Abstract
The brilliant red pigments prodiginines are natural secondary metabolites that are produced by select species of Gram-negative and Gram-positive bacteria. These molecules have received significant attention due to their reported antibacterial, antifungal, immunosuppressive, and anticancer activities. In this study, a Serratia marcescens SER1 strain was isolated and verified using 16s rDNA. The prodigiosin was purified using silica chromatography and was analyzed by 1H-NMR spectroscopy. The cell cytotoxic effects of the purified prodigiosin on multiple drug resistant cell lines that overexpress MDR1, BCRP, or MRP2 pumps were analyzed. Prodigiosin had nearly identical cytotoxic effects on the resistant cells in comparison to their parental lines. In agreement with the same prodigiosin cytotoxicity, FACS analysis of prodigiosin accumulation and efflux in MDR overexpressing cell lines also indicated that this pro-apoptotic agent operates independently of the presence of the MDR1, BCRP, or MRP transporter and may be a potential treatment for malignant cancer cells that overexpress multidrug resistance transporters.
The anticancer agent prodigiosin is not a multidrug resistance protein substrate. Prodigiosin, produced by Serratia marcescens, has cytotoxic effects on cells irrespective of whether the cells express MDR1, MRP2, or BCRP pumps of the multidrug resistance families. Therefore this drug may be potentially useful as a treatment for malignancies that overexpress the MDR pumps.
Introduction
Prodiginines are natural red pigmented heterocyclic tripyrroles that appear at later phases of the bacterial growth curve. Prodiginines consist of three broad structural classes. The first group is the linear tripyrroles group, which includes prodigiosin (isolated mainly from Serratia marcescens, Serratia plymuthica, Hahella chejuensis, Pseudomonas magnesiorubra, and Vibrio psychroerythreus) and undecylprodigiosin (isolated from Streptomyces longisporus ruber, Streptoverticillium rubrireticuli, Actinomadura madurae, Streptomyces coelicolor, and Saccharopolyspora sp.). The second group is the macrocyclic group, which is characterized by ring formation between pyrrole A and pyrrole C and includes cyclononylprodigiosin (isolated from Actinomadura pelletieri and Actinomadura madurae). Finally, the third is the cyclic group, which has a ring on pyrrole C and includes cycloprodigiosin (isolated from Vibrio gazogenes, Alteromonas rubra, and Pseudoalteromonas denitrificans) and butylcycloheptylprodiginine (isolated from Saccharopolyspora sp and Streptomyces coelicolor). Many of these alkaloids have been examined for their antibacterial, antifungal, antiprotozoal, antimalarial, algicidal, and insecticide characteristics (Bennett and Bentley, 2000; Harris et al., 2004; Williamson et al., 2006; Kim et al., 2008).
Although the physiological importance of these bioactive secondary metabolites is not fully understood, they have recently gained attention due to their immunosuppressive and anticancer effects on myeloma, T-cell leukemia, Burkitt's lymphoma, and breast, colon, liver, and lung cancers, with few adverse effects on noncancerous cells. Significant progress has been made in uncovering the mechanisms of these anticancerous properties. The observed cellular effects include oxidative DNA damage (Elahian et al., 2012), the induction of mitochondria-mediated apoptosis, inhibition of phosphatases, and disruption of the pH gradient in various cellular compartments, and DNA intercalation, cell cycle arrest in late G1, and caspase activation (Montaner and Perez-Tomas, 2003; Perez-Tomas et al., 2003; Francisco et al., 2007; Williamson et al., 2007).
Multidrug resistance (MDR) is one of the major challenges affecting the development of new chemotherapeutic agents. MDR pumps on cancer cells can efflux a variety of traditional anticancer drugs and reduce intracellular drug concentrations, thereby leading to escape from cytotoxic effects of the drugs. Recently, many attempts have been made to develop new drugs that are not substrates for any of the three different MDR pumps (Gottesman, 2002). Preliminary studies on breast cancer cells demonstrated that prodigiosin has similar cytotoxicity on both MCF-7 and MCF7MX (mitoxantrone resistant counterpart) cells. These data suggest that prodigiosin may not be a substrate for the breast cancer resistance protein (BCRP) pump (Soto-Cerrato et al., 2004). The present study focused on the prodigiosin sensitivity of parental human gastric and ovarian cancer lines versus their resistant counterparts that overexpress different MDR pumps. If prodiginines circumvent MDR-mediated resistance, they will be good agents for cancer chemotherapy. In addition, designing and discovering more potent anticancer prodiginines that are not substrates for MDR pumps could help to reduce the failure of chemotherapy regimens and reduce cancer mortality.
Materials and Methods
Chemicals and media
Human cell culture media, trypsin, fetal bovine serum (FBS), penicillin, and streptomycin were purchased from Gibco (Grand Island, NY). MTT was obtained from Sigma–Aldrich (Sigma–Aldrich, Deisenhofen, Germany). Superfine Silica gel-60 powder, aluminum sheets of Silica TLC, Luria-Bertani media, and Muller-Hinton media were provided by Merck (Darmstadt, Germany). Primers were synthesized by the Cinaclone Company (Tehran, Iran). All other chemicals and solvents were of analytical grade and were purchased from commercial sources in Iran.
