Abstract
Purpose
Cell death is one of the most important endpoints of radiosensitivity. The tumor suppressor p53 participates not only in regulation of apoptosis, but also in autophagy mechanism. In this study, H1299-P53 (with wild-type p53) and H1299-175H (with mutant 175H) were used, and the effects of p53 on radiosensitivity were analyzed.
Methods
Cell models with different p53 status were established by gene engineering, and cell viability was examined by colony formation assay, and cell counting kit-8 (CCK-8), 3-Methyladenine, and Z-VAD were used to block autophagy and apoptosis, respectively. Western blot was used to detect protein expression; monodansylcadaverine (MDC) staining was used to analyze autophagy rate; DAPI/Propidium Iodide (PI) staining and flow cytometry were used to assess apoptosis and necrosis.
Results
In parental H1299, H1299-P53, and H1299-175H cells, radiosensitivity exhibited different by colony formation and CCK-8 assay (D0: 1.764 Gy, 1.407 Gy and 1.695 Gy; Dq: 2.977 Gy, 1.199 Gy and 2.312 Gy in turn). The radiosensitization of p53 was associated with the increase of MDM2 and P21 expression. The ionizing radiation (IR)-induced apoptosis was significant in H1299-P53 compared with in H1299 and H199-175H (p<0.05) by flow cytometry, and the expression of cleaved-caspase3 was increased in H1299-P53 cells. While the IR-induced autophagy was significant in H1299 cells (p<0.01) and decreased in H1299-P53 and H1299-175H cells (p<0.01) by MDC staining, the expression of MAPLC3II and Beclin-1 increased in H1299, but not in H1299-p53 and H199-175H cells. The IR-induced cell survival was significantly increased by Z-VAD-FMK and decreased by 3MA in H1299-P53 cells; IR- induced autophagy was significantly increased by Z-VAD-FMK in H1299-P53 cells (p<0.01), but not changed in H1299 cells.
Conclusion
p53 could regulate radiosensitivity by inhibiting autophagy and activating apoptosis; autophagy provides a prosurvival mechanism, and p53 potently abrogated the IR-induced autophagy, while mutant 175H shown no effect on radiosensitivity, suggesting that individual treatment strategies should be based on p53 status in patients.
Key words: p53, apoptosis, autophagy, lung cancer, radiosensitivity
Introduction
Lung cancer is still the most common cancer in worldwide; the mobility and mortality have been increasing and ranking first for decades.1 Non-small cell lung cancer (NSCLC) comprises 80%–85% of all lung cancers,2 and most patients present with metastasis, only ∼15% presenting with localized disease.3 The majority of NSCLC patients are not eligible for surgical resection, and ionizing radiation (IR) is one of the most commonly used and efficacious strategies.4 For radiation therapy, the radiosensitivity, representing the relative susceptibility of cells, tissues, or organs to the harmful effect of IR, determines the treatment efficacy. Cell death is the most important endpoint of radiosensitivity.
p53, a well-known tumor suppressor, becomes activated in response to a myriad of stresses, including DNA damage, oxidative stress, and IR, leading to diverse cellular responses, including cell cycle arrest, apoptosis, and senescence.5 It has been accepted that wt-p53 increases the sensitivity to radiation, but for mutants, the results are controversial.6 By searching the Chinese molecular epidemiology database, we found that most cancer-associated p53 mutations occur at exon 5, 6, 7, and 8, and p53 mutations occur mainly in or around amino acids 143, 175, 273, 281, etc. These mutants produce elevated levels of p53 protein, which has extended half-lives (1.5–7 hours) compared with wild-type p53 protein (20–30 minutes).7,8 175H mutant (Arg changes to His at position 175) has been documented to exert novel oncogenic functions, including the increase of tumorigenicity, metastatic potential, genomic instability, and therapy resistance of tumor cells.
Although apoptosis is the primary mechanism of radiation-induced cell death, an alternative cell death pathway, termed autophagic cell death (programmed cell death, type II), has emerged recently as an important mechanism of tumor cell death induced by radiation.9 Recently, increasing evidence proven the potential regulatory roles of p53 in the process of autophagy. More importantly, some investigations have demonstrated that the coregulation of both apoptosis and autophagy can participate in mammalian cell death,10 and apoptosis and autophagy may be interconnected and even simultaneously regulated by the same triggering factor.11 Under some circumstances, apoptosis and autophagy seem to be interconnected positively or negatively, and there might be a molecular switch between them. Undoubtedly, there are multiple connections between apoptotic and autophagic processes that can jointly seal the fate of tumor cells.12
In this study, we manage to elucidate the roles of p53 in the regulation of the radio sensitivity; if different p53 phenotypes would lead to different outcomes in the radiosensitivity or not, the results might contribute to the understanding of a potential regulatory mechanism of cell death induced by radiation and provide individual treatment aiming at p53 status and provide specific radiosensitizers for improving the efficacy of radiation therapy.
