Abstract
Background:
In light of recent controversy about the safety and efficacy of intracranial stenting, we sought to evaluate our experience with primary balloon angioplasty for symptomatic, high-grade intracranial stenosis.
Methods:
All intracranial angioplasty cases performed at Vanderbilt University Medical Center from 2006 to 2011 were retrospectively reviewed for degree of stenosis pre- and post-procedure. Immediate peri-procedural complications were evaluated as well as one-month and long-term outcomes.
Results:
A total of 26 patients were included in the study with a mean age of 63.0 years and a mean follow-up of 350.2 days. The average pre-procedure stenosis was 71.2%. The immediate, average post-procedure stenosis was 46.6%, and the average post-procedure stenosis at last angiographic follow-up was 44.5%. Retreatment was required in only 3.8% of patients. The primary end-point of major stroke or death at 30 days was observed in 11.5%, and the overall intra-procedural complication rate was 7.7%. The incidence of stroke or death at last follow-up was 15.4%, which is comparable to the one-year stroke or death rate in the medical arm of the SAMPRISS trial.
Conclusions:
In this retrospective series, primary balloon angioplasty was found to be effective as a treatment option for symptomatic intracranial stenosis with the risk of stroke or death at 30 days higher than the medical arm of SAMPRIS but lower than the stenting arm. The one-year risk of stroke was comparable to that reported for the one-year outcomes in the SAMPRISS medical arm.
Keywords: Angioplasty, cerebrovascular, intracranial stenosis, stenting, stenosis
INTRODUCTION
The results of the highly anticipated Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial revealed a 5.8% 30-day stroke or death rate in the medical treatment arm compared to 14.7% in the stent plus medical therapy arm.[1] In its wake, optimal treatment for symptomatic intracranial stenosis is unclear. Many experts continue to question whether certain high-risk populations might still benefit from endovascular intervention in the setting of severe intracranial stenosis.
Certainly there is still room for significant improvement in the management of these patients, as the projected stroke or death rate at one year approaches 13%, even in the SAMMPRIS medical arm.[1] Thus, for patients at highest risk for stroke, a more permissive role for primary angioplasty, especially in situations where stent navigation is technically challenging, has been advocated.[9]
In this retrospective review of our 5-year institutional experience, we analyzed patients treated with primary balloon angioplasty without stenting in order to compare these outcomes with the medically-treated and stented arms of the SAMMPRIS trial as well as other published series.
PATIENT SELECTION AND METHODS
Patient selection included all patients who had undergone primary intracranial balloon angioplasty at Vanderbilt University Medical Center in the period between January 2006 and January 2011. A retrospective review of these patients was performed with institutional review board (IRB) approval (#110220). All patients who underwent angioplasty exhibited high-grade, angiographic stenosis (>50%) with ischemic symptomatology in the corresponding vascular distribution.
Patients were excluded if angioplasties were performed in conjunction with stenting or for indications other than intracranial atherosclerosis: (e.g., vasospasm resulting from SAH). Patients who underwent angioplasty in the setting of acute stroke were also excluded. The decision to pursue angioplasty as opposed to stenting was ultimately determined by the interventionist at the time of patient presentation. Patient demographics and risk factors were compiled from a chart review, as well as from intra-procedural and post-procedural complications.
Digital subtraction angiography (DSA) images were reviewed by a blinded neuro-radiologist who quantified pre-and post-procedure stenosis. Because this study was performed retrospectively, an absolute measurement of vessel diameter was unobtainable due to the varying degrees of magnification in each radiologic projection. Therefore, a stenosis ratio was calculated. The narrowest diameter of each stenosed vessel at the site of the angioplasty was measured and divided by the diameter of the normal vessel just distal to the lesion. If no suitable vessel existed beyond the lesion for measurement, the vessel proximal to it was used. The resulting quotient, which we call the “stenosis ratio,” was subtracted from 1 to determine the degree of stenosis, which was presented as a percentage.
