Abstract
Myocardial infarction (MI) causes significant cell loss and damage to myocardium. Cell-based therapies for treatment of MI aim to remuscularize the resultant scar tissue, but the majority of transplanted cells do not survive or integrate with the host tissue. Scaffolds can improve cell retention following construct implantation, but often do little to enhance host-graft integration and/or show limited biodegradation. Fibrin is an ideal biomaterial for cardiac tissue engineering as it is a natural, biodegradable polymer that can induce neovascularization, promote cell attachment, and has tunable mechanical properties. Here we describe a novel, high-density microtemplated fibrin scaffold seeded with a tri-cell mixture of cardiomyocytes, endothelial cells (ECs), and fibroblasts to mimic native cardiac tissue in structure and cellular composition to improve cell retention and promote integration with the host tissue. Scaffolds were designed with uniform architecture of parallel 60 μm microchannels surrounded by an interconnected microporous network of 27-μm-diameter pores and mechanical stiffness comparable to native cardiac tissues (70–90kPa). Scaffold degradation was controlled with the addition of Factor XIII (FXIII) and/or protease inhibitor (aprotinin). Unmodified scaffolds had a fast degradation profile both in vitro (19.9%±3.9% stiffness retention after 10 days) and in vivo. Scaffolds treated with FXIII showed an intermediate degradation profile in vitro (45.8%±5.9%), while scaffolds treated with aprotinin or both FXIII and aprotinin showed significantly slowed degradation in vitro (60.9%±5.2% and 76.4%±7.6%, respectively, p<0.05). Acellular aprotinin scaffold myocardial implants showed decreased collagen deposition after 7 days. Unmodified and aprotinin implants could not be located by 14 days, while 2 of 8 FXIII implants were found, but were significantly degraded. Constructs supported seeded cell survival and organization in vitro, promoting EC-lined lumen structure formation in construct channels and colocalization of viable ECs and cardiomyocytes. In addition, constructs promoted extracellular matrix deposition by seeded cells, as shown by collagen staining within construct channels and by significant increases in construct stiffness over 10 days in vitro (209%±32%, p<0.05). The data suggest our fibrin scaffolds are ideally designed to promote graft cell survival and organization, thus improving chances of promoting construct integration with the host tissue upon implantation.
Introduction
Current cell-based therapies for myocardial infarction (MI) in human trials include direct injection of cells (such as bone marrow stem cells, skeletal myoblasts, or stem cell-derived cardiomyocytes) into infarcted myocardium or surrounding tissue or coronary vessels. The hope is that these delivered cells will home to damaged tissue and exert therapeutic effects. However, few cells reach the infarct with these methods and cell retention and survival remain extremely low (<10%).1–4 In addition, cells delivered in suspension can be disorganized and often lack cell–cell and cell–matrix contacts. Cells respond differently when grown in a 2D versus 3D environment, and the function of many cell types is anchorage-dependent. Sheets of cells have been stacked to form thicker tissue grafts, but these are limited by diffusion of nutrients and low mechanical strength.2,5 For cell-based therapies to successfully regenerate damaged myocardium, it is imperative to deliver enough viable cells to the infarct zone that survive and function like normal cardiac cells and integrate with the host tissue. For these reasons, biomaterials are desirable as delivery vehicles to help improve cell retention. However, issues with immune rejection, degradation, and mechanical integration have limited their success in cardiac repair.1,2
An effective cellular construct for the treatment of MI should (1) enhance cell attachment and promote cellular organization to increase the number of functional cells being delivered, (2) encourage infiltration of and integration with the host vasculature to improve construct survival upon implantation, (3) be nonimmunogenic, and (4) degrade into nontoxic byproducts. Additionally, constructs should have a mechanical strength similar to native myocardium to support grafted cell function and to avoid issues with mechanical mismatch upon implantation. Both synthetic and natural polymers are being investigated for use in cardiac tissue engineering applications. Many of these are used to form scaffolds mimicking an extracellular matrix (ECM)-like structure on which cells are grown before implantation of the construct.2
Fibrin is a natural protein used as an effective scaffolding material to grow cells and tissue constructs due to its cell adhesive properties and natural nontoxic degradation products.6–8 Fibrin is the major protein component of blood clots, and is formed when the precursor, fibrinogen, is cleaved by thrombin, which also activates the zymogen factor XIII (FXIII) to covalently crosslink the unstable fibrin network to form an insoluble fibrin clot. These clots are naturally degraded by plasmin into D-dimers and other degradation products that are easily cleared by the body.7,9 Fibrin hydrogels have been utilized to deliver cells,10–12 with Christman et al. being the first to demonstrate transplanted cell survival was improved when injected with fibrin hydrogels.13 Additionally, fibrin induces neovascularization within ischemic myocardium and reduces infarct expansion, with enhanced neovascularization in ischemic myocardium observed following injection of fibrin gels alone or in conjunction with cells.12–15 The observed neovascularization effects of fibrin fit with the well-established role of fibrin degradation products in the induction of angiogenesis,16 and further demonstrate the suitability of fibrin as a scaffold biomaterial.
The main issue with fibrin hydrogels for cardiac tissue engineering applications is that they are mechanically weak, limiting their ability to adequately support dynamically contracting cardiomyocytes and constantly working native myocardium. In contrast, high-concentration fibrin glues are too dense for cells to penetrate, limiting their use as cell delivery vehicles. Ideally, the scaffold–cell construct should have similar biomechanical properties to ventricular wall tissue. Recently, Linnes et al. developed a method of microtemplating high-density fibrin into an interconnected microporous architecture with controllable pore size and mechanical stiffness closer to cardiac tissue than fibrin gels.7 A sphere-templated microporous architecture with pores of equal size is ideal for promoting nutrient delivery, gas exchange, and neovascularization within the construct,4 but is limited by low-density cell-seeding and random cellular alignment. Madden et al. overcame these limitations by incorporating a microchannel network into a similar microporous scaffold construction, producing bimodal synthetic scaffolds for use as cardiac constructs.4
Here we describe a novel high-density templated fibrin scaffold with a microchanneled and microporous architecture that we successfully seeded with a tri-cell mixture of cardiomyocytes, endothelial cells (ECs), and cardiac fibroblasts. This cell-seeded scaffold is designed to mimic native cardiac tissue in both structure and composition to address many of the issues described above. The microarchitecture of our scaffold allows higher density cell-seeding, promotes cellular alignment and prevascular network formation within channels, and facilitates nutrient exchange and waste removal within constructs. In addition, the high-density fibrin material promotes cellular attachment, provides adequate and tunable mechanical strength for the grafted cells, and should promote construct integration with the host tissue upon implantation.
