Abstract
Wound management represents a major clinical challenge on what concerns healing enhancement and pain control. The selection of an appropriate dressing plays an important role in both recovery and esthetic appearance of the regenerated tissue. Despite the wide range of available dressings, the progress in the wound care market relies on the increasing interest in using natural-based biomedical products. Herein, a rat wound-dressing model of partial-thickness skin wounds was used to study newly developed chitosan/soy (cht/soy)-based membranes as wound-dressing materials. Healing and repair of nondressed, cht/soy membrane-dressed, and Epigard®-dressed wounds were followed macroscopically and histologically for 1 and 2 weeks. cht/soy membranes performed better than the controls, promoting a faster wound repair. Re-epithelialization, observed 1 week after wounding, was followed by cornification of the outermost epidermal layer at the second week of dressing, indicating repair of the wounded tissue. The use of this rodent model, although in impaired healing conditions, may enclose some drawbacks regarding the inevitable wound contraction. Moreover, being the main purpose the evaluation of cht/soy-based membranes' performance in the absence of growth factors, the choice of a clinically relevant positive control was limited to a polymeric mesh, without any growth factor influencing skin healing/repair, Epigard. These new cht/soy membranes possess the desired features regarding healing/repair stimulation, ease of handling, and final esthetic appearance—thus, valuable properties for wound dressings.
Introduction
Tissue repair and scar formation are main concerns of wound care. The appropriate dressing selection plays an important role both in the complete regeneration of the injured tissue and in the esthetic appearance.1 A wide range of dressings and bandages, adaptable to various types of wounds, are nowadays available in the wound care market, and are currently used in the clinics. The traditional wound dressing that simply covered and protected the wound2,3 has been replaced by alternative dressings that allow control of wound moisture4–7 and, more recently, by dressings with an (bio-) active role in the healing environment.8,9 However, most of the existing products need to be changed every few days after their application; in some cases, there is the need to replace the material to maintain/accelerate the ongoing healing process.10 Therefore, further developments that facilitate the healing process or address issues such as the control of the chemical environment and bacterial infection are required and desired.
Indiscriminately, synthetic,11–14 natural,8,15,16 or biological materials10,17,18 have been presented along the years, as the key elements for controlling/modulating the healing mechanisms and the outcomes of wound repair upon dressing.10 Among the recently proposed natural-origin materials for wound dressing, collagen2,9,19 chitosan,20–24 and silk25,26 occupy a central position due to their nature, which might present improved performance.
The positive impact of chitosan, a deacetylated derivative from chitin, concerning the healing mechanisms that include the inflammatory process, is well documented. The demonstration of the chitosan anti-inflammatory activity and potential in promoting wound healing was reported by Mori et al.27 Although chitosan activates macrophages, it also induces gradual macrophage apoptosis in vitro (about 50% programmed cell death in 6 h),27 thus preventing the possible occurrence of septic shock and death.28 Macrophage activation induced by chitosan resulted in increased metabolic activity, secretion of cytokines, growth factors and inflammatory mediators, and enhanced phagocytic activity.27 The production of macrophage inflammatory protein-2 (MIP-2) by chitosan-activated macrophages stimulates epithelial cell proliferation29 and thus skin healing. Additionally, a reduction on the influx of activated tissue macrophages, which in turn decreases angiogenesis, fibroplasia, and connective tissue deposition, was also attributed to chitosan.30 These features may substantiate the hemostatic properties preventing bleeding,30 as well as the antibacterial activity when applied in skin wounds22,24,31 that chitosan also displays. In a clinical trial, Azad et al.32 have shown a positive effect of chitosan both on the re-epithelialization and regeneration of the granular layer of the skin where chitosan-dressed wounds healed faster compared to controls. These data are in agreement with other studies demonstrating that chitosan induce the migration of polymorphonuclear neutrophils (PMNs) at the early stage of wound healing, when treating open skin wounds in dogs, enhancing the formation of granulation tissue and production of collagen by fibroblasts.33 In addition, we have previously demonstrated that PMNs are not activated by chitosan and, therefore, an acute inflammatory response will not persist at the wound site in the presence of chitosan.34
Soybean-rich foods have been recognized to decrease the risks of chronic diseases and protective effects against persistent inflammation.35 These features are mainly attributed to isoflavones.35 Among the isoflavones, genistein, a phytoestrogen, showed to have favorable effects on cutaneous wound healing36,37 through an estrogen receptor-independent mechanism.36 Additionally, it was demonstrated that genistein is able to signal, via mitogen-activated protein kinase (MAPK) activation in macrophages, reducing wound pro-inflammatory cytokines.36
Chitosan/soy (cht/soy) membranes were previously proposed for biomedical applications based on the observations of their in vitro capacity to enhance the proliferation of fibroblasts38 and their impaired ability to activate PMNs.34 Additionally, a normal host reaction was observed after subcutaneous implantation in rats.39 Other important features of these cht/soy membranes, critical for wound dressings, are their simple manipulation and their transparency. In this context and on the worldwide-recognized potential of chitosan and soy-based components for skin wounds regeneration, the aim of this study was to evaluate the suitability of newly developed cht/soy-based membranes as dressings for partial thickness skin wounds. Among the many different animal models to study wound healing and skin regeneration, excision of rat skin portions is a model widely used.8,17,22,40–45 Therefore, a wound-dressing rat model of partial-thickness skin wound, under impaired healing conditions, was used to assess the suitability of the cht/soy membranes to promote wound healing. Comparatively, Epigard®, a clinically accepted wound dresser, was used as control. Epigard® is also only a dressing without any further active substances.12,46 Thus, it is a clinically widely used dressing similar to the one studied here.
