Abstract
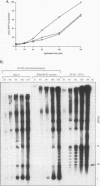
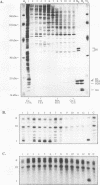
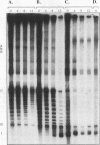
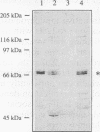
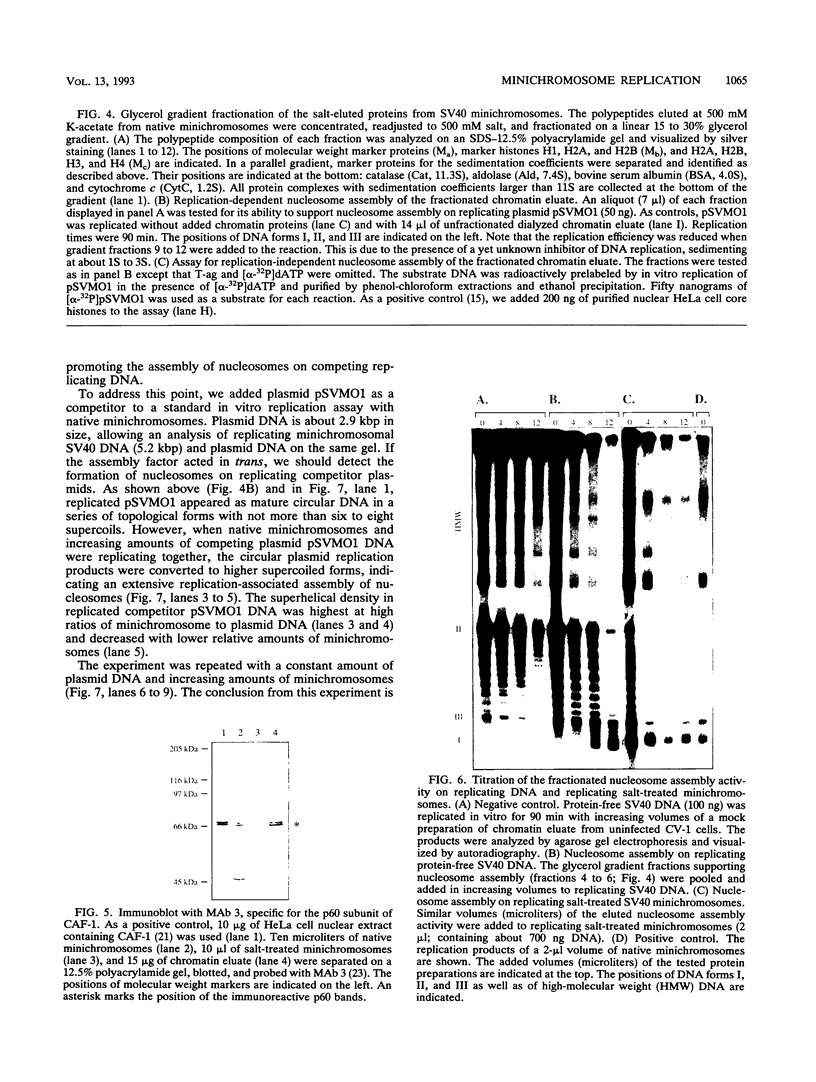
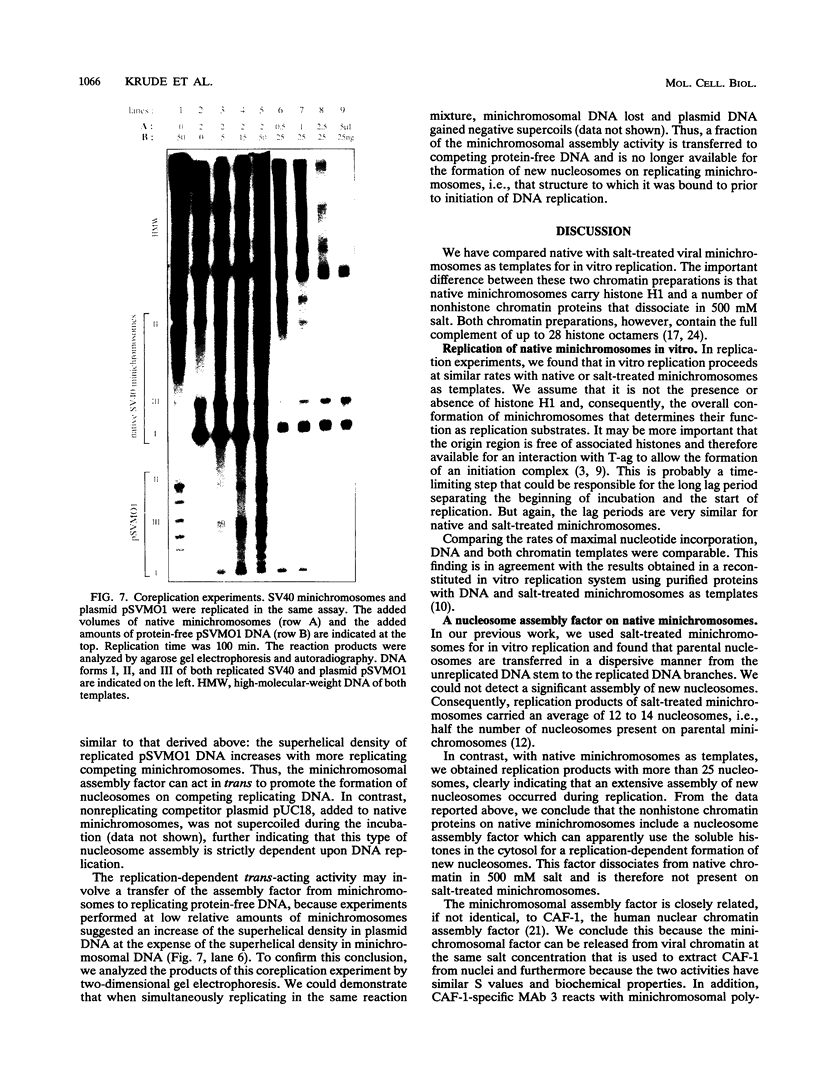
Using in vitro replication assays, we compared native with salt-treated simian virus 40 minichromosomes isolated from infected cell nuclei. Minichromosomes from both preparations contain the full complement of nucleosomes, but salt treatment removes histone H1 and a fraction of nonhistone chromatin proteins. Both types of minichromosomes served well as templates for in vitro replication, but the structures of the replication products were strikingly different. Replicated salt-treated minichromosomes contained, on average, about half the normal number of nucleosomes as previously shown (T. Krude and R. Knippers, Mol. Cell. Biol. 11:6257-6267, 1991). In contrast, the replicated untreated minichromosomes were found to be densely packed with nucleosomes, indicating that an assembly of new nucleosomes occurred during in vitro replication. Biochemical and immunological data showed that the fraction of nonhistone chromatin proteins associated with native minichromosomes includes a nucleosome assembly activity that appears to be closely related to chromatin assembly factor I (S. Smith and B. W. Stillman, Cell 58:15-25, 1989). Furthermore, this minichromosome-bound nucleosome assembly factor is able to exert its activity in trans to replicating protein-free competitor DNA. Thus, native chromatin itself contains the activities required for an ordered assembly of nucleosomes during the replication process.
