Abstract
The periosteum, a thin, fibrous tissue layer covering most bones, resides in a dynamic, mechanically loaded environment. The periosteum also provides a niche for mesenchymal stem cells. The mechanics of periosteum vary greatly between species and anatomical locations, indicating the specialized role of periosteum as bone's bounding membrane. Furthermore, periosteum exhibits stress-state-dependent mechanical and material properties, hallmarks of a smart material. This review discusses what is known about the multiscale mechanical and material properties of the periosteum as well as their potential effect on the mechanosensitive progenitor cells within the tissue. Furthermore, this review addresses open questions and barriers to understanding periosteum's multiscale structure–function relationships. Knowledge of the smart material properties of the periosteum will maximize the translation of periosteum and substitute periosteum to regenerative medicine, facilitate the development of biomimetic tissue-engineered periosteum for use in instances where the native periosteum is lacking or damaged, and provide inspiration for a new class of smart, advanced materials.
Introduction
As the population continues to age, trauma is expected to rise from the seventh to the third leading cause of disability in adults within the next decade.1 Bone fractures are a common result of trauma and accounted for 26% of total reported musculoskeletal injuries in 2004.2 Fractures or conditions resulting in critical size bone defects are especially debilitating, as such defects are incapable of healing without surgical intervention. Defects of this severity arise from trauma, tumor, infection, or congenital malformations.3
The healing of critical size bone defects is of great interest as such defects present one of the most challenging problems in orthopedic surgery. Current methods to heal critical size bone defects utilize surgical procedures such as the Ilizarov technique of distraction osteogenesis and typically involve use of bone graft and/or graft substitutes. In the Ilizarov technique, the distal and proximal ends of the injured bone are stabilized using an external cylindrical frame that is affixed to the stable bone ends as well as osteotomized bone segments via fixation wires. The transport segment is then distracted a millimeter or so per day (via a screw or motor on the external frame), which stimulates bone regeneration in the widening gap, until it reaches the proximal or distal segment on the other side of the defect zone.4 Hence, new bone regenerate emanates from the osteotomy gap, which is placed under tension through distraction of the fragment; cells and factors related to this new bone formation likely derive from the bone itself as well as the medullary niche, if an external fixator is used. Adaptations of the technique include stabilization and bone transport over an intramedullary nail.5–11 Distraction osteogenesis has several disadvantages, including long and labor-intensive treatment times, a high risk of complications, patient discomfort, and scarring. Furthermore, distraction osteogenesis requires significant technical expertise, limiting the number of surgeons qualified to perform the procedure and, hence, access for patients.
Recent research in the field of orthopedics has drawn attention to the power of periosteal tissue and periosteum-derived cells (PDCs) to heal bone defects.3,5,12–15 Specifically, the periosteum has been found to regenerate woven bone within a critical size defect within as little as 2 weeks from time of treatment.3,12,13 Periosteum tissue generation within the defect correlates significantly to mechanical loading as well as periosteal proximity. Furthermore, packing of the defect with morcellized autologous bone graft retards the ingression of PDCs and, thus, infilling of the defect with intramembranous bone. In absence of graft, infilling occurs from the periosteum, toward the surface of the implant, which stabilizes the femur and fills the medullary cavity. These data suggest that the biophysical and chemical environment of PDCs egressing from the periosteum into the critical-sized defect modulates tissue genesis (chondro- as well as osteogenesis) and healing. In addition, these biophysical and chemical effects are likely to interact at multiple length scales, that is, tissue-, cell-, and molecular-length scales. While previous studies have addressed specific aspects of the mechanobiological structure and function within the periosteum, for example, cellular changes in the periosteum subjected to mechanical loading,16–19 structural changes in developing20 and in aging periosteum,21 as well as mechanical properties of periosteum from different species,22 and anatomic sampling sites,23 relatively little is known with regard to periosteum's multiscale mechanobiology.
Yet, periosteum and other materials found in nature are heralded for their smart properties, which make them models to emulate when engineering tissues and designing new classes of advanced materials.24–27 An example at the length scale of the cell, the cytoskeleton is akin to a living bridge that restructures its architecture to minimize areas of stress concentration in high wind or traffic situations.27 Similarly, at the length scale of a tissue, the periosteum serves as bone's bounding membrane and harnesses endogenous biophysical cues to modulate environmental conditions on either side (within and outside of bone).14,27,29 The Intelligence Quotient of so-called smart materials' “is measured in terms of…‘responsiveness' to environmental stimuli and ‘agility’ (as in capacity for dynamic response).”30 In the case of tissues (living biomaterials), their living inhabitants (cells) actively modulate the dynamic response.
The following reviews the current state of periosteal mechanobiology from a molecule to a cell to a tissue length scale, in bone health and during healing of bone. Furthermore, open questions and barriers to understanding are addressed, both from the perspective of defining important future directions for the field and also from the perspective of identifying the technologies that are missing to elucidate and apply the smart properties of the periosteum with the ultimate goals to (1) maximize the translation of periosteum and substitute peristeum use in regenerative medicine, (2) facilitate the development of biomimetic tissue-engineered periosteum (TEP) for use in instances where native periosteum is lacking or damaged, and (3) provide inspiration for a new class of smart, advanced (bio)materials.
Structure–Function Relationships: Periosteum Tissue Mechanobiology
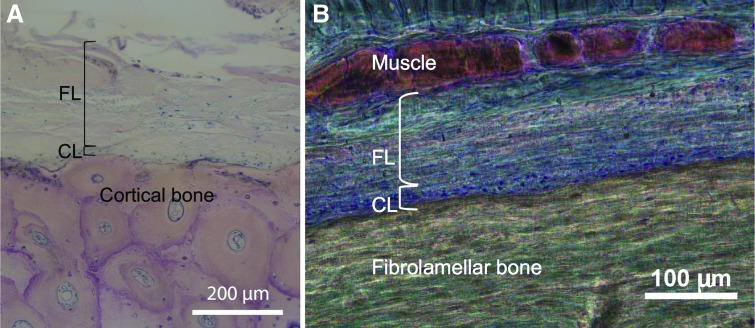
The periosteum is a bilayered, soft tissue sleeve that envelopes bone (Fig. 1). Periosteum is highly vascularized and provides at least 1/3 of the blood supply to cortical bone, with the remaining supply coming from the intramedullary niche.32 The tissue exhibits a composite structure comprised of an outer fibrous layer and an inner cambium layer. The outer fibrous layer contains fibroblasts, collagen and elastin fibers, and microvessels.20 Histological data reveal axially aligned collagen fibers33 and a high density of elastin34,35 throughout mid-diaphyseal periosteum, likely providing mechanical (structural) strength to the tissue.36 The periosteum provides a niche for adult mesenchymal stem cells (periosteum-derived mesenchymal stem cells [MSCs], PDCs, which can give rise to osteoblasts, adipocytes, and chondroblasts,37 as reviewed and compared to bone marrow-derived MSCs [BMSCs] recently38), as well as osteochondroprogenitors,39 and osteoblasts.40 PDCs have been shown to play a major role in bone fracture healing by constantly building34 and repairing bone.12,15,41–43 Adjacent PDCs are joined together by tight junctions,44 hallmarks of epithelial membrane morphology that allow for vectorial transport to apical and basal sides of the tissue.45 The progenitor cells proliferate and then differentiate into osteoblastic and chondroblastic cells, respectively driving the process of bone repair via either direct intramembranous bone formation or indirect endochondral mechanisms.39
FIG. 1.
Histological images of periosteum from skeletally mature bone (A) of an aged human and middle aged sheep (B). (A) In a cross section of the mid-diaphysis of the human tibia (patient age at death: 69 years). Image used with permission.31 (B) In a cross section of the mid-diaphysis of the ovine femur (∼4 years of age). Image adapted and used with permission.29 CL, cambium layer; FL, fibrous layer. Color images available online at www.liebertpub.com/teb
The periosteum is anchored to the bone by Sharpey's fibers, strong fibers with a high collagen content. Sharpey's fibers serve as a link between the exterior musculature and the interior skeleton21 and allow the periosteum to remain intact and attached to the bone, even after severe trauma occurs. In certain bones, Sharpey's fibers anchor tendons and ligaments to the bone. Periosteum is absent at sites of tendon attachments. As tendon and ligament attachments vary by bone, periosteum morphology is highly variable between bones and even within bones.35
The periosteum is often thought to behave as a mechanically stabilizing boundary membrane, which functions in skeletal growth and musculoskeletal biomechanics.21,22 Damage to or absence of the periosteum is known to cause developmental abnormalities. For example, in bones without periosteum, the development of a substantial layer of regenerated bone adjacent to intact cortical bone is unlikely.46 The anatomy of the periosteum, as well as its osteoinductive and nutrient transport and inductive capacities,47 are well described. Characterization of the the periosteum's material and mechanical properties will allow for a better understanding of the periosteum's role as the barrier membrane, which bounds all bones, “serving as a gatekeeper for flow of fluids and hence mass and cell transport into and out of [the organ] bone.”29 Furthermore, characterization of these properties will improve our understanding of the PDCs native environment, including biophysical and mechanically modulated factors, such as chemical gradients. Finally, characterization of the mechanical environment of PDCs will provide knowledge valuable for the fabrication of biomimetic TEP. Ultimately, knowledge of periosteal mechanobiology will allow the use of native and tissue engineered periosteum (TEP) to become commonplace in regenerative medicine.