Materials and Methods
Materials
Cht with a deacetylation degree of about 85% was purchased from Sigma, and the soy protein isolate (SI) was provided by Loders Crocklaan. All other reagents were analytical grade and used as received. Cht/soy protein-blended membranes (average thickness of 84 μm and 17 mm of diameter) were prepared by solvent casting according to a procedure described elsewhere.38 Briefly, chitosan was dissolved in an aqueous acetic acid 2% (v/v) solution at a concentration of 4 wt%. A soy suspension (1 wt%) was prepared by slowly dispersing the soy protein powder, under constant stirring, in distilled water with glycerol. After adjusting the pH to 8.0±0.3 with 1 M sodium hydroxide, the dispersion was heated in a water bath at 50°C for 30 min. The cht and the SI solutions were mixed at a weight ratio of 75/25% cht/soy (CS75). After homogenization, the CS75 solution was casted into Petri dishes and dried at room temperature for 6 days. The neutralization of the membranes was obtained by immersion in 0.1 M sodium hydroxide for about 10 min. Membranes were washed with distilled water to remove all traces of alkali and then dried at room temperature. The materials were sterilized under standard conditions with ethylene oxide.47
Animals
Twenty male Sprague-Dawley rats weighting between 230 and 280 g were used for the study. Three groups were investigated: membranes (wound directly covered with the cht/soy-based membranes); positive control (wound directly covered with Epigard®—Biovision GmbH); and negative control (no direct coverage of the wound). Epigard® is composed of a nontextile outer layer of polytetrafluorethylene and an inner layer of soft elastic polyurethane that forms an open matrix to which adsorbs the exudate from the wound bed. This dressing was chosen as positive control because it is extensively used in the clinical practice as a short-term wound dressing without growth factors.12,46
Each animal was anesthetized with an intramuscular injection of 90 mg/kg of ketamine combined with 5 mg/kg xylazine after induction with 3%–3.5% isofluorane and 7 L/min of air for 2–3 min. After shaving the skin, the back of the animals was disinfected and two paravertebral wounds (17 mm in diameter) were created by excision, leaving the skin smooth muscle layer (panniculus carnosus) intact. The test conditions were randomly distributed among the animals (Table 1). After dressing (except the negative control), the wounds were protected with Bactigras® (Smith & Nephew) and then covered with Opsite Flexigrid® (Smith & Nephew). Bactigras is a paraffin gauze dressing containing 0.5% of chlorhexidine acetate; being soothing and nonadhesive, it allows the wound to drain freely into an absorbent secondary dressing.32 Opsite Flexigrid®, a vapor-permeable adhesive film dressing that is the standard in moist wound healing48 was used as a secondary dressing. The whole abdomen of each animal was protected with stretching bandages to prevent the removal of the whole set of dressings by scratching and biting (Fig. 1). At days 0 (surgery day) and 7, methylprednisolone acetate (Depo-Medrol®; Pfizer) was subcutaneously injected (20 mg/kg BW) to impair wound healing49 and inhibit hair growth.
Table 1.
Summary of the Random Distribution of the Study Groups for Each Animal, and the Total Number of Samples Considered for Each Group, at 1 and 2 Weeks of Implantation
| Animal number | Left lesion | Right lesion | Healing time period | Total A | Total B | Total C |
|---|---|---|---|---|---|---|
| 1 | A | C | 1 week | 6 | 6 | 6 |
| 2 | B | A | ||||
| 3 | C | B | ||||
| 4 | A | B | ||||
| 5 | B | C | ||||
| 6 | C | A | ||||
| 7 | A | B | ||||
| 8 | B | C | ||||
| 9 | C | A | ||||
| 10 | A | C | 2 weeks | 6 | 6 | 6 |
| 11 | B | A | ||||
| 12 | C | B | ||||
| 13 | A | B | ||||
| 14 | B | C | ||||
| 15 | C | A | ||||
| 17 | A | B | ||||
| 17 | B | C | ||||
| 18 | C | A |
A, wounds treated with chitosan/soy-based membranes; B, wounds treated with Epigard® (positive control); C, nontreated wounds (negative control).
FIG. 1.
Macroscopic appearance of the wounds created by skin excision and before dressing (A). Aspect of the animal with the whole set of dressings and bandages (B). Color images available online at www.liebertpub.com/tea
The animals were kept separately and received daily analgesia with metamizole sodium (200 μg/g BW) and sedation with diazepam (2.5 mg/125 mL water) in drinking water.
The bandages were changed every 3–4 days. Macroscopic analysis of the wounds was carried out at days 3, 7, and 14 and the images taken used for the planimetric evaluation of the healing process. The evaluation was performed with the LUCIA G® version 4.8 (Laboratory Imaging Ltd.) software by two independent researchers blinded to the experimental condition.
After 1 and 2 weeks, the animals were anesthetized with isoflurane and then euthanized by and intracardial overdose of ketamin/xylazine. The wound area and the surrounding healthy skin were explanted. Central wound cross sections were performed and samples were fixed for histological analysis, in 3.7% formalin, and then paraffin embedded, sectioned, and stained according to a routine hematoxylin and eosin protocol.
The histological samples were analyzed using an Axioplan Imager Z1 microscope (Zeiss) and included the measurement of the length of the wound, allowing the establishment of a correlation with the planimetric assessment of the wound areas. The planimetric assessment comprises the wound area. This area is diminished during healing by contraction and epithelialization. These entities cannot be separated, but are present in all the groups. The full wounded area and the healthy margins images were then obtained after the composition of multiple standardized histological pictures.
Statistical analysis
Data from the planimetry and from the wound length measurements50 were analyzed by a single-factor ANOVA test and the significance value was set at p<0.05.
Results
Macroscopic analysis
The macroscopic characterization of the wounds during the observation period was based on the planimetric analysis of the wounded area. A qualitative evaluation of the healing area and interface regions between healthy and newly formed tissue was also performed.
