without a short course of MTX at 10 mg/m2 on day +1, then 5 mg/m2 on days +3 and +6 were used.
Supportive care
Supportive care, including prophylactic antibiotics, antifungal therapy, total parenteral nutrition, hematopoietic growth factors, immune globulin replacement, and treatment of mucositis and neutropenic fever was administered in accordance with institutional standard practice guidelines23. Monitoring for CMV viremia was performed twice a week from day +21 to day +100, using the shell vial method and/or PCR and patients received pre-emptive ganciclovir at onset of cytomegalovirus reactivation.
Transplant cell doses
Determinations of CD34+ cells were performed on unseparated PBSC by flow cytometry according to the ISAHGE protocol. Total infused lymphocyte doses were calculated as follows: (% differential of the lymphocyte/100 x total WBC count of the PBSC graft) /recipient weight in Kg. We did not include CD3+ cell dose in this analysis since the CD3+ count became our standard only recently and the information was available in a limited number of patients within this cohort.
Clinical evaluation
Engraftment
Neutrophil engraftment was defined as the first of three consecutive days with an absolute neutrophil count (ANC) of 0.5x109/L or above. Platelet engraftment was defined as the first of seven consecutive days when the platelet count exceeded 20 x 109/L without transfusion support.
GVHD
Acute GVHD was graded 0 to IV, according to standard criteria24. Chronic GVHD was assessed in patients surviving more than 90 days after transplantation and defined as limited or extensive25.
Statistics
Analysis was performed on 181 patients, with a median follow-up time of 28 months (range: 6.5–59 months) for surviving patients. The probabilities of overall survival (OS), disease-free survival (DFS), relapse, acute and non-relapse mortality (NRM) were calculated with the Kaplan-Meier method26, comparing the groups with use of the log-rank test (Mantel-Haentszel)27. We used the Wilcoxon rank-sum test to compare the means of time to engraftment. In the univariate and multivariate analyses of risk factors for acute GVHD and prognostic factors for engraftment, the logistic regression model was used for the binary outcomes28. When analyzing prognostic factors for chronic GVHD, TRM, survival, relapse, and DFS, the Cox regression model was used29. Factors significant at the 10% level in the univariate analysis were entered into the multivariate analyses. The following factors were analyzed: total mononucleated cell (MNC) dose, lymphocyte dose, CD34+ cell dose, disease risk status, recipient CMV serology, conditioning regimen (full intensity versus reduced intensity), patient age, and donor/recipient sex (female donor to male recipient versus other combinations), HLA match status (10 out of 10 match versus others).
Results
Overall outcomes
Of the 181 patients with a median follow up of 28 months (range: 6.5–59), 89 patients are alive. The two-year overall survival (OS), disease-free survival (DFS), relapse, and 100-day transplant-related mortality (TRM) probabilities were 52%±4%, 47%±4%, 29%±4%, and 15±3% respectively. Grade II-IV acute GVHD occurred in 64% of patients (grade 3–4: 30%). Chronic GVHD was observed in 59% of evaluable patients (52% extensive).
Characteristics of infused products
Median (range) CD34+ cell, lymphocyte, and MNC doses were 6.7 (0.6–28) x106/kg, 4.4 (4.0–26.4) x108/kg, and 6.9 (5.0–50.6) x108/kg, respectively (Table 1). There was a strong correlation between Ly and MNC doses (r=0.88, p<0.0001), and less significant correlations between CD34+ cell and Ly (r=0.23, p=0.035) or MNC (r=0.24, p=0.029). For subsequent analysis, the cases were grouped into four categories according to the CD34+ cell doses in quartiles (≤ percentile 25 [p25]: percentile 25–50 [p25–50], percentile 50–75 [p50–75], and percentile ≥ 75 [p75]). The cutoff values for p25, p50, and p75 were 4.2x106/kg, 6.7x106/kg, and 9.5x106/kg, respectively.
Impact of infused CD34+ cell dose and transplant outcome
Hematopoietic recovery
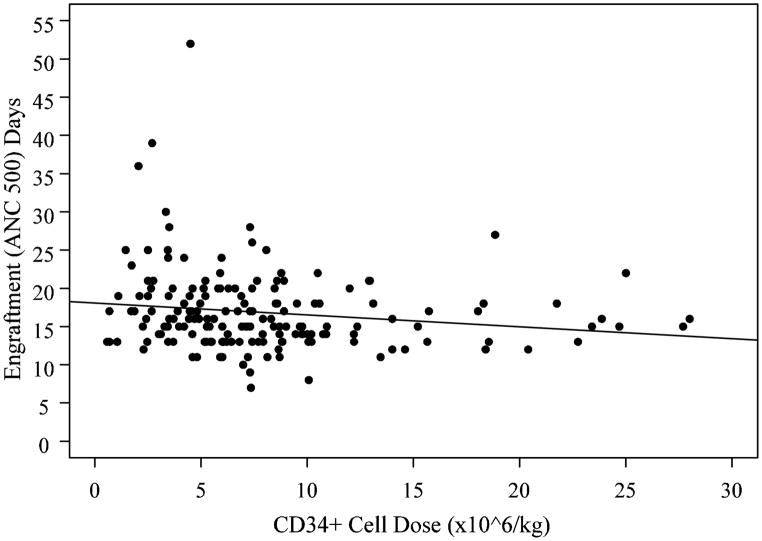
All patients engrafted with the median time to ANC>500/uL at 16 days (range: 7–52) and platelet counts >20,000/ul at 20 days (range: 12–98). There was a significant inverse relationship between higher CD34+ cell dose (log scale) and faster neutrophil engraftment (r= −0.16, p=0.035, Figure 1). A similar trend was observed for engraftment of platelets without reaching a statistical significance (r= −0.19, p=0.13).