Materials and Methods
Fibrin scaffold construction
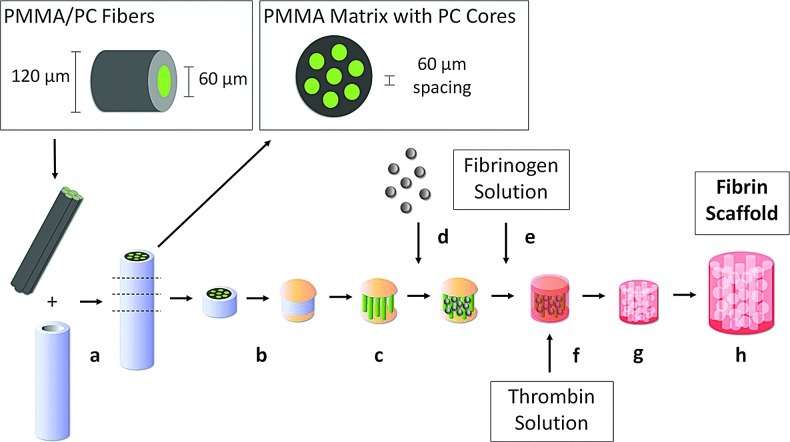
Scaffolds were constructed according to published methods with modifications,4,7 summarized in Figure 1. Briefly, optical fibers (Paradigm Optics, Vancouver, WA) with a 60-μm-diameter inner polycarbonate (PC) core and a 30-μm-thickness poly(methyl methacrylate) (PMMA) outer shell were bundled in Teflon shrink tubing and sintered at 145°C overnight to form a solid PMMA matrix containing PC cores spaced 60 μm apart. After sectioning into 1–3-mm-length disks and immobilizing the ends of the PC cores with cyanoacrylate, the PMMA matrix was selectively dissolved with xylene washes over 5 days. The resulting void space around the PC cores was then filled with PMMA microbeads (27 μm diameter, Microbeads, Skedsmokorset, Norway) in a close-packed arrangement via sonic sifting. The beads were sintered in place at 180°C for 24 h to obtain a pore neck diameter 50% of the bead diameter. The polymer template was infiltrated with a 200 mg/mL fibrinogen solution (bovine fibrinogen Type 1-S, Sigma-Aldrich, St. Louis, MO; in 0.9% NaCl) via centrifugation. A concentrated thrombin solution (13.25 U/mL thrombin, Sigma, St. Louis, MO; 8.3 mM CaCl2; DMEM, Gibco, Grand Island, NY) was used to polymerize the fibrinogen into fibrin around the polymer template overnight at room temperature. After scraping the exterior of the scaffolds to remove excess fibrin, the polymer template was dissolved with two 24 h washes in a 90% dichloromethane/10% hexanes solution followed by a 24-h acetone wash on an orbital shaker at room temperature. Scaffolds were treated with 100% ethanol rinses for 1 week before rehydration with a graded ethanol series into sterile phosphate-buffered saline (PBS).
FIG. 1.
Microtemplated fibrin scaffold construction. (a) Bundle poly(methyl methacrylate) (PMMA)/polycarbonate (PC) fibers & sinter to form PMMA matrix with PC cores spaced 60 μm apart, (b) section & immobilize ends of PC cores, (c) selectively remove PMMA matrix, (d) pack PMMA beads around PC cores and sinter, (e) add fibrinogen solution via centrifugation, (f) polymerize with thrombin solution, (g) remove synthetic polymers, and (h) rehydrate fibrin scaffold. Color images available online at www.liebertpub.com/tea
Scaffold modifications
Additional crosslinking of fibrin scaffolds was achieved by adding the human Factor XIII (FXIII; Innovative Research, Novi, MI) at 100 μg/mL to the fibrinogen solution before centrifugation into polymer templates. Inhibition of scaffold degradation by proteases was tested by adding the serine protease inhibitor aprotinin (3000 U/mL; Sigma-Aldrich, St. Louis, MO) to the fibrinogen solution. These scaffold modifications were tested alone and in combination with each other to determine their effects on stiffness and in vitro degradation rates of the fibrin scaffolds.
Architecture evaluation
Scaffold architecture was evaluated with scanning electron microscopy (SEM). Briefly, scaffolds were fixed in 0.5% glutaraldehyde overnight at room temperature followed by dehydration in a graded ethanol series and critical point drying to maintain pore and channel structures. Samples were cut to different depths and Au/Pd sputter-coated for 60 s before imaging with an FEI (Hillsboro, OR) Sirion field-emission microscope under high-vacuum conditions at an accelerating voltage of 10 kV.
Mechanical stiffness and in vitro degradation analysis
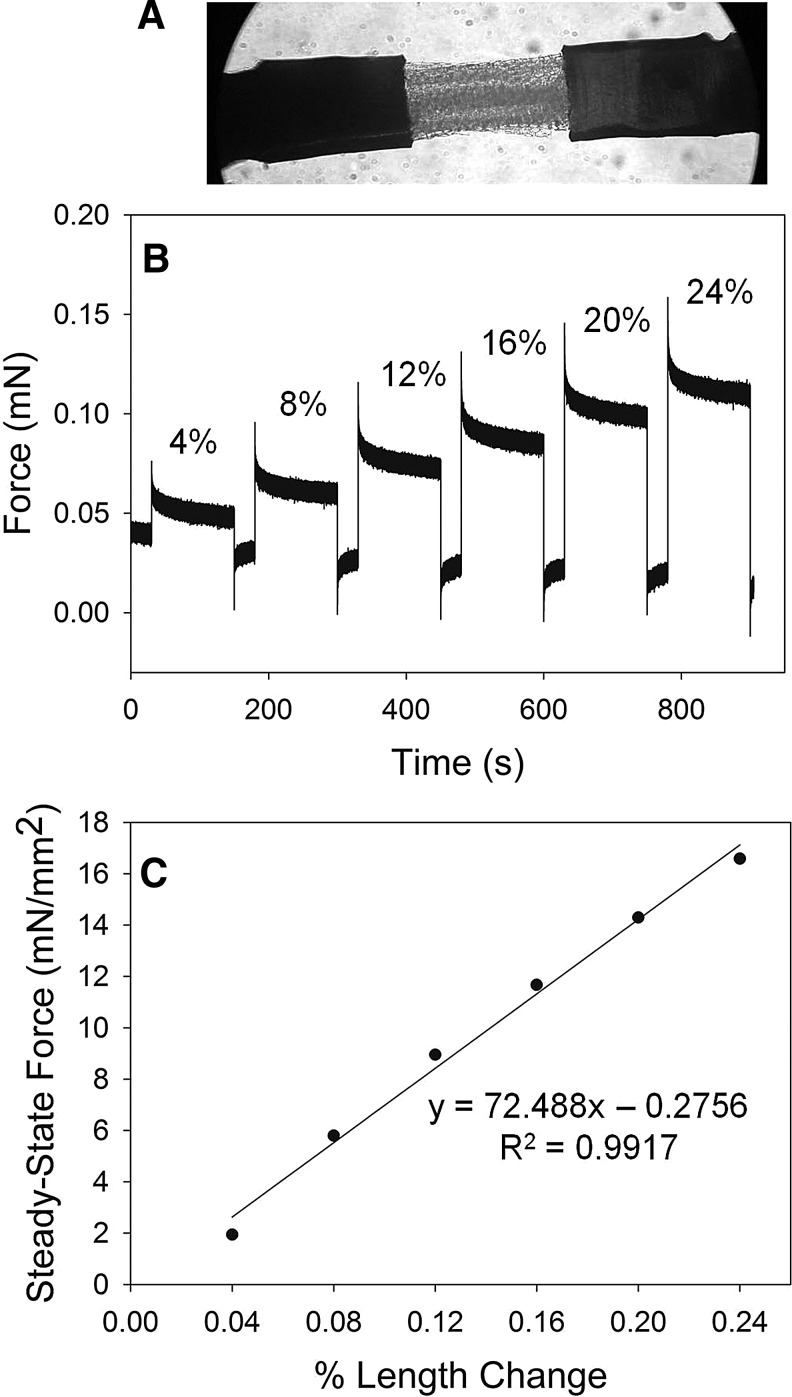
To assess the tensile strength and degradation time-course of the fibrin scaffolds, scaffold stiffness was measured using a custom-built biomechanical analysis setup in the Regnier lab.17–19 Briefly, scaffolds were cut into strips (0.2–0.4 mm diameter×1.0 mm length) and mounted with aluminum foil T-clips between a force transducer and a servomotor tuned for a 300 μs step response (Fig. 2A). Step changes in length (4%–24% of scaffold strip length) and resulting changes in tension (Fig. 2B) were measured and recorded by our custom LabVIEW software program. Resulting tension (mN) and force normalized to a cross-sectional area (mm2) were plotted against percent length change, and the slope of the resulting linear regression line was used as a measure of stiffness in kPa (Fig. 2C). Scaffolds were measured at different time points (0, 3, 6, & 10 days) after incubation at 37°C in sterile PBS (for acellular degradation studies) or in culture medium (for cell-seeded scaffolds).
