Abstract
Dedifferentiated human chondrocytes severely limit successful hyaline cartilage repair in clinical practice. The primary interest of this study is to evaluate the naturally obtained cell-derived matrix (CDM) as a physical microenvironment for chondrocyte re-differentiation. Once different cell types were cultured for 6 days and decellularized using detergents and enzymes, the fibroblast-derived matrix (FDM), preosteoblast-derived matrix (PDM), and chondrocyte-derived matrix (CHDM) were obtained. From scanning electron microscope observation, each CDM was found to resemble a fibrous mesh with self-assembled fibrils. Both the FDM and PDM showed a more compact matrix structure compared to the CHDM. For compositional analysis, sodium dodecyl sulfate–polyacrylamide gel electrophoresis displayed numerous matrix proteins, which were quite different from each CDM in quantity and type. Specific matrix components, such as fibronectin, type I collagen (Col I), and laminin, were detected using immunofluorescent staining. In addition, the water contact angle suggests that the FDM is more hydrophilic than the PDM or CHDM. The proliferation of rat primary chondrocytes growing on CDMs was better than those growing on a plastic coverslip (control) or gelatin. Meanwhile, synthesis of glycosaminoglycan (GAG) was more effective for passaged chondrocytes (P4) cultivated on CDMs, and the difference was significant compared to cells grown on the control or on gelatin. As for the gene expression of cartilage-specific markers, CDMs exhibited good chondrocyte re-differentiation with time: the dedifferentiating marker, Col I was restrained, whereas the ratio between Col II and Col I, and between aggrecan and Col I, as an indicator of re-differentiation, was greatly improved. In addition, immunofluorescence of Col II showed a very positive signal in chondrocytes cultivated for 2 weeks on the CDMs. In an additional study, when three-dimensional cell pellets made from either plate-grown or matrix-grown dedifferentiated chondrocytes (P5) were cultured for 4 weeks, the results of Safranin-O staining, immunohistochemistry of Col II, and total GAG assay suggested that matrix-grown cells were significantly better in the induction of chondrocyte re-differentiation, than those grown on the plate. This work suggests that the naturally occurring matrix, CDM, can provide a favorable surface texture for cell attachment, proliferation, and more importantly, a chondroinductive microenvironment for the re-differentiation of dedifferentiated chondrocytes.
Introduction
Articular cartilage is an avascular, hypocellular tissue with little capability of self-repair, once damaged by traumatic injuries or osteoarthritis. Currently, a variety of surgical approaches have been developed to manage and treat cartilage lesions: arthroscopic abrasion, subchondral drilling, perichondral, periosteal grafts, total knee arthroplasty, and autologous chondrocyte transplantation (ACT).1,2 Primarily focused on ACT as a regenerative medicine, monolayer culture is the gold standard for rapid cell expansion to obtain a sufficient number of therapeutic cells. During this process, however, chondrocytes dedifferentiate and lose their phenotype,3,4 and this has been a persistent problem in the mass production of cells. Successful ACT-based cartilage repair is closely associated with the capability of transplanted chondrocytes to maintain their phenotype and to synthesize a hyaline cartilage-like extracellular matrix (ECM). Therefore, a proper remedy is necessary for the prevention of dedifferentiation of chondrocytes or to maintain a differentiation potential during in vitro cell expansion.
Numerous studies have investigated different ways of stimulating chondrocyte re-differentiation, focusing mainly on a proper substrate in which chondrocytes directly attach and proliferate. The use of ECM proteins, such as collagen and gelatin, or synthetic polymers was able to help passaged chondrocytes regain their chondrogenic characteristics. Kino-Oka et al. showed that a type I collagen (Col I)-coated substrate offered a monolayer culture system in expanding chondrocytes with a differentiation potential.5 Yang et al. documented that dedifferentiated human articular chondrocytes re-differentiated on a chemically crosslinked copolymer hydrogel surface.6 Another approach suggests that medium supplements, for example, the fibroblast growth factor 2 or insulin-transferrin-selenium, can contribute to protecting chondrocyte phenotypes during expansion in a monolayer culture.7,8 On the other hand, culturing cells in a three-dimensional (3D) environment has been considered effective in suppressing the phenotypic change of chondrocytes. Encapsulation of dedifferentiated chondrocytes in alginate beads or the collagen gel is a common method in re-differentiating cells by promoting the synthesis of cartilage matrix proteins.9,10 Chondrocyte culture in 3D, however, leads to a low rate of cell division. In addition, there are many reports that a dynamic culture environment is also helpful in maintaining the chondrocyte phenotype. A spinner-flask culture is useful in the induction of re-differentiation of dedifferentiated chondrocytes, or in the expansion of microcarrier-attached chondrocytes.11,12 Furthermore, a hypoxic culture condition with a low oxygen content is also believed to be a factor in promoting chondrocyte re-differentiation.13
In this study, we obtained cell-derived matrices (CDMs) from in vitro-cultured cells—fibroblasts, preosteoblasts, and chondrocytes—and utilized them as a culture template to evaluate the effect on the induction of re-differentiation of dedifferentiated chondrocytes in a 2D monolayer culture and 3D pellet culture. The hypothesis is that these CDMs can provide chondrocytes with a favorable surface condition for cell adhesion and proliferation, and more importantly, a naturally developed matrix microenvironment may act as a physical cue of cell signaling in upregulating chondrocyte re-differentiation. In fact, most cells of multicellular organisms interact with neighboring ECM molecules, and such an in vivo ECM environment possesses both physical and chemical signals that are critical for cell adhesion, migration, differentiation, and apoptosis.14,15 The mechanism of how ECM can regulate cell behavior is still poorly understood due to the complexity of cell-ECM or ECM-ECM interaction. The present CDM showed a distinct surface texture formed by self-assembled nanofibrils. These matrices retained key ECM proteins, such as fibronectin (FN), Col I, and laminin, and greatly improved chondrocyte attachment and proliferation. Evidence of chondrocyte re-differentiation was supported in the upregulating gene expression of cartilage-specific markers, production of glycosaminoglycan (GAG), and biosynthesis of Col II. More interestingly, when chondrocytes were subcultured on a large scale, using either a matrix-covered plate or tissue culture plastic, and those cells were subjected to a cell pellet culture, the results of the 3D pellet culture was in good agreement with those of the 2D monolayer culture. This work will significantly enhance our understanding of dedifferentiated chondrocyte responses to matrix-based culture platforms in both 2D and 3D configurations.