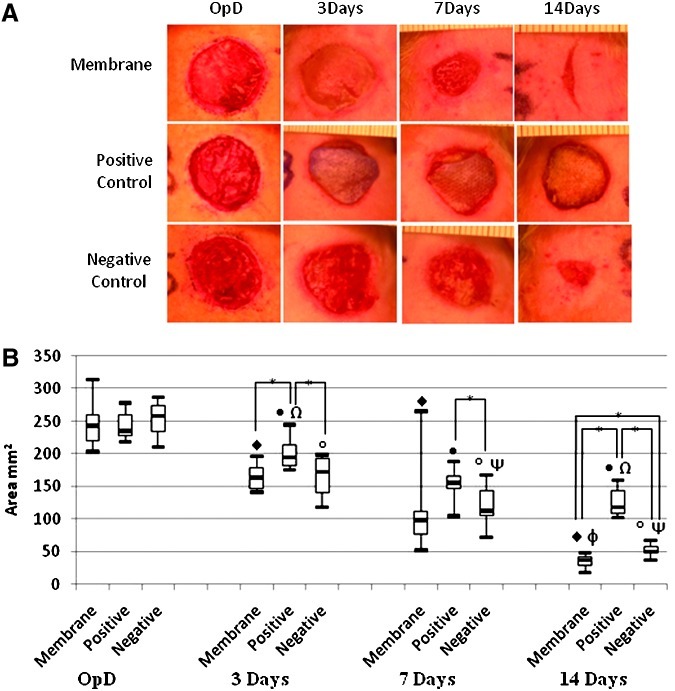
At day 3 after wounding and dressing, clear macroscopic differences between the groups were observed (Fig. 2A). The cht/soy membrane-dressed wounds showed a significant infiltration of granulation tissue that led to membrane lifting and allowed wound observation. Conversely, the nondressed wounds (negative control) showed bleeding, which is a sign of impaired healing. Epigard (positive control) had completely adhered to the wound bed, making it impossible to be removed without further wounding (Fig. 2A). At this stage, the planimetric analysis revealed that the areas of the undressed (negative control) and of the cht/soy membrane-dressed wounds were not significantly different (p>0.05) (Fig. 2B). The wounded area of the negative control was significantly smaller (p<0.05) than that of the positive control (Fig. 2B).
FIG. 2.
Representative images of the macroscopic aspect of the excisional wounds at the operation day (OpD) and subsequent healing at days 3, 7, and 14 after dressing with the chitosan/soy-based membranes, and in comparison with the negative and positive controls (A). Follow-up of the wound area determined by planimetric analysis (B). *Differences statistically significant; ♦, •, and °p<0.05 versus OpD; Ψp<0.05 versus negative control at day 3 of dressing; Ωp<0.05 versus positive control at day 7 of dressing; φp<0.05 versus membrane dressing at day 7. Color images available online at www.liebertpub.com/tea
Despite some scratching of the secondary bandages, after 7 days of dressing no signs of infection were detected in any of the test groups (Fig. 2A). After this time period, the new epithelial tissue started to replace the granulation tissue in the cht/soy membrane-dressed wounds, and the membranes were easily lifted from the wound bed without bleeding. An improved healing was observed for the cht/soy membrane-covered wounds versus the negative control, where some granulation tissue was still present (Fig. 2A). In contrast Epigard® completely adhered to the wound bed, avoiding new epidermis formation, and an attempt to remove it led to bleeding (Fig. 2A). Compared to both controls, the wounds dressed with the cht/soy membranes showed thinner margins with an almost complete healing and new tissue formation replacing the excised epidermis (Fig. 2A). The wound of the negative control revealed a significant degree of contraction. Despite the smaller wound area of the cht/soy membrane group, at day 7 the planimetric evaluation did not reveal significant differences in comparison to both controls (p>0.05) (Fig. 2B).