FIG. 2.
Mechanical stiffness testing of fibrin scaffolds. (A) Strip of scaffold mounted between a force transducer and a motor (10× magnification). (B) Step length changes (4%–24%) with resulting tension measured as force (mN). (C) Normalized steady-state force (mN/mm2) is plotted against % length change, and the slope of the resulting regression line is the material stiffness (kPa).
Acellular scaffold myocardial implants
After rehydration and equilibration in sterile PBS, acellular fibrin scaffolds (unmodified, FXIII-treated, or aprotinin-treated) were cut into 300–400-μm-diameter×1–2-mm-long strips and kept in sterile PBS at 4°C until implantation. All animal procedures were conducted in accordance with the US National Institutes of Health Policy on Humane Care and Use of Laboratory Animals and were approved by the University of Washington (UW) Animal Care Committee. Rats were housed in the Department of Comparative Medicine at UW and cared for in accordance with the UW Institutional Animal Care and Use Committee (IACUC) procedures. Adult male Fischer 344 rats (200–300 g) were sedated with isoflurane before implanting scaffold strips into the wall of the left ventricle as described previously by our group.4 At endpoints of 1, 7, and 14 days, rats were euthanized via pentobarbital overdose (120–150 mg/kg) administered via IP injection, followed by pneumothorax before rapidly dissecting the hearts. This method of euthanasia is in accordance with recommendations of the Panel on Euthanasia of the American Veterinary Medical Association.
Cell-seeding and culture of fibrin constructs
Primary neonatal rat ventricular cardiomyocytes (NRVCs) and neonatal rat cardiac fibroblasts (NRCFs) were isolated from 1–3-day-old Fischer 344 rats using standard protocols reported previously by our group.19 Cardiomyocytes were separated from fibroblasts by preplating the primary cell suspension, and the two cell types were kept separately in tissue culture flasks for 2–4 days before seeding. Cardiomyocytes were heat shocked at 42°C for 40 min, 24 h before seeding, as this has been shown to enhance cell survival after transplantation, and we believe this treatment will be important for our system due to the stress of seeding and the high density of cells being used.19,20 Rat aortic endothelial cells (RAECs, a gift from Dr. Nicosia, VA hospital Seattle, WA) or human umbilical vein endothelial cells (HUVECs, Lonza, Basel, Switzerland) were grown in culture. For some constructs, cardiomyocytes were labeled with a fluorescent lipophilic tracer dye (CM-DiI, Vybrant Cell-Labeling Solutions; Molecular Probes, Eugene, OR) during heat shock treatment 24 h before seeding. After rehydration into sterile PBS, scaffolds were cut using 2-mm-diameter biopsy punch tubes in which the scaffolds remained for the duration of seeding. Cells were seeded at a 4:2:1 ratio (NRVCs:ECs:NRCFs) at a density of 2×106 cells per scaffold (2 mm diameter×2–3 mm length). The tri-cell mixture was pipetted into the biopsy punch tubes and centrifuged into the scaffolds over 3–4 rounds of centrifugation at ∼7 g for 5 min per round. After seeding, scaffolds were removed from the biopsy punch tubes, placed in 12-well tissue culture plates, and kept in an incubator (37°C, 5% CO2) for 15–30 min to allow cells to adhere to scaffolds before pipetting 2 mL of plating media21 around each scaffold. Cell-seeded scaffolds were maintained in static culture with media changes every other day. Live cell-seeded constructs containing CM-DiI-labeled cardiomyocytes were imaged under fluorescent light.
Histological analysis
Hearts from acellular implant studies were sliced into 2-mm sections beginning with a cut through the center of the implant site, and then immersion-fixed in Methyl Carnoy's (MC) fixative. Cell-seeded constructs were immersion-fixed in MC fixative at different time points from 0–14 days after seeding. Samples were processed, embedded in paraffin, and sectioned (5 μm) for histology. Serial sections of samples were stained with H&E, Masson's trichrome stain, and with immunohistochemical staining using hematoxylin as the nuclear counterstain. Immunostaining was performed with primary antibodies against sarcomeric myosin (clone MF-20, Developmental Studies Hybridoma Bank), rat endothelial cell antigen (RECA-1, mouse anti-rat (1:10); AbDSerotec, Raleigh, NC), human EC marker (CD31, mouse anti-human (1:100); Dako, Carpinteria, CA), desmin (rabbit anti-rat (1:20); Dako, Carpinteria, CA), leukocyte marker CD45 (mouse anti-rat (1:100); BD Biosciences, San Jose, CA), and macrophage marker CD68 (mouse anti-rat (1:100); AbDSerotec, Raleigh, NC). Samples were then labeled with secondary antibodies using either a biotinylated horse anti-mouse (1:400) secondary antibody (Vector, Burlingame, CA) and developed with DAB (Sigma-Aldrich, St. Louis, MO), or with fluorescently labeled secondary antibodies (Alexa Fluor 488 goat anti-mouse (1:100) or Alexa Fluor 594 goat anti-rabbit (1:100), Molecular Probes, Eugene, OR) and counterstained with 4′,6-diamidino-2-phenylindole, dilactate (DAPI, dilactate, 300 nM, Molecular Probes, Eugene, OR).
Statistical analysis
All values are reported as mean±S.E.M. When comparing stiffness values, statistical significance from unmodified scaffold values was determined with a One-Way ANOVA test using the Dunnett method. Statistical significance between other scaffold modifications was tested using a 2-sided Student's t-test (α=0.05). Differences at a p-value<0.05 were considered statistically significant.
Results
Fibrin scaffold construction
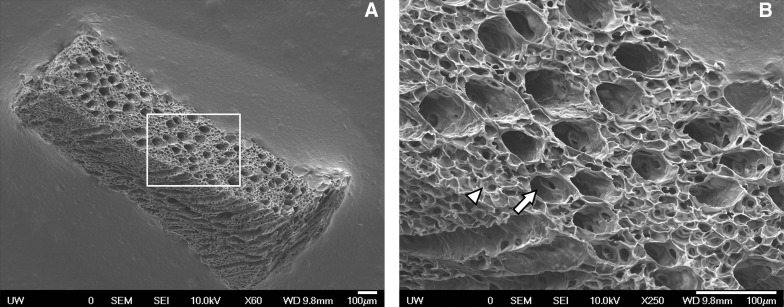
High-density (200 mg/mL) fibrin scaffolds were constructed using the polymer templates described in the Methods section. Success of fibrin scaffold formation was assessed with SEM imaging at different depths through the scaffold, confirming an interconnected microporous network (27-μm-diameter pores) surrounding open microchannels (60 μm diameter) that spanned the length of the construct. SEM analysis confirmed consistent formation of this microtemplated architecture with regularly shaped and evenly spaced channels and pores throughout the fibrin scaffold (Fig. 3).