Bacterial screening, identification, and prodigiosin production
Soil samples were collected from the Zanjan University of Medical Sciences yard, located in northwest Iran, during the spring of 2011. The soil filtrates were cultured on either nutrient agar plates or selective media containing caprylate-thallous (Starr et al., 1976). Red colonies were serially diluted and subcultured on nutrient plates to achieve pure pigment producer bacteria. The desired clones were genomic DNA extracted and genotypically verified using 16 s rDNA amplification with universal primers (27F 5′-AGATTTGATCMTGGCTCAG-3′ and 1492R 5′-TACGGYTACCTTGTTACGACTT-3′) and then sequencing (Andreazza et al., 2010; Mirzaei et al., 2010). The sequences were blasted using the NCBI BLAST Website and submitted to GenBank using the NCBI Sequin software, version 11. Verified strains were cultivated in 2% peanut media (2% w/v fine peanut powder in 100 mM potassium phosphate with a pH of 7.0) for pigment production. A modified chromatographic approach was developed for the purification steps (Giri et al., 2004; de Araujo et al., 2010). The purified pigment was quantified spectrophotometrically (TECAN, infinite-M200; Grodig, Austria) by measuring the absorbance at 535 nm using a standard curve and was structurally determined using 1H-NMR (Bruker-400MH; Rheinstetler, Germany).
Cell culture
A2780RCIS (MRP1, 2 overexpressing human epithelial ovarian cancer cell line), EPG85-257RNOV (BCRP overexpressing human gastric carcinoma cell line), EPG85-257RDB (MDR1 overexpressing human gastric carcinoma cell line), and their parental lines were generously provided by Professor Herman Lage (Molecular pathology department, Charite Campus Mitte, Berlin, Deutschland). A2780 and EPG85-257 were cultured in RPMI-1640 media supplemented with 2 mM L-glutamine, 10% (v/v) heat-inactivated FBS, 50 IU/mL penicillin, and 50 μg/mL streptomycin at 37°C in a humidified CO2 incubator. The media for the RCIS, RNOV, and RDB resistant cell lines was also supplemented with 33.21 μM cisplatin, 0.386 μM mitoxantrone, and 4.74 μM daunorubicin, respectively (Materna et al., 2005; Lage et al., 2010).
Cell proliferation assay
To determine cell proliferation characteristics, 1000 cells were plated into each well of 96-well plates in 200 μL of growth medium. The culture medium was refreshed every 2 days. The growth rate evaluated during 7-day incubation using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Formazan crystal production was determined using the TECAN microplate reader after dissolving the crystals in dimethyl sulfoxide (DMSO) (Elahian et al., 2009).
Cell cytotoxicity assay
The cells were seeded at 1000 cells per well in 96-well plates and incubated at 37°C for 24 h. A stock solution of 10 mM prodigiosin in 10% DMSO was prepared and stored in aliquots at −20°C. The stock was freshly diluted with culture media before use and added to microplates at the defined concentrations from 0–100 μM. Appropriate controls containing DMSO were used to ensure that the solvent had no intrinsic effect in the cytotoxicity assays at these concentrations. Cell viability was evaluated after a 5-day incubation of the treated cells using MTT assay. IC50 was determined as the concentration of drug that reduced 50% of the surviving fraction of cells in each well as compared to the control. The IC50 values of the cytotoxic drugs (cisplatin, daunorubicin, and mitoxantrone) on the resistant cells and their corresponding parental cells were also analyzed in the same way. All experiments were performed three independent times in triplicate (Elahian et al., 2010).
Detection of prodigiosin accumulation and efflux
FACS analysis of prodigiosin accumulation and efflux was performed to determine whether this agent could be a substrate for MDR transporters. To analyze drug transport, approximately 1×105 cells of EPG85-257, EPG85-257RDB, EPG85-257RNOV, A2780, or A2780RCIS were obtained by trypsinization (van Hattum et al., 2002; Kowalski et al., 2005). All cells were incubated in 1 μM prodigiosin either with or without a specific MDR pump inhibitor (Verapamil (10 μM) as the specific MDR1 inhibitor, novobiocin (200 μM) as the specific BCRP inhibitor, or indomethacin (100 μM) as the specific multidrug resistance-associated protein [MRP] inhibitor) for 30 min at 37°C. Daunorubicin (1 μM) was used as the positive control for MDR1 and MRP transporters activity. Mitoxantrone (3 μM) was applied as the positive control for BCRP activity. Following adequate incubation periods, aliquots of the cells were removed from each sample, washed twice with ice-cold phosphate-buffered saline (PBS), and kept in PBS for analysis of fluorescent drug uptake. Following the uptake period and centrifugation, the supernatant was removed and the cells were washed twice with ice-cold PBS and then the cells were resuspended in drug-free medium either with or without verapamil, indomethacin, or novobiocin and were incubated for 1 h at 37°C. Cells were then washed twice with ice-cold PBS, placed in PBS at 4°C, and kept in the dark for analysis by flow cytometry (Marbeuf-Gueye et al., 2000; Elahian et al., 2010).
Flow cytometric analysis
The intracellular prodigiosin, daunorubicin, or mitoxantrone fluorescence intensities were determined using a BD Biosciences FACSCalibure™ flow cytometer. Samples were gated on forward/side scatter to exclude cell debris and clumps. A total of 10,000 cells were measured per sample. The autofluorescence of the cells was elucidated by measuring the fluorescence of cells in PBS alone. Cells were excited at 488 nm with an argon laser and the emission recorded via a 580 nm band-pass filter (FL2) for prodigiosin and daunorubicin fluorescence, and 670 nm band-pass filter (FL3) for mitoxantrone fluorescence. Flow cytometry data were processed and analyzed using WinMDI version 2.8. All assays were performed in at least three independent experiments (Elahian et al., 2010).
Statistical analysis
All experiments were carried out in triplicate at least three independent times and average values±standard errors were reported. These data were analyzed using Student's t-test. p-values less than 0.05 were considered to be statistically significant.
