Abstract
The purpose of this study is to evaluate the dose–volume metrics under different heterogeneity corrections and the factors associated with local recurrence (LR) after stereotactic body radiation therapy (SBRT) for non-small-cell lung cancer (NSCLC). Eighty-three patients who underwent SBRT for pathologically proven stage I NSCLC were reviewed retrospectively. The prescribed dose was 48 Gy in four fractions at the isocenter (IC) under heterogeneity correction with the Batho power law (BPL). The clinical plans were recalculated with Eclipse (Varian) for the same monitor units under the BPL and anisotropic analytical algorithm (AAA) and with no heterogeneity correction (NC). The dose at the IC, dose that covers 95% of the volume (D95), minimum dose (Min), and mean dose (Mean) of the planning target volume (PTV) were compared under each algorithm and between patients with local lesion control (LC) and LR. The IC doses under NC were significantly lower than those under the BPL and AAA. Under the BPL, the mean PTV D95, Min and Mean were 8.0, 9.4 and 7.4% higher than those under the AAA, and 9.6, 9.2 and 4.6% higher than those under NC, respectively. Under the AAA, all dose–volumetric parameters were significantly lower in T1a patients than in those with T1b and T2a. With a median follow-up of 35.9 months, LR occurred in 18 patients. Between the LC and LR groups, no significant differences were observed for any of the metrics. Even after stratification according to T-stage, no significant difference was observed between LC and LR.
Keywords: lung cancer, stereotactic body radiation therapy, dose–volume metrics, heterogeneity correction, local control
INTRODUCTION
Stereotactic body radiotherapy (SBRT) is an innovative technique using multiple narrow beams with high precision, which enables tumor irradiation with a high, limited, focal dose [1, 2]. SBRT is an effective treatment option for patients with stage I non-small-cell lung cancer (NSCLC) who are inoperable or who refuse surgery. Many single-institution reports have indicated that SBRT for stage I NSCLC can achieve a high local control (LC) rate with minimal toxicity [3–6]. The reported 3-year LC rate ranges from 80 to 95% [3–7]. The recently disclosed results of prospective multicenter phase II trials [8–10] have also shown the efficacy of lung SBRT.
Despite these high LC rates, some patients experience local progression after SBRT. Several authors have indicated that the delivered dose is one of the factors affecting the LC rate after SBRT [7, 11] as well as the tumor diameter [6, 12]. Onishi et al. [7] showed a correlation between biologically effective dose (BED) at the isocenter (IC) and LC. Wulf et al. [11] concluded that the peripheral dose to the planning target volume (PTV) was a significant factor in LC.
Since we started SBRT for NSCLC in 1998, most patients have been treated with a uniform dose-fractionation schedule of 48 Gy in four fractions, equivalent to a BED10Gy of 105.6 Gy at the IC. However, the dose coverage at the PTV varies among patients. Additionally, all the SBRT plans were calculated with pencil-beam convolution with heterogeneity correction using the Batho power law (BPL), which uses the tissue–air ratio as a collection factor and only partly accounts for the scattering around the inhomogeneity regions [13].Therefore, the calculated dose distributions under the BPL are inaccurate, especially at the periphery of the PTV.
To calculate dose distribution more accurately, a newer method of heterogeneity correction has been developed. The anisotropic analytical algorithm (AAA), which is available in Eclipse (Varian Medical Systems, Palo Alto, CA, USA), is a more accurate algorithm for heterogeneity correction. Several reports have shown the superiority of the AAA over the BPL in the inhomogeneity area [14–16]. However, most of the published SBRT results were based on earlier dose-calculation algorithms. In the Radiation Therapy Oncology Group (RTOG) 0236 phase II trial, the prescribed dose was 60 Gy in three fractions to cover 95% of the volume of the PTV (PTV D95), calculated under no heterogeneity correction (NC). The Japan Clinical Oncology Group (JCOG) 0403 phase II trial used a simple one-dimensional equivalent path correction as the inhomogeneity correction algorithm for the IC prescription, and the ongoing phase I dose-escalation study of SBRT for T2N0M0 NSCLC prescribed to the PTV D95 using superposition/convolution algorithms. When interpreting these outcomes, we need to consider the effect of the heterogeneity correction method and how great the difference is.