Materials and Methods
Preparation of CDM
NIH3T3 fibroblasts (ATCC) and rat primary chondrocytes were separately loaded at the cell density of 2×104 cells/cm2 into a plastic coverslip, and were cultured for 6 days in the Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. MC3T3 preosteoblasts (ATCC) were also cultured in the alpha minimum essential medium (α-MEM) containing 10% FBS and 100 U/mL penicillin and 100 μg/mL streptomycin. At the time of confluence, the cell-loaded coverslips were washed twice with phosphate-buffered saline (PBS), incubated briefly in a detergent solution containing 0.25% Triton X-100 and 10 mM NH4OH at 37°C, and were then treated with 50 U/mL DNase I and 50 μg/mL RNase A (Invitrogen) for 2 h. After the decellularization process, the specimens were washed, treated with 0.1 M glycine in PBS, and were stored at −20°C before use. The resultant CDM was assigned: fibroblast-derived matrix (FDM), preosteoblast-derived matrix (PDM), and chondrocyte-derived matrix (CHDM).
Characterization of CDM
Surface morphology of each CDM was observed using a scanning electron microscope (SEM; Hitachi S-410, Tokyo, Japan). Each CDM was rinsed twice with PBS, fixed with 3% ρ-formaldehyde (pH 7.4) for 30 min, dehydrated in a series of different concentrations of ethanol and air-dried. The samples were put on the metal stub and were sputter-coated with gold–palladium and then examined using SEM. With the SEM image on hand, the pore size in the matrix and matrix fibril thickness were manually calculated using image analysis software (ImageJ program, NIH). Five different areas were randomly selected on each CDM, and individual fibril thickness was determined using the scale bar as shown in SEM, the values for which were then averaged. The same rule was applied to the pore size on average that was determined using multiple pore areas (n=5) over the matrix. For the analysis of matrix proteins, sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was conducted. Samples of each matrix were soluble in the SDS-PAGE sample buffer: 125 mM Tris-HCl (pH 6.8), 10% glycerol, 1% β-mercaptoethanol, 2% SDS, and 2M urea. Once the supernatants were collected after centrifugation at 18,000 g for 10 min, the protein concentration was determined by Bradford assay. As the samples were loaded and run in a 10% polyacrylamide gel until the bromophenol blue dye marker reached the bottom of the gel, the gel was taken out from the vertical slab gel apparatus (Bio-Rad Laboratories, Inc.), and was stained with a coomassie-blue or silver staining kit (Sigma-Aldrich).
In addition, to identify specific components in the matrix, immunofluorescent staining of each CDM was carried out using primary antibodies of Santa Cruz Biotechnology: mouse monoclonal anti-FN (SC-8422), goat polyclonal anti-Col I (SC-25974), goat polyclonal anti-Col II (SC-7763), and goat polyclonal anti-laminin (SC-16588), diluted in 1% bovine serum albumin (BSA) (1:50). Each sample was incubated with primary antibodies overnight at 4°C, washed several times, and was then incubated for 1 h with a secondary antibody: Alexa Fluor® 488-conjugated goat anti-mouse IgG (A11017; Invitrogen) or Alexa Fluor 594-conjugated donkey anti-goat IgG (705-516-147; Jackson Immunoresearch), diluted in 1% BSA (1:200). The fluorescent image was visualized using a confocal microscope (FluoView™ FV1000; Olympus). The decellularized matrix was also examined using DNA-specific 4′,6-diamidino-2-phenylindole (DAPI; Sigma) staining. Meanwhile, the water contact angle of CDM was also examined using a contact angle machine (VCA Optima XE Video Contact Angle System; Crest Technology Pte Ltd.) (n=3, each).
Isolation of rat chondrocytes and in vitro culture
Hyaline articular cartilage was harvested from the femoral condyles in the knee of 6-week-old male Wistar rats (130–150 g). The animal experiment was conducted under ethical approval from the institute (KIST). Once the condylar cartilage was cut into thin slices, they were washed with PBS several times and incubated with moderate shaking overnight using 0.1% (w/v) collagenase (Worthington Biochemical Corp.) supplemented with 5% FBS. After the cartilage pieces were fully digested, the chondrocyte suspension was filtered using a nylon cell strainer (BD) and was centrifuged at 250 g for 10 min. The cell pellet was then resuspended in the DMEM and centrifuged again. The resuspended cells were then seeded on a culture plate with the DMEM supplemented with 10% FBS and 1% P/S and were incubated in a humidified incubator at 37°C with 5% CO2. The culture medium was replaced twice each week. When chondrocytes reached 80% confluence, they were harvested using 0.25% trypsin/EDTA solution (Gibco BRL), washed with PBS, counted, and replated. A subculture of rat chondrocytes was repeated several times to not only obtain sufficient number of cells, but also to induce chondrocyte dedifferentiation.
Examination of chondrocyte morphology and proliferation
For the comparison of early cell morphology, chondrocytes were seeded on different substrates: plastic coverslip (control), gelatin, FDM, PDM, and CHDM, and were imaged using Field emission scanning electron microscopy at 3 and 24 h postseeding, respectively. For gelatin coating, gelatin type B (G-9391; Sigma) from bovine skin was dissolved in distilled water, and the solution (0.5%, w/v) was sterilized in an autoclave at 120°C for 15 min. Coverslips were added with a 2 mL gelatin solution and were incubated for more than 15 min at 37°C. After the remaining gelatin solution was removed, the gelatin-coated coverslip was washed with PBS twice before cell seeding. The cell-loaded specimens were washed with PBS, fixed with 2.5% glutaraldehyde, then dehydrated using 70%, 80%, 90%, and 100% alcohol, respectively, and air-dried before observation. Meanwhile, to evaluate cell proliferation with time, chondrocytes (P4) were put on each substratum at a density of 5000 cells/cm2 and a chondrogenic medium was added: the DMEM supplemented with 5% FBS, 1% ITS+ premix (6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 μg/mL selenium, 5.33 μg/mL linoleic acid, and 1.25 μg/mL BSA), 50 μg/mL ascorbate-2-phosphate, 100 μg/mL sodium pyruvate, 40 μg/mL proline, 100 U/mL penicillin, and 100 μg/mL streptomycin. Initial cell attachment was counted at 6 h postseeding and a cell proliferation assay was performed using a Cell Counting Kit-8 (CCK-8; Dojindo) at 1 and 2 weeks, respectively. A sample aliquot of 100 μL was transferred to a 96-well plate and a 10% CCK-8 solution was then added to each well and incubated for 2 h at 37°C before absorbance measurement at 450 nm using a micro plate reader (Thermo Scientific).