Results
Screening, purification, and prodigiosin identification
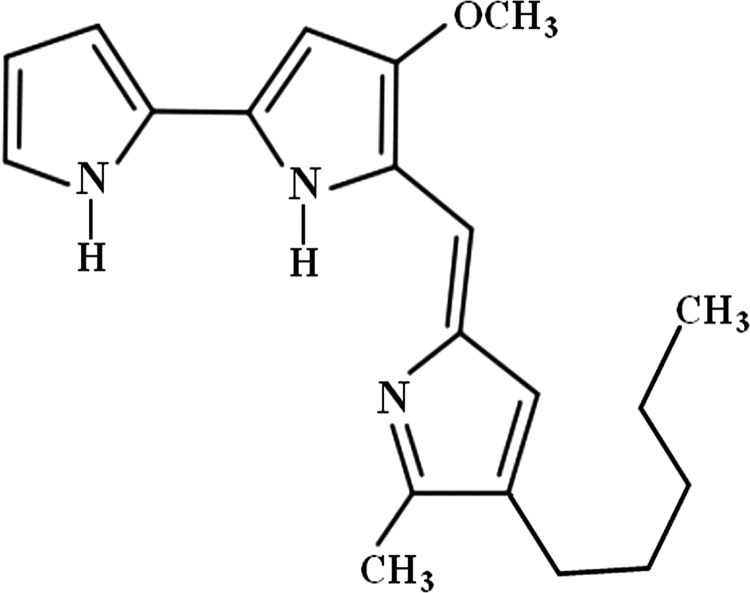
Samples from ecological niches such as soil and water were cultured on nutrient or caprylate-thallous plates to isolate pigment producing Serratia marcescens, and the best prodigiosin producer was named S. marcescens SER1 and verified using 16s rDNA amplification. The sequencing results were subjected to a homology search using BLAST (ftp://ftp.ncbi.nih.gov), and the homology and the phylogenetic tree were identified. The new 1429 bp sequence was submitted to GenBank under the accession number GI: 379327963. Fermentation at the optimal conditions yielded approximately 5 mg/mL crude prodigiosin after 2 days. Pure, red, and needle-shaped crystals were obtained after three sequential chromatographic steps. The pigments eluted from the final step were found to be a homogeneous single band by TLC. According to the Bear–Lambert equation, the molar extinction coefficient of prodigiosin production was calculated to be 0.1397 mM−1 cm−1 (0.4311 L mg−1 cm−1), as determined from the slope of the plot of ΔA535 versus pigment concentration. The maximum UV absorbance was observed at 535 nm, and the 1H-NMR spectroscopic data verified the pigment to be prodigiosin (Fig. 1). The 1H-NMR data were summarized as 1HNMR (CD2Cl2, 400 MHz) δ 6.5 (m, 7H, pyrrole-H, pyrrole-NH), 3.9 (s, 3H, OCH3), 2.25 (m, 2H, Ar-CH2), 1.8 (s, 3H, Ar-CH3), 0.85 (m, 9H, CH2-CH2-CH2-CH3), which confirmed the structure of prodigiosin (5[(3-methoxy-5-pyrrol-2-ylidene-pyrrol-2-ylidene)-methyl]-2-methyl-3-pentyl-1Hpyrrole).
FIG. 1.
Prodigiosin structure according to 1H-NMR spectroscopy.
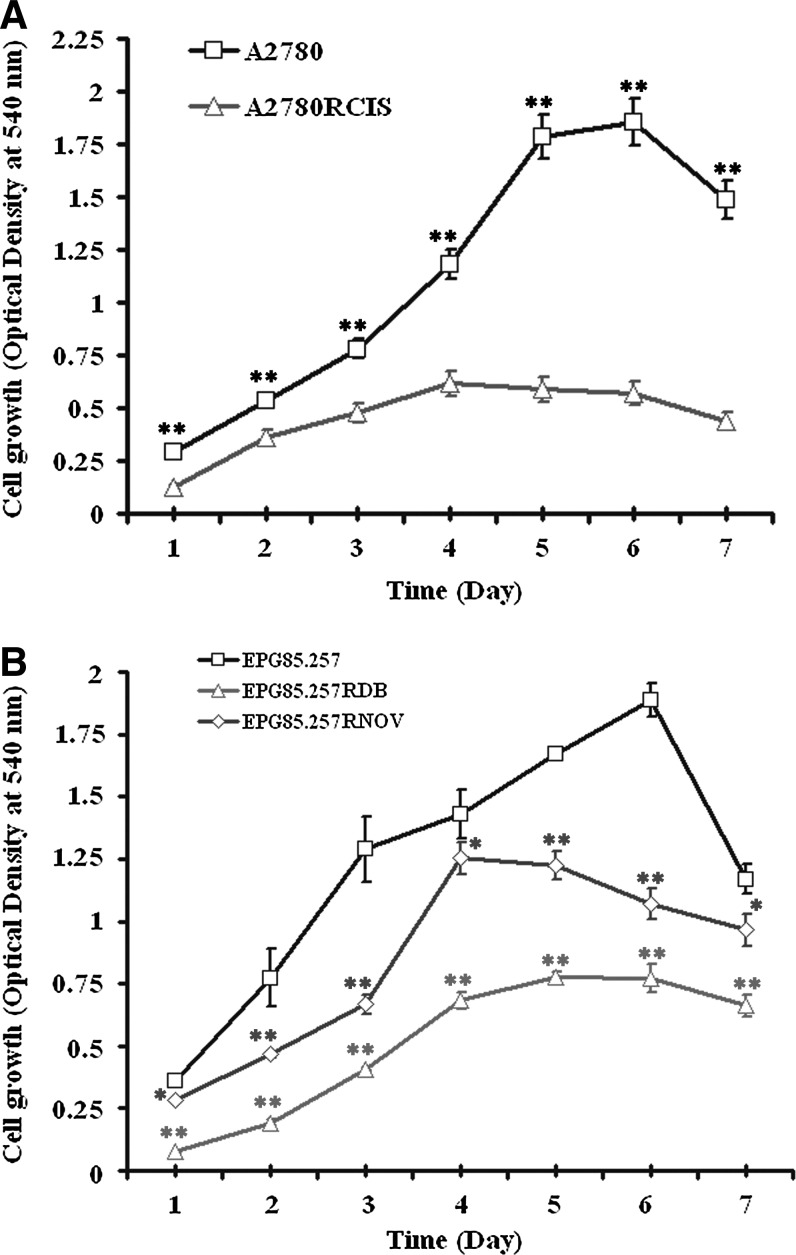
Growth characteristics of A2780 and EPG85-257 cells
The cells grew as a monolayer attached to the bottom of the flask and appear to be small round clumps in shape. The growth characteristics of A2780 and EPG85-257 lines and their resistant counterparts were shown in Figure 2. The difference between growth rates in parent versus resistant cells was highly statistically significant (p<0.05). Figure 2 also illustrates the point-by-point difference between A2780 and EPG85-257 cells and their resistant counterparts.