Figure 1.
Neutrophil recovery is associated with CD34+ cell dose. There was a significant inverse relationship between higher CD34+ cell dose (log scale) and faster neutrophil engraftment (r= −0.16, p=0.035).
Time-to-event outcomes
Table 2 shows the results of univariate Cox analysis for factors associated with transplant outcomes. Significant prognostic variables associated with improved OS were 10/10 match status and greater Ly dose (as continuous variable), and significant variables for improved DFS included these two factors and low-risk disease status.
Table 2.
Univariate analysis for factors associated with transplant outcomes
| OS HR (95% CI) | DFS HR (95% CI) | Relapse HR (95% CI) | TRM HR (95% CI) | |
|---|---|---|---|---|
| Age (continuous) | 1.00 (0.99–1.01) p = 0.87 |
1.00 (0.99–1.02) p = 0.48 |
1.02 (1.00–1.04)
p = 0.0315 |
1.01 (0.99–1.02) p = 0.47 |
| CD34 +dose (continuous) | 0.99 (0.95–1.03) p = 0.60 |
0.98 (0.94–1.01) p = 0.22 |
0.94 (0.87–1.00)
p = 0.0533 |
0.99 (0.94–1.04) p = 0.82 |
| CD34+ ≤p25 (baseline) vs. >p25 | 0.91 (0.73–1.17) p = 0.46 |
0.84 (0.68–1.05) p = 0.12 |
0.67 (0.49–0.91)
p = 0.0126 |
1.01 (0.75–1.41) p = 0.94 |
| Ly dose (continuous) |
0.41 (0.16–0.96)
p = 0.0396 |
0.40 (0.16–0.90)
p = 0.0249 |
0.37 (0.09–1.25) p = 0.12 |
0.48 (0.15–1.28) p = 0.16 |
| Ly ≤p25 (baseline) vs. >p25 | 0.88 (0.70–1.11) p = 0.26 |
0.89 (0.72–1.12) p = 0.31 |
0.93 (0.67–1.35) p = 0.70 |
0.90 (0.68–1.22) p = 0.49 |
| MNC dose (continuous) | 0.99 (0.61–1.45) p = 0.95 |
0.88 (0.54–1.28) p = 0.54 |
0.55 (0.21–1.16) p = 0.13 |
1.05 (0.59–1.59) p = 0.86 |
| MNC ≤p25 (baseline) vs. >p25 | 1.05 (0.84–1.35) p = 0.67 |
1.01 (0.81–1.28) p = 0.94 |
0.87 (0.63–1.22) p = 0.40 |
1.09 (0.81–1.51) p = 0.59 |
| Disease risk good (baseline) vs. poor risk | 1.62 (0.94–3.06) p = 0.0868 |
1.75 (1.03–3.21)