Biology of Periosteum Derived Cells (PDCs)
PDCs are MSCs that are isolated from the periosteum, in contrast with BMSCs that are derived from the bone marrow.38,48–50 “… [M]ost studies have found PDCs to be comparable to, if not superior to, BMSCs with regard to bone healing and regeneration.”38,51–53 PDCs are commonly used for bone and cartilage tissue engineering applications due to their ability to differentiate into tissues of mesodermal origin, specifically bone and cartilage.16–19,54–56 Current methods for isolating PDCs from periosteum include enzymatic digestion or explant culture.38 The choice of isolation protocol has not only practical, but also potentially important mechano-chemo-biological consequences; digestion liberates cells from the entire periosteum and exposes cells to collagenase (with unknown downstream effects) and explant culture favors the isolation of motile cells, which are capable of egressing from the cambium layer.38
Few studies have characterized surface antigens unique to PDCs and some studies report conflicting data. Knowledge of such surface markers will be important, going forward, to account for the specific population of cells isolated from the periosteum and to have a basis from which to compare PDC mechanobiology data within and between studies (Table 1 summarizes the current state of the art). In addition, like other stem cells, the number of PDCs residing in the cambium layer is known to decline with age,19 but changes in the proliferative capacity of PDCs with age have not yet been well characterized. PDCs play an important role in modulating bone and cartilage formation during growth and healing.44,53
Table 1.
Compilation of Data from Published Studies Showing Positive and Negative Surface Markers for Periosteum-Derived Cells
| BMSC | PDC | References | |
|---|---|---|---|
| Integrins | |||
| CD29 | + | + | 57,58 |
| CD49e | + | 57 | |
| Adhesion molecules | |||
| CD31 | − | − | 57 |
| CD44 | + | + | 57 |
| CD106 | (+)(−) | (+)(−) | 57,59 |
| CD166 | + | (+)+ | 57,58,60 |
| CD54 | + | + | 57 |
| MHC class | |||
| HLA-DR | + | − | 57 |
| HLA-ABC | + | + | 57 |
| Hematopoietic markers | |||
| CD14 | − | − | 57 |
| CD33 | − | 57 | |
| CD34 | (+)(−) | − | 57,60 |
| CD38 | − | 57 | |
| CD45 | − | − | 57,58–60 |
| CD133 | − | 57 | |
| Additional markers | |||
| CD9 | (+)(−) | 57,60 | |
| CD13 | + | + | 57,59 |
| CD73 | + | + | 57–60 |
| CD90 | + | + | 57,58,60 |
| CD105 | + | (+)+ | 57–60 |
| CD117 | − | 57 | |
| SH2 | + | + | 60 |
| SH3 | + | + | 60 |
| SH4 | + | + | 60 |
| TNAP (ALP) | − | 59 | |
| CD140b | (+) | 58 | |
| D7-FIB | + | 59 | |
| LNGFR | − | 59 | |
| CD146 | + | (−) | 59 |
Symbols are indicative of marker quality, where+indicates a strongly positive marker, (+) a weakly positive marker, − a strongly negative, (−) a weakly negative. Color coding indicates the specificity of the markers for PDCs, that is, red indicates a strong PDC negative marker, blue indicates a marker showing variable (positive and negative) results, and green indicates a strongly PDC-positive marker.
BMSC, bone marrow-derived mesenchymal stem cell; PDCs, periosteum-derived cells.
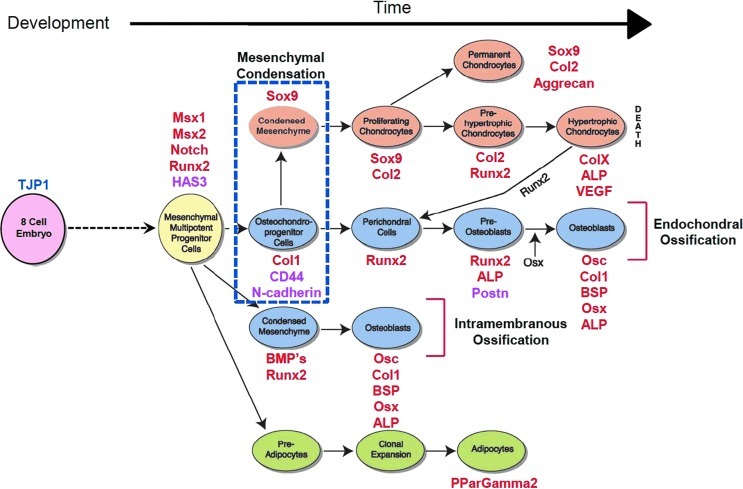
Measures of gene transcription by rtPCR provide a snapshot of not only PDC activity, but also emergent fate at a given moment in time and in a given biophysical and/or biochemical environment (Fig. 2).44 This approach has been used successfully to relate changes in the mesenchymal stem cell shape and emergent fate decisions,61–64 in essence to “map the mechanome”.65 More recently, the approach has been used to demonstrate the feasibility of engineering emergent tissue architectures by presenting adult PDCs with recombinant N-cadherin, a cell–cell junctional protein, on a functionalized solid supported lipid bilayer mimicking the plasma membrane of the cell.44 This and other contemporary approaches have focused on identifying the mechanobiological properties of both PDCs and the tissue in which they reside, as well as the role of molecular factors at the cellular and tissue length scales, to understand structure–function relationships as well as the emergence of fate and tissue architectures throughout growth and development.
FIG. 2.
Postnatal bone healing recapitulates processes of bone formation in utero. Mesenchymal condensation is a seminal event marking the initiation of skeletogenesis (blue dotted square in schematic). By tracking relative gene expression, one can assess the relative stage and/or path of lineage commitment for uncommitted pluripotent cell-like periosteum-derived cells. Red font depicts gene markers for pre-, peri-, and postmesenchymal condensation. Magenta font indicates gene markers for the formation of cell–cell and cell–matrix adhesions, key for self-aggregation of cells and emergent tissue architecture. Mesenchymal stem cells can commit to chondrogenic (orange), osteogenic (blue), and adipogenic (green) path lineages. Figure adapted and used with permission.39 Color images available online at www.liebertpub.com/teb
Mechanosensitivity and Mechanoadaptation of PDCs
A number of published studies describe and classify the structural adaptation of MSCs in response to fluid flow induced shear and normal stresses,39,61,62,65–68 substrate stiffness,69 and seeding density.63,70,71 Substrate stiffness and cell shape are known to control MSC fate decisions, including self renewal and lineage commitment. Topographic changes also influence MSC fate and exogenous dilatational (volume changing) and deviatoric (shape changing) stresses modulate changes in MSC gene expression indicative of early fate decisions.27,61–66,71 Furthermore, mechanical signals are known to affect growth and differentiation of MSCs. Mechanical stimuli are increasingly recognized as key regulators of cell structure and function.27,39,65,66,70 The ability of a cell to sense mechanical forces, transmit them internally or to other cells, and transduce mechanical signals into biochemical signals forms the concept of cellular mechanobiology39,70,72 and recent studies have begun to map the mechanome, delineating a reference library of mechanical cues to direct lineage commitment (Fig. 3) independent of or in concert with biochemical signals.65
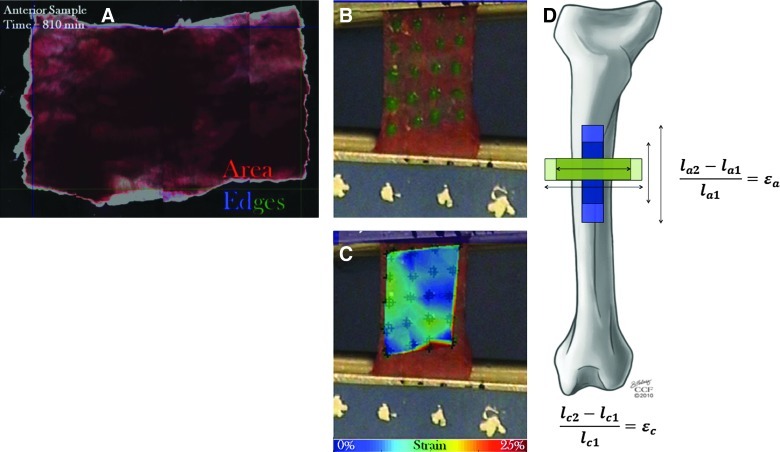
FIG. 3.
Characterization of periosteum's mechanical properties. (A) Periosteal shrinkage as measured previously.14 The left and top edges are shown in blue and the right and bottom edges are shown in green. Image correlation was used to determine change in axial length (difference between top and bottom edges) and change in circumferential length (difference between left and right edges). (B, C) The elastic modulus of ovine femoral periosteum was determined using mechanical testing and high-resolution strain mapping.14 (D) Periosteal strain is calculated as the change in length of periosteum samples from when they are attached to bone to their length initially upon removal from the bone. Prestress is calculated by multiplying the change in strain by the elastic modulus of the tissue. Portions of this figure used with permission.14 Color images available online at www.liebertpub.com/teb
Although both are subpopulations of MSCs, PDCs and BMSCs reside in distinct niches with unique, intrinsic biochemical, and biophysical cues. In contrast to BMSCs, PDCs are currently only broadly characterized and little is known about their morphology. Research indicates that the phenotypical and morphological properties of PDCs differ from those of BMSCs in response to similar environments.38 PDCs and BMSCs cultured in the same osteoinductive medium have shown different growth patterns, with PDCs reaching confluence 5–7 days earlier than BMSCs.48 Furthermore, PDCs and BMSCs have been shown to differentiate along different lineages when cultured on the same roughened titanium surface, suggesting different pathways or mechanisms for mechanotransduction for MSCs from different niches.49,50 It is therefore important to investigate periosteal cell mechanics independently of MSC mechanics as well as to design studies to mimic the endogenous environment of the cells.