In this study, we evaluated the dose–volume metrics in clinical SBRT plans under the BPL and AAA, and NC, and then identified factors associated with LR after SBRT for Stage I NSCLC.
MATERIALS AND METHODS
Patients
From September 2003 to March 2009, 89 patients treated with SBRT for histologically confirmed stage I NSCLC in our institution were reviewed retrospectively. Of these, six patients without LR who were observed for less than 12 months after the completion of SBRT were excluded. Consequently, this study evaluated 83 patients (61 males, 22 females; median age 77 (range 63–88) years). The histology was adenocarcinoma in 39 patients, squamous cell carcinoma in 33, large-cell carcinoma in one and non-small-cell carcinoma (not specified) in 10. The clinical T-stage was T1a in 30 patients, T1b in 25 and T2a in 28 according to the seventh edition of the TNM Classification of Malignant Tumors. The mean tumor diameter was 25.2 (range 10–43) mm. The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics (n = 83)
| Characteristics | All | LC | LR | P-values |
|---|---|---|---|---|
| n = 83 | n = 65 | n = 18 | LC vs. LR | |
| Age (years) | 0.989 | |||
| Median | 77 | 77 | 76.5 | |
| [Range] | [63–88] | [63–87] | [63–88] | |
| Gender | 0.642 | |||
| Male | 61 | 47 | 14 | |
| Female | 22 | 18 | 4 | |
| Histology | 0.532 | |||
| Adenocarcinoma | 39 | 33 | 6 | |
| Squamous cell carcinoma | 33 | 24 | 9 | |
| Large cell carcinoma | 1 | 1 | 0 | |
| NSCLC (not specified) | 10 | 7 | 3 | |
| T-stage | 0.535 | |||
| T1a (≤20 mm) | 30 | 25 | 5 | |
| T1b (>20 to ≤30 mm) | 29 | 23 | 6 | |
| T2a (>30 to ≤50 mm) | 24 | 17 | 7 | |
| Tumor diameter (mm) | 0.336 | |||
| Mean | 25.2 | 24.7 | 26.7 | |
| [Range] | [10–43] | [10–43] | [11–37] | |
| PTV volume (cm3) | 0.780 | |||
| Mean | 36.1 | 35.8 | 37.1 | |
| [Range] | [9.9–86.6] | [9.9–86.6] | [12.6–60.4] |
NSCLC, non-small-cell lung cancer; LC, patients with local lesion control; LR, patients who developed local recurrence.
SBRT procedure
The details of the setup and treatment planning at our institution have been reported previously [17, 18]. The treatment plans were made using Eclipse (Varian Medical Systems) and calculated under heterogeneity correction using the BPL. The prescribed dose was 48 Gy in four fractions at the IC. The patients were immobilized with a Stereotactic Body Frame (Elekta, Stockholm, Sweden) before April 2008; thereafter, with the BodyFix system (Medical Intelligence, Schwabmunchen, Germany). The internal target volume (ITV) was contoured using computed tomography (CT) with a slow-scan technique with a rotation time of 4 s, considering the tumor motion assessed using X-ray fluoroscopy. Since October 2006, four-dimensional (4D) CT was available, thereafter 4DCT images were also acquired to determine ITV. The PTV was defined as the ITV with an additional 5-mm margin for setup uncertainty. The treatment beam was collimated to the PTV with a 5-mm margin using a multileaf collimator to ensure the peripheral dose of the PTV. In all cases, irradiation was applied with five to eight noncoplanar static beams with a 6-MV X-ray using a Clinac 2300 C/D (Varian Medical Systems) for patients treated before April 2008 and a Novalis system (BrainLAB, Feldkirchen, Germany) thereafter. All patients were set up with skeletal anatomy using megavoltage portal imaging or the Novalis kilovoltage imaging system.