The macroscopic analysis showed that the wound area significantly decreased from the operation day to the final excision time point (14 days) in all the tested conditions, which is an indication of neoepithelialization and replacement of the wounded tissue (Figs. 2A, B) by newly formed tissue. A significant and consecutive reduction of the wound area was gradually observed in both negative and positive controls, although with a significantly delayed wound closure (Figs. 2A, B). While comparing the contour and dimensions of the wounds at day 14 (Figs. 2A, B), it was evident that the re-epithelialization and healing was also more efficient in the wounds dressed with the cht/soy-based membranes.
Histological analysis
After the first and second week of dressing, the wounds and surrounding healthy skin were excised, together with the attached dressing in the case of the positive controls, and histological analysis was performed. As during the process of sectioning and staining, the adherent Epigard® did not detach from the wound bed, the analysis of the explants included its integration with the growing tissue.
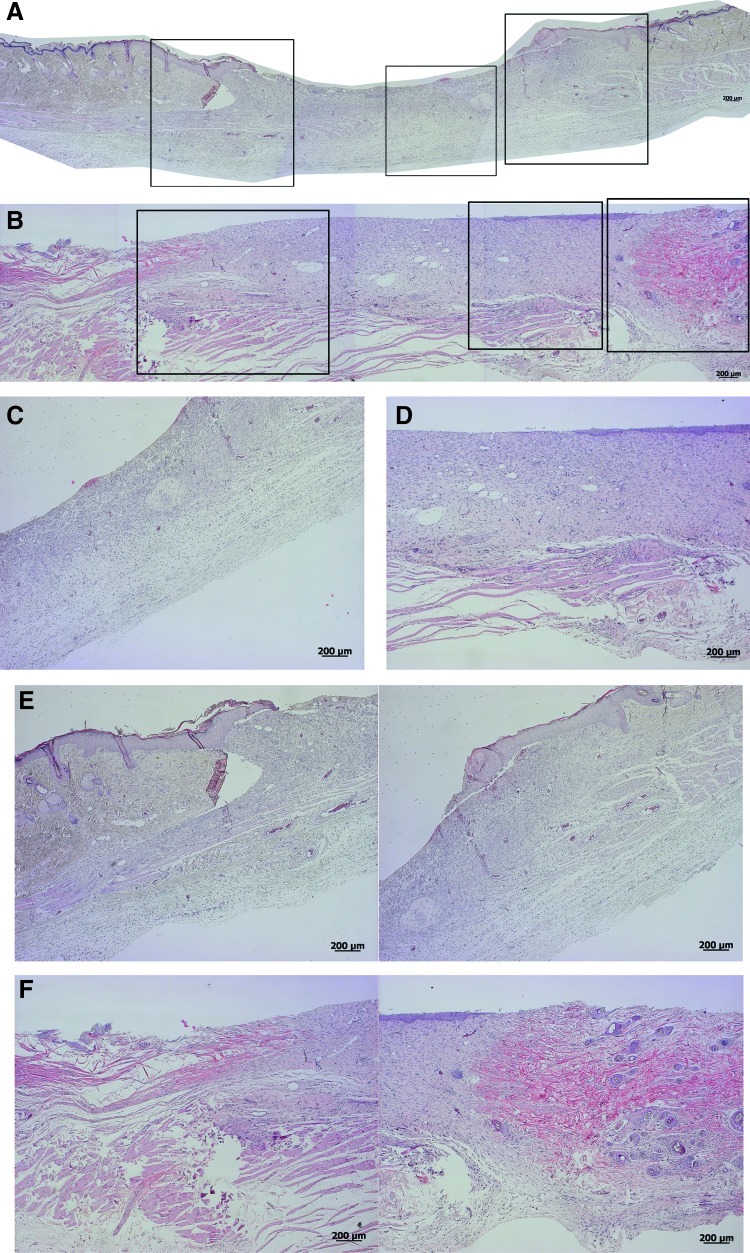
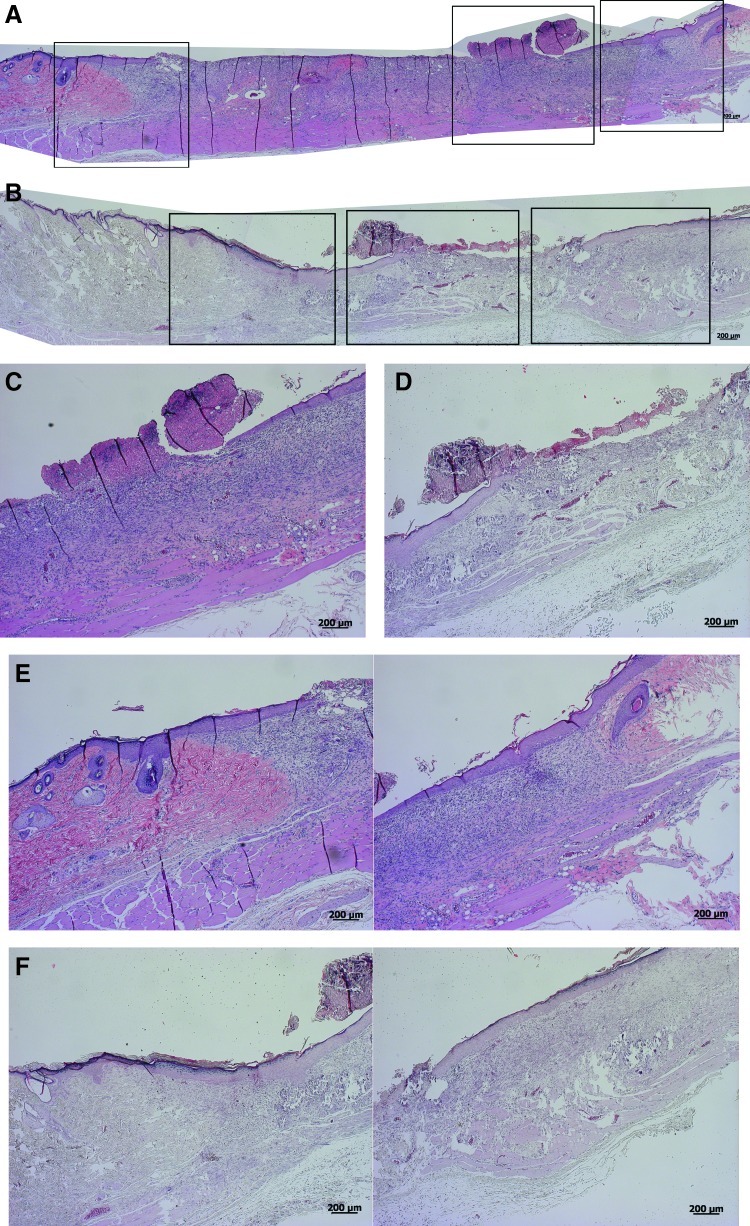
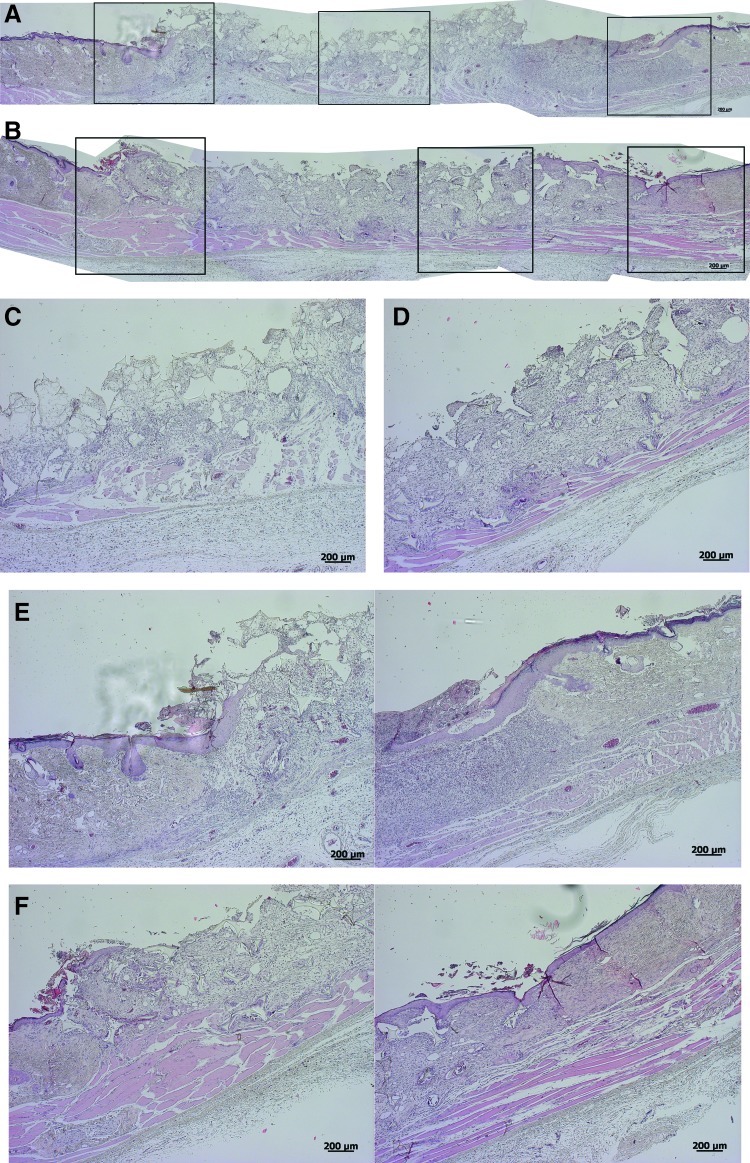
One week after dressing, the cht/soy-based membranes (Fig. 3A, C, E) seemed to enhance the formation of granulation tissue, in comparison to the negative control (undressed wounds) (Fig. 4A, C, E). Furthermore, the margins of the cht/soy membrane-dressed wounds presented a continuity of the repairing tissue (Fig. 3E) that was not observed in the wound margins of the negative (Fig. 4E) and positive (Fig. 5E) controls. At this stage of healing, it was already possible to identify some stratification of the tissue in the membrane-dressed wounds (Fig. 3C). These features were not detectable either in the negative (Fig. 4C) or in the positive controls (Fig. 5C). A disorganized mesh of cells, including a large amount of inflammatory cells, some fibroblasts, and collagen fibers, were observed in both groups. Furthermore, necrosis was detected in the nondressed wounds (Fig. 4C) but not in the Epigard®-dressed wounds (Fig. 5C). In fact, the material adhered to the wound bed and formed an intimate network composed by the polymer matrix and the inflammatory cells. This network started to vascularize, indicating a good integration of the material into the wound bed (Fig. 5C). During the first week of healing the presence of foreign body giant cells was not detected in all test groups.