The native environment of PDCs is mechanically regulated by a combination of tension and shear (given that the periosteum itself exhibits different moduli of elasticity in the longitudinal and circumferential directions, discussed below). The intracellular tension PDCs experience is suggested to regulate long bone growth.17,19 Surgical release of the inherent tension within the periosteum alters the local mechanical environment of PDCs. Periosteal release has been shown to increase proliferation of cambium layer PDCs as early as 1 h after periosteal release as measured by mRNA expression for BMP-2,19 a growth factor commonly expressed during bone and cartilage development. Histological analysis indicates that four-sided periosteal release results in a greater bone formation compared to two-sided periosteal release, indicating that cells are sensitive to the magnitude, and potentially to the direction, of mechanical stimulus.19
PDCs' capacity to carry intracellular tension through their active microfilament network has been postulated to regulate a signaling cascade which, in turn, is responsible for the expression of soluble factors that modulate cartilage growth. The stiffness of the culture surface determines the magnitude of intracellular tension placed on the actin microfilament network. PDCs were cultured on substrates of varying stiffnesses which allowed cells to generate various magnitudes of intracellular tension.17 The conditioned medium from these cell cultures was added to cultures of intact or periosteum stripped embryonic chick tibiotarsi to evaluate the growth response. The culture medium from cells grown on low-stiffness substrates (3 kPa) resulted in a greater cartilage growth of periosteum stripped bones compared to a medium from stiffer substrates (80 kPa).34 Furthermore, culture of stripped tibiotarsi in a medium taken from cells cultured on 80 kPa substrates and treated with cytochalasin D to disrupt the actin microfilament network showed significantly increased cartilage growth. This indicates a mechanobiological feedback mechanism between growing cartilage and tension in the periosteum in which expression of soluble growth inhibitors is regulated by the intracellular tension of PDCs.
In addition to being regulated by tension in the local microenvironment, PDCs respond to the topographical surface on which they are cultured, culture density, shear fluid flow, dynamic fluid pressure (DFP), and mechanical strain. PDCs cultured on machined and acid etched titanium surfaces proliferate at significantly different rates, with cells cultured on the machined surface exhibiting increased proliferation.49 The acid etched surface was shown to promote chondroblastic differentiation of PDCs, while suppressing osteoblastic differentiation.49 Culture density of PDCs has been found to affect the level of type II collagen mRNA expression, a marker for chondrogenesis.73 Studies using a model MSC cell line demonstrate that increasing cell seeding density per se exerts volume changing dilatational stress on the cell as a whole and shape changing deviatoric stress on the nucleus of stem cells.63 In the PDC density study, the level of type II collagen mRNA was significantly higher in PDCs cultured in a high-density, micromass culture system compared to cells cultured in a monolayer.73 Furthermore, PDCs cultured in a micromass culture system in the absence of TGF-β3, a chondrogenic growth factor also exhibited type II collagen expression, indicating that mechanical signaling alone can cause PDCs to differentiate.73 Culture of periosteal explants in spinner flask bioreactors resulted in a fourfold increase in cartilage yield compared to static culture conditions.54 The majority of tissue growth occurred in the longitudinal direction, indicating PDCs are sensitive to the direction of applied shear stress. Results of shear flow studies on stem cells must be interpreted with caution as consistency of flow and minimization of turbulent flow are often of concern.54,67,68
DFP has been shown to induce PDC proliferation in vitro.18 Periosteal explants are subjected to cyclic hydrostatic pressure during culture using a pneumatically driven membrane chamber, which creates dynamic pressurization in the gas phase. Upon comparison to control explants, samples cultured under DFP expressed a 60% higher DNA content after 3 days of culture, representative of an increase in cell proliferation. Cell proliferation, highest in the fibrous layer on day two of culture, increases in the cambium layer by day three. Immunostaining with proliferating cell nuclear antigen confirmed that, on day four of culture, explants exposed to DFP showed a significantly greater number of proliferating cells localized in the cambium layer of the periosteum.18 Thus, PDC proliferation during periosteal chondrogenesis can be stimulated by DFP. In addition, small magnitude strains comparable to those experienced by the periosteum in vivo have been found to stimulate PDC proliferation.74 Small magnitude mechanical strains have also been found to upregulate Runx2 and ColIa1, transcription factors critical for osteoblast differentiation.75 One hypothesis to explain this increase in cell proliferation is that a paracrine signaling mechanism exists in which cells in the fibrous layer release soluble factors in response to mechanical stimuli, stimulating the PDCs in the cambium layer to divide and differentiate.18 More recent studies point to the role of soluble extracellular factors, including ATP and UTP, released upon mechanical stimulation of tissue, in activating Runx2, a fundamental transcription factor implicated in osteoblastic differentiation.76
PDCs reside in a mechanobiologically dynamic environment. Model MSCs (C3H3T1/2, a cell line derived from the mesoderm of the murine embryo and capable of differentiating along multiple lineages, including bone, cartilage, and fat) have been shown to be more than 1000 times more sensitive to exogenous mechanical stimuli than terminally differentiated cells, changing their baseline gene expression significantly after exposure to short-term (30 min) and small-magnitude shear stresses (0.1 dyn/cm2).61 Pluripotent cells,39 including PDCs,74,75 have been shown to exhibit mechanical sensitivity as well. Mechanical stimulation of PDCs is thought to stimulate paracrine signaling pathways,17–19 leading to the absence of production of soluble factors that stimulate cell proliferation and differentiation. The specific cells stimulated (PDCs deriving from the cambium versus fibrous layer) remain to be elucidated. Further research is necessary to identify specific soluble factors released via different means of mechanical stimulation of PDCs and how these stimuli and soluble factors interact to influence cell proliferation, cell migration (e.g., egression from the periosteum), and lineage commitment.
Periosteum Tissue Mechanics
Understanding the mechanical properties of the periosteum at the tissue level is necessary to predict the environment of the pluripotent and osteochondroprogenitor cells known to reside within the periosteum.14 Furthermore, knowledge of periosteum mechanical properties may be the key to development of novel materials designed to replace periosteal function or to mimic periosteum's smart properties. Since the periosteum exhibits a composite structure at multiple length scales and viscoelastic behavior over a range of time scales, the measurement and description of a given property should always reference the length and time scale of interest.
It is understood that PDCs taken from a variety of animals and bones have the capacity to generate bone de novo.3,5,77–79 Research has shown that net changes in periosteal strain during stance shift loading after surgery correlate to rapid de novo bone generation in critically sized defects.13 Therefore, mechanical signaling at the tissue level may be responsible for the initiation of bone regeneration at the cellular level. Determination of the mechanobiological environment in which PDCs reside will give insight into how the regenerative capacities of these cells are turned on to form new bone.
Ex vivo periosteal tissue mechanics have been tested primarily using one of two testing methods. In the first method, periosteal mechanics are investigated in situ, while the tissue is still anchored to the bone. The second, more commonly used method involves harvesting the periosteum from intact bones and investigating the tissue mechanics alone.
The periosteum stabilizes bones mechanically during failure. To investigate the biomechanical capacity of the periosteum in intact long bones, femora and tibiae of Wistar rats were subjected to a destructive three-point-bending test protocol. The ultimate strength, stiffness, energy absorption, and deflection were measured in bones stripped of periosteum and bones with intact periosteum. For the femora, all parameters measured exhibited a statistically significant difference, with periosteum-covered femora displaying higher values compared to periosteum-stripped femora. In the tibia, only energy absorption and deflection were significantly higher, indicating that the mechanical role of periosteum varies between long bones.36 In bones with a preserved periosteum, fractured bone ends remained in close apposition and the periosteum remained intact at the conclusion of the bending test. In contrast, periosteum-stripped bones fractured completely, indicating that periosteum plays a mechanical role in stabilizing bone fractures.36
Mechanical loading of periosteal tissue stimulates changes in behavior of the cells within the tissue. The mechanical response of the periosteum to cyclic loading and the effects of loading frequency were studied in the ulnae of adult female rats subjected to dynamic loading at frequencies of 1, 5, and 10 Hz for 360 cycles/day with peak loads ranging from 4.3 to 18 N. After 2 weeks of loading, bone formation on the periosteal and endocortical surfaces was measured. Periosteal bone formation increased in a dose-dependent manner with peak load and compressive strain. No significant bone formation was seen on the endocortical surface for any loading frequency. To determine the effect of loading frequency on periosteal bone formation, a mathematical model was developed in which osteogenesis was assumed to be proportional to the bone tissue strain rate, which in turn was proportional to the extracellular fluid shear stress divided by the extracellular stiffness. This model effectively predicted the periosteal bone formation rate at all loading frequencies, showing that mechanical loading at the tissue level modulates the cellular level via fluid flow induced stresses.80 In a separate study using the same rat ulna end loading model to induce fatigue fracture, rapid proliferative woven bone from the periosteum was evident at 1 week after fracture and had consolidated completely to heal the fracture within 1 month.81
Further studies demonstrate the effects of the tissue level mechanical loading on cell behavior. Cyclic bending loads have been applied to stimulate bone regeneration from the periosteum.77 In one study, a vascularized flap of periosteum was resected from the rabbit tibia and subsequently sutured to the medial side of the knee. The knee underwent mechanical loading as rabbits were allowed to move spontaneously outside their cages for 1 h/day. Four days after surgery, chondrogenic differentiation of mesenchymal precursor cells was observed. The initial cartilage generated was replaced by fibrous tissue and bone, 15 to 30 days postsurgery. At 30 days, a segment of long bone comparable in size to the implanted periosteal flap had formed along the medial side of the knee.77 The results of this study once again show that mechanical loading at the tissue level is transduced to the cellular level, resulting in the formation of new bone from periosteal tissue.
