Abstract
Tendon and ligament injury is a worldwide health problem, but the treatment options remain limited. Tendon and ligament engineering might provide an alternative tissue source for the surgical replacement of injured tendon. A bioreactor provides a controllable environment enabling the systematic study of specific biological, biochemical, and biomechanical requirements to design and manufacture engineered tendon/ligament tissue. Furthermore, the tendon/ligament bioreactor system can provide a suitable culture environment, which mimics the dynamics of the in vivo environment for tendon/ligament maturation. For clinical settings, bioreactors also have the advantages of less-contamination risk, high reproducibility of cell propagation by minimizing manual operation, and a consistent end product. In this review, we identify the key components, design preferences, and criteria that are required for the development of an ideal bioreactor for engineering tendons and ligaments.
Introduction
Tendons and ligaments have an important function in transferring force from muscle to bone or bone to bone. Tendons also help store elastic energy while walking, increasing locomotion efficiency. However, sudden, excessive strain of ligaments and tendons from athletic or recreational activities can cause acute traumatic injury to these tissues, which can range from small, partial tears to complete ruptures. Furthermore, repetitive loadings over long periods of time can lead to similar tissue injuries, when the fatigue damage within the tendon exceeds the capacity of the tissue to repair itself. In the United States, injuries to tendons and ligaments represent about half of the 33 million musculoskeletal injuries.1 Each year more than 33,000 tendon reconstructions occur in the United States, costing $30 billion USD2,3 and in Australia, $250 million is spent annually just on rotator cuff repair.4 However, despite the high prevalence of tendon injury and associated tendinopathy worldwide, treatment options remain poorly defined.
Autograft and allograft transplantations are the common surgical treatments for tendon and ligaments that are injured or degenerated. However, the risks of damage to the donor site from which the autografts are taken, and the potential immune reactions for allografts are the major concerns.5–7 A promising translational approach to the treatment of tendon/ligament injury or degeneration is through the use of engineered autologous grafts made available through the development of bioreactors that generate tendon/ligament tissue in vitro. One common view is that the key to a successful bioreactor is being able to recreate, in vitro, the cell microenvironments that are experienced by cells in vivo. The cell microenvironments can be defined using cellular morphological information with data from molecular biology, biochemistry, and biomechanics. This review aims to clarify the requirements for a successful bioreactor that may be used for tendon/ligament engineering, and to provide an overview of the range of components found in tendon/ligament bioreactors, including custom-made and commercial products. We will also discuss the studies that have involved the application of tendon/ligament bioreactors.
Key Elements for Tendon/Ligament Formation and Regeneration
Tendons and ligaments are force-transferring tissues from muscle to bone and bone to bone, respectively. They consist of collagens, cells, proteoglycans, elastin, glycolipids, and water. Although roughly 65%–70% of the total weight is water,8 tendon is a highly organized structure. Collagen type I is the main structural/functional component and comprises around 70%–80% of the dry weight. Type III collagen is mainly present in the endotenon and epitenon, but is also present in the early phase of tendon repair.9,10 Tendons and ligaments are relatively hypovascular and hypocellular tissues, with their cells (tenocytes and fibroblasts) comprising <5% of the total volume. The morphology of tenocytes and fibroblasts is sharp and usually elongated along collagen fibers in normal tissues, but some rounded tenoblasts are found occasionally.11–14 Based on the composition and function of tendon/ligament tissue, one must consider the four basic elements for their successful regeneration: the cell source, the characteristics of the scaffold matrix, and establishing an appropriate chemical and physical cellular microenvironment.
An ideal cell source should meet the following three requirements: availability, rapid proliferation, and the ability to differentiate into in situ cells.15 Stem cells, dermal fibroblasts, and tenocytes have all been tested as potential cell sources for tendon repair. In the past decade, promising results have been achieved using mesenchymal stem cells (MSCs) in tendon/ligament engineering.16–23 However, the potential for MSCs to also differentiate into osteoblastic cells leading to ectopic bone formation raises concerns for their application in tendon repair. Likewise, bone marrow-derived MSCs that have been used in various studies24–28 also pose a potential risk of ectopic bone formation.29 A recent study demonstrated that embryonic stem cells (ESCs) can be induced to exhibit a tenocyte-like morphology and express tendon-related gene markers if exposed to an appropriate biomechanical environment.2 Unfortunately, the clinical application of ESCs is restricted due to their limited availability (depending on the policy of different countries) and the complexity of cell manipulation required. Dermal fibroblasts have also been shown to form tendon tissue,30–32 although further research has suggested that the healing process using skin-derived fibroblasts is suppressed with a lack of tenocyte markers and histopathologic correlations.33,34 Being native cell sources, tenocytes and in situ fibroblasts are perhaps the most ideal cell sources for engineered tendon and ligament tissue, respectively. Preclinical and early clinical studies using these native cell sources are promising.35–42 In the most recent clinical trial of autologous tenocyte therapy, a total of 25 patients with recalcitrant lateral epicondylitis were treated with fibroblasts isolated from biopsied anterior cruciate ligament tissue using a patented technique developed by our laboratory. We found a 60% or greater improvement in both the Quick DASH and pain assessment scores.43,44
Apart from the selection of the cell source, cell seeding also plays an essential role in the development of engineered tendon and ligament. Several reports indicate that sufficient cell number and a uniform distribution throughout the scaffold are desirable for achieving a homogeneous extracellular matrix (ECM) deposition in vitro.45,46 Compared to lower initial cell-seeding density, high seeding density has been shown to result in increasing ECM deposition rate, a higher final cell number and better cellular morphology.47,48 However, overseeding also has potential for negative effects on nutrient delivery, and consequently cellular metabolism and cell viability. Nutrient depletion at high cell-seeding densities could lead to spatially in homogenous ECM production.49 A study by Issa et al. showed that mechanostimulated human umbilical veins seeded with 3 million cells/mL had better cellular proliferation rates than other groups.28 However, optimal seeding density will vary depending on the cell source and the bioscaffold physical and chemical properties, as these properties will affect nutrient transport and rates of consumption, as well as the mechanical and chemical stimuli provided to the attached cells.49
The construct scaffold plays on important role in engineering the new tendon/ligament tissue. Ideally, the scaffold should be able to provide substantial initial mechanical strength for its immediate postimplantation functional role, while providing a suitable biological environment for cell migration and proliferation. Furthermore, the degradation rate of the biomaterial needs to be comparable with the rate of tissue synthesis, to allow the eventual replacement of the starting scaffold with neotissue.
Both synthetic and natural biomaterials are commonly used in tendon/ligament engineering. Synthetic polymer scaffolds have the advantage of reproducible mechanical and chemical properties, and they are relatively easy to fabricate into different sizes.24,25,40,41,50 However, their rapid degradation rate and potential risk of releasing acidic byproducts or toxic polyesters during degradation have limited their application in clinical trials. Given these disadvantages, more researchers have turned their focus on exploring natural biomaterials.19–21,37,51–54 Being the main component of native tendon, collagen type I is the most obvious choice of material. Although the biocompatibility is excellent, the poor mechanical properties of reconstituted type I collagen scaffolds has limited their further development as a load-bearing material. Silk fibroin, on the other hand, has similar biocompatibility as collagen scaffolds and comparable mechanical properties as native tendon/ligament. In several in vivo studies, silk fibroin-based engineered ligaments have been proven their ability to restore the function of injured ligament.16,17,26 Another option is the decellularised tendon/ligament construct. Although the mechanical and biological properties are a better match to native tissue than any other currently available scaffolds, donor cells may remain in the allograft, even with strict sterilization and cleaning, and thus they can potentially cause inflammatory responses.55,56
After choosing an appropriate cell source and scaffold type, the tissue needs to be encouraged to develop the properties of native tissue by providing an appropriate biochemical and biomechanical environment to stimulate ECM synthesis. Regarding the biochemical environment, several growth factors have been found to play an important role in tendon/ligament formation and healing. These include Insulin-like growth factor-I, vascular endothelial growth factor, platelet-derived growth factor, basic fibroblast growth factor, transforming growth factor (TGFβ), and growth differentiation factor 5 (GDF-5). The roles of TGFβ and GDF-5 seem to be particularly prominent. TGFβ remains active throughout tendon/ligament healing,57 and is able to regulate cell migration, proteinase expression, fibronectin-binding interactions, cell proliferation, and collagen production.57–60 Recent studies demonstrated that tendon and ligament formation was impaired in TGFβ2 and TGFβ3 knockout mouse embryos, reinforce the importance of TGFβs in tendon development and homeostasis.61 GDF-5 regulates cell growth and differentiation, with a lack of GDF-5 causing delayed tendon healing, irregular collagen type I fibrils, and weakened fibril mechanical properties.62–64
How these various biochemical factors should be introduced into the tissue bioreactor system to shape the chemical environment is an extremely challenging and open question. Specifically, at what concentrations, in what combinations, and at what sequence or timing should they be made available? Studies have shown that the expression of these factors in tendon repair changes over periods of days.65–67 Presumably, as the engineered tissue progresses, cells begin to control their own biochemical environment, and the role of the bioreactor is to now provide the building block nutrients and expected systemic signals, along with a mechanical stimulus. The early stage in the tissue-engineered tendon is likely to be the most critical in establishing tendon development along a pathway to resemble native tendon. Finding the correct combination of factors is daunting due to the complexity arising from the multitude of possible combinations and interactions. Systematically, varying experimental conditions, coupled with computational modeling of transport processes and signaling molecule pathways leading to cell responses, provide the only conceivable means to both understanding and efficiently optimizing the tissue bioreactor system.