While all the participating interventionists used heparin to obtain a target ACT of 2-3 times baseline (or a target ACT of 250-300 s), the use of anti-platelets was more variable. Almost universally, patients were already taking either aspirin, clopidogrel or both, at the time of the procedure [Table 1]. Assays to determine anti-platelet activity (e.g., VerifyNow, Accumetrics, San Diego, CA) were not routinely utilized.
Table 1.
Anti-platelet therapy at time of procedure
At least one of the interventionists (RJS) gave a bolus dose of a GP-IIbIIIa inhibitor intra-procedurally before inflating the balloon. The specific nuances of balloon-type, balloon sizing, and inflation method (e.g., rapidity of inflation, number of times the balloon was inflated, duration of angioplasty) were not standardized among the various practitioners.
ILLUSTRATIVE CASES
Example patient 1
A 71-year-old male with a one-month history of intermittent, waxing and waning, right-sided weakness, slurred speech, and confusion presented to an outside hospital. He underwent MRI and MRA, and was noted to have a severe stenosis of the proximal basilar artery. He was transferred to our institution for consideration of interventional therapy. He was placed on a medical regimen of aspirin, a statin, an ACE inhibitor, as well as a diuretic for management of his hypertension. He was discharged initially but returned to the ED eight days later with similar symptoms. It was decided that intervention would be appropriate at this time [Figures 1–3].
Figure 1.
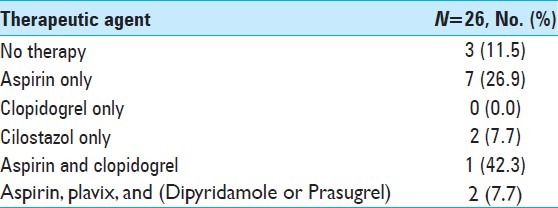
Example patient 1's pre-intervention angiography. Pre-intervention angiography of proximal basilar artery demonstrating critical stenosis
Figure 3.
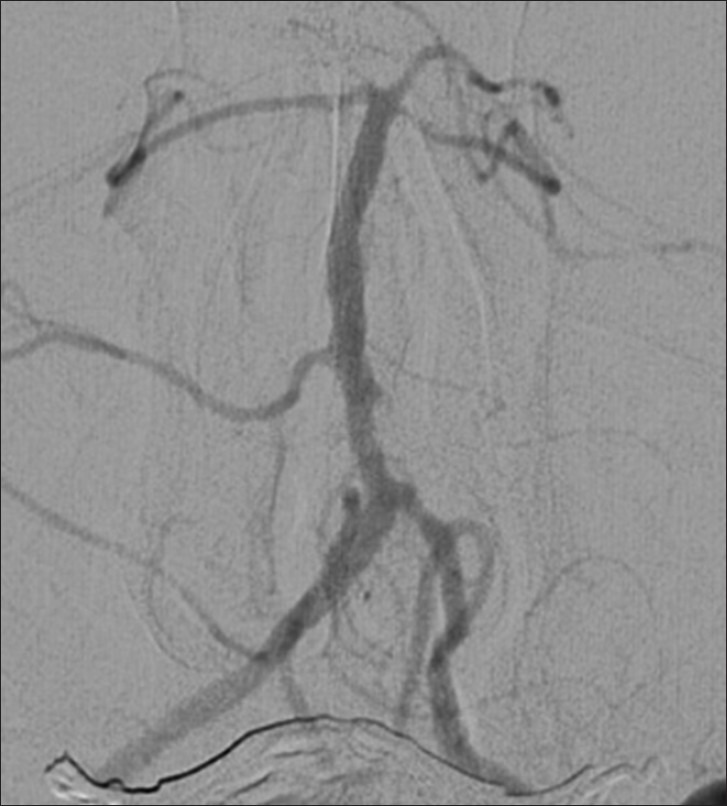
Example patient 1's follow-up angiography. Follow-up angiography nearly five years post-procedure demonstrating impressive remodeling of the stenosis and a durable result
Figure 2.