Recently, new methods were developed to measure the prestress of healthy periosteum in situ. Studying fresh ovine femoral periosteum resected from the mid-diaphysis of the femur, prestress was measured as shrinkage of the periosteal tissue upon release from the underlying bone. While it has been known for some time that the periosteum retracts upon release from the underlying bone,19 this study quantified the amount of retraction for the first time. By measuring shrinkage after release of the periosteum from the Sharpey's fibers that anchor the tissue to the bone, it was possible to estimate the amount of endogenous tension (prestress) present in native periosteum. Periosteum from all aspects of the bone experienced an ∼50% decrease in area immediately upon release from the underlying bone. Significantly, more shrinkage occurred in the axial direction, parallel to the axis of the bone, compared to the circumferential or transverse direction for all aspects.14 Hence, not only is the periosteum anisotropic (shows different mechanical behavior dependent on the direction of loading), but it also resides in a mechanically loaded state in vivo (prestress). Idealizing the periosteum as an elastic material, it is then possible to estimate the endogenous prestress by accounting for the previously measured elastic moduli (see next section for details) and the measured shrinkage (strain, described above) in the longitudinal and circumferential directions. Based on this approximation, the periosteum from the anterior of the skeletally mature ovine femur exhibits 12.06±0.40 MPa longitudinal prestress and 0.77±0.43 MPa circumferential prestress (Fig. 3).
The long bones of vertebrates are subjected to substantial mechanical loads during daily activities such as walking, running, and jumping. Bones and the attached periosteum experience increased mechanical stresses during fracture and limb lengthening. The mechanical properties of resected periosteum under different loading conditions have been characterized using a number of experimental protocols. Experimentally determined stress–strain behavior of resected periosteum from ovine femora, avian tibiotarsus, bovine tibia, and swine metacarpus has been reported in the literature14,22,23,82 (Table 2). In each of these studies, the bones from which the periosteum was to be obtained were resected immediately upon death of the animal. Overlying muscle and tendon insertions were cleaned from the bone before periosteal removal. In certain instances, bones with intact periosteum14 or resected periosteum sheets22,23 were cryopreserved until the time of experiment. In some instances of mechanical testing, the periosteum was removed using a template that allowed for the tissue to be kept at its precise in vivo length.14,82 In other studies, observable tissue shrinkage occurred once the tissue was removed from the bone before insertion in the test fixture.22,23 Samples resected from the axial14,22,23,82 and circumferential14,22,23 orientations of long bones have been tested. In all cases, samples were secured in a set of grips designed to prevent the tissue from slipping during testing. A controlled loading rate ranging from a high speed of 0.42 mm/s23 to a low speed of 0.004 mm/s14 was applied until samples reached failure. In one instance, samples were preconditioned for 3–4 cycles before loading to failure.22 The force experienced by the tissue due to loading was measured by a load cell in all instances.
Table 2.
Elastic Moduli of Resected Periosteum from Different Bones and Animals
| Region | E toe (MPa) | E axial (MPa) | E circ (MPa) | References |
|---|---|---|---|---|
| Ovine femur | 1.93 (0.79, 3.06) | 25.67 (18.8, 32.5) | 4.41 (3.19, 5.62) | 14 |
| Porcine metacarpus | N/A | 79 (42, 116) | 96 (64, 128) | 22 |
| Chick tibiotarsus | 3.4 (1.5, 5.2) | 230 (140, 320) | N/A | 82 |
| Bovine tibia | 0.43 (0.16, 0.7) | 51 (15, 87) | 72 (22, 122) | 23 |
Confidence intervals for the elastic moduli are listed in parentheses.
N/A, not available.
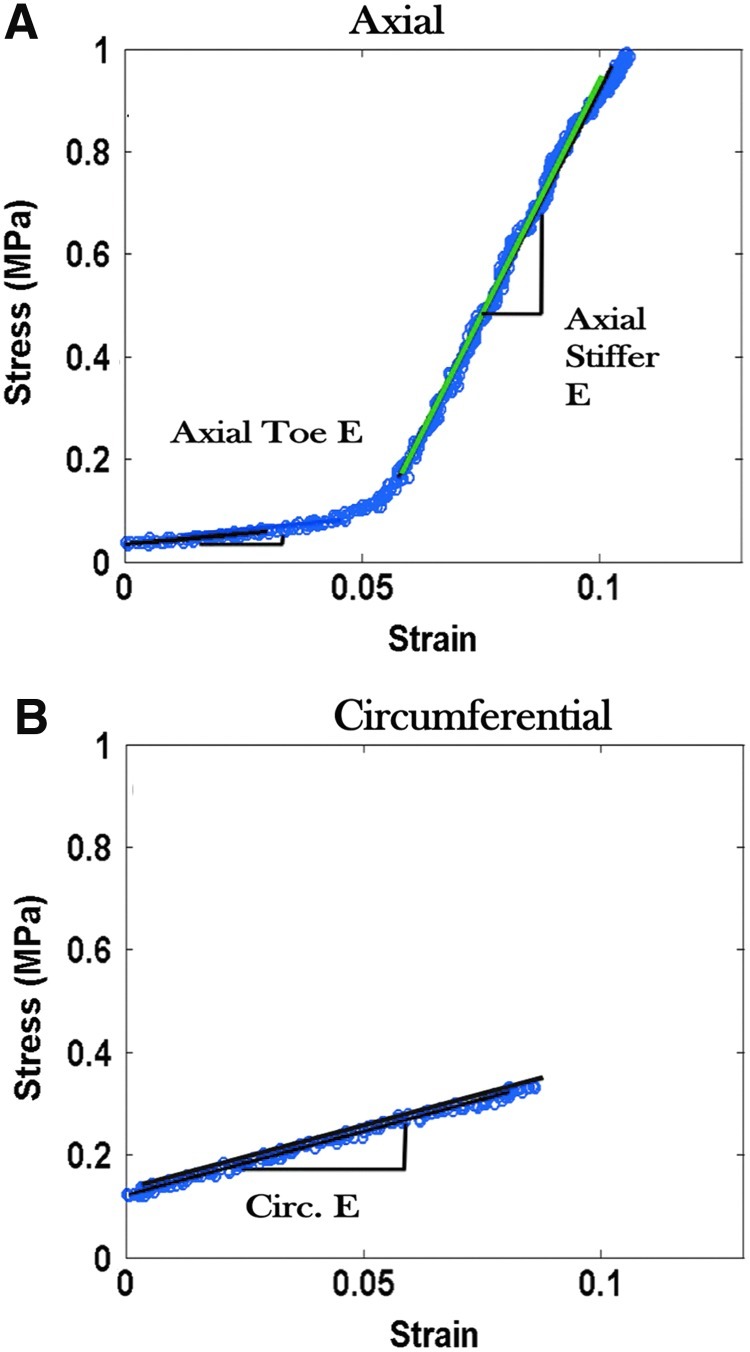
Periosteal stress-strain curves were created from test data (Fig. 4). For all animals and bones, periosteal stress–strain curves obtained for axial samples generally exhibit a compliant toe region followed by a much stiffer elastic region (Table 2, Fig. 4). The elastic modulus for axial samples varies depending on the animal and bone from which the sample was obtained. Circumferential samples exhibit highly variable behavior dependent upon the bone and animal of origin (Table 2).
FIG. 4.