FIG. 3.
Representative composition of the histological micrographs of the wounded skin (A, B) and detailed micrographs of the wound beds (C, D) and margins (E, F), respectively, 1 and 2 weeks after dressing with the chitosan/soy (cht/soy) membrane. Boxes in Figs. A and B represent the higher magnifications of Figs. C–F. Color images available online at www.liebertpub.com/tea
FIG. 4.
Representative composition of the histological micrographs of the wounded skin (A, B) and detailed micrographs of the wound beds (C, D) and margins (E, F), respectively, 1 and 2 weeks after dressing with the Epigard® (positive control). Boxes in Figs. A and B represent the higher magnifications of Figs. C–F. Color images available online at www.liebertpub.com/tea
FIG. 5.
Representative composition of the histological micrographs of the wounded skin (A, B) and detailed micrographs of the wound beds (C, D) and margins (E, F), respectively, 1 and 2 weeks left undressed (negative control). Boxes in Figs. A and B represent the higher magnifications of Figs. C–F. Color images available online at www.liebertpub.com/tea
At the second week of dressing, the healing of the wounds covered by the cht/soy membranes (Fig. 3B, D, F) was enhanced in comparison to the negative (Fig. 4B, D, F) and positive (Fig. 5B, D, F) controls. The wounds decreased in size (Fig. 3B), the margins were thinner (Fig. 3F) and the previously observed granulation tissue was replaced by a more stratified regenerated epidermis (Fig. 3D). Cornification of the outermost epidermal layer, although still near the margin of the wound, was observed (Fig. 3B, D), demonstrating clear signs of re-epithelialization. The negative controls showed a severe epidermis macerating effect that was translated in extensive necrosis of the tissue (Fig. 4B, D, F). Regarding Epigard®-dressed wounds, the matrix network detected at the first week of healing was still present but with a higher infiltration of inflammatory cells (Fig. 5D). Underneath the network, a significant layer of muscle and collagen fibers was observed (Fig. 5D).
The microscopic measurement of the length of the wounds in the histological samples did not show significant statistical differences (p>0.05) among the groups after 1 and 2 weeks (Fig. 6). At the first week of healing the wounds dressed with the cht/soy membranes showed a significant decrease in length, in comparison to both controls (p<0.05) (Fig. 6). Conversely, the controls did not significantly differ in the length of the wounds (p>0.05) (Fig. 6). Two weeks after healing, the positive and negative controls showed significantly different wound lengths (p<0.05) (Fig. 6). In contrast, the cht/soy membranes-dressed wounds showed lengths comparable to the negative control (Fig. 6).
FIG. 6.
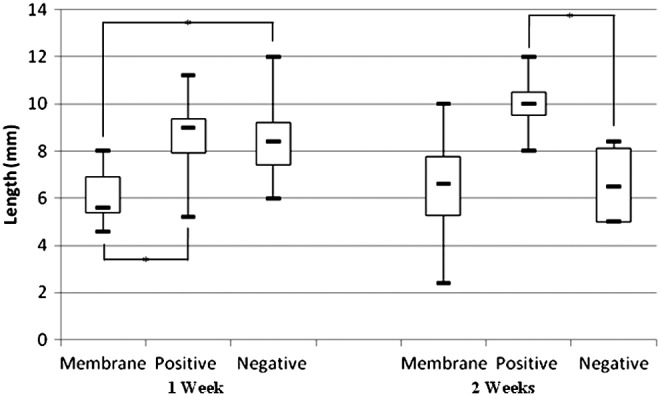
Representation of the length of the wound 1 and 2 weeks after dressing with the cht/soy membrane (Membrane), the Epigard (Positive), and undressed (Negative). Measurements were obtained from the histological micrographs of the wounded region of the skin. *Statistically significant differences.
Discussion
Excisional wound animal models are the most frequently used models to investigate the performance of skin wound dressings.51 These wounds are useful to assess re-epithelialization after implantation of different devices, such as wound dressings, topical formulations, and growth factors,51 when a considerable volume of skin is removed. However, one drawback of using rats as a model is the characteristic contraction during wound healing. Nevertheless, this occurred in all groups investigated and is thus part of the wound healing in these types of models. Many research groups have reported the successful use of different types of chitosan-based materials for wound-dressing applications.32,48,52–54 Taking into consideration some of those reports and previous knowledge on the behavior of cht/soy-based membranes,34 a wound-dressing rat model of partial-thickness skin wound was used to assess the suitability of these membranes as wound dressings.
This study demonstrates that the cht/soy membranes, in a rodent model under impaired healing conditions induced by corticosteroid treatment,49 are suitable wound dressings, allowing the progress of a normal healing path and a faster replacement of the excised epidermis in comparison to an undressed wound (negative control). The presence of granulation tissue at earlier stages of healing was indicative of an accelerated tissue reaction55 toward the repair of the injured site. In contrast, an early inflammatory reaction and bleeding were observed in the negative control. In fact, for both negative and positive controls, a disorganized mesh of cells, with a large amount of inflammatory cells, some fibroblasts, and collagen fibers were observed after the first week of healing, which is in contrast to what is observed in the cht/soy membrane-dressed wounds. Therefore, the normal healing cascade was not occurring in both control conditions.