Full text
PDF


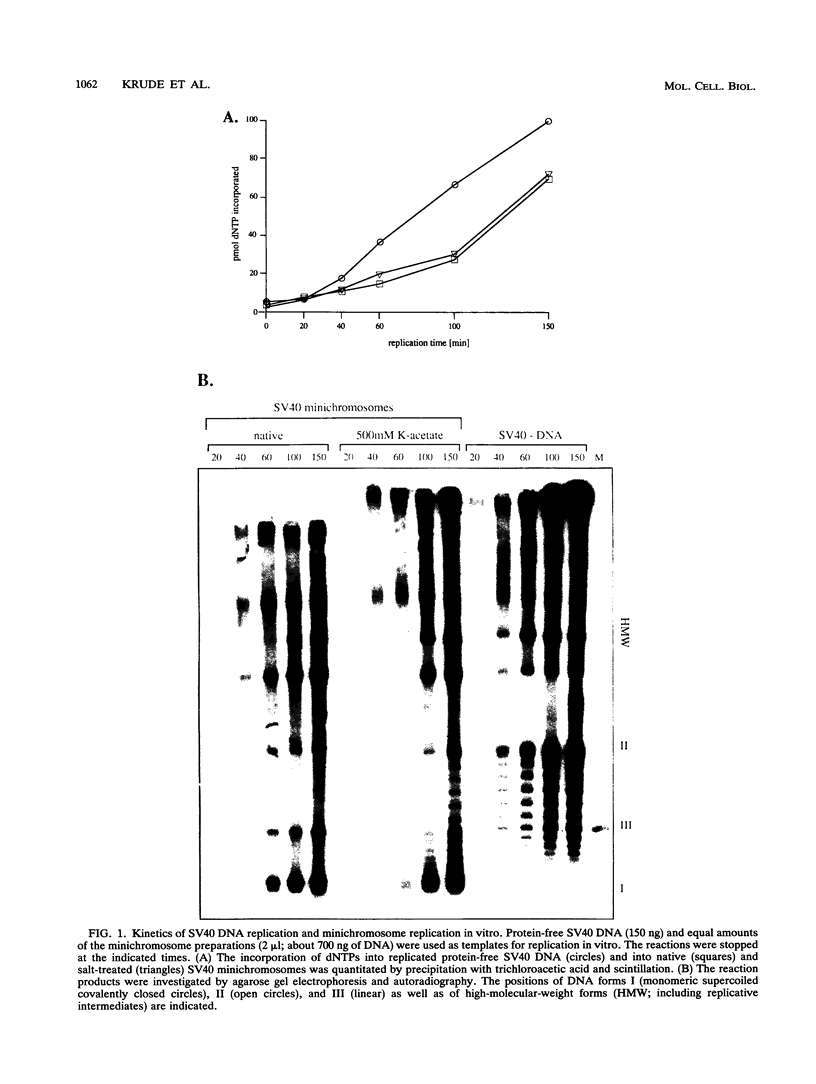
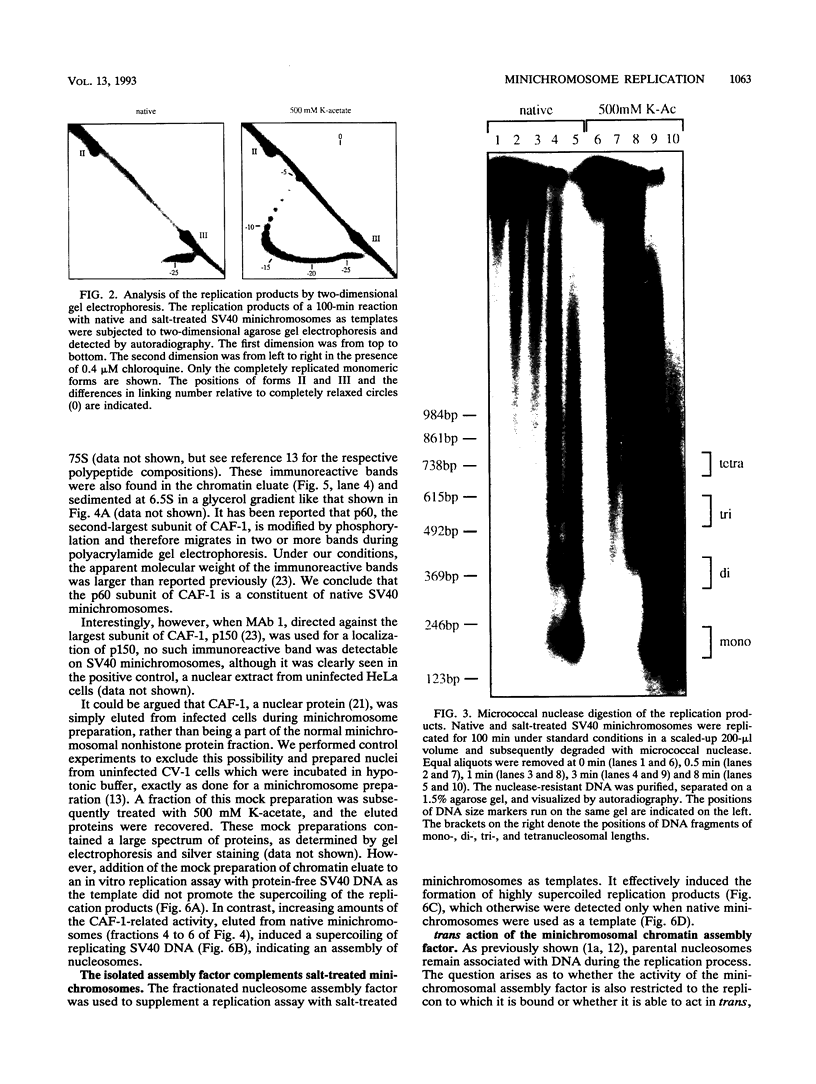
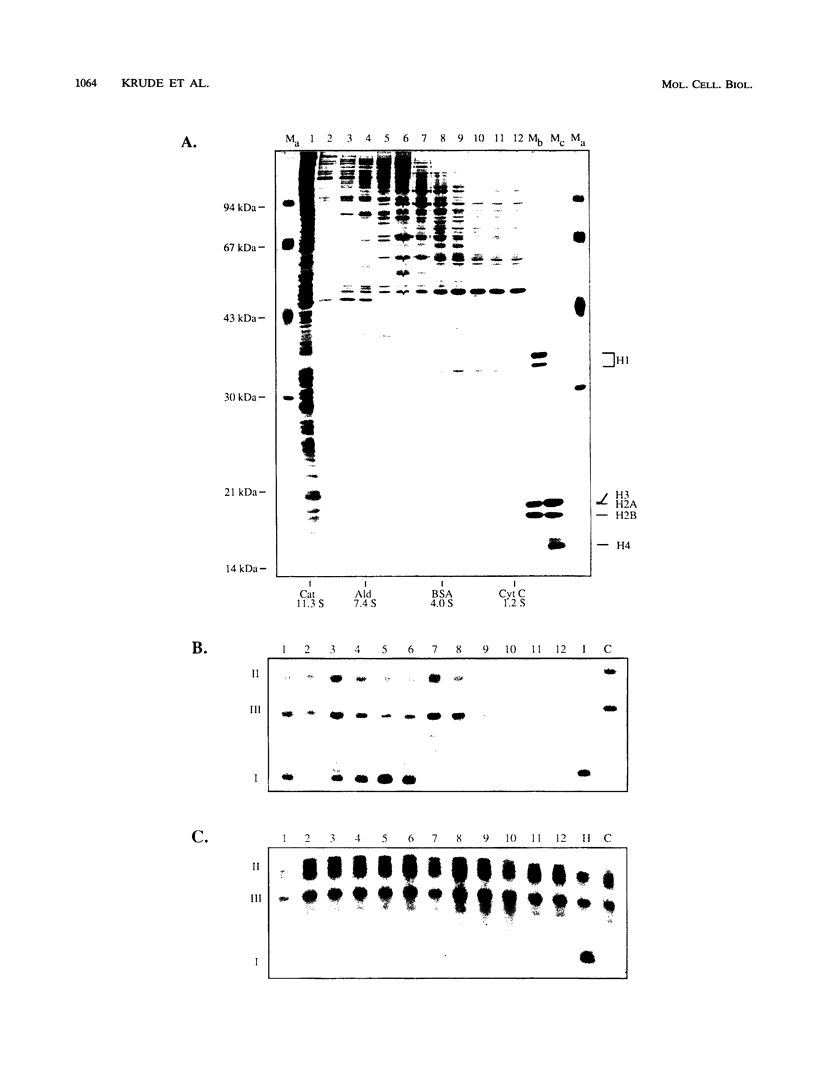


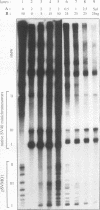
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonne-Andrea C., Wong M. L., Alberts B. M. In vitro replication through nucleosomes without histone displacement. Nature. 1990 Feb 22;343(6260):719–726. doi: 10.1038/343719a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheng L., Kelly T. J. Transcriptional activator nuclear factor I stimulates the replication of SV40 minichromosomes in vivo and in vitro. Cell. 1989 Nov 3;59(3):541–551. doi: 10.1016/0092-8674(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Decker R. S., Yamaguchi M., Possenti R., Bradley M. K., DePamphilis M. L. In vitro initiation of DNA replication in simian virus 40 chromosomes. J Biol Chem. 1987 Aug 5;262(22):10863–10872. [PubMed] [Google Scholar]
- Fotedar R., Roberts J. M. Association of p34cdc2 with replicating DNA. Cold Spring Harb Symp Quant Biol. 1991;56:325–333. doi: 10.1101/sqb.1991.056.01.039. [DOI] [PubMed] [Google Scholar]
- Fotedar R., Roberts J. M. Multistep pathway for replication-dependent nucleosome assembly. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6459–6463. doi: 10.1073/pnas.86.17.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimi Y. Preincubation of T antigen with DNA overcomes repression of SV40 DNA replication by nucleosome assembly. J Biol Chem. 1992 May 25;267(15):10910–10913. [PubMed] [Google Scholar]