Stress–strain properties of ovine femoral periosteum removed from the (A) axial and (B) circumferential orientations of the bone. Axial periosteum exhibits a high stiffness (E=25.67 MPa) in the linear region of the stress–strain curve. Used with permission from Ref.14 Color images available online at www.liebertpub.com/teb
To date, the only report of anisotropic mechanical properties in periosteum are from measurements of the ovine femur. Interestingly, bone denuded of periosteum shows robust regeneration of periosteum over time, but the thickness of regenerated periosteum does not correlate to the loading history to which the tissue is exposed.64 Periosteal thickness varies significantly depending on the bone, site on the surface of a particular bone, within and between animals of experimental cohorts.29 Similar to the structural properties, the mechanical properties of the periosteum are site specific, depending on the bone, location on the surface of the bone, and the species and age of animal of origin. Given structure–function relationships in biomaterials, the varying mechanical properties of long bone periosteum likely indicate varying tissue composition and collagen fiber alignment.14,33,82
In addition to the long bone periosteum, the mechanical properties of swine zygomatic arch and mandible22 and human nasal83 periosteum have been characterized. The mechanical properties of periosteum from swine zygomatic arch and mandible showed no directional differences as compared to the swine metacarpal in which directional differences were noted.22 Furthermore, shrinkage of the tissue along the long axis varied greatly between the two bones, while shrinkage in the circumferential direction did not vary. Human nasal periosteum has been tested and found to be less extensible than fascia. Furthermore, nasal periosteum exhibits a higher tensile strength than fascia, indicating it may be suitable for implant fixation.83 These results further indicate the variability of the mechanical properties of periosteum among bones. A limitation to consider is that the above-mentioned studies characterize periosteum from bones that do not experience a significant amount of mechanical loading, although it may be argued that these bones, especially the mandible, experience loading of varied direction, on a daily basis.
The degree to which the periosteal tissue serves as a functional interface between muscle and bone is reflected by greatly varying elastic moduli and failure properties of periosteum in varying animals and anatomical locations. This evidence as a whole suggests that the periosteum is a highly specialized, mechanosensitive tissue. The degree to which periosteum serves as a functional barrier between the muscle and bone is an area of intense current interest.28,29 Whereas the periosteum is sometimes assumed to exhibit a complete barrier function, acting as an outer seal to bone,84 it has been postulated that the robust regenerative capacity of the periosteum to infill defects derives from pluripotent cells that ingress, via the periosteum, to the defect site.3,12,15 Recent studies indicate that the periosteum may exhibit directionally dependent permeability and that permeability is highly dependent on the stress-state of the tissue.28,29
The mechanical response of the periosteum to injury may greatly vary dependent upon the location and severity of injury. For example, a mild to moderate tibial fracture may result in fracture of the bone, but allow the periosteum to remain intact, thus keeping the two ends of the fractured bone in close apposition. In this example, the periosteum would operate in the linear elastic region of the stress–strain curve14 and only small stress signals would be sent to PDCs, initiating proliferation and differentiation. In a more severe case, such as a fractured femur, the periosteum as well as the bone may fracture. The strain stiffening incurred by the periosteum just before fracture would correlate to the linear region of the stress–strain curve14 (Fig. 4). The large strains experienced would be transduced to PDCs residing in the inner cambium layer and stimulate rapid proliferation and differentiation of all periosteal progenitor cells from local as well as surrounding areas.
Bridging the Cell-Tissue Level: Tissue Engineered Periosteum
Although a number of studies have used native periosteum to heal critical size bone defects,5,77–79 alternatives are sought for the case when periosteum availability is limited. In many cases, native periosteum contains too few PDCs or has been damaged therefore limiting the tissue available for use. It is therefore desirable to create a TEP for use in these instances.
Many attempts to create a successful TEP have occurred over the last two decades. Early attempts at designing TEP focused on creating a confluent sheet of cells mechanically robust enough to be removed from the tissue culture plastic without tearing.85 Few studies have characterized the mechanical properties of TEP. Current TEP is often intended for use in oral applications, where it would experience significantly less mechanical stress and strain than in a dynamically loaded environment such as the femur. It is imperative to consider the mechanical properties of the native tissue to design TEP to be used in mechanically loaded environments such as the long bones of the leg.
TEP has been created using a variety of natural and synthetic biomaterials in combination with BMSCs and PDCs (Table 3). Porcine small intestinal submucosa (SIS) seeded with BMSCs is one popular combination for creating TEP.85,86 Use of a natural scaffold, such as SIS, eliminates concerns related to biodegradability and toxicity that are often associated with synthetic polymer biomaterials. SIS periosteum membranes were 100–200 μm thick after 7 days of growth. The membranes were somewhat permeable and allowed for the diffusion of nutrients from interstitial fluid to the cells. Membrane mechanics were characterized qualitatively and found to be of a flexible texture similar to natural bone membrane.85,86
Table 3.
| References | Animal | Source | Cell type | Scaffold | Application |
|---|---|---|---|---|---|
| 85 | Rabbit | Femur: medullary cavity | BMSC | SIS | Radial defects |
| 86 | Rabbit | Femur: medullary cavity | BMSC | SIS | N/A |
| 87 | Rabbit | Iliac crest | BMSC | N/A | Mandibular fracture |
| 88 | Human | N/A | BMSC | Collagen membranes | Skull defects |
| 89 | Rat | Femur and tibia: medullary cavity | BMSC | Acellular human dermis | Mandibular defects |
| 47 | Human | Mandible | PDC | Collagen membrane | N/A |
| 90 | Calf | Humerus | PDC | Polyglycolic acid | Femoral defects in rats |
| 91 | Rat | Tibia | PDC | SIS | Critical-sized calvarial defects |
| 92 | Human | Mandible | PDC | Collagen membrane with platelet-rich fibrin | N/A |
| 93 | Rabbit | Tibia | PDC | Fibrin gel or Ethisorb-fleece cylinders | Ulnar defects |
| 94 | Human | Tibia | PDC | Ethisorb polymer scaffolds | N/A |
| 95 | Dog | Mandible | PDC | Either no scaffold or PTFE | Mandibular defects |
| 96 | Human | Mandible | PDC | Hydroxyapatite blocks | Periodontal defects |
| 97 | Human | Mandible | PDC | N/A | Periodontal defects |
| 98 | Ovine | Femur | PDC | Elastomeric membrane±collagen sheets (±PDCs) or periosteal strips | Critical-sized femoral defects |
| 99 | Human | Mandible | PDC | Porous poly(L-lactic acid) membranes | N/A |
| 100 | Human | Mandible | PDC | Polydioxanone/pluronic F127 | Porcine mandibular defects |
SIS, small intestinal submucosa.
Scaffold-free TEP has been grown from BMSCs cultured in osteogenic media.87 At 10 days of culture, a thin membrane with poor resistance to external forces had formed. Further culture until day 17 resulted in a mechanically robust sheet with a tissue structure similar to the cambium layer of the native periosteum.87 In the above studies, the mechanical properties of the TEP were not characterized quantitatively.
In addition to BMSCs, PDCs from a variety of sources have been used to engineer TEP.47,90–100 PDCs have been used to construct TEP with and without the use of scaffolds. Rat femoral shaft defects were successfully healed using TEP constructed from PDCs seeded on a polyglycolic acid scaffold.90 TEP constructs constructed from porcine SIS and PDCs were not as effective as autologous bone grafts in healing rat calvarial critical size defects.91 A TEP construct composed of an elastomer surgical membrane filled with autologous periosteum strips was found to promote healing of critically sized ovine femoral defects.98 The membrane allowed for the directional delivery of periosteal factors to the defect. Studies are currently in progress to determine the material and mechanical properties of the elastomer membrane.
Scaffolds used to create TEP are designed to influence cell differentiation via cell–matrix interactions.101,102 It is therefore important to classify the mechanical properties of TEP to match those of the native tissue. Furthermore, creating TEP with variable stiffness and elastic moduli is desirable as the local mechanical environment in which the tissue is implanted varies from bone to bone. In a load-bearing bone, such as the femora or tibia, TEP with a high-elastic modulus would be desired, whereas a TEP with a lower elastic modulus could be used in a nonmechanically loaded environment such as the mandible.
Future Directions
As the behavior of PDCs in response to tissue-level mechanical forces is further characterized, it will become possible to create a biomimetic TEP. In addition to the tensile mechanical properties of the tissue, it is important to characterize other mechanical and material properties, such as the fatigue life of the tissue and the permeability, pore size, and transport gradients. Together with what is already known about the periosteum, knowledge of these properties will allow for an understanding of the mechanobiological environment in which PDCs reside. The importance of the mechanical and material properties of periosteum should not be underestimated when formulating design criteria for TEP. Current research shows that periosteum resides in a mechanobiological environment. The mechanical environment is highly regulated and varies from bone to bone. Further research is necessary to determine the factors responsible for the variety of periosteal mechanical properties. However, it is hypothesized that the local microstructure of the periosteum and collagen content of the tissue contributes directly to ultimate tensile strength of the tissue.14,33
Mechanical stimulation of the intact periosteum surrounding a bone defect leads to new bone formation. The PDCs within the periosteum are regulated by the mechanical environment in which they reside. Release of the inherent tension in periosteum stimulates PDCs to proliferate and differentiate. Further characterization of PDC behavior in response to substrate stiffness, shear fluid flow, and cell seeding density is necessary to fully understand how mechanical signaling regulates cell growth and development. It is hypothesized that mechanical loads applied at the tissue level send signals to PDCs which, in turn, release soluble factors responsible for regulating tissue growth and development.17 TEP must therefore be designed with the mechanical properties at the forefront of the design criteria. A successful TEP will be able to withstand the forces experienced by the native periosteum to allow for proper bone regeneration. Furthermore, the location of the periosteum to be replaced must also be considered when designing TEP.