FIG. 2.
Growth rate of A2780 (A), EPG85-257 (B) cells and their resistant counterpart. Cells were seeded in 96-well plates at 1000 cells/well in RPMI-1640 culture medium. Cells were then counted using MTT assay during 7 days of seeding. Data are mean±SE of three independent experiments each in triplicate. The symbols (
 ) and (
) and ( ) represent the mean absorbance difference between parental and resistant cells with p<0.001 and p<0.05, respectively.
) represent the mean absorbance difference between parental and resistant cells with p<0.001 and p<0.05, respectively.
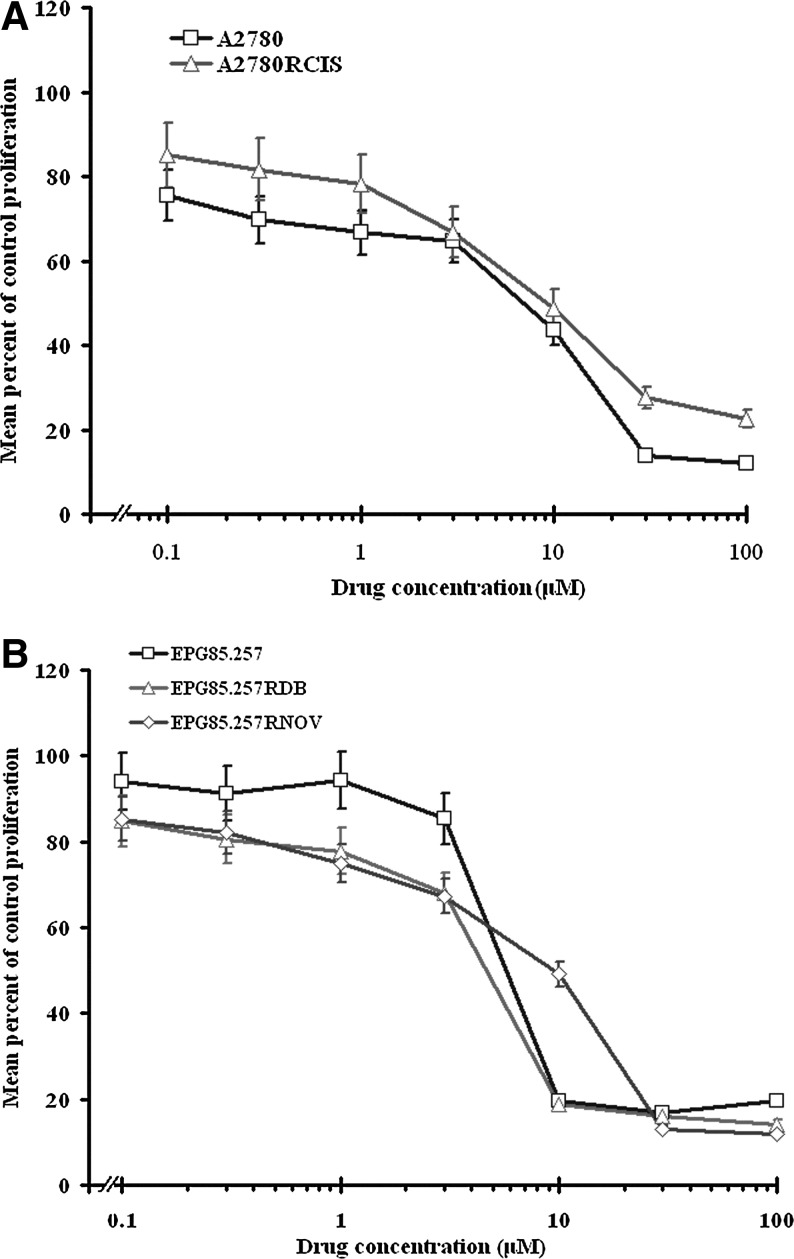
Effects of prodigiosin on parental cells and their resistant counterparts' proliferation
To investigate the effect of prodigiosin on cell survival, parental and resistant A2780 and EPG85-257 cells were treated with prodigiosin (0–100 μM). A dose–response curve was fitted to our data, and the IC50 values were calculated after 5 days of exposure (Fig. 3). Treatment with different concentrations of cisplatin (0–100 μM), daunorubicin (0–100 nM or 0–100 μM), or mitoxantrone (0–2000 nM) were also performed, and the IC50 values were calculated after 5 days of exposure. Despite significant difference in toxicity of cisplatin, daunorubicin, and mitoxantrone (p<0.05), prodigiosin had nearly identical cytotoxicity on both parental and the resistant cells (p>0.05) (Table 1).
