Abstract
The freshwater crustacean Daphnia is an emerging model system in the biology of aging. Diversity in aging patterns is thought to be caused by ecological variation in selection on age-specific performance. Previous work in Daphnia has shown a strong correspondence between selective differences and genetic variation in aging in the Daphnia pulex species complex. However, recent evidence suggests obligate asexuality could account for the more rapid aging found in pond genotypes compared with lake genotypes without invoking differences in selection. Evolutionary biologists have to date assumed equivalent operation of neutral processes when comparing aging across populations, but a shift in the breeding system changes the basic dynamics of neutral evolution. To test the hypothesis that the breeding system could explain the short lifespans of pond-dwelling Daphnia, we compared aging of sexual and asexual Daphnia clones from temporary ponds. Our data contradict the breeding system hypothesis. Differences in aging between the breeding systems were slight, and trended in the opposite direction from that predicted: asexual clones had longer lifespans and appeared to age more slowly than sexual clones. We conclude that divergent selection between habitats remains the best explanation for differences in aging between Daphnia species.
Keywords: senescence, mutation accumulation, sex, life history, Daphnia
INTRODUCTION
Aging is the degradation of fitness, including both survival and reproduction, as organisms grow older. The evolutionary theory of aging has been a very successful explanation for the existence of aging. The proposition that aging evolves due to a weakening of natural selection as organisms grow older forms the core of the theory (Medawar, 1952; Williams, 1957; Hamilton, 1966). Simply put, alleles with deleterious late-age effects are passed on to the next generation before they are exposed to selection. Numerous experimental tests have supported the theory, and both primary genetic mechanisms, mutation accumulation and antagonistic pleiotropy, have been shown to be involved (e.g. Rose and Charlesworth, 1980; Hughes et al., 2002; reviewed in Hughes and Reynolds, 2005). However, many fewer tests have applied the theory to the problem of why there is great diversity in longevity and rates of aging among taxa. The relevant extension of the evolutionary theory of aging argues that ecological variation can create selective differences among populations in how sharply the force of natural selection declines with age (Edney and Gill, 1968). These selective differences lead to different balances between mutations with age-specific effects and selection against them. At this point, conflicting results from different model systems suggest that explaining the diversity of rates of aging will be more complex than explaining the existence of aging (Keller and Genoud, 1997; Dudycha, 2001; Reznick et al., 2004; Morbey et al., 2005).
Part of the difficulty may be that in field-based comparisons of aging it is difficult to distinguish situations where selective regime varies from those where the neutral evolutionary context varies. Ecologically based comparisons of aging have implicitly assumed that neutral processes operated equivalently in compared populations, despite the possibility for relevant variation, for example, in effective population size (Tatar et al., 1997; Dudycha, 2001; Reznick et al., 2004). It is nonetheless important to consider the context for neutral processes because one of the two genetic mechanisms by which aging can evolve depends explicitly on neutral evolution. Under the antagonistic pleiotropy mechanism, alleles that are advantageous early in life are selected, despite deleterious later effects, and aging arises as a byproduct of positive selection (Williams, 1957). Aging arises because the deleterious effect of an allele at an old age is genetically locked to its beneficial effect at a young age and selection places a premium on youth. Under the mutation accumulation mechanism, alleles with a deleterious effect at late ages accumulate via the inexorable clicking of Muller's Ratchet, but are entirely neutral with respect to early-life phenotypic effects (Hamilton, 1966). The mutation accumulation mechanism follows neutral evolutionary dynamics because there are no effects on fitness before they are passed on to the next generation. Thus, situations which alter the process of mutation accumulation, such as differences in the breeding system, may alter patterns of aging. The theoretical development of these mechanisms is discussed in detail in Rose (Rose, 1991) and Charlesworth (Charlesworth, 1994). With either mechanism, rates of aging have been generally thought to diverge because of differences in selection; the difference between the mechanisms is a focus on early- versus late-life traits.
