Abstract
Context:
There is a need for identifying risk factors aggravating development of acute renal failure after attaining trauma and defining new parameters for better assessment and management. Aim of the study was to determine the incidence of acute renal failure among trauma patients, and its correlation with various laboratory and clinical parameters recorded at the time of admission and in-hospital mortality.
Subjects and Methods:
The retrospective cohort study included admitted 208 trauma patients over a period of one year. 135 trauma patients at the serum creatinine level >2.0 mg/dL were enrolled in under the group of acute renal failure. 73 patients who had normal creatinine level made the control group. They were further assessed with clinical details and laboratory investigations.
Results:
Incidence of acute renal failure was 3.1%. There were 118 (87.4%) males and average length of stay was 9 (1, 83) days. Severity of injury (ISS, GCS) was relatively more among the renal failure group. Renal failure was transient in 35 (25.9%) patients. They had higher incidence of bone fracture (54.0%) (P= 0.04). Statistically significant association was observed between patients with head trauma and mortality 72 (59.0%) (P= 0.001). Prevalence of septic 24 (59.7%) and hemorrhagic 9 (7.4%) shock affected the renal failure group.
Conclusion:
Trauma patients at the urea level >50 mg/dL, ISS >24 on the first day of admission had 23 times and 7 times the risk of developing renal failure. Similarly, patients with hepatic dysfunction and pulmonary dysfunction were 12 times and 6 times. Patients who developed cardiovascular dysfunction, hematological dysfunction and post-trauma renal failure during the hospital stay had risk for mortality 29, 7 and 8 times, respectively. The final prognostic score obtained was: 14*hepatic dysfunction + 11*cISS + 18*cUrea + 12*cGlucose + 10*pulmonary dysfunction. Optimal score cut-off for prediction of renal failure was found to be ≥25 with specificity, sensitivity and positive likelihood ratio to be 84.9%, 78.4% and 3.9, respectively.
Keywords: Acute renal failure, risk factors, trauma
INTRODUCTION
Post-trauma renal failure first came into cognition after Bywaters and Beal revealed its high incidence during World War II.[1] It causes high mortality ranging from 7% to 83% among trauma cases.[2–6] Various studies have been done to articulate its definition and recently acute dialysis quality initiative (ADQI) has proposed RIFLE criteria, classifying acute renal failure (ARF) in five different classes, namely, R (Risk), I (injury), F (failure), L (loss) and E (end stage kidney disease).[7] Renal failure in trauma patients is multifaceted and its aetiology has been diverse, renal ischemia being the most common cause. Although various studies have established that hypotension, rhabdomyolysis (crush syndrome), vascular thrombosis, acidosis, shock, bacterial and viral infections have been contributing factors.[8–10]
Previous studies have used different criteria to define post-trauma renal failure[11–14] making it ambiguous to compare and assess results for concluding risk and predictive factors. Our aim was to identify clinical factors affecting incidence of post-trauma renal failure and to analyze whether associated other organ dysfunction had any effect on this condition and to establish a relationship between laboratory parameters, hospital mortality, severity of trauma and length of stay in patients with post-trauma renal failure. This study had two outcomes, first being the development of renal failure in trauma patients during their hospital stay and second being in-hospital mortality.
SUBJECTS AND METHODS
Study design
Retrospective cohort analysis of hospitalized trauma patients over a period of one year (January to December 2009) was performed. A total of 4396 trauma patients were hospitalized during this period, of which 135 patients with their serum creatinine level >2.0 mg/dL during the hospital stay, comprised the study group, including patients with blunt and penetrating injury and also those who developed transient or persistent renal dysfunction post-trauma. Patients with previous history of chronic kidney disease were excluded from the study. Trauma patients admitted during the first month of the study period with serum creatinine level ≤2.0 mg/dL and did not develop renal failure throughout their hospital stay comprised the control group. The post-trauma renal failure group was compared with the control group. Patients were further subdivided based on immediate outcome (survivors and non-survivors), and both the groups were studied.
Statistical variables
Patients’ clinical and laboratory records were reviewed from the computerized patient record system (CPRS) database at trauma center. Patients’ demographics, site and type of trauma (blunt or penetrating), presence of bone fracture, injury severity score (ISS), Glasgow coma score (GCS) and systolic BP, at the time of admission were extracted. Length of stay, hospital mortality, focus assessment with sonography for trauma (FAST) analysis, incidence of shock and need for dialysis were recorded. American Association for the Surgery of Trauma (AAST) grade was recorded for patients with renal injury in post-trauma renal failure group.
Patients’ hematology, biochemistry and blood transfusion profile was assessed and observations for some critical parameters like urea, creatinine, sodium, potassium and phosphate were recorded from the time of admission of the patient till their discharge or death during the hospital stay. Patients with transient post-trauma renal failures were also taken into account. Days from the time of admission to the onset of renal failure, duration and recovery along with the incidence of multiple organ dysfunctions were recorded.
