Abstract
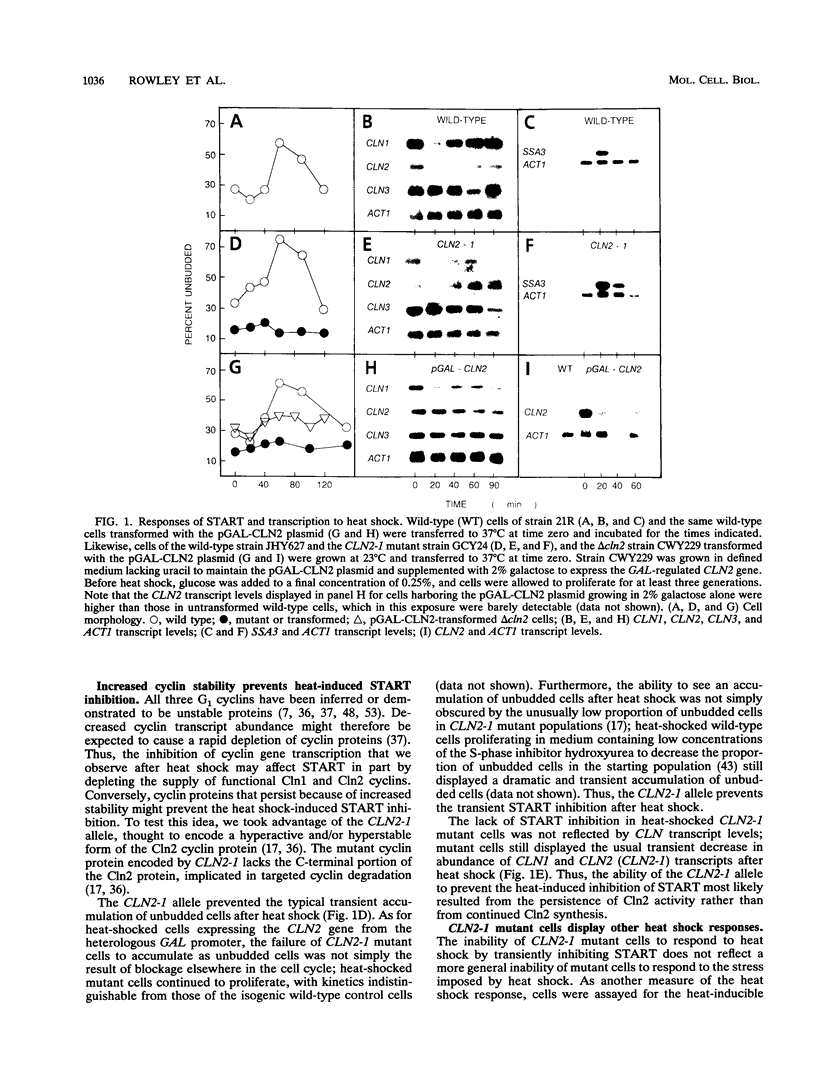
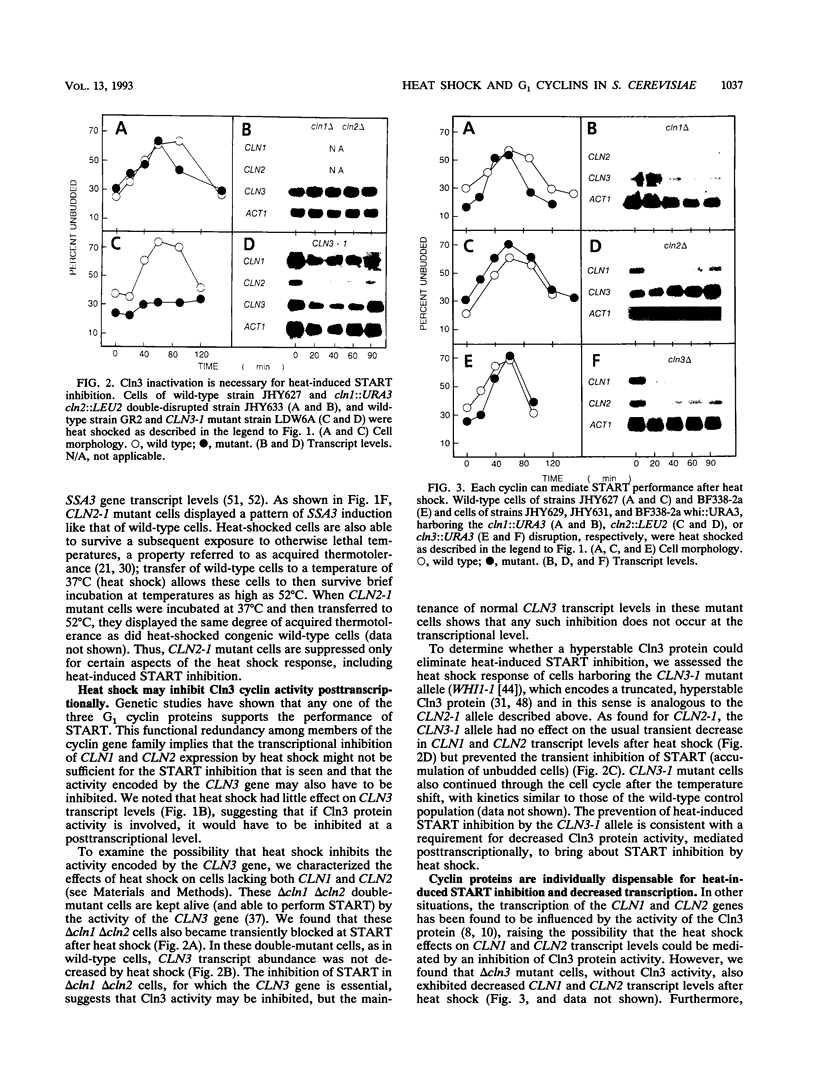
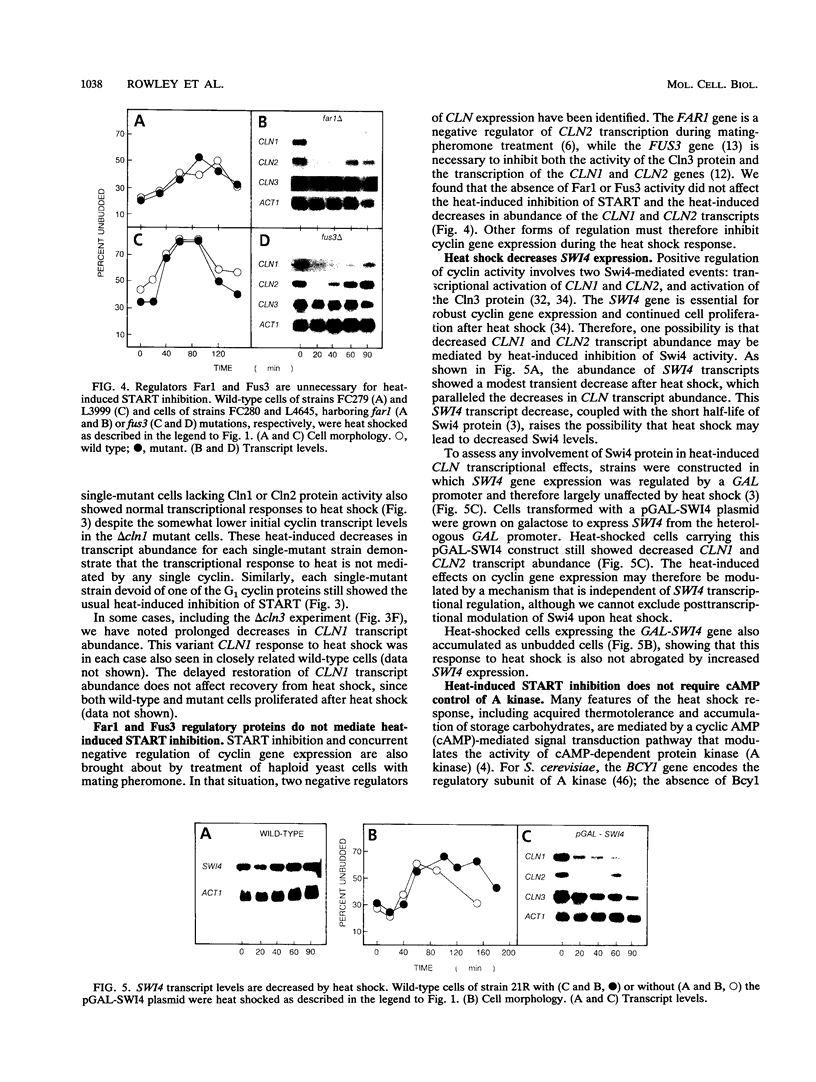
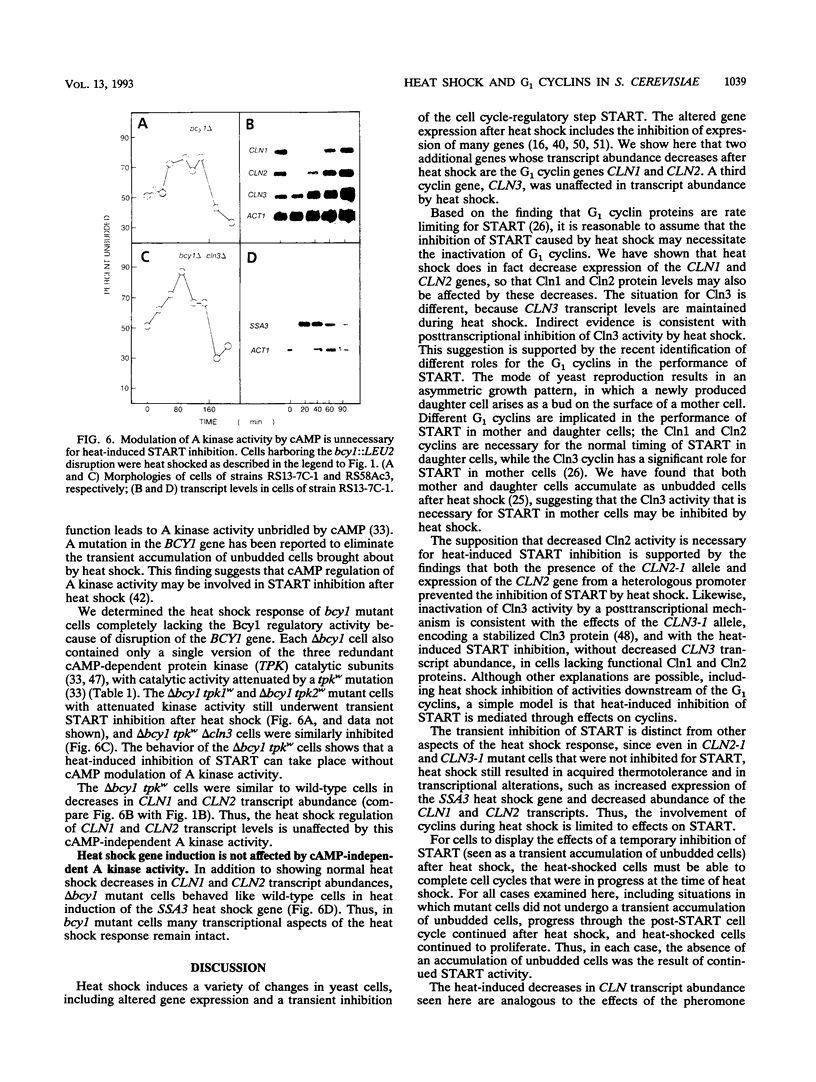
For cells of the yeast Saccharomyces cerevisiae, heat shock causes a transient inhibition of the cell cycle-regulatory step START. We have determined that this heat-induced START inhibition is accompanied by decreased CLN1 and CLN2 transcript abundance and by possible posttranscriptional changes to CLN3 (WHI1/DAF1) cyclin activity. Persistent CLN2 expression from a heterologous promoter or the CLN2-1 or CLN3-1 alleles that are thought to encode cyclin proteins with increased stability eliminated heat-induced START inhibition but did not affect other aspects of the heat shock response. Heat-induced START inhibition was shown to be independent of functions that regulate cyclin activity under other conditions and of transcriptional regulation of SWI4, an activator of cyclin transcription. Cells lacking Bcy1 function and thus without cyclic AMP control of A kinase activity were inhibited for START by heat shock as long as A kinase activity was attenuated by mutation. We suggest that heat shock mediates START blockage through effects on the G1 cyclins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Induction of galactokinase in Saccharomyces cerevisiae: kinetics of induction and glucose effects. J Bacteriol. 1972 Aug;111(2):308–315. doi: 10.1128/jb.111.2.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes C. A., Johnston G. C., Singer R. A. Thermotolerance is independent of induction of the full spectrum of heat shock proteins and of cell cycle blockage in the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4352–4358. doi: 10.1128/jb.172.8.4352-4358.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeden L., Mikesell G. E. Cell cycle-specific expression of the SWI4 transcription factor is required for the cell cycle regulation of HO transcription. Genes Dev. 1991 Jul;5(7):1183–1190. doi: 10.1101/gad.5.7.1183. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Deschenes R. J. The function of ras genes in Saccharomyces cerevisiae. Adv Cancer Res. 1990;54:79–139. doi: 10.1016/s0065-230x(08)60809-x. [DOI] [PubMed] [Google Scholar]