Follow-up and local assessment
The regular follow-up visits and chest CT were performed as reported [6]. LR was diagnosed on the basis of enlargement of the local tumor on CT that continued for at least 6 months or histologic confirmation. 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) was recommended when LR was suspected, but this was not mandatory. For patients who could tolerate salvage surgery, LR was confirmed at surgical resection.
Dose–volume evaluation
The SBRT plans that were used clinically were recalculated using Eclipse ver. 8.6 using the monitor units irradiated clinically under the following three different conditions of heterogeneity correction: (1) pencil beam convolution with heterogeneity correction using BPL version 8.6.15; (2) AAA with heterogeneity correction version 8.6.15; and (3) pencil-beam convolution with NC version 8.6.15. First, the dose–volume metrics of the PTV under each algorithm were compared. The evaluated parameters were the dose at the IC (IC dose), PTV D95, the minimum dose of the PTV (PTV Min) and the mean dose of the PTV (PTV Mean). Then, these dose–volumetric parameters were compared between LC and LR groups under each algorithm. This comparison was performed for whole patients and for stratified groups according to the T-stage.
Statistical analysis
All statistical analysis was performed using IBM SPSS Statistics version 19 (IBM, Armonk, NY, USA). Tukey's honestly significant difference test was used to assess the differences among the three algorithms. Student's t-test and Pearson's chi-square test were used to assess difference between the LC and LR groups. When the P-value was <0.05, the difference was deemed statistically significant.
RESULTS
Comparison of the dose–volume metrics under different algorithms
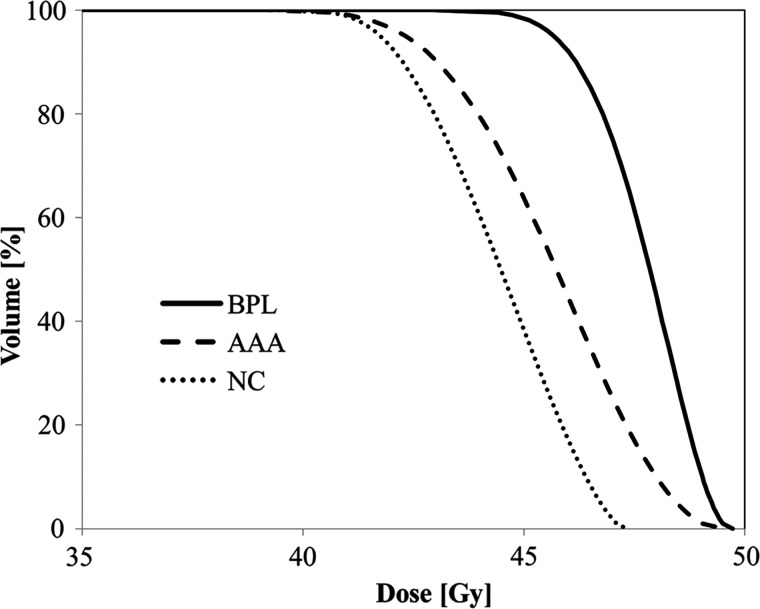
The averaged dose–volume histograms (DVHs) for the PTV under the three algorithms are plotted in Fig. 1, and the mean values and ranges of the dose–volume metrics under each algorithm are shown in Table 2.
Fig. 1.
The averaged dose–volume histogram for the PTV under BPL, AAA and NC
BPL, under heterogeneity correction with the Batho power law; AAA, under heterogeneity correction using the anisotropic analytical algorithm; NC, no heterogeneity correction.
Table 2.
Mean values [range] of the dose–volume metrics under each algorithm
| Metrics | BPL | AAA | NC |
P-values |
||
|---|---|---|---|---|---|---|
| BPL-AAA | BPL-NC | AAA-NC | ||||
| IC dose (Gy) | 48.2 [47.9–48.5] | 48.4 [43.1–49.6] | 44.9 [41.7–48.2] | 0.464 | <0.001 | <0.001 |
| PTV D95 (Gy) | 45.7 [40.9–46.8] | 42.3 [36.1–46.3] | 41.7 [37.9–45.4] | <0.001 | <0.001 | 0.034 |
| PTV Min (Gy) | 42.9 [36.2–45.3] | 39.2 [30.9–43.4] | 39.3 [33.5–43.7] | <0.001 | <0.001 | 0.726 |
| PTV Mean (Gy) | 47.7 [46.9–48.4] | 45.6 [38.8–49.1] | 44.4 [41.1–47.1] | <0.001 | <0.001 | <0.001 |
IC dose, the dose at the isocenter; PTV D95, the dose that covers 95% of the PTV; PTV Min, the minimum dose of the PTV; PTV Mean, the mean dose of the PTV. The other abbreviations are as in Fig. 1.