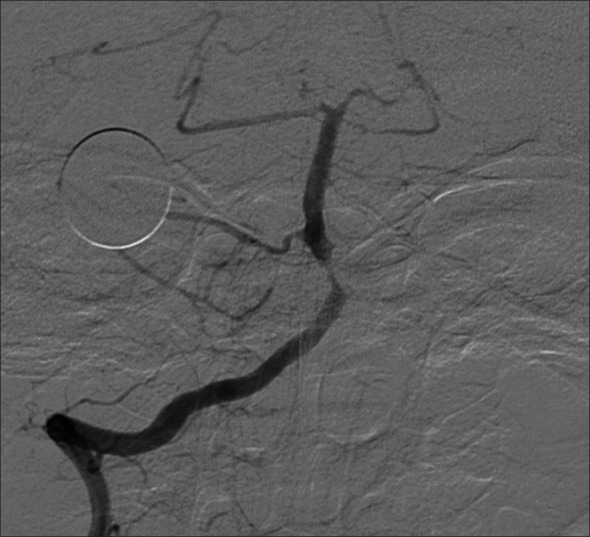
Example patient 1's post-intervention angiography. Post-intervention angiography of proximal basilar artery demonstrating significant improvement in caliber of stenotic lumen
Example patient 2
A 52-year-old male was working outside in the heat when he developed weakness and lost his balance. Two weeks later, he developed an episode of numbness involving his left thumb and left side of the tongue, as well as slurred speech and drooling. He visited an outside hospital where an MRI revealed a sub-acute, sub-cortical cerebral infarction involving the right fronto-parietal area. Subsequent MRA revealed high-grade focal stenosis within the M1 segment of the right middle cerebral artery. He was referred to our institution where he underwent angiography and subsequent angioplasty [Figures 4–6].
Figure 4.
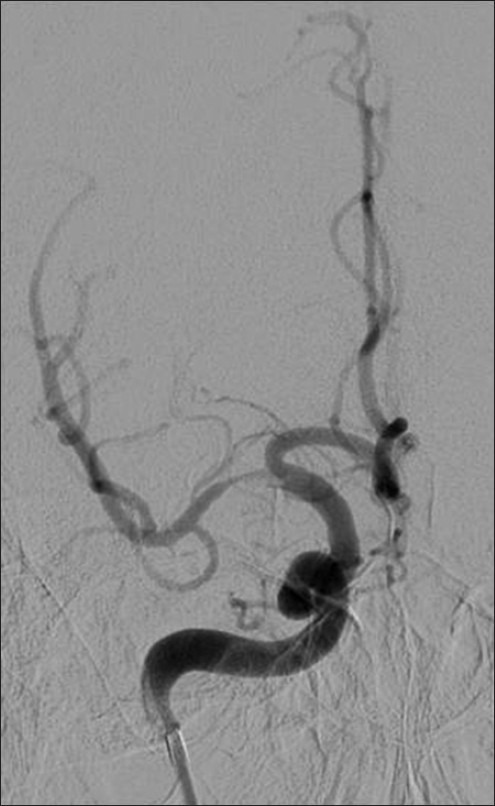
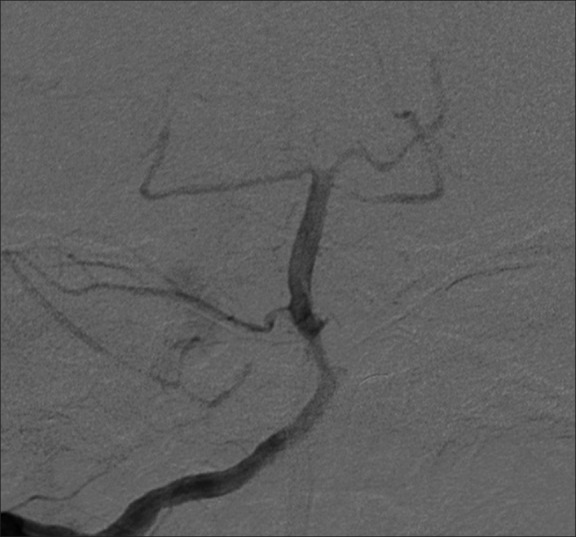
Example patient 2's pre-intervention angiography. Pre-intervention angiography of proximal right M1 middle cerebral artery demonstrating severe focal stenosis
Figure 6.