Tendon's primary function is mechanical. It operates in a varying load environment, both on short timescales (e.g., walking and running) and on longer timescales (e.g., changes in body size with age). Tendon responds to its mechanical environment through changes in ECM biosynthesis and degradation. Unsurprisingly, given its functional role, a suitable mechanical stimulus is vital for tendon/ligament homeostasis. In fact, it has been shown that after 4 weeks in a load-free culture environment, tenocytes lose their native elongated morphology, become increasingly rounded, and the collagen fiber becomes more crimped.68 In cell-free reconstructed collagen fibril network systems, a tensile load has been shown to be protective to degradation by MMP-8.69 Furthermore, cyclic stretching has been shown to produce an up to nine-fold increase in the cell number of an engineered tendon compared with a static culture over a 2-week period.23 Finally, an appropriate mechanical environment could help guide collagen fiber formation, that is, along the direction of loading, which is able to enhance or optimize the mechanical properties, including stiffness, elastic modulus, maximum tensile stress, and maximum force.19,31,36
Rather than being two separate signals, there is a crosstalk between mechanical and chemical signals. Recent studies have shown that gradual and temporary loss of tensile loading leads to reversible loss of Scleraxis (Scx) expression, which is a transcription factor specific for tenocytes and their progenitors. In addition, it has been shown that TGFβ directly induced the expression of Scx in cultured tenocytes isolated from mice.70 In Scx−/− mice, a disordered limb tendon phenotype was observed,71 and a similar phenomenon happened in TGFβ type II receptor gene knockout (Tgfbr2−/−) mice with dramatic loss of Scx expression.61
Providing a suitable biomechanical signal is clearly an important component for the success of tendon/ligament engineering. Generating a suitable mechanical signal within the bioreactor system is critically important for tendon/ligament tissue engineering.
Bioreactor Design for Tendon/Ligament Engineering
Despite the increasing appreciation of tendon/ligament biology and function, conventional culture methods do not seem to meet the biochemical and biomechanical requirements to generate bioengineered tendon/ligament in vitro. A bioreactor system that subjects the cell culture to dynamic loading, mimicking the physiological conditions of tendon/ligament in vivo, while allowing cellular proliferation, differentiation, and matrix production in a mechanical environment, may provide a solution.
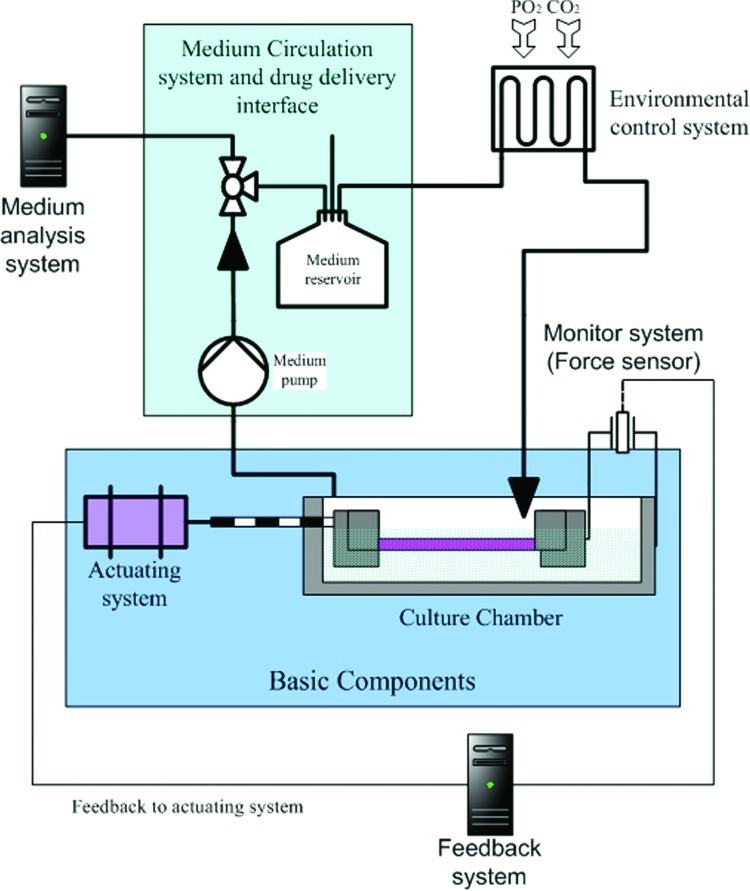
Bioreactors for tendon/ligament engineering are different to the systems that have been used in various other tissue-engineering fields in the past decades, that is, systems for the muscle,72 liver,73 and bone.74,75 Compared to other bioreactors, the main task of the bioreactor for tendon/ligament engineering is to provide the proper biomechanical and biochemical environment specific to tendon/ligament formation. To achieve this, certain basic components are required, that is, the actuating system and the culture chamber, which can provide the construct's mechanical stimulation and controlled culture environment, respectively. Furthermore, the bioreactor may also include a medium circulation system, monitoring system, feedback system, and a medium analysis system, depending on the operational requirements (Fig. 1). With these facts in mind, several custom-made bioreactors have been developed for tendon/ligament engineering (see Table 1), and the aforementioned components of these are now are discussed in detail.
FIG. 1.
Schematic demonstration of connection between different components of tendon/ligament bioreactor system. Color images available online at www.liebertpub.com/teb
Table 1.
Components of Custom-Made Bioreactor Systems
| Reference | Actuating system | Culture chamber | Monitor system | Feedback system | Medium circulation system | Medium analysis system |
|---|---|---|---|---|---|---|
| 76 | Biaxial | Multiple | × | × | ✓ | × |
| 42 | Uniaxial | Single chamber, multiple samples | × | × | × | × |
| 19,20,21,3 | Uniaxial | Multiple | Displacement | × | × | × |
| 35 | Uniaxial | Multiple | Force | × | × | × |
| 22 | Uniaxial | Single | Force | × | × | × |
| 23 | Uniaxial | Multiple | × | × | ✓ | × |
| 83 | Uniaxial | Single chamber, multiple samples | Displacement Force | × | × | ✓ |
| 2 | Uniaxial | Multiple | × | × | × | × |
| 27 | Uniaxial | Multiple | Displacement | × | × | × |
| 82 | Uniaxial | Multiple | Displacement Force | ✓ | × | × |
Actuator and Culture Chamber Design
The actuating system is the main component for providing different mechanical stimulation to engineered tissue. Pneumatic actuators,19–21 linear motors,22,27 and step motor-ball screws (SMBSs)2,42,76 are the most common actuators used in tendon/ligament bioreactors. Pneumatic actuators have several advantages, including the ease of maintenance, cleanliness, low cost, and high power-to-weight ratio.77 Unfortunately, by using air as a medium, pneumatic actuators are subject to high friction. The sensitivity and response to an input signal are relatively slow because of the dead band and dead time caused by stiction and air compressibility. Due to these nonlinearities, it is difficult to achieve accurate position control with pneumatic actuators.77 Typically, the accuracy of pneumatic actuators is ∼±0.1 mm, which is not insignificant when typical tendon bioreactors require <5% strain on 1–5-cm tissues. Compared with pneumatic actuators, electrical actuators are more expensive, but the level of accuracy in their positional control is much higher. A direct-drive linear motor is able to provide high-speed/high-accuracy linear motion by eliminating mechanical transmission,78–80 and the accuracy is the highest of all three actuators, that is, ∼±1 μm. SMBS transmission systems are based on a ball-screw linkage and a crank-slider mechanism. In general, SMBSs transfer the rotation of the crank to a reciprocating motion of the screw.81 By choosing different ball screws, the optimal speed range and output force can be selected using Equation (1), that is,
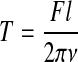 |
(1) |
where T is torque applied to screw; F is linear force; l is ball screw lead; and ν is ball screw efficiency. Although SMBS is not as accurate as a linear motor, a positional accuracy of around±5 μm can still be achieved. In a multichamber-shared loading system, SMBS is widely used given its high accuracy and relative high loading capacity.35,42,76 In independent loading multichamber systems, linear motors are more popular due to their small size and relatively simple mechanical arrangement.22,82
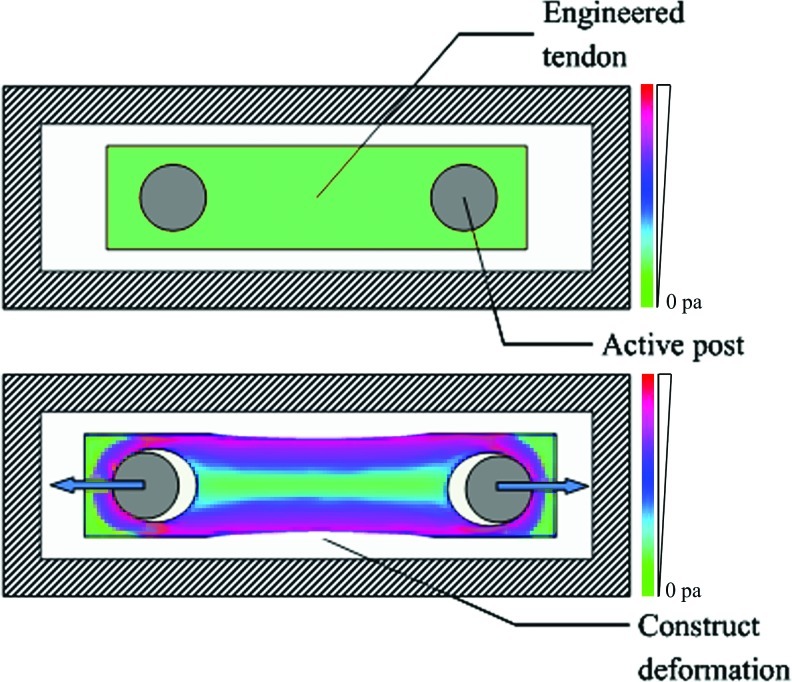
In the addition to the actuator, the connection between the mechanical input and the tissue is vital and often presents a major challenge. During dynamic loading of the tissue in culture, an even distribution of force throughout the entire sample is critical, otherwise tissue integrity is compromised by overloading the mechanical connection regions and/or by inhomogeneous mechanical stimulation. Different strategies of applying mechanical loads have been adopted based on different construct dimensions. In a study by Chen et al., cell-seeded knitted silk–collagen sponge scaffolds were fixed on stainless rings and connected to sample hooks.2 The bioreactor system by Juncosa-Melvin et al. applied two posts to fix the construct, punching through the scaffold as shown in Figure 2.19 However, nonuniform construct deformation is clearly apparent. Tensile force is focused on the side of constructs, which causes uneven distribution of mechanical stimulation. Although tissue clamps are the most popular and relatively effective method for holding tendon contructs,22,42,82 the potential for damage at the clamping region needs to be considered. The method of reproducibly applying uniform loads to soft tissue without tissue damage or slippage is a critical problem in need of a satisfactory solution. It is our opinion that a robust clamping region should be designed along with the artificial bioscaffold to ensure the proper connection between the sample and the mechanical load.