p = 0.0390 |
2.03 (0.88–5.91) p = 0.10 |
1.37 (0.71–2.99) p = 0.37 |
| Sex mismatch: F to M (baseline) vs. other combinations | 1.08 (0.83–1.38) p = 0.55 |
1.07 (0.83–1.35) p = 0.59 |
1.13 (0.75–1.59) p = 0.54 |
1.09 (0.77–1.47) p = 0.61 |
| Full (baseline) vs. reduced-intensity conditioning | 1.02 (0.68–1.54) p = 0.93 |
1.18 (0.80–1.76) p = 0.40 |
1.34 (0.73–2.53) p = 0.35 |
0.43 (0.90–2.69) p = 0.11 |
| Recipient CMV serology Neg (baseline) vs. Pos | 1.01 (0.82–1.25) p = 0.89 |
0.96 (0.78–1.18) p = 0.70 |
1.0 (0.72–1.37) p = 0.98 |
0.94 (0.71–1.23) p = 0.66 |
| 10/10 HLA match (baseline) vs. others |
2.11 (1.40–3.26)
p = 0.0004 |
1.87 (1.26–2.80)
p = 0.0018 |
1.19 (0.64–2.19) p = 0.57 |
2.25 (1.33–3.93)
p = 0.0024 |
Ly: lymphocyte, MNC: mononuclear cell
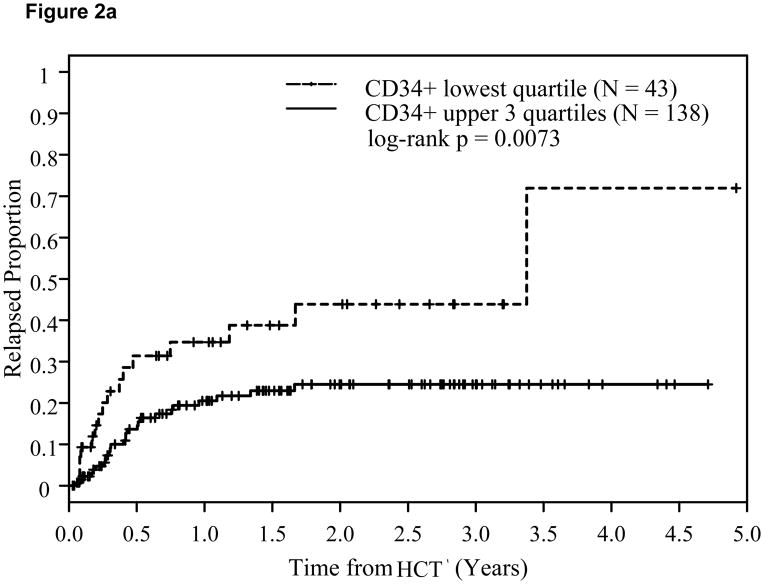
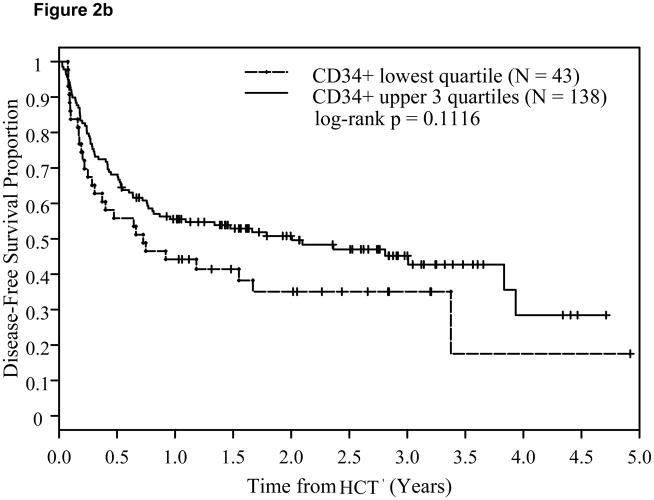
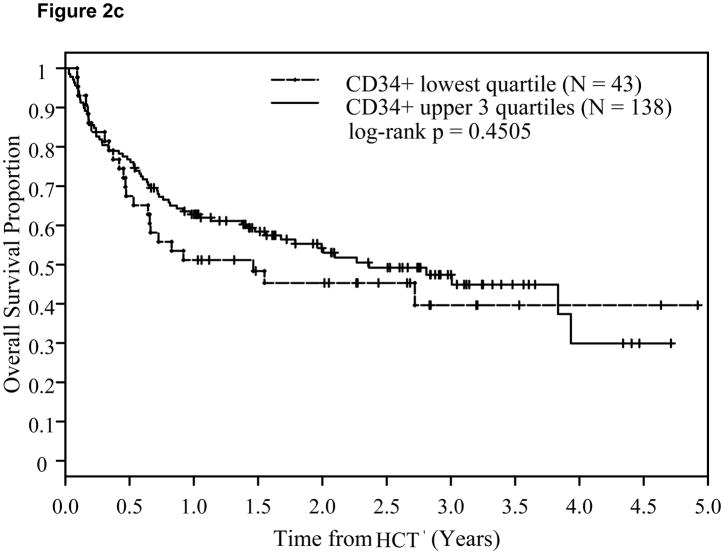
A CD34+ cell dose 4.2x106/kg (p25) or above was associated with significantly lower relapse risk (Hazard Ratio: HR=0.67; p=0.013) and a trend for corresponding improvement in DFS (HR=0.84; p=0.12), but not in OS (HR=0.91; p=0.46). This time-to-relapse difference was also evident by the Kaplan-Meier estimates and the log-rank test. At two years post-transplant, CD34+ cell dose <4.2x106/kg (q25) was associated with significantly higher relapse risk at 44% compared with 24% among patients with CD34+ dose in the upper 3 quartiles (log-rank p=0.0073, Figure 2a). A trend for better DFS (p=0.11, Figure 2b) was also noted. This was, however, not accompanied by an associated improvement in OS (p=0.45, Figure 2c). The impact of CD34+ cell dose on relapse was also significant when it was analyzed as a continuous variable (Table 3).
Figure 2.
Effect of CD34+ cell dose on relapse (2a), disease-free survival (2b), and overall survival (2c).
Table 3.