Daphnia, a common freshwater crustacean whose normal life cycle is cyclic parthenogenesis, is a useful model system for investigating the diversity of aging (Martinez-Jeronimo et al., 1994; Dudycha and Tessier, 1999; Dudycha, 2001, 2003; Sarma et al., 2002; Nandini and Sarma, 2006; Bouchnak and Steinberg, 2010; Pietrzak et al., 2010; Pietrzak, 2011). Dudycha and Tessier (Dudycha and Tessier, 1999) demonstrated that Daphnia pulex ages much more rapidly and has a much shorter lifespan than Daphnia pulicaria, a closely related and morphologically similar sister species. Longevity in D. pulicaria was twice that of D. pulex, and correspondingly large differences were seen in mortality rate parameters and rates of fecundity decline. Further work showed that the genetic differences between species were robust both at high food levels and under caloric restriction (Dudycha, 2003). Daphnia pulex live in temporary ponds, whereas D. pulicaria live in deep temperate lakes, though they appear to be named ecotypes rather than reproductively isolated true species (Dudycha, 2004; Heier and Dudycha, 2009). Dudycha and Tessier (Dudycha and Tessier, 1999) argued that the differences in aging evolved as a result of differing selection pressures between ponds and lakes, and it was later shown that the per-capita average daily extrinsic mortality rate was much higher in ponds than in lakes (Dudycha, 2001, 2004). In addition, there was evidence that genetic differences in aging were maintained despite substantial gene flow between habitat types (Dudycha, 2004). Taken together, this is strong evidence that selective differences between habitats are associated with divergence of aging between Daphnia species.
Dudycha and Tessier (Dudycha and Tessier, 1999) reported modest evidence for antagonistic pleiotropy (rapidly senescing populations had superior juvenile growth and early reproduction) but drew no particular inference about the role of mutation accumulation. Recent evidence has suggested that a potentially confounding factor may lead to higher rates of mutation accumulation in the pond populations of D. pulex than in the lake populations of Daphnia pulicaria irrespective of selective differences. This means that it is possible that the previous results could be explained by variation in the operation of neutral processes rather than in selective differences.
The potentially confounding factor is variation in the breeding system. The typical life cycle of Daphnia is cyclic parthenogenesis, where generations of clonal reproduction are predictably interrupted by sexual generations brought on by adverse environmental conditions. However, in some populations of D. pulex, females are obligately asexual and can produce the normally sexual dormant eggs parthenogenetically (Hebert and Crease, 1980). An important consequence of this is that completely asexual populations harbor a greater genetic load of deleterious mutations in comparison to periodically sexual populations (Paland et al., 2005; Paland and Lynch, 2006). Thus, if D. pulex populations are obligately asexual, they may age more rapidly than D. pulicaria simply due to a reproductive mode more prone to accumulation of deleterious mutations rather than differences in selection pressures.
Paland et al. (Paland et al., 2005; Paland and Lynch, 2006) provided the strongest evidence for geographically widespread obligate asexuality and its consequences for mutation accumulation in D. pulex. Indeed, some of the original D. pulex populations used to show genetic differences in rates of aging by Dudycha (Dudycha, 2001, 2003) were analyzed by Paland et al. (Paland et al., 2005; Paland and Lynch, 2006) and they found some to be obligately asexual (e.g. OL3). Thus, we were interested in whether obligate asexuality could explain the differences in senescence between D. pulex and D. pulicaria through its effect on mutation accumulation in D. pulex. If obligate asexuality is a viable explanation for the large differences between the D. pulex and D. pulicaria ecotypes, we predicted that sexual clones would show greater longevity and markedly slower rates of aging than obligately asexual clones. To test the breeding system/mutation accumulation hypothesis, we conducted a life table experiment comparing aging of Daphnia clones from temporary ponds known to be sexual with aging of clones known to be obligately asexual.