FIG. 3.
Effects of prodigiosin on the survival of A2780 (A), EPG85-257 (B) cells and their resistant counterpart. Cells were cultured for 5 days with increasing doses of prodigiosin from 0 to 100 μM. Cell survival was measured by MTT assay. The values represent the means of three independent experiments performed in triplicate (Mean±SE). p>0.05 indicates that prodigiosin had nearly identical cytotoxicity on A2780 and EPG85-257 cells and their resistant counterpart.
Table 1.
IC50 Values of Prodigiosin and Anticancer Agents for the Normal and Corresponding Resistant Cells
| Cells | Prodigiosin IC50±SE (μM)a | Cisplatin IC50±SE (μM) | Daunorubicin IC50±SE (μM) | Mitoxantrone IC50±SE (μM) |
|---|---|---|---|---|
| A2780 | 8.92±0.272 | 1.97±0.084 | ND | ND |
| A2780RCIS | 9.07±0.361 | 45.25±1.985 | ND | ND |
| EPG85-257 | 8.45±0.216 | ND | 0.07±0.002 | 0.01±0.004 |
| EPG85-257RDB | 9.27±0.780 | ND | 9.06±0.136 | ND |
| EPG85-257RNOV | 9.08±0.136 | ND | ND | 0.95±0.023 |
Drug concentration required for 50% inhibition of cell growth after 5 days of drug exposure.
ND, not done.
Data represent the mean±standard error of three individual experiments.
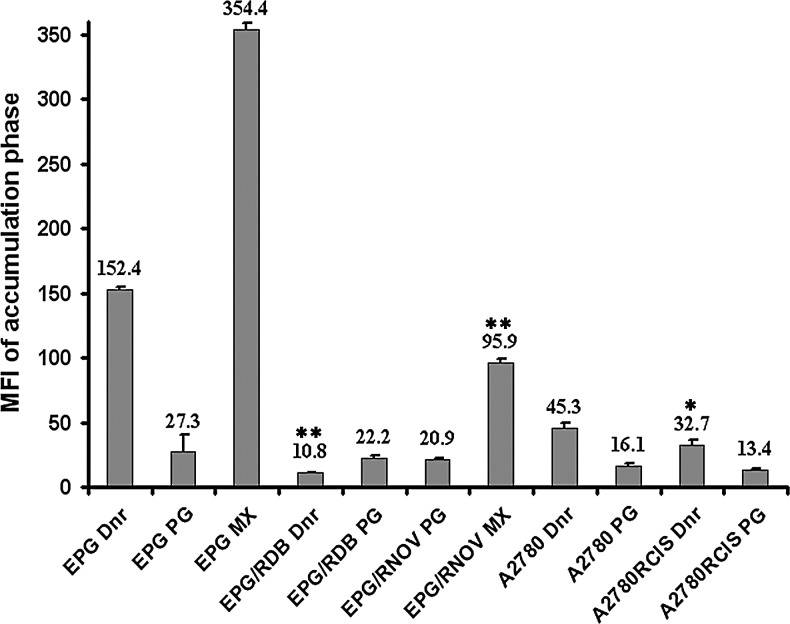
Prodigiosin uptake in MDR cells
The cell samples used in this experiment included the parental EPG85-257 (no MDR1, no BCRP), EPG85-257RDB (high levels of MDR1), EPG85-257RNOV (high levels of BCRP), A2780 (high levels of MRP1), and MRP1, 2 overexpressing A2780RCIS cells. As expected, the EPG85-257 and A2780 parent cells accumulated higher levels of mitoxantrone and daunorubicin after the uptake period than resistant cells (p<0.001 and p<0.05). EPG85-257RDB, EPG85-257RNOV, and A2780RCIS cells showed high levels of functionally active MDR1, BCRP, and MRP, as they accumulated much less mitoxantrone and daunorubicin than parental cells. By contrast, prodigiosin accumulated at similar levels in these resistant cells, compared with the parental cells (p>0.05). These results indicated that prodigiosin is not a substrate for MDR1, BCRP, and MRP (Fig. 4). Additional data are given in Supplementary Figures S1 and S2 (Supplementary Data are available online at www.liebertpub.com/dna).
FIG. 4.
FACS analysis of accumulation of daunorubicin, mitoxantrone, or prodigiosin in three individual experiments in a panel of parental and their resistant cell lines. Values are presented as the mean±SE. The symbols (
 ) and (
) and ( ) represent the mean fluorescence difference between parental and resistant cells with p<0.001 and p<0.05, respectively. Dnr, daunorubicin; PG, prodigiosin; MX, mitoxantrone.
) represent the mean fluorescence difference between parental and resistant cells with p<0.001 and p<0.05, respectively. Dnr, daunorubicin; PG, prodigiosin; MX, mitoxantrone.