Evaluation of chondrocyte re-differentiation
To assess the re-differentiation of passaged chondrocytes (P4), they were seeded at 5×103 cells/cm2 onto each substrate and cultivated for up to 2 weeks in the chondrogenic medium. Each sample was recovered at specific time points. For histological staining of proteoglycans, specimens were fixed in 10% buffered formalin for 2 days, rinsed with PBS, and were then subjected to a routine protocol of Safranin-O staining using Safranin-O (0.1%, w/v) and Fast green solution (0.001%, w/v). In addition, total GAG contents for different sets of the chondrocyte-loaded samples were also determined by using the 1,9-dimethylmethylene blue (DMB) (Serve) method,16 with the use of shark chondroitin sulfate C (Sigma) as a standard. When an aliquot of each sample was gently mixed with a fresh DMB solution in a glass tube, the mixture was immediately transferred to a quartz cuvette, and the absorbance was read using a plate reader at 530 nm wavelength. In addition, gene expression of cartilage-specific markers was investigated using total RNA of each sample isolated from the cultured chondrocytes following the manufacturer's instruction (TRIzol® RNA Isolation Reagents; Invitrogen). Each RNA pellet was washed with 75% ethanol and was dried before dissolving in RNase- and DNase-free distilled water. The RNA sample was then reverse transcribed for 60 min at 45°C, and then for 5 min at 95°C using a Maxime RT PreMix Kit (Intron). Target genes of polymerization chain reaction were type I, II, X collagen, and aggrecan, along with a housekeeping gene, glyceraldehydes-3-phosphate dehydrogenase (GAPDH). The primers used in this study are listed in Table 1. The amplification profile consisted of denaturation at 95°C for 30 s, annealing at 50°C for 40 s, and extension at 72°C for 40 s. The number of cycles was 17 for Col I and 30 for the other markers. The amplification product (5 μL) of each PCR reaction was separated in a 1.2% agarose gel, stained with SYBR Green, and visualized by ultraviolet exposure. The band intensity in the gel image was analyzed using a Gel dock system (ATTO E-graph, AE-9000; Takara), which was equipped with CS Analyzer 3 program. As for an indication of chondrocyte re-differentiation, the ratio between Col II or aggrecan and Col I was determined based on the intensity of each band determined by the CS Analyzer program.
Table 1.
Sequences of PCR Primers of Target Marker Genes
| Target gene | Primer (5′-3′) | bp |
|---|---|---|
| GAPDH | Forward: atggtgaaggtcggtgtgaacg | 560 |
| Reverse: tccacagtcttctgagtggcag | ||
| Collagen type I | Forward: tgacgcatggccaagaagaca | 315 |
| Reverse: ccatcatctccgttcttgcca | ||
| Collagen type II | Forward: tcatcgccacggtcctacaatg | 623 |
| Reverse: acctctgtgacccttgacacca | ||
| Collagen type X | Forward: gagattctgtacaacaggcagc | 307 |
| Reverse: atcctgagaaggacgagtgga | ||
| Aggrecan | Forward: ccagaccatgacaactcactg | 480 |
| Reverse: gatgatggcgctgttctgaag |
GAPDH, glyceraldehydes-3-phosphate dehydrogenase.
Immunofluorescent staining of Col II
A hyaline cartilage maker protein Col II was examined using immunofluorescence. Chondrocyte (P4)-seeded samples were recovered at 2 weeks, washed three times with PBS, and blocked for 1 h with 3% BSA. After they were incubated overnight with a mouse monoclonal anti-Col II (CP18; Calbiochem) (1:50) at 4°C, the samples were washed three times with PBS, incubated for 1 h with Alexa Fluor 488 goat anti-mouse IgG (A11017; Invitrogen) (1:200) at room temperature, and then rinsed with PBS. The fluorescence-tagged samples were further subjected to DAPI staining for nucleic detection of chondrocytes. The fluorescent signal of cell nuclei and Col II was visualized using a confocal microscope.
Pellet culture of dedifferentiated chondrocyte
For further investigation of the effect of matrix on chondrocyte re-differentiation, the PDM was prepared on a large scale using a tissue culture plate (TCP; 10 cm in diameter), following the same protocol as that mentioned earlier. The matrix-deposited TCP is assigned as a PDM plate. Once primary rat chondrocytes were subcultured to passage 3 (P3) using the TCP, expanded chondrocytes were divided into two groups, seeded on either TCP or PDM plates, and were then cultivated in the DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin. When cells were confluent, they were isolated and reseeded on the same condition. Once this process was repeated one more time, passaged chondrocytes (P5) were collected from TCP and PDM plates, and were then counted. For pellet cultures, suspended chondrocytes were obtained from TCP and PDM plates, respectively, and those cells (1×106) were then centrifuged in a 15-mL Falcon tube for 10 min at 1200 rpm. Subsequently, the tubes were placed in the incubator and each pellet was cultured for 4 weeks in the DMEM containing 10% FBS, 50 μg/mL ascorbate-2-phosphate, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Evaluation of chondrocyte re-differentiation
By the time pellets were recovered, the pellet size was compared between matrix-grown chondrocyte pellets (MGCP) and TCP-grown chondrocyte pellets (PGCP). Analyses, such as Safranin-O staining, immunohistochemistry, and DMB assay, were carried out to monitor the re-differentiation of chondrocytes in the two different 3D culture environments. Each specimen was fixed for 24 h in 10% neutral-buffered formalin (BBC Biochemical), embedded in paraffin, and cut into 4-μm sections, after which they were subjected to Safranin-O staining. For immunohistochemistry of Col II, endogenous peroxidase activity was inactivated using 3% hydrogen peroxide in methanol for 30 min. The thin sections were then washed with PBS and immunostained with a mouse monoclonal Col II (Santa Cruz Biotechnology, Inc.). The antigen–antibody complex was visualized using an avidin–biotin–peroxidase complex (ABC) solution in the ABC kit (Invitrogen) with 3,3-diamino benzidine (Invitrogen). In addition, the total GAG content in each pellet was measured using the DMB method as described. Each sample was frozen in liquid nitrogen, and broken into tiny particulates that were then collected for DMB assay.