In partially thickness wounds the formed granulation tissue leads to wound contraction and re-epithelialization, which closes the wounded area, allowing the regeneration of the epidermis with its different layers and annexes.43,51 Furthermore, as the subcutaneous tissue with the portion of the panniculus carnosus muscle in the backs of the animals is left intact,56 the re-epithelialization of the wound is expected to start not only from the margins of the wound containing healthy and intact skin, but also from the wound bed.43,56 After 1 week the wounds dressed with the cht/soy membrane already presented some stratification. The smaller wound area and the thinner margins, with an almost complete repair of all the layers of the excised epidermis after 14 days of dressing, confirmed the normal progress of re-epithelialization and a better healing after dressing with the cht/soy membrane. In contrast and despite the decreased area, the undressed wounds showed severe necrosis and failed in re-epithelialization. Additionally, the undressed wound margins showed a significant depression lacking a smooth transition from the healthy skin to the injured area. As stated before, in all groups some contraction could be observed but was similar in all groups. Therefore, observed wound healing was mainly due to epithelialization.
According to a previous report,34 PMNs did not secrete reactive oxygen species (ROS) after direct contact with cht/soy membranes. Furthermore, the membranes exerted an anti-inflammatory effect by inhibiting ROS production by PMNs when stimulated with phorbol 12-myristate 13-acetate (PMA) or formyl-methionyl-leucyl-phenylalanine (fMLP).34 It is therefore likely that the inflammatory cells present at the wound bed of undressed wounds were secreting ROS, responsible for destroying the surrounding cells and tissues and thus explaining the observed necrosis at the wound bed. In addition, the observed necrosis at the wound bed of the negative controls may be explained by the lack of stimulation of the wound bed tissue to heal. In fact it is well known that, as the growth from the wound margins is slower, the absence of nutrients in the wound bed leads to cell death.43 In contrast, cht/soy membranes exerted their anti-inflammatory potential by inhibiting the activation of the inflammatory cells and avoiding a toxic environment in the wound bed, which lead to normal wound healing process.
In this study, an unexpected reaction was observed for the Epigard®, independently of the time periods tested. Being a clinically used wound dressing, the observed impaired repair was not predicted. Of note, this dressing is not clinically used for wounds up to the muscle layer. However, it was important to use this dressing as it is a dressing without any biologically active substances. Therefore, it was in our opinion the best suitable means for comparison. The different healing rates between rats and humans and the faster metabolism of rats, although in impaired wound conditions, may have led to an exacerbated reaction and to the formation of a vascularized network of cell-embedded matrix might justify the results with Epigard®. Conversely to the cht/soy membranes that were easily detached from the wounds, without additional trauma and without removing the granular tissue, the Epigard® integrated within the wound tissue, thus delaying the resolution of the inflammatory process. The continued presence of the Epigard® within the wound showed that the host was still combating the foreign body and thus was unable to proceed with the re-epithelialization and the closure of the wound.
The overall results showed that the cht/soy-based membranes induced the formation of new tissue with good histological features in a short period of time although, in comparison to the standard controls, the wound area seems similar at the end of the experiment. The fact that cht/soy membranes were completely loose on the wound seemed to facilitate the spatial and increased progression of the newly formed epithelium. It is also critical to emphasize that, besides wound closure, wound dressing must promote the formation of quality tissue, ideally without contraction. The cht/soy-based membranes appear not only to be able to regulate the wound moisture, which at extreme levels would impair epidermis repair, but also to provide the adequate coverage that does not physically constrain the formation of new tissue.
Despite the relevance of the observations included in this study, the use of a rodent model in wound-dressing evaluation, although in impaired healing conditions, may enclose some drawbacks regarding the inevitable wound contraction. Furthermore, since the main purpose was the evaluation of cht/soy-based membranes' performance without growth factors, the choice of a clinically relevant positive control was limited to a polymeric mesh, without any growth factor (Epigard®), which had a detrimental influence in wound healing and cutaneous repair.
Nevertheless, the present work strongly suggests that the newly developed cht/soy-based membranes produced by solvent casting methodology that had proven to promote low in vitro activation of human PMNs isolated from circulating blood decrease the healing time period of partial-thickness skin wounds in impaired healing rats. Moreover, these cht/soy-based membranes showed an enhanced performance as compared to the negative and positive controls, inducing fast re-epithelialization and wound closure.
Acknowledgments
The author Tírcia C. Santos acknowledges the Portuguese Foundation for Science and Technology (FCT) for her PhD grant (SFRH/BD/40861/2007). This work was developed under the scope of the European Network of Excellence EXPERTISSUES (NMP3-CT-2004-5000283).
Disclosure Statement
The authors state no conflicts of interest.