Material and Methods
Cell culture and transfection
H1299 cells were cultured at 37°C in a 5% CO2 incubator and maintained in the Dulbecco's modified Eagle's medium (DMEM; GIBCO) culture medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Invitrogen). To establish the H1299-p53 and H1299-175H stable cell lines, H1299 cells were transfected with pCDNA3.1-p53 and PCDNA3.1-175H plasmid constructed in our laboratory. Forty-eight hours post-transfection using Lipofectamine 2000 (Invitrogen), positive stable clones were selected by growing cells with G418 (400 μg/mL) for 2 weeks.
Reagents
FBS, Cell Counting Kit (CCK-8; Dojin Laboratories), and 3-Methyladenine (3-MA) monodansylcadaverine (MDC) were purchased from Sigma Chemical. Z-VAD was purchased from Enzo LifeSciences (Enzo). (Sigma-Aldrich, Inc.) Rabbit polyclonal antibodies against Bcl-2, caspase-3, MAPLC3, p53, and p21 were purchased from Abcam mouse polyclonal antibodies against Beclin 1,MDM2, GAPHD, AKT, and secondary antibodies were purchased from Santa Cruz Biotechnology.
Radiation
180-KVp X-ray generator (Model XSZ-Z20/20; Dandong) was utilized to deliver radiation at a dose rate of 0.41 Gy/min (200 kV; 18 mA).
Cell viability assay
CCK-8 (Dojin Laboratories) was used to detect living cells. Five thousand cells were seeded in a 96-well plate. Cells were treated with irradiation in 0GY, 4GY, and 8GY, and 16 hours later, 10 μL of a solution from CCK-8 was added for each well, and then the plate was incubated for 2 hours at 37°C in a humidified CO2 incubator. The absorbance was measured on a microplate reader (Synergy HT, Bio-Tek) at 450 nm. The percent of surviving cells at each concentration was plotted against the untreated group using the DMEM as a blank.
Colony formation assay
Cells were irradiated, trypsinized, counted, and plated into 60-mm Petri dishes using a standard culture medium, then irradiated by different doses (0, 1,2, 4, 6, and 8 Gy) using a 180-KVp X-ray generator at a dose rate of 0.41 Gy/min (200 kV; 18 mA). Two weeks later, cells were stained with crystal violet, and surviving colonies of >50 cells were scored under a dissection microscope. The surviving fraction for a given treatment dose was calculated as the plating efficiency of the irradiated samples relative to that of the sham-irradiated ones. For each dose level in three groups, three independent experiments were done. Multitarget click model of GraphPad Prism 5.0 (Systat Software) was used to fit cell survival curves. The dose quasithreshold (Dq) and mean lethal dose (D0) were calculated.
Apoptosis assay
The morphological observation of apoptosis was measured by DAPI staining. Cells were seeded in 6-well flat-bottomed plate, and the cells were treated with or without irradiation, and 16 hours later, cells were fixed with 4% paraformaldehyde for 10 minutes at room temperature. After cells were washed with phosphate-buffered saline (PBS) for three times, 1 μL DAPI (1 μg/mL) was added for 5 minutes, and then cells were visualized by fluorescence microscopy (Olympus IX71). Cells with condensed chromatin and fragmented nuclei were taken as apoptotic cells.
Apoptotic cells were also detected by flow cytometry (FCM) using FACScan (BD Biosciences). An annexin V/propidium iodide (PI) detection kit (Invitrogen) was used according to the manufacturer's protocol. Briefly, 16 hours after irradiation, 0.5×106 cells were incubated at room temperature with annexin V and PI for 30 minutes. Data were analyzed with CellQuest (BD Biosciences) and FlowJo software (Tree Star Inc.).
Autophagy assay
Cells were cultured on coverslips, and autophagic vacuoles were labeled with 0.05 mM MDC in PBS at 37°C for 10 minutes. Cells were then washed three times with PBS and immediately analyzed by fluorescence microscopy using an inverted microscope (Nikon Eclipse TE 300) equipped with a filter system (excitation filter V-2A: 380–420 nm, barrier filter: 450 nm). Images were obtained with a CCD camera (Orca I, Hamamatsu) and processed using the program Meta View, version 4.5 (Universal Images Corporation). 3-MA was added 2 hours before irradiation. The percentage of MDC-positive cells expressing punctuate staining were analyzed under a fluorescence microscope.