Statistical analysis
Data are expressed as mean±standard deviation. Statistical analysis was carried out using one-way analysis of variance with a post hoc, Newman–Keuls, using GraphPad Prismx 5 (GraphPad Software). In the statistics, one group was compared with the other groups at each time point or with the same group at different time points. Statistical significance was indicated in *p<0.05, **p<0.01, and ***p<0.001, respectively.
Results
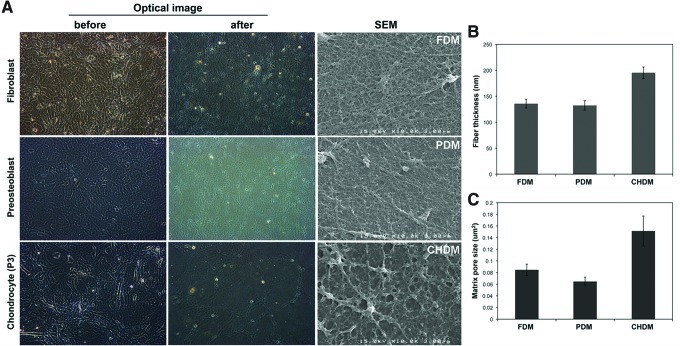
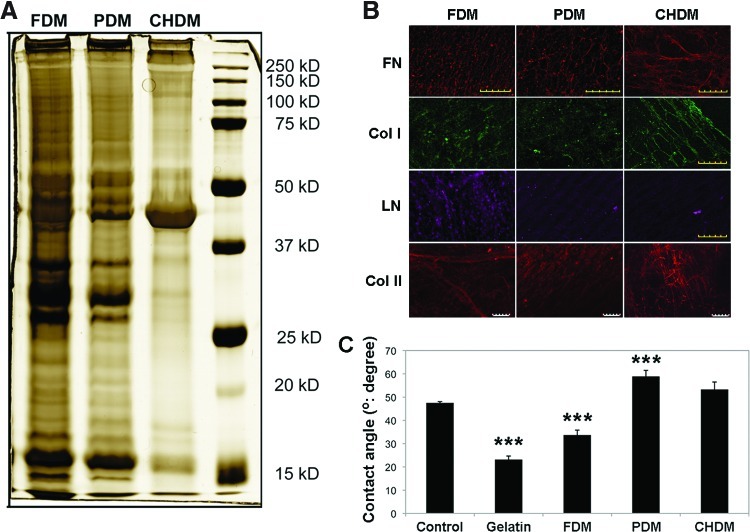
Optical images of fibroblasts, preosteoblasts, and chondrocytes presented a confluent morphology just before decellularization (Fig. 1A). After the process, distinct cell morphologies were removed, leaving a naturally derived matrix from each cell type. SEM images more clearly visualized the surface texture of each matrix—FDM, PDM, and CHDM. Each CDM looked like a fibrous mesh, with a number of fibrils interconnected. Interestingly, both the FDM and PDM exhibited a more compact matrix structure compared with the CHDM. When the thickness of matrix fibrils and pore areas were measured, they ranged from 100 to 200 nm and from 0.06 to 0.16 μm2, respectively (Fig. 1B, C). These parameters were fairly higher in the CHDM than those from the FDM and PDM. For compositional analysis, the image of SDS-PAGE displayed that there was a notable difference in the protein concentration and type as indicated by the band intensity and its location at each molecular marker (Fig. 2A). It seemed that matrix proteins were more diverse and abundant in the FDM, and in particular, the band pattern of the CHDM was significantly different from the other CDMs. In addition, to identify the type of specific ECM components, immunofluorescence of FN, Col I, Col II, and laminin (LN) exhibited that all of the fluorescent signals were positive over the matrix and some of the target proteins (Col I, LN) appeared to be relatively more abundant in the FDM (Fig. 2B). Measurement of water contact angle showed that the FDM (34°±2°) was more hydrophilic than the PDM (59°±2°) and CHDM (53°±3°), as well as the control (culture plastic) (47°±1°) (Fig. 2C). That of gelatin was 23°±2°.
FIG. 1.
Cell morphology before decellularization and surface morphology of each cell-derived matrix (CDM) after the process (A). Once distinct cell morphology was removed, scanning electron microscope (SEM) images clearly showed the surface texture of each matrix—fibroblast-derived matrix (FDM), preosteoblast-derived matrix (PDM), and chondrocyte-derived matrix (CHDM). Each CDM looked like a fibrous mesh, with a number of fibrils interwoven. Both FDM and PDM exhibited a more compact matrix structure compared with CHDM. When the thickness of fibrils and the matrix pore size were measured and averaged (n=5), they ranged from 100 to 200 nm and from 0.06 to 0.16 μm2, respectively (B, C). Color images available online at www.liebertpub.com/tea
FIG. 2.
Characterization of each CDM: sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (A), immunofluorescence (B), and water contact angle (C). The SDS-PAGE displayed that there was a notable difference in protein concentration and type, as indicated by the band intensity and its location at each molecular marker. In addition, specific extracellular matrix components were identified using immunofluorescence of fibronectin (FN), type I collagen (Col I), and LN. The fluorescent signal was positive over the matrix. The scale bar is 100 μm. Measurement of water contact angle showed that FDM was much more hydrophilic than other types of CDMs. Statistical significance (***p<0.001) compared to the control. Color images available online at www.liebertpub.com/tea
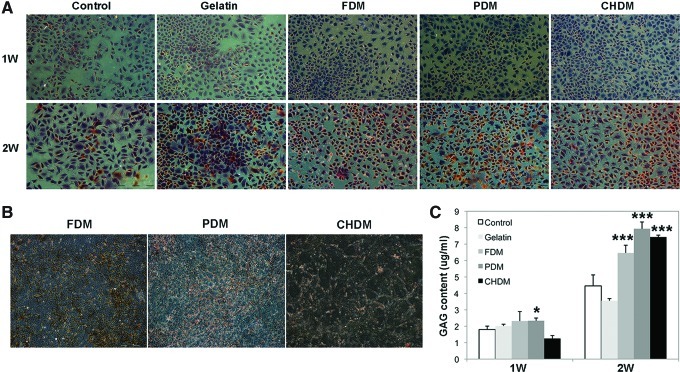
When early cell morphology of chondrocytes were examined at 3 and 24 h using SEM, cell morphology on the CDMs was more rounded compared with the cells on gelatin or on the control, but the difference of cell morphology was barely noticeable among CDMs (Fig. 3). Meanwhile, optical images of cell adhesion exhibited that the number of cells on the CDMs was very populated at day 3 compared to those of the control and gelatin specimens, suggesting that the matrix greatly improved cell division (Fig. 4A). When the proliferation of passaged chondrocytes was observed for 2 weeks, the results presented that each CDM was as effective as gelatin in expanding chondrocytes (Fig. 4B). When cell proliferation was evaluated, a significant difference (p<0.05) at 1 week was observed only between the control and FDM, but the difference at 2 weeks was even more significant (p<0.001) between the control and each CDM. As for the evaluation of cell re-differentiation from dedifferentiated chondrocytes (P4), Safranin-O staining of each group showed newly synthesized GAG around chondrocytes with time, and the positive stains appear to be mostly localized in a narrow pericellular region, as indicated by the orange color (Fig. 5A). In addition, the background of each CDM itself was negative for Safranin-O staining demonstrating that there was little pre-existing GAG in the matrix (Fig. 5B). Quantitatively, DMB assay revealed that the GAG contents of cell-growing matrices were comparable to each other at 1 week, but that the difference was obvious at 2 weeks: GAG deposition was much better with cells grown on the CDMs than those cultured on the control and gelatin (Fig. 5C). It was notable that the chondrocytes raised on the CHDM showed a 5-fold increase of GAG synthesis in between weeks 1 and 2.