Patients with hypotension, arrhythmia and those on vasopressors were considered to have cardiovascular dysfunction. Patients with the aspartate transaminase (AST) level >80 IU/L or serum bilirubin level >3 mg% were considered to have hepatic dysfunction. Central nervous system dysfunction was defined as patients with severe head injury, i.e., GCS <8. Pulmonary dysfunction was defined as the presence of bilateral lung infiltrate or acute respiratory distress syndrome (ARDS) or central venous pressure <18 mm Hg and mechanical ventilation. Patients with coagulopathy (PT/or aPTT ≥1.5 × times the control) were considered to have hematology dysfunction.[15,16]
Statistical analysis
Statistical analysis was performed for the comparison of parameters for two outcomes : f0 irstly, renal failure and non-renal failure group and secondly, survivors and non-survivors during the hospital stay. Data was compiled and managed on excel spreadsheet. Continuous variables were expressed as mean ± SD or median (interquartile range) and categorical variables were represented as frequency (percentage). Analysis was performed using STATA 11.0 statistical software (USA). Univariate analysis of the continuous variables between two groups was done using t-test/Wilcoxon's rank sum test and categorical data were analyzed using Pearson Chi-Square test/Fishers test. To find the risk factors/predictors of PTRF, univariate logistic regression analysis followed by multivariate logistic regression analysis of risk factors with step wise regression for significant parameters in both categories of patients was done to obtain a logistic model. Results expressed as odds ratio (OR) and 95% confidence interval (95% CI). P value of <0.05 was considered to be statistically significant. We then devised a prognostic score using logistic regression coefficients of variables derived from the logistic model. ROC curve was obtained, and goodness-of-fit test was performed for evaluation of discriminatory power of prognostic score. Cut-offs for the score and estimated specificity, sensitivity and positive likelihood ratio were also obtained.
RESULTS
Comparison of clinical and laboratory parameters with renal failure as an outcome
Total 4396 patients were admitted during the study period and an incidence rate of 3.1% (n = 135) renal failure was observed, out of which 118 (87.4%) were males. There were73 patients in the control group of which 56 (76.7%) were males. The mean ± SD age in the control and renal failure group was 38.5 ± 16.6 years and 42.3 ± 18.7 years, respectively. The difference in the length of stay between the groups was found to be significant (P = 0.001) [Table 1]. Mean ± SD ISS of 20.4 ± 8.5 in the renal failure group was significantly higher than 13.3 ± 6.0 in the control group (P < 0.001). ISS was a clinically significant parameter for illusion of development of renal failure (unadjusted OR-8.1, 95% CI: 3.3-20.0). Severity of head injury was higher in the renal failure group with mean±SD GCS of 9.8 ± 4.4 than control group (11.2 ± 4.4) (P = 0.03) [Table 1].
Table 1.
Comparison of laboratory and clinical parameters between trauma patients with (study group) and without acute renal failure (control group)
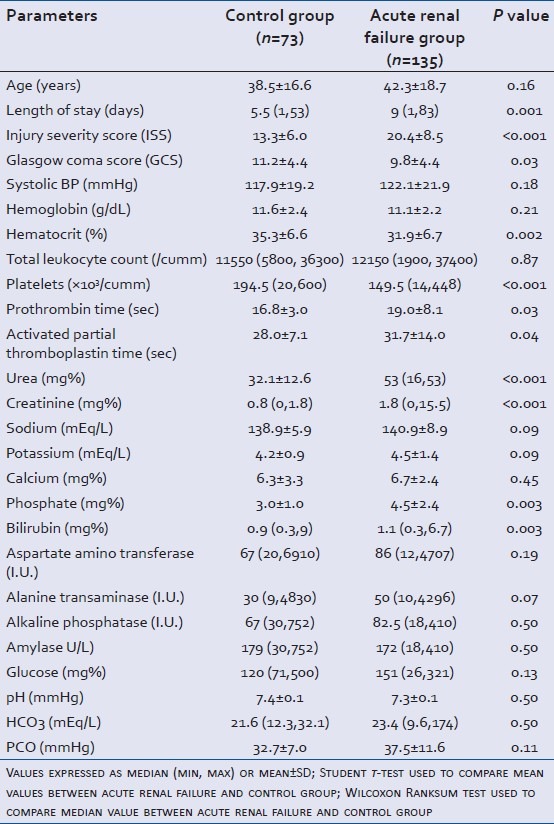
Levels of urea (P < 0.001), creatinine (P < 0.001), phosphate (P = 0.003) and bilirubin (P = 0.003) on the first day-after admission were found to be significantly higher in patients with renal failure as an outcome [Table 1]. In control group 17 (41.5%) patients had glucose levels in the normal range (70-110 mg/dL) than 13 (13.0%) patients in renal failure group (P < 0.001). Hematocrit was found significant with mean±SD value of 35.3 ± 6.6 (%) in control and 31.9 ± 6.7 (%) in the renal failure group (P = 0.002). Mean ± SD prothrombin time (PT) was 16.8 ± 3.0 s and 19.0 ± 8.1 s in control and renal failure group, respectively (P = 0.003). Activated partial thromboplastin time (aPTT) was 28.0 ± 7.1 s in control and 31.7 ± 14.0 s in the renal failure group with P = 0.004 [Table 1]. On the first day-after admission, hematocrit level (unadjusted OR-2.5, 95% CI: 1.3-4.7), bilirubin level (unadjusted OR-2.5, 95% CI: 1.4-4.6) and phosphate level (unadjusted OR-4.5, 95% CI: 1.5-13.4) between the control and renal failure group were statistically significant [Table 2].