Western blot analysis
The total proteins were extracted with an RIPA lysis buffer [HEPES (50 mM), NaCl (150 mM), EDTA (1 mM), EGTA (2.5 mM), NaF (10 mM), DTT (1 mM), SV (1 mM), PMSF (1 mM), NP-40 (1%), sodium dodecyl sulfate (SDS; 0.1%)], and 2 mL aliquot was mixed with 20 μL protease inhibitor cocktail. Unless indicated, 40 μg of total protein was separated by SDS–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane, and analyzed by immunoblotting using the chemiluminescence (Santa Cruz). The primary antibodies used were DRAM1 (1:300; Abcam), LC3 I/II (1:300), and CASPASE-3 (1:500; Cell Signaling), GAPDH (1:1000), BECN1 (1:1000), and peroxidase-conjugated anti-mouse IgG or peroxidase-conjugated anti-rabbit IgG (1:1000; Santa Cruz). The intensity of protein bands was quantified using ImageJ software, and the ratio of specific band to control was analyzed.
Statistical analysis
All the presented data and results were confirmed in three independent experiments. The data are expressed as means±SD. Statistical comparisons were made by Student's t-test. p<0.05 was considered statistically significant.
Results
H1299, H1299-P53, and H1299-175H cells exhibit different radiosensitivity
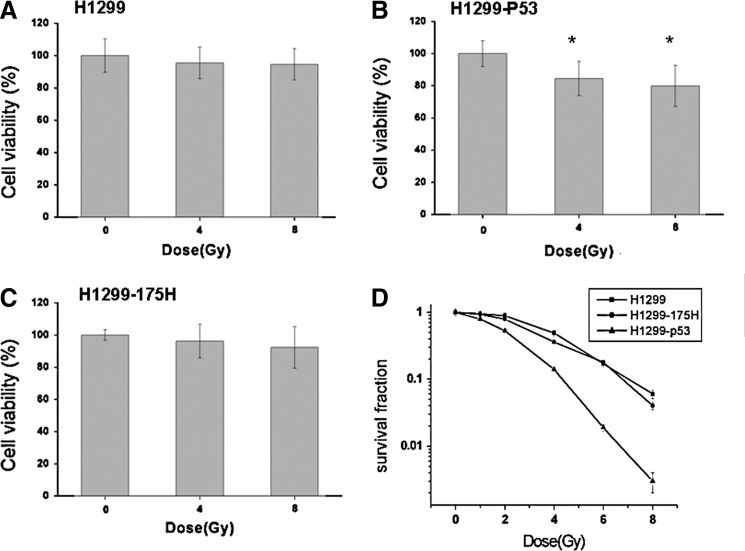
Cell models with different p53 phenotypes were established by introducing wild-type p53 construct or mutant p53 construct pCDNA3.1–175 H, and western blotting was used to confirm the expression of wt p53 and 175H (data not shown). H1299, H1299-p53, and H1299-175H cells were exposed to various doses of radiation (0, 1, 2, 4, 6, or 8 Gy), and cell viability was measured by CCK-8 assay and colony formation assay. As shown in Figure 1A, B, and C, radiation decreased the cell viability only in the H1299-p53 group, and no changes in parental H1299 and H1299-175H groups. Radiation suppressed the survival fraction more significantly in H1299-P53 than in H1299 and H1299-175H (p<0.05), and as shown in Figure 1D, the D0 and Dq values were as follows: for H1299-P53, D0=1.407 Gy, Dq=1.199 Gy; for H1299 D0=1.764 Gy, Dq=2.977 Gy; for H1299-175H D0=1.695 Gy, Dq=2.312 Gy. These results showed that the introduction of p53 increased radiosensitivity in H1299 lung cancer cells.
FIG. 1.
The radiosensitivity in lung cancer cell H1299 with different p53 phenotypes. Cell models with different p53 phenotypes were established by introducing pCDNA3.1-p53 or pCDNA3.1–175 H; western blotting was used to confirm the expression of wt p53 and 175H (data not shown). (A) Cell counting kit-8 (CCK-8) assay was used to detect the short-term cell viability in parental H1299 cells; (B) CCK-8 assay was used to detect the short-term cell viability in H1299-p53 cells, *p<0.05, versus sham-irradiated (0 Gy); (C) CCK-8 assay was used to detect the short-term cell viability in H1299-175H cells; (D) Colony formation assay was used to detect the long-term dose–survival curve, and Multitarget click model of GraphPad Prism 5.0 (Systat Software) was used to fit cell survival curves. H1299: D0=1.764, Dq=2.977; H1299-175H: D0=1.695, Dq=2.312; H1299-P53: D0=1.407, Dq=1.199.