METHOD
We obtained six clones of Daphnia from independent temporary ponds for our experiment. Two (OL3 and LIN) were reported to be obligately asexual and we confirmed this by inducing the production of dormant eggs in the absence of males and successfully hatching them. The OL3 clone is from southwest Michigan, USA (42°36′N, 85°24′W), and the LIN clone is from southern Ontario, Canada (43°32′N, 80°45′W). Three clones were thought to be sexual (RW1, WEST5 and BW16) and we confirmed this by breeding them and genotyping the resulting offspring with allozymes and microsatellites (Heier and Dudycha, 2009). RW1 is from southwest Michigan (42°18′N, 85°19′W), ∼40 km south of OL3, and WEST5 (40°02′ N, 87°57′ W) and BW16 (40°07′ N, 88°12′ W) originated from ponds ∼20 km apart in central Illinois, USA. The sixth clone (TRE, 40°08′N, 88°57′W; central Illinois) was D. obtusa, another species in the same subgenus as D. pulex and D. pulicaria. Daphnia obtusa is a temporary pond species very similar to D. pulex, with no clear ecological axis differentiating the ponds they inhabit. Daphnia obtusa is always sexual, and therefore including it constitutes an additional test of longevity in a sexual pond dweller. If pond populations have short lifespans due to selection pressures, all three categories (cyclically parthenogenetic D. pulex and D. obtusa and obligately asexual D. pulex) should have rapid aging. Conversely, if the short lifespans previously observed in D. pulex were driven by mutation accumulation caused by obligate asexuality, D. obtusa and the cyclically parthenogenetic D. pulex should show greater longevity than the asexual D. pulex clones.
Life tables were conducted using the protocol described in detail by Dudycha and Tessier (Dudycha and Tessier, 1999). For each genotype, we kept 15 beakers of females at a low density (3 individuals per 200 mL filtered lakewater) for three generations prior to the experimental generation to minimize maternal effect variance. These animals, and the experimental animals, were fed a satiating concentration of Scenedesmus obliquus (200 000 cells mL−1) daily, and transferred to fresh filtered (to 1 μm) lakewater on alternate days. All animals were kept in a controlled environment chamber at 20°C on a 12:12 L:D photoperiod cycle. When most of the third generation had passed its second clutch, we collected offspring born in a 12-h window to begin life tables with an even-aged cohort. Our starting numbers for each genotype varied considerably due to variation in production and some male offspring, and since we had only two known obligately asexual genotypes, we sought to have larger numbers in these cohorts. Thus, our life tables began with the following cohort sizes: asexual D. pulex: LIN N = 191, OL3 N = 162; sexual D. pulex: RW1, WEST5, BW16 N = 80 each and sexual D. obtusa: TRE N = 140. We recorded survivorship daily until all animals died, and collected offspring every other day. Based on the offspring size, we could identify whether offspring had been born the day prior to collection.
Juvenile mortality was low (0–5 per clone) and excluded from analyses because aging is an adult phenomenon. Only two observations of adult mortality were censored. Mortality models were fitted to the data via maximum likelihood in WinModest v. 1.0.2 (Pletcher, 1999) starting at the onset of maturity (age = 8 d). A number of mortality models have been proposed, differing in complexity, the number of parameters estimated and whether they are based on biological mechanisms or phenomenological data descriptions. For our clones, WinModest recommended either a Gompertz or logistic model be fit to the data.
The simplest model we considered, a two-parameter Gompertz model, is a common exponential model used to describe demographic aging, and its parameters have a straightforward biological interpretation. The Gompertz equation is 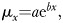 where x is the age, μx is the age-specific mortality rate, a is a baseline, age-independent mortality rate and b represents age-dependent mortality. A positive b indicates the physiological deterioration of aging and greater values indicate faster aging.
where x is the age, μx is the age-specific mortality rate, a is a baseline, age-independent mortality rate and b represents age-dependent mortality. A positive b indicates the physiological deterioration of aging and greater values indicate faster aging.