Significant factors associated with OS, DFS, relapse, and TRM by multivariate analysis (Cox regression)
| OS | HR | p-values |
|---|---|---|
| 10/10 HLA match (baseline) vs. others | 2.23 (95%CI 1.46–3.43) | 0.0002 |
| Lymphocyte dose in the graft (continuous) | 0.20 (95%CI 0.05–0.64) | 0.0045 |
| MNC dose in the graft: p25 (baseline) vs. others | 1.34 (95%CI 1.01–1.82) | 0.0388 |
|
| ||
| DFS | ||
|
| ||
| 10/10 HLA match (baseline) vs. others | 1.91 (95%CI 1.28–2.87) | 0.0014 |
| Lymphocyte dose in the graft (continuous) | 0.35 (95%CI 0.14–0.81) | 0.0123 |
| Good risk disease (baseline) vs. others | 1.73 (95%CI 1.01–3.17) | 0.044 |
|
| ||
| Relapse | ||
|
| ||
| CD34+ quartile: p25 (baseline) vs. others | 0.64 (95%CI 0.47–0.88) | 0.0077 |
| Recipient age (continuous) | 1.02 (95%CI 1.00–1.05) | 0.019 |
|
| ||
| TRM | ||
|
| ||
| 10/10 HLA match (baseline) vs. others | 2.46 (95%CI 1.44–4.30) | 0.0009 |
| Full-intensity (baseline) vs. reduced-intensity conditioning | 1.76 (95%CI 1.03–3.10) | 0.038 |
MNC: mononuclear cell
CD34+ cell doses ≥ 6.7x106/kg (p50: median) was associated with reduced relapse risk (HR= 0.52; p=0.042) but this result did not extend to DFS, OS, or TRM. A CD34+ cutoff at 9.5 x106/kg (p75) had no significant impact on OS, DFS, relapse, or TRM.
There was no significant association between the CD34+ cell dose (either as a continuous or categorized variable) and acute GVHD (grade II–IV or III–IV). We observed no significant association between the CD34+ dose and chronic GVHD when the CD34+ cell dose was analyzed as a continuous variable (p = 0.19). When the CD34+ dose was categorized in quartiles, CD34+ cell dose < median was associated with increased chronic GVHD (p=0.006, HR: 0.42 [CI: 0.23 – 0.78]).
In multivariate analysis, cellular composition of the PBSC grafts remained significant in influencing transplant outcomes (Table 3), independent of other variables such as conditioning regimen or disease risk status. Similar to the univariate analysis, the greater lymphocyte dose (as continuous variable) was associated with improved OS and DFS. A greater CD34+ cell dose ≥ p25 was associated with reduced relapse without improved OS or DFS independent of disease risk status or conditioning regimens (reduced intensity or full-intensity). Total MNC dose infused also affected OS when grouped as <p25 versus ≥p25, in which the higher MNC dose was associated with inferior survival. As expected, patients who received transplant from a 10/10 matched donor had a significantly better OS and DFS associated with reduced TRM. We found that the increased patient age was associated with slightly higher risk (HR: 1.02, P=0.03) of relapse in our cohort. We observed that reduced-intensity conditioning was associated with increased TRM, likely due to underlying comorbidities such as age, organ function, and performance status.
Association between CD34+ cell dose and post-transplant lymphocyte recovery
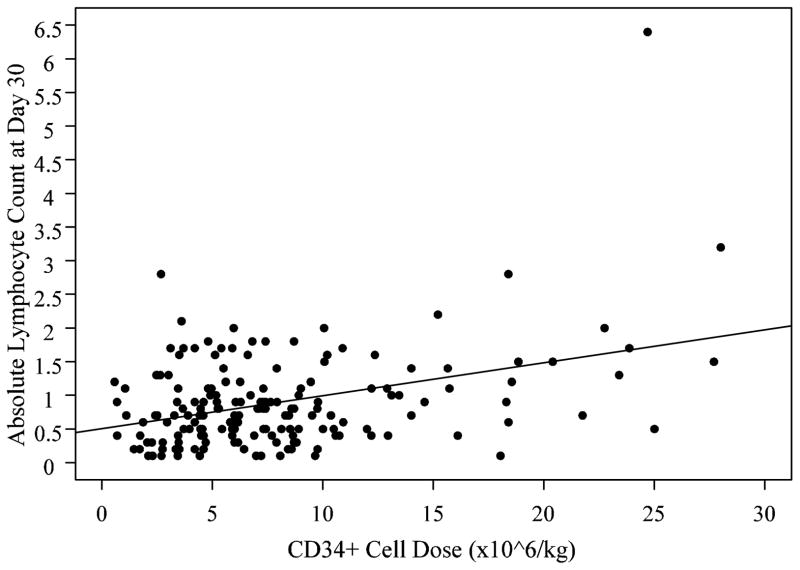
We evaluated a possibility that the protective effect of CD34+ cell dose on relapse may be immune-mediated. We found a highly significant correlation between CD34+ cell dose infused and absolute lymphocyte count (ALC) on day 30 post-transplant (r=0.378 p<0.0001, Figure 3), suggesting that the higher CD34+ cell dose contributes to faster lymphoid recovery. The relationship between infused NMC and ALC was less significant (p=0.027). There was no significant association between infused lymphocyte dose and ALC (p=0.1).
Figure 3.
Absolute lymphocyte count on day +30 post-transplant is associated with CD34+ cell dose. There was a highly significant correlation between CD34+ cell dose infused and absolute lymphocyte count (ALC) on day 30 post-transplant (r=0.378 p<0.0001).