Table 2.
Assessment of clinical and laboratory parameters in trauma patients with acute renal failure as the outcome (Y/N): Results of bivariate and multivariate logistic regression analysis
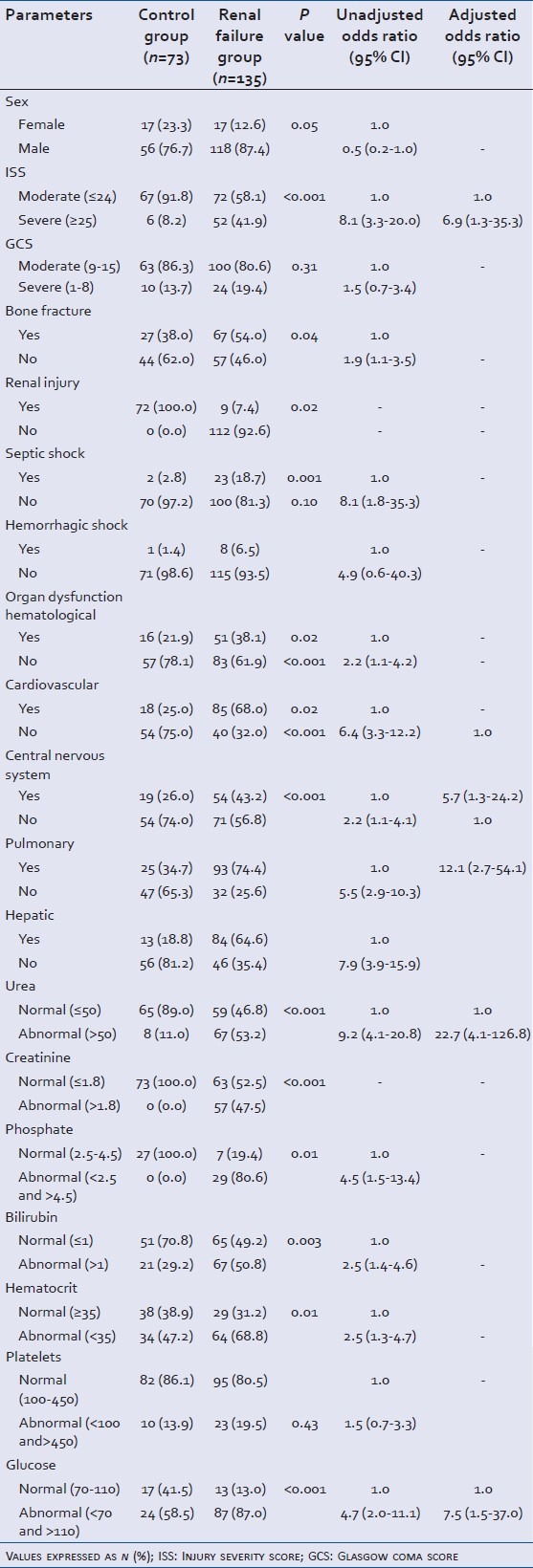
Incidence of bone fracture was statistically significant with 67 (54.0%) patients in renal failure group and 27 (38.0%) in the control group (P = 0.04) (unadjusted OR-1.9, 95% CI: 1.1-3.5) [Table 2]. Head was the major site of trauma with 64 (52.5 %) patients in the renal failure group (P = 0.30) and 31 (43.7%) patients in the control group [Figure 1].
Figure 1.
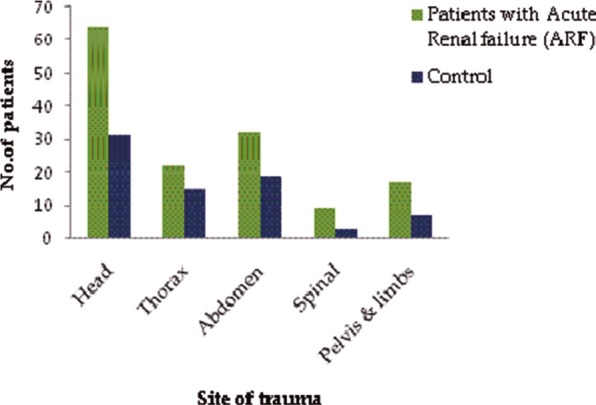
Sites of trauma in renal failure and control patients
Significantly higher occurrence of multiple organ dysfunctions was observed in the renal failure group as compared to the control group : 0 with hematological dysfunction (P = 0.02) (unadjusted OR-2.2, 95% CI: 1.1-4.2), cardiovascular dysfunction (P < 0.001) (unadjusted OR-6.4, 95% CI: 3.3-12.2), pulmonary dysfunction (P < 0.001) unadjusted OR-5.5, 95% CI: 2.9-20.3), hepatic dysfunction (P < 0.001) (unadjusted OR-7.9, 95% CI: 3.9-15.9) and central nervous system dysfunction (P = 0.002) (unadjusted OR-2.2, 95% CI: 1.1-4.1) [Table 2]. Also septic shock (unadjusted OR-8.1, 95% CI: 1.8-35.3) was statistically significant.