FIG. 3.
Scanning electron microscopy images of high-density microtemplated fibrin confirming scaffold architecture with a uniform 27-μm porous network (arrowhead) surrounding evenly spaced 60-μm channels (arrow) at (A) 60×and (B) 250× magnification. Scale bars=100 μm.
Acellular scaffold myocardial implants
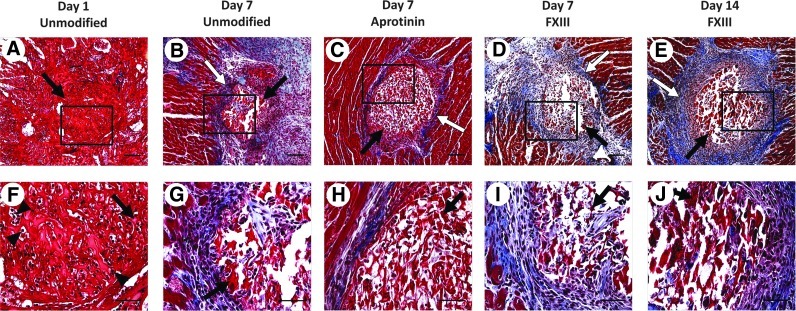
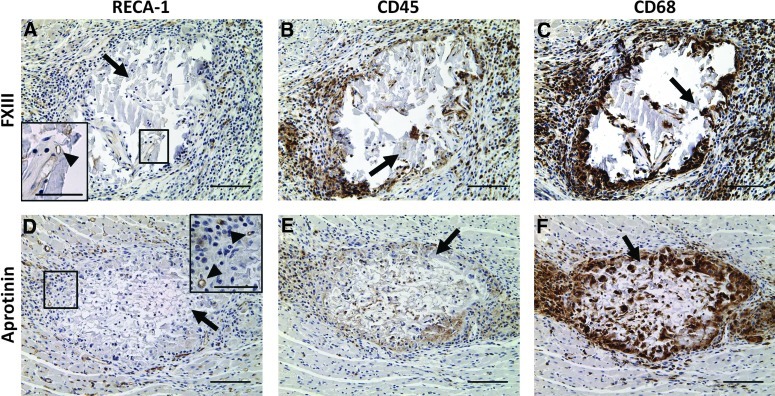
An initial study was performed to determine how the host cardiac tissue would respond to the scaffold material and to assess the time course of degradation of scaffolds in vivo. Unmodified, FXIII-treated, and aprotinin-treated fibrin scaffolds were implanted in the ventricular wall of adult rat hearts. Scaffolds located at the site of implantation were completely infiltrated with host cells after 1 day (Fig. 4A, F). Histological analysis indicated these were mainly neutrophils, suggesting a normal early inflammatory response to the fibrin scaffolds as expected for this early time point. Scaffolds were significantly degraded by 7 days postimplantation (Fig. 4b–D, G–I). Histological analysis of day 7 samples showed an area of inflammation immediately surrounding the implants, characterized by RECA-1+ EC-lined lumens (Fig. 5A, D), CD45+ leukocytes (Fig. 5B, E) and CD68+ macrophages (Fig. 5C, F), and an area of newly deposited loose collagen (Fig. 4B–D, G–I), consistent with a normal wound-healing response. Unmodified and FXIII-treated scaffolds showed a similar host response at the 7-day time point. Aprotinin-treated scaffolds appeared to have less collagen deposition in the area of implantation at the 7-day time point (Fig. 4C, H), and 3 of 6 aprotinin-treated implants were located after 7 days (as compared to 1 of 6 unmodified, and 2 of 6 FXIII-treated implants). Unmodified and aprotinin-treated scaffolds could not be located 14 days postimplantation, suggesting complete scaffold degradation by this time. Although significantly degraded, 2 of 8 FXIII-treated scaffolds were located at the site of implantation after 14 days (Fig. 4E, J) versus 0 of 5 unmodified scaffolds and 0 of 6 aprotinin-treated scaffolds, suggesting that FXIII modification may slow fibrin scaffold degradation in vivo.
FIG. 4.
Acellular fibrin scaffold implants in adult rat myocardium. Masson's trichrome staining of scaffold implants in cross section. Unmodified scaffolds after (A, F) 1 day and (B, G) 7 days, aprotinin scaffolds after (C, H) 7 days, and FXIII scaffolds after (D, I) 7 days and (E, J) 14 days of implantation. Black arrows indicate fibrin implant material, white arrows indicate loose collagen deposition, arrowheads indicate examples of infiltrated neutrophils. Scale bars=100 μm in (A–E), 50 μm (F–J). Color images available online at www.liebertpub.com/tea
FIG. 5.
Acellular fibrin scaffold implants in adult rat myocardium. Immunohistological staining of FXIII (A–C) and aprotinin (D–F) scaffold implants in cross section after 7 days of implantation. Rat endothelial cell antigen (RECA)-1 (A, D), CD45 (B, E), and CD68 (C, F) staining indicate a normal wound-healing response to implants. Inset images in (A) and (D) show RECA-1+ cells (indicated by arrowheads) within and adjacent to fibrin scaffold. Black arrows in all panels indicate fibrin implant material. Scale bars=100 μm (50 μm in insets). Color images available online at www.liebertpub.com/tea
Acellular scaffold mechanical stiffness and in vitro degradation analysis
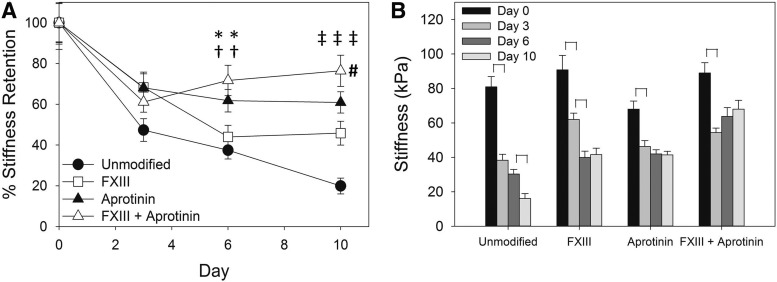
Following the in vivo study, we wanted to determine whether scaffold treatments that may slow degradation in vivo would affect the material stiffness, as we believe it will be important to maintain an initial scaffold stiffness comparable to that of native tissues (neonatal rat myocardium=39±9 kPa; adult rat myocardium=273±100 kPa).19 Scaffolds were mechanically tested in vitro to determine material stiffness and degradation rates with and without material modifications (±FXIII,±aprotinin). The stiffness and degradation time course for each treatment tested is summarized in Figure 6 and material stiffness values are reported in Table 1. Unmodified fibrin scaffolds had an average stiffness of 80.9±6.0 kPa, a value comparable to native cardiac tissues. This stiffness rapidly decreased to 38.3±3.5 kPa (47.3%±5.6% stiffness retention, significantly different from day 0, p<0.05) after 3 days and to 30.3±2.7 kPa (37.5%±4.3%) after 6 days, with a continuing significant drop in stiffness to 16.1±2.9 kPa (19.9%±3.9%, p<0.05) after 10 days in sterile PBS at 37°C. During testing of the day 10 samples, 10 of the unmodified scaffold strips tore before they could be measured due to a high extent of degradation. The stiffness value reported for the day 10 unmodified scaffolds includes these 10 samples as zero passive force. Without the inclusion of these samples, the day 10 stiffness was 22.8±3.1 kPa (28.2%±4.4% stiffness retention). This data in combination with the results of the in vivo implant study indicate a fast degradation time course for our unmodified fibrin scaffolds. Addition of FXIII did not significantly change initial scaffold stiffness (90.8±8.4 kPa), but did significantly decrease the overall extent of scaffold degradation (45.8%±5.9% after 10 days, p<0.05) compared with unmodified scaffolds. A significant decrease in FXIII scaffold stiffness was observed between the 3- and 6-day time points (68.3%±7.5% down to 43.9%±5.8%, p<0.05). Addition of aprotinin to the fibrin material also did not significantly change initial material stiffness (68.0±4.7 kPa), and significantly decreased the rate of scaffold degradation in vitro (60.9%±5.2% after 10 days, p<0.05) compared to unmodified scaffolds, with no significant decrease in stiffness observed past the 3-day time point. The combination of FXIII with aprotinin produced a scaffold with a stiffness of 89.0±5.9 kPa and, as with aprotinin alone, significantly decreased the extent of scaffold degradation (76.4%±7.6% after 10 days, p<0.05), with no significant decrease in stiffness past the 3-day time point. The degradation rates of aprotinin with FXIII and aprotinin-only scaffolds were not significantly different. These data demonstrate our ability to tune the material properties of our fibrin scaffolds in terms of initial stiffness and rate of degradation to target the desired scaffold properties for in vivo implants.