Subset analysis: The CD34+ dose effect is observed in myeloid cancers, reduced-intensity conditioning, high-risk diseases, patients with an iKIRL mismatch in the GVH direction, and those who lack iKIRL
It has been known that NK cells largely contribute to the ALC recovery early post-transplant30. Since we observed a close relationship between infused CD34+ cell dose and faster ALC recovery, we hypothesized that the protective effect against relapse in the higher CD34+ cell dose may be linked to an NK cell-mediated graft-versus-leukemia/lymphoma (GVL) effect. In this retrospective study, we had no saved blood samples that we could directly analyze NK cell numbers and functions. Instead, we evaluated the CD34+ cell dose effect in the following subsets of patients: 1) myeloid cancers versus lymphoid cancers, 2) reduced-intensity conditioning versus full-intensity conditioning, 3) donor-recipient pairs with iKIRL mismatch in the GVH direction versus the pairs without iKIRL mismatch in the GVH direction, and 4) patients lacking iKIRL. We expected to see an enhanced CD34+ cell dose effect in myeloid patients, pairs with KIR mismatch in the GVH direction, and patients lacking iKIRL.
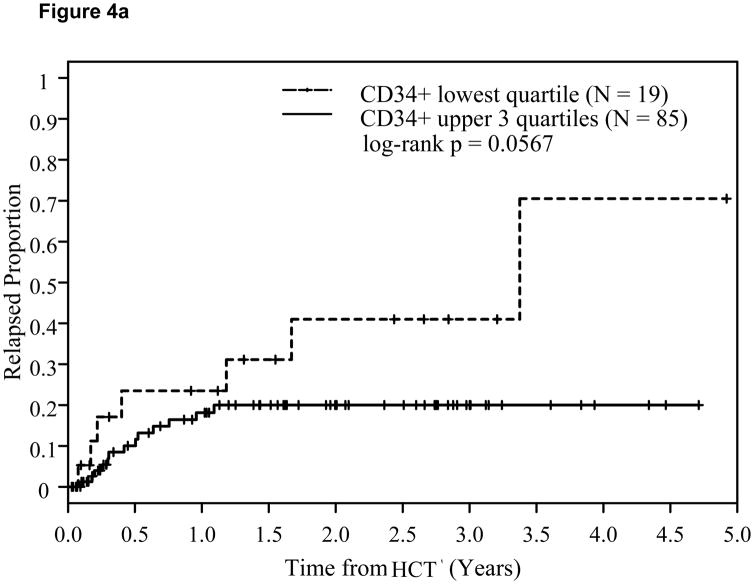
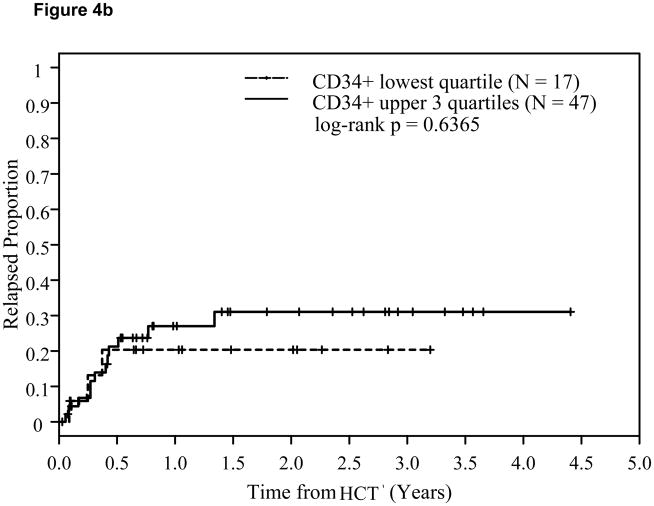
In a subgroup of 104 myeloid cancer patients (AML, CML, and MDS), there were no statistically significant differences between the upper 3 quartiles of CD34+ cell dose versus the lowest quartile with respect to OS or DFS. However, the log-rank p-value for comparing time to relapse in the myeloid subgroup was p=0.0567. Among the 64 patients in the lymphoid malignancy subgroup (ALL, NHL, HD, and CLL), there were no statistically significant differences with respect to any time-to-event endpoints when we compared the lowest quartile of CD34+ cell dose versus the upper three quartiles.
We also analyzed the impact of CD34+ cell dose separately in reduced-intensity conditioning (n=98) and full-intensity conditioning (n=83). The log-rank p-value for comparing time to relapse between the upper 3 quartiles of CD34+ cell dose versus the lowest quartile in the reduced-intensity subgroup was p=0.0192 (47.6% versus 24.7%), while it was not significant in the full-intensity subgroup. In the reduced-intensity subgroup, however, there was no corresponding significant improvement in OS or DFS with higher CD34+ cell doses.