- Cannon J. F., Gitan R., Tatchell K. Yeast cAMP-dependent protein kinase regulatory subunit mutations display a variety of phenotypes. J Biol Chem. 1990 Jul 15;265(20):11897–11904. [PubMed] [Google Scholar]
- Chang F., Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990 Nov 30;63(5):999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- Cross F. R. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol Cell Biol. 1990 Dec;10(12):6482–6490. doi: 10.1128/mcb.10.12.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Tinkelenberg A. H. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell. 1991 May 31;65(5):875–883. doi: 10.1016/0092-8674(91)90394-e. [DOI] [PubMed] [Google Scholar]
- Denis C. L., Kemp B. E., Zoller M. J. Substrate specificities for yeast and mammalian cAMP-dependent protein kinases are similar but not identical. J Biol Chem. 1991 Sep 25;266(27):17932–17935. [PubMed] [Google Scholar]
- Dirick L., Nasmyth K. Positive feedback in the activation of G1 cyclins in yeast. Nature. 1991 Jun 27;351(6329):754–757. doi: 10.1038/351754a0. [DOI] [PubMed] [Google Scholar]
- Drebot M. A., Barnes C. A., Singer R. A., Johnston G. C. Genetic assessment of stationary phase for cells of the yeast Saccharomyces cerevisiae. J Bacteriol. 1990 Jul;172(7):3584–3589. doi: 10.1128/jb.172.7.3584-3589.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., Brill J. A., Fink G. R. FUS3 represses CLN1 and CLN2 and in concert with KSS1 promotes signal transduction. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9392–9396. doi: 10.1073/pnas.88.21.9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion E. A., Grisafi P. L., Fink G. R. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell. 1990 Feb 23;60(4):649–664. doi: 10.1016/0092-8674(90)90668-5. [DOI] [PubMed] [Google Scholar]