Knowledge of the mechanical properties of the periosteum and the mechanosensitivity of PDCs is also important for incorporation in multiscale models. The ability to predict stresses, strains, and fluid flow at the tissue and cellular level will allow for determination of the effects of these variables on the organ-scale structure of bone during growth, health, healing, trauma, and disease. Multiscale models will prove valuable for the development of tissue-engineered strategies in healing bone as well as physical therapy protocols to improve outcome postsurgery. The mechanobiological properties of the periosteum are of increasing importance to understand bone's micromechanical milieu and fully harness the regenerative potential of the periosteum in critical size defect healing. Finally, an understanding of the periosteum's smart properties provides inspiration for a new class of advanced (bio)materials that harness and change their behavior based on the mechanical cues inherent to their environment. Alas, the identification and assessment of smart properties alone does not pave a straight path for the development of advanced materials; for a hallmark of smart properties is their emergent nature, where the whole is much greater than the sum of the parts, making reverse engineering of the property inefficient at best. In contrast, application of bottom-up approaches and the use of first principles to engineer emergence is a promising although relatively unstudied pathway for advanced materials innovation.
Acknowledgments
Parts of this work were supported by funding from the AO Research Fund F-07-99K. In addition, financial support was provided by the National Science Foundation, Grant #CMMI-0826435 and the Alexander von Humboldt Foundation.
Disclosure Statement
The intellectual property behind the periosteum substitute membrane described (from the corresponding author's laboratory, M.K.T.) has been declared through an IDA and is a pending patent. This does not change M.K.T.'s adherence to the journal's best practices and publishing guidelines.
References
- 1.Bishop G.B. Einhorn T.A. Current and future clinical applications of morphogenetic proteins in orthopaedic trauma surgery. Int Orthop. 2007;31:721. doi: 10.1007/s00264-007-0424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Academy of Orthopedic Surgeons. The Burden of Musculoskeletal Diseases in the United States: Musculoskeletal Injuries. 2008. www.boneandjointburden.org/pdfs/BMUS_chpt6_injuries.pdf www.boneandjointburden.org/pdfs/BMUS_chpt6_injuries.pdf
- 3.Knothe Tate M.L. Ritzman T.F. Schneider E. Knothe U.R. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum for the repair of long-bone defects. J Bone Joint Surg Am. 2007;89:307. doi: 10.2106/JBJS.E.00512. [DOI] [PubMed] [Google Scholar]
- 4.Alonso J.E. Regazzoni P. Bridging bone gaps with the Ilizarov technique. Biologic principles. Clin Plast Surg. 1991;18:497. [PubMed] [Google Scholar]
- 5.Knothe U.R. Springfield D.S. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh C.W. Song H.R. Roh J.Y. Oh J.K. Min W.K. Kyung H.S. Kim J.W. Kim P.T. Ihn J.C. Bone transport over an intramedullary nail for reconstruction of long bone defects in tibia. Arch Orthop Trauma Surg. 2008;128:801. doi: 10.1007/s00402-007-0491-8. [DOI] [PubMed] [Google Scholar]
- 7.Isaksson H. Comas O. van Donkelaar C.C. Mediavilla J. Wilson W. Huiskes R. Ito K. Bone regeneration during distraction osteogenesis: mechano-regulation by shear strain and fluid velocity. J Biomech. 2007;40:2002. doi: 10.1016/j.jbiomech.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Vidyadhara S. Rao S.K. A novel approach to juxta-articular aggressive and recurrent giant cell tumours: resection arthrodesis using bone transport over an intramedullary nail. Int Orthop. 2007;31:179. doi: 10.1007/s00264-006-0150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon D.K. Dougall T.W. Pool R.D. Simonis R.B. Augmentative Ilizarov external fixation after failure of diaphyseal union with intramedullary nailing. J Orthop Trauma. 2002;16:491. doi: 10.1097/00005131-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Carrington N.C. Smith R.M. Knight S.L. Matthews S.J.E. Ilizarov bone transport over a primary tibial nail and free flap: a new technique for treating Gustilo grade 3b fractures with large segmental defects. Injury. 2000;31:112. doi: 10.1016/s0020-1383(99)00225-9. [DOI] [PubMed] [Google Scholar]
- 11.Li G. Berven S. Athanasou N.A. Simpson A.H.R.W. Bone transport over an intramedullary nail: A case report with histologic examination of the regenerated segment. Injury. 1999;30:525. doi: 10.1016/s0020-1383(99)00112-6. [DOI] [PubMed] [Google Scholar]
- 12.Knothe U.R. Dolejs S. Miller R.M. Knothe Tate M.L. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect healing. J Biomech. 2010;43:2728. doi: 10.1016/j.jbiomech.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 13.McBride S.H. Dolejs S. Brianza S. Knothe U.R. Knothe Tate M.L. Net change in periosteal strain during stance shift loading after surgery correlates to rapid de novo bone generation in critically sized defects. Ann Biomed Eng. 2011;39:1570. doi: 10.1007/s10439-010-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride S.H. Evans S.F. Knothe Tate M.L. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J Biomech. 2011;44:1954. doi: 10.1016/j.jbiomech.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knothe Tate M.L. Dolejs S. McBride S.H. Miller R.M. Knothe U.R. Multiscale mechanobiology of de novo bone generation, remodeling and adaptation of autograft in a common ovine femur model. J Mech Behav Biomed Mater. 2011;4:829. doi: 10.1016/j.jmbbm.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foolen J. van Donkelaar C.C. Murphy P. Huiskes R. Ito K. Residual periosteum tension is insufficient to modulate bone growth. J Biomech. 2009;42:152. doi: 10.1016/j.jbiomech.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Foolen J. van Donkelaar C.C. Ito K. Intracellular tension in periosteum/perichondrium cells regulates long bone growth. J Orthop Res. 2011;29:84. doi: 10.1002/jor.21224. [DOI] [PubMed] [Google Scholar]
- 18.Saris D.B.F. Sanyal A. An K.N. Fitzsimmons J.S. O'Driscoll S.W. Periosteum responds to dynamic fluid pressure by proliferating in vitro. J Orthop Res. 1999;17:668. doi: 10.1002/jor.1100170508. [DOI] [PubMed] [Google Scholar]
- 19.Simon T.M. Van Sickle D.C. Kunishima D.H. Jackson D.W. Cambium cell stimulation from surgical release of the periosteum. J Orthop Res. 2003;21:470. doi: 10.1016/S0736-0266(02)00206-1. [DOI] [PubMed] [Google Scholar]
- 20.Ellender G. Feik S.A. Carach B.J. Periosteal structure and development in a rat caudal vertebra. J Anat. 1988;158:173. [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Qtaitat A. Shore R.C. Aaron J.E. Structural changes in the ageing periosteum using collagen III immuno-staining and chromium labeling as indicators. J Musculoskelet Neuronal Interact. 2010;10:112. [PubMed] [Google Scholar]
- 22.Popowics T.E. Zhu Z. Herring S.W. Mechanical properties of the periosteum in the pig, Sus scrofa. Arch Oral Biol. 2002;47:733. doi: 10.1016/s0003-9969(02)00065-1. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama E. Yamakoshi K. Sasaki T. Measurement of mechanical characteristics of tibial periosteum and evaluation of local differences. J Biomech Eng. 1998;120:85. doi: 10.1115/1.2834311. [DOI] [PubMed] [Google Scholar]
- 24.Knothe Tate M.L. Smart body armor inspired by flow in bone. Smart Struct Syst. 2011;7:1. [Google Scholar]
- 25.Knothe Tate M.L. Anderson E.J. Flow directing materials and systems, US Patent No. 12106748. 2008.