The transcriptional activation of the p53 gene exists in H1299-P53 cells
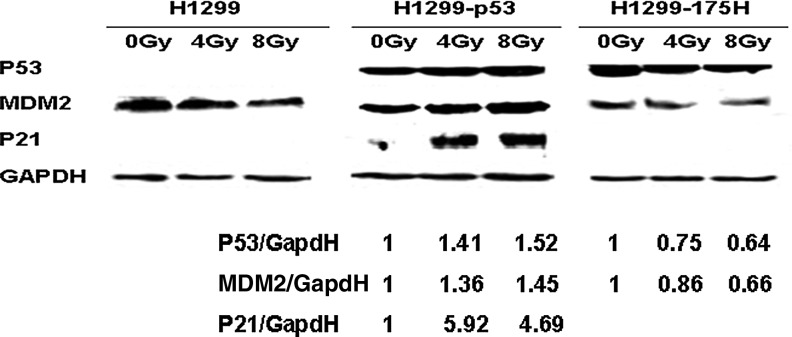
To detect whether the exogenous P53 was transcriptionally active, the expression of MDM2, p53, and p21 was examined in H1299, H1299-P53, and H1299-175H cells. As shown in Figure 2, the MDM2 expression increased, with concomitant increase of p53 and p21 expression in H1299-P53 cells. In H1299 and H1299-175H cells, p21 expression was undetectable. These findings indicate that p53 transcriptional function exists in H1299-P53, but not in H1299 and H1299-175H, cells.
FIG. 2.
The activity of exogenous p53 and mutant 175H after the treatment of irradiation. Western blotting was used to detect the expression of p53, MDM2, and p21; the increase of MDM2 and p21 was visible after irradiation. GAPDH was used as loading control.
Radiation-induced apoptosis in H1299, H1299-P53, and H1299-175H cells
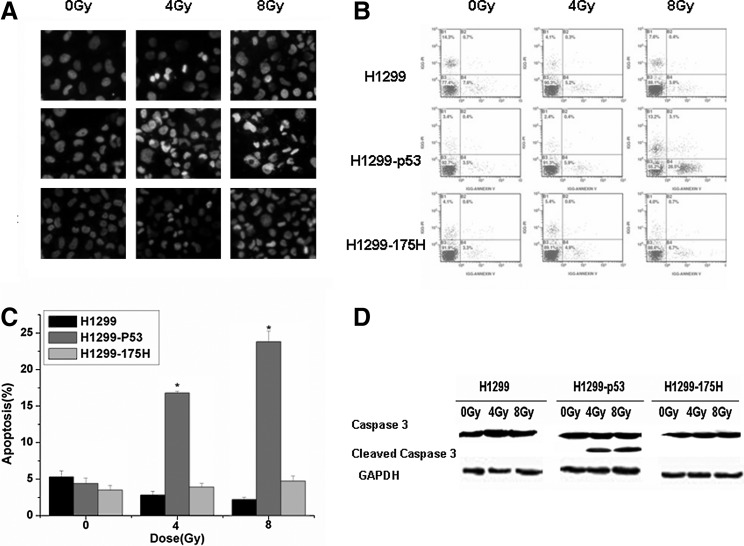
To determine whether the growth inhibition by irradiation was associated with apoptotic cell death, H1299, H1299-p53, and H1299-175H cells were exposed to various doses of radiation (0, 4, and 8 Gy), and apoptotic cell death was measured 24 hours after irradiation by DAPI nuclear staining (cells with condensed and fragmented nuclei were considered to be apoptotic) as shown in Figure 3A. FCM was also used to detect the apoptosis and necrosis quantitively by double staining with Annexin V and PI at 24 hours after irradiation. As shown in Figure 3B and C, there was significant increase of apoptotic cells in the H1299-P53 group after treatment with 4 and 8 Gy of radiation (1.7 times and 8.1 times vs sham-irradiation group, respectively, p<0.05), while there was no change of apoptosis in the H1299 and H1299-175H group after radiation. These results indicated that IR-induced apoptosis was here p53 dependent, and the apoptosis mechanism did not exist in H1299 cells and H1299-175H cells. We also found that the expression of cleaved Caspase3 (specific apoptosis marker) increased after exposure to radiation in H1299-P53 cells, which was absent in H1299 and H1299-175H cells, indirectly proved the occurrence of apoptosis. These results point to the association between apoptosis and growth inhibition, and suggest that the cell death induced in H1299-P53 cells by irradiation is mainly apoptotic.
FIG. 3.