In the logistic model, age-dependent mortality increases to an asymptote, and is described by b and s in the equation 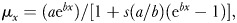 where b is the exponential increase in mortality rates in “early” adulthood and s is the deceleration of mortality rate increase at “old” ages (Vaupel, 1990). The structure of the equations does not allow for direct comparison of parameters between models; the b of the Gompertz model is not interchangeable with the b of the logistic model and the simpler Gompertz model is not nested within the more complex logistic model. Unlike the Gompertz model, the logistic model is a statistical description of data rather than a model of a biological mechanism, and is heavily influenced by a few long-lived individuals. Furthermore, the asymptotic mortality it was designed to describe may in fact be an artifact of stochastic heterogeneity (Service, 2000). Thus, the logistic model is primarily useful in actuarial contexts and other situations where interest is focused on the supposed late-life mortality plateaus. Such is not the case in ecological comparisons since these late ages do not generally occur outside of the protected environment of the laboratory.
where b is the exponential increase in mortality rates in “early” adulthood and s is the deceleration of mortality rate increase at “old” ages (Vaupel, 1990). The structure of the equations does not allow for direct comparison of parameters between models; the b of the Gompertz model is not interchangeable with the b of the logistic model and the simpler Gompertz model is not nested within the more complex logistic model. Unlike the Gompertz model, the logistic model is a statistical description of data rather than a model of a biological mechanism, and is heavily influenced by a few long-lived individuals. Furthermore, the asymptotic mortality it was designed to describe may in fact be an artifact of stochastic heterogeneity (Service, 2000). Thus, the logistic model is primarily useful in actuarial contexts and other situations where interest is focused on the supposed late-life mortality plateaus. Such is not the case in ecological comparisons since these late ages do not generally occur outside of the protected environment of the laboratory.
We compared fit of the models using Akaike's Information Criterion (AIC) rather than a likelihood-ratio test because the models are not nested (Burnham and Anderson, 1998). We calculated the AIC from the –(log) likelihood scores estimated in WinModest, using the formula 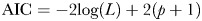 where L is the likelihood and p is the number of parameters in the model.
where L is the likelihood and p is the number of parameters in the model.
The proposition that the breeding system, rather than selective differences, caused previously reported variation in Daphnia lifespan (Dudycha and Tessier, 1999; Dudycha, 2003) leads to a one-tailed prediction about lifespans in the data reported here: that sexual lifespans will be longer than asexual lifespans. The test of our hypothesis is one tailed because we are seeking to understand a previous empirical result that establishes a direction. Applying a two-tailed test would unnecessarily diminish the power of the test and, in fact, bias the comparison in favor of our previous interpretation that selection drives divergence of aging in Daphnia. Since we are trying in this experiment to determine whether alternate explanations are plausible, we want to avoid biasing the analysis in favor of the interpretation offered in our prior work. Conventional approaches to comparing medians (or means) cannot be used for lifespan, due to the inherent non-independence of successive observations of whether an individual has yet to die, but failure time analyses that account for that non-independence necessarily treat individuals as the experimental unit. In our case, the population-of-origin is the appropriate experimental unit and so we report the log-rank test of survival differences, which we conducted in the survival package of R 2.15.1.
Survival and reproduction are complementary components of fitness subject to trade-offs, and analyses of aging should include an assessment of their joint contribution to the state of an average individual but rarely do. We therefore report the intrinsic value, ix, of the populations, a life history statistic that quantifies the age-specific contribution to expected lifetime reproduction (Dudycha and Tessier, 1999). The intrinsic value is calculated as
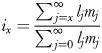 |
It is analogous to the reproductive value, which measures the contribution to r, but the intrinsic value is uninfluenced by factors extrinsic to the average individuals' state, in particular population growth. A steeper decline in the intrinsic value is interpreted as more rapid general aging.