References
- 1.White R. Morris C. Mepitel: a non-adherent wound dressing with Safetac technology. Br J Nurs. 2009;18:58. doi: 10.12968/bjon.2009.18.1.93582. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez O.M. Mertz P.M. Eaglstein W.H. The effect of occlusive dressings on collagen synthesis and re-epithelialization in superficial wounds. J Surg Res. 1983;35:142. doi: 10.1016/0022-4804(83)90136-1. [DOI] [PubMed] [Google Scholar]
- 3.Nathan P. MacMillan B.G. Holder I.A. In situ production of a synthetic barrier dressing for burn wounds in rats. Infect Immun. 1975;12:257. doi: 10.1128/iai.12.2.257-260.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geronemus R.G. Robins P. The effect of two new dressings on epidermal wound healing. J Dermatol Surg Oncol. 1982;8:850. doi: 10.1111/j.1524-4725.1982.tb03992.x. [DOI] [PubMed] [Google Scholar]
- 5.James J.H. Watson A.C. The use of Opsite, a vapour permeable dressing, on skin graft donor sites. Br J Plast Surg. 1975;28:107. [PubMed] [Google Scholar]
- 6.Wood R.A. Hughes L.E. Silicone foam sponge for pilonidal sinus: a new technique for dressing open granulating wounds. Br Med J. 1975;4:131. doi: 10.1136/bmj.4.5989.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tavis M.J. Thornton J.W. Harney J.H. Woodroof E.A. Bartlett R.H. Graft adherence to de-epithelialized surfaces: a comparative study. Ann Surg. 1976;184:594. doi: 10.1097/00000658-197611000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Damour O. Gueugniaud P.Y. Berthin-Maghit M. Rousselle P. Berthod F. Sahuc F., et al. A dermal substrate made of collagen—GAG—chitosan for deep burn coverage: first clinical uses. Clin Mater. 1994;15:273. doi: 10.1016/0267-6605(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 9.Burget A. Nathan P. Holder I.A. Macmillan B.G. The effect of a collagen dressing on contaminated surgical wounds in rats. Langenbecks Arch Chir. 1976;343:69. doi: 10.1007/BF01261571. [DOI] [PubMed] [Google Scholar]
- 10.Ovington L.G. Advances in wound dressings. Clin Dermatol. 2007;25:33. doi: 10.1016/j.clindermatol.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Nathan P. Macmillan B.G. Holder I.A. Effect of a synthetic dressing formed on a burn wound in rats: a comparison of allografts, collagen sheets, and polyhydroxyethylmethacrylate in the control of wound infection. Appl Microbiol. 1974;28:465. doi: 10.1128/am.28.3.465-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone H.A. Edelman R.D. McGarry J.J. Epigard: a synthetic skin substitute with application to podiatric wound management. J Foot Ankle Surg. 1993;32:232. [PubMed] [Google Scholar]
- 13.Szycher M. Lee S.J. Modern wound dressings: a systematic approach to wound healing. J Biomater Appl. 1992;7:142. doi: 10.1177/088532829200700204. [DOI] [PubMed] [Google Scholar]
- 14.Nathan P. Law E.J. MacMillan B.G. Murphy D.F. Ronel S.H. D'Andrea M.J., et al. A new biomaterial for the control of infection in the burn wound. Trans Am Soc Artif Intern Organs. 1976;22:30. [PubMed] [Google Scholar]
- 15.Fulton J.E., Jr The stimulation of postdermabrasion wound healing with stabilized aloe vera gel-polyethylene oxide dressing. J Dermatol Surg Oncol. 1990;16:460. doi: 10.1111/j.1524-4725.1990.tb00065.x. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto Y. Shibazaki K. Minami S. Matsuhashi A. Tanioka S. Shigemasa Y. Evaluation of chitin and chitosan on open would healing in dogs. J Vet Med Sci. 1995;57:851. doi: 10.1292/jvms.57.851. [DOI] [PubMed] [Google Scholar]
- 17.King W.W. Lam P.K. Liew C.T. Ho W.S. Li A.K. Evaluation of artificial skin (Integra) in a rodent model. Burns. 1997;23(Suppl 1):S30. doi: 10.1016/s0305-4179(97)90098-x. [DOI] [PubMed] [Google Scholar]
- 18.Leipziger L.S. Glushko V. DiBernardo B. Shafaie F. Noble J. Nichols J., et al. Dermal wound repair: role of collagen matrix implants and synthetic polymer dressings. J Am Acad Dermatol. 1985;12:409. doi: 10.1016/s0190-9622(85)80004-9. [DOI] [PubMed] [Google Scholar]
- 19.Norton L. Chvapil M. Comparison of newer synthetic and biological wound dressings. J Trauma. 1981;21:463. [PubMed] [Google Scholar]
- 20.Keong L.C. Halim A.S. In vitro models in biocompatibility assessment for biomedical-grade chitosan derivatives in wound management. Int J Mol Sci. 2009;10:1300. doi: 10.3390/ijms10031300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W. Lin S.Q. Xiao Y.C. Huang Y.D. Tan Y. Cai L., et al. Acceleration of diabetic wound healing with chitosan-crosslinked collagen sponge containing recombinant human acidic fibroblast growth factor in healing-impaired STZ diabetic rats. Life Sci. 2008;82:190. doi: 10.1016/j.lfs.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Wang C.C. Su C.H. Chen C.C. Water absorbing and antibacterial properties of N-isopropyl acrylamide grafted and collagen/chitosan immobilized polypropylene nonwoven fabric and its application on wound healing enhancement. J Biomed Mater Res Part A. 2008;84A:1006. doi: 10.1002/jbm.a.31482. [DOI] [PubMed] [Google Scholar]
- 23.Qin Y.M. The preparation and characterization of chitosan wound dressings with different degrees of acetylation. J Appl Polym Sci. 2008;107:993. [Google Scholar]
- 24.Ong S.Y. Wu J. Moochhala S.M. Tan M.H. Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 25.Okabayashi R. Nakamura M. Okabayashi T. Tanaka Y. Nagai A. Yamashita K. Efficacy of polarized hydroxyapatite and silk fibroin composite dressing gel on epidermal recovery from full-thickness skin wounds. J Biomed Mater Res B Appl Biomater. 2009;90:641. doi: 10.1002/jbm.b.31329. [DOI] [PubMed] [Google Scholar]
- 26.Schneider A. Wang X.Y. Kaplan D.L. Garlick J.A. Egles C. Biofunctionalized electrospun silk mats as a topical bioactive dressing for accelerated wound healing. Acta Biomater. 2009;5:2570. doi: 10.1016/j.actbio.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori T. Murakami M. Okumura M. Kadosawa T. Uede T. Fujinaga T. Mechanism of macrophage activation by chitin derivatives. J Vet Med Sci. 2005;67:51. doi: 10.1292/jvms.67.51. [DOI] [PubMed] [Google Scholar]