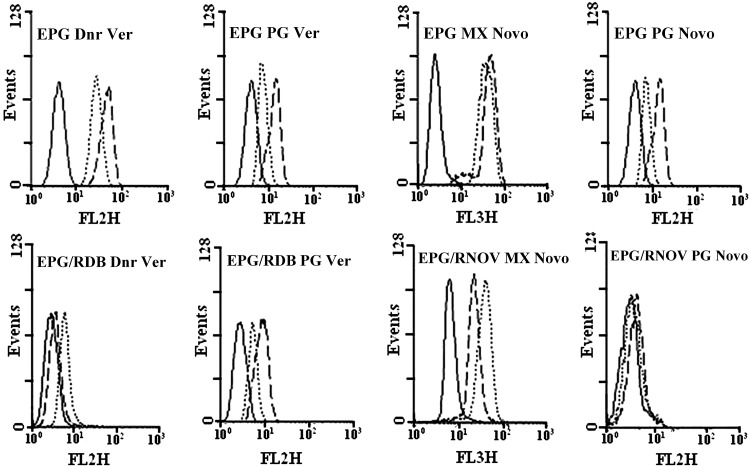
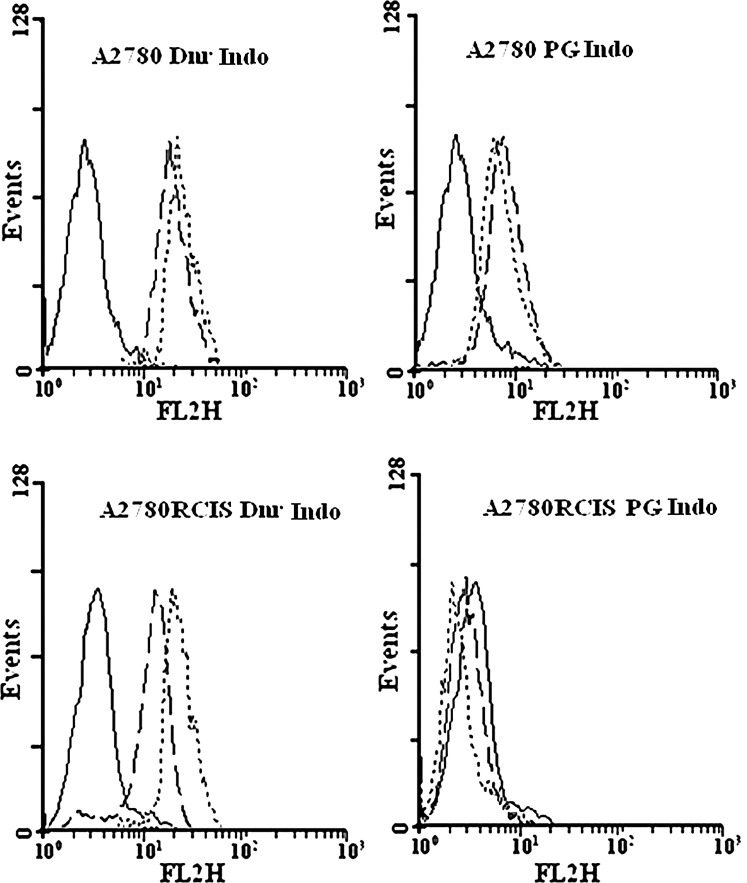
No prodigiosin transportation by MDR1, BCRP, and MRP
EPG85-257 parent cells do not express any of MDR1 or BCRP. Therefore, the specific MDR inhibitors like verapamil or novobiocin did not inhibit the efflux for daunorubicin, mitoxantrone, and prodigiosin, during the efflux period (Fig. 5). However, EPG85-257RDB, EPG85-257RNOV, A2780, or A2780RCIS cells showed high levels of functionally active MDR1, BCRP, MRP1, or MRP2 and after 60 min of the efflux period, the accumulation of the daunorubicin was enhanced in the presence of verapamil (p<0.01) or indomethacin (p<0.05 for A2780, and p<0.001 for A2780RCIS) and the accumulation of mitoxantrone was enhanced in the presence of novobiocin (p<0.05) (Figs. 5 and 6 and Table 2). In contrast, some decrease in fluorescence levels of prodigiosin was noted in the presence of verapamil, novobiocin, or indomethacin after the efflux period (Figs. 5 and 6 and Table 2). This decrease is not statistically meaningful except in EPG parental cell. It seems that the MDR pumps inhibitors compete with the cells' uptake of daunorubicin, mitoxantrone, or prodigiosin and lead to reduction of fluorescence after the efflux period (Table 2 and Supplementary Figs. S1 and S2). These results indicate that prodigiosin is not a substrate for MDR1, BCRP, or MRP. Therefore, it is unlikely that the efficiency of this antineoplastic agent will be affected by the presence of these MDR transporters in tumor cells.
FIG. 5.
Efflux analysis of daunorubicin, mitoxantrone, and prodigiosin in EPG85-257 cells. The upper panel is EPG85-257 parent cells and the lower panel is EPG85-257RDB and EPG85-257RNOV resistant cells. No drug (autofluorescence; solid line), fluorescence levels after 60 min of efflux in the absence (dashed line) or presence (dotted line) of the inhibitors. Ver, verapamil; Novo, novobiocin.
FIG. 6.
Efflux analysis of daunorubicin and prodigiosin in A2780 cells. The upper panel is A2780 parent cells and the lower panel is A2780RCIS resistant cells. No drug (autofluorescence; solid line), fluorescence levels after 60 min of efflux in the absence (dashed line) or presence (dotted line) of the inhibitor. Indo, indomethacin.
Table 2.