RESULTS
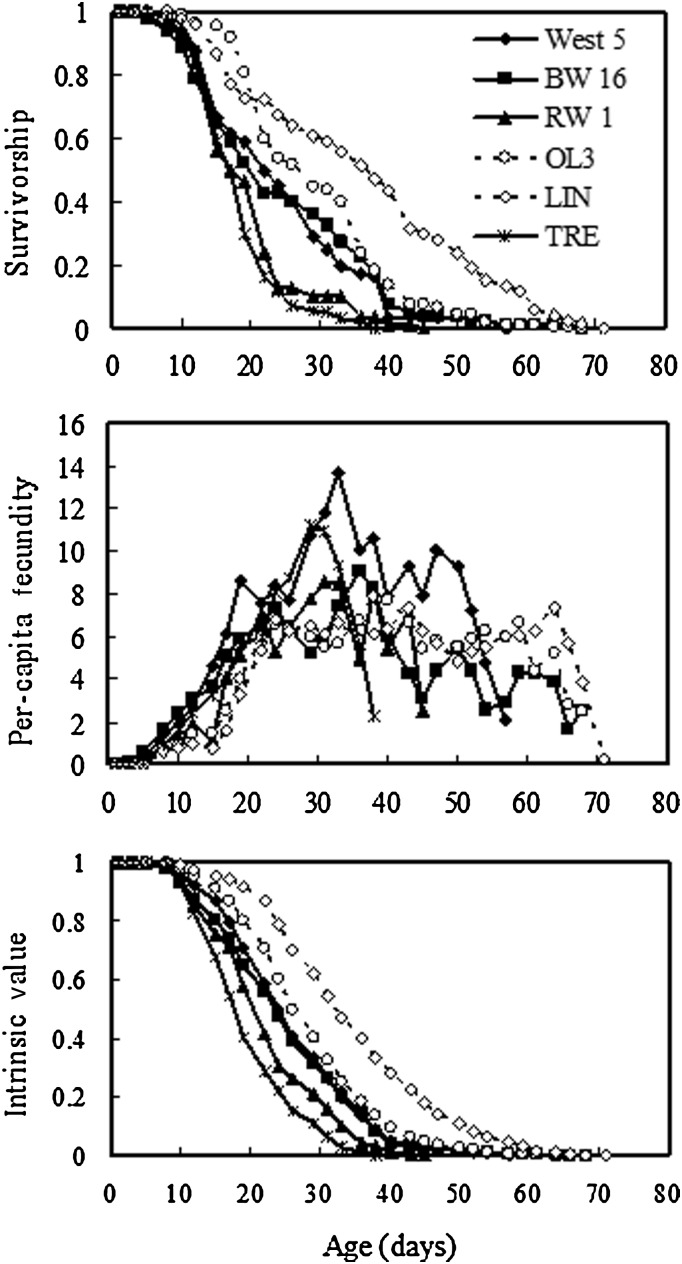
Our data showed that lifespans of sexual clones are not longer than those of asexual clones (Fig. 1, top). Survivorship curves show that both asexual clones had better survival than the sexual clones, and visual inspection indicates this is particularly true prior to age 15 days, i.e. during early adulthood (Fig. 1). Median lifespans in all three sexual D. pulex clones (BW16 = 19 days, RW1 = 17 days, WEST5 = 22 days) were in fact shorter than that of either asexual clone (LIN = 36 days, OL3 = 26days). The sexual D. obtusa clone also had a short median lifespan (17 days). Because all sexual median lifespans were unexpectedly shorter than the asexual lifespans, no meaningful statistical test of our one-tailed prediction that sexuals should live longer than asexuals is possible. To determine whether the longer lifespans of asexuals were a chance result, we conducted two-tailed log-rank tests for pairwise comparisons between the asexual and sexual clones of D. pulex. In one comparison, OL3 versus BW16, there was no support for the hypothesis that lifespans differed between the two clones (χ2 = 1.04, df = 1, P = 0.3080). In all other comparisons, there was statistical evidence that the asexual clones were actually longer lived than the sexual clones (LIN versus WEST5 χ2 = 38.5, df = 1, P < 0.0001; LIN versus BW16 χ2 = 26.5, df = 1, P < 0.0001; LIN versus RW1 χ2 = 97.1, df = 1, P < 0.0001; OL3 versus WEST5 χ2 = 5.20, df = 1, P = 0.0226; OL3 versus RW1 χ2 = 60.6, df = 1, P < 0.0001). However, the magnitude of the differences is not large, and statistical significance is driven by the high levels of within-clone replication.
Fig. 1.