- 28.Cerami A. Inflammatory cytokines. Clin Immunol Immunopathol. 1992;62:S3. doi: 10.1016/0090-1229(92)90035-m. [DOI] [PubMed] [Google Scholar]
- 29.Driscoll K.E. Hassenbein D.G. Howard B.W. Isfort R.J. Cody D. Tindal M.H., et al. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol. 1995;58:359. doi: 10.1002/jlb.58.3.359. [DOI] [PubMed] [Google Scholar]
- 30.Diegelmann R.F. Dunn J.D. Lindblad W.J. Cohen I.K. Analysis of the effects of chitosan on inflammation, angiogenesis, fibroplasia, and collagen deposition in polyvinyl alcohol sponge implants in rat wounds. Wound Repair Regen. 1996;4:48. doi: 10.1046/j.1524-475X.1996.40109.x. [DOI] [PubMed] [Google Scholar]
- 31.Deng C.M. He L.Z. Zhao M. Yang D. Liu Y. Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr Polym. 2007;69:583. [Google Scholar]
- 32.Azad A.K. Sermsintham N. Chandrkrachang S. Stevens W.F. Chitosan membrane as a wound-healing dressing: characterization and clinical application. J Biomed Mater Res B Appl Biomater. 2004;69:216. doi: 10.1002/jbm.b.30000. [DOI] [PubMed] [Google Scholar]
- 33.Ueno H. Yamada H. Tanaka I. Kaba N. Matsuura M. Okumura M., et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- 34.Santos T.C. Marques A.P. Silva S.S. Oliveira J.M. Mano J.F. Castro A.G., et al. In vitro evaluation of the behaviour of human polymorphonuclear neutrophils in direct contact with chitosan-based membranes. J Biotechnol. 2007;132:218. doi: 10.1016/j.jbiotec.2007.07.497. [DOI] [PubMed] [Google Scholar]
- 35.Coward L. Barnes N.C. Setchel K.D.R. Barnes S. Genistein, Daidzein, and their &glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian Diets. J Agric Food Chem. 1993;41:1961. [Google Scholar]
- 36.Emmerson E. Campbell L. Ashcroft G.S. Hardman M.J. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol Cell Endocrinol. 2010;321:184. doi: 10.1016/j.mce.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Marini H. Polito P. Altavilla D. Irrera N. Minutoli L. Calò M., et al. Genistein aglycone improves skin repair in an incisional model of wound healing: a comparison with raloxifene and oestradiol in ovariectomized rats. Br J Pharmacol. 2010;160:1185. doi: 10.1111/j.1476-5381.2010.00758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silva S.S. Santos M.I. Coutinho O.P. Mano J.F. Reis R.L. Physical properties and biocompatibility of chitosan/soy blended membranes. J Mater Sci Mater Med. 2005;16:575. doi: 10.1007/s10856-005-0534-z. [DOI] [PubMed] [Google Scholar]
- 39.Santos T.C. Marques A.P. Silva R.M. Silva S.S. Mano J.F. Castro A.G., et al. Chitosan improves the biological performance of soy-based biomaterials. Tissue Eng Part A. 2010;16:2883. doi: 10.1089/ten.TEA.2010.0114. [DOI] [PubMed] [Google Scholar]
- 40.Hong H.J. Jin S.E. Park J.S. Ahn W.S. Kim C.K. Accelerated wound healing by smad3 antisense oligonucleotides-impregnated chitosan/alginate polyelectrolyte complex. Biomaterials. 2008;29:4831. doi: 10.1016/j.biomaterials.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 41.Burkatovskaya M. Castano A.P. Demidova-Rice T.N. Tegos G.P. Hamblin M.R. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noorjahan S.E. Sastry T.P. An in vivo study of hydrogels based on physiologically clotted fibrin-gelatin composites as wound-dressing materials. J Biomed Mater Res B Appl Biomater. 2004;71:305. doi: 10.1002/jbm.b.30094. [DOI] [PubMed] [Google Scholar]
- 43.Laplante A.F. Germain L. Auger F.A. Moulin V. Mechanisms of wound reepithelialization: hints from a tissue-engineered reconstructed skin to long-standing questions. FASEB J. 2001;15:2377. doi: 10.1096/fj.01-0250com. [DOI] [PubMed] [Google Scholar]
- 44.Kweon D.K. Song S.B. Park Y.Y. Preparation of water-soluble chitosan/heparin complex and its application as wound healing accelerator. Biomaterials. 2003;24:1595. doi: 10.1016/s0142-9612(02)00566-5. [DOI] [PubMed] [Google Scholar]
- 45.Burkatovskaya M. Tegos G.P. Swietlik E. Demidova T.N. P Castano A. Hamblin M.R. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth R.R. Winton G.B. A synthetic skin substitute as a temporary dressing in Mohs surgery. J Dermatol Surg Oncol. 1989;15:670. doi: 10.1111/j.1524-4725.1989.tb03607.x. [DOI] [PubMed] [Google Scholar]
- 47.Reis R.L. Mendes S.C. Cunha A.M. Bevis M.L. Processing and in vitro degradation of starch/EVOH thermoplastic blends. Polym Int. 1997;43:347. [Google Scholar]
- 48.Yusof N.L. Wee A. Lim L.Y. Khor E. Flexible chitin films as potential wound-dressing materials: wound model studies. J Biomed Mater Res A. 2003;66:224. doi: 10.1002/jbm.a.10545. [DOI] [PubMed] [Google Scholar]
- 49.Wicke C. Halliday B. Allen D. Roche N.S. Scheuenstuhl H. Spencer M.M., et al. Effects of steroids and retinoids on wound healing. Arch Surg. 2000;135:1265. doi: 10.1001/archsurg.135.11.1265. [DOI] [PubMed] [Google Scholar]
- 50.Kirkwood B. Sterne J. Essential Medical Statistics. 2nd. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 51.Davidson J.M. Animal models for wound repair. Arch Dermatol Res. 1998;290(Suppl):S1. doi: 10.1007/pl00007448. [DOI] [PubMed] [Google Scholar]
- 52.Ishihara M. Nakanishi K. Ono K. Sato M. Kikuchi M. Saito Y., et al. Photocrosslinkable chitosan as a dressing for wound occlusion and accelerator in healing process. Biomaterials. 2002;23:833. doi: 10.1016/s0142-9612(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 53.Khan T.A. Peh K.K. A preliminary investigation of chitosan film as dressing for punch biopsy wounds in rats. J Pharm Pharm Sci. 2003;6:20. [PubMed] [Google Scholar]
- 54.Ueno H. Mori T. Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- 55.Atala A. Lanza R. Thomson J. Nerem R. Principles of Regenerative Medicine. London, UK: Academic Press; 2008. [Google Scholar]
- 56.Saulis A. Mustoe T.A. Models of wound healing in growth factor studies. In: Souba W.W., editor; Wilmore D.W., editor. Surgery Research. San Diego, CA: Academic Press; 2001. p. 857. [Google Scholar]