Only 9 (7.4%) patients of the renal failure group had a physical injury to the kidney. Focus assessment with sonography for trauma (FAST) was found to be positive in 33 (26.2%), and hematuria was observed in 14 (12.5%) patients of the renal failure group. 33 (26.6%) PTRF patients underwent dialysis. PTRF was transient in 35 (25.9%) patients, its median (IQR) duration being 3 (1-19) days.
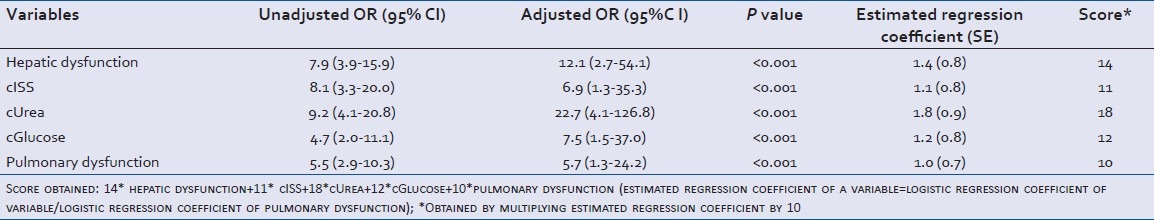
Step wise regression was applied to the significant factors obtained. Trauma patients at the urea level >50 mg/dL on admission had 23 times (95% CI: 4.1-126.8) the risk of developing renal failure. Severity of trauma also affected the outcome, patients with ISS >24 were 7 times (95% CI: 1.3-35.3) more prone to develop renal failure. A similar result was observed for patients with the abnormal glucose level, with risk of developing renal failure increasing by eight times (95% CI: 1.5-37.0). Pulmonary and hepatic dysfunctions were also identified as major risk factors increasing the chances of renal failure by six times (95% CI: 1.1-24.2) and 12 times (95% CI: 2.7-54.1), respectively.
Prognostic score for prediction of acute renal failure in trauma patients
Five variables found to be significant in the logistic model were used to calculate the prognostic score. The final score obtained was: 14*hepatic dysfunction + 11*cISS + 18*cUrea + 12*cGlucose + 10*pulmonary dysfunction (estimated regression coefficient of a variable = logistic regression coefficient of variable/logistic regression coefficient of pulmonary dysfunction*10) [Table 3]. Variables, hepatic and pulmonary dysfunction assigned a value of ‘1’ if present and ‘0’ if absent. cISS assigned ‘0’ if ISS ≤ 24 and ‘1’ if ISS ≥ 25. cUrea assigned a value of ‘0’ if urea levels ≤50 mg/dL and ‘1’ if >51 mg/dL. cGlucose assigned ‘0’ if the glucose level lies between 70-110 mg/dL and ‘1’ if <70 and >110 mg/dL.
Table 3.
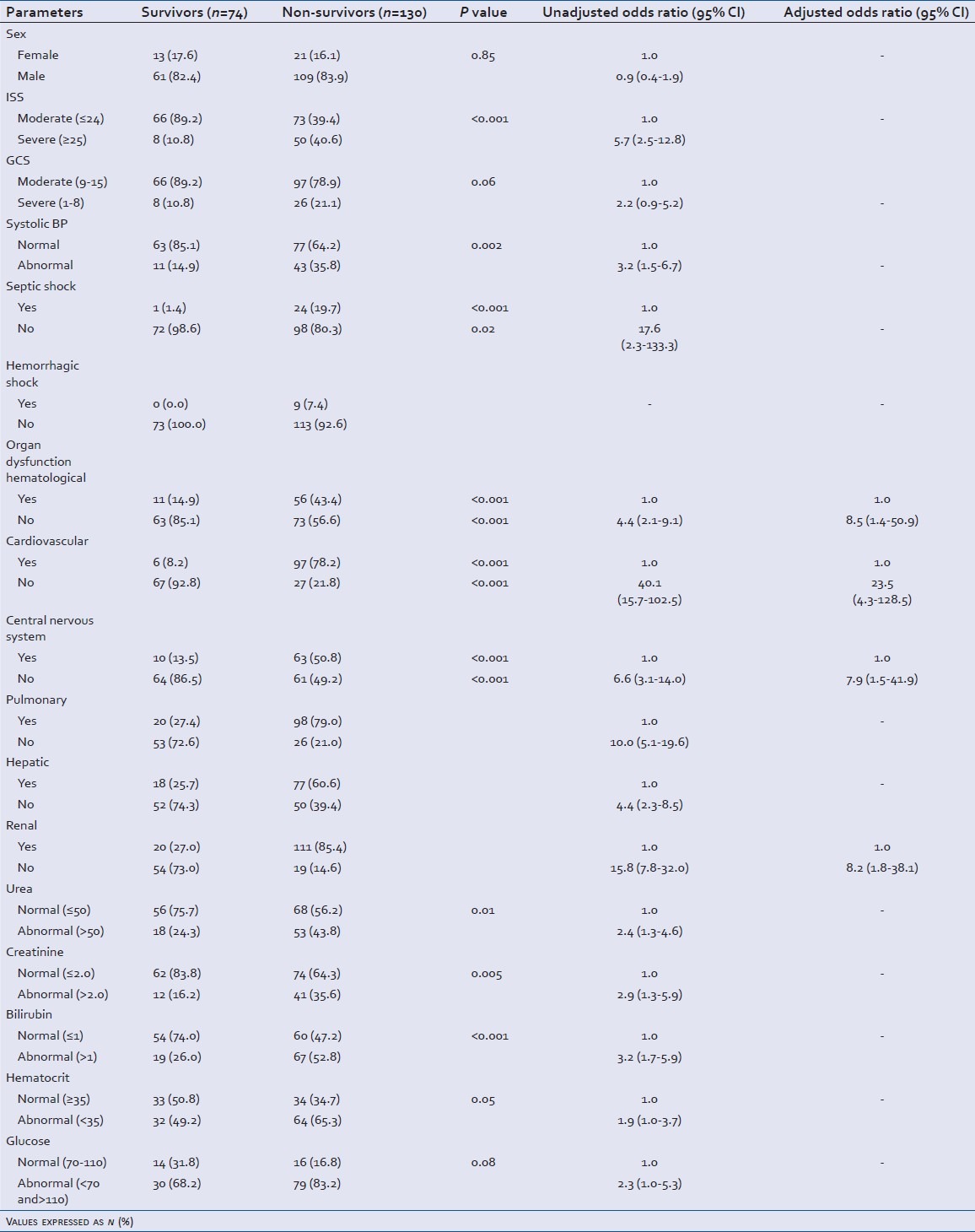