FIG. 3.
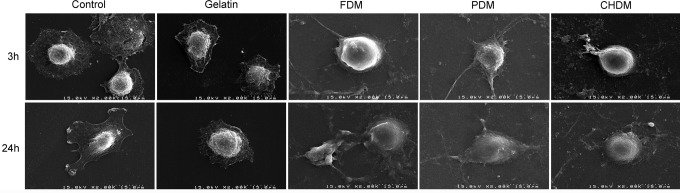
Observation of early chondrocyte morphology attached on different substrates by using SEM. Chondrocytes appeared more readily spread with time on the culture plastic (control) and gelatin-coated surface as compared to those on the CDMs. The cell morphology difference among CDMs was not found to be significant.
FIG. 4.
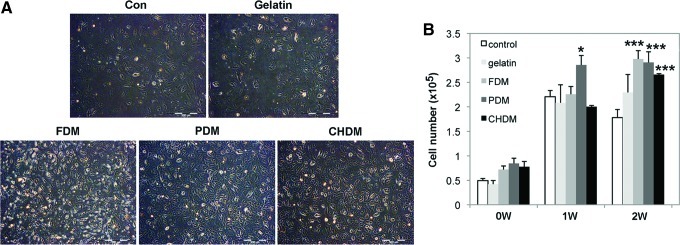
Cell adhesion and proliferation of chondrocytes on different substrates (n=3). Optical images displayed that the number of cells was more populated at day 3 on the CDMs than on the control or gelatin (A). When proliferation of passaged chondrocytes (P4) was investigated on the given substrates for 2 weeks, each CDM was as effective as gelatin in expanding chondrocytes. The difference was statistically significant between control and PDM at 1 week (*p<0.05) or CDMs at 2 weeks (***p<0.001) (B). Color images available online at www.liebertpub.com/tea
FIG. 5.
Histology and measurement of total glycosaminoglycan (GAG): Safranin-O staining (A, B) and total GAG content (C). The staining exhibited the newly synthesized proteoglycans around chondrocytes with time and the positive stains appear to be mostly localized in a narrow pericellular region, as indicated by the orange color (Magnification is ×40). In addition, a background staining of CDMs was negative, demonstrating that there were little pre-existing proteoglycans. Quantitative 1,9-dimethylmethylene blue assay revealed that the amount of total GAG was comparable each other at 1 week, but that the statistical difference was obvious at 2 weeks (***p<0.001) compared to the control. GAG deposition was much better with cells grown on the CDMs than those cultured on the control or on gelatin. Color images available online at www.liebertpub.com/tea
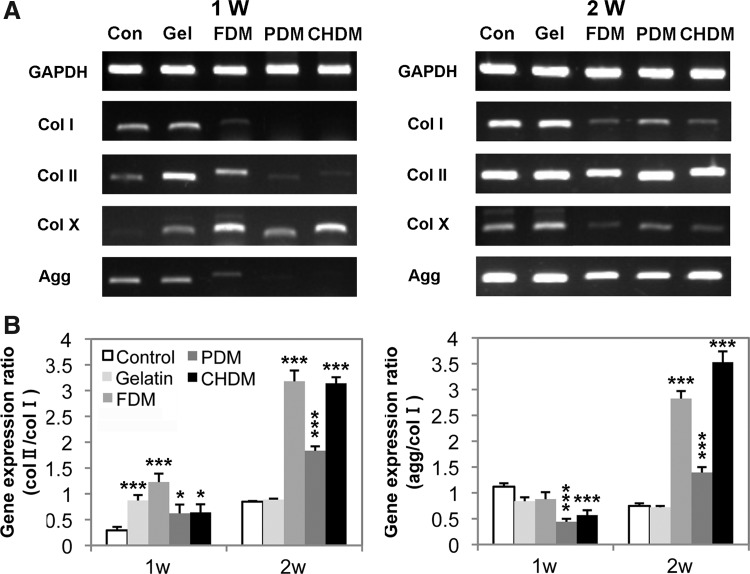
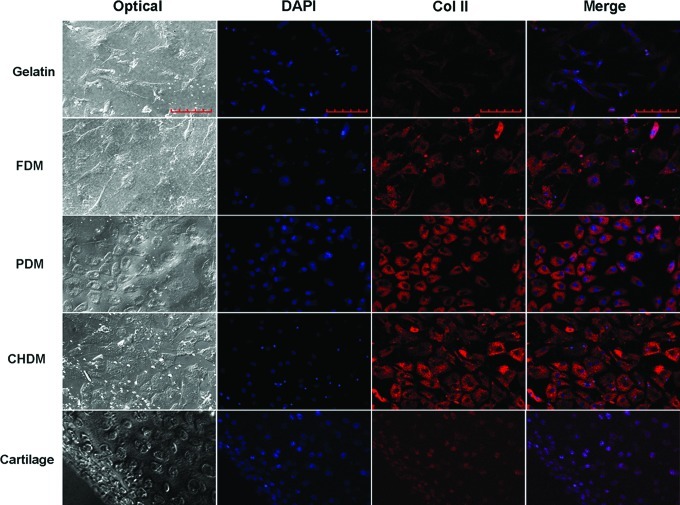
On the other hand, gene expression of cartilage-specific markers was also screened using passaged chondrocytes (P4). For the cells on the CDMs, the expression of differentiation markers, Col II and aggrecan, was greatly upregulated with time (Fig. 6A). In contrast, it was notable that the chondrocytes grown on the control and gelatin simultaneously exhibited a strong dedifferentiation marker (Col I) as well as the differentiation markers at 2 weeks. For a semiquantitative analysis of gene expression levels, the ratios of Col II to Col I or aggrecan to Col I as an indicator of chondrocyte re-differentiation significantly climbed with CDMs compared to those of control or gelatin, and the difference was statistically significant (Fig. 6B). Interestingly, those ratios for the FDM and CHDM remained in a relatively higher level over the PDM. Furthermore, the protein expression level of Col II was also investigated at 2 weeks postseeding of dedifferentiated chondrocytes (P4) using immunofluorescence (Fig. 7). As the target protein of Col II was visible as a red color, it was clear that the signal of gelatin was weaker than those of each CDM. The signal of a normal articular cartilage was also positive. Notably, when the images of DAPI (blue) and Col II were merged, Col II stains on the CDMs were largely detected in the cytoplasmic region of chondrocytes, based on its proximity to the DAPI-stained cell nuclei.