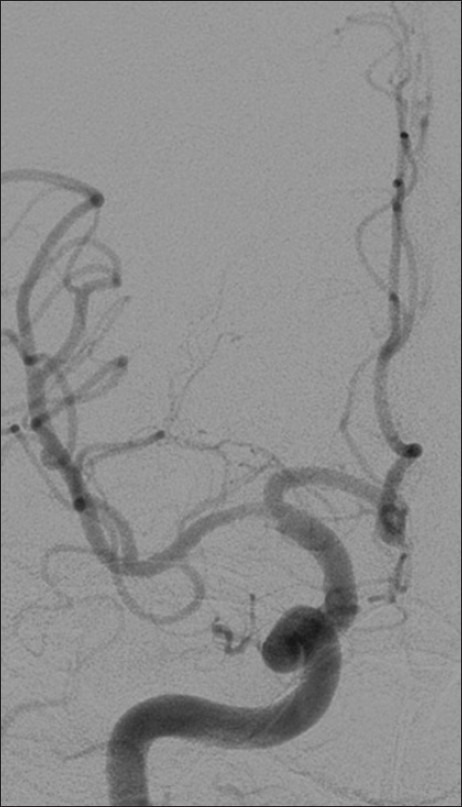
Example patient 2's follow-up angiography. Follow-up angiography approximately 2 months post-procedure demonstrating significantly improved luminal caliber despite moderate residual proximal focal stenosis
Figure 5.
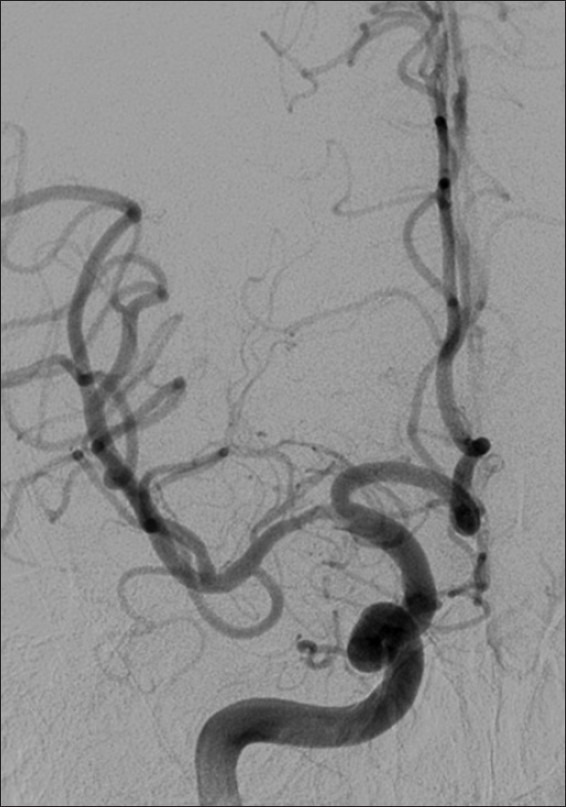
Example patient 2's post-intervention angiography. Post-intervention angiography of right M1 middle cerebral artery demonstrating successful angioplasty of the stenotic lumen with a widely patent result
RESULTS
A total of 26 patients were included in the study, with a mean age of 63.0 years and a mean length of follow-up of 350.2 days. The most common risk factor for intracranial stenosis present in the study population was hypertension (84.6%). Other risk factors included hyperlipidemia, diabetes mellitus, coronary artery disease, COPD, and atrial fibrillation. The mean amount of time from initial clinical presentation to date of procedure was 13.3 days (range 1 to 61 days, std. dev. 15.1 days). The anatomic location of the stenotic lesion in each case is recorded in Table 2. The Gateway (Boston Scientific, Natick, MA) represented the most commonly used, identifiable balloon type in the series [Table 3].