The mean IC dose under BPL, AAA and NC was 48.2, 48.4 and 44.9 Gy, respectively. Under NC, the IC dose was significantly lower than under the other two algorithms (P < 0.001), whereas there was no significant difference in the IC dose between BPL and AAA (P = 0.464). The PTV D95 under BPL was significantly higher than that under AAA and NC (P < 0.001), whereas the PTV D95 under AAA was higher than that under NC (P = 0.034), although the difference was small (mean value 42.3 vs. 41.7 Gy, respectively). For PTV Min, the value under BPL was significantly higher than under AAA and NC (P < 0.001), whereas there was no significant difference between AAA and NC. The PTV Mean under BPL was the highest of the three algorithms, and the value under AAA was higher than that under NC (P < 0.001). Under BPL, the mean PTV D95, PTV Min and PTV Mean were 8.0, 9.4 and 7.4% higher than under AAA, and 9.6, 9.2 and 4.6% higher than under NC, respectively.
Under AAA, the dose–volume metrics were related to the tumor diameter. Dividing the patients according to T-stage, the IC dose, PTV D95, PTV Min and PTV Mean were significantly lower in the T1a patients than those values in patients with larger tumors, 47.8 vs. 48.7 Gy (P = 0.002), 41.2 vs. 43.0 Gy (P < 0.001), 38.1 Gy vs. 39.7 (P = 0.001) and 44.4 Gy vs. 46.3 Gy (P < 0.001), respectively. No significant difference was observed in those parameters between T1b and T2a. Accordingly, the difference in the dose–volume metrics between BPL and AAA was larger in smaller tumors; the mean PTV D95, PTV Min and PTV Mean calculated under BPL were 11.4, 13.3 and 12.8% higher than the respective values under AAA in the T1a patients.
Status of local control
With a median follow-up of 35.9 (range 13.6–81.9) months, LR was observed in 18 patients (21.7%). The median time to LR was 17.5 (range 7.4–55.0) months. LR was confirmed at salvage surgery in six patients, diagnosed radiologically with CT and FDG-PET in six patients, and diagnosed with CT only in six patients. The patient characteristics in the LR and LC groups are shown in Table 1. Tumor diameter and PTV volume in LR tended to be greater than in LC, but no significant difference was observed in the clinical factors between the LC and LR groups.