Age-specific life history of sexual and asexual clones of pond-dwelling Daphnia. Solid lines and filled symbols represent cyclically parthenogenetic (sexual) clones, and dashed lines and open symbols represent obligately asexual clones. Clones are D. pulex, except TRE, which is D. obtusa. Survivorship and intrinsic value are proportions that range from 1.0 to 0.0. Per-capita fecundity is given as offspring per female per day.
For all six clones, the AIC was lower for the simpler Gompertz model than for the logistic model, indicating that the more complex logistic did not provide a significantly better fit. Mortality rate analyses do not suggest that the rate of aging, measured in terms of survival, is slower in the sexuals relative to the asexuals. Overall, clonal b estimates from the Gompertz model ranged from 0.30 to 0.42 for D. pulex (Table I). Confidence intervals (CIs) on b encompass 0.35–0.41 for all five D. pulex clones, with the lowest pairwise overlap between the two asexual clones, and no significant difference between sexual and asexual parameter estimates (t = 0.7419, df = 3, P = 0.5119). These estimates are in line with prior estimates for genetically heterogeneous D. pulex cohorts under similar environmental conditions (0.35–0.50; Dudycha, 2003) and considerably higher than estimates for the long-lived D. pulicaria (0.09–0.15; Dudycha, 2003). In addition, the sexual D. obtusa clone exhibited the highest b, 0.63 (95% CI: 0.51–0.77).
Table I:
Estimates of the Gompertz b parameter, a measure of the rate of aging, and its 95% CI
| Clone | b | CI lowerbound | CI upperbound | AICG | AICL |
|---|---|---|---|---|---|
| Asexuals | |||||
| LIN | 0.34 | 0.28 | 0.41 | −806.99 | −804.99 |
| OL3 | 0.42 | 0.35 | 0.51 | −612.84 | −564.34 |
| Sexual D. pulex | |||||
| WEST5 | 0.35 | 0.24 | 0.51 | −272.69 | −269.77 |
| BW16 | 0.30 | 0.25 | 0.43 | −287.76 | −284.49 |
| RW1 | 0.39 | 0.26 | 0.58 | −225.80 | −171.28 |
| Sexual D. obtusa | |||||
| TRE | 0.63 | 0.51 | 0.77 | −398.49 | −323.12 |
AICG and AICL give the AIC for the Gompertz and Logistic models, respectively.
Fecundity is noisy in time, and not readily amenable to statistical analysis that will accurately detect differences in the rate of reproductive senescence. However, it is clear that the fecundity of asexuals is not declining more rapidly than the sexuals (Fig. 1, middle), as predicted by the breeding-system/mutation-accumulation hypothesis. In fact, fecundity of the asexuals is broadly stable for ∼40 days, declining notably only at the very end. In contrast, the fecundity of sexuals rises to a peak and then falls, though this is less pronounced in the BW16 clone than in the others.
Our data show that sexuals do not have a particularly slow decline in the intrinsic value with respect to age (Fig. 1, bottom). Indeed, it appears that the sexuals decline more rapidly than the asexuals, though this trend is slight. The period where intrinsic value declines from 0.8 to 0.2 is approximately linear in all clones and its duration serves as an indicator of the rate of adult senescence. In the data presented here, 0.8–0.2 represents the longest duration that is approximately linear across all clones and thus should represent adult (rather than juvenile) performance without being substantially influenced by individual long-lived outliers within each clone. For the sexual D. pulex, this duration averaged 16.7 days for the asexuals it averaged 17.5 days; this difference is not statistically significant (t = 0.4060, df = 3, P = 0.7120).