FIG. 6.
Gene expression of cartilage-specific markers (A) and plot of expression ratio between differentiation and dedifferentiation marker (B). For the cells on the CDMs, the expression of differentiating markers, Col II and aggrecan was greatly upregulated with time. It was notable that the chondrocytes grown on the control and gelatin simultaneously exhibited a strong dedifferentiation marker (Col I) as well as differentiation markers at 2 weeks. For a semiquantitative analysis of gene expression, the ratio of Col II to Col I, or of aggrecan to Col I, indicative of chondrocyte re-differentiation, significantly improved with CDMs than those of the control or gelatin. The difference was statistically significant, (*p<0.05, ***p<0.001) compared to the control.
FIG. 7.
Immunofluorescence of Col II at 2 weeks postseeding of dedifferentiated chondrocytes (P4). As the target protein was visible as a red color, it was clear that the signal of gelatin was weaker compared with each CDM. The signal of normal articular cartilage was also positive. Notably, when the images of DAPI (blue) and Col II were merged, Col II stains on the CDMs were largely detected in the cytoplasmic region of chondrocytes, based on its proximity to the DAPI-stained cell nuclei. The scale bar is 100 μm. Color images available online at www.liebertpub.com/tea
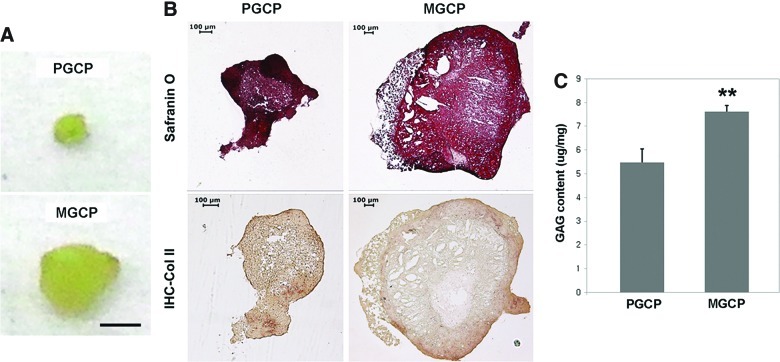
For a further investigation of the effect of CDMs on chondrocyte re-differentiation in a 3D environment, the cells were subcultured to P5 using either a culture plate or PDM, harvested, and were then subjected to a pellet culture for 4 weeks (details in Materials and Methods). When the size of each pellet was compared, the MGCP was significantly larger compared with PGCP (Fig. 8A). For histological analysis, both pellets showed that cartilage-specific GAG was distributed over the entire area of the pellet, as identified using Safranin-O staining, but the color intensity was quite different between the interior and peripheral area of the pellets (Fig. 8B). In addition, the immunohistochemistry of Col II revealed that MGCP had a better sign of cartilaginous matrix with more positive staining around the peripheral area compared to PGCP that showed a poorly organized matrix. The measurement of the GAG content also exhibited that matrix-cultured chondrocytes were able to synthesize and accumulate more GAG than plate-cultured ones, as the raw data were normalized by the wet weight of each pellet (Fig. 8C). The difference of the GAG content between MGCP and PGCP was statistically significant.
FIG. 8.
Comparative study of matrix-grown chondrocytes and plate-grown ones in pellet cultures. The size of the matrix-grown chondrocyte pellet (MGCP) was significantly larger compared with the plate-grown chondrocyte pellet (PGCP) (A). The scale bar is 1 mm. Both pellets showed that GAG was distributed over the entire area of the pellet, as identified using Safranin-O staining (B). The immunohistochemistry of Col II revealed that MGCP had a better cartilaginous matrix, with more positive staining around peripheral area compared to PGCP that showed a poorly organized matrix. Measurement of GAG content also revealed that matrix-cultured chondrocytes were able to synthesize and accumulate more GAG, and that the difference between MGCP and PGCP was statistically significant (**p<0.01) (C). Color images available online at www.liebertpub.com/tea
Discussion
The cell–matrix interaction is a prerequisite of normal cell function and survival. Therefore, the ECM structure, composition, physical, and mechanical properties are key factors in providing cells with proper adhesion sites, signaling factors, and architectural support. The purpose of this study is to evaluate whether the current matrix environment can provide an effective signaling cue in boosting dedifferentiated chondrocytes for the recovery of the cell phenotype. Upon the hypothesis that a naturally occurring matrix may be beneficial in chondrocyte re-differentiation, the present data demonstrate that the CDM can significantly upregulate the chondrogenic activity of dedifferentiated chondrocytes. The use of the CDM was not only determined to be beneficial in improving the expansion rate of chondrocytes, but was also found to be very competitive in promoting chondrocyte re-differentiation. Additionally, the results of using 2D matrix environments further supported those of 3D pellet culture with matrix-grown chondrocytes.