- 26.Mukherjee S. Ganguli R. A dragonfly inspired flapping wing actuated by electroactive polymers. Smart Struct Syst. 2010;6:867. [Google Scholar]
- 27.Knothe Tate M.L. Top down and bottom up engineering of bone. J Biomech. 2011;44:304. doi: 10.1016/j.jbiomech.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Evans S.F. Parent J. Knothe Tate M.L. Periosteum permeability exhibits mechanically responsive and direction dependent behavior. Trans ORS. 2012;36:1355. [Google Scholar]
- 29.Evans S.F. Parent J. Lasko C. Zhen C. Knothe U. Lemaire T. Knothe Tate M.L. Periosteum, bone's “smart” bounding membrane, exhibits direction dependent permeability. J Bone Miner Res. 2012 Sep 27; doi: 10.1002/jbmr.1777. [DOI] [PubMed] [Google Scholar]
- 30.Cao W. Cudney H.H. Waser R. Smart materials and structures. Proc Natl Acad Sci U S A. 1999;96:8330. doi: 10.1073/pnas.96.15.8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moore S.R. Milz S. Knothe Tate M.L. Relation of periosteal thickness and cellularity to loading history. Trans ORS. 2013;38:0733. [Google Scholar]
- 32.Brookes M. Revell W.J. Blood supply of bone. Scientific aspects. Springer: Verlag; 1998. [Google Scholar]
- 33.Foolen J. Van Donkelaar C. Nowlan N. Murphy P. Huiskes R. Ito K. Collagen orientation in periosteum and perichondrium is aligned with preferential directions of tissue growth. J Orthop Res. 2008;26:1263. doi: 10.1002/jor.20586. [DOI] [PubMed] [Google Scholar]
- 34.Allen M.R. Burr D.B. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 2005;36:311. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Allen M.R. Hock J.M. Burr D.B. Periosteum: biology, regulation and response to osteoporosis therapies. Bone. 2004;35:1003. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 36.Yiannakopoulos C.K. Kanellopoulos A.D. Trovas G.P. Dontas I.A. Lyritis G.P. The biomechanical capacity of the periosteum in intact long bones. Arch Orthop Trauma Surg. 2008;128:117. doi: 10.1007/s00402-007-0433-5. [DOI] [PubMed] [Google Scholar]
- 37.Dominici M. Le Blanc K. Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. 2003;8:315. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 38.Chang H. Knothe Tate M.L. The periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Transl Med. 2012;1:480. doi: 10.5966/sctm.2011-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knothe Tate M.L. Falls T. McBride S.H. Atit R. Knothe U.R. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40:2720. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aubin J. Triffitt J. Mesenchymal stem cells and osteoblast differentiation. In: Bilezikian J., editor; Raisz L.G., editor; Rodan G.A., editor. Principles of Bone Biology. San Diego: Academic Press; 2002. p. 59. [Google Scholar]
- 41.Zhang X. Naik A. Xie C. Reynolds D. Palmer J. Lin A. Awad H. Guldberg R. Schwarz E. O'Keefe R. Periosteal stem cells are essential for bone revitalization and repair. J Musculoskelet Neuronal Interact. 2005;5:360. [PubMed] [Google Scholar]
- 42.Knothe Tate M.L. Dolejs S. Miller R.M. Knothe U.R. Role of mechanical loading in healing of massive bone autografts. J Orthop Res. 2010;28:1657. doi: 10.1002/jor.21190. [DOI] [PubMed] [Google Scholar]
- 43.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans S.F. Docheva D. Bernecker A. Colnot C. Richter R.P. Knothe Tate M.L. Solid supported lipid bilayers as a novel platform to engineer emergence of stem cell fate and tissue architecture using periosteum derived progenitor cells. Biomaterials. 2013;234:1878. doi: 10.1016/j.biomaterials.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 45.Schneeberger E.E. Lynch R.D. Structure, function and regulation of cellular tight junctions. Am J Physiol Lung Cell Mol Physiol. 1992;262:L647. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- 46.Li M. Amizuka N. Oda K. Tokunaga K. Ito T. Takeuchi K. Takagi R. Maeda T. Histochemical evidence of the initial chondrogenesis and osteogenesis in the periosteum of a rib fracture model: implications of osteocyte involvement in periosteal chondrogenesis. Microsc Res Tech. 2004;64:330. doi: 10.1002/jemt.20088. [DOI] [PubMed] [Google Scholar]
- 47.Warnke P.H. Douglas T. Sivananthan S. Wiltfang J. Springer I. Becker S.T. Tissue engineering of periosteal cell membranes in vitro. Clin Oral Implants Res. 2009;20:761. doi: 10.1111/j.1600-0501.2009.01709.x. [DOI] [PubMed] [Google Scholar]
- 48.Solchaga L.A. Cassiede P. Caplan A.I. Different response to osteo-inductive agents in bone marrow- and periosteum derived cell preparations. Acta Orthop Scand. 1998;69:426. doi: 10.3109/17453679808999061. [DOI] [PubMed] [Google Scholar]
- 49.Kubo K. Att W. Yamada M. Ohmi K. Tsukimura N. Suzuki T. Maeda H. Ogawa T. Microtopography of titanium suppresses osteoblastic differentiation but enhances chodroblastic differentiation of rat femoral periosteum-derived cells. J Biomed Mater Res A. 2008;87A:380. doi: 10.1002/jbm.a.31791. [DOI] [PubMed] [Google Scholar]
- 50.Takeuchi K. Saruwatari L. Nakamura H.K. Yang J.M. Ogawa T. Enhanced intrinsic biomechanical properties of osteoblastic mineralized tissue on roughened titanium surface. J Biomed Mater Res A. 2004;72A:296. doi: 10.1002/jbm.a.30227. [DOI] [PubMed] [Google Scholar]
- 51.Merritt F. Erinc A. Knothe Tate M.L. Periosteum regenerates on periosteum-denuded, transported bone segment. Trans ORS. 2012;36:1570. [Google Scholar]
- 52.Agata H. Ashahina I. Yamazaki Y. Effective bone engineering with periosteum derived cells. J Dent Res. 2007;86:79. doi: 10.1177/154405910708600113. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi O. Katsube Y. Hirose M. Comparison of osteogenic ability of rat mesenchymal stem cells from bone marrow, periosteum and adipose tissue. Calcif Tissue Int. 2008;82:238. doi: 10.1007/s00223-008-9112-y. [DOI] [PubMed] [Google Scholar]
- 54.Tarng Y.W. Casper M.E. Fitzsimmons J.S. Stone J.J. Bekkers J. An K.N. Su F.C. O'Driscoll S.W. Reinholz G.G. Direstional fluid flow enhances in vitro periosteal tissue growth and chondrogenesis on poly-ɛ-caprolactone scaffolds. J Biomed Mater Res A. 2010;95:156. doi: 10.1002/jbm.a.32830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amsdorf E.J. Jones L.M. Carter D.R. Jacobs C.R. The periosteum as a cellular source for functional tissue engineering. Tissue Eng Part A. 2009;15:2637. doi: 10.1089/ten.tea.2008.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsushima S. Isogai N. Jacquet R. Lowder E. Tokui T. Landis W.J. The nature and role of periosteum in bone and cartilage regeneration. Cells Tissues Organs. 2011;194:320. doi: 10.1159/000324642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi Y.-S. Noh S.-E. Lim S.-M, et al. Multipotency and growth characteristic of periosteum-derived progenitor cells for chondrogenic, osteogenic, and adipogenic differentiation. Biotechnol Lett. 2008;30:593. doi: 10.1007/s10529-007-9584-2. [DOI] [PubMed] [Google Scholar]
- 58.Alexander D. Schäfer F. Olbrich M., et al. MSCA-1/TNAP selection of human jaw periosteal cells improves their mineralization capacity. Cell Physiol Biochem. 2010;26:1073. doi: 10.1159/000323985. [DOI] [PubMed] [Google Scholar]
- 59.De Bari C. Dell'Accio F. Vanlauwe J., et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 60.Lim S.M. Choi Y.S. Shin H.C., et al. Isolation of human periosteum-derived progenitor cells using immunophenotypes for chondrogenesis. Biotechnol Lett. 2005;27:607. doi: 10.1007/s10529-005-3625-5. [DOI] [PubMed] [Google Scholar]
- 61.McBride S.H. Knothe Tate M.L. Modulation of stem cell shape and fate B: mechanical modulation of cell shape and gene expression. Tissue Eng Part A. 2008;14:1573. doi: 10.1089/ten.tea.2008.0113. [DOI] [PubMed] [Google Scholar]
- 62.Song M.J. Dean D. Knothe Tate M.L. In situ spatiotemporal mapping of flow fields around seeded stem cells at the subcellular length scale. PLoS One. 2010;5:e12796. doi: 10.1371/journal.pone.0012796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zimmerman J.A. Knothe Tate M.L. Structure-function relationships in the stem cell's mechanical world A: seeding protocols as a means to control shape and fate of live stem cells. Mol Cell Biomech. 2011;8:275. [PubMed] [Google Scholar]
- 64.Chang H. Knothe Tate M.L. Structure-function relationships in the stem cell's mechanical world B: emergent anisotropy of the cytoskeleton correlates to volume and shape changing stress exposure. Mol Cell Biomech. 2011;8:297. [PMC free article] [PubMed] [Google Scholar]
- 65.Song M.J. Brady-Kalnay S. Phillips-Mason P. Dean D. Knothe Tate M.L. Mapping the mechanome of live stem cells: using a novel method to measure local strain fields in situ at the fluid-cell interface exposed to controlled exogenous forces. Novel methods to decipher the stem cell's mechanome. PLoS One. 2012;7:e43601. doi: 10.1371/journal.pone.0043601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson E.J. Knothe Tate M.L. Design of tissue engineering scaffolds as delivery devices for mechanical and mechanically modulated signals. Tissue Eng. 2007;13:2525. doi: 10.1089/ten.2006.0443. [DOI] [PubMed] [Google Scholar]