FIG. 2.
Stem cell-seeded Collagen sponge deformation during mechanical stimulation. Modified from reference 19. Color images available online at www.liebertpub.com/teb
The culture chamber is an essential part of whole bioreactor system. The high humidity of culturing conditions (99% humidity, 37°C, and 5% CO2) and chemistry of the culture medium are corrosive to many materials. Corrosion products may in turn be toxic to the tissue. Therefore, noncorrosive and autoclavable materials such as stainless steel, polymethylemethacrylate, polyoxymethylene, polycarbonate, glass, and silicon are preferred and widely used in culture chamber design.2,23,27,76,83
The chamber structure is an important consideration in the bioreactor design, with most currently available culture chambers divided into two groups: integrated42,83 and separated chambers.2,3,19–27,35,76,82 In integrated chambers, multiple samples are cultured while sharing the same culture medium. Conversely, separated chambers can provide separate culture environments for each sample. Although the complexity of design and manufacturing costs may be higher in a separated chamber system, reduced cross-contamination and the option of independent environmental control are distinct advantages.
During tissue culture, sufficient air exchange within the culture chamber is critical. Air exchange in conventional cell culture incubators is through the integrated hydrophobic filter of the culture flask and the gap between the leak and the culture dish/well plate. Therefore, the ideal design for the culture chamber should be similar. Like conventional cell culture, an unsealed chamber bioreactor connects to the outside environment through various ways,19,22,27,35 such as the hydrophobic filter leak42 and the labyrinth channel (Fig. 3).82 In a study by Webb et al.,42 a modified tissue culture flask was used as an integrated culture chamber, and could culture up to four samples simultaneously. Although there are potential risks of cross-contamination from different samples and toxicity from autoclaving the culture flask, the integrated hydrophobic filter leak can ensure adequate gas exchange without inducing contamination. In the bioreactor system used in Parent et al.'s research,82 a labyrinth channel was added to improve the air exchange and eliminate contamination as shown as Figure 3. However, in closed chambers, air exchange mostly depends on medium circulation, which is now discussed in the following section.
FIG. 3.
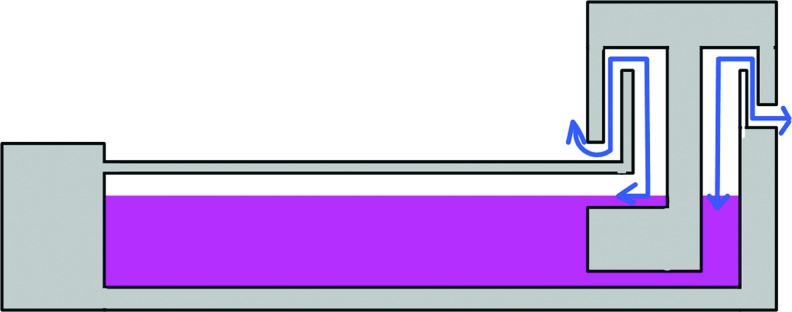
Schematic drawings of air exchange through the labyrinth channel in the culture chamber. Modified from reference 82. Color images available online at www.liebertpub.com/teb
Environmental Control and Medium Circulation Systems
Although to the best of our knowledge, no contamination has been reported in any bioreactor study, transportation of the bioreactor and opening of chamber for medium exchange every 3 days, especially for multichamber systems, are still a potential contamination risk. Therefore, a medium circulation system can be introduced to improve the efficiency and minimize these risks. In addition to the advantages of reduced contamination, a circulating medium may be better able to infiltrate into cultured tissue. For example, perfusion bioreactors used in bone engineering circulate the culture medium for better nutrient delivery and subsequent improved cell numbers.84–89 Similarly, human umbilical vein cultured under a circulating medium had approximately three times the cellular number as those cultured using a quiescent medium.23
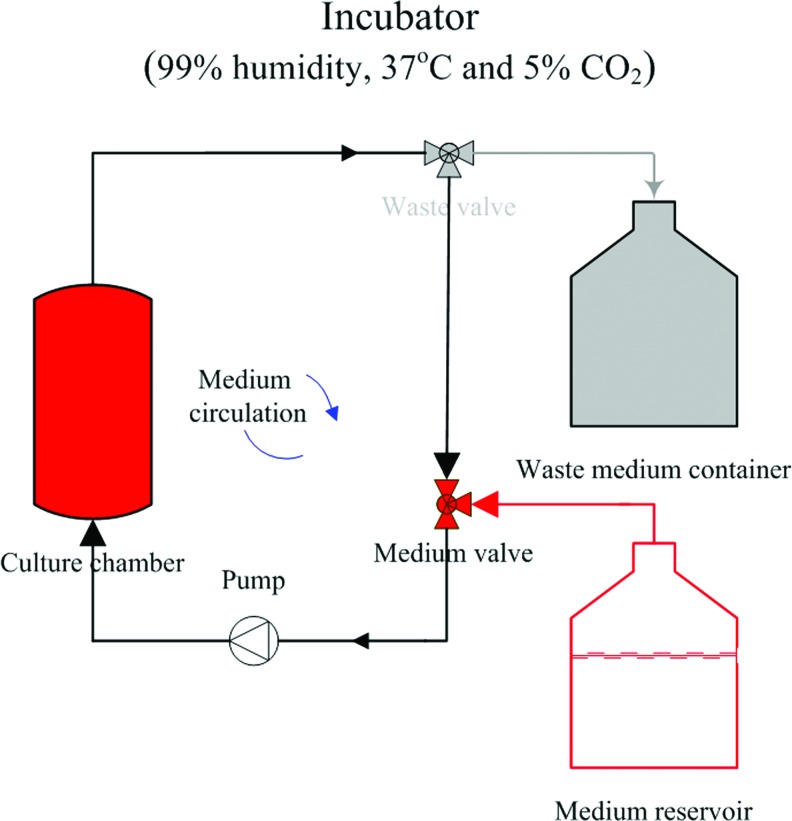
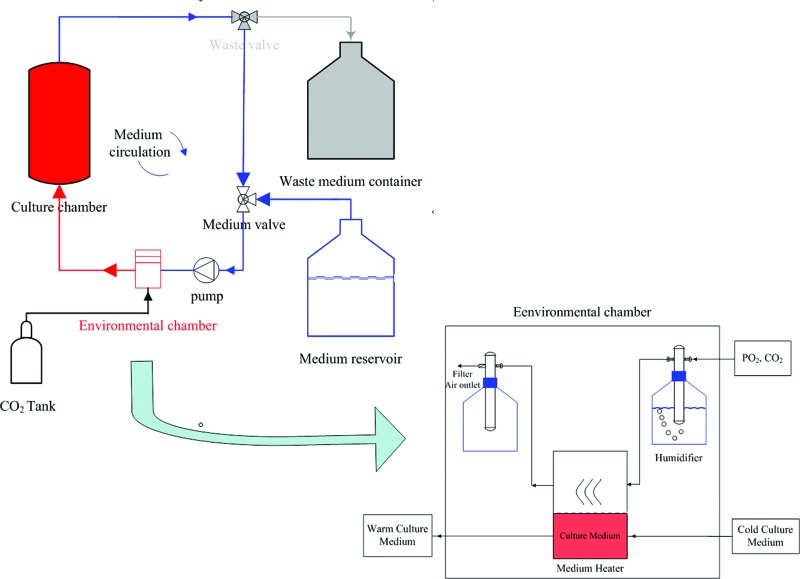
A traditional incubator-based bioreactor and/or independent bioreactor can be used for tendon/ligament engineering. In an incubator bioreactor system, air exchange is through the hydrophobic filter leak of the medium reservoir, and proper CO2 and temperature level is controlled by the incubator. Then, by circulating the culture medium, suitable conditions can be applied to engineered tendon/ligament, as shown in Figure 4.23 However, an independent bioreactor system, as shown Figure 5, does not rely on an incubator to control the culture environment. The percentage of different gases (PO2, CO2, and N2) are controlled by air valves,76 and the mixed gas is humidified first, and then passed into a medium heater. Waste gas emission is transported through a filter in the case of contamination. The prepared, warm culture medium is circulated through the bioreactor culture chamber. For certain tissues such as cartilage, some specific culture conditions are required. For example, under a low-oxygen environment, engineered cartilage displays faster matrix glycosaminoglycan deposition rate and better cellular morphology, but with less dedifferentiation.90–92 The environmental chamber allows researchers to manipulate different culture conditions, thereby enabling a systematic study of cell growth and differentiation into functional tissue.