The current investigation harnessed CDMs obtained from different cell sources along with the test of culture plastic and gelatin-coated substrates. SEM images exhibited that cells were able to create a self-assembled texture of the ECM. In terms of fiber size and fiber assembly, the surface texture of the FDM was analogous to that of an electrospun nanofibrous polymer mesh, where the morphology of poly (L-lactic acid) nanofibers was randomly oriented and the diameter of fibers ranged from 400 to 600 nm,17 larger than present matrix fibers. In fact, the real tissue observed was a well-organized network of nano-sized fibrils. For a nanotopographical analysis of intact tissue, Liliensiek et al. found an ultrastructure of the vascular basement membrane that is composed of a complex meshwork consisting of pores and fibers in the submicron (100–1000 nm) and nanoscale (1–100 nm) range.18 Characterization of CDMs reveals that each CDM shares not only a compositional homology, but also a variation. A significant difference of matrix compositions in CDMs might be partly due to the different potentials of each cell type in matrix production and/or retention. For example, proteoglycans are supposed to be abundant, especially in the CHDM, but they are barely detectable in the polyacrylamide gel. There is also a chance that proteoglycans could leach out of the matrix during the decellularization process. The identified proteins in this study are FN, Col I, Col II, and LN, as confirmed by immunofluorescence. Macromolecule candidates in the matrix include vitronectin and Col IV, as detected by using Western blot analysis (data not shown). Early investigations showed that the ECM composition was quite different depending on the type of cells. For human fetal lung fibroblasts, versican, tenascin-C, decorin, FN, Col I, IV, and VI were the components in the CDM.19 Analysis of the marrow stromal cell-derived ECM found that FN, LN, Col I, III, decorin, and biglycan were the major components in the CDM.20
The advantages of the CDM appear to be due to the role of the matrix as a physical cue in stimulating chondrocyte re-differentiation. Although the nature of the signaling cue is hard to identify at this time, current results suggest that the matrix environment holds a very promising impact on chondrocyte differentiation compared to a conventional culture format that lacks a proper microenvironment. The fluorescent signal of the hyaline cartilage protein, Col II, was much stronger with the chondrocytes growing on the CDMs than those on gelatin (Fig. 7). The fact that the Col II signal on the CDMs was greater than that in natural cartilage is explained by the size difference of the cytoplasm in chondrocytes compared to chondrocytes in normal cartilage, as those on the CDMs have a larger cytoplasmic region that is positively stained for Col II. Therefore, the signal of Col II was stronger with CDMs compared with natural cartilage, which has a normal cytoplasmic size. Taken together, it should be addressed that deposition of Col II around chondrocytes is a more favorable indicative of chondrocyte re-differentiation, but the present result (Fig. 7) provides a weak evidence of Col II in the extracellular domain. Meanwhile, for the analysis of gene expression, even though the signal of Col II and aggrecan in CDMs is not quite different at 2 weeks compared to other groups, there is a notable increase of Col I expression in the control and gelatin specimens (Fig. 6). In fact, Col I expression is an indicative of chondrocyte dedifferentiation, whereas the relative suppression of Col I signal on the CDMs is a very encouraging indicator of chondrocyte redifferentiation. Furthermore, the ratios between Col II or aggrecan and Col I, an indicative of chondrogenic differentiation,21 also significantly increased with matrix-grown chondrocytes over time compared to control- or gelatin-cultured chondrocytes (Fig. 6B). It is recognized that while the use of real-time PCR may give a better quantitative indication of specific gene expression, many studies also use semiquantitative PCR. The results of gene expression are supported by the other experimental data, such as histology, DMB assay, and immunofluorescence. In addition, the finding that the PDM was inferior to the FDM or the CHDM in upregulating the gene expression of chondrogenic markers may partly be supported by an ongoing study of in vitro induction of chondrogenesis of human mesenchymal stem cells (hMSCs): the expression of chondrogenic genes was significantly upregulated in FDM-grown hMSCs compared to PDM-cultivated ones after 4 weeks of culture in the chondrogenic medium (data not shown). Since cells reside in extracellular environments that are unique, but different from each other in composition and structure, matrices obtained from fibroblasts or chondrocytes may present a more suitable microenvironment than the matrix derived from preosteoblasts. There are numerous reports of the use of collagen, gelatin, and alginate for chondrocyte culture, but few studies investigated the effect of the chondrocyte response using the ECM derived from in vitro cultured cells. Pei and He showed that the ECM deposited by synovium-derived stem cell (SDSC) enhanced chondrocytes re-differentiation using chondrocytes at P0, P2, and P6 in a pellet culture for 2 weeks.22 They adopted the ratio of CD 90 to CD 105 as a marker of re-differentiation capacity of dedifferentiated chondrocytes and considered the SDSC-derived matrix as a 3D environment. Hoshiba et al. prepared ECMs derived from chondrocytes, fibroblasts, and MSCs, and found that those ECMs exhibited little difference on chondrocyte differentiation.23
The benefit of a matrix was even more encouraging in the 3D environment of MGCP, without the use of any chondrogenic growth factors in the culture medium. The difference of pellet size was obvious between MGCP and PGCP. Biochemical, histological, and immunohistochemical analysis supported the re-differentiation potential of dedifferentiated chondrocytes in MGCP. It is plausible that the matrix components incorporated in the MGCP should play a role in determining a chondroinductive microenvironment for chondrocyte re-differentiation. On the other hand, upon the possibility that the cell type-dependent ECM may carry a different chondrogenic potential, we postulated that the CHDM might be more competitive than the FDM or PDM in eliciting the re-differentiation activity of dedifferentiated chondrocytes. The present data, however, indicated that it is difficult to draw a solid line regarding the chondrogenic activity of three different CDMs. This might be explained by the limitation of using 2D culture conditions, because it is generally accepted that chondrocyte re-differentiation favors a 3D environment. Further comparative study of MGCP using different types of matrices may provide an answer. In fact, previous reports emphasized the relationship between the matrix source and cell differentiation. Zhang et al. demonstrated that when the surface was coated with various decellularized ECM solutions, the specific ECM promoted the proliferation and differentiation of the cells of tissue origin.24 Philp et al. reported that ECMs facilitated the differentiation of embryonic stem cells into the differentiated cells, and that the structures were similar to the tissue from which the matrix was derived.25 For example, an extract of cartilage, containing multiple cartilage components prompted chondrogenesis in vivo.