- Ishimi Y., Sugasawa K., Hanaoka F., Kikuchi A. Replication of the simian virus 40 chromosome with purified proteins. J Biol Chem. 1991 Aug 25;266(24):16141–16148. [PubMed] [Google Scholar]
- Krude T., Knippers R. Replication of SV40 minichromosomes in vitro. Chromosoma. 1992;102(1 Suppl):S83–S92. doi: 10.1007/BF02451790. [DOI] [PubMed] [Google Scholar]
- Krude T., Knippers R. Transfer of nucleosomes from parental to replicated chromatin. Mol Cell Biol. 1991 Dec;11(12):6257–6267. doi: 10.1128/mcb.11.12.6257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lässle M., Richter A., Knippers R. Comparison of replicative and non-replicative chromatin assembly pathways in HeLa cell extracts. Biochim Biophys Acta. 1992 Aug 17;1132(1):1–10. doi: 10.1016/0167-4781(92)90045-2. [DOI] [PubMed] [Google Scholar]
- Müller U., Zentgraf H., Eicken I., Keller W. Higher order structure of simian virus 40 chromatin. Science. 1978 Aug 4;201(4354):406–415. doi: 10.1126/science.208155. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Energetics of B-to-Z transition in DNA. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6206–6210. doi: 10.1073/pnas.80.20.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura A., Tremethick D., Worcel A. Characterization of the repressed 5S DNA minichromosomes assembled in vitro with a high-speed supernatant of Xenopus laevis oocytes. Mol Cell Biol. 1988 Oct;8(10):4257–4269. doi: 10.1128/mcb.8.10.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simanis V., Lane D. P. An immunoaffinity purification procedure for SV40 large T antigen. Virology. 1985 Jul 15;144(1):88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B. Immunological characterization of chromatin assembly factor I, a human cell factor required for chromatin assembly during DNA replication in vitro. J Biol Chem. 1991 Jun 25;266(18):12041–12047. [PubMed] [Google Scholar]
- Smith S., Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989 Jul 14;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Smith S., Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. EMBO J. 1991 Apr;10(4):971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell. 1986 May 23;45(4):555–565. doi: 10.1016/0092-8674(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa K., Murakami Y., Miyamoto N., Hanaoka F., Ui M. Assembly of nascent DNA into nucleosome structures in simian virus 40 chromosomes by HeLa cell extract. J Virol. 1990 Oct;64(10):4820–4829. doi: 10.1128/jvi.64.10.4820-4829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]