Table 2.
Patient and lesion characteristics
Table 3.
Balloon catheter types

The average pre-procedure stenosis was 71.2% (range 51% to 90%, std. dev. 10.2%). The average immediate post-procedure stenosis was 46.6% (range 26% to 75%, std. dev. 12.2%), and the average post-procedure stenosis at last angiographic follow-up was 44.5% (range 44-92%, std. dev. 14%). Retreatment was required in only 3.8% of patients, secondary to recurrence of clinical ischemia. The primary end-point of major stroke or death at 30 days was observed in 11.5% of patients, and the intra-procedural complication rate was 7.7% [Tables 4 and 5].
Table 4.
Average stenosis and patient outcomes
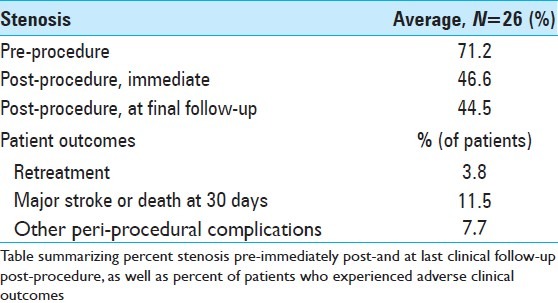
Table 5.
Peri-procedural complications
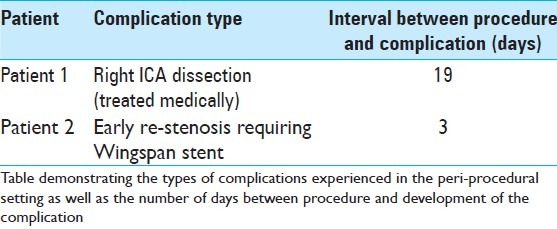
Of the 26 patients examined, 19 patients underwent some type of radiographic follow-up performed using either conventional angiography, CTA, or MRA. Twelve patients underwent DSA as primary method of follow-up, with an average follow-up interval of 167 days (range 3-742 days, std. dev. 237.7). Six patients underwent CTA as their primary method of follow-up, with an average follow-up interval of 178.4 days (range 3-587 days, std. dev. 179.4). One patient underwent MRA as the primary method of follow-up seen 396 days post-procedure. At last clinical follow-up of all patients, there was a 15.4% incidence of treatment-related stroke (11.5% of patients) or death (3.8% of patients).
DISCUSSION
The results of the recently published SAMMPRIS trial suggest that medical management may be superior to stenting for symptomatic, high-grade intracranial stenosis.[1,2,5] In the wake of these findings, there has been renewed interest in primary balloon angioplasty in this patient population.[9] This institutional series demonstrates the feasibility and relative safety of primary balloon angioplasty, and supports the incorporation of this technique into future trials that seek to identify high-risk populations that may benefit from angioplasty in conjunction with aggressive medical therapy for high-grade, symptomatic intracranial atherosclerosis.