FIG. 6.
(A) Degradation curves (% stiffness retention) of fibrin scaffold formulations in vitro. Statistically significant difference from unmodified scaffolds on day 6 (*) or on day 10 (‡) (One-Way ANOVA, Dunnett's method, p<0.05); statistically significant difference from FXIII scaffolds on day 6 (†) or on day 10 (#) (2-sided Student's t-test, p<0.05). (B) Stiffness values of scaffold formulations over 10 days in vitro. Brackets indicate significant differences between time points within each group (2-sided Student's t-test, p<0.05).
Table 1.
Stiffness (kPa) of Fibrin Scaffold Formulations Over 10 Days In Vitro
| |
Stiffness in kPa (n) |
|||
|---|---|---|---|---|
| Scaffold treatment | Day 0 | Day 3 | Day 6 | Day 10 |
| Unmodified | 80.9±6.0 (40) | 38.3±3.5 (35) | 30.3±2.7 (37) | 16.1±2.9 (34)a |
| FXIII | 90.8±8.4 (35) | 62.0±3.7 (35) | 39.9±3.7 (35) | 41.6±3.7 (35) |
| Aprotinin | 68.0±4.7 (49) | 46.3±3.4 (35) | 42.0±2.4 (48) | 41.4±2.1 (35) |
| FXIII+Aprotinin | 89.0±5.9 (35) | 54.4±2.6 (36) | 63.8±5.1 (35) | 68.0±5.1 (34) |
Unmodified day 10 stiffness value includes 10 preps that yielded zero passive force, which did not occur in other groups.
Tri-cell seeding of fibrin scaffolds
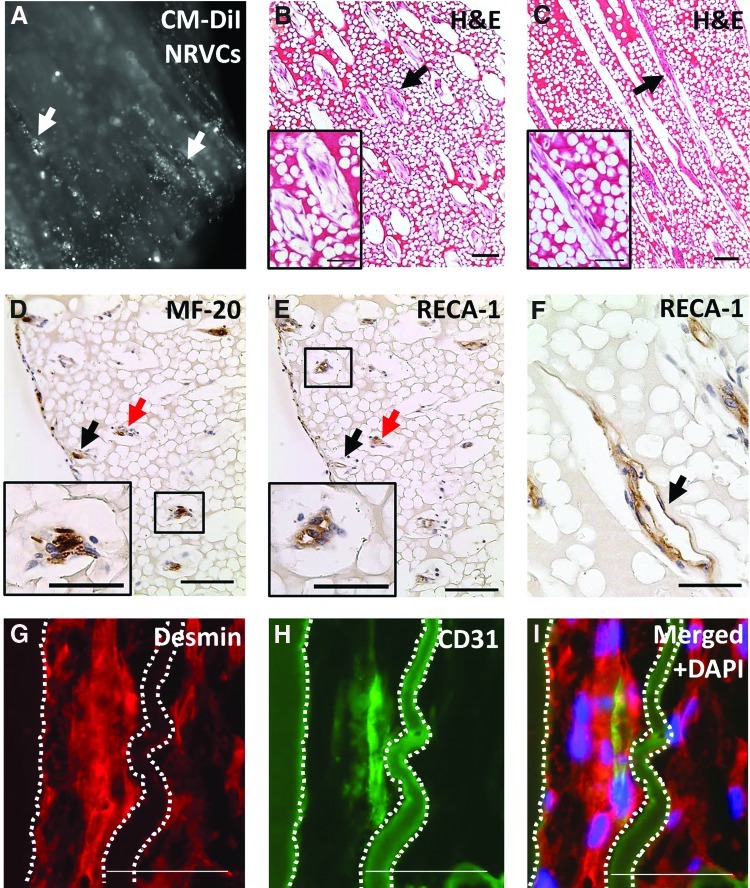
Tri-cell seeding of scaffolds with NRVCs, RAECs or HUVEC, and NRCFs via centrifugation resulted in constructs containing viable cells, as indicated by images of fluorescently labeled (CM-DiI) cardiomyocytes 2 hours after seeding into scaffolds (Fig. 7A). Histological analysis showed viable cells seeded through the width of the constructs (Fig. 7B) and down the length of the microchannels (Fig. 7C) in 8-day constructs. Cardiomyocytes were seen contracting within microchannels 2–4 days after seeding (Supplementary Information), as were nonseeded cardiomyocytes on the bottom of culture dishes (data not shown), demonstrating cardiac cell survival and viability. Constructs seeded with only cardiomyocytes or ECs resulted in poor cell survival (data not shown). Histological analysis of tri-cell constructs indicated cardiomyocytes (sarcomeric myosin, MF-20) survived seeding and were localized in scaffold channels (Fig. 7A, D, G), and that they elongated within channels over time in culture (Fig. 7G). This analysis suggests that the cardiomyocytes may be beginning to form columnar structures through the construct parallel to the microchannel orientation. ECs (RECA-1 or CD31) also survived seeding, became elongated within channels, and consistently formed lumen structures within and aligned parallel with channels, as indicated by histological staining both in cross-sectional (Fig. 7E) and in longitudinal sections (Fig. 7F, H). Fluorescent double-staining (desmin & CD31, Fig. 7G–I) of a tri-cell-seeded scaffold in the longitudinal section confirmed these cells were colocalized and aligned within scaffold channels. Taken together, these results indicate the scaffold microarchitecture is promoting EC and cardiomyocyte organization and orientation within constructs and the development of a prevascular cell network adjacent to columns of elongated and aligned cardiomyocytes throughout the parallel construct channels, mimicking the cell orientation present in native cardiac tissue.