- 67.Anderson E.J. Knothe Tate M.L. Open access to novel dual flow chamber technology for in vitro cell mechanotransduction, toxicity and pharamacokinetic studies. Biomed Eng Online. 2007;6:46. doi: 10.1186/1475-925X-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Anderson E.J. Falls T.D. Sorkin A.M. Knothe Tate M.L. The imperative for controlled mechanical stresses in unraveling cellular mechanisms of mechanotransduction. Biomed Eng Online. 2006;5:27. doi: 10.1186/1475-925X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;18:310. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 70.Li D. Zhou J. Chowdhury F. Cheng J. Wang N. Wang F. Role of mechanical factors in fate decisions of stem cells. Regen Med. 2011;6:229. doi: 10.2217/rme.11.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McBride S.H. Knothe Tate M.L. Modulation of stem cell shape and fate A: the role of density and seeding protocol on nucleus shape and gene expression. Tissue Eng Part A. 2008;14:1561. doi: 10.1089/ten.tea.2008.0112. [DOI] [PubMed] [Google Scholar]
- 72.Carpenter R.D. Carter D.R. The mechanobiological effects of periosteal surface loads. Biomech Model Mechanobiol. 2008;7:227. doi: 10.1007/s10237-007-0087-9. [DOI] [PubMed] [Google Scholar]
- 73.de Mara C.S. Sartori A.R. Duarte A.S. Andrade A.L.L. Pedro M.A.C. Coimbra I.B. Periosteum as a source of mesenchymal stem cells: the effects of TGF-β3 on chondrogenesis. Clinics. 2011;66:487. doi: 10.1590/S1807-59322011000300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones D.B. Nolte H. Scholübbers J.G. Turner E. Veltel D. Biochemical signal transduction of mechanical strain in osteoblast-like cells. Biomaterials. 1991;12:101. doi: 10.1016/0142-9612(91)90186-e. [DOI] [PubMed] [Google Scholar]
- 75.Kanno T. Takahashi T. Ariyoshi W. Tsujisawa T. Haga M. Nishihara T. Tensile mechanical strain up-regulates Runx2 and osteogenic factor expression in human periosteal cells: implications for distraction osteogenesis. J Oral Maxillofac Surg. 2005;63:499. doi: 10.1016/j.joms.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 76.Costessi A. Pines A. D'Andrea P, et al. Extracellular nucleotides activate Runx2 in the osteoblast-like HOBIT cell line: a possible molecular link between mechanical stress and osteoblasts' response. Bone. 2005;36:418. doi: 10.1016/j.bone.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 77.Moukoko D. Pourquier D. Pithioux M. Chabrand P. Influence of cyclic bending loading on in vivo skeletal tissue regeneration from periosteal origin. Orthop Traumatol Surg Res. 2010;96:833. doi: 10.1016/j.otsr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 78.Kanou M. Ueno T. Kagawa T. Fujii T. Sakata Y. Ishida N. Fukunaga J. Sugahara T. Osteogenic potential of primed periosteum graft in the rat calvarial model. Ann Plast Surg. 2005;54:71. doi: 10.1097/01.sap.0000139562.42726.dd. [DOI] [PubMed] [Google Scholar]
- 79.Reynders P. Becker J. Broos P. The osteogenic potential of free periosteal autografts in tibial fractures with severe soft tissue damage: an experimental study. Acta Orthop Belg. 1998;64:184. [PubMed] [Google Scholar]
- 80.Hsieh Y.F. Turner C.H. Effects of loading frequency on mechanically induced bone formation. J Bone Miner Res. 2001;16:918. doi: 10.1359/jbmr.2001.16.5.918. [DOI] [PubMed] [Google Scholar]
- 81.Tami A. Nasser P. Schaffler M.B. Knothe Tate M.L. A novel, non-invasive fatigue fracture model of the rat ulna. J Orthop Res. 2003;21:1018. doi: 10.1016/S0736-0266(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 82.Bertram J.E.A. Polevoy Y. Cullinane D.M. Mechanics of avian fibrous periosteum: tensile and adhesion properties during growth. Bone. 1998;22:669. doi: 10.1016/s8756-3282(98)00035-0. [DOI] [PubMed] [Google Scholar]
- 83.Zeng Y.Y. Sun X.P. Yang J. Wu W.H. Xu X.H. Yan Y.P. Mechanical properties of nasal fascia and periosteum. Clin Biomech. 2003;18:760. doi: 10.1016/s0268-0033(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 84.Tami A. Schaffler M.B. Knothe Tate M.L. Probing the tissue to subcellular level structure underlying bone's molecular sieving function. Biorheology. 2003;40:577. [PubMed] [Google Scholar]
- 85.Zhao L. Zhao J.L. Wan L. Wang S.K. The study of the feasibility of segmental bone defect repair with tissue-engineered bone membrane: a qualitative observation. Strategies Trauma Limb Reconstr. 2008;3:57. doi: 10.1007/s11751-008-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao L. Zhao J. Wang S. Xia Y. Liu J. He J. Wang X. Evaluation of immunocompatibility of tissue-engineered periosteum. Biomed Mater. 2011;6:015005. doi: 10.1088/1748-6041/6/1/015005. [DOI] [PubMed] [Google Scholar]
- 87.Ma D. Yao H. Tian W. Chen F. Liu Y. Mao T. Ren L. Enhancing bone formation by transplantation of a scaffold-free tissue-engineered periosteum in a rabbit model. Clin Oral Implants Res. 2011;22:1193. doi: 10.1111/j.1600-0501.2010.02091.x. [DOI] [PubMed] [Google Scholar]
- 88.Fan W. Crawford R. Xiao Y. Enhancing in vivo vascularized bone formation by cobalt chloride–treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials. 2010;31:3580. doi: 10.1016/j.biomaterials.2010.01.083. [DOI] [PubMed] [Google Scholar]
- 89.Schönmeyr B. Clavin N. Avraham T. Longo V. Mehrara B.J. Synthesis of a tissue-engineered periosteum with acellular dermal matrix and cultured mesenchymal stem cells. Tissue Eng A. 2009;15:1833. doi: 10.1089/ten.tea.2008.0446. [DOI] [PubMed] [Google Scholar]
- 90.Puelacher W.C. Vacanti J.P. Ferraro N.F. Schloo B. Vacanti C.A. Femoral shaft reconstruction using tissue-engineered growth of bone. Int J Oral Maxillofac Surg. 1996;25:223. doi: 10.1016/s0901-5027(96)80035-x. [DOI] [PubMed] [Google Scholar]
- 91.Keskin M. Kelly C.P. Moreira-Gonzalez A. Lobocki C. Yarim M. Kaplan S. Jackson I.T. Repairing critical-sized rat calvarial defects with a periosteal cell-seeded small intestinal submucosal layer. Plast Reconstr Surg. 2008;122:400. doi: 10.1097/PRS.0b013e31817d6206. [DOI] [PubMed] [Google Scholar]
- 92.Gassling V. Douglas T. Warnke P.H. Acil Y. Wiltfang J. Becker S.T. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res. 2010;21:543. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 93.Perka C. Schultz O. Spitzer R.S. Lindenhayn K. Burmester G.R. Sittinger M. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000;21:1145. doi: 10.1016/s0142-9612(99)00280-x. [DOI] [PubMed] [Google Scholar]
- 94.Arnold U. Lindenhayn K. Perka C. In vitro-cultivation of human periosteum derived cells in bioresorbable polymer-TCP composites. Biomaterials. 2002;23:2303. doi: 10.1016/s0142-9612(01)00364-7. [DOI] [PubMed] [Google Scholar]
- 95.Ribeiro F.V. Suaid F.F. Gonzales K. Ruiz S. Rodrigues T.L. Carvalho M.D. Nociti F.H. Sallum E.A. Casati M.Z. Peri-implant reconstruction using autologous periosteum-derived cells and guided bone regeneration. J Clin Periodontol. 2010;37:1128. doi: 10.1111/j.1600-051X.2010.01635.x. [DOI] [PubMed] [Google Scholar]
- 96.Kawase T. Okuda K. Kogami H. Nakayama H. Nagata M. Sato T. Wolff L.F. Yoshie H. Human periosteum-derived cells combined with superporous hydroxyapatite blocks used as an osteogenic bone substitute for periodontal regenerative therapy: an animal implantation study using nude mice. J Periodontol. 2010;81:420. doi: 10.1902/jop.2009.090523. [DOI] [PubMed] [Google Scholar]
- 97.Yamamiya K. Okuda K. Kawase T. Hata K. Wolff L.F. Yoshie H. Tissue-engineered cultured periosteum used with platelet-rich plasma and hydroxyapatite in treating human osseous defects. J Periodontol. 2008;79:811. doi: 10.1902/jop.2008.070518. [DOI] [PubMed] [Google Scholar]
- 98.Knothe Tate M.L. Chang H. Moore S.R. Knothe U.R. Surgical membranes as directional delivery devices to generate tissue: testing in an ovine critical sized defect model. PLoS One. 2011;6:e28702. doi: 10.1371/journal.pone.0028702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawase T. Tanaka T. Nishimoto T. Okuda K. Nagata M. Burns D.M. Yoshie H. Improved adhesion of human cultured periosteal sheets to a porous poly(L-lactic acid) membrane scaffold without the aid of exogenous adhesion biomolecules. J Biomed Mater Res A. 2011;98A:100. doi: 10.1002/jbm.a.33074. [DOI] [PubMed] [Google Scholar]
- 100.Lee J.H. Kim J.H. Oh S.H. Kim S.J. Hah Y.S. Park B.W. Kim D.R. Rho G.Y. Maeng G.H. Jeon R.H. Lee H.C. Kim J.R. Kim G.C. Kim U.K. Byun J.H. Tissue-engineered bone formation using periosteal-derived cells and polydioxanone/pluronic F127 scaffold with pre-seeded adipose tissue-derived CD146 positive endothelial cells. Biomaterials. 2011;32:5033. doi: 10.1016/j.biomaterials.2011.03.081. [DOI] [PubMed] [Google Scholar]
- 101.Hutmacher D.W. Sittinger M. Periosteal cells in bone tissue engineering. Tissue Eng. 2003;9S:S45. doi: 10.1089/10763270360696978. [DOI] [PubMed] [Google Scholar]
- 102.Reichert J.C. Cipitria A. Epari D.R., et al. A tissue engineering solution for segmental defect regeneration in load-bearing long bones. Sci Transl Med. 2012;4:4. doi: 10.1126/scitranslmed.3003720. [DOI] [PubMed] [Google Scholar]