FIG. 4.
Schematic illustration of an incubator bioreactor system. Suitable temperature, humidity, and CO2 level of the culture medium are maintained by the incubator in the medium reservoir. The medium is circulated by a pump. The waste valve is closed normally, and it will open during medium exchange. Color images available online at www.liebertpub.com/teb
FIG. 5.
Schematic diagram of an independent bioreactor system. Suitable temperature, humidity, and CO2 level of the culture medium are controlled by the environmental chamber. Cold culture medium is pumped to the environmental chamber for heating and then circulated through the culture chamber. The waste valve is closed normally, and it will open during medium exchange. Color images available online at www.liebertpub.com/teb
Monitoring and Feedback Systems
The biomechanical properties of the final engineered tendon/ligament should be a central concern of bioreactor design, as the tendon/ligament's functional role in the body is primarily mechanical. The maximum load and elastic modulus are essential mechanical properties to evaluate the suitability of engineered tendon/ligament. However, in most studies, mechanical tests are performed only at the end of tissue culture. For example, the stiffness of the constructs at different time points during culture is rarely recorded or assessed. The correlation between stiffness and tissue maturation may provide a better understanding about how the cell differentiates into functional tissue, and for this reason, online stiffness monitoring is likely to be invaluable.
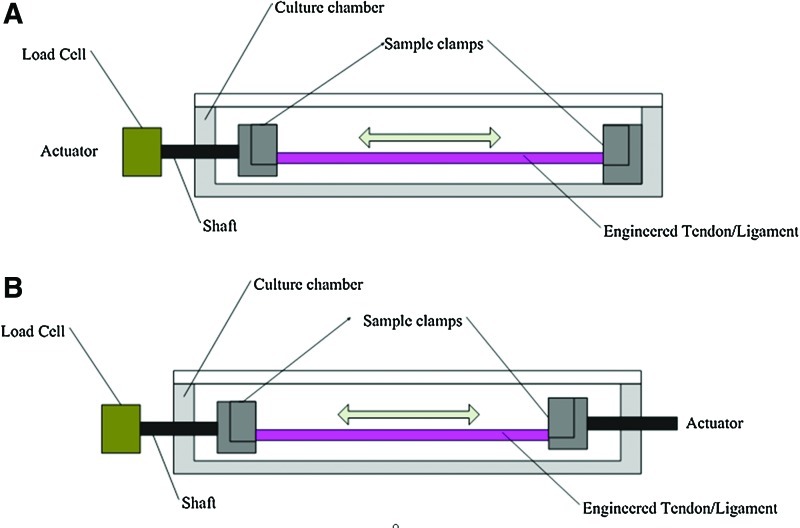
In tendon/ligament bioreactors, force measurement is enabled using sensors called load cells,22,82,93 which are located differently in various bioreactor designs. Load cells located between the actuator and sample clamps,83 shown in Figure 6A, requires high manufacturing accuracy, as the friction between the shaft and culture chamber can induce error. For fragile materials such as biological tissues, this friction might be higher than the applied load. Conversely, a load cell placed at the end of the culture chamber, shown in Figure 6B, can minimize the fiction between the actuator and culture chamber and also the fluid resistance during stimulation. To acquire accurate data, load cell selection should be based on the initial mechanical properties of the constructs. Working with the sensitivity of the load cell, extra attention is needed when manipulating the construct so as to avoid causing damage to the load cell through overloading.
FIG. 6.
Load cell position in different bioreactor systems. (A) Load cell is located between the actuator and sample clamps. (B) A separate load cell placed at the end of culture chamber system. Color images available online at www.liebertpub.com/teb
In addition to force monitoring, tissue displacement is another important variable that requires monitoring. Linear variable differential transformers and optical decoders are commonly used position sensors.19,82 Although the motion of actuating systems is preprogrammed, overload of the actuator and manual misoperation can cause desynchronization between the program and actual stimulation. The real-time displacement monitor can produce a full record of stimulation position, which then allows researchers to track if there are any unusual features in the results. Another function of the position sensor is to provide feedback of the displacement information to the actuator control system to correct for any desynchronization.82 Critically, by monitoring load and displacement, real-time stiffness during engineered tendon/ligament culture can be estimated.
Lastly, imaging the construct might be a potentially important monitoring method that can be adopted in bioreactor systems. Two imaging modalities, the confocal microscope and optical coherence tomography, are potential candidates.94,95 However, sample deformation induced by mechanical stimulation can cause image shift resulting in poor focus or loss of image. Imaging dynamic sample is extremely difficult in conventional bioreactors, as features of interest (e.g., cells) soon leave the field of view. Techniques need to be developed to move the sample and microscope together. This can become difficult, especially, when doing in situ imaging of a tendon tissue culture.
Commercial Bioreactor Systems for Tendon/Ligament Engineering
Recently, commercial tendon-/ligament-engineering bioreactor systems have become available. As discussed above, these basic principles are applied to customized bioreactors; however, with greater design input and advanced manufacturing techniques, commercial products are able to provide more accurate and complex environments for tendon/ligament culture. To our knowledge, two relatively complete commercial bioreactor systems have been developed recently: The Bose® ElectroForce® BioDynamic® system and the LigaGen system.
The Bose ElectroForce BioDynamic test instrument provides an accurate programmable uniaxial stretching stimulation and a controllable medium circulation environment to engineered tendon/ligament, which theoretically can be adjusted to mimic the in vivo biomechanical environment. With a load cell and optional laser micrometer, this bioreactor system is able to monitor the force/strain curve of engineered tendon/ligament during the culture period. Two different force and displacement ranges are available. Single-chamber and multiple-chamber systems with shared or independent loading are optional. As there are culture chambers compatible to the Bose testing devices such as ElectroForce 3200, biomechanical tests can be done at different time points without disruption of the tissue culture (www.bose-electroforce.com).
The LigaGen system is a lightweight (<3 kg) incubator-compatible bioreactor. It is capable of applying a maximum force of 40 N to the tissue sample and simulating complex, and presumably more physiologically realistic, loading patterns. Two systems are available from LigaGen. L30-1×is a single-culture model, which has a 23-mL-internal-volume chamber for single-tissue culture, and the L30-4C is a multiple-culture model with an 80-mL chamber for shared dynamic culture on two or four samples. The standard medium circulation system can reduce contamination risk during medium exchange. Rather than being a comprehensive system, the bioreactor is extensible to suit individual needs, by adding various components to the (universal) basic model as required. With an accessory tissue-monitoring sensor, this system is able to achieve real-time measurements of the sample stiffness during culture. If flow control is necessary, extra control systems can be installed (www.tissuegrowth.com/).
However, there are some disadvantages in the commercial bioreactors. First, the fixation mechanism to stabilize the tendon tissue in the bioreactor cannot be adjusted. Tissue clamps provided in the commercial bioreactor systems can become less effective when it comes to the use of a cylindrical scaffold. Secondly, capacities of the chamber and mechanical input are limited, and they can only host small-animal tissues. This may restrict clinical development. Moreover, some ligaments, such as the anterior cruciate ligament, are not only subjected to tensile force, but also to rotational loading, and none of the commercial bioreactor systems provide the addition of torsional loading. Lastly, full-scale commercial bioreactor systems are expensive, and for most of the time, not all of the functions they provide are commonly used in every study.
Ideal Bioreactors for Tendon/Ligament Engineering
The ideal bioreactor should be able culture tendon- and ligament-like constructs, which are well-organized, cell-seeded assemblies of collagen bundles with mechanical properties functionally similar to the native tissue. Autologous cell-seeded constructs are biologically compatible, and able to provide mechanical support similar to native tissue, and consequently, they are widely studied in tendon/ligament engineering. However, induction of cell-directed collagen fiber reorganization and assembly of collagen bundles are two important impediments to this approach. Mechanical tensile loads can help provide the necessary signals to cells to increase collagen synthesis, spatially organize of the collagen along the primary stress direction, and stabilize collagen from collagenase degradation.22,96,97 Moreover, proper mechanical stimulation can upregulate different proteoglycans, such as decorin, biglycan, fibromodulin, and fibronectin, which help the cells organize the parallel collagen fibrils forming bundles.42,98–101
The host body is in many ways the ultimate bioreactor for all engineered tissues. The study of Juncosa-Melvin et al. indicated that the maximum force of engineered patellar tendons increased more than 3000 times after 2 weeks of implantation in rabbits,19 and to date, none of the bioreactors have been able to accomplish this outcome. Therefore, an ideal bioreactor should aim to mimic the dynamic biochemical and biophysical environments in vivo. In musculoskeletal tissue engineering, various bioreactors have been developed. For instance, muscle tissues are not only subjected to mechanical stretching, but also able to receive the electrical impulses to simulate inputs from the central nervous system,29 and mechanical and electrical stimulation bioreactors have been developed based on mimicking the in vivo environment.102,103 Compared to muscle, the in vivo environment is comparatively less complex in tendon/ligament and appears to require only passive mechanical input.