Mean Fluorescent Intensity of Prodigiosin, Daunorubicin, and Mitoxantrone in the Presence and Absence of Specific Multidrug Resistance Inhibitors After Efflux Period
|
Mean fluorescent intensity (MFI)±SE | |||||
|---|---|---|---|---|---|
| EPG85-257 | EPG85-257RDB | EPG85-257RNOV | A2780 | A2780RCIS | |
| Prodigiosin | 31.7±2.31 | 15.8±0.59 | 8.1±0.15 | 15.1±0.28 | 4.1±0.14 |
| Prodigiosin+Ver | 14.3±2.01 | 11.0±0.83 | ND | ND | ND |
| Prodigiosin+Indo | ND | ND | ND | 13.3±0.62 | 3.4±0.15 |
| Prodigiosin+Novo | 7.4±0.53 | ND | 6.7±0.35 | ND | ND |
| Daunorubicin | 134.1±4.11 | 5.6±0.61 | ND | 50.4±0.62 | 24.7±1.64 |
| Daunorubicin+Ver | 66.4±3.98 | 11.4±0.95 | ND | ND | ND |
| Daunorubicin+Indo | ND | ND | ND | 59.2±0.62 | 60.6±2.45 |
| Mitoxantrone | 185.5±5.12 | ND | 59.9±2.51 | ND | ND |
| Mitoxantrone+Novo | 111.9±2.91 | ND | 117.5±3.83 | ND | ND |
ND, not done.
Discussion and Conclusion
Since its discovery in 1960 (Rapoport and Holden, 1962), prodigiosin has been the subject of much anticancer research. These promising studies provided valuable information about prodigiosin and its mechanisms of action. For example, it was shown that in addition to the anticancer effects of prodigiosin, it can also circumvent drug resistance by inducing apoptosis via p53-dependent or independent mechanisms (Montaner et al., 2000). Activation of apoptotic factors in both the cytoplasmic and the mitochondrial pathway is an alternate method by which prodigiosin exerts anticancer effects and escapes drug resistance (Campas et al., 2003). Research has also shown that BCRP (a transporter involved in MDR mainly belongs to the family of ATP binding cassette (ABC) transporters) did not play a role in prodigiosin resistance (Soto-Cerrato et al., 2004). Among the various ABC-transporters, the following members have been recognized as the most significant for clinical MDR: the classical P-glycoprotein (P-gp, MDR1, or ABCB1), the breast cancer resistance protein (BCRP, ABCG2, MXR, or ABCP), and MRPs that represent the MDR-associated proteins (Glavinas et al., 2004). The present study was designed to prove and expand upon these studies by investigating a variety of ABC transporters. Different parental and resistant cell lines that overexpress three main classes of ABC transporters were chosen, and the cytotoxic effects of prodigiosin were investigated. These experiments were also performed for daunorubicin and mitoxantrone, the standard substrates for the ABC pumps. Although the cytotoxic effects of prodigiosin were less than those of cisplatin, daunorubicin, and mitoxantrone on the parental cells, no meaningful differences were observed for prodigiosin cytotoxicity on the resistant and parental cells. However, cytotoxicity of the aforementioned ABC pumps' substrates on their resistant lines dramatically differed from the cytotoxicity on the parental cells. FACS analysis of accumulation and efflux of prodigiosin in MDR1, BCRP, or MRP overexpressing cells also confirmed the results obtained from cytotoxicity of prodigiosin on the parental and their resistant cells. These data demonstrate that despite the ABC pumps' ability to effectively export daunorubicin or mitoxantrone, they could not efflux prodigiosin out of the human gastric carcinoma and human epithelial ovarian cancer cells. Thus, prodigiosin is not an ABC transporter substrate and could circumvent this mechanism of cancer drug resistance in these cells. Therefore, chemical or biological modifications of prodigiosin could uncover more potent derivatives, leading us closer to the ultimate goal of finding improved chemotherapeutics for the treatment and management of multidrug resistant cancers and reducing cancer mortality. In conclusion, these data suggest that prodigiosin is a novel potential apoptotic agent, which may be effective irrespective of the presence of MDR transporter molecules compared to routine anticancer agents like mitoxantrone, cisplatin, and daunorubicin.
Supplementary Material
Acknowledgments
The authors are grateful for financial support from Zanjan University of Medical Sciences (grant No. 19/3-3/3426). We also thank Professor Herman Lage (Charite Campus Mitte, Berlin, Deutschland) for his valuable help.
Disclosure Statement
No competing financial interests exist.