There are several potential benefits to primary balloon angioplasty over stenting in the intracranial circulation.[8] Tortuous anatomy may make stent navigation technically challenging and increase procedural risk. Lesion location, lesion length, vessel size, and adjacent vascular architecture may also argue for balloon angioplasty. The costs associated with balloon angioplasty are lower, and no foreign body is left in the circulation. Furthermore, the use of stents obligates the patient to dual anti-platelet therapy and involves the possibility of in-stent re-stenosis, which can be problematic and difficult to treat.[3] While several authors have opined on the theoretical advantages of stenting over primary angioplasty in preventing early elastic recoil, the existing data is sparse and not clearly corroborative.[12,13]
The contemporary literature includes several reports of primary balloon angioplasty for intracranial atherosclerosis, using a wide variety of balloons mostly marketed for coronary use and revealing a success rate and risk profile largely comparable to stenting. A recent multi-institutional retrospective review reported a 92% success rate for primary balloon angioplasty and a three-month stroke or death rate of 8.5% in a series of 74 patients. Either Gateway (Boston Scientific) or Maverick balloon catheters (Boston Scientific, Fremont CA) were used in all cases, and the mean pre-treatment stenosis was 79% ± 14%.[8] One of the largest multi-center studies to compare primary angioplasty to stenting for symptomatic intracranial stenosis showed no difference in survival at 2 years follow-up. The 30-day rate of major stroke or death was 8.4% in the angioplasty group (8/95) versus 9.2% in the stenting group (9/98). Fifteen percent of the angioplasty arm as compared to only 4% of the stenting arm had significant post-procedural residual stenosis, although it was not clear how often this necessitated re-treatment.[11] In our series of 26 patients treated over the last five years at a single institution, we found an 11.5% rate of major stroke or death at one month, which is comparable to the existing literature on this topic.
It is also worth consideration that the one-year stroke or death rate in our series is 15.4%, which is comparable to the one-year stroke or death rate in the medical arm of SAMPRISS. This raises the question of whether angioplasty confers up-front risk with a pay-off of protection from stroke as follow-up increases. Certainly, further studies as to the long-term durability of this treatment method are wanting.
As we continue to add to the collective experience with primary balloon angioplasty for intracranial stenosis, it will be important to study whether certain patient populations or lesions with particular angiographic characteristics are more favorable for interventional treatment. A patient with a critical left M1 stenosis and “active ischemia” who becomes aphasic and hemiplegic with upright posture or a drop in blood pressure must be approached from a different perspective than a patient who presents with a TIA or minor stroke in the distribution of stenosis but has no active or reproducible symptoms. The type of index event (i.e., TIA vs. stroke) and the interval between the index event and procedure may also affect risk profile and outcome; however, the literature has yet to show a consistent relationship.[6,7,9,14] With regard to the angiographic characteristics of the lesion in question, a systematic review of 31 studies dealing with over 1,100 procedures (stenting and primary balloon angioplasty) revealed the complication rate in the posterior circulation to be almost double that in the anterior circulation.[4] While it has been suggested that lesion length, degree of stenosis, anatomic location, and the presence of tandem lesions might also affect outcomes, the role that these factors play remains largely unknown.
The senior author routinely administers a glycoprotein IIb/IIIa inhibitor (e.g., integrillin) intra-procedurally. Perhaps the use of this agent might decrease the rates of acute thrombus formation. This effect may be especially pertinent in those patients who exhibit some degree of resistance to aspirin, clopidogrel, or both, as some studies show this population is at higher risk for thromboembolic complications from neurointerventions.[10] This practice is not standardized, and no studies have documented the impact of GP-IIb/IIIa inhibitor use at time of angioplasty on patient outcomes. Certainly, there exists a concern regarding increased hemorrhage rates in patients receiving these agents; however, none of the patients in this series experienced a hemorrhage as a result of the procedure.
The limitations of this study arise from the small sample size, retrospective design, and absence of a valid comparison arm. It should be noted that only a prospective-randomized study will be able to establish the usefulness of this treatment.
CONCLUSIONS
In this retrospective series, primary balloon angioplasty was found to be a relatively safe and effective treatment option for symptomatic intracranial stenosis. Further study may elucidate specific clinical factors or anatomic sites or patterns of stenosis, which make a patient particularly amenable to balloon angioplasty.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2013/4/1/18/107542
Contributor Information
Luke Tomycz, Email: luke.tomycz@vanderbilt.edu.
Neil K. Bansal, Email: neil.k.bansal@vanderbilt.edu.
Tim Lockney, Email: dennis.t.lockney@vanderbilt.edu.