Summarizing, the ideal tendon-/ligament-engineering bioreactor that enables systemic research should integrate all aforementioned components (Fig. 1). The bioreactor should have culture chambers and an actuating system, but also be fitted with a medium circulation system, an environmental system, a monitoring system, a feedback system, and a waste medium analysis system. This bioreactor should first be able to provide not only a multiple, suitably sized. and sterilized chambers for tissue culture, but also accurate and programmable mechanical stimulation. Tensile strain and rotation are needed to mimic different in vivo tendon/ligament loads. Second, the circulated medium should infiltrate into tissue better than a static medium configuration, and the circulation system needs to reduce the risks of contamination during medium exchange and drug delivery. Moreover, environmental control, such as PO2, CO2, and pH level, allows researchers to explore the impact of different culture conditions on tissue maturation. Third, a monitoring system should provide the real-time status of cultured tendon/ligament, such as force and displacement, and based on these data, adjustment of mechanical stimulation by use of a feedback system. Fourth, through analysis of the waste medium, nutrient consumption needs to be evaluated, which may enable the changing of the medium base at different stages as required, rather than fixed, regular medium exchange every 3 days. Finally and importantly, the best patterns of mechanical stimulation for culturing engineered tendon/ligaments need to be defined.
Investigation of Evidence
Since the first 3D engineered tendon/ligament bioreactor system published by Altman et al. in 2002, the effect of mechanical stimulation on the engineered tendon/ligament has drawn a lot of research attention. In the past decade, dynamic loading of culture in bioreactor systems has been proven to have been a significant development in producing an engineered tendon/ligament. Indeed, various studies have been performed using bioreactor systems and have met with considerably success, and these are summarized in Table 2. Compared with static culture, the tissue produced using dynamic mechanical stimulation has a better cell morphology, including elongated cellular morphology and increased cell density. The mechanical properties of engineered tendon/ligament, such as tensile strength and elastic modulus, are also greatly improved by cyclic loading of the tissue culture, as is the microstructure of the extracellular matrix, such as collagen fiber alignment. Gene expression is also positively influenced by cyclic mechanical stimulation. For instance, collagen I expression under dynamic loading is three times higher than static culture in 2 weeks.83 Although cyclic stretching has been proven to be an effective way to stimulate the engineered tendon/ligament culture, the optimal stimulation pattern is still unknown. Nirmalanandhan et al. revealed that a 2.4% strain cycle consisting of 3000 cycles per day produced the best linear stiffness in rabbit MSCs seeded in type I collagen sponge.21 However, from the perspective of Butler et al., the stimulation pattern should be adjusted based on maturation of the engineered tendon/ligament, with higher dose loading applied at the later stage of tissue culture.83
Table 2.
Studies Using Bioreactor Systems for Tendon/Ligament Engineering
| First author | Bioreactor type (Company) | Parameters of mechanical stimulation | Scaffold material (dimensions) | Cell source | Effect | Reference |
|---|---|---|---|---|---|---|
| Altman (2002) | Custom-made step motor bioreactor with environmental chamber | Cyclic stretching 2-mm, 90o rotation, 0.0167 Hz, 21 days | Collagen type I gels, Bombyx mori silkworm silk fiber matrices (length:30 mm) | Human bone marrow stromal cells (hBMSC) | Elongation of hBMSCs, cross-section cell density↑ | 76 |
| Juncosa-Melvin (2006) | Custom-made pneumatic cylinder bioreactor with LVDT for displacement monitoring | Cyclic stretching 2.4% strain, 0.0033 Hz, 8 h/day for 2 weeks | Type I collagen sponge (23±0.8 mm×9±0.8 mm ×3±0.1 mm) | Rabbit MSCs | Maximum force↑, linear stiffness↑, maximum stress↑, linear modulus↑ | 19 |
| Webb (2006) | Custom-made step motor bioreactor | Cyclic stretching 10% strain, 0.25 Hz, 8 h/day for 7 days | Polyurethane construct (20×10×2 mm) | Human tracheal fibroblasts | Type I collagen↑, TGFβ-1↑, CTGF, Elastin↑, alpha I↑, Procollagen↑, Fibronectin↑, MMP-1↑, elastic modulus↑ | 42 |
| Androjna (2007) | Custom-made bioreactor with load- displacement measure system | Cyclic stretching 9% strain, twice daily for 30 min each period separated by 8-h rest for 2 weeks | Small-intestine submucosa (3×5×95 μm) | Dog tenocytes | Cell density↑, stiffness↑ | 35 |
| Nirmalanandhan (2008) | Custom-made pneumatic cylinder bioreactor | Cyclic stretching 2.4% and 1.2% strain, 1 Hz, stimulation period: 8 h/day, 100 and 3000 cycles/day for 12 days | Type I collagen sponge | Rabbit iliac-crest MSCs | The stimulation pattern of 2.4% strain, 3000 cycles/day, 1 Hz has the best effect on increasing stiffness. | 21 |
| Nirmalanandhan (2008) | Custom-made pneumatic cylinder bioreactor | Cyclic stretching 2.4% strain, 0.0033 Hz, 8 h/day for 12 days | Type I purified bovine collagen gel, type I collagen sponges (length: 11 and 51 mm) | Rabbit MSCs | Stiffness↑ | 20 |
| Nguyen (2009) | Custom-made linear actuator bioreactor with load cell for force measurement | Preloaded with 0.05 N, cyclic stretching 10%, 0.5 Hz, 2 h stimulation—2 h rest—2 h stimulation—18 h rest for 5 days | Porcine small intestine submucosa (length: 2 cm and width: 1 cm) | Rabbit MCL fibroblasts | Improved fiber orientation, fiber angular dispersion↓, better organized collagen fiber, elongated cell morphology, | 22 |
| Abousleiman (2009) | Custom-made linear actuator bioreactor with medium circulation system | Cyclic stretching 2% strain, 1 h/day, 0.0167 Hz for 1 and 2 weeks. | Human umbilical veins (wall thickness: 0.75 mm, outer diameter: 6.75±0.25 mm, length: 8.5 cm) | Wistar Rat bone marrow MSCs | better cell distribution, more elongated cell morphology, cell proliferation↑, ultimate stress↑, elastic modulus↑ | 23 |
| Butler 2009) | Custom-made pneumatic cylinder bioreactor with LVDT for displacement monitoring | Cyclic stretching 2.4% strain, 0.0033 Hz, 8 h/day for 2 weeks | Type I collagen sponge (94% pore volume; 62-mm mean pore diameter) | Mouse mesenchymal stem cell | Type I collagen↑, linear stiffness↑ | 83 |
| Chen(2010) | Custom-made step motor bioreactor | Cyclic stretching 10% strain, 2 h/day, 1 Hz for 14 days | Knitted silk-collagen sponge scaffold (5×0.5×0.2 cm) | Human embryonic stem cell | Collagen I↑, Collagen III↑, Epha4↑, Scx↑, Sox9↓, Myosin↑, Integrin α1↑, Integrin α2↑, Integrin β1↑, Collagen content↑, Collagen diameter↑, better cell alignment | 2 |
| Doroski (2010) | Custom-made linear motor bioreactor | Cyclic stretching 10% strain (5% offset, 5% amplitude), 1 Hz, 3 h/day, 1. 7, 14, and 21 days | Poly(ethylene glycol)-based hydrogel material oligo(poly(ethylene glycol) fumarate) (12.5×9.5×1.6 mm) | MSCs (PT-2510; Lonza) | Collagen I↑, Collagen III↑, TNC↑, Tenascin-C↑ | 27 |
| Saber (2010) | Ligagen L30-4C (Tissue Growth Technologies) | Cyclic stretching 1.25 N, 1 cycle/min, 1 h/day for 5 days | Acellular rabbit hindpaw tendon (length: 5 cm) | Rabbit tenocytes | Ultimate tensile stress↑ (closed to fleshly harvested tendon), elastic modulus↑ | 36 |
| Issa (2011) | Custom-made bioreactor | Cyclic stretching 2% strain, 1 h/day, 0.0167 Hz for 1 and 2 weeks. | Human umbilical veins (wall thickness: 0.41 mm) with Wharton's jelly matrix as central portion (total thickness: 0.75 mm) | Wistar rat bone marrow MSCs | Cell proliferation↑, tensile strength↑ | 28 |
| Woon (2011) | Ligagen L30-1C & Ligagen L30-4C (Tissue Growth Technologies) | L30-1C: dynamic loading, 10 N, 1 h/day, 0.0167 Hz for 5 days L30-4C: dynamic loading, 0.625 N 1.25 N 2.5 N, 1 h/day, 0.0167 Hz for 3, 5, and 8 days |
Acellular human flexor tendon scaffolds | Human dermal fibroblasts | Ultimate tensile stress↑, elastic modulus↑ | 31 |
LVDT, linear variable differential transformers; ↑, up regulation; ↓, down regulation.