Assessment of clinical and laboratory parameters in trauma patients within.hospital mortality as the outcome (survivors/non.survivors): Results of bivariate and multivariate logistic regression analysis
Based on ROC curve analysis, optimal score cut-off for prediction of renal failure was found to be ≥25 [Table 4] with specificity, sensitivity and positive likelihood ratio to be 84.9%, 78.4% and 3.9, respectively. ROC curve was plotted [Figure 2], with area under curve (AUC) being 0.91 (95% CI: 0.86-0.96) showing excellent discriminatory power of score.
Table 4.
Distribution of trauma patients with respect to prognostic score obtained
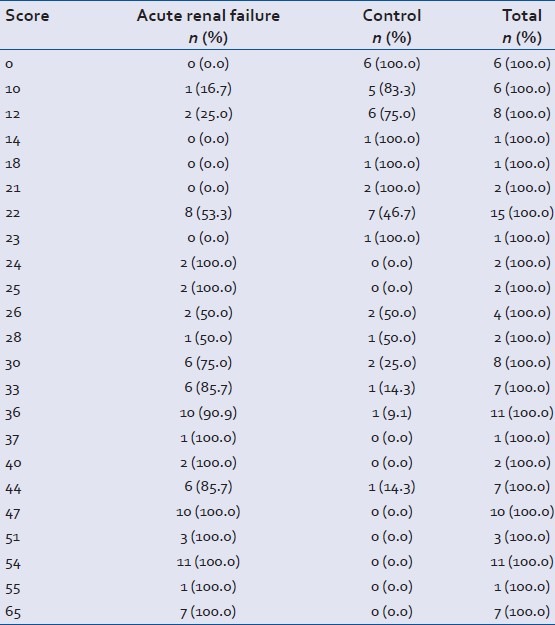
Figure 2.
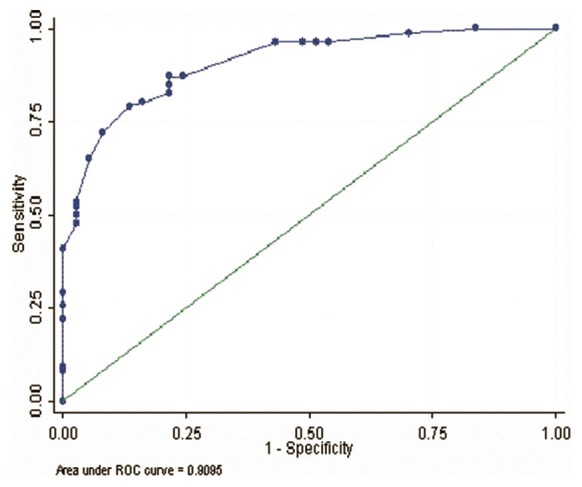
ROC curve for prognostic score. Area under curve (91%) and goodness of fit value (P = 0.84)
Comparison of clinical and laboratory parameters with mortality as an outcome
While comparing parameters between the survivors and the non-survivors at the end of hospital stay, the severity of trauma was a major factor for a high mortality rate. 50 (40.6%) non-survivors had severe trauma, i.e., ISS score >24 as compared to eight (10.8%) patients who survived (P < 0.001). Head trauma was statistically significant (P = 0.001) with higher occurrence in non-survivors 72 (59.0%) than survivors 23 (32.4%) [Figure 3]. Severe head injury (GCS<8) was significantly associated to mortality with 26 (21.1%) non-survivors as compared to only eight (10.8%) in survivors [Table 5]. Bone fracture did not display significant association with in hospital mortality. Of the nine renal failure patients inflicted by physical injury to the kidney, five survived and four died. Minimum and maximum American Association for the Surgery of Trauma (AAST) grade observed was 2 and 5 in these patients. For the patients who had transient renal failure, 18 patients survived whereas 17 patients died (P < 0.001). Out of the 33 patients with ARF who underwent dialysis only one survived and 32 died.