- Garrett S., Broach J. Loss of Ras activity in Saccharomyces cerevisiae is suppressed by disruptions of a new kinase gene, YAKI, whose product may act downstream of the cAMP-dependent protein kinase. Genes Dev. 1989 Sep;3(9):1336–1348. doi: 10.1101/gad.3.9.1336. [DOI] [PubMed] [Google Scholar]
- Garrett S., Menold M. M., Broach J. R. The Saccharomyces cerevisiae YAK1 gene encodes a protein kinase that is induced by arrest early in the cell cycle. Mol Cell Biol. 1991 Aug;11(8):4045–4052. doi: 10.1128/mcb.11.8.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Periodic density fluctuation during the yeast cell cycle and the selection of synchronous cultures. J Bacteriol. 1970 Dec;104(3):1280–1285. doi: 10.1128/jb.104.3.1280-1285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle K. J., Karamuz J. E., Leeper D. B. Induction of thermotolerance in Chinese hamster ovary cells by high (45 degrees) or low (40 degrees) hyperthermia. Cancer Res. 1978 Mar;38(3):570–574. [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. RNA synthesis and control of cell division in the yeast S. cerevisiae. Cell. 1978 Aug;14(4):951–958. doi: 10.1016/0092-8674(78)90349-5. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A. Ribosomal precursor RNA metabolism and cell division in the yeast Saccharomyces cerevisiae. Mol Gen Genet. 1980;178(2):357–360. doi: 10.1007/BF00270484. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Marini N. J., Reed S. I. Different G1 cyclins control the timing of cell cycle commitment in mother and daughter cells of the budding yeast S. cerevisiae. Cell. 1992 Apr 17;69(2):317–327. doi: 10.1016/0092-8674(92)90412-6. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- McAlister L., Finkelstein D. B. Heat shock proteins and thermal resistance in yeast. Biochem Biophys Res Commun. 1980 Apr 14;93(3):819–824. doi: 10.1016/0006-291x(80)91150-x. [DOI] [PubMed] [Google Scholar]
- Nash R., Tokiwa G., Anand S., Erickson K., Futcher A. B. The WHI1+ gene of Saccharomyces cerevisiae tethers cell division to cell size and is a cyclin homolog. EMBO J. 1988 Dec 20;7(13):4335–4346. doi: 10.1002/j.1460-2075.1988.tb03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K., Dirick L. The role of SWI4 and SWI6 in the activity of G1 cyclins in yeast. Cell. 1991 Sep 6;66(5):995–1013. doi: 10.1016/0092-8674(91)90444-4. [DOI] [PubMed] [Google Scholar]