There were 18 patients with an iKIRL mismatch in the GVH direction. Among this subgroup, the log-rank p-value for comparing the lowest quartile (p25) of CD34+ cell dose versus those who received higher doses was p<0.0001 with respect to time to relapse. This is in contrast with a log-rank p-value of p=0.041 among those patients who did not have an iKIRL mismatch in the GVH direction. Whereas the CD34+ cell dose in patients without iKIRL mismatch showed no statistically significant difference in DFS, the log-rank p-value for DFS was significant in those with a iKIRL mismatch in the GVH direction (p=0.0053). iKIRL mismatch stratified by CD34+ cell dose was not statistically significant on OS.
We also performed a stratified analysis in patients lacking iKIRL versus not. There were 56 patients lacking iKIRL and 125 without lacking iKIRL. When stratified by CD34+ cell dose, time-to-relapse was not significant among the patients who did not lack iKIRL but was significant in patients lacking iKIRL (log-rank p=0.0030). The impact of the higher dose of CD34+ cells in patients lacking iKIRL did not extend to improved DFS or OS, however.
Further subset analysis of standard risk group (n=35; Table 1) versus high-risk disease (n=146) showed that the CD34+ dose ≥ p25 was significantly associated with reduced relapse in high-risk disease (p=0.015), whereas the p-value was not significant in low-risk disease (p=0.5).
Discussion
There have been several studies demonstrating the impact of CD34+ cell dose on transplant outcomes1–6. However, the data are limited to sibling donor transplants or unrelated donor bone marrow transplant. In our MUD-PBSC cohort, we observed a faster neutrophil and platelet recovery with higher CD34+ cell dose, which is consistent with the earlier reports.
Importantly, we found that patients who received higher numbers of CD34+ cells had a lower relapse rate. Subset analysis suggests that this may be more important in myeloid diseases that are typically associated with a stronger GVL effect. In addition, the beneficial effect of higher CD34+ cell dose in reducing relapse was more evident in high-risk diseases than in low-risk diseases consistent with an earlier study8.
In our cohort, the significance of CD34+ cell dose was highest when the group was separated by the CD34+ cell dose at the lowest quartile (p25) versus the others (cutoff: 4.2x106/kg) rather than by median or at p75. It is thus possible that the CD34+ cell dose level above which there is no further benefit for reducing relapse may lie around the dose of 4–5x106/kg.
The protective effect of higher CD34+ cell dose for relapse resulted in a trend for improved DFS but did not translate to a better OS. Although we observed no significant difference in TRM or acute GVHD according to the CD34+ cell dose, there is a possibility that the higher CD34+ cell dose may negatively impact on TRM without reaching a statistical significance due to the size of this study. Contrary to a few previously published studies in sibling donor PBSCT10,11, we observed a reduced risk of chronic GVHD when higher CD34+ cell doses were infused.
The mechanism in which the higher CD34+ dose reduces relapse is not known. One possible mechanism is that the higher CD34+ cell dose may improve immune recovery, which induces a stronger GVL effect. In the early post-transplant period, the majority of peripheral blood lymphocytes are NK cells30 which can mediate cytotoxicity without prior sensitization and may be responsible for early GVL effects31. A study by Savani et al. showed that higher CD34+ doses established an earlier donor NK cell population, resulting in superior antileukemic activity post-transplant17. In agreement with that work, we found a significant association between CD34+ cell dose and early lymphocyte recovery. The possibility of NK cell-mediated GVL is further suggested by the findings that the higher CD34+ cell dose was associated with 1) greater reduction in relapse in myeloid malignancies compared to lymphoid malignancies; 2) greater reduction in relapse when there is an iKIRL-mismatch in the GVH direction; and 3) greater reduction in relapse when there is a lack of iKIRL. Our subset analysis on reduced-intensity transplants was also consistent with a possility that the CD34+ cell dose may be more important in reduced-intensity transplants as they likely rely more on GVL.
Allospecific interactions between donor NK cells and recipient leukemic cells, was originally demonstrated in the clinical setting of T-cell–depleted HCT from MHC haplotype–disparate related donors13. The pivotal structural elements underlying this mechanism are KIR, which are clonally expressed on the surface of NK cells or certain T-cell subsets, and membrane-bound MHC class I molecules as their natural ligands on target cells32–34. Since our study was limited to retrospective analysis, it was not possible to demonstrate correlative in-vitro data for NK-mediated GVL. A prospective study of evaluating NK cell recovery and function post-transplant is currently underway in our institution.
Our data need to be interpreted with caution. Confounding factors in our study include various regimens for GVHD prophylaxis and conditioning, various degrees of HLA matching, as well as combining patients with heterogeneous diagnoses, although multivariate analysis showed an independent effect of CD34+ doses. We cannot definitively differentiate the effect of higher Ly dose resulting in greater GVL versus a GVL effect from higher CD34+ dose. In this study, there was a correlation between CD34+ dose and Ly dose in the PBSC grafts. Lymphocyte recovery after allogeneic PBSCT can be affected not only by CD34+ cells but also by lymphocyte content of the graft35. However, higher lymphocyte dose in our study was associated with better OS and DFS but not with decreased relapse, suggesting that the GVL effect was more closely linked to the CD34+ cell dose than Ly dose. Multivariate analysis also indicated the effect of higher CD34+ cell doses in reducing relapse was independent of the Ly dose.