Figure 3.
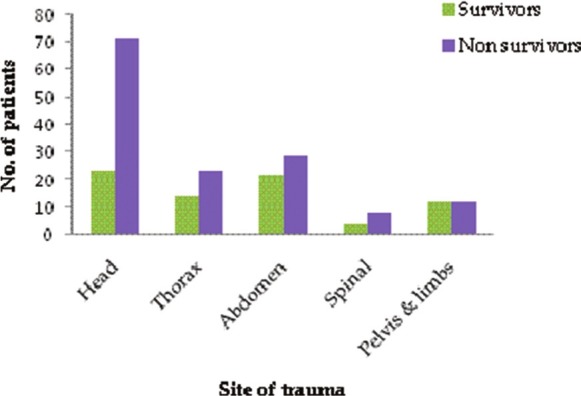
Sites of trauma in survivors and non survivors of acute renal failure group
Table 5.
Logistic regression analysis of variables related to acute renal failure in trauma patients
Incidence of septic shock was also significantly correlated with death (P < 0.001) with 24 (59.7%) patients in this group. Hemorrhagic shock (P = 0.02) was observed only in 9 (7.4%) non-survivors with renal failure [Table 3]. It was observed that septic shock (unadjusted OR-17.6, 95% CI: 2.3-133.3), hematological dysfunction (unadjusted OR-4.4, 95% CI: 2.1-9.1), cardiovascular dysfunction (unadjusted OR-40.1, 95% CI: 15.7-102.5), central nervous system dysfunction (unadjusted OR-6.6, 95% CI: 3.1-14.0), pulmonary dysfunction (unadjusted OR-10.0, 95% CI: 5.1-19.6), hepatic dysfunction (Unadjusted OR-4.4, 95% CI: 2.3-8.5) and renal failure (unadjusted OR-15.8, 95% CI: 7.8-32.0) statistically correlated with in-hospital mortality.
In the survivor group 19 (26.0%) patients had a bilirubin level >1 mg/dL as compared to the 67 (52.8%) of non-survivors (P < 0.001) (unadjusted OR-3.2, 95% CI: 1.7-5.9). Also, urea level (unadjusted OR-2.4, 95% CI: 1.3-4.6) and creatinine level (unadjusted OR-2.9, 95% CI: 1.3-5.9) were established as significant parameters for the prognosis of adverse outcomes in trauma patients [Table 3].
When step wise regression was performed on significant parameters, mortality was found to be 29 times (95% CI: 6.2-138.9) higher for patients with cardiovascular dysfunction. Presence of hematological dysfunction was observed to augment the risk of mortality by seven times (95% CI: 1.5-33.8) in non-survivors than survivors. Patients with post-trauma renal failure had eight times (95% CI: 1.9-32.3) higher chances of mortality during their hospital stay [Table 3].
DISCUSSION
Despite high mortality in trauma cases due to renal failure,[2–6] its obscure definition proves to be a hindrance in assessing its deleterious effects. Also the absence of reliability factors for its early diagnosis along with complications like sepsis, hypotension, shock and severity of trauma has a role in increasing the risk.[8–10] Our retrospective study comprising of 208 patients of which 73 formed control group, was aimed at elucidating the risk factors for renal failure and also analyzing the association of mortality with severity of trauma and length of stay. In our study, we used serum creatinine >2.0 mg/dL as the definition for renal failure. Matas, et al.[17] established that mortality was high in renal failure, death occurring mainly due to complications associated with it. He concluded that sepsis was one of the main factors. We also found the incidence of septic shock and hemorrhagic shock to be high in the patients who died. In renal failure group 23 (18.7%) patients had a septic shock as compared to only two patients in control group.
In a study by Tierney, et al.[18] mortality was found to be higher among patients with hip fracture who had End Stage Renal Disease (ESRD) as compared to those who had ESRD but no hip fracture. Also, Matas, et al.[17] concluded higher incidence of renal failure post-blunt trauma in patients with large bone fractures, but found it difficult to predict the development of the complication. In our study, 27 (38.0%) had a bone fracture in control and 67 (54.0%) in the renal failure group (P = 0.04). For mortality as an outcome, bone fracture incidence was similar in both discharged (48.6%) and the dead group (48.0%).
Series of studies done to establish a relation between mortality dependence on LOS (length of stay) and severity of trauma was concordant with our results.[3,5,6,12,19] We found that median (IQR) LOS was 5.5 (1-53) days (P = 0.001) in the control group as compared to 9 (1, 83) days in the renal failure group. Also ISS was found to be higher in control group (20.4 ± 8.5) than in the non-renal failure groups (13.3 ± 6.0) with P value < 0.001. Similarly mean ± SD GCS of 11.2 ± 4.4 and 9.8 ± 4.4 (P = 0.03) was observed in control and renal failure group.