DISCUSSION
Daphnia populations from lakes have been reported to live about twice as long as those from ponds, and to age much more slowly (Dudycha and Tessier, 1999; Dudycha, 2001, 2003). This is thought to be driven by selective differences between ponds and lakes (Dudycha, 2001, 2004). However, later work has shown this habitat difference is partially confounded by differences in the breeding system (Paland et al., 2005; Paland and Lynch, 2006), and we do not know if the clones used in the initial life history reports were based on sexual or asexual pond clones, calling into question the earlier interpretation of habitat-based differences in selection as the driver of life history differentiation. If differences between the aging patterns of lake and pond populations of Daphnia were substantially explained by differences in the breeding system among populations, we would expect to find that sexual clones from ponds had longer lifespans and aged much more slowly than obligately asexual clones from ponds. This was not the case. There was no strong difference between breeding systems, and the trend ran opposite to that predicted based on the action of mutation accumulation in obligate asexuals. Though the results here are based on clones from a small number of populations, the complete absence of the predicted large differences mean that shifts in the breeding system are not altering the accumulation of deleterious age-specific mutations in a manner that would substantially explain the strong divergence that has been reported among ecotypes. Rather, the interpretation that selective differences between habitats drive reported life history divergence between D. pulex and D. pulicaria stands.
Some readers will no doubt be curious about the apparent longer lifespan of the sexual clones. We know of no mechanism that would lead to such an association, and note that the magnitude of the differences is small in the broader context of Daphnia life history variation. We therefore caution against interpreting the observed differences beyond the obvious conclusion that large differences in the opposite direction were not evident.
We do not mean to imply that neutral processes play no role in the evolution of aging in Daphnia. Mutation accumulation could be an important mechanism underlying shared aspects of aging across Daphnia populations, and mutations whose only consequence is a reduction of late-life performance could still escape selection in ponds. A recent theoretical treatment (Baudisch, 2005) has suggested that mutation accumulation may be relatively unimportant in the evolution of aging patterns. Despite this, and the absence of the predicted effect in our data, we recommend that workers in other model systems evaluate the potential for variation in neutral processes to influence the evolutionary divergence of aging. It remains to be seen whether the divergence that occurs in nature is due to antagonistic pleiotropy, mutation accumulation or both.
ACKNOWLEDGEMENTS
S. Paland kindly provided the clones for this experiment. M. Wagner provided help confirming breeding systems and E. Morrin assisted with the life tables. Members of the M. Lynch laboratory provided helpful insight and advice.
FUNDING
This study was supported by NIH grant R01-AG037969 to J.L.D. and National Science Foundation grant EF-0328516 to M. Lynch.
REFERENCES
- Baudisch A. Hamilton's indicators of the force of selection. Proc. Natl Sci. USA. 2005;102:8263–8268. doi: 10.1073/pnas.0502155102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchnak R., Steinberg C. E. W. Modulation of longevity in Daphnia magna by food quality and simultaneous exposure to dissolved humic substances. Limnologica. 2010;40:86–91. [Google Scholar]
- Burnham K. P., Anderson D. R. Model Selection and Inference. New York: Springer-Verlag; 1998. [Google Scholar]
- Charlesworth B. Evolution in Age-structured Populations. 2nd edn. Cambridge, UK: Cambridge University Press; 1994. [Google Scholar]
- Dudycha J. L. The senescence of Daphnia from risky and safe habitats. Ecol. Lett. 2001;4:102–105. [Google Scholar]
- Dudycha J. L. A multi-environment comparison of senescence between sister species of Daphnia. Oecologia. 2003;135:555–563. doi: 10.1007/s00442-003-1230-7. [DOI] [PubMed] [Google Scholar]