Comparison of the dose–volume metrics between the LC and LR groups
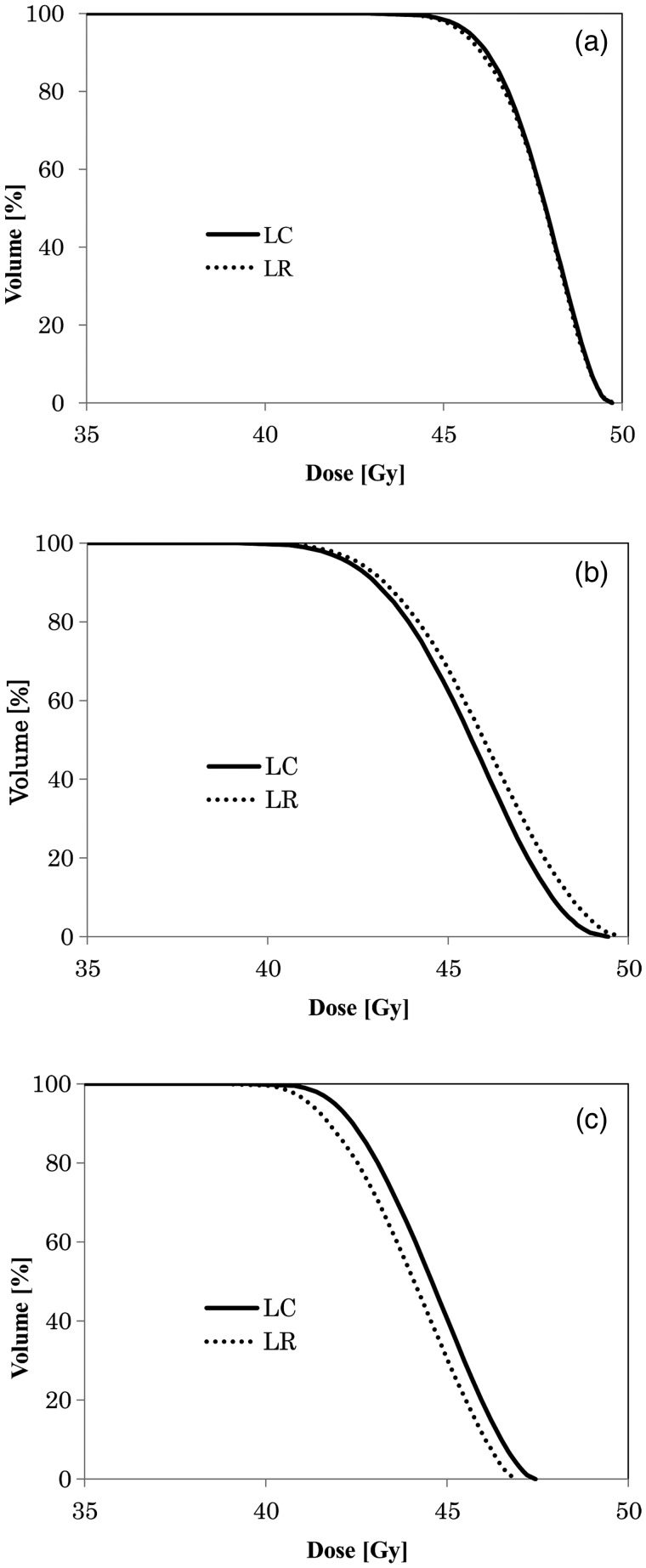
The averaged DVHs for the PTV in the LC and LR groups under the three algorithms are plotted in Fig. 2, and the mean values and ranges of the dose–volume metrics are shown in Table 3. No significant difference between the LC and LR groups was observed in any of the metrics. Consequently, we stratified the patients into T1a, T1b and T2a and re-evaluated the dose–volume parameters between the LC and LR groups; however, none of the dose–volume metrics differed significantly.
Fig. 2.
Averaged dose–volume histogram under each heterogeneity correction status in LC and LR under BPL (a), AAA (b) and NC (c).
LC, patients with local lesion control; LR, patients who developed local. The other abbreviations are same as in Fig. 1.
Table 3.
The mean values [range] of the dose–volume metrics in the LC and LR groups
| Algorithms | Metrics | LC (n = 67) | LR (n= 16) | P-values LC vs. LR |
|---|---|---|---|---|
| BPL | IC dose (Gy) | 48.2 [47.9–48.5] | 48.2 [47.9–48.4] | 0.386 |
| PTV D95 (Gy) | 45.7 [43.9–46.8] | 45.5 [40.9–46.5] | 0.604 | |
| PTV Min (Gy) | 42.9 [37.7–45.3] | 42.8 [36.2–44.7] | 0.881 | |
| PTV Mean (Gy) | 47.7 [46.9–48.4] | 47.7 [46.9–48.2] | 0.550 | |
| AAA | IC dose (Gy) | 48.3 [43.1–49.6] | 48.6 [47.4–49.5] | 0.288 |
| PTV D95 (Gy) | 42.3 [36.1–46.3] | 42.5 [39.5–45.2] | 0.598 | |
| PTV Min (Gy) | 39.1 [30.9–43.4] | 39.4 [36.4–42.4] | 0.596 | |
| PTV Mean (Gy) | 45.5 [38.8–49.1] | 45.9 [43.2–48.5] | 0.450 | |
| NC | IC dose (Gy) | 45.0 [41.8–48.2] | 44.5 [41.7–47.9] | 0.239 |
| PTV D95 (Gy) | 41.9 [38.4–44.6] | 41.2 [37.9–45.4] | 0.120 | |
| PTV Min (Gy) | 39.5 [33.6–42.6] | 38.8 [33.5–43.7] | 0.221 | |
| PTV Mean (Gy) | 44.5 [41.1–47.1] | 44.0 [41.1–47.1] | 0.214 |
The abbreviations are as in Tables 1 and 2.