Conclusion
The goal should be to create a construct with similar microstructure and mechanical properties, as native tissue, using bioscaffolds and autologous tenocytes. However, a tendon-like uniorientated collagen structure cannot be achieved without a mechanical stimulus within the culture environment. Traditional culture techniques do not provide this mechanical stimulation. The use of a bioreactor system is able to bridge the gap between in vitro and in vivo systems by creating suitable biochemical and biomechanical environments. Although it is clearly very difficult to reproduce the in vivo microenvironmental conditions exactly within the bioreactor, the goal is to mimic the in vivo biomechanical condition as closely as possible. The essential components of a tendon-/ligament-engineering bioreactor are the actuating system and culture chamber, which are responsible for the mechanical stimulation and providing sterilized environment for tissue culture. For better manipulation of the culture environment, accurate stimulation and more precise reporting of mechanical maturation of engineered tendon/ligament, an environmental control system, medium circulation system, monitor, and feedback system need to be included. As bioreactors nowadays are becoming more focused on preclinical research, using these clinically in the future still presents a substantial challenge. Tissue engineering requires substantial system optimization to achieve a reproducible functional engineered tendon/ligament consistently. This process is difficult and expensive in animal models, let alone in humans. However, there are additional problems in moving from small-animal models to humans related to the physical size of human tissue samples. Issues of nutrient transport, cell source, and spatial heterogeneity of scaffold properties and cell stimulation become more prominent in these larger tissues.
However, all the evidence suggests that by using bioreactors as described above, it will be possible to successfully produce engineered tendons and ligaments. When this occurs, we will be able to manufacture basic multichamber bioreactors with accurate and appropriate patterns of mechanical stimulation, which are affordable, and have low maintenance costs that can be used in commercial settings.
Acknowledgments
This work is supported by the Australia Research Council Linkage Grant (LP110100581). We would like to thank our fellow group members, in particular Ms. Euphemie Landao and Mr. Robert Day for helpful discussion.
Disclosure Statement
No competing financial interests exist.
References
- 1.Huang H.H. Qureshi A.A. Biundo J.J., Jr Sports and other soft tissue injuries, tendinitis, bursitis, and occupation-related syndromes. Curr Opin Rheumatol. 2000;12:150. doi: 10.1097/00002281-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Chen J.L. Yin Z. Shen W.L. Chen X. Heng B.C. Zou X.H. Ouyang H.W. Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials. 2010;31:9438. doi: 10.1016/j.biomaterials.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Butler D.L. Gooch C. Kinneberg K.R. Boivin G.P. Galloway M.T. Nirmalanandhan V.S. Shearn J.T. Dyment N.A. Juncosa-Melvin N. The use of mesenchymal stem cells in collagen-based scaffolds for tissue-engineered repair of tendons. Nat Protoc. 2010;5:849. doi: 10.1038/nprot.2010.14. [DOI] [PubMed] [Google Scholar]
- 4.Chen J. Xu J. Wang A. Zheng M. Scaffolds for tendon and ligament repair: review of the efficacy of commercial products. Expert Rev Med Devices. 2009;6:61. doi: 10.1586/17434440.6.1.61. [DOI] [PubMed] [Google Scholar]
- 5.Cerullo G. Puddu G. Gianni E. Damiani A. Pigozzi F. Anterior cruciate ligament patellar tendon reconstruction: it is probably better to leave the tendon defect open! Knee Surg Sports Traumatol Arthrosc. 1995;3:14. doi: 10.1007/BF01553519. [DOI] [PubMed] [Google Scholar]
- 6.Coupens S.D. Yates C.K. Sheldon C. Ward C. Magnetic resonance imaging evaluation of the patellar tendon after use of its central one-third for anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:332. doi: 10.1177/036354659202000317. [DOI] [PubMed] [Google Scholar]
- 7.Harner C.D. Olson E. Irrgang J.J. Silverstein S. Fu F.H. Silbey M. Allograft versus autograft anterior cruciate ligament reconstruction: 3- to 5-year outcome. Clin Orthop Relat Res. 1996;324:134. doi: 10.1097/00003086-199603000-00016. [DOI] [PubMed] [Google Scholar]
- 8.Lin T.W. Cardenas L. Soslowsky L.J. Biomechanics of tendon injury and repair. J Biomech. 2004;37:865. doi: 10.1016/j.jbiomech.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Jarvinen T.A. Jarvinen T.L. Kannus P. Jozsa L. Jarvinen M. Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J Orthop Res. 2004;22:1303. doi: 10.1016/j.orthres.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Buckwalter J.A. Hunziker E.B. Orthopaedics. Healing of bones, cartilages, tendons, and ligaments: a new era. Lancet. 1996;348(Suppl 2):sII18. doi: 10.1016/s0140-6736(96)98028-9. [DOI] [PubMed] [Google Scholar]
- 11.Hildebrand K.A. Frank C.B. Hart D.A. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11:368. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- 12.Bray R.C. Rangayyan R.M. Frank C.B. Normal and healing ligament vascularity: a quantitative histological assessment in the adult rabbit medial collateral ligament. J Anat. 1996;188(Pt 1):87. [PMC free article] [PubMed] [Google Scholar]
- 13.Lo I.K. Ou Y. Rattner J.P. Hart D.A. Marchuk L.L. Frank C.B. Rattner J.B. The cellular networks of normal ovine medial collateral and anterior cruciate ligaments are not accurately recapitulated in scar tissue. J Anat. 2002;200:283. doi: 10.1046/j.1469-7580.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manske P.R. Flexor tendon healing. J Hand Surg Br. 1988;13:237. doi: 10.1016/0266-7681_88_90077-0. [DOI] [PubMed] [Google Scholar]
- 15.Arnsdorf E.J. Jones L.M. Carter D.R. Jacobs C.R. The periosteum as a cellular source for functional tissue engineering. Tissue Eng Part A. 2009;15:2637. doi: 10.1089/ten.tea.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan H. Liu H. Wong E.J. Toh S.L. Goh J.C. In vivo study of anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold. Biomaterials. 2008;29:3324. doi: 10.1016/j.biomaterials.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Fan H. Liu H. Toh S.L. Goh J.C. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30:4967. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Fuentes M. Meinel A.J. Hilbe M. Meinel L. Merkle H.P. Silk fibroin/hyaluronan scaffolds for human mesenchymal stem cell culture in tissue engineering. Biomaterials. 2009;30:5068. doi: 10.1016/j.biomaterials.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Juncosa-Melvin N. Shearn J.T. Boivin G.P. Gooch C. Galloway M.T. West J.R. Nirmalanandhan V.S. Bradica G. Butler D.L. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006;12:2291. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 20.Nirmalanandhan V.S. Rao M. Shearn J.T. Juncosa-Melvin N. Gooch C. Butler D.L. Effect of scaffold material, construct length and mechanical stimulation on the in vitro stiffness of the engineered tendon construct. J Biomech. 2008;41:822. doi: 10.1016/j.jbiomech.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Nirmalanandhan V.S. Shearn J.T. Juncosa-Melvin N. Rao M. Gooch C. Jain A. Bradica G. Butler D.L. Improving linear stiffness of the cell-seeded collagen sponge constructs by varying the components of the mechanical stimulus. Tissue Eng Part A. 2008;14:1883. doi: 10.1089/ten.tea.2007.0125. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen T.D. Liang R. Woo S.L. Burton S.D. Wu C. Almarza A. Sacks M.S. Abramowitch S. Effects of cell seeding and cyclic stretch on the fiber remodeling in an extracellular matrix-derived bioscaffold. Tissue Eng Part A. 2009;15:957. doi: 10.1089/ten.tea.2007.0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abousleiman R.I. Reyes Y. McFetridge P. Sikavitsas V. Tendon tissue engineering using cell-seeded umbilical veins cultured in a mechanical stimulator. Tissue Eng Part A. 2009;15:787. doi: 10.1089/ten.tea.2008.0102. [DOI] [PubMed] [Google Scholar]
- 24.Sahoo S. Ouyang H. Goh J.C. Tay T.E. Toh S.L. Characterization of a novel polymeric scaffold for potential application in tendon/ligament tissue engineering. Tissue Eng. 2006;12:91. doi: 10.1089/ten.2006.12.91. [DOI] [PubMed] [Google Scholar]
- 25.Sahoo S. Cho-Hong J.G. Siew-Lok T. Development of hybrid polymer scaffolds for potential applications in ligament and tendon tissue engineering. Biomed Mater. 2007;2:169. doi: 10.1088/1748-6041/2/3/001. [DOI] [PubMed] [Google Scholar]