Conclusions
In this study, the CDM was successfully prepared and characterized. CDMs were effective in creating a suitable microenvironment for dedifferentiated chondrocytes. The effect of CDMs on chondrocyte re-differentiation was positive, as supported by the results of histology, GAG content, gene expression of cartilage-specific markers, and synthesis of Col II. Interestingly, 3D pellet cultures of dedifferentiated chondrocytes were also very supportive of the data obtained from 2D matrix environments. In conclusion, naturally occurring matrix templates can offer a chondroinductive microenvironment in eliciting the re-differentiation of dedifferentiated chondrocytes.
Acknowledgments
This work was supported by the grant (#2011-0028796) from the National Research Foundation and by the grant 2E227 2E22710 (KIST) from the Ministry of Education, Science and Technology, Republic of Korea.
Disclosure Statement
No competing financial interests exist.
References
- 1.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2001;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 3.Darling E.M. Athanasiou K.A. Rapid phenotypic changes in passaged articular chondrocytes subpopulations. J Orthop Res. 2005;23:425. doi: 10.1016/j.orthres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Schnabel M. Marlovits S. Eckhoff G. Fichtel I. Gotzen L. Vecsei V. Schlegel J. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 5.Kino-Oka M. Yashiki S. Ota Y. Mushiaki Y. Sugawara K. Yamamoto T. Takezawa T. Taya M. Subculture of chondrocytes on a collagen type I-coated substrate with suppressed cellular dedifferentiation. Tissue Eng. 2005;11:597. doi: 10.1089/ten.2005.11.597. [DOI] [PubMed] [Google Scholar]
- 6.Yang J.J. Chen Y.M. Liu J.F. Kurokawa T. Gong J.P. Spontaneous redifferentiation of dedifferentiated human articular chondrocytes on hydrogel surfaces. Tissue Eng. 2010;16:2529. doi: 10.1089/ten.TEA.2009.0647. [DOI] [PubMed] [Google Scholar]
- 7.Martin I. Novakovic G.V. Yang J. Langer R. Freed L.E. Mammalian chondrocytes expanded in the presence of fibroblast growth factor 2 maintain the ability to differentiate and regenerate three-dimensional cartilaginous tissue. Exp Cell Res. 1999;253:681. doi: 10.1006/excr.1999.4708. [DOI] [PubMed] [Google Scholar]
- 8.Chua K.H. Aminuddin B.S. Fuzina N.H. Ruszymah B.H.I. Insulin-transferrin-selenium prevents human chondrocyte dedifferentiation and promotes the formation of high quality tissue engineered human hyaline cartilage. Euro Cell Mater. 2005;9:58. doi: 10.22203/ecm.v009a08. [DOI] [PubMed] [Google Scholar]
- 9.Masuda K. Sah R.L. Hejna M.J. Thonar E.J. A novel two-step method of the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi T. Ogasawara T. Asawa Y. Mori Y. Uchinuma E. Takato T. Hoshi K. Three-dimensional microenvironment retain chondrocyte phenotypes during proliferation culture. Tissue Eng. 2007;13:1583. doi: 10.1089/ten.2006.0322. [DOI] [PubMed] [Google Scholar]
- 11.Schrobback K. Klein T.J. Schuetz M. Upton Z. Leavesley D.I. Malda J. Adult human articular chondrocytes in a microcarrier-based culture system: expansion and differentiation. J Orthop Res. 2011;29:539. doi: 10.1002/jor.21264. [DOI] [PubMed] [Google Scholar]
- 12.Lee T.J. Bhang S.H. La W.G. Yang H.S. Seong J.Y. Lee H. Im G.I. Lee S.H. Kim B.S. Spinner-flask culture induces redifferentiation of dedifferentiated chondrocytes. Biotech Lett. 2011;33:829. doi: 10.1007/s10529-010-0488-1. [DOI] [PubMed] [Google Scholar]
- 13.Tan G.K. Dinnes D.L. Myers P.T. Cooper-White J.J. Effects of biomimetic surfaces and oxygen tension on redifferentiation of passaged human fibrochondrocytes in 2D and 3D cultures. Biomaterials. 2011;32:5600. doi: 10.1016/j.biomaterials.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 14.Hynes R. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berrier A.L. Yamada K.M. Cell-matrix adhesion. J Cell Phys. 2007;213:565. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 16.Farndale R.W. Buttle D.J. Barrett A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;833:173. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 17.Yin Z. Chen X. Chen J.L. Shen W.L. Hieu T.M. Nguyen L.G. Ouyang H.W. The regulation of tendon stem cell differentiation by the alignment of nanofibers. Biomaterials. 2010;31:2163. doi: 10.1016/j.biomaterials.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 18.Liliensiek S.J. Nealey P. Murphy C.J. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A. 2009;15:2643. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soucy P.A. Romer L.H. Endothelial cell adhesion, signaling, and morphogenesis in fibroblast-derived matrix. Matrix Biol. 2009;28:273. doi: 10.1016/j.matbio.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Lai Y. Sun Y. Skinner C.M. Son E.L. Lu Z. Tuan R.S. Jilka R.L. Ling J. Chen X. Reconstitution of marrow-derived extracellular matrix ex vivo: a robust culture system for expanding large-scale highly functional human mesenchymal stem cells. Stem Cell Dev. 2010;19:1095. doi: 10.1089/scd.2009.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arufe M.C. Fuente A.D. Fuentes I. Toro F.F. Blanco F.J. Chondrogenic potential of subpopulations of cells expressing mesenchymal stem cell markers derived from human synovial membranes. J Cell Biochem. 2010;111:834. doi: 10.1002/jcb.22768. [DOI] [PubMed] [Google Scholar]
- 22.Pei M. He F. Extracellular matrix deposited by synovium-derived stem cells delays replicative senescent chondrocyte dedifferentiation and enhances redifferentiation. J Cell Phys. 2012;227:2163. doi: 10.1002/jcp.22950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoshiba T. Lu H. Yamada T. Kawazoe N. Tateishi T. Chen G. Effects of extracellular matrices derived from different cell sources on chondrocyte function. Biotech Prog. 2011;27:788. doi: 10.1002/btpr.592. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y. He Y. Bharadwaj S. Hammam N. Carnagey K. Myers R. Atala A. Dyke M.V. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials. 2009;30:4021. doi: 10.1016/j.biomaterials.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Philp D. Chen S. Fitzerald W. Orenstein J. Margolis L. Kleinman H. Complex extracellular matrices promote tissue-specific stem cell differentiation. Stem Cells. 2005;23:288. doi: 10.1634/stemcells.2002-0109. [DOI] [PubMed] [Google Scholar]