- Nikawa J., Cameron S., Toda T., Ferguson K. M., Wigler M. Rigorous feedback control of cAMP levels in Saccharomyces cerevisiae. Genes Dev. 1987 Nov;1(9):931–937. doi: 10.1101/gad.1.9.931. [DOI] [PubMed] [Google Scholar]
- Ogas J., Andrews B. J., Herskowitz I. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell. 1991 Sep 6;66(5):1015–1026. doi: 10.1016/0092-8674(91)90445-5. [DOI] [PubMed] [Google Scholar]
- Penn M. D., Thireos G., Greer H. Temporal analysis of general control of amino acid biosynthesis in Saccharomyces cerevisiae: role of positive regulatory genes in initiation and maintenance of mRNA derepression. Mol Cell Biol. 1984 Mar;4(3):520–528. doi: 10.1128/mcb.4.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Richardson H. E., Wittenberg C., Cross F., Reed S. I. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989 Dec 22;59(6):1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- Rowley A., Singer R. A., Johnston G. C. CDC68, a yeast gene that affects regulation of cell proliferation and transcription, encodes a protein with a highly acidic carboxyl terminus. Mol Cell Biol. 1991 Nov;11(11):5718–5726. doi: 10.1128/mcb.11.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. E., Brown T. A., Trumpower B. L. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 1990 May 25;18(10):3091–3092. doi: 10.1093/nar/18.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D. Y., Matsumoto K., Iida H., Uno I., Ishikawa T. Heat shock response of Saccharomyces cerevisiae mutants altered in cyclic AMP-dependent protein phosphorylation. Mol Cell Biol. 1987 Jan;7(1):244–250. doi: 10.1128/mcb.7.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. A., Johnston G. C. Nature of the G1 phase of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):3030–3033. doi: 10.1073/pnas.78.5.