- 26.Chen X. Qi Y.Y. Wang L.L. Yin Z. Yin G.L. Zou X.H. Ouyang H.W. Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials. 2008;29:3683. doi: 10.1016/j.biomaterials.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 27.Doroski D.M. Levenston M.E. Temenoff J.S. Cyclic tensile culture promotes fibroblastic differentiation of marrow stromal cells encapsulated in poly(ethylene glycol)-based hydrogels. Tissue Eng Part A. 2010;16:3457. doi: 10.1089/ten.tea.2010.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Issa R.I. Engebretson B. Rustom L. McFetridge P.S. Sikavitsas V.I. The effect of cell seeding density on the cellular and mechanical properties of a mechanostimulated tissue-engineered tendon. Tissue Eng Part A. 2011;17:1479. doi: 10.1089/ten.TEA.2010.0484. [DOI] [PubMed] [Google Scholar]
- 29.Ross J.J. Duxson M.J. Harris A.J. Neural determination of muscle fibre numbers in embryonic rat lumbrical muscles. Development. 1987;100:395. doi: 10.1242/dev.100.3.395. [DOI] [PubMed] [Google Scholar]
- 30.Liu W. Chen B. Deng D. Xu F. Cui L. Cao Y. Repair of tendon defect with dermal fibroblast engineered tendon in a porcine model. Tissue Eng. 2006;12:775. doi: 10.1089/ten.2006.12.775. [DOI] [PubMed] [Google Scholar]
- 31.Woon C.Y. Kraus A. Raghavan S.S. Pridgen B.C. Megerle K. Pham H. Chang J. Three-dimensional-construct bioreactor conditioning in human tendon tissue engineering. Tissue Eng Part A. 2011;17:2561. doi: 10.1089/ten.TEA.2010.0701. [DOI] [PubMed] [Google Scholar]
- 32.Deng D. Liu W. Xu F. Yang Y. Zhou G. Zhang W.J. Cui L. Cao Y. Engineering human neo-tendon tissue in vitro with human dermal fibroblasts under static mechanical strain. Biomaterials. 2009;30:6724. doi: 10.1016/j.biomaterials.2009.08.054. [DOI] [PubMed] [Google Scholar]
- 33.Obaid H. Connell D. Cell therapy in tendon disorders: what is the current evidence? Am J Sports Med. 2010;38:2123. doi: 10.1177/0363546510373574. [DOI] [PubMed] [Google Scholar]
- 34.Chen J. Wang A. Xu J. Zheng M. In chronic lateral epicondylitis, apoptosis and autophagic cell death occur in the extensor carpi radialis brevis tendon. J Shoulder Elbow Surg. 2010;19:355. doi: 10.1016/j.jse.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 35.Androjna C. Spragg R.K. Derwin K.A. Mechanical conditioning of cell-seeded small intestine submucosa: a potential tissue-engineering strategy for tendon repair. Tissue Eng. 2007;13:233. doi: 10.1089/ten.2006.0050. [DOI] [PubMed] [Google Scholar]
- 36.Saber S. Zhang A.Y. Ki S.H. Lindsey D.P. Smith R.L. Riboh J. Pham H. Chang J. Flexor tendon tissue engineering: bioreactor cyclic strain increases construct strength. Tissue Eng Part A. 2010;16:2085. doi: 10.1089/ten.TEA.2010.0032. [DOI] [PubMed] [Google Scholar]
- 37.Chen J.M. Willers C. Xu J. Wang A. Zheng M.H. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue Eng. 2007;13:1479. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 38.Cooper J.A. Lu H.H. Ko F.K. Freeman J.W. Laurencin C.T. Fiber-based tissue-engineered scaffold for ligament replacement: design considerations and in vitro evaluation. Biomaterials. 2005;26:1523. doi: 10.1016/j.biomaterials.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Freeman J.W. Woods M.D. Laurencin C.T. Tissue engineering of the anterior cruciate ligament using a braid-twist scaffold design. J Biomech. 2007;40:2029. doi: 10.1016/j.jbiomech.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C.H. Shin H.J. Cho I.H. Kang Y.M. Kim I.A. Park K.D. Shin J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials. 2005;26:1261. doi: 10.1016/j.biomaterials.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 41.Moffat K.L. Kwei A.S. Spalazzi J.P. Doty S.B. Levine W.N. Lu H.H. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue Eng Part A. 2009;15:115. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Webb K. Hitchcock R.W. Smeal R.M. Li W. Gray S.D. Tresco P.A. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J Biomech. 2006;39:1136. doi: 10.1016/j.jbiomech.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 43.Zheng M. Wang A. Autologous Cell Therapy for Tendon Tissue Reconstruction. Sino-American Symposium on Clinical and Translational Medicine; Washington, DC. 2011. [Google Scholar]
- 44.Zheng M. Tenocyte cell culturing method, 2009. Jul 26, 2011. US7985408.
- 45.Freed L.E. Vunjak-Novakovic G. Langer R. Cultivation of cell-polymer cartilage implants in bioreactors. J Cell Biochem. 1993;51:257. doi: 10.1002/jcb.240510304. [DOI] [PubMed] [Google Scholar]
- 46.Freed L.E. Marquis J.C. Nohria A. Emmanual J. Mikos A.G. Langer R. Neocartilage formation in vitro and in vivo using cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1993;27:11. doi: 10.1002/jbm.820270104. [DOI] [PubMed] [Google Scholar]
- 47.Awad H.A. Butler D.L. Harris M.T. Ibrahim R.E. Wu Y. Young R.G. Kadiyala S. Boivin G.P. In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res. 2000;51:233. doi: 10.1002/(sici)1097-4636(200008)51:2<233::aid-jbm12>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 48.Wang L. Seshareddy K. Weiss M.L. Detamore M.S. Effect of initial seeding density on human umbilical cord mesenchymal stromal cells for fibrocartilage tissue engineering. Tissue Eng Part A. 2009;15:1009. doi: 10.1089/ten.tea.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L. Gardiner B.S. Smith D.W. Pivonka P. Grodzinsky A. A fully coupled poroelastic reactive-transport model of cartilage. Mol Cell Biomech. 2008;5:133. [PubMed] [Google Scholar]
- 50.Pham Q.P. Sharma U. Mikos A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 51.Derwin K.A. Baker A.R. Spragg R.K. Leigh D.R. Iannotti J.P. Commercial extracellular matrix scaffolds for rotator cuff tendon repair. Biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am. 2006;88:2665. doi: 10.2106/JBJS.E.01307. [DOI] [PubMed] [Google Scholar]
- 52.Kinneberg K.R. Nirmalanandhan V.S. Juncosa-Melvin N. Powell H.M. Boyce S.T. Shearn J.T. Butler D.L. Chondroitin-6-sulfate incorporation and mechanical stimulation increase MSC-collagen sponge construct stiffness. J Orthop Res. 2010;28:1092. doi: 10.1002/jor.21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert T.W. Stewart-Akers A.M. Simmons-Byrd A. Badylak S.F. Degradation and remodeling of small intestinal submucosa in canine Achilles tendon repair. J Bone Joint Surg Am. 2007;89:621. doi: 10.2106/JBJS.E.00742. [DOI] [PubMed] [Google Scholar]
- 54.Fleming B.C. Spindler K.P. Palmer M.P. Magarian E.M. Murray M.M. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37:1554. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Malcarney H.L. Bonar F. Murrell G.A. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. Am J Sports Med. 2005;33:907. doi: 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- 56.Zheng M.H. Chen J. Kirilak Y. Willers C. Xu J. Wood D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: possible implications in human implantation. J Biomed Mater Res B Appl Biomater. 2005;73:61. doi: 10.1002/jbm.b.30170. [DOI] [PubMed] [Google Scholar]
- 57.Chang J. Thunder R. Most D. Longaker M.T. Lineaweaver W.C. Studies in flexor tendon wound healing: neutralizing antibody to TGF-beta1 increases postoperative range of motion. Plast Reconstr Surg. 2000;105:148. doi: 10.1097/00006534-200001000-00025. [DOI] [PubMed] [Google Scholar]
- 58.Bennett N.T. Schultz G.S. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 59.Wojciak B. Crossan J.F. The effects of T cells and their products on in vitro healing of epitenon cell microwounds. Immunology. 1994;83:93. [PMC free article] [PubMed] [Google Scholar]
- 60.Marui T. Niyibizi C. Georgescu H.I. Cao M. Kavalkovich K.W. Levine R.E. Woo S.L. Effect of growth factors on matrix synthesis by ligament fibroblasts. J Orthop Res. 1997;15:18. doi: 10.1002/jor.1100150104. [DOI] [PubMed] [Google Scholar]
- 61.Pryce B.A. Watson S.S. Murchison N.D. Staverosky J.A. Dunker N. Schweitzer R. Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation. Development. 2009;136:1351. doi: 10.1242/dev.027342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clark R.T. Johnson T.L. Schalet B.J. Davis L. Gaschen V. Hunziker E.B. Oldberg A. Mikic B. GDF-5 deficiency in mice leads to disruption of tail tendon form and function. Connect Tissue Res. 2001;42:175. doi: 10.3109/03008200109005648. [DOI] [PubMed] [Google Scholar]
- 63.Mikic B. Schalet B.J. Clark R.T. Gaschen V. Hunziker E.B. GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res. 2001;19:365. doi: 10.1016/S0736-0266(00)90018-4. [DOI] [PubMed] [Google Scholar]