Mortality was also seen to be significantly higher in patients with ISS >24 and severe head injury (GCS <8) similar to the results of a study by Sipkins, et al., who concluded that trauma followed by ARF had dismal prognosis and patients with severe head trauma carried worst prognosis for this group.
Ala-Kokko, et al.[19] found trauma requiring intensive care, following adjustment for the severity of illness, age and sex, development of renal failure during the first 24 hours after ICU admission came out to be an independent predictor for hospital death. 3.5% of the patients developed renal failure in first 24 hours, and overall hospital mortality was 48.7%. A study by Ravindra, et al.[13] for developing regression model for hospital mortality in ARF showed that respiratory, liver, and hematological failure (determined by specific criteria) were predictor variables. They examined 851 patients with ARF and following variables were significantly associated with in-hospital death : a0 ge (odds ratio [OR], 1.02 per year), male gender (OR, 2.36), respiratory (OR, 2.62), liver (OR, 3.06), and hematologic failure (OR, 3.40), creatinine (OR, 0.71 per mg/dl), blood urea nitrogen (OR, 1.02 per mg/dl), log urine output (OR, 0.64 per log ml/d), and heart rate (OR, 1.01 per beat/min). Also, a study by Lins, et al.[20] devised as the prognostic score for hospital mortality of individual patients with ARF. They developed two Stuivenberg Hospital acute renal failure scores (SHARF scores), one at the time of diagnosis of ARF (T0) and the other 48 hours later (T48): SHARF T0 (7 × age) + (6 × alb0) + (3 × PTT0) + (39 × vent0) + (9 × heartf0) + 52 SHARF T48 (7 × age) + (6 × alb0) + (3 × PTT0) + (43 × vent48) + (16 × heartf48) + 52 age, albumin (alb0) and prothrombin time (PTT0) at T0 are expressed as categories, respiratory support (vent) and heart failure (heartf) at T0 and T48 are presented as absent (0) or present (1). Whereas in our study, prognostic score was established for development of renal failure, though hospital mortality as an outcome was also studied for which hematological, cardiovascular, CNS and renal dysfunctions were found to be significant variables. We found that patients at the urea level >50 mg/dL at the first day of admission were 23 times (95% CI: 4.1-126.8) more likely to develop renal failure. Severity of trauma also affected the outcome, patients with ISS >24 were seven times (95% CI: 1.3-35.3) more prone to develop renal failure. Pulmonary and hepatic dysfunctions were also identified as significant risk factors increasing the chances of renal failure by six times (95% CI: 1.1-24.2) and 12 times (95% CI: 2.7-54.1), respectively.
Zimmerman, et al.[4] established that respiratory failure complicated renal failure, affecting 83% of the patients having renal failure. In our study, pulmonary and hepatic dysfunctions were identified as major risk factors increasing the chances of renal failure by six times (95% CI: 1.1-24.2) and 12 times (95% CI: 2.7-54.1), respectively. Erek, et al.[3] in his study on patients with crush injuries due to earthquake associated mortality in ARF with thoracic and abdominal trauma and medical problems like DIC, ARDS, and respiratory failure associated with sepsis. In our study, patients with post-trauma renal failure had eight times (95% CI: 1.9-32.3) higher chances of mortality.
Carlos, et al.[2] found that, renal failure started in trauma patients at the mean± SD of 4 ± 7 days after admission, with peak serum creatinine occurring 7 ± 1 day after admission and only 56% of patients came to normalization before being discharged. In our trauma study, renal failure started at a median (IQR) of 2 (1-25) days and the duration of transient renal failure was 3 (1-19) days. His study also showed that patients who were hypotensive and had severe head injury died after developing renal failure. We found that head trauma had higher incidence of renal failure with 64 (52.5%) patients as compared to 31 (43.7%) patients in non-renal failure group. In a study by Erek, et al.,[3] patients with trauma due to earthquake injuries found that hyperkalemia, uremia, hypophosphatemia, elevated creatinine levels and hypocalcemia as major abnormalities associated with morbidity due to crush injuries. We observed significantly higher levels of mean urea and creatinine in the renal failure group (P < 0.001). Phosphate levels were also found significant (P = 0.01), along with increased bilirubin (P = 0.003) levels.
Since this was a retrospective the cases with incomplete data were excluded from the study. However, not much literature is available on renal failure as previous studies have considered ARF as an outcome of crush injuries, due to burn mishaps or physical kidney injury. In our study, we reviewed trauma patients comprising a wider group with different trauma sites and varying injury severity. A separate cohort study would be conducted for validation of the established prognostic score.