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P. E., Goodey A. R., Carter B. L. Genes which control cell proliferation in the yeast Saccharomyces cerevisiae. Nature. 1980 Nov 27;288(5789):401–404. doi: 10.1038/288401a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA transferred or dotted nitrocellulose paper. Methods Enzymol. 1983;100:255–266. doi: 10.1016/0076-6879(83)00060-9. [DOI] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Scott J. D., McMullen B., Hurwitz M., Krebs E. G., Wigler M. Cloning and characterization of BCY1, a locus encoding a regulatory subunit of the cyclic AMP-dependent protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Apr;7(4):1371–1377. doi: 10.1128/mcb.7.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Cameron S., Sass P., Zoller M., Wigler M. Three different genes in S. cerevisiae encode the catalytic subunits of the cAMP-dependent protein kinase. Cell. 1987 Jul 17;50(2):277–287. doi: 10.1016/0092-8674(87)90223-6. [DOI] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Nash R., Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992 May;11(5):1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veinot-Drebot L. M., Johnston G. C., Singer R. A. A cyclin protein modulates mitosis in the budding yeast Saccharomyces cerevisiae. Curr Genet. 1991 Jan;19(1):15–19. doi: 10.1007/BF00362082. [DOI] [PubMed] [Google Scholar]
- Veinot-Drebot L. M., Singer R. A., Johnston G. C. Heat shock causes transient inhibition of yeast rRNA gene transcription. J Biol Chem. 1989 Nov 25;264(33):19473–19474. [PubMed] [Google Scholar]
- Werner-Washburne M., Becker J., Kosic-Smithers J., Craig E. A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989 May;171(5):2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Stone D. E., Craig E. A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jul;7(7):2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg C., Sugimoto K., Reed S. I. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell. 1990 Jul 27;62(2):225–237. doi: 10.1016/0092-8674(90)90361-h. [DOI] [PubMed] [Google Scholar]