DISCUSSION
The dose–volume metrics differed significantly among the algorithms we used for heterogeneity correction. Task Group No. 65 (TG-65) of the Radiation Therapy Committee of the American Association of Physicists in Medicine [13] classified the algorithms for heterogeneity correction into Categories 1 to 4 according to the level of anatomy sampled for the scatter calculation and the inclusion or exclusion of electron transport. BPL is classified as a Category 1 algorithm (no electron transport and one-dimensional density sampling) and AAA as Category 4 (electron transport and three-dimensional density sampling). In a phantom study, Bragg and Conway [14] reported that the difference between the dose calculated using AAA and the dose measured experimentally was within 2.5% or 2 mm in the presence of heterogeneity. Ronde and Hoffmann [15] showed the superiority of AAA over BPL and demonstrated that the dose deviation in lung lesions under AAA was <3% for most plans. The TG-65 [13] recommends that a Category 4 algorithm be considered in order to ascertain the dosage at tumor/lung interfaces in radiation planning for the lung and that simplistic one-dimensional equivalent path corrections are reasonable only for point dose estimations for lung tumors.
To our knowledge, our report is the largest study to compare the dose–volume metrics under BPL, AAA and NC directly using clinical plans. Our results regarding the difference in dose–volume metrics with heterogeneity correction status concur with reports based on fewer cases. Xiao et al. [19] compared 20 plans in the RTOG protocol and the same plans calculated using superposition/convolution algorithms. The volume of the PTV receiving the prescribed dose decreased by >10%, and the PTV D95 decreased by 4.7 Gy with heterogeneity correction. Ding et al. [16] recalculated 10 lung SBRT treatment plans under AAA and the modified Batho, which was originally calculated under NC. The calculated dose distributions near the interface under AAA agreed with those from a Monte Carlo simulation and measured values. The difference between AAA and NC in the PTV D95 was within 10%, whereas there were differences of up to 45% in the PTV D95 between NC and the modified Batho. Schuring and Hurkmans [20] evaluated 26 plans optimized under NC and the equivalent path length (EPL) by recalculating them using superposition/convolution algorithms. They concluded that the dose to the PTV margin was overestimated under the EPL, and the overestimation of the dose increased with decreasing PTV size. As we found no significant difference in the IC dose between the BPL and AAA, the BPL is acceptable for calculating the IC dose for a tumor of a certain size. However, the BPL cannot accurately predict the peripheral dose of the PTV (Min or D95). In our study, the PTV D95 and PTV Min calculated under the BPL were overestimated compared with the AAA by 8.0% and 9.4% in all patients and by 11.4% and 13.3% in the T1a patients.
Although we evaluated IC dose and peripheral dose of the PTV, we could not find any correlation between either of the dose–volume metrics and the LC rate, even when reevaluated under AAA. The doses at the IC and PTV margin are thought to affect the LC rate after SBRT [7, 11]. Onishi et al. [7] retrospectively reviewed 257 patients with stage I NSCLC treated with SBRT using various dose-fractionation schedules and evaluated the LC rate according to BED at IC. The LR rate was significantly lower for patients with a BED10Gy ≥100 Gy compared with those with a BED10Gy <100 Gy (8.4 vs. 42.9%). Wulf et al. [11] evaluated the relationship between LC status and different dose-fractionation schedules in 81 patients with 92 lung lesions and determined the dose–response curve using BED. They figured out that the estimated 50% probability of tumor control dose with BED10Gy was 49.9 Gy at the periphery of PTV and 94.2 Gy at the IC. They also evaluated the data from several published studies and concluded that the peripheral dose to the PTV was the significant factor determining LC.