FIG. 7.
Tri-cell-seeded scaffolds in vitro. (A) Scaffold 2 hours after seeding showing fluorescently labeled (CM-DiI) cardiomyocytes (NRVCs) seeded in microchannels (white arrows), 5× magnification. Histological analysis of 8 day samples cut in cross-sectional (B, D, E) and longitudinal section (C, F–I). (B, C) H&E staining showing viable cells within construct channels (black arrows), scale bars=100 μm (50 μm in insets). (D) NRVCs (MF-20, sarcomeric myosin) and (E) endothelial cells (RECA-1) colocalized within channels (representative channels shown by black and red arrows), scale bars=100 μm (50 μm in insets). (E, F) Endothelial cells formed lumen structures within channels (black arrows), scale bar in (F)=50 μm. Fluorescent double staining with (G) desmin and (H) CD31 confirming cardiomyocytes and endothelial cells are colocalized and elongated within scaffold channels (channel walls outlined with dotted lines, fibrin material is autofluorescent). (I) Merged images with DAPI nuclear stain. Scale bars in (G–I)=50 μm. Color images available online at www.liebertpub.com/tea
Cell-seeded construct stiffness in vitro
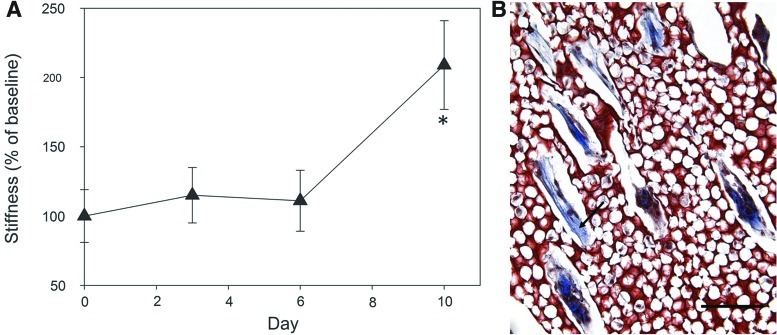
Aprotinin scaffolds were seeded with the tri-cell mixture and mechanically tested over 10 days to determine the effect of cell seeding on scaffold degradation. Stiffness of day 0 unseeded scaffolds was used as the baseline stiffness value. In contrast to the degradation profile of acellular scaffolds, the stiffness of cell-seeded scaffolds did not drop initially and significantly increased between days 6 and 10, up to 209%±32% (p<0.05) of initial stiffness (Fig. 8A). Masson's trichrome staining of tri-cell constructs confirmed the presence of newly deposited collagen by cells within construct channels after 8 days in vitro (Fig. 8B), providing a potential explanation of the observed increase in construct stiffness.
FIG. 8.
Cell-seeded construct mechanical properties. (A) Change in construct stiffness (% of baseline, Day 0 stiffness) of tri-cell-seeded aprotinin scaffolds over 10 days in vitro. * Statistically significant difference from Day 6 time point (2-sided Student's t-test, p<0.05). (B) Masson's trichrome staining of 8 day cell-seeded construct indicating newly deposited collagen (arrow) by cells within channels. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
Discussion
In this study, we describe the development of a tri-cell-seeded cardiac tissue construct using a high-density microchanneled and microporous fibrin scaffold that promoted cell survival and organization and had tunable stiffness and rates of degradation. We successfully seeded cardiomyocytes, ECs, and fibroblasts into the aligned channels of our scaffolds, creating a construct that mimics native cardiac tissue in structure, material properties, and cellular composition. Our constructs supported cardiomyocyte survival and the development of a prevascular EC network in vitro. In addition, our constructs promoted collagen deposition by seeded cells within construct channels. Taken together, these are all characteristics of a promising cardiac tissue construct that is engineered to improve graft survival and integration upon implantation.
Fibrin is a natural protein ideally suited for tissue engineering, as it has been shown to promote cellular adhesion, has tunable mechanical and degradation properties, and naturally degrades into nontoxic bioactive molecules that induce neovascularization. Linnes et al. developed a method of sphere-templating fibrin at a high density to produce stable and nontoxic porous scaffolds with mechanical stiffness that could be controlled by altering fibrinogen concentration, time in acetone, and time of exposure to an external crosslinker (genipin).7 Madden et al. developed a method of incorporating parallel microchannels within the porous network to align seeded cardiomyocytes within synthetic scaffolds.4 Modification of these two templating procedures allowed us to produce a high-density templated fibrin scaffold with 60-μm parallel microchannels surrounded by an interconnected 27-μm porous network. The choice of channel diameter was based on studies done by Madden et al. that determined 60-μm channels were optimal for cell-seeding, while minimizing mass transport issues within the channel, while channel spacing was chosen so that pores in the range of 20–40 μm diameter could be incorporated around the channels, as this range of pore sizes has been shown to induce a greater extent of host vascular infiltration and reduced fibrous capsule formation in myocardial implants.4,22
Our in vivo degradation studies confirmed a normal host response to our fibrin implants with no indications of calcification or foreign body giant cell formation. We observed an area of granulation tissue surrounding acellular implants after 1 and 2 weeks in vivo. We determined our acellular unmodified fibrin scaffold had a stiffness of 80.9±6.0 kPa, a value comparable to native cardiac tissues.19 However, our unmodified fibrin scaffolds had a rapid degradation profile both in vitro (likely due to contaminating levels of plasmin in the fibrinogen) and in vivo. We hypothesized that a slower degradation profile would be necessary to maintain structural support for grafted cells until they had deposited their own replacement ECM. This led us to test modifications to the fibrin material in an attempt to slow the degradation of our scaffolds, while maintaining the material stiffness within the range of native cardiac tissues.
During coagulation, fibrin clots are stabilized and protected from rapid fibrinolysis by FXIII crosslinking.7,9,23 We therefore tested the addition of FXIII to the fibrinogen solution at a concentration approximately 3–5×that normally found in mammalian plasma23,24 as a method to retain material stiffness. Contrary to other studies,23,25,26 we did not find a significant increase in material stiffness of our FXIII-treated scaffolds (90.8±8.4 kPa) compared to unmodified scaffolds (80.9±6.0 kPa). An explanation for this could be that the extremely high density of the fibrin (200 mg/mL) is masking moderate effects on stiffness resulting from addition of FXIII. Additionally, the fibrin scaffolds are exposed to a series of water-free solvents during production that are most likely further increasing material stiffness.7,27 The effect of FXIII treatment on scaffold degradation was unclear from our in vivo implant studies, but seemed to indicate it may be slowing scaffold degradation. Further investigation with in vitro degradation studies showed an intermediate level of stiffness retention (45.8%±5.9%) that was significantly improved from unmodified fibrin scaffolds (19.9%±3.9%) over 10 days in vitro.