- 64.Chhabra A. Tsou D. Clark R.T. Gaschen V. Hunziker E.B. Mikic B. GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res. 2003;21:826. doi: 10.1016/S0736-0266(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 65.Dahlgren L.A. Mohammed H.O. Nixon A.J. Temporal expression of growth factors and matrix molecules in healing tendon lesions. J Orthop Res. 2005;23:84. doi: 10.1016/j.orthres.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi M. Itoi E. Minagawa H. Miyakoshi N. Takahashi S. Tuoheti Y. Okada K. Shimada Y. Expression of growth factors in the early phase of supraspinatus tendon healing in rabbits. J Shoulder Elbow Surg. 2006;15:371. doi: 10.1016/j.jse.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Chamberlain C.S. Crowley E. Vanderby R. The spatio-temporal dynamics of ligament healing. Wound Repair Regen. 2009;17:206. doi: 10.1111/j.1524-475X.2009.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hannafin J.A. Arnoczky S.P. Hoonjan A. Torzilli P.A. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 69.Flynn B.P. Bhole A.P. Saeidi N. Liles M. Dimarzio C.A. Ruberti J.W. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8) PLoS One. 2010;5:e12337. doi: 10.1371/journal.pone.0012337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maeda T. Sakabe T. Sunaga A. Sakai K. Rivera A.L. Keene D.R. Sasaki T. Stavnezer E. Iannotti J. Schweitzer R. Ilic D. Baskaran H. Sakai T. Conversion of mechanical force into TGF-beta-mediated biochemical signals. Curr Biol. 2011;21:933. doi: 10.1016/j.cub.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lejard V. Brideau G. Blais F. Salingcarnboriboon R. Wagner G. Roehrl M.H. Noda M. Duprez D. Houillier P. Rossert J. Scleraxis and NFATc regulate the expression of the pro-alpha1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007;282:17665. doi: 10.1074/jbc.M610113200. [DOI] [PubMed] [Google Scholar]
- 72.Dennis R.G. Smith B. Philp A. Donnelly K. Baar K. Bioreactors for guiding muscle tissue growth and development. Adv Biochem Eng Biotechnol. 2009;112:39. doi: 10.1007/978-3-540-69357-4_3. [DOI] [PubMed] [Google Scholar]
- 73.Catapano G. Patzer J.F., 2nd Gerlach J.C. Transport advances in disposable bioreactors for liver tissue engineering. Adv Biochem Eng Biotechnol. 2010;115:117. doi: 10.1007/10_2008_34. [DOI] [PubMed] [Google Scholar]
- 74.Rauh J. Milan F. Gunther K.P. Stiehler M. Bioreactor systems for bone tissue engineering. Tissue Eng Part B Rev. 2011;17:263. doi: 10.1089/ten.TEB.2010.0612. [DOI] [PubMed] [Google Scholar]
- 75.Salter E. Goh B. Hung B. Hutton D. Ghone N. Grayson W.L. Bone tissue engineering bioreactors: a role in the clinic? Tissue Eng Part B Rev. 2012;18:62. doi: 10.1089/ten.TEB.2011.0209. [DOI] [PubMed] [Google Scholar]
- 76.Altman G.H. Lu H.H. Horan R.L. Calabro T. Ryder D. Kaplan D.L. Stark P. Martin I. Richmond J.C. Vunjak-Novakovic G. Advanced bioreactor with controlled application of multi-dimensional strain for tissue engineering. J Biomech Eng. 2002;124:742. doi: 10.1115/1.1519280. [DOI] [PubMed] [Google Scholar]
- 77.Varseveld R.B.v. Bone G.M. Accurate Position Control of a Pneumatic Actuator Using On/Off Solenoid Valves. Trans Mechatronics. 1997;2:195. [Google Scholar]
- 78.Alter D. Tsao T. Control of linear motors for machine tool feed drives: design and implementation of H1 optimal feedback control. ASME J Dyn Syst, Meas, Control. 1996;118:649. [Google Scholar]
- 79.Alter D. Tsao T. Dynamic stiffness enhancement of direct linear motor feed drives for machining. Proceedings of the American Control Conference; Baltimore, MD. 1994. [Google Scholar]
- 80.Braembussche P. Swevers J. Brussel H. Vanherck P. Accurate tracking control of linear synchronous motor machine tool axes. Mechatronics. 1996;6:507. [Google Scholar]
- 81.Liu J. Hsu M. Chen F. On the design of rotating speed functions to improve the acceleration peak value of ball-screw transmission mechanism. Mech Machine Theory. 2001;36:1035. [Google Scholar]
- 82.Parent G. Huppe N. Langelier E. Low stress tendon fatigue is a relatively rapid process in the context of overuse injuries. Ann Biomed Eng. 2011;39:1535. doi: 10.1007/s10439-011-0254-0. [DOI] [PubMed] [Google Scholar]
- 83.Butler D.L. Hunter S.A. Chokalingam K. Cordray M.J. Shearn J. Juncosa-Melvin N. Nirmalanandhan S. Jain A. Using functional tissue engineering and bioreactors to mechanically stimulate tissue-engineered constructs. Tissue Eng Part A. 2009;15:741. doi: 10.1089/ten.tea.2008.0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janssen F.W. Oostra J. Oorschot A. van Blitterswijk C.A. A perfusion bioreactor system capable of producing clinically relevant volumes of tissue-engineered bone: in vivo bone formation showing proof of concept. Biomaterials. 2006;27:315. doi: 10.1016/j.biomaterials.2005.07.044. [DOI] [PubMed] [Google Scholar]
- 85.Cartmell S.H. Porter B.D. Garcia A.J. Guldberg R.E. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197. doi: 10.1089/10763270360728107. [DOI] [PubMed] [Google Scholar]
- 86.Meinel L. Karageorgiou V. Fajardo R. Snyder B. Shinde-Patil V. Zichner L. Kaplan D. Langer R. Vunjak-Novakovic G. Bone tissue engineering using human mesenchymal stem cells: effects of scaffold material and medium flow. Ann Biomed Eng. 2004;32:112. doi: 10.1023/b:abme.0000007796.48329.b4. [DOI] [PubMed] [Google Scholar]
- 87.Uemura T. Dong J. Wang Y. Kojima H. Saito T. Iejima D. Kikuchi M. Tanaka J. Tateishi T. Transplantation of cultured bone cells using combinations of scaffolds and culture techniques. Biomaterials. 2003;24:2277. doi: 10.1016/s0142-9612(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 88.Holtorf H.L. Jansen J.A. Mikos A.G. Flow perfusion culture induces the osteoblastic differentiation of marrow stroma cell-scaffold constructs in the absence of dexamethasone. J Biomed Mater Res A. 2005;72:326. doi: 10.1002/jbm.a.30251. [DOI] [PubMed] [Google Scholar]
- 89.Olivier V. Hivart P. Descamps M. Hardouin P. In vitro culture of large bone substitutes in a new bioreactor: importance of the flow direction. Biomed Mater. 2007;2:174. doi: 10.1088/1748-6041/2/3/002. [DOI] [PubMed] [Google Scholar]
- 90.Saini S. Wick T.M. Effect of low oxygen tension on tissue-engineered cartilage construct development in the concentric cylinder bioreactor. Tissue Eng. 2004;10:825. doi: 10.1089/1076327041348545. [DOI] [PubMed] [Google Scholar]
- 91.Domm C. Schunke M. Christesen K. Kurz B. Redifferentiation of dedifferentiated bovine articular chondrocytes in alginate culture under low oxygen tension. Osteoarthritis Cartilage. 2002;10:13. doi: 10.1053/joca.2001.0477. [DOI] [PubMed] [Google Scholar]
- 92.Hansen U. Schunke M. Domm C. Ioannidis N. Hassenpflug J. Gehrke T. Kurz B. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 93.Butler D.L. Juncosa-Melvin N. Boivin G.P. Galloway M.T. Shearn J.T. Gooch C. Awad H. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008;26:1. doi: 10.1002/jor.20456. [DOI] [PubMed] [Google Scholar]
- 94.Goetz M. Thomas S. Heimann A. Delaney P. Schneider C. Relle M. Schwarting A. Galle P.R. Kempski O. Neurath M.F. Kiesslich R. Dynamic in vivo imaging of microvasculature and perfusion by miniaturized confocal laser microscopy. Eur Surg Res. 2008;41:290. doi: 10.1159/000148242. [DOI] [PubMed] [Google Scholar]
- 95.Drexler W. Morgner U. Kartner F.X. Pitris C. Boppart S.A. Li X.D. Ippen E.P. Fujimoto J.G. In vivo ultrahigh-resolution optical coherence tomography. Opt Lett. 1999;24:1221. doi: 10.1364/ol.24.001221. [DOI] [PubMed] [Google Scholar]
- 96.Yang G. Im H.J. Wang J.H. Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene. 2005;363:166. doi: 10.1016/j.gene.2005.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lavagnino M. Arnoczky S.P. Tian T. Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181. doi: 10.1080/03008200390215881. [DOI] [PubMed] [Google Scholar]
- 98.Cribb A.M. Scott J.E. Tendon response to tensile stress: an ultrastructural investigation of collagen:proteoglycan interactions in stressed tendon. J Anat. 1995;187(Pt 2):423. [PMC free article] [PubMed] [Google Scholar]
- 99.Scott J.E. The periphery of the developing collagen fibril. Quantitative relationships with dermatan sulphate and other surface-associated species. Biochem J. 1984;218:229. doi: 10.1042/bj2180229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vogel K.G. What happens when tendons bend and twist? Proteoglycans. J Musculoskelet Neuronal Interact. 2004;4:202. [PubMed] [Google Scholar]
- 101.Franchi M. Trire A. Quaranta M. Orsini E. Ottani V. Collagen structure of tendon relates to function. Sci World J. 2007;7:404. doi: 10.1100/tsw.2007.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharifpoor S. Simmons C.A. Labow R.S. Paul Santerre J. Functional characterization of human coronary artery smooth muscle cells under cyclic mechanical strain in a degradable polyurethane scaffold. Biomaterials. 2011;32:4816. doi: 10.1016/j.biomaterials.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 103.Donnelly K. Khodabukus A. Philp A. Deldicque L. Dennis R.G. Baar K. A novel bioreactor for stimulating skeletal muscle in vitro. Tissue Eng Part C Methods. 2010;16:711. doi: 10.1089/ten.TEC.2009.0125. [DOI] [PubMed] [Google Scholar]