Megan Strothers, Email: megan.strother@vanderbilt.edu.
John J. Connors, Email: buddy.connors@vanderbilt.edu.
Scott Shay, Email: scott.shay@vanderbilt.edu.
Robert J. Singer, Email: robert.singer@vanderbilt.edu.
REFERENCES
- 1.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–16. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 3.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–7. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 4.Gröschel K, Schnaudigel S, Pilgram SM, Wasser K, Kastrup A. A systematic review on outcome after stenting for intracranial atherosclerosis. Stroke. 2009;40:340–7. doi: 10.1161/STROKEAHA.108.532713. [DOI] [PubMed] [Google Scholar]
- 5.Khan M, Naqvi I, Bansari A, Kamal AK. Intracranial atherosclerotic disease. Stroke Res Treat. 2011;2011:282845. doi: 10.4061/2011/282845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurre W, Berkefeld J, Brassel F, Brüning R, Eckert B, Kamek S, et al. INTRASTENT Study Group. In-hospital complication rates after stent treatment of 388 symptomatic intracranial stenoses: Results from the INTRASTENT multicentric registry. Stroke. 2010;41:494–8. doi: 10.1161/STROKEAHA.109.568063. [DOI] [PubMed] [Google Scholar]
- 7.Nahab F, Lynn MJ, Kasner SE, Alexander MJ, Klucznik R, Zaidat OO, et al. NIH Multicenter Wingspan Intracranial Stent Registry Study Group. Risk factors associated with major cerebrovascular complications after intracranial stenting. Neurology. 2009;72:2014–9. doi: 10.1212/01.wnl.0b013e3181a1863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TN, Zaidat OO, Gupta R, Nogueira RG, Tariq N, Kalia JS, et al. Balloon angioplasty for intracranial atherosclerotic disease: Periprocedural risks and short-term outcomes in a multicenter study. Stroke. 2011;42:107–11. doi: 10.1161/STROKEAHA.110.583245. [DOI] [PubMed] [Google Scholar]
- 9.Qureshi AI, Al-Senani FM, Husain S, Janjua NA, Lanzino G, Lavados PM, et al. Intracranial angioplasty and stent placement after stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) trial: Present state and future considerations. J Neuroimaging. 2012;22:1–13. doi: 10.1111/j.1552-6569.2011.00685.x. [DOI] [PubMed] [Google Scholar]
- 10.Ryu DS, Hong CK, Sim YS, Kim CH, Jung JY, Joo JY. Anti-platelet drug resistance in the prediction of thromboembolic complications after neurointervention. J Korean Neurosurg Soc. 2010;48:319–24. doi: 10.3340/jkns.2010.48.4.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiq F, Vazquez G, Memon MZ, Suri MF, Taylor RA, Wojak JC, et al. Comparison of primary angioplasty with stent placement for treating symptomatic intracranial atherosclerotic diseases: A multicenter study. Stroke. 2008;39:2505–10. doi: 10.1161/STROKEAHA.108.515361. [DOI] [PubMed] [Google Scholar]
- 12.Taylor RA, Kasner SE. Treatment of intracranial arterial stenosis. Expert Rev of Neurother. 2006;6:1685–94. doi: 10.1586/14737175.6.11.1685. [DOI] [PubMed] [Google Scholar]
- 13.Terada T, Tsuura M, Matsumoto H, Masuo O, Tsumoto T, Yamaga H, et al. Endovascular therapy for stenosis of the petrous or cavernous portion of the internal carotid artery: Percutaneous transluminal angioplasty compared with stent placement. J Neurosurg. 2003;98:491–7. doi: 10.3171/jns.2003.98.3.0491. [DOI] [PubMed] [Google Scholar]
- 14.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, et al. NIH Multi-center Wingspan Intracranial Stent Registry Study Group. The NIH registry on use of the Wingspan stent for symptomatic 70-99% intracranial arterial stenosis. Neurology. 2008;70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]