It should also be noted that the graft cell dose (CD34+ cell, Ly) may not necessarily be the prime determinant of leukemia relapse. These parameters may be surrogates for other measures of T-cell and NK-cell function, antigen-presenting cells, engraftment, or pathologic processes that ultimately result in relapse of residual disease.
In summary, we demonstrate that the graft cell dose affects on transplant outcome in MUD-PBSCT, and that higher CD34+ cell doses reduce relapse rates, in particular, for high-risk diseases. The data also suggest that the protective effect of higher CD34+ cell dose may be immune-mediated, possibly through NK cell recovery. Further detailed characterization of the graft content and functional assessment of emerging NK cells post-transplant are necessary to better understand the mechanism, and to improve outcomes after MUD-PBSCT.
Figure 4.
Effect of CD34+ cell dose on relapse in myeloid cancers (4a) and lymphoid cancers (4b).
Acknowledgments
The authors would like to acknowledge our transplant coordinators, unrelated donor coordinators, and transplant nurses for their dedicated care of our patients, and all the members of the Hematopoietic Cell Transplant Team for their constant support of the program. We also thank clinical research associates for their support on data management and collections.
Footnotes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conception and design: Nakamura R, Palmer J, Smith DD, Nademanee A, Forman SJ. Provision of study materials or patients: Nakamura R, Nademanee A, Sun JY, Senitzer D, Pullarkat V, Parker P, Rodriguez R, Stein A, Rosenthal J, Wang S, Schriber J, Karanas C, Gaal K, Forman SJ. Administrative, technical, or logistic support: Nakamura R, Nademanee A, Palmer J, Senitzer D, Forman S Collection and assembly of data: Nakamura R, Nademanee A, Sun JY, Senitzer D, Pullarkat V, Parker P, Rodriguez R, Stein A, Rosenthal J, Wang S, Schriber J, Karanas C, Gaal K, Gaal K, Forman SJ. Analysis and interpretation of data: Nakamura R, Palmer J, Smith AD, Nademanee A, Sun JY, Senitzer D, Schriber J, Pullarkat V, Forman S. Drafting of the article: Nakamura R, Smith DD, Nademanee A, Sun J, Senitzer D, Schriber J, Forman SJ
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mavroudis D, Read E, Cottler-Fox M, et al. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88:3223–3229. [PubMed] [Google Scholar]
- 2.Nakamura R, Bahceci E, Read EJ, et al. Transplant dose of CD34(+) and CD3(+) cells predicts outcome in patients with haematological malignancies undergoing T cell-depleted peripheral blood stem cell transplants with delayed donor lymphocyte add-back. Br J Haematol. 2001;115:95–104. doi: 10.1046/j.1365-2141.2001.02983.x. [DOI] [PubMed] [Google Scholar]
- 3.Ringden O, Barrett AJ, Zhang MJ, et al. Decreased treatment failure in recipients of HLA-identical bone marrow or peripheral blood stem cell transplants with high CD34 cell doses. Br J Haematol. 2003;121:874–885. doi: 10.1046/j.1365-2141.2003.04364.x. [DOI] [PubMed] [Google Scholar]
- 4.Dominietto A, Lamparelli T, Raiola AM, et al. Transplant-related mortality and long-term graft function are significantly influenced by cell dose in patients undergoing allogeneic marrow transplantation. Blood. 2002;100:3930–3934. doi: 10.1182/blood-2002-01-0339. [DOI] [PubMed] [Google Scholar]
- 5.Sierra J, Storer B, Hansen JA, et al. Transplantation of marrow cells from unrelated donors for treatment of high-risk acute leukemia: the effect of leukemic burden, donor HLA-matching, and marrow cell dose. Blood. 1997;89:4226–4235. [PubMed] [Google Scholar]
- 6.Bittencourt H, Rocha V, Chevret S, et al. Association of CD34 cell dose with hematopoietic recovery, infections, and other outcomes after HLA-identical sibling bone marrow transplantation. Blood. 2002;99:2726– 2733. doi: 10.1182/blood.v99.8.2726. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Simon JA, Diez-Campelo M, Martino R, et al. Impact of CD34+ cell dose on the outcome of patients undergoing reduced-intensity-conditioning allogeneic peripheral blood stem cell transplantation. Blood. 2003;102:1108–1113. doi: 10.1182/blood-2002-11-3503. [DOI] [PubMed] [Google Scholar]
- 8.Gorin NC, Labopin M, Boiron JM, et al. Results of Genoidentical Hemopoietic Stem Cell Transplantation With Reduced Intensity Conditioning for Acute Myelocytic Leukemia: Higher Doses of Stem Cells Infused Benefit Patients Receiving Transplants in Second Remission or Beyond--The Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24:3959– 3966. doi: 10.1200/JCO.2006.05.5855. [DOI] [PubMed] [Google Scholar]
- 9.Przepiorka D, Smith TL, Folloder J, et al. Risk factors for acute graft-versus-host disease after allogeneic blood stem cell transplantation. Blood. 1999;94:1465–70. [PubMed] [Google Scholar]