The changes in apoptosis after the treatment of irradiation. (A) DAPI staining was used to detect the morphologic changes of apoptosis; (B) Flow cytometry was use to quantitate the apoptotic rate; (C) statistical analysis of apoptotic rate based on flow cytometry results was expressed as the percentage of untreated cells, mean±SE of thrice. *p<0.05, versus sham-irradiated (0 Gy); (D) Western blot was used to detect caspase 3 and cleaved caspase 3; the increase of cleaved caspase 3 suggested the apoptosis occurrence. GAPDH served as a loading control. Experiment was performed in triplicate.
Radiation-induced autophagy in H1299, H1299-P53, and H1299-175H cells
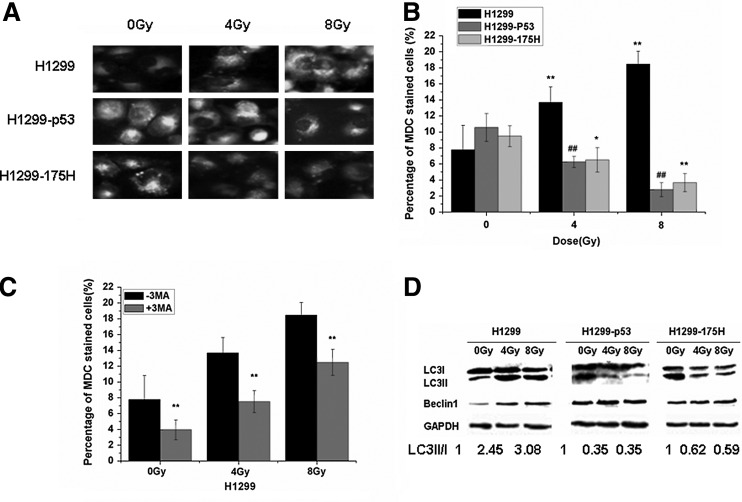
Next, we sought to explore whether IR affects the autophagic mechanism. Therefore, MDC staining was used, and MDC is specific dye for autophagosomes and accumulates in mature autophagic vacuoles (AVs) as autophagolysosomes, but not in the early endosome compartment. Under a fluorescence microscope, AVs stained by MDC appear as distinct dot-like structures distributed within the cytoplasm or localized in the perinuclear regions. We found that the autophagy was significantly enhanced after exposure to different doses of IR in H1299 cells (p<0.01). However, the autophagic rate declined after radiation in H1299-P53 and H1299-175H (p<0.01), as shown in Figure 4A and B. To verify the role of irradiation in induction of autophagy in H1299 cells, we used 3-MA to block autophagy. Preincubation of H1299 cells with 3-MA (2.5 mM) led to inhibition of IR-induced autophagy (p<0.01, Fig. 4C). Although the tendency of autophagy was almost the same with or without radiation, suggesting that the PI3KIII level had little effect on the occurrence of autophagy, considering the fact that activation of class III PI3K in complex with the autophagy-associated protein Beclin-1 promotes autophagy, the expression of Beclin-1 was detected, and the increase of Beclin-1 was found in a dose-dependent manner after radiation exposure in H1299 cells, but no change in H1299-P53 and H1299-175H cells (Fig. 4D). During autophagy development, MAPLC3-I cytoplasmic protein is converted into an LC3-II-lapidated form, which is incorporated onto the autophagosome. Consistent with MDC results, we found that different doses of IR resulted in a dose-dependent increase of the LC3-I/LC3-II ratio in the H1299 cell line. In contrast, this ratio showed dose-dependent descent in H1299-p53 and H1299-175H cells after exposure to same doses of IR. Collectively, these results indicate that p53 inhibits the IR-induced autophagy in lung cancer cells.
FIG. 4.
The changes in autophagy after the treatment of irradiation. (A) Monodansylcadaverine (MDC) staining was used to detect the morphologic changes of autophagy; (B) Statistical analysis of autophagic rate based on MDC staining, Mean±SE of ten vision fields. *p<0.05, **,##p<0.01, versus sham-irradiated (0 Gy); (C) statistical analysis of autophagic rate in parental H1299 cells based on MDC staining, with or without autophagy inhibitor, 3-Methyladenine (3-MA) (2.5 mM); mean±SE of ten vision fields. *p<0.05 versus untreated 3-MA group (0 Gy); (D) Western blot was used to detect MAPLC3 and Beclin 1, and the increase of MAPLC3II and Beclin 1 suggested the autophagy occurrence.