Our negative result in correlation between LC and dose–volume factors is attributed to several factors. First, the variations in dose distribution might be too small to find any differences affecting clinical outcome. Both Onishi et al. [7] and Wulf et al. [11] showed the effect of dose–volume metrics on LR rate comparing different dose-fractionation schedules. In the Onishi study, BED10Gy ranged from 57.6 Gy to 180 Gy at IC. Similarly, in the Wulf study, BED10Gy ranged from 93.6 Gy to 262.5 Gy at IC and from 64 Gy to 180 Gy at PTVD95. On the other hand, our study used a uniform dose-fractionation of 48 Gy in four fractions at the IC under BPL. Under AAA, the IC dose ranged from 43.1 Gy to 49.6 Gy, the equivalent of 89.5 Gy to 111.1 Gy in BED10Gy, and PTV D95 ranged from 36.1 Gy to 46.3 Gy, the equivalent of 68.7 Gy to 99.9 Gy in BED10Gy, respectively.
Second, the calculated dose distributions have been different from the delivered dose distributions in the patients, even using more advanced algorithms. Although AAA is superior to BPL for heterogeneity correction, it still has a calculation error [14, 15]. In our previous report, most LR occurred within 3 years and LR rate was 13.2% at both 3 and 5 years [6]. This means it takes at least 3 years to evaluate the clinical outcome of LR rate appropriately. To assess the relationship between dose–volume metrics and LR, the patients who were treated several years ago were also included in this study and the median follow-up duration in our study reached 35.9 months. At that time, a more correct calculation algorithm, the Monte Carlo simulation, was not available in a commercial radiation treatment planning system, and it is difficult to recalculate dose distribution in another calculation system with Monte Carlo.
Third, there are limitations to evaluating the dose distribution of lung tumors, which displace and deform intrafractionally due to the respiratory motion, and also from day to day. The range of respiration-induced lung tumor motion is sometimes more than 3 cm [21]. To compensate for the influence of this intrafractional tumor motion, Guckenberger et al. tried to evaluate the dose to target volume with 4D dose calculation and stated that the 3D dose at PTV margins underestimated the 4D dose [22]. Then, with the quotients between 4D doses to the clinical target volume (CTV) D95 and 3D doses to PTV D95, they also evaluated the relationship between CTV dose and LR rate comparing different dose-fractionation schedules and concluded that BED10Gy to the CTV >100 Gy resulted in excellent local control based on 4D dose calculation [23]. In addition, the day-to-day positional errors also affect the dose distribution. Michalski et al. [21] also assessed the intrafractional reproducibility of respiratory motion using 23 pairs of repeated 4DCT tumor motion, and showed that three cases did not exhibit similarity of tumor motion on different days. Galerani et al. [24] investigated the dosimetric impact of online cone-beam CT (CBCT) guided positional correction. They showed that without CBCT-guided positional correction the target dose reduced with respect to those of the planning, and the means and standard deviations of the reduction in GTV D99 and GTV D95 were –3.2 ± 4.9% and –2.1 ± 4.4%, respectively.
These errors may have obscured the small difference in the dose distribution between the LC and LR groups, and led to the negative result of this study. We might need a more adaptive dose evaluation system to identify the factors in the dose–volume metrics that affect the LC rate in the future.
FUNDING
This work was supported by the Japan Society for the Promotion of Science (JSPS) through the ‘Funding Program for World-leading Innovative R&D on Science and Technology’ (FIRST Program).