- 10.Zaucha JM, Gooley T, Bensinger WI, et al. CD34 cell dose in granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cell grafts affects engraftment kinetics and development of extensive chronic graft-versus-host disease after human leukocyte antigen-identical sibling transplantation. Blood. 2001;98:3221– 3227. doi: 10.1182/blood.v98.12.3221. [DOI] [PubMed] [Google Scholar]
- 11.Mohty M, Bilger K, Jourdan E, et al. Higher doses of CD34+ peripheral blood stem cells are associated with increased mortality from chronic graft-versus-host disease after allogeneic HLA-identical sibling transplantation. Leukemia. 2003;17:869–75. doi: 10.1038/sj.leu.2402909. [DOI] [PubMed] [Google Scholar]
- 12.Remberger M, Ringden O, Blau I-W, H, et al. No difference in graft-versus-host disease, relapse, and survival comparing peripheral stem cells to bone marrow using unrelated donors. Blood. 2001;98:1739– 1745. doi: 10.1182/blood.v98.6.1739. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 14.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 15.Davies SM, Ruggieri L, DeFor T, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants: killer immunoglobulin-like receptor. Blood. 2002;100:3825–3827. doi: 10.1182/blood-2002-04-1197. [DOI] [PubMed] [Google Scholar]
- 16.Miller JS, Cooley S, Parham P, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. 2007;109:5058–5061. doi: 10.1182/blood-2007-01-065383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savani BN, Rezvani K, Mielke S, et al. Factors associated with early molecular remission after T cell-depleted allogeneic stem cell transplantation for chronic myelogenous leukemia. Blood. 2006;107:1688– 1695. doi: 10.1182/blood-2005-05-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaffer M, Olerup O. HLA-AB typing by polymerase-chain reaction with sequence-specific primers: more accurate, less errors, and increased resolution compared to serological typing. Tissue Antigens. 2001;58:299–307. doi: 10.1034/j.1399-0039.2001.580503.x. [DOI] [PubMed] [Google Scholar]
- 19.Sun JY, Gaidulis L, Miller MM, Goto RM, Rodriguez R, Forman SJ, Senitzer D. Development of a multiplex PCR-SSP method for Killer-cell immunoglobulin-like receptor genotyping. Tissue Antigens. 2004;64:462–468. doi: 10.1111/j.1399-0039.2004.00303.x. [DOI] [PubMed] [Google Scholar]
- 20.Giebel S, Locatelli F, Lamparelli T, et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- 21.Beelen DW, Ottinger H, Ferencik S, et al. Genotypic inhibitory killer immunoglobulin-like receptor ligand incompatibility enhances the long-term antileukemic effect of unmodified allogeneic hematopoietic stem cell transplantation in patients with myeloid leukemias. Blood. 2005;105:2594–2600. doi: 10.1182/blood-2004-04-1441. [DOI] [PubMed] [Google Scholar]
- 22.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blume KG, Forman SJ, Appelbaum FR. Thomas’ Hematopoietic Cell Transplantation. 3. Malden, MA: Blackwell Scientific Publications; Oxford; 2004. [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 25.Shulman HM. Graft-versus-host disease. Surv Synth Pathol Res. 1984;3:233–234. doi: 10.1159/000156928. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan G, Meier P. Non-parametric estimations from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Peto R, Peto J. Asymptotically efficient rank invariant test procedures (with discussion) J R Stat Soc A. 1972;135:195–206. [Google Scholar]
- 28.Hauck WW, Donner A. Wald’s test as applied to hypotheses in logit analysis. J Am Stat Assoc. 1977;72:851–853. [Google Scholar]
- 29.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 30.Jiang YZ, Barrett AJ, Goldman JM, Mavroudis DA. Association of natural killer cell immune recovery with a graft-versus-leukemia effect independent of graft-versus host disease following allogeneic bone marrow transplantation. Ann Hematol. 1997;74:1–6. doi: 10.1007/s002770050246. [DOI] [PubMed] [Google Scholar]
- 31.Niederwieser D, Gastl G, Rumpold H, Marth C, Kraft D, Huber C. Rapid reappearance of large granular lymphocytes (LGL) with concomitant reconstitution of natural killer (NK) activity after human bone marrow transplantation (BMT) Br J Haematol. 1987;65:301–305. doi: 10.1111/j.1365-2141.1987.tb06857.x. [DOI] [PubMed] [Google Scholar]
- 32.Boyington JC, Sun PD. A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors. Mol Immunol. 2002;38:1007–1021. doi: 10.1016/s0161-5890(02)00030-5. [DOI] [PubMed] [Google Scholar]
- 33.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. J Exp Med. 1995;181:1133–1144. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumperz JE, Barber LD, Valiante NM, et al. Conserved and variable residues within the Bw4 motif of HLA-B make separable contributions to recognition by the NKB1 killer cell-inhibitory receptor. J Immunol. 1997;158:5237–5241. [PubMed] [Google Scholar]
- 35.Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol. 2004;125:217–224. doi: 10.1111/j.1365-2141.2004.04891.x. [DOI] [PubMed] [Google Scholar]