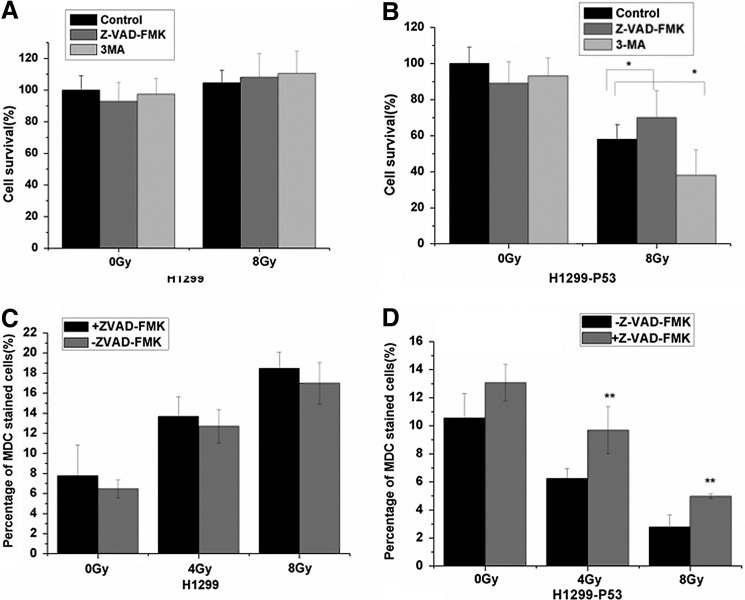
Effects of autophagy and apoptosis inhibition on radiosensitivity
To determine the role of autophagy and apoptosis in radio sensitivity, 3-MA and Z-VAD-FMK were used to block autophagy and apoptosis, respectively, followed by 8 Gy of radiation. As shown in Figure 5B, the inhibition of autophagy significantly reduced the cell viability of H1299-P53 cells, while inhibition of apoptosis significantly increased the cell viability of H1299-P53 cell after radiation. However, there was no change in H1299 cells (Fig. 5A). As shown in Figure 5C and D, inhibition of apoptosis by Z-VAD-FMK significantly increased autophagy in H1299-P53 cells, but not in H1299 cells. These findings indicate that autophagy might play a prosurvival role in H1299-P53 lung cancer cells.
FIG. 5.
Effects of inhibitors for autophagy and apoptosis on the cell survival rate. (A) The changes of cell survival rate in parental H1299 cells in the presence or absence of 3-MA (2.5 mM) or Z-VAD (20 μM), with or without indicated IR at 24 hours; (B) the changes of cell survival rate in H1299-p53 cells in the presence or absence of 3-MA (2.5 mM) or Z-VAD (20 μM), with or without indicated IR at 24 hours; mean±SE of thrice. *p<0.05 versus Control group (8 Gy); (C) the changes of autophagic rate in parental H1299 cells in the presence or absence of Z-VAD (20 μM), with or without indicated IR at 24 hours; (D) the changes of autophagic rate in H1299-p53 cells in the presence or absence of Z-VAD (20 μM), with or without indicated IR at 24 hours; mean±SE of thrice. **p<0.01, versus untreated-Z-VAD group.
Discussion
Although analysis of human cancers reveals the fundamental role for p53 in tumor suppression, p53 mutation is very common in lung cancer cell lines and reported in 40%–90% of NSCLC tumors, different p53 mutants presenting different functions such as conserved, dominant negative, loss of function, and gain of function might have different effects on radiosensitivity from the wild-type p53 gene. p53 mutants commonly found in human tumors include 175H, 248W, and 273H. p53(175H) increased genomic instability, resulting from an inhibition of G1 arrest and abnormal amplification of centrosomes.
In this study, the p53-deficient human lung cancer cell line H1299 was used, and cell models with different p53 backgrounds were established by introducing wt-p53 or 175H constructs. H1299, H1299-p53, and H1299-175H cells were exposed to various doses of radiation (0,1, 2, 4, 6, or 8 Gy). Colony formation assay and CCK-8 assay confirmed that IR suppressed the survival fraction more significantly in H1299-P53 than in H1299, H1299-175H, suggesting that H1299-P53 was more radiosensitive than H1299 and H1299-175H cells, which is consistent with previously reported promotion of radiosensitivity by wt-p53.13
Although the p53 and mutant 175H had been introduced into cells, their functional activity still needed to be verified. Considering the fact that only the functional p53 leads to induction and activation of downstream target genes,14 in other words, though dominant-negative mutants showed increased stabilization, they would lead to the loss of expression of the downstream genes. Therefore, p21Waf1 was detected in this study next. In the presence of the p53 gene, that is, in H1299-p53 cells, the exogenous p53 could trigger p21WAF1/CIP1 transcription and expression (Fig. 2), While in H1299 and H1299-175H cells, p21 expression was undetectable, indicating that the normal wt-p53 transcriptional function was disrupted in H1299-175H. MDM2 is an oncogene and generally negatively regulates p53 expression, consequently, form a p53-MDM2-p53 feedback loop mechanism. Activation of p53 promotes the expression of MDM2, while MDM2 combines with p53 to inhibit p53 function and promote its degradation.15 Our results showed that IR increased MDM2 expression in H1299-P53 cells (Fig. 2).