The addition of the protease inhibitor aprotinin also significantly reduced scaffold degradation in our constructs (60.9%±5.2%) over 10 days in vitro, and appeared to decrease collagen deposition in vivo 7 days after implantation (Fig. 4). Aprotinin is commonly incorporated in fibrin glue-based tissue sealants for surgical applications to increase the material durability.25 In addition, aprotinin has been indicated as an anti-inflammatory agent that reduces activation and chemotaxis of neutrophils and macrophages, therefore lessening the release of inflammatory cytokines and the resulting cell and tissue damage they cause.28,29 We wanted to determine whether aprotinin would remain in our high-density fibrin material and, most importantly, retain its activity after undergoing the scaffold processing required to remove the polymer template. The slowing of degradation seen in our in vitro study suggests aprotinin is still present and is not denatured by the scaffold processing procedures. As hypothesized, the combination of FXIII with aprotinin also had a significant effect on slowing scaffold degradation compared to the unmodified and FXIII-only groups, maintaining 76% of scaffold stiffness after 10 days in vitro. However, this was not significantly different from the degradation rate of the aprotinin-only group (Fig. 6). Based on these in vitro results, it was expected that aprotinin implants would perform equally as well as or better than FXIII implants. However, while more aprotinin implants were located after 7 days than in the unmodified and FXIII groups, no aprotinin implants could be located at the 14-day time point. One possible explanation for this may be that the dose of aprotinin delivered with the implant was sufficient to prolong the scaffold life for 7 days, but not for 14 days due to the relatively small size of the implants. Since our degradation studies showed most of the retained stiffness occurs with aprotinin scaffolds for up to 10 days in vitro, we propose that the addition of aprotinin to our high-density fibrin scaffolds will extend the scaffold life in vivo in the presence of cell seeding and the inflammatory environment of the infarct when a larger scaffold size is implanted. Optimal degradation time will depend on a variety of factors, such as how quickly the construct mechanically integrates, forms vascular anastomoses, and how much fibrin needs to degrade to promote neovascularization. These questions are beyond the scope of this study, but the tunable properties of our novel construct in regard to both stiffness and degradation will allow careful assessment of these questions in the future.
For future animal studies, it will be essential for grafted cardiomyocytes within a scaffold to survive after implantation in infarcted myocardium and become rapidly vascularized by the host. By using fibrin as our scaffold material and by developing a prevascular network within our constructs in vitro, we hope to encourage rapid anastomosis of our graft with the host vasculature and improve the overall chances of graft survival. Important components of healthy heart tissue are mature cardiomyocytes coupled with an extensive microvascular network. Thus, a cardiac repair construct should contain a high density of cardiomyocytes and the components of microvessels, specifically ECs and supporting cells. It is well established that coculture of ECs and fibroblasts or mesenchymal stem cells generates stable vascular networks in 3D cultures.30,31 Studies by the Davis group have shown that the neovessel formation is a complex process requiring a sequential set of events modulated by cell–cell and cell–matrix interactions.32 When 3D tri-cell constructs were implanted in vivo, they exhibited continuous differentiation and were invaded by host blood vessels.33–36 Fibroblasts stabilized neovessels by reducing EC death and increasing EC proliferation, and capillaries improved cardiomyocyte proliferation.36,37 Fibrin constructs seeded with cardiomyocytes alone showed poor cell survival in our system (data not shown). Conversely, within 8 days of in vitro culture our tri-cell constructs consistently developed EC-lined lumen structures within scaffold microchannels, and were colocalized with viable cardiomyocytes oriented parallel to scaffold channels (Fig. 7). In addition, seeded cells began depositing their own ECM in the form of collagen within construct microchannels during this time period, as indicated by histological analysis and resulting in increased construct stiffness over 10 days (Fig. 8). This is a desirable attribute for a degradable cellular construct, as the newly deposited ECM will provide structural support to the cells and assist in maturation of the tissue as the scaffold is degraded. The rate of scaffold degradation and ECM deposition should be balanced to prevent transfer to the newly deposited matrix too soon.
With the design of our scaffold architecture, we are directing the organization of cultured cells by seeding them into parallel microchannels to more closely resemble the organized alignment found in vivo. In addition, this architecture allows for a greater extent of cell seeding, alignment, and organization than other traditional methods of seeding cells into scaffolds. The use of our tri-cell mixture and the architecture of our scaffolds are designed to provide guidance for the formation of a prevascular network within our constructs to promote a quick anastomosis of grafted cells with the host myocardium upon implantation. The microporous network consisting of 27-μm pores that surround the channels aids in exchange of nutrients and waste removal, and may also elicit further host angiogenesis and a weaker or more prohealing inflammatory response when constructs are implanted into hearts.1,4,38–40 Future studies will focus on further improving the density of cell-seeding, organization of cardiomyocytes and prevascular network formation within constructs, and assessing construct integration with the host tissue and effects on heart function. The combination of the natural polymer fibrin in a microtemplated architecture with our tri-cell seeding strategy has allowed the development of a prevascularized and aligned cardiac tissue construct with tunable mechanical properties that will promote integration with host myocardium for the improvement of cardiac function following MI.
Supplementary Material
Acknowledgments
The authors sincerely thank Sarah Dupras for performing animal implant surgeries, Galina Flint for performing cell isolations, Maria Razumova for instruction in mechanical testing, Veronica Muskheli, Kareen Kreutziger, Nathaniel Tulloch, and Christopher Hargrave for assistance with histology, Michael Linnes, Lauran Madden, and Derek Mortisen for instruction in scaffold construction, and Nathanial Tulloch, Anthony Rodriguez, Scott Lundy, Gabrielle Robinson, and Claire McLeod for useful discussions. This work was supported by a National Science Foundation Graduate Research Fellowship (K.S.T.), an award from the American Heart Association (K.S.T.), and by a NIH NHLBI grant 5R01HL064387 (M.R., M.S., B.D.R.).
Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Laflamme M.A. Murry C.E. Heart regeneration. Nature. 2011;473:326. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jawad H. Lyon A. Harding S. Ali N. Boccaccini A. Myocardial tissue engineering. Br Med Bull. 2008;87:31. doi: 10.1093/bmb/ldn026. [DOI] [PubMed] [Google Scholar]
- 3.Stevens K. Pabon L. Muskheli V. Murry C. Scaffold-free human cardiac tissue patch created from embryonic stem cells. Tissue Eng Part A. 2009;15:1211. doi: 10.1089/ten.tea.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madden L.R. Mortisen D.J. Sussman E.M. Dupras S.K. Fugate J.A. Cuy J.L., et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sasagawa T. Shimizu T. Sekiya S. Haraguchi Y. Yamato M. Sawa Y., et al. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed T.A. Dare E.V. Hincke M. Fibrin: a Versatile Scaffold for Tissue Engineering Applications. Tissue Eng Part B Rev. 2008;14:199. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- 7.Linnes M.P. Ratner B.D. Giachelli C.M. A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials. 2007;28:5298. doi: 10.1016/j.biomaterials.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barsotti M.C. Felice F. Balbarini A. Di Stefano R. Fibrin as a scaffold for cardiac tissue engineering. Biotechnol Appl Biochem. 2011;58:301. doi: 10.1002/bab.49. [DOI] [PubMed] [Google Scholar]
- 9.Monroe D.M. Hoffman M. What does it take to make the perfect clot? Arterioscler Thromb Vasc Biol. 2006;26:41. doi: 10.1161/01.ATV.0000193624.28251.83. [DOI] [PubMed] [Google Scholar]
- 10.Chen X. Aledia A.S. Ghajar C.M. Griffith C.K. Putnam A.J. Hughes C.C., et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng Part A. 2009;15:1363. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud M.N. Ayuni E. Cook S. Siepe M. Carrel T.P. Tevaearai H.T. Hydrogel-based engineered skeletal muscle grafts normalize heart function early after myocardial infarction. Artif Organs. 2008;32:692. doi: 10.1111/j.1525-1594.2008.00595.x. [DOI] [PubMed] [Google Scholar]
- 12.Ryu J.H. Kim I.K. Cho S.W. Cho M.C. Hwang K.K. Piao H., et al. Implantation of bone marrow mononuclear cells using injectable fibrin matrix enhances neovascularization in infarcted myocardium. Biomaterials. 2005;26:319. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 13.Christman K.L. Fok H.H. Sievers R.E. Fang Q. Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 14.Chekanov V. Akhtar M. Tchekanov G. Dangas G. Shehzad M.Z. Tio F., et al. Transplantation of autologous endothelial cells induces angiogenesis. Pacing Clin Electrophysiol. 2003;26:496. doi: 10.1046/j.1460-9592.2003.00080.x. [DOI] [PubMed] [Google Scholar]
- 15.Huang Y.C. Khait L. Birla R.K. Contractile three-dimensional bioengineered heart muscle for myocardial regeneration. J Biomed Mater Res A. 2007;80:719. doi: 10.1002/jbm.a.31090. [DOI] [PubMed] [Google Scholar]
- 16.Takei A. Tashiro Y. Nakashima Y. Sueishi K. Effects of fibrin on the angiogenesis in vitro of bovine endothelial cells in collagen gel. In Vitro Cell Dev Biol Anim. 1995;31:467. doi: 10.1007/BF02634260. [DOI] [PubMed] [Google Scholar]
- 17.Kreutziger K.L. Piroddi N. Belus A. Poggesi C. Regnier M. Ca2+-binding kinetics of troponin C influence force generation kinetics in cardiac muscle. Biophys J. 2007;92:477a. [Google Scholar]
- 18.Gillis T.E. Martyn D.A. Rivera A.J. Regnier M. Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle. J Physiol. 2007;580:561. doi: 10.1113/jphysiol.2007.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moreno-Gonzalez A. Korte F.S. Dai J. Chen K. Ho B. Reinecke H., et al. Cell therapy enhances function of remote non-infarcted myocardium. J Mol Cell Cardiol. 2009;47:603. doi: 10.1016/j.yjmcc.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robey T.E. Saiget M.K. Reinecke H. Murry C.E. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez A.G. Han S.J. Regnier M. Sniadecki N.J. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys J. 2011;101:2455. doi: 10.1016/j.bpj.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galperin A. Long T.J. Ratner B.D. Degradable, thermo-sensitive poly(N-isopropyl acrylamide)-based scaffolds with controlled porosity for tissue engineering applications. Biomacromolecules. 2010;11:2583. doi: 10.1021/bm100521x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagoly Z. Koncz Z. Hársfalvi J. Muszbek L. Factor XIII, clot structure, thrombosis. Thromb Res. 2012;129:382. doi: 10.1016/j.thromres.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 24.Takagi J. Kasahara K. Sekiya F. Inada Y. Saito Y. Subunit B of factor XIII is present in bovine platelets. Thromb Res. 1988;50:767. doi: 10.1016/0049-3848(88)90337-4. [DOI] [PubMed] [Google Scholar]
- 25.Hickerson W.L. Nur I. Meidler R. A comparison of the mechanical, kinetic, and biochemical properties of fibrin clots formed with two different fibrin sealants. Blood Coagul Fibrinolysis. 2011;22:19. doi: 10.1097/MBC.0b013e32833fcbfb. [DOI] [PubMed] [Google Scholar]
- 26.Standeven K.F. Carter A.M. Grant P.J. Weisel J.W. Chernysh I. Masova L., et al. Functional analysis of fibrin {gamma}-chain cross-linking by activated factor XIII: determination of a cross-linking pattern that maximizes clot stiffness. Blood. 2007;110:902. doi: 10.1182/blood-2007-01-066837. [DOI] [PubMed] [Google Scholar]
- 27.Akpalo E. Bidault L. Boissière M. Vancaeyzeele C. Fichet O. Larreta-Garde V. Fibrin-polyethylene oxide interpenetrating polymer networks: new self-supported biomaterials combining the properties of both protein gel and synthetic polymer. Acta Biomater. 2011;7:2418. doi: 10.1016/j.actbio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 28.McEvoy M.D. Reeves S.T. Reves J.G. Spinale F.G. Aprotinin in cardiac surgery: a review of conventional and novel mechanisms of action. Anesth Analg. 2007;105:949. doi: 10.1213/01.ane.0000281936.04102.9f. [DOI] [PubMed] [Google Scholar]
- 29.Bull D.A. Maurer J. Aprotinin and preservation of myocardial function after ischemia-reperfusion injury. Ann Thorac Surg. 2003;75:S735. doi: 10.1016/s0003-4975(02)04702-1. [DOI] [PubMed] [Google Scholar]
- 30.Melero-Martin J.M. De Obaldia M.E. Kang S.Y. Khan Z.A. Yuan L. Oettgen P., et al. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley J.R. Balaji S. Narmoneva D.A. Complex temporal regulation of capillary morphogenesis by fibroblasts. Am J Physiol Cell Physiol. 2010;299:C444. doi: 10.1152/ajpcell.00572.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis G.E. Koh W. Stratman A.N. Mechanisms controlling human endothelial lumen formation and tube assembly in three-dimensional extracellular matrices. Birth Defects Res C Embryo Today. 2007;81:270. doi: 10.1002/bdrc.20107. [DOI] [PubMed] [Google Scholar]
- 33.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C., et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 34.Kaully T. Kaufman-Francis K. Lesman A. Levenberg S. Vascularization-the conduit to viable engineered tissues. Tissue Eng Part B Rev. 2009;15:159. doi: 10.1089/ten.teb.2008.0193. [DOI] [PubMed] [Google Scholar]
- 35.Stevens K. Kreutziger K. Dupras S. Korte F. Regnier M. Muskheli V., et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci U S A. 2009;106:16568. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tulloch N.L. Muskheli V. Razumova M.V. Korte F.S. Regnier M. Hauch K.D., et al. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ Res. 2011;109:47. doi: 10.1161/CIRCRESAHA.110.237206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caspi O. Lesman A. Basevitch Y. Gepstein A. Arbel G. Habib I.H., et al. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ Res. 2007;100:263. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 38.Brauker J. Martinson L.A. Hill R.S. Young S.K. Carr-Brendel V.E. Johnson R.C. Neovascularization of immunoisolation membranes: the effect of membrane architecture and encapsulated tissue. Transplant Proc. 1992;24:2924. [PubMed] [Google Scholar]
- 39.Brauker J.H. Carr-Brendel V.E. Martinson L.A. Crudele J. Johnston W.D. Johnson R.C. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517. doi: 10.1002/jbm.820291208. [DOI] [PubMed] [Google Scholar]
- 40.Marshall A.J. Irvin C.A. Barker T. Sage E.H. Hauch K.D. Ratner B.D. Biomaterials with tightly controlled poresize that promote vascular in-growth. Polym Preprints. 2004;45:100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.