References
- Andreazza R. Pieniz S. Wolf L. Lee M.K. Camargo F.A. Okeke B.C. Characterization of copper bioreduction and biosorption by a highly copper resistant bacterium isolated from copper-contaminated vineyard soil. Sci Total Environ. 2010;408:1501–1507. doi: 10.1016/j.scitotenv.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Bennett J.W. Bentley R. Seeing red: the story of prodigiosin. Adv Appl Microbiol. 2000;47:1–32. doi: 10.1016/s0065-2164(00)47000-0. [DOI] [PubMed] [Google Scholar]
- Campas C. Dalmau M. Montaner B. Barragan M. Bellosillo B. Colomer D. Pons G. Perez-Tomas R. Gil J. Prodigiosin induces apoptosis of B and T cells from B-cell chronic lymphocytic leukemia. Leukemia. 2003;17:746–750. doi: 10.1038/sj.leu.2402860. [DOI] [PubMed] [Google Scholar]
- de Araujo H.W. Fukushima K. Takaki G.M. Prodigiosin production by Serratia marcescens UCP 1549 using renewable-resources as a low cost substrate. Molecules. 2010;15:6931–6940. doi: 10.3390/molecules15106931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elahian F. Kalalinia F. Behravan J. Dexamethasone downregulates BCRP mRNA and protein expression in breast cancer cell lines. Oncol Res. 2009;18:9–15. doi: 10.3727/096504009789745674. [DOI] [PubMed] [Google Scholar]
- Elahian F. Kalalinia F. Behravan J. Evaluation of indomethacin and dexamethasone effects on BCRP-mediated drug resistance in MCF-7 parental and resistant cell lines. Drug Chem Toxicol. 2010;33:113–119. doi: 10.3109/01480540903390000. [DOI] [PubMed] [Google Scholar]
- Elahian F. Sepehrizadeh Z. Moghimi B. Mirzaei S.A. Human cytochrome b5 reductase: structure, function, and potential applications. Crit Rev Biotechnol. 2012 doi: 10.3109/07388551.2012.732031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Francisco R. Perez-Tomas R. Gimenez-Bonafe P. Soto-Cerrato V. Gimenez-Xavier P. Ambrosio S. Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol. 2007;572:111–119. doi: 10.1016/j.ejphar.2007.06.054. [DOI] [PubMed] [Google Scholar]
- Giri A.V. Anandkumar N. Muthukumaran G. Pennathur G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol. 2004;4:11. doi: 10.1186/1471-2180-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavinas H. Krajcsi P. Cserepes J. Sarkadi B. The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv. 2004;1:27–42. doi: 10.2174/1567201043480036. [DOI] [PubMed] [Google Scholar]
- Gottesman M.M. Mechanisms of cancer drug resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- Harris A.K. Williamson N.R. Slater H. Cox A. Abbasi S. Foulds I. Simonsen H.T. Leeper F.J. Salmond G.P. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology. 2004;150:3547–3560. doi: 10.1099/mic.0.27222-0. [DOI] [PubMed] [Google Scholar]
- Kim D. Kim J.F. Yim J.H. Kwon S.K. Lee C.H. Lee H.K. Red to red - the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J Microbiol Biotechnol. 2008;18:1621–1629. [PubMed] [Google Scholar]
- Kowalski P. Surowiak P. Lage H. Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, MRP2, and BCRP. Mol Ther. 2005;11:508–522. doi: 10.1016/j.ymthe.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Lage H. Duarte N. Coburger C. Hilgeroth A. Ferreira M.J.U. Antitumor activity of terpenoids against classical and atypical multidrug resistant cancer cells. Phytomedicine. 2010;17:441–448. doi: 10.1016/j.phymed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Marbeuf-Gueye C. Salerno M. Quidu P. Garnier-Suillerot A. Inhibition of the P-glycoprotein- and multidrug resistance protein-mediated efflux of anthracyclines and calceinacetoxymethyl ester by PAK-104P. Eur J Pharmacol. 2000;17:207–216. doi: 10.1016/s0014-2999(00)00047-9. [DOI] [PubMed] [Google Scholar]
- Materna V. Liedert B. Thomale J. Lage H. Protection of platinum–DNA adduct formation and reversal of cisplatin resistance by anti-MRP2 hammerhead ribozymes in human cancer cells. Int J Cancer. 2005;115:393–402. doi: 10.1002/ijc.20899. [DOI] [PubMed] [Google Scholar]
- Mirzaei S.A. Yazdi M.T. Sepehrizadeh Z. Secretory expression and purification of a soluble NADH cytochrome b5 reductase enzyme from Mucor racemosus in Pichia pastoris based on codon usage adaptation. Biotechnol Lett. 2010;32:1705–1711. doi: 10.1007/s10529-010-0348-z. [DOI] [PubMed] [Google Scholar]
- Montaner B. Navarro S. Pique M. Vilaseca M. Martinell M. Giralt E. Gil J. Perez-Tomas R. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131:585–593. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner B. Perez-Tomas R. The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Targets. 2003;3:57–65. doi: 10.2174/1568009033333772. [DOI] [PubMed] [Google Scholar]
- Perez-Tomas R. Montaner B. Llagostera E. Soto-Cerrato V. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem Pharmacol. 2003;66:1447–1452. doi: 10.1016/s0006-2952(03)00496-9. [DOI] [PubMed] [Google Scholar]
- Rapoport H.H. Holden K.G. The synthesis of prodigiosin. J Am Chem Soc. 1962;84:635. [Google Scholar]
- Soto-Cerrato V. Llagostera E. Montaner B. Scheffer G.L. Perez-Tomas R. Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol. 2004;68:1345–1352. doi: 10.1016/j.bcp.2004.05.056. [DOI] [PubMed] [Google Scholar]
- Starr M.P. Grimont P.A. Grimont F. Starr P.B. Caprylate-thallous agar medium for selectively isolating Serratia and its utility in the clinical laboratory. J Clin Microbiol. 1976;4:270–276. doi: 10.1128/jcm.4.3.270-276.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hattum A. Hoogsteen I.J. Schlüper H.M. Maliepaard M. Scheffer G.L. Scheper R.J. Kohlhagen G. Pommier Y. Pinedo H.M. Boven E. Induction of breast cancer resistance protein by the camptothecin derivative DX-8951f is associated with minor reduction of antitumour activity. Br J Cancer. 2002;87:665–672. doi: 10.1038/sj.bjc.6600508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson N.R. Fineran P.C. Gristwood T. Chawrai S.R. Leeper F.J. Salmond G.P. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007;2:605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- Williamson N.R. Fineran P.C. Leeper F.J. Salmond G.P. The biosynthesis and regulation of bacterial prodiginines. Nat Rev Microbiol. 2006;4:887–899. doi: 10.1038/nrmicro1531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.