Autophagy, apoptosis, and necrosis were analyzed in our study, since previous studies have reported that the rate of apoptosis and autophagy was variable after treatment of cells with different p53 status.13 Apoptosis was illustrated by DAPI staining, FCM, and western blotting. The results illustrated that Cleaved Caspase3 (specific apoptosis marker) was increased significantly in H1299-P53 cells, suggesting the high occurrence of apoptosis in H1299-P53 cells. Liu et al. reported that H1299 cells treated with C-beam with AdCMV-p53 or C-beam only underwent changes typical of apoptosis.16
Other than apoptosis, autophagy is a dynamic process of protein degradation characterized by the formation of prominent double-membrane cytoplasmic vesicles called autophagosomes.17 The specific markers of autophagy include the expression and the relocalization of MAPLC3, the autophagy regulatory gene, and so on. During autophagosome formation, MAPLC3I is converted to MAPLC3II, which represents a key event in autophagy machinery.18 Our results showed that both MAPLC3II and Beclin-1 increased significantly in H1299 cells after radiation, and the expression of MAPLC3 II decreased in H1299-p53 cells, suggesting the occurrence of autophagy in H1299 cells. To explore the association between autophagy and radiosensitivity, 3-MA, an inhibitor of autophagy, was added before radiation treatment in H1299 cells. The results illustrated that 3-MA inhibited autophagy. Ruth Scherz et al. reported that wt-p53 is required for MAPLC3 mRNA downregulation in chronically starved HCT116 cells, and p53 may promote the degradation of mature MAPLC3 mRNA through its RNA-binding activity.19 Thus, mutant p53, which lost wild-type p53 function, cannot be efficiently degraded, and thereby recycling of autophagosomes is aberrant.
It has been reported that in certain cancer cells, autophagy induced by chemotherapy may prevent cells from undergoing apoptosis,20 implying an interlink modulation between autophagy and apoptosis. Current data about the effects of p53 on autophagy regulation suggested that the relationship between p53 and radiosensitivity was conditional, depending on the p53 status, cell types, chemicals, surrounding environment, and underlying mechanism, and so on. Our results showed that in H1299-p53 cells, the inhibition of apoptosis by Z-VAD could promote the occurrence of autophagy and increase the survival rate, suggesting the molecular switch from apoptosis to autophagy and the protective mechanism of autophagy; while the fact that the inhibition of autophagy by 3-MA decreases the survival rate also suggested the protective mechanism of autophagy in the presence of p53. Necrosis was also detected by immunofluorescent double staining in this study, Necrosis also increased in H1299-P53 cells (data not shown). Functional wt-p53 is neither merely a positive nor negative regulator of autophagy and maintains autophagic homeostasis and adjusts the rate of autophagy according to the changing circumstances.19,21 Autophagy plays an active role in radiosensitization in several cancer cell types.22,23 Recent evidence suggests that the ability to enhance or suppress autophagy by regulating autophagy protein MAPLC3 allows p53 signaling to provide the most appropriate cell survival strategy during nutrient starvation. Thus, cells with low basal autophagic rates show a p53-dependent increase in autophagy in response to starvation, whereas cells with high basal autophagic rate show a p53-dependent decrease in autophagy in response to the same conditions—the end result in both scenarios is promotion of cell survival.19
Taken together, our findings point out the significance of p53 in determining the radiosensitivity of lung cancer cells by regulating autophagy and apoptosis, and there might be a molecular switch between autophagy and apoptosis. Since p53 is frequently deleted or mutated in most cancer patients, our above-mentioned results suggest that individual treatment strategies should be based on the p53 status in patients.
Acknowledgments
This study was supported by NSFC grants (30770649 and 30970682), Research Fund for the Doctoral Program of Higher Education of China (20100061110070), Program for New Century Excellent Talents in University, and the Fundamental Research Funds for the JiLin Universities.
Disclosure Statement
None of the authors have any conflict of interest to be disclosed regarding this study.