- Dudycha J. L. Mortality dynamics of Daphnia in contrasting habitats and their role in ecological divergence. Freshwater Biol. 2004;49:505–514. [Google Scholar]
- Dudycha J. L., Tessier A. J. Natural genetic variation of life span, reproduction, and juvenile growth in Daphnia. Evolution. 1999;53:1744–1756. doi: 10.1111/j.1558-5646.1999.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Edney E. B., Gill R. W. Evolution of senescence and specific longevity. Nature. 1968;220:281–282. doi: 10.1038/220281a0. [DOI] [PubMed] [Google Scholar]
- Hamilton W. D. Moulding of senescence by natural selection. J. Theor. Biol. 1966;12:12–45. doi: 10.1016/0022-5193(66)90184-6. [DOI] [PubMed] [Google Scholar]
- Hebert P. D. N., Crease T. J. Clonal coexistence in Daphnia pulex (Leydig)—another planktonic paradox. Science. 1980;207:1363–1365. [Google Scholar]
- Heier C. R., Dudycha J. L. Ecological speciation in a cyclic parthenogen, sexual capability of experimental hybrids between Daphnia pulex and Daphnia pulicaria. Limnol. Oceanogr. 2009;54:492–502. [Google Scholar]
- Hughes K. A., Alipaz J. A., Drnevich J. M., et al. A test of evolutionary theories of aging. Proc. Natl Sci. USA. 2002;99:14286–14291. doi: 10.1073/pnas.222326199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K. A., Reynolds R. M. Evolutionary and mechanistic theories of aging. Ann. Rev. Ent. 2005;50:421–445. doi: 10.1146/annurev.ento.50.071803.130409. [DOI] [PubMed] [Google Scholar]
- Keller L., Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of ageing. Nature. 1997;389:958–960. [Google Scholar]
- Martinez-Jeronimo F., Villasenot R., Rios G., et al. Effect of food type and concentration on the survival, longevity and reproduction of Daphnia magna. Hydrobiologia. 1994;287:207–214. [Google Scholar]
- Medawar P. B. An Unsolved Problem of Biology. London: H. K. Lewis; 1952. [Google Scholar]
- Morbey Y. E., Brassil C. E., Hendry A. P. Rapid senescence in Pacific salmon. Am. Nat. 2005;166:556–568. doi: 10.1086/491720. [DOI] [PubMed] [Google Scholar]
- Nandini S., Sarma S. S. S. Ratio of neonate to adult size explains life history characteristics in cladoceran zooplankton. Acta Hydrochim. Hydrobiol. 2006;34:474–479. [Google Scholar]
- Paland S., Colbourne J. K., Lynch M. Evolutionary history of contagious asexuality in Daphnia pulex. Evolution. 2005;59:800–813. [PubMed] [Google Scholar]
- Paland S., Lynch M. Transitions to asexuality result in excess amino acid substitutions. Science. 2006;311:990–992. doi: 10.1126/science.1118152. [DOI] [PubMed] [Google Scholar]
- Pietrzak B. Interclonal differences in age-specific performance in Daphnia magna. J. Limnol. 2011;70:345–352. [Google Scholar]
- Pietrzak B., Bednarska A., Grzesiuk M. Longevity of Daphnia magna males and females. Hydrobiologia. 2010;643:71–75. [Google Scholar]
- Pletcher S. D. Model fitting and hypothesis testing for age-specific mortality data. J. Evol. Biol. 1999;12:430–439. [Google Scholar]
- Reznick D. N., Bryant M. J., Roff D., et al. Effect of extrinsic mortality on the evolution of senescence in guppies. Nature. 2004;431:1095–1099. doi: 10.1038/nature02936. [DOI] [PubMed] [Google Scholar]
- Rose M. Evolutionary Biology of Aging. New York: Oxford University Press; 1991. [Google Scholar]
- Rose M., Charlesworth B. A test of evolutionary theories of senescence. Nature. 1980;287:141–142. doi: 10.1038/287141a0. [DOI] [PubMed] [Google Scholar]
- Sarma S. S. S., Nandini S., Gulati R. D. Cost of reproduction in selected species of zooplankton (rotifers and cladocerans) Hydrobiologia. 2002;481:89–99. [Google Scholar]
- Service P. M. Heterogeneity in individual mortality risk and its importance for evolutionary studies of senescence. Am. Nat. 2000;156:1–13. doi: 10.1086/303371. [DOI] [PubMed] [Google Scholar]
- Tatar M., Gray D. W., Carey J. R. Altitudinal variation for senescence in Melanoplus grasshoppers. Oecologia. 1997;111:357–364. doi: 10.1007/s004420050246. [DOI] [PubMed] [Google Scholar]
- Vaupel J. W. Relative risks, frailty models of life history data. Theor. Pop. Biol. 1990;37:220–234. [Google Scholar]
- Williams G. C. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]