CONCLUSION
Morbidity and mortality in renal failure were associated with length of stay and severity of trauma. Mortality was increased by presence of septic shock and multiple organ dysfunctions. Patients with the urea level >50 mg/dL at the first day of admission were 23 times (95% CI: 4.1-126.8) more likely to develop renal failure. Severity of trauma also affected the outcome, patients with ISS >24 were seven times (95% CI: 1.3-35.3) more prone to develop renal failure. Pulmonary and hepatic dysfunctions were also identified as significant risk factors increasing the chances of renal failure by six times (95% CI: 1.1-24.2) and 12 times (95% CI: 2.7-54.1), respectively. Patients with post-trauma renal failure had eight times (95% CI: 1.9-32.3) higher chances of mortality. The final prognostic score obtained was: 14*hepatic dysfunction + 11*cISS + 18*cUrea + 12*cGlucose + 10*pulmonary dysfunction. Optimal score cut-off for prediction of renal failure was found to be ≥25 with specificity, sensitivity and positive likelihood ratio to be 84.9%, 78.4% and 3.9, respectively.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Bywaters EG, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1:427–32. doi: 10.1136/bmj.1.4185.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CV, Dubose JJ, Hadjizacharia P, Yanar H, Salim A, Inaba K, et al. Natural history and outcomes of renal failure after trauma. J Am Coll Surg. 2008;206:426–31. doi: 10.1016/j.jamcollsurg.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Erek E, Sever MS, Serdengeçti K, Vanholder R, Akoğlu E, Yavuz M, et al. An overview of morbidity and mortality in patients with acute renal failure due to crush syndrome: The Marmara earthquake experience. Nephrol Dial Transplant. 2002;17:33–40. doi: 10.1093/ndt/17.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman JE. Respiratory failure complicating post-traumatic acute renal failure: Etiology, clinical features and management. Ann Surg. 1971;174:12–8. doi: 10.1097/00000658-197107010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beitland S, Moen H, Os I. Acute kidney injury with renal replacement therapy in trauma patients. Acta Anaesthesiol Scand. 2010;54:833–40. doi: 10.1111/j.1399-6576.2010.02253.x. [DOI] [PubMed] [Google Scholar]
- 6.Mehta RL, Pascual MT, Soroko S, Chertow GM PICARD Study Group. Diuretics, mortality, and nonrecovery of renal function in acute renal failure. JAMA. 2002;288:2547–53. doi: 10.1001/jama.288.20.2547. [DOI] [PubMed] [Google Scholar]
- 7.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whelton A. Post-traumatic acute renal failure. Bull N Y Acad Med. 1979;55:150–62. [PMC free article] [PubMed] [Google Scholar]
- 9.Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viek NF, Uhlman R, Howard JH. The etiology and treatment of acute post-traumatic renal failure. J Natl Med Assoc. 1961;53:252–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Venkataraman R, Kellum JA. Defining acute renal failure: The RIFLE criteria. J Intensive Care Med. 2007;22:187–93. doi: 10.1177/0885066607299510. [DOI] [PubMed] [Google Scholar]
- 12.Gomes E, Antunes R, Dias C, Araújo R, Costa-Pereira A. Acute kidney injury in severe trauma assessed by RIFLE criteria: A common feature without implications on mortality? Scand J Trauma Resusc Emerg Med. 2010;18:1. doi: 10.1186/1757-7241-18-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. J Am Soc Nephrol. 2002;13:1350–7. doi: 10.1097/01.asn.0000014692.19351.52. [DOI] [PubMed] [Google Scholar]
- 14.Shariat SF, Trinh QD, Morey AF, Stage KH, Roehrborn CG, Valiquette L, et al. Development of a highly accurate nomogram for prediction of the need for exploration in patients with renal trauma. J Trauma. 2008;64:1451–8. doi: 10.1097/TA.0b013e3181271b77. [DOI] [PubMed] [Google Scholar]
- 15.Hoste EA, Clermont G, Kersten A, Venkataraman R, Angus DC, De Bacquer D, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: A cohort analysis. Crit Care. 2006;10:R73. doi: 10.1186/cc4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baue AE. MOF, MODS, and SIRS: What is in a name or an acronym? Shock. 2006;26:438–49. doi: 10.1097/01.shk.0000228172.32587.7a. [DOI] [PubMed] [Google Scholar]
- 17.Matas AJ, Payne WD, Simmons RL, Buselmeier TJ, Kjellstrand CM. Acute renal failure following blunt civilian trauma. Ann Surg. 1977;185:301–6. doi: 10.1097/00000658-197703000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tierney GS, Goulet JA, Greenfield ML, Port FK. Mortality after fracture of the hip in patients who have end-stage renal disease. J Bone Joint Surg Am. 1994;76:709–12. doi: 10.2106/00004623-199405000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Ala-Kokko T, Ohtonen P, Laurila J, Martikainen M, Kaukoranta P. Development of renal failure during the initial 24 h of intensive care unit stay correlates with hospital mortality in trauma patients. Acta Anaesthesiol Scand. 2006;50:828–32. doi: 10.1111/j.1399-6576.2006.01082.x. [DOI] [PubMed] [Google Scholar]
- 20.Lins RL, Elseviers M, Daelemans R, Zachée P, Zachée P, Gheuens E, et al. Prognostic value of a new scoring system for hospital mortality in acute renal failure. Clin Nephrol. 2000;53:10–7. [PubMed] [Google Scholar]