References
- 1.Ferlay J. Shin HR. Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Hayat MJ. Howlader N. Reichman ME, et al. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 3.Jett JR. Midthun DE. Screening for lung cancer: Current status and future directions: Thomas A. Neff lecture. Chest. 2004;125(5 Suppl):158S. doi: 10.1378/chest.125.5_suppl.158s. [DOI] [PubMed] [Google Scholar]
- 4.Pfister DG. Johnson DH. Azzoli CG, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: Update 2003. J Clin Oncol. 2004;22:330. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Vousden KH. Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 6.Okaichi K. Wang LH. Sasaki J, et al. A point mutation of human p53, which was not detected as a mutation by a yeast functional assay, led to apoptosis but not p21Waf1/Cip1/Sdi1 expression in response to ionizing radiation in a human osteosarcoma cell line, Saos-2. Int J Radiat Oncol Biol Phys. 1999;45:975. doi: 10.1016/s0360-3016(99)00285-0. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH. Seo WD. Ryu HW, et al. Anti-tumor effects by a synthetic chalcone compound is mediated by c-Myc-mediated reactive oxygen species production. Chem Biol Interact. 2010;188:111. doi: 10.1016/j.cbi.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Hinds PW. Finlay CA. Quartin RS, et al. Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: A comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1990;1:571. [PubMed] [Google Scholar]
- 9.Zois CE. Koukourakis MI. Radiation-induced autophagy in normal and cancer cells: Towards novel cytoprotection and radio-sensitization policies? Autophagy. 2009;5:442. doi: 10.4161/auto.5.4.7667. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Y. Qiu F. Tashiro S, et al. ERK and JNK mediate TNFalpha-induced p53 activation in apoptotic and autophagic L929 cell death. Biochem Biophys Res Commun. 2008;376:483. doi: 10.1016/j.bbrc.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 11.Xue L. Fletcher GC. Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: An alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 12.Liu B. Cheng Y. Zhang B, et al. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Lett. 2009;275:54. doi: 10.1016/j.canlet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Chiu SJ. Hsaio CH. Tseng HH, et al. Rosiglitazone enhances the radiosensitivity of p53-mutant HT-29 human colorectal cancer cells. Biochem Biophys Res Commun. 2010;394:774. doi: 10.1016/j.bbrc.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 14.Kaneuchi M. Yamashita T. Shindoh M, et al. Induction of apoptosis by the p53-273L (Arg —> Leu) mutant in HSC3 cells without transactivation of p21Waf1/Cip1/Sdi1 and bax. Mol Carcinog. 1999;26:44. [PubMed] [Google Scholar]
- 15.Ard PG. Chatterjee C. Kunjibettu S, et al. Transcriptional regulation of the mdm2 oncogene by p53 requires TRRAP acetyltransferase complexes. Mol Cell Biol. 2002;22:5650. doi: 10.1128/MCB.22.16.5650-5661.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu B. Zhang H. Duan X, et al. Adenovirus-mediated wild-type p53 transfer radiosensitizes H1299 cells to subclinical-dose carbon-ion irradiation through the restoration of p53 function. Cancer Biother Radiopharm. 2009;24:57. doi: 10.1089/cbr.2008.0514. [DOI] [PubMed] [Google Scholar]
- 17.Kondo Y. Kondo S. Autophagy and cancer therapy. Autophagy. 2006;2:85. doi: 10.4161/auto.2.2.2463. [DOI] [PubMed] [Google Scholar]
- 18.Liu XW. Su Y. Zhu H, et al. HIF-1alpha-dependent autophagy protects HeLa cells from fenretinide (4-HPR)-induced apoptosis in hypoxia. Pharmacol Res. 2010;62:416. doi: 10.1016/j.phrs.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Scherz-Shouval R. Weidberg H. Gonen C, et al. p53-dependent regulation of autophagy protein LC3 supports cancer cell survival under prolonged starvation. Proc Natl Acad Sci U S A. 2010;107:18511. doi: 10.1073/pnas.1006124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosner J. Mummenbrauer T. Bauer C, et al. Negative feedback regulation of wild-type p53 biosynthesis. EMBO J. 1995;14:4442. doi: 10.1002/j.1460-2075.1995.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boya P. Gonzalez-Polo RA. Casares N, et al. Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol. 2005;25:1025. doi: 10.1128/MCB.25.3.1025-1040.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim KW. Mutter RW. Cao C, et al. Autophagy for cancer therapy through inhibition of pro-apoptotic proteins and mammalian target of rapamycin signaling. J Biol Chem. 2006;281:36883. doi: 10.1074/jbc.M607094200. [DOI] [PubMed] [Google Scholar]
- 23.Daido S. Yamamoto A. Fujiwara K, et al. Inhibition of the DNA-dependent protein kinase catalytic subunit radiosensitizes malignant glioma cells by inducing autophagy. Cancer Res. 2005;65:4368. doi: 10.1158/0008-5472.CAN-04-4202. [DOI] [PubMed] [Google Scholar]