REFERENCES
- 1.Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–70. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 2.Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer. 1998;82:1062–70. doi: 10.1002/(sici)1097-0142(19980315)82:6<1062::aid-cncr8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 3.Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for early stage non-small cell lung cancer: results of a prospective trial. Lung Cancer. 2010;68:72–7. doi: 10.1016/j.lungcan.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–82. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 5.Kopek N, Paludan M, Petersen J, et al. Co-morbidity index predicts for mortality after stereotactic body radiotherapy for medically inoperable early-stage non-small cell lung cancer. Radiother Oncol. 2009;93:402–7. doi: 10.1016/j.radonc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;79:1104–11. doi: 10.1016/j.ijrobp.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 8.Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290–6. doi: 10.1200/JCO.2008.21.5681. [DOI] [PubMed] [Google Scholar]
- 9.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–6. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagata Y, Hiraoka M, Shibata T, et al. A phase ii trial of stereotactic body radiation therapy for operable T1N0M0 non-small cell lung cancer: Japan Clinical Oncology Group (JCOG0403) Int J Radiat Oncol Biol Phys. 2010;78:S27–8. doi: 10.1016/j.ijrobp.2015.07.2278. [DOI] [PubMed] [Google Scholar]
- 11.Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. 2005;77 doi: 10.1016/j.radonc.2005.09.003. 8–7. [DOI] [PubMed] [Google Scholar]
- 12.Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45:787–95. doi: 10.1080/02841860600904862. [DOI] [PubMed] [Google Scholar]
- 13.Papanikolaou N, Battista JJ, Boyer AL, et al. AAPM Report No. 85: Tissue Inhomogeneity Corrections for Megavoltage Photon Beams - Report of Task Group No. 65 of the Radiation Therapy Committee of the American Association of Physicists in Medicine. Madison: Medical Physics Publishing; 2004 [Google Scholar]
- 14.Bragg CM, Conway J. Dosimetric verification of the anisotropic analytical algorithm for radiotherapy treatment planning. Radiother Oncol. 2006;81:315–23. doi: 10.1016/j.radonc.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Ronde HS, Hoffmann L. Validation of Varian's AAA algorithm with focus on lung treatments. Acta Oncol. 2009;48:209–15. doi: 10.1080/02841860802287108. [DOI] [PubMed] [Google Scholar]
- 16.Ding GX, Duggan DM, Lu B, et al. Impact of inhomogeneity corrections on dose coverage in the treatment of lung cancer using stereotactic body radiation therapy. Med Phys. 2007;34:2985–94. doi: 10.1118/1.2745923. [DOI] [PubMed] [Google Scholar]
- 17.Takayama K, Nagata Y, Negoro Y, et al. Treatment planning of stereotactic radiotherapy for solitary lung tumor. Int J Radiat Oncol Biol Phys. 2005;61:1565–71. doi: 10.1016/j.ijrobp.2004.12.066. [DOI] [PubMed] [Google Scholar]
- 18.Hiraoka M, Matsuo Y, Takayama K. Stereotactic body radiation therapy for lung cancer: achievements and perspectives. Jpn J Clin Oncol. 2010;40:846–54. doi: 10.1093/jjco/hyq134. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I–II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;73:1235–42. doi: 10.1016/j.ijrobp.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuring D, Hurkmans CW. Developing and evaluating stereotactic lung RT trials: what we should know about the influence of inhomogeneity corrections on dose. Radiat Oncol. 2008;3:21. doi: 10.1186/1748-717X-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michalski D, Sontag M, Li F, et al. Four-dimensional computed tomography-based interfractional reproducibility study of lung tumor intrafractional motion. Int J Radiat Oncol Biol Phys. 2008;71:714–24. doi: 10.1016/j.ijrobp.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 22.Guckenberger M, Wilbert J, Krieger T, et al. Four-dimensional treatment planning for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2007;69:276–85. doi: 10.1016/j.ijrobp.2007.04.074. [DOI] [PubMed] [Google Scholar]
- 23.Guckenberger M, Wulf J, Mueller G, et al. Dose-response relationship for image-guided stereotactic body radiotherapy of pulmonary tumors: relevance of 4D dose calculation. Int J Radiat Oncol Biol Phys. 2009;74:47–54. doi: 10.1016/j.ijrobp.2008.06.1939. [DOI] [PubMed] [Google Scholar]
- 24.Galerani AP, Grills I, Hugo G, et al. Dosimetric impact of online correction via cone-beam CT-based image guidance for stereotactic lung radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1571–8. doi: 10.1016/j.ijrobp.2010.02.012. [DOI] [PubMed] [Google Scholar]